Abstract
Objective:
MicroRNAs (miRNAs) have been found to play important roles in the development of non-small cell lung carcinoma (NSCLC). The aim of this study was to analyze the expression and clinical value of serum miR-185 in NSCLC.
Methods:
Serum miR-185 levels were detected in 146 NSCLC patients, 50 patients with carcinoma in situ, 25 patients with non-malignant lung diseases (NMLD), and 80 healthy controls using quantitative reverse transcription PCR. The correlation between serum miR-185 level and clinical status of NSCLC was explored.
Results:
The results revealed that serum miR-185 expression was progressively decreased in healthy controls, patients with NMLD, patients with carcinoma in situ and NSCLC patients. In addition, compared to carcinoembryonic antigen (CEA), serum miR-185 demonstrated better diagnostic accuracy for discriminating patients with carcinoma from healthy controls, NSCLC patients from healthy controls and NSCLC patients from patients with carcinoma in situ. In addition, serum miR-185 levels were significantly elevated in post-treated samples compared to the pre-treated samples. Moreover, reduced serum miR-185 was closely associated with unfavorable clinicopathological parameters and worse survival. Univariate and multivariate cox regression analysis confirmed that serum miR-185 was an independent prognostic indicator for NSCLC.
Conclusions:
Collectively, our findings have demonstrated that serum miR-185 might serve as a promising and robust biomarker for the early detection and prognosis prediction of NSCLC.
Keywords: serum miR-185, non-small cell lung cancer, biomarker, prognosis, diagnosis
Introduction
Non-small-cell lung cancer (NSCLC) accounts for about 80-85% of lung cancer, which is the most frequent cause of cancer-related death worldwide.1,2 In 2018, it was reported that there were more than 2 million new cases and more than 1.7 million new deaths of lung cancer.3 As no obvious symptoms can be detected or observed at the early stages, most of NSCLC patients are diagnosed at the advanced stages when local/distant metastasis has occurred. Despite clinical treatment strategies have been significantly improved over the past few decades, the overall 5-year survival rate of NSCLC remains very dismal.4,5 Thus, it is urgent to identify novel and reliable biomarkers for the diagnosis and prognosis prediction of NSCLC.
MicroRNAs (miRNAs) are a class of single stranded small non-coding RNAs that regulate gene expression at the post-transcriptional level by binding to the 3’-untranslated region (3’-UTR) of target mRNAs, resulting in translational suppression or degradation.6,7 MiRNAs are found to involve in various biological processes, such as cell proliferation, growth, differentiation, apoptosis, transformation and cell metabolism.8 Accumulating evidence have shown that miRNAs can be stably detected in the blood samples, and are promising candidate biomarkers for NSCLC diagnosis, treatment and prognosis prediction. For instance, Wang et al found that serum miR-411 levels were highly expressed in patients with NSCLC. In addition, serum miR-411 upregulation predicted poor prognosis of NSCLC.9 Conversely, Sun et al reported that low serum miR-770 expression occurred more frequently in NSCLC. Downregulation of serum miR-770 was significantly associated with shorter survival and aggressive clinical parameters.10
MiR-185 have been previously identified as a tumor suppressive miRNA in NSCLC.11-14 However, currently little information is available for the potential clinical value of serum miR-185 in NSCLC. Here, the aim of this study was to measure the expression level of serum miR-185 in NSCLC, and then further explore its potential diagnostic and prognostic value.
Material and Methods
Ethics Statement
This study was approved by the Ethics Committee of Tianjin Medical University General Hospital (Approval number: 2015066) and written informed consent was obtained from each participant. All specimens were handled and made anonymous according to the ethical and legal standards.
Study Population and Clinical Samples
The current study included 146 patients with NSCLC, 50 patients with carcinoma in situ, 25 patients with non-malignant lung diseases (NMLD) and 80 healthy controls. The cases in the above 4 groups were age and gender matched. All NSCLC patients were staged based on American Joint Committee on Cancer (AJCC) tumor-node-metastasis (TNM) staging system. Patients who had received any treatment prior to first-time blood sample collection were excluded from this study. 115 NSCLC patients received surgery and chemo/radiotherapy, while the remaining 31 cases received chemo/radiotherapy. The demographic characteristics of NSCLC patients were shown in Table 1. Clinical follow-up was available for all patients. Overall survival (OS) was defined as the time from diagnosis to death. Recurrent free survival (RFS) was defined as the time from diagnosis to recurrence.
Table 1.
The Correlations Between Serum miR-185 Expression and Clinical Parameters in NSCLC.
| Parameters | Number | Serum miR-185 expression | P | |
|---|---|---|---|---|
| Low | High | |||
| Gender | 0.169 | |||
| Male | 94 | 51 | 43 | |
| Female | 52 | 22 | 30 | |
| Age | 0.615 | |||
| <60 | 85 | 44 | 41 | |
| ≥60 | 61 | 29 | 32 | |
| Smoking status | 0.376 | |||
| Never | 47 | 21 | 26 | |
| Ever | 99 | 52 | 47 | |
| Histology | 0.499 | |||
| AD | 88 | 42 | 46 | |
| SCC | 58 | 31 | 27 | |
| Differentiation | 0.052 | |||
| Well | 11 | 4 | 7 | |
| Moderate | 84 | 36 | 44 | |
| Poor | 51 | 33 | 18 | |
| Lymph node metastasis | 0.003 | |||
| Negative | 68 | 25 | 43 | |
| Positive | 78 | 48 | 30 | |
| Tumor size | 0.021 | |||
| <5 cm | 110 | 49 | 61 | |
| ≥ 5 cm | 36 | 24 | 12 | |
| TNM stage | <0.001 | |||
| I/II | 92 | 35 | 57 | |
| III/IV | 54 | 38 | 16 | |
AD: adenocarcinoma; SCC: squamous cell carcinoma.
Up to 5 mL whole blood samples were obtained from all the participants. For the NSCLC patients receiving surgery resection + chemo/radiotherapy, the post-treated blood samples were collected 4 weeks after the surgery. For the NSCLC patients receiving only chemo/radiotherapy, the post-treated blood samples were collected after 4 chemotherapy cycles. The blood samples were centrifugated at 2500 g for 10 min. Then the supernatants were collected and stored at −80°C until further analysis.
RNA Isolation and Quantitative Reverse Transcription PCR
Total RNA was extracted from 200 µL serum sample using mirVana miRNA isolation Kit (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s protocol. The RNA purity and concentration were determined with a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). Reverse transcription was performed using The PrimeScript RT reagent kit (Takara, Dalian, China). Cycle amplification was carried out using SYBR Premix Ex TaqTM II (Takara) with ABI PRISM 7900 Sequence Detection System (Applied Biosystems). The conditions of PCR amplification were: denaturation at 95°C for 10 min, followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min. During the RNA isolation procedure, cel-miR-39 was added to each sample as a spike-in control. Each sample was measured in triplicate, and the relative serum miR-185 expression was calculated using 2–ΔΔCt method. The primers were as follows: miR-185 forward: 5′-CAATGGAGAGAAAGGCAGTTCC-3′, miR-185 reverse: 5′-AATCCATGAGAGATCCCTACCG-3′; cel-miR-39 forward: 5′-UCACCGGGUGUAAAUC AGCUUG-3′. The reverse primer of cel-miR-39 was a universal reverse primer.
Carcinoembryonic Antigen (CEA) Assay
Serum levels of CEA were detected using Human Carcino Embryonic Antigen ELISA Kit (Abcam, Cambridge, MA, USA) based on the manufacturer’s instructions. Each sample was repeated 3 times.
Statistical Analysis
Statistical analysis was performed with the GraphPad Prism 8.0.2 (GraphPad Software, San Diego, CA, USA) and MedCalc 19.0.7 (MedCalc Software, Ostend, Belgium). The serum miR-185 levels in different groups were analyzed by the Mann-Whitney U test or Kruskal-Wallis test. The chi-square test was performed to evaluate the association between serum miR-185 levels and clinicopathological characteristics. The area under the receiver operating characteristic (ROC) curve (AUC) value was used to evaluate the diagnostic efficacy of serum miR-185. OS and RFS rates were analyzed by the Kaplan-Meier analysis, and log-rank test was used to compare the differences. Univariate and multivariate s were performed to identify the independent prognostic indicators. P < 0.05 was considered to indicate a statistically significant difference.
Results
Serum miR-185 Expression in NSCLC Patients and Controls
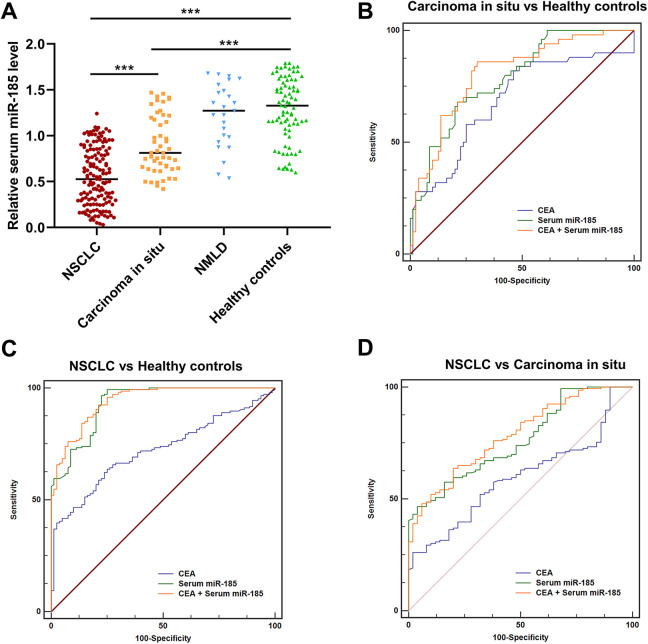
The qRT-PCR was performed to detect serum miR-185 expression levels in all participants. The serum miR-185 expression levels were progressively reduced in healthy controls, patients with NMLD, patients with carcinoma in situ and patients with NSCLC. However, no significant difference was observed between patients with NMLD and healthy controls (***P < 0.001, Figure 1A). Compared to CEA (AUC = 0.687, 95%CI = 0.600-0.766, sensitivity = 82.0%, specificity = 53.7%), serum miR-185 exhibited better performance for discriminating patients with carcinoma in situ from healthy controls, with an AUC value of 0.790 (95%CI = 0.709-0.856, sensitivity = 68.0%, specificity = 78.7%). Combing serum miR-185 and CEA slightly increased the AUC value to 0.808 (95%CI = 0.730-0.872, sensitivity = 86.0%, specificity = 70.0%) (Figure 1B). Similarly, the AUC values of CEA, serum miR-185 and CEA + serum miR-185 for discriminating NSCLC patients from healthy controls were 0.721 (95%CI = 0.657-0.778, sensitivity = 63.7%, specificity = 75.0%), 0.932 (95%CI = 0.892-0.962, sensitivity = 97.3%, specificity = 75.0%) and 0.942 (95%CI = 0.903-0.969, sensitivity = 92.5%, specificity = 78.7%), respectively (Figure 1C). The AUC values of CEA, serum miR-185 and CEA + serum miR-185 for differentiating NSCLC patients from patients with carcinoma in situ were 0.594 (95%CI = 0.521-0.663, sensitivity = 22.0%, specificity = 98.0%), 0.762 (95%CI = 0.696-0.820, sensitivity = 46.6%, specificity = 96.0%) and 0.784 (95%CI = 0.720-0.839, sensitivity = 63.7%, specificity = 80.0%), respectively (Figure 1D).
Figure 1.
Serum miR-185 was reduced in NSCLC. (A) The expression level of serum miR-185 was progressively decreased in healthy controls, patients with carcinoma in situ and patients with NSCLC. (B) The diagnostic values of CEA, serum miR-185, and CEA + serum miR-185 for differentiating patients with carcinoma in situ from healthy controls. (C) The diagnostic values of CEA, serum miR-185, and CEA + serum miR-185 for identifying NSCLC patients from healthy controls. (D) The diagnostic values of CEA, serum miR- 185, and CEA + serum miR-185 for identifying NSCLC patients from patients with carcinoma in situ.
Serum miR-185 Expression and Clinical Variables in NSCLC
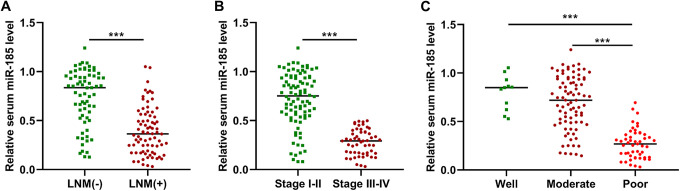
Compared to the respective controls, the expression levels of serum miR-185 were significantly lower in the NSCLC patients with lymph node metastasis, at the advanced stage or with poor differentiation (Figure 2A-C, P < 0.001). The association between serum miR-185 expression and clinical parameters of NSCLC was then analyzed. All NSCLC samples were divided into low serum miR-185 expression group (n = 73) and high serum miR-185 expression group (n = 73) according to the median serum miR-185 level. As shown in Table 1, serum miR-185 expression was associated with tumor size (P = 0.021), lymph node metastasis (P = 0.003) and TNM stage (P < 0.001). However, no significant correlation was observed between serum miR-185 expression and other clinicopathological features, such as gender (P = 0.169), age (P = 0.615), smoking status (P = 0.376), histology (P = 0.499) and differentiation (P = 0.052).
Figure 2.
The correlation between serum miR-185 and clinical parameters of NSCLC. (A-C) The expression level of serum miR-185 was dramatically lower in the NSCLC cases with lymph node metastasis, at the advanced stage or with poor differentiation.
Serum miR-185 Level Was Increased Following Treatments
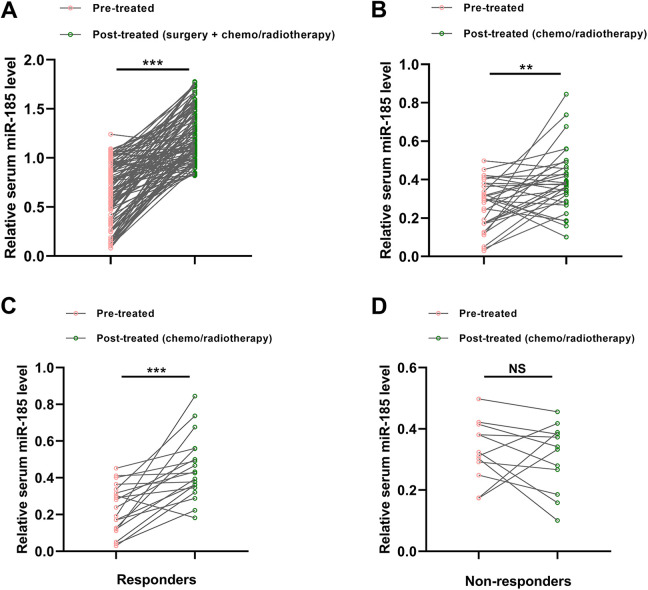
The expression levels of serum miR-185 were compared between paired pre- and post-treated samples. Our results showed that the serum miR-185 levels were markedly increased in the post-treated samples from the patients receiving surgery and chemo/radiotherapy (***P < 0.001, Figure 3A). The expression level of serum miR-185 was also increased in those receiving only chemo/radiotherapy (**P < 0.01, Figure 3B), but to a lesser extent. In the patients receiving only chemo/radiotherapy, 19 cases were sensitive to the treatments, while the remaining 12 cases were resistant to the therapy. For responders, the level of serum miR-185 was significantly increased following chemo/radiotherapy (***P < 0.001, Figure 3C). However, no significant different was found for serum miR-185 between the pre-treated and post-treated serum samples for non-responders (Figure 3D).
Figure 3.
Serum miR-185 was increased in NSCLC patients following treatment. (A) Serum miR-185 level was significantly upregulated in the patients receiving surgery and chemo/radiotherapy. (B) The expression level of serum miR-185 was also increased in the NSCLC cases receiving only chemo/radiotherapy, but to a lesser degree. (C) For responders, the level of serum miR-185 was significantly increased following chemo/radiotherapy. (D) No significant different was found for serum miR-185 between the pre-treated and post-treated serum samples for non-responders.
Prognostic Value of Serum miR-185 Expression in NSCLC
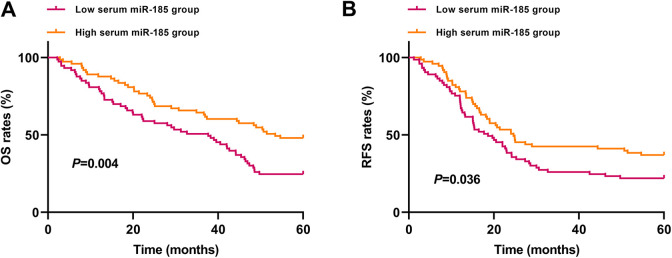
Kaplan-Meier survival analysis demonstrated that the OS rate was higher in the high serum miR-185 expression group than in the low serum miR-185 expression group (P = 0.004, Figure 4A). Similarly, NSCLC patients in the low serum miR-185 expression group had shorter RFS compared to those in the high serum miR-185 expression group (P = 0.036, Figure 4B).
Figure 4.
The association between serum miR-185 and survival of NSCLC. (A-B) The patients in the low serum miR-185 expression group had significantly shorter OS and RFS than those in the high serum miR- 185 expression group.
Univariate analysis showed that serum miR-185 expression (HR = 3.83, 95% CI = 1.57-6.32, P = 0.011), lymph node metastasis (HR = 3.12, 95% CI = 1.33-5.18, P = 0.019), and TNM stage (HR = 4.27, 95% CI = 1.76-7.03, P = 0.006) significantly affected OS of NSCLC patients. Multivariate analysis confirmed that serum miR-185 expression (HR = 3.62, 95% CI = 1.43-6.08, P = 0.013), lymph node metastasis (HR = 3.45, 95% CI = 1.40-5.66, P = 0.015), and TNM stage (HR = 4.67, 95% CI = 1.88-7.60, P = 0.002) were independently associated with the OS (Table 2). Similarly, serum miR-185 expression (HR = 2.47, 95% CI = 1.08-4.25, P = 0.039) and TNM stage (HR = 3.16, 95% CI = 1.32-5.37, P = 0.014) were the independent prognostic factors for the RFS of NSCLC (Table 3).
Table 2.
Univariate and Multivariate Analyses for OS of NSCLC Patients.
| Variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Serum miR-185 | 3.83 | 1.57-6.32 | 0.011 | 3.62 | 1.43-6.08 | 0.013 |
| Lymph node metastasis | 3.12 | 1.33-5.18 | 0.019 | 3.45 | 1.40-5.66 | 0.015 |
| TNM stage | 4.27 | 1.76-7.03 | 0.006 | 4.67 | 1.88-7.60 | 0.002 |
Table 3.
Univariate and Multivariate Analyses for RFS of NSCLC Patients.
| Variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Serum miR-185 | 2.81 | 1.21-4.82 | 0.021 | 2.47 | 1.08-4.25 | 0.039 |
| TNM stage | 3.55 | 1.41-6.19 | 0.007 | 3.16 | 1.32-5.37 | 0.014 |
Discussion
To the best of our knowledge, this is the first study to assess the diagnostic and prognostic value of serum miR-185 in NSCLC. We have demonstrated that serum miR-185 expression was significantly lower in NSCLC and showed good performance for the early detection of NSCLC. In addition, serum miR-185 levels were remarkably increased following treatments. Moreover, reduced serum miR-185 expression was strongly associated with worse clinical parameters and shorter survival. Serum miR-185 expression was an independent prognostic indicator for NSCLC. These data suggest that serum miR-185 might serve as a promising diagnostic and prognostic biomarker for NSCLC.
Our findings were in line with previous studies. For instance, miR-185 expression was significantly decreased both in NSCLC tissues and cell lines. Enforced miR-185 expression greatly inhibited cancer cell proliferation, invasion and migration in vitro and attenuated tumor growth in vivo by targeting SOX9 and AKT1.11,12 Likewise, Zhou et al revealed that miR-185 was markedly downregulated in cancerous tissues and cell lines. Overexpression of miR-185 significantly inhibited carcinogenesis of NSCLC and increased the chemo-sensitivity of cancer cells through regulating SOX13.13 Zhao et al showed that reduced miR-185 expression was correlated with lymph node metastasis. Restoration of miR-185 suppressed the epithelial-mesenchymal transition (EMT) process in vitro and restrained tumor growth in the xenograft model.14
MiR-185 have also been demonstrated to play a tumor suppressive role in many other cancer types. Reduced miR-185 expression was found in breast cancer tissues. In addition, miR-185 overexpression greatly restrained cell proliferation and stimulated cell apoptosis by degrading c-Met expression.15 MiR-185 expression was decreased both in hepatocellular carcinoma (HCC) tissues and cell lines. Reduced miR-185 expression was closely associated with poor prognosis of HCC. Enforced expression of miR-185 markedly suppressed the tumorigenesis of HCC by targeting Six2, CDC42 or DNMT1, and vice versa.16-18 Similarly, miR-185 was reduced in gastric cancer, and its downregulation was correlated with worse clinical variables. In vitro and in vivo evidence revealed that enforced miR-185 expression dramatically attenuated cell proliferation, metastasis and promoted cell apoptosis.19,20 Jing et al showed that miR-185 levels were significantly lower in esophageal squamous cell carcinoma (ESCC) patients compared to controls. Ectopic expression of miR-185 inhibited the oncogenic behaviors of ESCC cells in vitro and in vivo through regulating RAGE.21 Qu and colleagues demonstrated that upregulation of miR-185 not only suppressed the invasion and migration of prostate cancer cell in vitro, but also inhibited tumorigenicity in vivo.22 In colorectal cancer (CRC), miR-185 expression was remarkably decreased in CRC cell lines. In vitro analysis showed that enforced miR-185 expression significantly inhibited tumorigenesis of CRC and enhanced radiosensitivity of CRC cells.23,24 Based on these results, miR-185 might function as a tumor suppressor in different types of cancers.
In conclusion, serum miR-185 might serve as a reliable and non-invasive biomarker for the early detection and prognosis prediction of NSCLC. However, the sample size of this study was relatively small. Therefore, further studies with larger sample size are required to validate its clinical application.
Acknowledgments
This study was supported by Tianjin Medical University General Hospital Youth Incubation Fund (No. ZYYFY2017024)
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics statement: This study was approved by the Ethics Committee of Tianjin Medical University General Hospital (Approval number: 2015066).
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Sen Wei, MD  https://orcid.org/0000-0002-7874-4099
https://orcid.org/0000-0002-7874-4099
References
- 1. Gompelmann D, Eberhardt R, Herth FJ. Advanced malignant lung disease: what the specialist can offer. Respiration. 2011;82(2):111–123. [DOI] [PubMed] [Google Scholar]
- 2. Katlic MR, Facktor MA, Berry SA, McKinley KE, Bothe A, Jr, Steele GD., Jr ProvenCare lung cancer: a multi-institutional improvement collaborative. CA Cancer J Clin. 2011;61(6):382–396. [DOI] [PubMed] [Google Scholar]
- 3. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 4. Scagliotti GV, Novello S. Adjuvant therapy in completely resected non-small-cell lung cancer. Curr Oncol Rep. 2003;5(4):318–325. [DOI] [PubMed] [Google Scholar]
- 5. Reungwetwattana T, Weroha SJ, Molina JR. Oncogenic pathways, molecularly targeted therapies, and highlighted clinical trials in non-small-cell lung cancer (NSCLC). Clin Lung Cancer. 2012;13(4):252–266. [DOI] [PubMed] [Google Scholar]
- 6. Perron MP, Provost P. Protein interactions and complexes in human microRNA biogenesis and function. Front Biosci. 2008;13:2537–2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ambros V. The functions of animal microRNAs. Nature. 2004;431(7006):350–355. [DOI] [PubMed] [Google Scholar]
- 8. Kloosterman WP, Plasterk RH. The diverse functions of microRNAs in animal development and disease. Dev Cell. 2006;11(4):441–450. [DOI] [PubMed] [Google Scholar]
- 9. Wang SY, Li Y, Jiang YS, Li RZ. Investigation of serum miR-411 as a diagnosis and prognosis biomarker for non-small cell lung cancer. Eur Rev Med Pharmacol Sci. 2017;21(18):4092–4097. [PubMed] [Google Scholar]
- 10. Sun B, Liu HF, Ding Y, Li Z. Evaluating the diagnostic and prognostic value of serum miR-770 in non-small cell lung cancer. Eur Rev Med Pharmacol Sci. 2018;22(10):3061–3066. [DOI] [PubMed] [Google Scholar]
- 11. Lei Z, Shi H, Li W, et al. MiR 185 inhibits non small cell lung cancer cell proliferation and invasion through targeting of SOX9 and regulation of Wnt signaling. Mol Med Rep. 2018;17(1):1742–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li S, Ma Y, Hou X, et al. MiR-185 acts as a tumor suppressor by targeting AKT1 in non-small cell lung cancer cells. Int J Clin Exp Pathol. 2015;8(9):11854–11862. [PMC free article] [PubMed] [Google Scholar]
- 13. Zhou CW, Zhao WJ, Zhu YG, Zhao XD. MiR-185 inhibits tumor growth and enhances chemo-resistance via targeting SRY-related high mobility group box transcription factor 13 in non-small-cell carcinoma. Am J Transl Res. 2018;10(8):2600–2609. [PMC free article] [PubMed] [Google Scholar]
- 14. Zhao L, Zhang Y, Liu J, et al. MiR-185 inhibits cell proliferation and invasion of non-small cell lung cancer by targeting KLF7. Oncol Res. 2018;27(9):1015–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fu P, DU F, Yao M, Lv K, Liu Y. MicroRNA-185 inhibits proliferation by targeting c-Met in human breast cancer cells. Exp Ther Med. 2014;8(6):1879–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhu SM, Chen CM, Jiang ZY, Yuan B, Ji M, Wu FH, Jin J. MicroRNA-185 inhibits cell proliferation and epithelial-mesenchymal transition in hepatocellular carcinoma by targeting Six2. Eur Rev Med Pharmacol Sci. 2016;20(9):1712–1719. [PubMed] [Google Scholar]
- 17. Zhang Q, Chen Y, Liu K. MiR-185 inhibits cell migration and invasion of hepatocellular carcinoma through CDC42. Oncol Lett. 2018;16(3):3101–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Qadir XV, Han C, Lu D, Zhang J, Wu T. MiR-185 inhibits hepatocellular carcinoma growth by targeting the DNMT1/PTEN/Akt pathway. Am J Pathol. 2014;184(8):2355–2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tan Z, Jiang H, Wu Y, Xie L, Dai W, Tang H, Tang S. MiR-185 is an independent prognosis factor and suppresses tumor metastasis in gastric cancer. Mol Cell Biochem. 2014;386(1-2):223–231. [DOI] [PubMed] [Google Scholar]
- 20. Fan L, Tan B, Li Y, et al. Upregulation of miR 185 promotes apoptosis of the human gastric cancer cell line MGC803. Mol Med Rep. 2018;17(2):3115–3122. [DOI] [PubMed] [Google Scholar]
- 21. Jing R, Chen W, Wang H, et al. Plasma miR-185 is decreased in patients with esophageal squamous cell carcinoma and might suppress tumor migration and invasion by targeting RAGE. Am J Physiol Gastrointest Liver Physiol. 2015;309(9):G719–G729. [DOI] [PubMed] [Google Scholar]
- 22. Qu F, Cui X, Hong Y, et al. MicroRNA-185 suppresses proliferation, invasion, migration, and tumorigenicity of human prostate cancer cells through targeting androgen receptor. Mol Cell Biochem. 2013;377(1-2):121–130. [DOI] [PubMed] [Google Scholar]
- 23. Dong-Xu W, Jia L, Su-Juan Z. MicroRNA-185 is a novel tumor suppressor by negatively modulating the Wnt/β-catenin pathway in human colorectal cancer. Indian J Cancer. 2015;52(Suppl 3):E182–E185. [DOI] [PubMed] [Google Scholar]
- 24. Afshar S, Najafi R, Sedighi Pashaki A, et al. MiR-185 enhances radiosensitivity of colorectal cancer cells by targeting IGF1 R and IGF2. Biomed Pharmacother. 2018;106:763–769. [DOI] [PubMed] [Google Scholar]