Abstract
Interactions between solvents and solutes are a cornerstone of physical organic chemistry and have been the subject of investigations over the last century. In recent years, a renewed interest in fundamental aspects of solute–solvent interactions has been sparked in the field of supramolecular chemistry in general and that of supramolecular polymers in particular. Although solvent effects in supramolecular chemistry have been recognized for a long time, the unique opportunities that supramolecular polymers offer to gain insight into solute–solvent interactions have become clear relatively recently. The multiple interactions that hold the supramolecular polymeric structure together are similar in strength to those between solute and solvent. The cooperativity found in ordered supramolecular polymers leads to the possibility of amplifying these solute–solvent effects and will shed light on extremely subtle solvation phenomena. As a result, many exciting effects of solute–solvent interactions in modern physical organic chemistry can be studied using supramolecular polymers. Our aim is to put the recent progress into a historical context and provide avenues toward a more comprehensive understanding of solvents in multicomponent supramolecular systems.
Introduction
The interactions between solvents and their solutes are widely regarded as one of the most important topics in organic chemistry and are central in controlling solubility, reactivity, and structure.1 Although chemist use solvents every day in their laboratory to dissolve molecules, it is still an educated guess to select which solvent for which solute. Whereas the role of individual solvents with solutes has become well-documented, special attention is needed for solvent combinations. In many mixtures, solvent–solvent mesoscale phase separation occurs, for instance, in aqueous solutions of THF or alcohols, which influences chemical reactions and dissolution of solutes especially at the composition where these phase transitions occur. Several such examples are discussed in this Perspective. Through decades of detailed studies in physical organic chemistry, chemists arrived at a high level of understanding on the role of solvent properties, chemical structure, and the outcome of chemical reactions. The many exciting effects of solvents in supramolecular polymerizations make these “mostly forgotten” details worth revisiting.
The learning curve that led to the physical organic framework of solvent effects on chemical structure and reactivity of organic molecules was also present in polymer science. It is well-known that the solution-state conformations of synthetic polymers are strongly determined by the solvent quality. Small globular particles are present in bad solvents, and more extended chains are present in good solvents. A special place is occupied by theta solvents, in which the interactions between the solvent and polymer solute are of equal energy as the solute–solute interactions. The outcome of common procedures in material processing, such as annealing, molding, and electrospinning, strongly depends on solute–solvent interactions. As such, the noncovalent chemistry between solvents and their solutes is of crucial importance in many areas of industrial chemistry and materials science.
Solute–solvent interactions are of crucial importance in biology as well.2 The stability of the tertiary structure of folded biomacromolecules, such as polypeptides3 or polynucleotides,4 depends critically on interactions of the biomacromolecule with water both in the interior and at the exterior of the structure. Of more recent date are the exciting consequences of liquid–liquid phase transitions as observed in cellular systems,5 while the stabilizing role of water to balance dipoles in proteins is still a topic of discussion today.6 However, this Perspective will not cover these fascinating biochemical subjects but will focus on mostly synthetic supramolecular systems with the main emphasis on supramolecular polymers, as their cooperative nature opens the door to study subtle effects that are expressed at a mesoscopic level.
After their first intellectual conception as “Hochmolekulare Verbindungen” by Staudinger in 1920,7 macromolecules dominated the first century of polymer science. At the end of the last century, however, reports started to emerge on polymeric aggregates of repeating monomeric units that were not held together by covalent bonds but rather by supramolecular, noncovalent interactions.8−11 These examples marked the birth of a new branch in polymer science, the field of supramolecular polymers, with seminal contributions by Lehn, Aida, Stupp, and many others.12
In supramolecular polymers, the repeating monomeric units of the polymer are held together by one or more noncovalent interactions, such as hydrogen bonding, π-stacking, charge-transfer interactions, metal–ligand coordination, ionic interactions, and hydrophobic or solvophobic effects.13,14 The strength of these interactions is typically in the range of 10–100 kJ/mol, rather than the hundreds of kJ/mol characteristic for covalent bonds. Contrasting to macromolecules or covalent polymers, the backbone of supramolecular polymers is highly dynamic, with typical lifetimes of an intermonomeric bond between 1 ms and 1 min.15 This dynamic nature of the noncovalent interactions renders supramolecular polymers highly responsive to various external factors and stimuli, such as temperature, solvent and concentration.13,14 This stimuli-responsiveness facilitates straightforward processing of these materials by simply changing, for example, temperature or concentration. Additionally, the dynamic nature of the noncovalent interactions typically renders supramolecular polymers self-healing and enables easy recycling.16
Supramolecular polymers are commonly prepared and studied in solution. In contrast to macromolecules, the intermonomeric and polymer–solvent interactions in supramolecular polymers are of similar strength, and hence, the structure and properties are even more determined by the solvent than in covalent macromolecules. The dynamic nature of supramolecular polymers gives these systems a strong dependency on the experimental conditions under which they are analyzed. Consequently, studying the interactions between supramolecular polymers and their solvents is challenging but may also provide attractive opportunities to exploit these interactions to design material properties that cannot be realized in macromolecules.
In this Perspective, the physical and organic chemistry of solute–solvent interactions is reviewed with a focus on supramolecular polymerizations. First, the effects of solvents on covalent chemistry are discussed; more specifically the impact of these effects on reactivity and the structure of synthetic polymers are reviewed. Next, the general principles of supramolecular polymers are briefly introduced. Then, solvent effects in supramolecular chemistry and supramolecular polymers are discussed in detail, focusing on solvent-dependent stability, kinetics, pathway selection, and structure. Lastly, solvent effects in supramolecular polymerizations in water are briefly reviewed. We finalize this Perspective by providing an outlook toward a rebirth of the physical organic chemistry of solvent interactions in supramolecular chemistry.
The Role of Solvents in Covalent Chemistry
The main difference between covalent and noncovalent chemistry is the intrinsically dynamic nature of the bonds in supramolecular chemistry. The effect of solvents is well studied in organic chemistry with a focus on chemical reactivity, while in polymer chemistry, the studies are more focused on solubility.
Solvent Effects in Chemical Reactivity
Typically, chemical reactions are carried out in a solvent medium, which strongly impacts reactivity. For instance, SN1 reactions typically proceed faster in polar, protic solvents, which stabilize the charge developing during the reaction, while SN2 reactions are facilitated by more apolar, aprotic solvents, which properly solvate the transition state of the reaction.17 Many of these effects have been studied thoroughly halfway through the previous century18,19 and have become textbook knowledge.20 Yet, solvent effects on chemical reactivity and selectivity remain an active area of research up to this day.21
Although many different interactions are
balanced in solvation effects in chemical reactions, the effects of
solvents on reaction rates of elementary reactions can be efficiently
described through linear relationships.22,23 Winstein and
Grunwald22 showed that the rate of a chemical
reaction in reference to the rate in 80 vol % ethanol in water mixture
can be expressed with 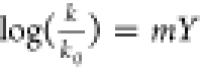 , with k being the reaction
rate, k0 the rate in the ethanol–water
mixture, m a parameter indicating the reaction sensitivity
to solvent ionizing power, and Y a measure for ionizing
power of the solvent.24 Interestingly,
the often observed linear dependency of reaction rates on solvent
composition originates from a complex interplay between enthalpic
and entropic variations as the solvent composition changes (Figure 1a).25 Later, alternative methods, which are easier to determine
than Winstein’s Y-scale, have been developed
by among others Kosower26 and Dimroth and
Reichardt.27 More detailed discussions
on solvent effects on chemical reactivity can be found in several
excellent reviews and textbooks.28−30
, with k being the reaction
rate, k0 the rate in the ethanol–water
mixture, m a parameter indicating the reaction sensitivity
to solvent ionizing power, and Y a measure for ionizing
power of the solvent.24 Interestingly,
the often observed linear dependency of reaction rates on solvent
composition originates from a complex interplay between enthalpic
and entropic variations as the solvent composition changes (Figure 1a).25 Later, alternative methods, which are easier to determine
than Winstein’s Y-scale, have been developed
by among others Kosower26 and Dimroth and
Reichardt.27 More detailed discussions
on solvent effects on chemical reactivity can be found in several
excellent reviews and textbooks.28−30
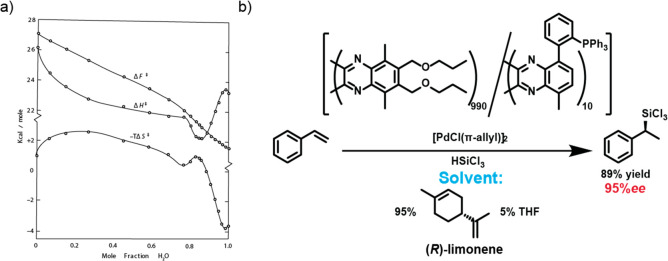
Figure 1.
(a) Changes in transition state Gibbs free energy (ΔF⧧), enthalpy (ΔH⧧), and entropy (ΔS⧧) of the solvolysis of t-butyl chloride in EtOH–H2O mixtures. Image adapted with permission from ref (25). Copyright 1957 American Chemical Society. (b) Poly(quinoxaline-2,3-diyl)s adopt a preferred helicity due to chiral solvent and transfer the chirality of the solvent through their catalytic activity to the silylated product of the reaction. Scheme adapted from ref (57) with permission from the American Chemical Society.
Solvent effects are not only observed in reactions of small molecules; also covalent polymerizations are known to be strongly influenced by solvents. Typically, high purity solvents are critical to obtain high molecular weight polymers, and impurities can dramatically impact the polymerization. Other solvent effects are also known to impact polymerization reactions. For example, the propagation rate is strongly correlated to the stability of the solvent-separated ion pair that is formed during anionic polymerization.31−33 Similar effects have been observed in cationic polymerizations34 and free radical polymerizations35−37 and are reviewed elsewhere.38 In controlled radical polymerizations, where the concentration of reactive chain ends is very low, complexation of the chain end with the solvent is less important in determining the rate of polymerization. Nonetheless, linear free energy relationships similar to those developed by Winstein, for example, have been reported for controlled radical polymerizations.39,40 Thus, solvent effects not only are important in reactions of small molecules but also play a prime role in the synthesis of covalent polymers.
Solvation in Covalent Polymers
Aside from affecting the rate of polymerization, solvents also greatly influence the conformational flexibility of dissolved polymers. Some of the earliest models describing the influence of solvents on macromolecular structures were developed by Flory41 and Huggins42 and have since become textbook knowledge.43 These models, in which solvents are qualified as good solvents, causing swelling of the polymer chains, bad solvents, causing a collapse of the polymers, or θ-solvents, in which the polymer adopts random walk statistics, rely on the Flory–Huggins parameter, χ, and are rather qualitative. A more quantitative description of polymer–solvent interactions was developed by Hildebrand, who defined the Hildebrand solubility parameter as the square root of the cohesive energy density44 of the solvent.45,46 Later, this model was extended by Hansen,47 whose Hansen solubility parameter has found widespread use in paint and coating technologies48,49 but also found some applications in supramolecular systems.50
More intricate solvent effects on covalent macromolecules, such as induction of a single helical handedness in helical polymers, have also been observed. In a series of seminal papers, Green showed the subtle influence of chiral solvent on macromolecular conformation.51,52 Later, similar effects with small molecule solvents or cosolvents were observed by among others the groups of Yashima53,54 and Suginome.55,56 Moreover, the group of Suginome reported recently that a solvent-induced helical handedness in catalytically active polymers can be transferred to enantiospecific hydrosilylation (Figure 1b).57 In this way, a unique connection between the covalent chemical reaction and the noncovalent interaction between the macromolecule and solvent is established. These examples highlight that in covalent systems, solvents can play a profound role in polymer conformation and function.
Supramolecular Polymers
Supramolecular polymers are long, typically one-dimensional aggregates of repeating monomeric units held together by supramolecular, noncovalent interactions. As a result of the noncovalent interactions, these polymers are in a dynamic equilibrium with free monomers in the surrounding solution. In natural systems, such one-dimensional aggregates of repeating monomeric units held together by noncovalent interactions have long been known. Examples thereof are polymerization of tubulin to form microtubules, actin filaments, and intermediate filaments. Some of the earlier models to describe protein aggregation into one-dimensional aggregates were pioneered by Oosawa already in the 1960s.58,59 Still, it was not until the late 20th century that the first reports of synthetic supramolecular polymers emerged8−11 and over the last two decades, the field of supramolecular polymers has expanded considerably.
Like their covalent analogues, supramolecular polymers can form via different mechanisms: an isodesmic mechanism, analogous to covalent step-growth polymerization, or a cooperative mechanism, analogous to covalent chain-growth polymerization (Figure 2). In an isodesmic supramolecular polymerization, the association between monomers and polymers to form polymer chains is characterized by a single equilibrium constant. As a result, the degree of polymerization of isodesmic polymers depends weakly on concentration and temperature. Well-known examples of such polymers include tethered ureidopyrimidinone-based systems (Figure 3a)60 and linked host–guest complexes.61,62 Interestingly, the degree of polymerization for isodesmic polymerizations follows Carothers’ equation for step-growth polymerizations, with the fraction of aggregated monomers substituting chemical conversion.63 Isodesmic polymerizations are characterized by an equilibrium between monomers and mostly small oligomers, while long polymers only form when almost all monomers are aggregated.
Figure 2.
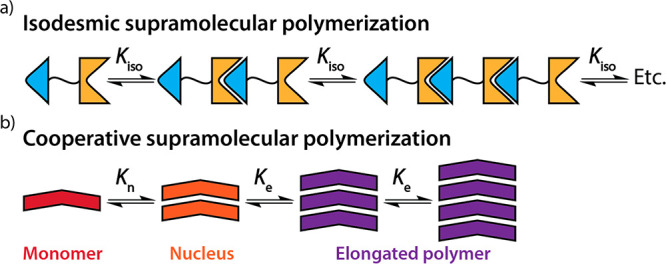
Cartoon representations of (a) an isodesmic supramolecular polymerization and (b) a cooperative, or nucleated, supramolecular polymerization.
Figure 3.
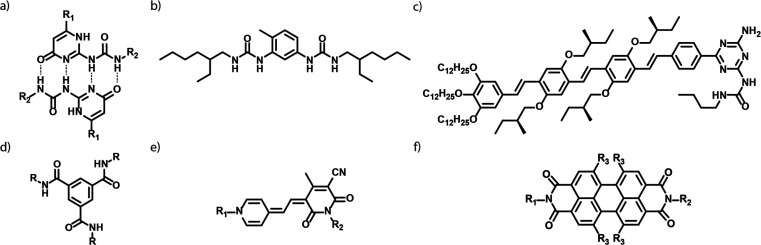
(a) Chemical structure of the dimerized UPy motif often used in isodesmic, telechelic supramolecular polymers. (b–d) Chemical structures of monomer platforms that form cooperative supramolecular polymers: (b) 2,4-di(N′-2-ethylhexylureido)toluene (EHUT), (c) an oligo(p-phenylene vinylene) derivative, as well as examples of the platforms of (d) the benzene-1,3,5-tricarboxamide, (e) merocyanine, and (f) perylenebisimide.
Another mechanism through which supramolecular polymers form is the cooperative, or nucleated, mechanism. In cooperative supramolecular polymerizations, a thermodynamically unstable nucleus first forms, after which this nucleus elongates into long one-dimensional polymers. The nucleus formation is characterized by an equilibrium constant of nucleation, Kn, which is typically lower than the equilibrium constant of the subsequent elongation into long polymers, Ke. The degree of cooperativity is characterized by the cooperativity parameter, σ = Kn/Ke.63 The degree of polymerization and molar mass dispersity are strongly affected by σ. In contrast to isodesmic polymerizations and analogous to chain-growth polymerizations, cooperative polymerizations form polymers with high degrees of polymerization already at low fractions of aggregated monomers. These polymerizations are thus characterized by an equilibrium between free monomers and long polymers already at low degrees of aggregation. Among the first reported examples of such cooperative polymerizations are 2,4-di(N′-2-ethylhexylureido)toluene (EHUT)64 and oligo(p-phenylene vinylene)s (OPVs).65 In addition, detailed investigations on the cooperative polymerizations of among others benzene-1,3,5-tricarboxamides (BTAs),66,67 merocyanins,68,69 and perylene bis/diimides (PBIs or PDIs)70,71 followed (Figure 3b-d). The stereochemical control and the highly ordered structures of the supramolecular polymers are characteristic for polymers formed through cooperative mechanisms.
Recently, more analogies between covalent and supramolecular polymerizations have been put forward. Examples thereof include impressive progress toward developing supramolecular analogues of living supramolecular polymerizations,72−74 as well as various copolymerizations.75 Other interesting developments toward application of supramolecular polymers, mostly as biomaterials76,77 but also for optoelectronical applications,78 show that these materials are reaching technological maturity.
The Role of Solvents in Noncovalent Chemistry
The strength of noncovalent interactions between molecules and atoms is strongly dependent on the solvent. For instance, stabilizing effects of ion–dipole interactions between ions and water facilitate the dissolution of many salts in aqueous solutions, while these solids are generally insoluble in alkanes, which lack those stabilizing interactions. The formation of supramolecular aggregates in solution is therefore special: it requires a careful balance of solubilizing properties of the solvent while also retaining the binding properties of the solute molecules to form the supramolecular aggregates. Well-known examples, in which this balance is realized, are the formation of micelles by surfactants and the DNA double helix.
Solvents in Supramolecular Chemistry
Already before the conception of supramolecular polymers, solvent effects in supramolecular host–guest systems were systematically studied.79−81 Typically, host–guest complexation is driven by enthalpy gain upon realization of noncovalent interactions, such as hydrogen bonds, and comes at an entropic cost. The solvent plays a crucial role in stabilizing or destabilizing the bound and unbound host and guest molecules, thereby altering the equilibrium constant.82 Besides properties of the solutes, the most important factors in stabilizing or destabilizing supramolecular complexes are cohesive forces and dispersive interactions.44 These can be macroscopically related through linear free energy relationships (LFERs) to Reichardt’s solvent polarity parameter, ET(30),83,84 which is in turn related to Winstein’s Y-scale.85 Both enthalpic and entropic contributions to the free energy of the aggregate depend linearly on the solvent polarity. Here, polar solvents typically show the strongest release in enthalpy and entropy upon complexation.86 Interestingly, when several effects, such as dipolar interactions and solvophobic effects, drive the aggregation, two regimes in the LFER, where each respective driving force is dominant, are observed.87,88 More elaborate analyses established intricate relationships to the permittivity of the solvent, rather than the empirical ET(30).89 The group of Hunter showed that, contrasting to such bimodal solvent dependencies, local effects due to high effective molarities may be independent of the solvent polarity.90,91 Despite the complexity of many interactions driving supramolecular complexation, interaction strength and solvent dependency can be calculated rather straightforwardly92,93 using literature-derived parameters.94 As such, solvation influences supramolecular aggregation in numerous ways and many cooperative effects can emerge.
To increase the stability of supramolecular complexes, several interactions can be combined to obtain a cooperative enhancement of binding properties.95 Such a cooperatively enhanced binding between molecules is particularly interesting in solvents of intermediate polarity or in solvents that have competitive (hydrogen-bonding) interactions.82 As a result, the unbound states are stabilized and supramolecular association can be decreased in these solvents. By engineering cooperative effects, such as arrays of hydrogen bond donors and acceptors, high to moderate association constants in a range of solvents have been realized. A typical example thereof is the self-complementary ureidopyrimidinone (UPy) moiety, which shows strong hydrogen bonding in CHCl3 and also dimerizes when DMSO, a competitive solvent, is added.96 Through cooperative effects, the UPy motif, which comprises two neighboring hydrogen bond donating and neighboring accepting groups, shows a considerable increase in association constant (Ka > 106 M–1 in CHCl3)96 compared to analogues with only 2 or 3 hydrogen bond donating and accepting groups, which show association constants of approximately 84 M–1 and between 400 and 900 M–1, respectively.97 Further engineering of such hydrogen-bonding complexes has led to the development of hydrogen-bonding motifs with very high association constants (Ka > 1012 M–1 in CHCl3).98,99 Due to their ability to link molecules together in a wide range of polar and apolar solvents, the development of hydrogen-bonding motifs, like UPy, has greatly facilitated the development of diverse supramolecular polymers in various applications.
Solvents in Supramolecular Polymers
For the formation of long supramolecular polymers, high association constants between the monomers are necessary. When complementary hydrogen-bonding motifs with high association constants are tethered together, isodesmic supramolecular polymers form. The UPy motif is one of the most widely used hydrogen-bonding motifs for such supramolecular polymers,60 but also other motifs, including Hamilton wedges,100 host–guest complexes of cyclodextrins,101 and many others, have been reported. The effects of solvents on these polymers are largely dictated by the effect these solvents have on the hydrogen-bonding strength between the dimerizing groups and are therefore mostly analogous to solvation effects in host–guest chemistry.
For polymers composed of discotic monomers, which typically polymerize cooperatively, the effects of solvents are more subtle. To form supramolecular polymers in solution, the polymer should be well-soluble, while poor solubility of the monomer is required to drive the aggregation. To combine good and poor solubility in a single monomer, these molecules often show amphiphilic or dichotomous character. Typically, a poorly soluble (aromatic) core is solubilized by soluble and flexible chains. The insolubility of the central parts of the monomer facilitates the one-dimensional precipitation, leading to the formation of fibrous supramolecular polymers. Thus, a delicate balance in solubilizing properties of the solvent determines whether monomers are insoluble, form supramolecular polymers or dissolve as free monomers without forming polymers. As such, the solvent is critical in determining the stability and length of supramolecular polymers. However, rational engineering of the monomer structure to tune the polymer stability has remained very challenging. Moreover, since the thermodynamic stability of the polymer is dictated by the solvent, pure solvents offer few ways to tailor the properties of supramolecular polymers. To obtain more control over the stability and length of supramolecular polymers, a combination of solvents can be exploited.
Solvent-Dependent Stability of Ordered Supramolecular Polymers
The solvent in which monomers are dissolved dictates whether supramolecular polymers will form. If a solvent does not solubilize the soluble parts of the monomer well enough, the compound is insoluble, while good solvation of the rigid, poorly soluble parts of the monomer dissolve the polymer. Although this effect has been known since the dawn of supramolecular polymers, systematic and quantitative studies have started to be reported only relatively recently.102 The most typical way to describe the effect of a good solvent (e.g., CHCl3) on a supramolecular polymer in a poor solvent (e.g., methylcyclohexane, MCH) is through a LFER:
| 1 |
with ΔGf being the Gibbs free energy of the polymerization at a fraction of good solvent f, ΔG° the Gibbs free energy of polymerization in the poor solvent, and fgood-solvent the volume fraction of the good solvent. A schematic depiction of these LFERs and how they change the Gibbs free energies of various aggregation pathways in a competitive supramolecular polymerization with a nucleated and an isodesmic pathway is given in Figure 4a. The use of such an LFER is well-established for the denaturation of folded proteins by urea and guanidinium chloride.103 Here, a growing consensus emerges that these polar additives stabilize the denatured protein through the formation of hydrogen bonds with specific protein residues.104 For urea denaturation of proteins, typical m-values range between 0.8–8 kJ·mol–1·(M urea)−1 with the exact magnitude being correlated to the protein’s solvent-accessible surface area.105
Figure 4.
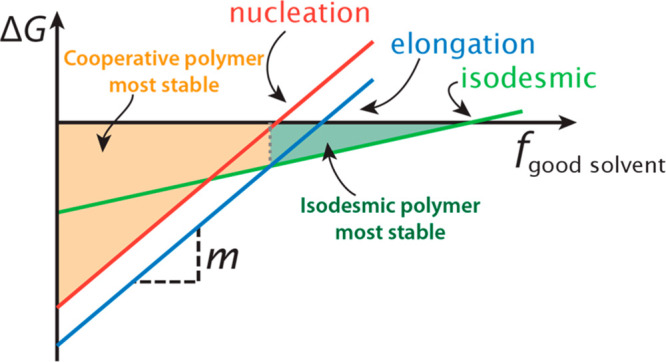
Schematic representation of the changes in Gibbs free energies (ΔG) upon the addition of a good solvent of the various aggregation pathways in a competitive supramolecular polymerization involving a cooperative and isodesmic pathway. As a fraction of good solvent, fgood-solvent, is added, the change in stability of the aggregates is given by their m-value. When the elongation or isodesmic pathway is lower in ΔG, the cooperative or isodesmic polymers, respectively, are the most stable polymers, as indicated by the shaded areas and dashed lines.
In supramolecular polymers, m-values have been reported for only a handful of systems. Typical m-values for CHCl3 cosolvents in MCH around 60 kJ/mol have been obtained for oligo(p-phenylene vinylene) (OPV), ureidotriazines,102 perylenes,102,106 and a BTA,107 while for a series of metallosupramolecular polymers108 and benzene-1,3,5-trithioamide,102m-values were on the order of 30 kJ/mol. Also for water-compatible BTA derivatives in acetonitrile/water mixtures, similar values have been reported.109 Taking the molar mass and density of CHCl3 into account, these values correspond to 4.8 kJ·mol–1·(M CHCl3)−1 and 2.4 kJ·mol–1·(M CHCl3)−1. In contrast, for several other systems, much larger m-values have been obtained. In a selenoderivative of BTA, an m-value of 600 kJ/mol was observed,110 while porphyrin-based polymers showed m-values between 100 and 200 kJ/mol.111 Folded bismerocyanins showed m-values on the order of 150 kJ/mol upon destabilization by THF.112 Interestingly, for the porphyrin-based system, which polymerized into both nucleated and isodesmic aggregates, the m-value of the isodesmic pathway was considerably lower, with values reported around 40 kJ/mol. Thus, for many systems, the destabilizing effect of CHCl3 on supramolecular polymers in MCH solvents is of comparable magnitude to the denaturation of proteins by urea, but general trends appear to be lacking.
Despite the analogies between destabilization of supramolecular polymers by good solvents and of proteins by urea and guanidinium, the molecular interactions leading to dissolution of the polymers by the good solvent are only poorly understood. Several theoretical reports suggest that good solvents stabilize the monomers, rather than destabilize the polymers.113,114
Although the use of LFERs to quantify the effect of a good solvent on supramolecular polymer stability has been well-established, these approaches do not give a molecular picture of destabilizing solvent interactions. Through a series of studies, some light on the molecular basis for solvent-dependent destabilization of supramolecular polymers has been shed for several well-studied systems, including EHUTs and BTAs (Figure 3b,d).
Solvent-Dependent Stability in Ethylhexylureidotoluenes
The system in which solvent effects have been studied most systematically are the N,N′-diethylhexylureidotoluenes, developed by Bouteiller.115 These molecules assemble into filaments or strongly nucleated tubular structures in a wide range of apolar organic solvents, ranging from chloroform to dodecane.116 Tuning of the aliphatic substituents forces the urea groups away from the aromatic plane, increasing the hydrogen bond strength and permitting the formation of polymers in polar solvents, such as ethyl acetate and tetrahydrofuran.117 Conversely, tuning the balance between peripheral hydrophobic and hydrophilic groups allowed for the formation of supramolecular polymers in both organic and aqueous media.118 By carefully controlling the molecular geometry of the solvent and interactions with the tubular or fibrillar structures, interesting control over the properties of the supramolecular polymers was obtained. Changing the surface area of the solvent, leading to a worse or better fit of the solvent into the hollow filaments, leads to a considerable change in viscosity of the solutions.119,120 Most strikingly, EHUT solutions in p-xylene were more than 200 times more viscous than solutions in o-xylene.115 Similar effects of solvent size on transition temperatures and polymer lengths were observed for an ester-derived EHUT, which forms nontubular assemblies with weak solvent dependencies.121 The enthalpy differences between the alkyl- and ester-EHUT are only approximately 1 kcal/mol, highlighting that subtle energy differences can have profound impacts on competitive supramolecular polymerizations. The competition between tubular and fibrillar EHUT polymers has been exploited in several other studies122,123 to determine very small energetic contributions and show that competition can be a valuable tool to determine subtle variations in solvation energies.
In another study,124 Bouteiller and co-workers showed that the cohesive energy44 induces slower local dynamics in the solvent. As a result, despite shorter polymers in solvents with low dynamics, the viscosity of the solutions increases. The authors specifically note that this effect originates from the solvent structure and not from the presence of trace amounts of water, which are known to affect EHUT.125 Finally, the interactions between peripheral halides on the EHUT alkyl chains and alkyl halide or toluene solvents also influence the transition temperature between the fibrillar and tubular supramolecular polymer.126
Solvent-Dependent Stability in Benzene-1,3,5-tricarboxamides
The cooperativity of supramolecular polymerizations of benzene-1,3,5-tricarboxamides has been attributed to the formation of macrodipoles along the helix axis.67,127 This cooperativity shows remarkable solvent dependency and is slightly more cooperative in n-heptane than in MCH, which was attributed to changes in the dihedral angles between the planes of the amides and the benzene core.128 Similar subtle solvent-dependent dihedral angles have also been reported for other BTA derivatives.129,130 MD simulations have shown that the solvent-induced energetic differences are minute and the general structure of the supramolecular polymers remains comparable, with two of the three amides pointing in the same direction.130,131
Aside from BTA systems that have the amides attached directly or through a one-carbon long spacer to the central phenyl core, extended BTA systems have been investigated by the group of Sánchez. These systems, bearing a phenylethynyl moiety between the central phenyl ring and the amides, show cooperative polymerizations in alkane solvents, while isodesmic polymers are formed in CHCl3 and aqueous environments.109,132 Compared to their smaller analogues, polymers of these extended BTAs have similar stability in MCH but show increased destabilization upon addition of CHCl3, presumably due to better interactions between CHCl3 and the extended aromatic surface.107 This effect is analogous to the trend observed in solvent-accessible surface area for protein denaturation by urea. A last example by the group of Sánchez is an extended BTA system that is appended with phenylalanine (Phe) groups at the amides.133 The Phe groups can transfer their helical preference to the supramolecular polymer, which forms in various solvents ranging from acetonitrile to CCl4. Remarkably, the addition of chloroform to CCl4 solutions of the Phe-decorated BTAs destabilized the polymers. In CCl4, relatively low stabilities around 25 kJ/mol are found, with m-values for chloroform in CCl4 of 58 kJ/mol. These strong differences between these related solvents highlight the intricate energetic balances and molecular interactions operating in supramolecular polymerizations.
Solvent-Dependent Stability in Other Supramolecular Polymers
In some of the first studies on cooperative supramolecular polymerizations, a remarkable odd–even effect of the solvent structure on the elongation temperature and aggregate size of OPV-based polymers was reported.65 This suggests that ordered solvent molecules around the polymer play a pivotal role in stabilizing the aggregate.134
In an elegant design, Yagai and co-workers employed SAXS and SANS measurements simultaneously to show that polymers from naphthalene-based supramolecular polymers showed varying degrees of solvent penetration into the toroidal superstructures these monomers formed.135 The denser naphthalene derivatives, showing the least solvent penetration, exhibited the highest elongation temperatures.
Furthermore, similar to the solvent geometry dependency observed by Bouteiller, the group of Ghosh observed that in linear alkanes, monomers based on naphthalene-diimide (NDI) form polymers through a highly cooperative mechanism, with longer alkanes forming less stable polymers.136 In cyclic alkanes, however, the NDI-based monomers polymerize isodesmically, and only upon the addition of seeds, long fibrils are formed. This difference is attributed to better participation of linear alkanes in ordering of the supramolecular polymer. Kulkarni et al. observed a similar difference between linear and cyclic solvents in a helix inversion.137 In addition, helix inversion was correlated to bulk solvent properties, such as the crystallization temperature. Furthermore, the proposed interdigitation of the solvent in the pockets of the polymers was supported by molecular dynamics simulations.
Kinetic Aspects of Solvent Interactions
Besides influencing the thermodynamic aspects of supramolecular polymerizations, solvents also directly affect the kinetic properties of supramolecular polymerizations. Most recent reports relate to kinetic effects of solvents in seeded polymerizations, which are inherently kinetically controlled, but some of the earliest reports are on thermodynamically controlled supramolecular polymerizations.
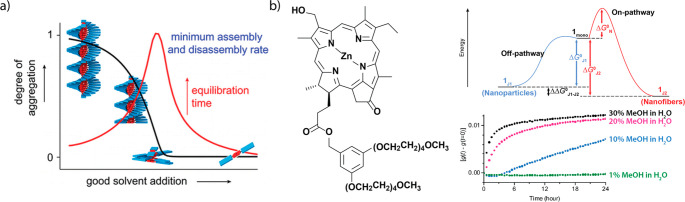
Korevaar et al. showed that supramolecular polymers disassemble when an increasing volume fraction of good solvent is added to the polymers.102 At the critical solvent fraction, above which the polymers are unstable, the disassembly rate is lowest (Figure 5a). When a good solvent is added to a final volume fraction below the critical solvent fraction, the monomers that are liberated from long polymers aggregate in new, shorter polymers. The rate of formation of new nuclei and small polymers, and thereby the overall equilibration rate, decreases as an increasing amount of good solvent is added. When a good solvent is added to a final volume fraction that is larger than the critical fraction, the liberated monomers remain free and do not form new aggregates. The equilibration rate is in this case dominated by the depolymerization rate, which increases as more good solvent is added. These two effects, that dominate the equilibration kinetics below and above the critical solvent fraction, respectively, lead to a counterintuitive effect, where equilibration rates are lowest at the critical solvent fraction. The long equilibration times at the critical solvent fraction, where the nucleated polymers elongate, has also been observed in competitive porphyrin-based polymers.111 Here, the porphyrin-based monomers could assemble into either an isodesmic or a nucleated polymer. The isodesmic polymers, which are not characterized by a sharp dependency of the fraction of polymerized material on solvent quality, formed at rates that were too high to measure. The presence of the isodesmic pathway, however, did not appear to impact the equilibration kinetics of the nucleated polymer. Thus, also in competitive polymerizations, where multiple polymer states are possible, equilibration times for the formation of the polymers is maximum at the solvent fraction where those polymers elongate. These long equilibration times can be circumvented through gradual addition of good or bad solvent.138 Moreover, the dependency of the equilibration kinetics on changes in solvent composition may also reveal detailed mechanistic information on competitive polymerization pathways.139
Figure 5.
(a) Cartoon depiction of the solvent-dependent equilibration time of OPV derivatives (Figure 3c), as measured by Korevaar et al. Reprinted with permission from ref (102). Copyright 2012 American Chemical Society. (b) Solvent-dependent interconversion between kinetically trapped off-pathway aggregates (low signal, bottom figure) and thermodynamically stable on-pathway aggregates of a zinc-chlorin model system. Image adapted from ref (146). Published by The Royal Society of Chemistry.
Most commonly, however, poor solvents are found to install kinetic traps in supramolecular polymers. The group of Würthner was one of the first to recognize kinetic trapping of monomers in ill-defined aggregates in poor solvents.68 The addition of THF to MCH solutions of trapped merocyanine-based monomers induced appropriate dynamics in the system to allow the formation of supramolecular polymers. The group of Nolte showed similar results, where porphyrin-appended BTA derivatives could not show strong non-linear amplification of helical handedness in heptane, while full homohelical expression was observed in toluene.140 By synthesis of the helically ordered supramolecular polymers in toluene and subsequent evaporation of the solvent and redispersion in heptane, ordered supramolecular polymers were obtained in solvents that did not allow for rapid monomer exchange. Very recently, the group of Fernández showed that quenching of a supramolecular system with bad solvents may lead to the formation of kinetic traps that cannot be accessed through thermal quenching.141 Thus, although the dependency of the Gibbs free energy of a polymer on solvent quality is analogous to the temperature dependency (see eq 1), these results show that kinetic trapping via solvent or thermal quenching can be different.
In crystallization-driven self-assembly, the group of Manners142 showed that polymerization rates decrease when the solvent quality increased, analogous to the report of Korevaar et al. In addition, by careful choice of the solvents for sequential polymerizations, triblock copolymers could be generated in a perylene-based system.143 Similarly, Sugiyasu and co-workers showed that supramolecular block copolymers of porphyrin-based monomers could be prepared when seeds in a good solvent were added to preformed polymers in a bad solvent.144 Ogi et al. showed that the temperature range in which perylene-based monomers could be trapped in metastable, off-pathway aggregates could be controlled by the toluene volume fraction in methylcyclohexane.145 They later showed that solvent quality determines the polymerization kinetics in a zinc chlorin system, which polymerizes in polar solvents (Figure 5b).146 Furthermore, Sánchez and co-workers showed that different pathways in living supramolecular polymerizations can be accessed in different solvents.147,148N-Heterotriangulenes were shown to polymerize either into J-type or H-type aggregates in pure CCl4 or mixtures of MCH and toluene, respectively. This last example shows that solvents not only affect the stability and rate of supramolecular polymerizations but can also lead to the formation of different aggregates by changing the energetic balance between different aggregation pathways. On this topic, most effects of solvents have been reported.
Pathway Selection and Morphology Driven by Solvation
Solvent-dependent polymer morphologies have been reported for many systems, but the discussions have been mostly limited to empirical descriptions of observed effects, rather than molecular explanations. One of the first examples of solvent-dependent polymer morphologies was reported by Lehn in 2007.149 Here, melamine and cyanuric acid were shown to polymerize into either linear or branched aggregates in toluene or THF, respectively. However, most reported systems showing various pathways consist of extended π-surfaces, with PDI- and NDI-based systems being the most reported, and the breadth of different types of behavior is rather wide.
Rybtchinski reported fluorinated, amphiphilic PDIs that formed polymers through increasingly cooperative pathways as the volume fraction water in water–THF mixtures increased.150 Diverse pathways were also observed in the aqueous polymerization of N-phenylalanyl decorated PDIs.151 In aqueous solutions containing 10 vol % THF, the monomers assembled into concentric rings of left-handed supramolecular polymers due to the poor solubility of the growing polymers. In contrast, in THF, long fibers with a right-handed helicity were obtained, as the growing polymers remained soluble in the more apolar solvent. When PDIs were tethered with sugars, the self-assembly into either right- or left-handed helices could be controlled through the volume fraction of DMF in water.143 Similar sugar-appended PDIs were also shown to form different polymer morphologies when self-assembled in water–THF mixtures or CHCl3–n-octane mixtures. The sugar group, pointing outward or inward of the polymers when they are formed in the aqueous or organic solvent mixtures, respectively, could be used to control water contact angles of solid substrates. Similar effects have also been observed for other PDI systems with quantum dots152 and a series of NDI-based polymers.153−155 As such, these examples highlight the potential of solvent-engineered supramolecular structures for material applications.
Besides PDI- and NDI-based systems, solvent dependencies of several other systems have also been reported. The group of Nolte showed that porphyrin-appended BTAs can be deposited on surfaces as ordered supramolecular polymers when processed from CHCl3, where the polymers form during the evaporation process.156 When the polymers are preformed and deposited from hexane, however, disordered arrays were observed on the surface. Cantekin et al. observed more subtle effects in C3-symmetrical BTAs and showed that the introduction of a single deuterium, rendering the side chains of N-alkyl substituted BTAs chiral, induces different helical preferences in the supramolecular polymer using either linear or cyclic alkane solvents.157 In a different C3-symmetrical system, Das et al. reported that oxadiazole-containing monomers formed H- or J-aggregated supramolecular polymers depending on the aliphatic or aromatic nature of the solvent, respectively.158 This different aggregating behavior is attributed to the breaking up of the π-stacked H-aggregates by aromatic solvents. This hypothesis is supported by control experiments in which more bulky aromatic solvents show decreased J-aggregation. The group of Yagai very recently showed that the aromatic or aliphatic nature of the solvent can also drive the selection of polymer morphology by balancing the kinetic and thermodynamic aspects of several polymerization pathways of a cyanuric acid-based monomer.159 By use of solvent mixtures, a myriad of structures could readily be prepared, and some of these could otherwise only be obtained with slow cooling in pure solvents. In a related report, Yagai and co-workers showed that the cyanuric acid-derived monomers can even form supramolecular polycatenanes.160 The yield of the polycatenanes, which form through secondary nucleation events, was highest in cyclic aliphatic solvents. In these least polar solvents, the increased favorability of the elongation pathway leads to long fibers, rather than circular polymers. The morphological changes of supramolecular polymers upon changing solvents, highlighted by the above examples, have also been observed in triarylamines,161,162 metallosupramolecular polymers,163,164 azobenzenes,165 and pyrenes,166 showing that pathway selection through solvent effects is a general phenomenon in supramolecular polymerizations.
Lastly, solvents can be used to distinguish not only between several different polymer morphologies but also between several levels of hierarchical self-assembly. The group of Ajayaghosh reported chiral oligo(phenylene ethynylene)s that assemble into helical supramolecular polymers, which in turn assemble into superhelices of opposite handedness (Figure 6a).167 By careful control of the amount of CHCl3 in n-decane, the degree of superhelical twisting could be controlled.
Figure 6.
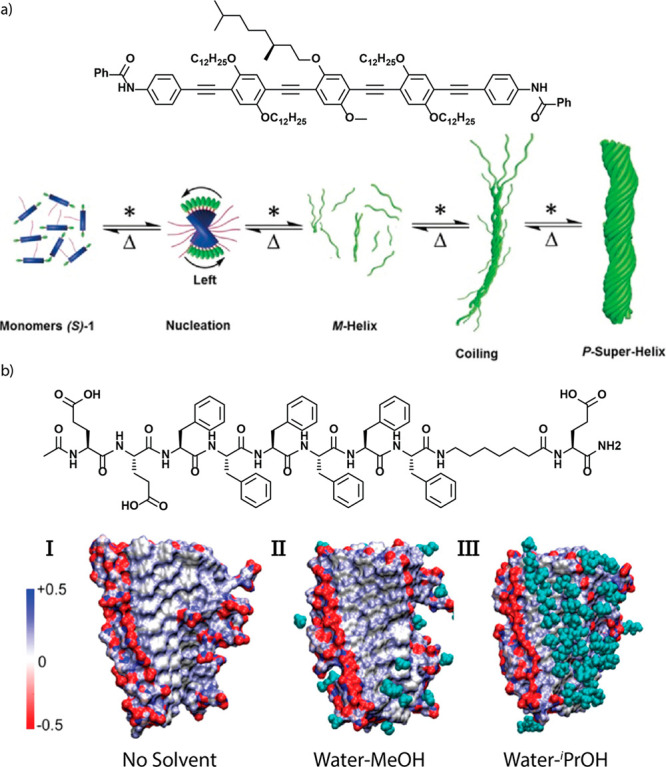
(a) Cartoon representation of the supramolecular polymerization and subsequent superhelix formation of oligo(phenylene ethynylene) derivatives. Reproduced with permission from ref (167). Copyright 2017 John Wiley and Sons. (b) Space filling model of the peptide amphiphile studied by Stevens and co-workers, showing how various alcohol cosolvents solvate the amphiphile surface.168 Image reproduced from ref (168). Copyright 2019 the American Chemical Society.
Despite the numerous reported examples, very few studies into the molecular origins of solvation-dependent pathways have been reported. Valera et al. recently reported an extensive study into solvent-directed stereomutation of N-heterotriangulenes.147 Most importantly, VCD analyses indicated that the thermodynamic state of the polymers in toluene is better packed than the kinetically controlled product in CCl4. In another recent report, Stevens and co-workers using a combination of several experimental techniques and molecular dynamics simulations studied the solvent-dependent aggregation into nanosheets and fibrils of peptide amphiphiles (Figure 6b).168 Relatively apolar organic solvents were found to solvate the aliphatic parts of the amphiphiles better, leading to the formation of one-dimensional fibrils. In polar solvents, these aliphatic domains remain poorly solvated, which promoted aggregation, leading to the formation of 2D-nanosheets.
Solvent-Induced Structure in Supramolecular Polymers
Besides a change in energetic balance, leading to the population of various polymer states, solvents can also directly direct supramolecular polymer structure and morphology. The use of optically active solvents to bias helical preference when achiral monomers form supramolecular polymers is most frequently reported. Recently, also a number of reports in which a solvent or cosolvent acts as a structural component of the supramolecular system have been published.
Chiral Solvents and Solvent-Induced Helicity in Supramolecular Polymers
Palmans et al. first reported helical induction by chiral solvents and showed that supramolecular polymers of achiral bipyridine-decorated BTAs in (S)-2,6-dimethyloctane show circular dichroism (CD) intensity of similar magnitude as the (S)-enantiomeric monomer.169 Helical direction of supramolecular structures by alkane solvents was later also shown for limonene in several EHUT and PBI-based systems. In case of the EHUT solutions, solvation by limonene resulted in the same expression of helicity as observed for the enantiomeric homopolymers in achiral solvent.170 Furthermore, a linear dependency of the CD intensity on the enantiomeric excess of the solvent was observed. Similarly, in dilute solutions, chiral solvents induced a screw sense excess close to 100% in PBI-based polymers.171 In contrast, in the gel state, the chiral solvent could only dictate up to 20% screw sense excess. Later, it was found that helical induction by chiral solvents in a living supramolecular polymerization only affects the supramolecular polymer, while the nanoparticle seeds remain unaffected.172
The induction of helical structures by chiral solvents on solid substrates, as observed by Würthner and co-workers,173 was also observed by De Feyter and co-workers.174 Here, partial chiral expression on the solid substrate could be observed for several chiral apolar alcohols, with the exact degree of chirality on the surface being dependent on the exact chemical structure of the solvent. Complete helical induction on the surface was observed for triarylamine-based polymers.175 Remarkably, the helicity of the formed superhelices in this system was also directed by the configuration of the chiral limonene solvent.
In contrast to the helical induction by chiral aliphatic alkanes, such as limonene and dimethyloctane, George et al. reported that solvent-induced helicity in OPV derivatives only occurs when the solvent contains hydrogen-bonding moieties (Figure 7a).176 This suggests that the helical induction in the OPV systems is based on enthalpic interactions, which contrasts to the lack of any directional interactions in aliphatic solvents, indicating that in these cases, helical organization originates from entropic interactions. The entropic nature of directing interactions of chiral solvents was also confirmed by Liu and co-workers, who showed that in both the presence and absence of alcohol moieties, chiral solvents could direct the helicity of polymers of non-amidated C3-symmetrical monomers.177
Figure 7.
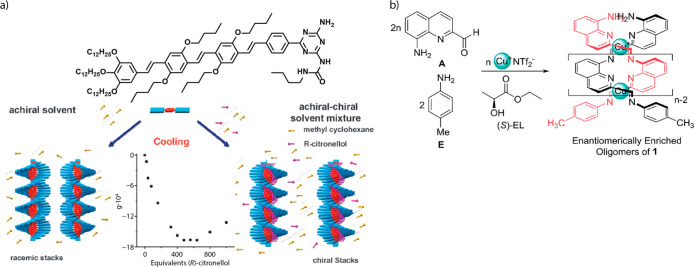
(a) (R)-Citronellol as cosolvent in MCH induces the formation of supramolecular polymers of OPV derivatives (Figure 2b) of a preferred handedness. Reproduced from ref (176) with permission from The Royal Society of Chemistry. (b) Chiral (S)-ethyl lactate ((S)-EL) solvent induces the formation of metallosupramolecular polymers of a single handedness. Figure adapted from ref (179). Copyright 2018 the American Chemical Society.
Recently, we reported that the supramolecular polymerization of chiral triphenylene derivatives in chiral solvents can shed an unprecedented light on the enantiomeric mirror symmetry breaking by chiral solvents.178 By combination of experiments with mathematical modeling, the enantiospecific contribution of the chiral solvent in the polymer stability was obtained. We anticipate that the study of chiral monomers for polymerizations in chiral solvents will be a fruitful strategy to elucidate detailed aspects of solvent-induced helicity in supramolecular polymerizations. Detailed studies on the molecular mechanisms of this helicity induction by chiral solvents are rare, however. In one example, the group of Nitschke studied the formation of metallosupramolecular polymers in achiral and chiral solvents and found partial induction of helicity resulting from interactions of the (S)-ethyl lactate solvent (Figure 7b).179 Using a statistical mechanical model, the authors determined the energetic difference between P and M helices, as induced by the solvent, at 0.36kBT, while the difference between the two helicities for enantiomerically pure monomers, 2.15kBT, was considerably larger. A more extensive recent review of solvent-induced helicity in supramolecular polymers is published elsewhere.180
Solvents or Cosolvents as Structural Components in Supramolecular Polymers
Besides helical induction by chiral solvents, several reports in which solvents play an active structural role in supramolecular polymerizations have been published recently.
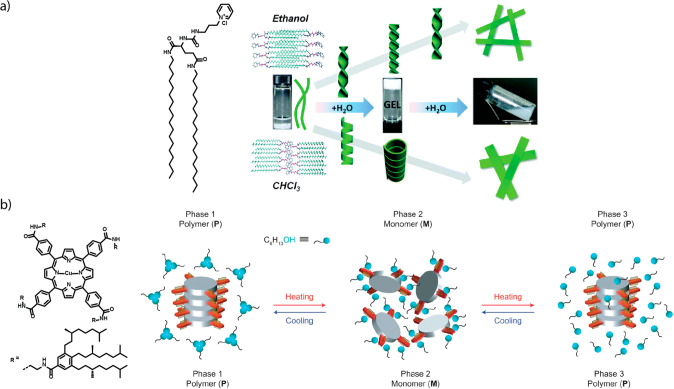
Although water is recognized as a structural component in natural chlorophyll assemblies since the 1980s,181,182 its role in synthetic supramolecular polymers was only uncovered in the past decade. The first suggestion of water as a structural component of supramolecular polymers was made by Liu and co-workers for a pyridinium-based monomer that polymerizes into helical ribbons in the presence of water in several organic solvents (Figure 8a).183 However, in this report, no strong evidence for the incorporation of water in the supramolecular polymer was given. Around the same time, Johnson et al. reported a water-dependent helical switch in hydrogen-bonded rosettes.184 The different helical states of the polymers in water or methanol were explained using a computational model, but experimental evidence for water incorporation was not presented. Bouteiller and co-workers later showed that the presence of water has a strong effect on the viscoelastic properties of EHUT gels.125 In the presence of water, the polymers became considerably shorter due to presumed chain capping by water molecules. The effect of alcohols as chain capping agents, reducing the length of the polymers, was also observed for other, organic alcohols, although general destabilizing effects due to the polar cosolvents cannot be ruled out.185 Later, the group of Yagai reported PBI-based monomers in which the thermodynamic parameters of the polymerization of the oligo(ethylene glycol) decorated monomers were strongly characterized by the release of water molecules hydrated to the ethylene glycol-based side chains of the free monomers.186
Figure 8.
(a) Chemical structure of the glutamide-based amphiphile used by Liu et al.,183 and cartoon representations of the different polymer morphologies formed in different solvents. Reproduced from ref (183) with permission from The Royal Society of Chemistry. (b) Schematic depiction of the thermally bisignate supramolecular polymerization developed by the group of Aida.191 Adapted with permission from Springer Nature from ref (191), copyright 2017.
In BTA derivatives decorated with 18-crown-6, hydration of the crown ethers showed very pronounced changes in the bulk properties of this adhesive material.187 Through a combination of infrared spectroscopy, broadband dielectric spectroscopy and density functional theory calculations, the authors convincingly show that water is a structural comonomer in these systems. The binding energy of water in these systems is almost equal to the hydrogen bond energy of water in ice lattices, highlighting the release of considerable enthalpy upon binding of water.
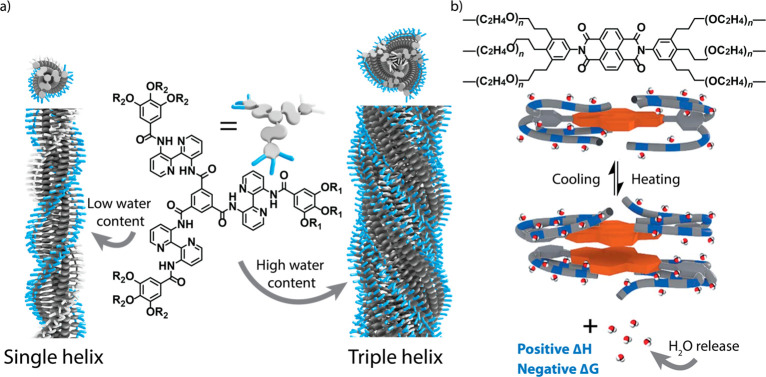
We recently reported several studies in which we revealed that water can be a structurally important component of supramolecular polymerizations in alkanes. Our first observation of the structural role of water in supramolecular polymerizations was in the supramolecular polymerization of a chiral biphenyl tetracarboxamide (BPTA) derivative.188 When dissolved in MCH, the BPTA derivative can form three structurally different supramolecular polymers, of which two are dependent on codissolved water molecules. By combining experimental results with a mathematical model, we showed that incorporation of water releases considerable enthalpic energy of the monomeric water molecules to overcome the entropic penalty for organizing these components in the polymer. In the course of this investigation, we noticed that the helical inversion of triarylamines, reported by Adelizzi et al.,189 was also dependent on humidity, and also in this system, water can act as a structural component in the supramolecular polymer. A third, more intricate, example of water in supramolecular polymerizations is provided by a porphyrin derivative.190 Here, the stability of the supramolecular polymers is influenced by the presence of the components of a Michael reaction. It appeared that the destabilizing influence of the reaction components was critically mediated by codissolved water, and in the absence of water, no destabilization was observed. Through combined spectroscopic and scattering experiments and atomic force microscopy, water was proposed to bind to the helix macrodipole and thereby facilitate chain capping of the supramolecular polymer by the various reaction components. The complexation of the reaction components to the chain ends then eventually destabilizes the supramolecular polymer. Thus, these and other examples that are currently underway show that water also plays a role in directing supramolecular structure in dilute alkane solutions.
Besides water, alcohols have also been used to direct supramolecular polymer structure. In a report by the group of Aida, a thermally bisignate polymerization was realized in highly thermally stable, octa-amidated porphyrin-based polymers (Figure 8b).191 In the presence of small amounts of alcohol cosolvent in alkane solutions, the hydrogen bonding between the monomers is effectively disrupted at intermediate temperatures by competitive hydrogen bonding with the alcohol. Upon increase of the temperature, the alcohol–amide hydrogen bonds are weakened, resulting in the formation of supramolecular polymers upon heating. In contrast, cooling of a solution of the dissolved monomers leads to clustering of the alcohols, and the equilibrium between solvated monomers and polymers is restored to the polymers, leading to a polymerization upon both heating and cooling. Later, it was shown with a combination of experiments and mathematical modeling that in the polymerization upon heating, the hydrogen bonds in the supramolecular polymer are formed one-by-one in a sequential manner, while in the polymerization upon cooling, the hydrogen bonds in the polymer are formed all at once in a synchronous fashion.192 Together, these results show that solvents and cosolvents can act as structural components in supramolecular polymers and that enthalpy release through hydrogen bonding is often the driving force for the solvent effects observed. This insight may facilitate the rational design and tailoring of other specific functions in various supramolecular systems.
Solvent Effects on Supramolecular Polymers in Aqueous Systems
Water, a special solvent with its large dipole moment and small molecular weight, introduces specific requirements for the design of supramolecular systems in this solvent. The specific role of water in functional supramolecular polymers in aqueous environments has been excellently reviewed elsewhere,193 but several recent developments are worthwhile to discuss.
In general, supramolecular polymers in aqueous solutions rely on the hydrophobic collapse of apolar moieties attached to a central core to drive the aggregation of the monomers into polymers. In fact, in contrast to systems in organic solvents, only a few systems have been reported that show thermodynamically controlled nucleation and elongation behavior in aqueous media.194,195 The reliance of most aqueous supramolecular polymers on hydrophobic collapse rather than directional interactions to drive aggregation renders their dependency on temperature and other external properties more cryptic than in organic media. As a result, the properties of these systems are to a larger extent determined by thermodynamic aspects of the solvents or solvent mixtures, such as phase diagrams and transitions.
One of the first interesting correlations between supramolecular structure and phase diagrams of water–cosolvent mixtures was observed by Gillissen et al.196 The formation of triple helical superstructures of bipyridine decorated BTAs in water–isopropanol mixtures was found to be strongly correlated to the enthalpy of mixing of these solvents (Figure 9a). Second, the folding of single chain polymeric nanoparticles (SCPNs) decorated with bipyridine-BTAs was shown to be dependent on the volume fraction of THF, a good solvent, in water.197 Interestingly, optimal bipyridine-BTA stacking in the SCPNs was obtained at approximately 40 vol % THF in water. At this solvent composition, microscopic THF–water clusters exist, while in mixtures containing a higher volume fraction of THF, domains of pure THF form.198 Interestingly, at this solvent composition, PDI-based monomers also show a diverse set of structural features.199 Similar correlations between the phase diagram of a solvent mixture and properties of a supramolecular system were also observed in the supramolecular polymerization of water-soluble BTA derivatives.200 Here, the critical volume fraction of 15 vol % acetonitrile in water, above which the polymers are destabilized, coincides with the volume fraction of acetonitrile above which microscopic clusters rich in acetonitrile and water start to form. A similar effect was also found for platinum metallosupramolecular polymers, although the structural transitions between 10 and 25 vol % acetonitrile were attributed to different stabilization of the dipoles in the various polymer morphologies.201 As such, these examples strongly indicate that the formation of microscopically phase-separated domains in aqueous mixtures may be an important aspect of stabilizing or destabilizing interactions in supramolecular polymerizations in water.
Figure 9.
(a) Bipyridine–BTA platform from Gillissen et al. Image adapted with permission from ref (196). Copyright 2014, American Chemical Society. (b) Water-soluble naphthalene bisimides developed by the group of Würthner, which polymerize upon heating due to release of water. Image adapted from ref (204). Published by The Royal Society of Chemistry.
In addition, the strong hydrogen-bonding abilities of water allow the design of fundamentally different behavior and properties of supramolecular systems. This is most exploited through temperature-dependent desolvation of a hydrophilic periphery to induce assembly. The group of Würthner demonstrated this strategy in the supramolecular polymerization of ethylene glycol decorated PBIs upon heating. (Figure 9b).202−204 Interestingly, their earlier work202 showed that the aggregates of these molecules have considerably increased stability in aqueous solutions containing less than 40 vol % THF, as was also observed for bipyridine-BTA-based SCPNs. Besides the strong entropy gains of the release of solvated water upon aggregation, the entropic penalty of a decrease in flexibility of the side chains may lead to counterintuitive, strong differences in subtly different monomers.205 Besides stability, hydrogen bonded water can also influence the dynamic behavior of supramolecular polymers. In sugar-decorated, water-soluble BTA derivatives, hydrogen bonding of water was found to increase the dynamic behavior of the supramolecular polymers.206 In a series of phthalonitriles functionalized with linear peptides, the balance between hydrogen bonding and hydrophobicity directed the flexibility but not length of the supramolecular polymers.207
Taken together, the recent literature shows that the strong enthalpic components introduced by the hydrogen bonding and dipolar interactions of water with supramolecular polymers introduce behavior in these materials that is unique to aqueous systems. As a result of the directionality and strong enthalpic contributions in these interactions, solvent effects on supramolecular polymer are often fundamentally different from many of the effects observed in organic solvents.
Future Perspectives and Outlook
The interactions between solvents and their solutes are among the most fundamental interactions in chemistry and drive many technologically and biologically relevant phenomena. Although long studied, the intricate aspects of these ubiquitous interactions still have many unsolved challenges. Over the past decade, the understanding of solvent interactions in supramolecular systems has steadily gained attention. Through the application of rationally designed solvent interactions, promising examples of very interesting material behavior have been described. To unravel unexplored mechanisms in solute–solvent interactions, supramolecular systems, and in particular supramolecular polymers, offer interesting opportunities with much untapped potential. The many recent examples presented here, show that the solute–solvent interactions are as important as the solute–solute interactions. Especially with the use of chiral solvents and (the properties) of solvent mixtures, unprecedented insights can be obtained. We anticipate a rediscovery of the physical organic chemistry that fueled the discovery of many solvent effects in chemical reactivity and the structure of covalent polymers in the 1960s and 1970s. From this, a nuanced view of the balance between solute–solute, solute–solvent, and solvent–solvent interactions in supramolecular materials will emerge.
To arrive at a level of complexity in supramolecular structures as seen in biology, it is essential that the energetic contributions to the stability of the supramolecular aggregate can be deconstructed to the submolecular level, that is, the various functional groups. Thorough and systematic studies in combination with detailed computational investigations that take all solute–solute, solute–solvent, and solvent–solvent interactions into account are essential to arrive at this level of understanding.
Although the number of components is still limited in the studies published so far, the emergence of molecular complexity using multistep noncovalent synthesis requires careful choice of the solvent combinations used. Challenges foreseen are in the dynamic synthesis of an initial structure of a complex in one solvent, followed by passivation in another solvent, before the second step in the noncovalent synthesis is started. Like in a multistep synthesis using covalent bonds, the need to optimize temperature, concentration, and solvents used is equally important.208
We envision that through a thorough understanding of the intricate role of the solvent in supramolecular systems, novel adaptive systems can be synthesized. The recent insights into the role of hydrogen-bonded cosolvents in supramolecular systems in aliphatic solvents shines an intriguing light on the role of structural water in lipophilic protein interiors. As such, we foresee that the insights gained in the supramolecular chemistry between solvents and solutes may have consequences in distant fields such as structural biology and advanced electronics.
Acknowledgments
We acknowledge Rienk Eelkema (TU Delft) for a critical reading of our manuscript. We acknowledge financial support from NWO (TOP-PUNT Grant 10018944) and the Dutch Ministry of Education, Culture and Science (Gravitation program 024.001.035). This Perspective is dedicated to the memory of Professor Saul Winstein of UCLA for his eminent contributions to physical organic chemistry.
The authors declare no competing financial interest.
References
- Marcus Y. The Properties of Organic Liquids That Are Relevant to Their Use as Solvating Solvents. Chem. Soc. Rev. 1993, 22 (6), 409–416. 10.1039/cs9932200409. [DOI] [Google Scholar]
- Ball P. Water as an Active Constituent in Cell Biology. Chem. Rev. 2008, 108 (1), 74–108. 10.1021/cr068037a. [DOI] [PubMed] [Google Scholar]
- Dill K. A.; Bromberg S.; Yue K.; Chan H. S.; Ftebig K. M.; Yee D. P.; Thomas P. D. Principles of Protein Folding - A Perspective from Simple Exact Models. Protein Sci. 1995, 4 (4), 561–602. 10.1002/pro.5560040401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinoco I.; Bustamante C. How RNA Folds. J. Mol. Biol. 1999, 293 (2), 271–281. 10.1006/jmbi.1999.3001. [DOI] [PubMed] [Google Scholar]
- Banani S. F.; Lee H. O.; Hyman A. A.; Rosen M. K. Biomolecular Condensates: Organizers of Cellular Biochemistry. Nat. Rev. Mol. Cell Biol. 2017, 18 (5), 285–298. 10.1038/nrm.2017.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H.-X.; Pang X. Electrostatic Interactions in Protein Structure, Folding, Binding, and Condensation. Chem. Rev. 2018, 118 (4), 1691–1741. 10.1021/acs.chemrev.7b00305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudinger H. Über Polymerisation. Ber. Dtsch. Chem. Ges. B 1920, 53 (6), 1073–1085. 10.1002/cber.19200530627. [DOI] [Google Scholar]
- Aida T.; Takemura A.; Fuse M.; Inoue S. Synthesis of a Novel Amphiphilic Porphyrin Carrying Water-Soluble Polyether Side Chains of Controlled Chain Length. Formation of a Cofacial Molecular Assembly in Aqueous Media. J. Chem. Soc., Chem. Commun. 1988, (5), 391–393. 10.1039/c39880000391. [DOI] [Google Scholar]
- Ducharme Y.; Wuest J. D. Use of Hydrogen Bonds to Control Molecular Aggregation. Extensive, Self-Complementary Arrays of Donors and Acceptors. J. Org. Chem. 1988, 53 (24), 5787–5789. 10.1021/jo00259a037. [DOI] [Google Scholar]
- Fouquey C.; Lehn J.-M.; Levelut A.-M. Molecular Recognition Directed Self-Assembly of Supramolecular Liquid Crystalline Polymers from Complementary Chiral Components. Adv. Mater. 1990, 2 (5), 254–257. 10.1002/adma.19900020506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerkowski J. A.; Seto C. T.; Wierda D. A.; Whitesides G. M. The Design of Organic Structures in the Solid State: Hydrogen-Bonded Molecular “Tapes”. J. Am. Chem. Soc. 1990, 112 (24), 9025–9026. 10.1021/ja00180a083. [DOI] [Google Scholar]
- Aida T.; Meijer E. W. Supramolecular Polymers – We’ve Come Full Circle. Isr. J. Chem. 2020, 60 (1–2), 33–47. 10.1002/ijch.201900165. [DOI] [Google Scholar]
- Brunsveld L.; Folmer B. J.; Meijer E. W.; Sijbesma R. P. Supramolecular Polymers. Chem. Rev. 2001, 101 (12), 4071–4098. 10.1021/cr990125q. [DOI] [PubMed] [Google Scholar]
- De Greef T. F. A.; Smulders M. M. J.; Wolffs M.; Schenning A. P. H. J.; Sijbesma R. P.; Meijer E. W. Supramolecular Polymerization. Chem. Rev. 2009, 109 (11), 5687–5754. 10.1021/cr900181u. [DOI] [PubMed] [Google Scholar]
- Stupp S. I.; Palmer L. C. Supramolecular Chemistry and Self-Assembly in Organic Materials Design. Chem. Mater. 2014, 26 (1), 507–518. 10.1021/cm403028b. [DOI] [Google Scholar]
- Aida T.; Meijer E. W.; Stupp S. I. Functional Supramolecular Polymers. Science 2012, 335 (6070), 813–817. 10.1126/science.1205962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayden J.; Greeves N.; Warren S.. Organic Chemistry, 2nd ed.; Oxford University Press: Oxford, 2012. [Google Scholar]
- Taft R. W. Sigma Values From Reactivities. J. Phys. Chem. 1960, 64 (12), 1805–1815. 10.1021/j100841a003. [DOI] [Google Scholar]
- Wells P. R. Linear Free Energy Relationships. Chem. Rev. 1963, 63 (2), 171–219. 10.1021/cr60222a005. [DOI] [Google Scholar]
- Anslyn E. V.; Dougherty D. A.. Modern Physical Organic Chemistry, 1st ed.; University Science Books: Sausalito, 2006. [Google Scholar]
- Hayama N.; Kobayashi Y.; Sekimoto E.; Miyazaki A.; Inamoto K.; Kimachi T.; Takemoto Y. A Solvent-Dependent Chirality-Switchable Thia-Michael Addition to α,β-Unsaturated Carboxylic Acids Using a Chiral Multifunctional Thiourea Catalyst. Chem. Sci. 2020, 11 (21), 5572–5576. 10.1039/D0SC01729A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunwald E.; Winstein S. The Correlation of Solvolysis Rates. J. Am. Chem. Soc. 1948, 70 (2), 846–854. 10.1021/ja01182a117. [DOI] [Google Scholar]
- Fainberg A. H.; Winstein S. Correlation of Solvolysis Rates. III. 1 t-Butyl Chloride in a Wide Range of Solvent Mixtures. J. Am. Chem. Soc. 1956, 78 (12), 2770–2777. 10.1021/ja01593a033. [DOI] [Google Scholar]
- Alder R. W.; Baker R.; Brown J. M.. Mechanisms in Organic Chemistry; John Wiley & Sons: London, 1971. [Google Scholar]
- Winstein S.; Fainberg A. H. Correlation of Solvolysis Rates. IV. Solvent Effects on Enthalpy and Entropy of Activation for Solvolysis of t-Butyl Chloride. J. Am. Chem. Soc. 1957, 79 (22), 5937–5950. 10.1021/ja01579a027. [DOI] [Google Scholar]
- Kosower E. M. The Effect of Solvent on Spectra. I. A New Empirical Measure of Solvent Polarity: Z-Values. J. Am. Chem. Soc. 1958, 80 (13), 3253–3260. 10.1021/ja01546a020. [DOI] [Google Scholar]
- Dimroth K.; Reichardt C.; Siepmann T.; Bohlmann F. Über Pyridinium-N-Phenol-Betaine Und Ihre Verwendung Zur Charakterisierung Der Polarität von Lösungsmitteln. Justus Liebigs Ann. Chem. 1963, 661 (1), 1–37. 10.1002/jlac.19636610102. [DOI] [Google Scholar]
- Parker A. J. Protic-Dipolar Aprotic Solvent Effects on Rates of Bimolecular Reactions. Chem. Rev. 1969, 69 (1), 1–32. 10.1021/cr60257a001. [DOI] [Google Scholar]
- Gutmann V. Solvent Effects on the Reactivities of Organometallic Compounds. Coord. Chem. Rev. 1976, 18 (2), 225–255. 10.1016/S0010-8545(00)82045-7. [DOI] [Google Scholar]
- Reichardt C.; Welton T.. Solvents and Solvent Effects in Organic Chemistry, 4th ed.; Wiley-VCH Verlag: Weinheim, Germany, 2011. [Google Scholar]
- Dainton F. S.; East G. C.; Harpell G. A.; Hurworth N. R.; Ivin K. J.; LaFlair R. T.; Pallen R. H.; Hui K. M. The Kinetics of Anionic Polymerization of Styrene and α-Methylstyrene. Effects of Counter-Ion and Solvent. Die Makromol. Makromol. Chem. 1965, 89 (1), 257–262. 10.1002/macp.1965.020890119. [DOI] [Google Scholar]
- van Beylen M.; Bhattacharyya D. N.; Smid J.; Szwarc M. Solvent Effects in Anionic Polymerization. The Behavior of Living Polystyrene in Tetrahydrofuran-Dioxane Mixtures. J. Phys. Chem. 1966, 70 (1), 157–161. 10.1021/j100873a024. [DOI] [Google Scholar]
- Shimomura T.; Smid J.; Szwarc M. Reactivities of Contact and Solvent-Separated Ion Pairs. Anionic Polymerization of Styrene in Dimethoxyethane. J. Am. Chem. Soc. 1967, 89 (23), 5743–5749. 10.1021/ja00999a001. [DOI] [Google Scholar]
- Overberger C. G.; Kamath V. G. Solvent Effects in Cationic Polymerization and Copolymerization. J. Am. Chem. Soc. 1963, 85 (4), 446–449. 10.1021/ja00887a017. [DOI] [Google Scholar]
- Kamachi M.; Liaw D. J.; Nozakura S. Solvent Effect on Radical Polymerization of Phenyl Methacrylate. Polym. J. 1977, 9 (3), 307–316. 10.1295/polymj.9.307. [DOI] [Google Scholar]
- Kamachi M.; Satoh J.; Liaw D. J.; Nazakura S. Solvent Effects on Radical Polymerization of Vinyl Benzoate and Phenyl Methacrylate. Macromolecules 1977, 10 (2), 501–502. 10.1021/ma60056a054. [DOI] [Google Scholar]
- Kamachi M.; Liaw D. J.; Nozakura S. Solvent Effect on Radical Polymerization of Vinyl Acetate. Polym. J. 1979, 11 (12), 921–928. 10.1295/polymj.11.921. [DOI] [Google Scholar]
- Plochocka K. Effect of the Reaction Medium on Radical Copolymerization. J. Macromol. Sci., Polym. Rev. 1981, 20 (1), 67–148. 10.1080/00222358108080015. [DOI] [Google Scholar]
- Braunecker W. A.; Tsarevsky N. V.; Gennaro A.; Matyjaszewski K. Thermodynamic Components of the Atom Transfer Radical Polymerization Equilibrium: Quantifying Solvent Effects. Macromolecules 2009, 42 (17), 6348–6360. 10.1021/ma901094s. [DOI] [Google Scholar]
- Jiang X.; Fleischmann S.; Nguyen N. H.; Rosen B. M.; Percec V. Cooperative and Synergistic Solvent Effects in SET-LRP of MA. J. Polym. Sci., Part A: Polym. Chem. 2009, 47 (21), 5591–5605. 10.1002/pola.23689. [DOI] [Google Scholar]
- Flory P. J. Thermodynamics of High Polymer Solutions. J. Chem. Phys. 1941, 9 (8), 660–660. 10.1063/1.1750971. [DOI] [Google Scholar]
- Huggins M. L. Solutions of Long Chain Compounds. J. Chem. Phys. 1941, 9 (5), 440–440. 10.1063/1.1750930. [DOI] [Google Scholar]
- Rubinstein M.; Colby R. H.. Polymer Physics, 1st ed.; Oxford University Press: Oxford, UK, 2003. [Google Scholar]
- Otto S. The Role of Solvent Cohesion in Nonpolar Solvation. Chem. Sci. 2013, 4 (7), 2953–2959. 10.1039/c3sc50740h. [DOI] [Google Scholar]
- Hildebrand J.; Scott R. L.. The Solubility of Nonelectrolytes, 3rd ed.; Reinhold: New York, 1950. [Google Scholar]
- Hildebrand J.; Scott R. L.. Regular Solutions, 1st ed.; Prentice-Hall: Englewood Cliffs, 1962. [Google Scholar]
- Hansen C. M.Hansen Solubility Parameters—A User’s Handbook, 2nd ed.; CRC Press: Boca Raton, 2007. [Google Scholar]
- Hansen C. M. 50 Years with Solubility Parameters—Past and Future. Prog. Org. Coat. 2004, 51 (1), 77–84. 10.1016/j.porgcoat.2004.05.004. [DOI] [Google Scholar]
- Lindvig T.; Michelsen M. L.; Kontogeorgis G. M. A Flory–Huggins Model Based on the Hansen Solubility Parameters. Fluid Phase Equilib. 2002, 203 (1–2), 247–260. 10.1016/S0378-3812(02)00184-X. [DOI] [Google Scholar]
- Raynal M.; Bouteiller L. Organogel Formation Rationalized by Hansen Solubility Parameters. Chem. Commun. 2011, 47 (29), 8271. 10.1039/c1cc13244j. [DOI] [PubMed] [Google Scholar]
- Green M. M.; Khatri C.; Peterson N. C. A Macromolecular Conformational Change Driven by a Minute Chiral Solvation Energy. J. Am. Chem. Soc. 1993, 115 (11), 4941–4942. 10.1021/ja00064a086. [DOI] [Google Scholar]
- Khatri C. A.; Pavlova Y.; Green M. M.; Morawetz H. Chiral Solvation as a Means to Quantitatively Characterize Preferential Solvation of a Helical Polymer in Mixed Solvents. J. Am. Chem. Soc. 1997, 119 (30), 6991–6995. 10.1021/ja9709637. [DOI] [Google Scholar]
- Yashima E.; Matsushima T.; Okamoto Y. Chirality Assignment of Amines and Amino Alcohols Based on Circular Dichroism Induced by Helix Formation of a Stereoregular Poly((4-Carboxyphenyl)Acetylene) through Acid–Base Complexation. J. Am. Chem. Soc. 1997, 119 (27), 6345–6359. 10.1021/ja964470y. [DOI] [Google Scholar]
- Maeda K.; Mochizuki H.; Watanabe M.; Yashima E. Switching of Macromolecular Helicity of Optically Active Poly(Phenylacetylene)s Bearing Cyclodextrin Pendants Induced by Various External Stimuli. J. Am. Chem. Soc. 2006, 128 (23), 7639–7650. 10.1021/ja060858+. [DOI] [PubMed] [Google Scholar]
- Yamada T.; Nagata Y.; Suginome M. Non-Hydrogen-Bonding-Based, Solvent-Dependent Helix Inversion between Pure P-Helix and Pure M-Helix in Poly(Quinoxaline-2,3-Diyl)s Bearing Chiral Side Chains. Chem. Commun. 2010, 46 (27), 4914–4916. 10.1039/c001564d. [DOI] [PubMed] [Google Scholar]
- Nagata Y.; Yamada T.; Adachi T.; Akai Y.; Yamamoto T.; Suginome M. Solvent-Dependent Switch of Helical Main-Chain Chirality in Sergeants-and-Soldiers-Type Poly(Quinoxaline-2,3-Diyl)s: Effect of the Position and Structures of the “Sergeant” Chiral Units on the Screw-Sense Induction. J. Am. Chem. Soc. 2013, 135 (27), 10104–10113. 10.1021/ja403391m. [DOI] [PubMed] [Google Scholar]
- Nagata Y.; Takeda R.; Suginome M. Asymmetric Catalysis in Chiral Solvents: Chirality Transfer with Amplification of Homochirality through a Helical Macromolecular Scaffold. ACS Cent. Sci. 2019, 5 (7), 1235–1240. 10.1021/acscentsci.9b00330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosawa F.; Kasai M. A Theory of Linear and Helical Aggregations of Macromolecules. J. Mol. Biol. 1962, 4 (1), 10–21. 10.1016/S0022-2836(62)80112-0. [DOI] [PubMed] [Google Scholar]
- Oosawa F. Size Distribution of Protein Polymers. J. Theor. Biol. 1970, 27 (1), 69–86. 10.1016/0022-5193(70)90129-3. [DOI] [PubMed] [Google Scholar]
- Sijbesma R. P.; Beijer F. H.; Brunsveld L.; Folmer B. J. B.; Hirschberg J. H. K. K.; Lange R. F. M.; Lowe J. K. L.; Meijer E. W. Reversible Polymers Formed from Self-Complementary Monomers Using Quadruple Hydrogen Bonding. Science 1997, 278 (5343), 1601–1604. 10.1126/science.278.5343.1601. [DOI] [PubMed] [Google Scholar]
- Xue M.; Yang Y.; Chi X.; Zhang Z.; Huang F. Pillararenes, A New Class of Macrocycles for Supramolecular Chemistry. Acc. Chem. Res. 2012, 45 (8), 1294–1308. 10.1021/ar2003418. [DOI] [PubMed] [Google Scholar]
- Barrow S. J.; Kasera S.; Rowland M. J.; del Barrio J.; Scherman O. A. Cucurbituril-Based Molecular Recognition. Chem. Rev. 2015, 115 (22), 12320–12406. 10.1021/acs.chemrev.5b00341. [DOI] [PubMed] [Google Scholar]
- Zhao D.; Moore J. S. Nucleation-Elongation: A Mechanism for Cooperative Supramolecular Polymerization. Org. Biomol. Chem. 2003, 1 (20), 3471–3491. 10.1039/B308788C. [DOI] [PubMed] [Google Scholar]
- Simic V.; Bouteiller L.; Jalabert M. Highly Cooperative Formation of Bis-Urea Based Supramolecular Polymers. J. Am. Chem. Soc. 2003, 125 (43), 13148–13154. 10.1021/ja037589x. [DOI] [PubMed] [Google Scholar]
- Jonkheijm P.; van der Schoot P.; Schenning A. P. H. J.; Meijer E. W. Probing the Solvent-Assisted Nucleation Pathway in Chemical Self-Assembly. Science 2006, 313 (5783), 80–83. 10.1126/science.1127884. [DOI] [PubMed] [Google Scholar]
- Cantekin S.; de Greef T. F. A.; Palmans A. R. A. Benzene-1,3,5-Tricarboxamide: A Versatile Ordering Moiety for Supramolecular Chemistry. Chem. Soc. Rev. 2012, 41 (18), 6125–6137. 10.1039/c2cs35156k. [DOI] [PubMed] [Google Scholar]
- Kulkarni C.; Meijer E. W.; Palmans A. R. A. Cooperativity Scale: A Structure–Mechanism Correlation in the Self-Assembly of Benzene-1,3,5-Tricarboxamides. Acc. Chem. Res. 2017, 50 (8), 1928–1936. 10.1021/acs.accounts.7b00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohr A.; Gress T.; Deppisch M.; Knoll M.; Würthner F. Synthesis of Merocyanine Dye Nanorods: The Importance of Solvent, Kinetic and Thermodynamic Control, and Steric Effects on Self-Assembly. Synthesis 2007, 2007 (19), 3073–3082. 10.1055/s-2007-983900. [DOI] [Google Scholar]
- Würthner F. Dipole–Dipole Interaction Driven Self-Assembly of Merocyanine Dyes: From Dimers to Nanoscale Objects and Supramolecular Materials. Acc. Chem. Res. 2016, 49 (5), 868–876. 10.1021/acs.accounts.6b00042. [DOI] [PubMed] [Google Scholar]
- Kaiser T. E.; Stepanenko V.; Würthner F. Fluorescent J-Aggregates of Core-Substituted Perylene Bisimides: Studies on Structure - Property Relationship, Nucleation - Elongation Mechanism, and Sergeants-and-Soldiers Principle. J. Am. Chem. Soc. 2009, 131, 6719–6732. 10.1021/ja900684h. [DOI] [PubMed] [Google Scholar]
- Würthner F.; Saha-Möller C. R.; Fimmel B.; Ogi S.; Leowanawat P.; Schmidt D. Perylene Bisimide Dye Assemblies as Archetype Functional Supramolecular Materials. Chem. Rev. 2016, 116 (3), 962–1052. 10.1021/acs.chemrev.5b00188. [DOI] [PubMed] [Google Scholar]
- Ogi S.; Sugiyasu K.; Manna S.; Samitsu S.; Takeuchi M. Living Supramolecular Polymerization Realized through a Biomimetic Approach. Nat. Chem. 2014, 6 (3), 188–195. 10.1038/nchem.1849. [DOI] [PubMed] [Google Scholar]
- Kang J.; Miyajima D.; Mori T.; Inoue Y.; Itoh Y.; Aida T. A Rational Strategy for the Realization of Chain-Growth Supramolecular Polymerization. Science 2015, 347 (6222), 646–651. 10.1126/science.aaa4249. [DOI] [PubMed] [Google Scholar]
- Wehner M.; Würthner F. Supramolecular Polymerization through Kinetic Pathway Control and Living Chain Growth. Nat. Rev. Chem. 2020, 4 (1), 38–53. 10.1038/s41570-019-0153-8. [DOI] [Google Scholar]
- Adelizzi B.; Van Zee N. J. J.; de Windt L. N. J.; Palmans A. R. A.; Meijer E. W. Future of Supramolecular Copolymers Unveiled by Reflecting on Covalent Copolymerization. J. Am. Chem. Soc. 2019, 141 (15), 6110–6121. 10.1021/jacs.9b01089. [DOI] [PubMed] [Google Scholar]
- Webber M. J.; Appel E. A.; Meijer E. W.; Langer R. Supramolecular Biomaterials. Nat. Mater. 2016, 15 (1), 13–26. 10.1038/nmat4474. [DOI] [PubMed] [Google Scholar]
- Goor O. J. G. M.; Hendrikse S. I. S.; Dankers P. Y. W.; Meijer E. W. From Supramolecular Polymers to Multi-Component Biomaterials. Chem. Soc. Rev. 2017, 46 (21), 6621–6637. 10.1039/C7CS00564D. [DOI] [PubMed] [Google Scholar]
- Ghosh S.; Praveen V. K.; Ajayaghosh A. The Chemistry and Applications of π-Gels. Annu. Rev. Mater. Res. 2016, 46 (1), 235–262. 10.1146/annurev-matsci-070115-031557. [DOI] [Google Scholar]
- Tabushi I. New Insights into the Host-Guest-Solvent Interaction of Some Inclusion Complexes. Inorg. Chim. Acta 1980, 40, X13–X14. 10.1016/S0020-1693(00)92048-6. [DOI] [Google Scholar]
- Schneider H.-J.; Philippi K.; Pöhlmann J. Structure- and Solvent-Dependence in the Complexation of Lipophilic Substrates in a Water-Soluble Azacyclophane. Angew. Chem., Int. Ed. Engl. 1984, 23 (11), 908–910. 10.1002/anie.198409081. [DOI] [Google Scholar]
- Ueno A.; Moriwaki F.; Matsue T.; Osa T.; Hamada F.; Murai K. Host-guest Complexation of Ferrocene-appended Β-cyclodextrin in Organic Solvents. Die Makromol. Makromol. Chem., Rapid Commun. 1985, 6 (4), 231–233. 10.1002/marc.1985.030060403. [DOI] [Google Scholar]
- Schneider H.-J. Binding Mechanisms in Supramolecular Complexes. Angew. Chem., Int. Ed. 2009, 48 (22), 3924–3977. 10.1002/anie.200802947. [DOI] [PubMed] [Google Scholar]
- Smithrud D. B.; Diederich F. Strength of Molecular Complexation of Apolar Solutes in Water and in Organic Solvents Is Predictable by Linear Free Energy Relationships: A General Model for Solvation Effects on Apolar Binding. J. Am. Chem. Soc. 1990, 112 (1), 339–343. 10.1021/ja00157a052. [DOI] [Google Scholar]
- Cubberley M. S.; Iverson B. L. 1 H NMR Investigation of Solvent Effects in Aromatic Stacking Interactions. J. Am. Chem. Soc. 2001, 123 (31), 7560–7563. 10.1021/ja015817m. [DOI] [PubMed] [Google Scholar]
- Reichardt C. Solvatochromic Dyes as Solvent Polarity Indicators. Chem. Rev. 1994, 94 (8), 2319–2358. 10.1021/cr00032a005. [DOI] [Google Scholar]
- Smithrud D. B.; Wyman T. B.; Diederich F. Enthalpically Driven Cyclophane-Arene Inclusion Complexation: Solvent-Dependent Calorimetric Studies. J. Am. Chem. Soc. 1991, 113 (14), 5420–5426. 10.1021/ja00014a038. [DOI] [Google Scholar]
- Chen Z.; Fimmel B.; Würthner F. Solvent and Substituent Effects on Aggregation Constants of Perylene Bisimide π-Stacks – a Linear Free Energy Relationship Analysis. Org. Biomol. Chem. 2012, 10 (30), 5845–5855. 10.1039/c2ob07131b. [DOI] [PubMed] [Google Scholar]
- Prentice G. M.; Pascu S. I.; Filip S. V.; West K. R.; Pantoş G. D. Aromatic Donor–Acceptor Interactions in Non-Polar Environments. Chem. Commun. 2015, 51 (39), 8265–8268. 10.1039/C5CC00507H. [DOI] [PubMed] [Google Scholar]
- Würthner F.; Yao S.; Debaerdemaeker T.; Wortmann R. Dimerization of Merocyanine Dyes. Structural and Energetic Characterization of Dipolar Dye Aggregates and Implications for Nonlinear Optical Materials. J. Am. J. Am. Chem. Soc. 2002, 124 (32), 9431–9447. 10.1021/ja020168f. [DOI] [PubMed] [Google Scholar]
- Hunter C. A.; Misuraca M. C.; Turega S. M. Solvent Effects on Chelate Cooperativity. Chem. Sci. 2012, 3 (2), 589–601. 10.1039/C1SC00635E. [DOI] [Google Scholar]
- Hunter C. A.; Misuraca M. C.; Turega S. M. Comparative Analysis of the Influence of H-Bond Strength and Solvent on Chelate Cooperativity in H-Bonded Supramolecular Complexes. Chem. Sci. 2012, 3 (8), 2462–2469. 10.1039/c2sc20358h. [DOI] [Google Scholar]
- Hunter C. A. Quantifying Intermolecular Interactions: Guidelines for the Molecular Recognition Toolbox. Angew. Chem., Int. Ed. 2004, 43 (40), 5310–5324. 10.1002/anie.200301739. [DOI] [PubMed] [Google Scholar]
- Driver M. D.; Williamson M. J.; Cook J. L.; Hunter C. A. Functional Group Interaction Profiles: A General Treatment of Solvent Effects on Non-Covalent Interactions. Chem. Sci. 2020, 11 (17), 4456–4466. 10.1039/D0SC01288B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biedermann F.; Schneider H.-J. Experimental Binding Energies in Supramolecular Complexes. Chem. Rev. 2016, 116 (9), 5216–5300. 10.1021/acs.chemrev.5b00583. [DOI] [PubMed] [Google Scholar]
- Hunter C. A.; Anderson H. L. What Is Cooperativity?. Angew. Chem., Int. Ed. 2009, 48 (41), 7488–7499. 10.1002/anie.200902490. [DOI] [PubMed] [Google Scholar]
- Beijer F. H.; Sijbesma R. P.; Kooijman H.; Spek A. L.; Meijer E. W. Strong Dimerization of Ureidopyrimidones via Quadruple Hydrogen Bonding. J. Am. Chem. Soc. 1998, 120 (27), 6761–6769. 10.1021/ja974112a. [DOI] [Google Scholar]
- Beijer F. H.; Sijbesma R. P.; Vekemans J. A. J. M.; Meijer E. W.; Kooijman H.; Spek A. L. Hydrogen-Bonded Complexes of Diaminopyridines and Diaminotriazines: Opposite Effect of Acylation on Complex Stabilities. J. Org. Chem. 1996, 61 (18), 6371–6380. 10.1021/jo960612v. [DOI] [PubMed] [Google Scholar]
- Blight B. A.; Hunter C. A.; Leigh D. A.; McNab H.; Thomson P. I. T. An AAAA–DDDD Quadruple Hydrogen-Bond Array. Nat. Chem. 2011, 3 (3), 244–248. 10.1038/nchem.987. [DOI] [PubMed] [Google Scholar]
- Leigh D. A.; Robertson C. C.; Slawin A. M. Z.; Thomson P. I. T. AAAA-DDDD Quadruple Hydrogen-Bond Arrays Featuring NH···N and CH···N Hydrogen Bonds. J. Am. Chem. Soc. 2013, 135 (26), 9939–9943. 10.1021/ja404504m. [DOI] [PubMed] [Google Scholar]
- Berl V.; Schmutz M.; Krische M. J.; Khoury R. G.; Lehn J. M. Supramolecular Polymers Generated from Heterocomplementary Monomers Linked through Multiple Hydrogen-Bonding Arrays - Formation, Characterization, and Properties. Chem. - Eur. J. 2002, 8 (5), 1227–1244. . [DOI] [PubMed] [Google Scholar]
- Harada A.; Takashima Y.; Yamaguchi H. Cyclodextrin-Based Supramolecular Polymers. Chem. Soc. Rev. 2009, 38 (4), 875–882. 10.1039/b705458k. [DOI] [PubMed] [Google Scholar]
- Korevaar P. A.; Schaefer C.; De Greef T. F. A. A.; Meijer E. W. Controlling Chemical Self-Assembly by Solvent-Dependent Dynamics. J. Am. Chem. Soc. 2012, 134 (32), 13482–13491. 10.1021/ja305512g. [DOI] [PubMed] [Google Scholar]
- England J. L.; Haran G. Role of Solvation Effects in Protein Denaturation: From Thermodynamics to Single Molecules and Back. Annu. Rev. Phys. Chem. 2011, 62 (1), 257–277. 10.1146/annurev-physchem-032210-103531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canchi D. R.; García A. E. Cosolvent Effects on Protein Stability. Annu. Rev. Phys. Chem. 2013, 64 (1), 273–293. 10.1146/annurev-physchem-040412-110156. [DOI] [PubMed] [Google Scholar]
- Myers J. K.; Nick Pace C.; Martin Scholtz J. Denaturant m Values and Heat Capacity Changes: Relation to Changes in Accessible Surface Areas of Protein Unfolding. Protein Sci. 1995, 4 (10), 2138–2148. 10.1002/pro.5560041020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buendía J.; Greciano E. E.; Sánchez L. Influence of Axial and Point Chirality in the Chiral Self-Assembly of Twin N-Annulated Perylenecarboxamides. J. Org. Chem. 2015, 80 (24), 12444–12452. 10.1021/acs.joc.5b02309. [DOI] [PubMed] [Google Scholar]
- García F.; Korevaar P. A.; Verlee A.; Meijer E. W.; Palmans A. R. A.; Sánchez L. The Influence of π-Conjugated Moieties on the Thermodynamics of Cooperatively Self-Assembling Tricarboxamides. Chem. Commun. 2013, 49 (77), 8674–8676. 10.1039/c3cc43845g. [DOI] [PubMed] [Google Scholar]
- Wang C.; Chen Z.; Liu M.; Zhong H.; Wang F. Cooperative Supramolecular Polymerization of Phosphorescent Alkynyl-Gold(I)–Isocyanide Complexes. Polym. Chem. 2019, 10 (23), 3210–3216. 10.1039/C9PY00548J. [DOI] [Google Scholar]
- Buendía J.; Sánchez L. Solvent-Dependent Disassembly of Amphiphilic OPE-Based Tricarboxamides. Org. Lett. 2013, 15 (22), 5746–5749. 10.1021/ol402788n. [DOI] [PubMed] [Google Scholar]
- Berrocal J. A.; Mabesoone M. F. J.; García Iglesias M.; Huizinga A.; Meijer E. W.; Palmans A. R. A. Selenoamides Modulate Dipole–Dipole Interactions in Hydrogen Bonded Supramolecular Polymers of 1,3,5-Substituted Benzenes. Chem. Commun. 2019, 55 (99), 14906–14909. 10.1039/C9CC08423A. [DOI] [PubMed] [Google Scholar]
- Mabesoone M. F. J.; Markvoort A. J.; Banno M.; Yamaguchi T.; Helmich F.; Naito Y.; Yashima E.; Palmans A. R. A.; Meijer E. W. Competing Interactions in Hierarchical Porphyrin Self-Assembly Introduce Robustness in Pathway Complexity. J. Am. Chem. Soc. 2018, 140 (25), 7810–7819. 10.1021/jacs.8b02388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitzler-Kunkel A.; Kirchner E.; Bialas D.; Simon C.; Würthner F. Spacer-Modulated Differentiation Between Self-Assembly and Folding Pathways for Bichromophoric Merocyanine Dyes. Chem. - Eur. J. 2015, 21 (42), 14851–14861. 10.1002/chem.201502434. [DOI] [PubMed] [Google Scholar]
- Obrzud M.; Rospenk M.; Koll A. Self-Aggregation Mechanisms of N-Alkyl Derivatives of Urea and Thiourea. Phys. Chem. Chem. Phys. 2014, 16 (7), 3209–3219. 10.1039/c3cp53582g. [DOI] [PubMed] [Google Scholar]
- Caturello N. A. M. S.; Csók Z.; Fernández G.; Albuquerque R. Q. Influence of Metal, Ligand and Solvent on Supramolecular Polymerizations with Transition-Metal Compounds: A Theoretical Study. Chem. - Eur. J. 2016, 22 (49), 17681–17689. 10.1002/chem.201603600. [DOI] [PubMed] [Google Scholar]
- Isare B.; Pensec S.; Raynal M.; Bouteiller L. Bisurea-Based Supramolecular Polymers: From Structure to Properties. C. R. Chim. 2016, 19 (1–2), 148–156. 10.1016/j.crci.2015.06.013. [DOI] [Google Scholar]
- Bouteiller L.; Colombani O.; Lortie F.; Terech P. Thickness Transition of a Rigid Supramolecular Polymer. J. Am. Chem. Soc. 2005, 127 (24), 8893–8898. 10.1021/ja0511016. [DOI] [PubMed] [Google Scholar]
- Ouhib F.; Raynal M.; Jouvelet B.; Isare B.; Bouteiller L. Hydrogen Bonded Supramolecular Polymers in Moderately Polar Solvents. Chem. Commun. 2011, 47 (38), 10683–10685. 10.1039/c1cc14590h. [DOI] [PubMed] [Google Scholar]
- Obert E.; Bellot M.; Bouteiller L.; Andrioletti F.; Lehen-Ferrenbach C.; Boué F. Both Water- and Organo-Soluble Supramolecular Polymer Stabilized by Hydrogen-Bonding and Hydrophobic Interactions. J. Am. Chem. Soc. 2007, 129 (50), 15601–15605. 10.1021/ja074296l. [DOI] [PubMed] [Google Scholar]
- Pinault T.; Isare B.; Bouteiller L. Solvents with Similar Bulk Properties Induce Distinct Supramolecular Architectures. ChemPhysChem 2006, 7 (4), 816–819. 10.1002/cphc.200500636. [DOI] [PubMed] [Google Scholar]
- Alvarenga B. G.; Bernardino K.; de Moura A. F.; Sabadini E. Two Different Pathways for Assembling Bis-Urea in Benzene and Toluene. J. Mol. Model. 2018, 24 (7), 154. 10.1007/s00894-018-3688-6. [DOI] [PubMed] [Google Scholar]
- Dirany M.; Ayzac V.; Isare B.; Raynal M.; Bouteiller L. Structural Control of Bisurea-Based Supramolecular Polymers: Influence of an Ester Moiety. Langmuir 2015, 31 (42), 11443–11451. 10.1021/acs.langmuir.5b02974. [DOI] [PubMed] [Google Scholar]
- Roman M.; Cannizzo C.; Pinault T.; Isare B.; Andrioletti B.; Van Der Schoot P.; Bouteiller L. Supramolecular Balance: Using Cooperativity to Amplify Weak Interactions. J. Am. Chem. Soc. 2010, 132 (47), 16818–16824. 10.1021/ja105717u. [DOI] [PubMed] [Google Scholar]
- Bouteiller L.; Van Der Schoot P. Probing Weak Intermolecular Interactions in Self-Assembled Nanotubes. J. Am. Chem. Soc. 2012, 134 (2), 1363–1366. 10.1021/ja210706v. [DOI] [PubMed] [Google Scholar]
- Alvarenga B. G.; Raynal M.; Bouteiller L.; Sabadini E. Unexpected Solvent Influence on the Rheology of Supramolecular Polymers. Macromolecules 2017, 50 (17), 6631–6636. 10.1021/acs.macromol.7b00786. [DOI] [Google Scholar]
- Louhichi A.; Jacob A. R.; Bouteiller L.; Vlassopoulos D. Humidity Affects the Viscoelastic Properties of Supramolecular Living Polymers. J. Rheol. 2017, 61 (6), 1173–1182. 10.1122/1.4997600. [DOI] [Google Scholar]
- Ayzac V.; Raynal M.; Isare B.; Idé J.; Brocorens P.; Lazzaroni R.; Etienne T.; Monari A.; Assfeld X.; Bouteiller L. Probing Halogen–Halogen Interactions in Solution. Phys. Chem. Chem. Phys. 2017, 19 (48), 32443–32450. 10.1039/C7CP06996K. [DOI] [PubMed] [Google Scholar]
- Kulkarni C.; Bejagam K. K.; Senanayak S. P.; Narayan K. S.; Balasubramanian S.; George S. J. Dipole-Moment-Driven Cooperative Supramolecular Polymerization. J. Am. Chem. Soc. 2015, 137 (11), 3924–3932. 10.1021/jacs.5b00504. [DOI] [PubMed] [Google Scholar]
- Nakano Y.; Hirose T.; Stals P. J. M.; Meijer E. W.; Palmans A. R. A. Conformational Analysis of Supramolecular Polymerization Processes of Disc-like Molecules. Chem. Sci. 2012, 3 (1), 148–155. 10.1039/C1SC00547B. [DOI] [Google Scholar]
- Nakano Y.; Markvoort A. J.; Cantekin S.; Filot I. A. W.; ten Eikelder H. M. M.; Meijer E. W.; Palmans A. R. A. Conformational Analysis of Chiral Supramolecular Aggregates: Modeling the Subtle Difference between Hydrogen and Deuterium. J. Am. Chem. Soc. 2013, 135 (44), 16497–16506. 10.1021/ja4073645. [DOI] [PubMed] [Google Scholar]
- Berrocal J. A.; Di Meo F.; García-Iglesias M.; Gosens R. P. J.; Meijer E. W.; Linares M.; Palmans A. R. A. Consequences of Conformational Flexibility in Hydrogen-Bond-Driven Self-Assembly Processes. Chem. Commun. 2016, 52 (72), 10870–10873. 10.1039/C6CC05593A. [DOI] [PubMed] [Google Scholar]
- Bejagam K. K.; Balasubramanian S. Supramolecular Polymerization: A Coarse Grained Molecular Dynamics Study. J. Phys. Chem. B 2015, 119 (17), 5738–5746. 10.1021/acs.jpcb.5b01655. [DOI] [PubMed] [Google Scholar]
- García-Iglesias M.; Mayoral M. J.; Serrano-Molina D.; Aparicio F.; Vázquez-González V.; González-Rodríguez D. Self-Assembly of Diacetylene-Bridged Phenylenevinylene Oligomers in Water and Organic Solvents. ChemPlusChem 2019, 84 (5), 488–492. 10.1002/cplu.201900207. [DOI] [PubMed] [Google Scholar]
- Buendía J.; García F.; Yélamos B.; Sánchez L. Transfer and Amplification of Chirality in Phe-Based C 3 -Symmetric Non-Ionic Amphiphiles. Chem. Commun. 2016, 52 (57), 8830–8833. 10.1039/C6CC04273B. [DOI] [PubMed] [Google Scholar]
- Pradeilles J. A.; Zhong S.; Baglyas M.; Tarczay G.; Butts C. P.; Myers E. L.; Aggarwal V. K. Odd–Even Alternations in Helical Propensity of a Homologous Series of Hydrocarbons. Nat. Chem. 2020, 12 (5), 475–480. 10.1038/s41557-020-0429-0. [DOI] [PubMed] [Google Scholar]
- Hollamby M. J.; Aratsu K.; Pauw B. R.; Rogers S. E.; Smith A. J.; Yamauchi M.; Lin X.; Yagai S. Simultaneous SAXS and SANS Analysis for the Detection of Toroidal Supramolecular Polymers Composed of Noncovalent Supermacrocycles in Solution. Angew. Chem., Int. Ed. 2016, 55 (34), 9890–9893. 10.1002/anie.201603370. [DOI] [PubMed] [Google Scholar]
- Kar H.; Ghosh G.; Ghosh S. Solvent Geometry Regulated Cooperative Supramolecular Polymerization. Chem. - Eur. J. 2017, 23 (44), 10536–10542. 10.1002/chem.201701299. [DOI] [PubMed] [Google Scholar]
- Kulkarni C.; Korevaar P. A.; Bejagam K. K.; Palmans A. R. A.; Meijer E. W.; George S. J. Solvent Clathrate Driven Dynamic Stereomutation of a Supramolecular Polymer with Molecular Pockets. J. Am. Chem. Soc. 2017, 139 (39), 13867–13875. 10.1021/jacs.7b07639. [DOI] [PubMed] [Google Scholar]
- Korevaar P. A.; Grenier C.; Markvoort A. J.; Schenning A. P. H. J.; De Greef T. F. A.; Meijer E. W. Model-Driven Optimization of Multicomponent Self-Assembly Processes. Proc. Natl. Acad. Sci. U. S. A. 2013, 110 (43), 17205–17210. 10.1073/pnas.1310092110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Zwaag D.; Pieters P. A.; Korevaar P. A.; Markvoort A. J.; Spiering A. J. H.; De Greef T. F. A.; Meijer E. W. Kinetic Analysis as a Tool to Distinguish Pathway Complexity in Molecular Assembly: An Unexpected Outcome of Structures in Competition. J. Am. Chem. Soc. 2015, 137 (39), 12677–12688. 10.1021/jacs.5b08138. [DOI] [PubMed] [Google Scholar]
- Veling N.; Van Hameren R.; Van Buul A. M.; Rowan A. E.; Nolte R. J. M.; Elemans J. A. A. W. Solvent-Dependent Amplification of Chirality in Assemblies of Porphyrin Trimers Based on Benzene Tricarboxamide. Chem. Commun. 2012, 48 (36), 4371–4373. 10.1039/c2cc31156a. [DOI] [PubMed] [Google Scholar]
- Matern J.; Kartha K. K.; Sánchez L.; Fernández G. Consequences of Hidden Kinetic Pathways on Supramolecular Polymerization. Chem. Sci. 2020, 11 (26), 6780–6788. 10.1039/D0SC02115F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boott C. E.; Leitao E. M.; Hayward D. W.; Laine R. F.; Mahou P.; Guerin G.; Winnik M. A.; Richardson R. M.; Kaminski C. F.; Whittell G. R.; Manners I. Probing the Growth Kinetics for the Formation of Uniform 1D Block Copolymer Nanoparticles by Living Crystallization-Driven Self-Assembly. ACS Nano 2018, 12 (9), 8920–8933. 10.1021/acsnano.8b01353. [DOI] [PubMed] [Google Scholar]
- Jarrett-Wilkins C. C.; He X.; Symons H. E.; Harniman R. L.; Faul C. F. J.; Manners I. Living Supramolecular Polymerisation of Perylene Diimide Amphiphiles by Seeded Growth under Kinetic Control. Chem. - Eur. J. 2018, 24 (58), 15556–15565. 10.1002/chem.201801424. [DOI] [PubMed] [Google Scholar]
- Jung S. H.; Bochicchio D.; Pavan G. M.; Takeuchi M.; Sugiyasu K. A Block Supramolecular Polymer and Its Kinetically Enhanced Stability. J. Am. Chem. Soc. 2018, 140 (33), 10570–10577. 10.1021/jacs.8b06016. [DOI] [PubMed] [Google Scholar]
- Ogi S.; Stepanenko V.; Sugiyasu K.; Takeuchi M.; Würthner F. Mechanism of Self-Assembly Process and Seeded Supramolecular Polymerization of Perylene Bisimide Organogelator. J. Am. Chem. Soc. 2015, 137 (9), 3300–3307. 10.1021/ja511952c. [DOI] [PubMed] [Google Scholar]
- Ogi S.; Grzeszkiewicz C.; Würthner F. Pathway Complexity in the Self-Assembly of a Zinc Chlorin Model System of Natural Bacteriochlorophyll J-Aggregates. Chem. Sci. 2018, 9 (10), 2768–2773. 10.1039/C7SC03725B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valera J. S.; Sánchez-Naya R.; Ramírez F. J.; Zafra J. L.; Gómez R.; Casado J.; Sánchez L. Solvent-Directed Helical Stereomutation Discloses Pathway Complexity on N-Heterotriangulene-Based Organogelators. Chem. - Eur. J. 2017, 23 (46), 11141–11146. 10.1002/chem.201702391. [DOI] [PubMed] [Google Scholar]
- Valera J. S.; Gómez R.; Sánchez L. Tunable Energy Landscapes to Control Pathway Complexity in Self-Assembled N -Heterotriangulenes: Living and Seeded Supramolecular Polymerization. Small 2018, 14 (3), 1702437. 10.1002/smll.201702437. [DOI] [PubMed] [Google Scholar]
- Sarazin D.; Schmutz M.; Guenet J.-M.; Petitjean A.; Lehn J. M. Structure of Supramolecular Polymers Generated via Self-Assembly through Hydrogen Bonds. Mol. Cryst. Liq. Cryst. 2007, 468 (1), 187–201. 10.1080/15421400701229990. [DOI] [Google Scholar]
- Krieg E.; Weissman H.; Shimoni E.; Bar On (Ustinov) A.; Rybtchinski B. Understanding the Effect of Fluorocarbons in Aqueous Supramolecular Polymerization: Ultrastrong Noncovalent Binding and Cooperativity. J. Am. Chem. Soc. 2014, 136 (26), 9443–9452. 10.1021/ja503906p. [DOI] [PubMed] [Google Scholar]
- Ahmed S.; Pramanik B.; Sankar K. N. A.; Srivastava A.; Singha N.; Dowari P.; Srivastava A.; Mohanta K.; Debnath A.; Das D. Solvent Assisted Tuning of Morphology of a Peptide-Perylenediimide Conjugate: Helical Fibers to Nano-Rings and Their Differential Semiconductivity. Sci. Rep. 2017, 7 (1), 9485. 10.1038/s41598-017-09730-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi M.; Masuo S. Colloidal Quantum Dot Arrangement Assisted by Perylene Bisimide Self-Assembly. Chem. - Eur. J. 2019, 25 (1), 167–172. 10.1002/chem.201805119. [DOI] [PubMed] [Google Scholar]
- Wagalgave S. M.; DucLa D.; Bhosale R. S.; Kobaisi M. Al; Jones L. A.; Bhosale S. V.; Bhosale S. V. Fabrication of Diverse Nano-Architectures through the Self-Assembly of a Naphthalene Diimide Derivative Bearing Four Carbamates. New J. Chem. 2018, 42 (9), 6785–6793. 10.1039/C7NJ04503D. [DOI] [Google Scholar]
- Ghosh G.; Ghosh S. Solvent Dependent Pathway Complexity and Seeded Supramolecular Polymerization. Chem. Commun. 2018, 54 (45), 5720–5723. 10.1039/C8CC02832J. [DOI] [PubMed] [Google Scholar]
- Markiewicz G.; Smulders M. M. J.; Stefankiewicz A. R. Steering the Self-Assembly Outcome of a Single NDI Monomer into Three Morphologically Distinct Supramolecular Assemblies, with Concomitant Change in Supramolecular Polymerization Mechanism. Adv. Sci. 2019, 6 (16), 1900577. 10.1002/advs.201900577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hameren R.; Van Buul A. M.; Castriciano M. A.; Villari V.; Micali N.; Schön P.; Speller S.; Scolaro L. M.; Rowan A. E.; Elemans J. A. A. W.; Nolte R. J. M. Supramolecular Porphyrin Polymers in Solution and at the Solid-Liquid Interface. Nano Lett. 2008, 8 (1), 253–259. 10.1021/nl072563f. [DOI] [PubMed] [Google Scholar]
- Cantekin S.; Nakano Y.; Everts J. C.; Van der Schoot P.; Meijer E. W.; Palmans A. R. A. A Stereoselectively Deuterated Supramolecular Motif to Probe the Role of Solvent during Self-Assembly Processes. Chem. Commun. 2012, 48 (32), 3803–3805. 10.1039/c2cc17284d. [DOI] [PubMed] [Google Scholar]
- Prabhu D. D.; Sivadas A. P.; Das S. Solvent Assisted Fluorescence Modulation of a C3-Symmetric Organogelator. J. Mater. Chem. C 2014, 2 (34), 7039–7046. 10.1039/C4TC01008F. [DOI] [Google Scholar]
- Isobe A.; Prabhu D. D.; Datta S.; Aizawa T.; Yagai S. Effect of an Aromatic Solvent on Hydrogen-Bond-Directed Supramolecular Polymerization Leading to Distinct Topologies. Chem. - Eur. J. 2020, 26 (41), 8997–9004. 10.1002/chem.202001344. [DOI] [PubMed] [Google Scholar]
- Datta S.; Kato Y.; Higashiharaguchi S.; Aratsu K.; Isobe A.; Saito T.; Prabhu D. D.; Kitamoto Y.; Hollamby M. J.; Smith A. J.; Dalgliesh R.; Mahmoudi N.; Pesce L.; Perego C.; Pavan G. M.; Yagai S. Self-Assembled Poly-Catenanes from Supramolecular Toroidal Building Blocks. Nature 2020, 583 (7816), 400–405. 10.1038/s41586-020-2445-z. [DOI] [PubMed] [Google Scholar]
- Seo H.; Go M.; Choi H.; Kim K. Y.; Choi Y.; Lee S. S.; Jung S. H.; Jung J. H. Peculiar Triarylamine-Based Co-Assembled Supramolecular Polymers That Exhibit Two Transition Temperatures in the Formation of a Coiled Helical Bundle. Chem. - Asian J. 2018, 13 (19), 2847–2853. 10.1002/asia.201800961. [DOI] [PubMed] [Google Scholar]
- Kim K. Y.; Kim C.; Choi Y.; Jung S. H.; Kim J. H.; Jung J. H. Helicity Control of Triphenylamine-Based Supramolecular Polymers: Correlation between Solvent Properties and Helicity in Supramolecular Gels. Chem. - Eur. J. 2018, 24 (45), 11763–11770. 10.1002/chem.201802086. [DOI] [PubMed] [Google Scholar]
- Chan M. H.-Y.; Leung S. Y.-L.; Yam V. W.-W. Controlling Self-Assembly Mechanisms through Rational Molecular Design in Oligo(p -Phenyleneethynylene)-Containing Alkynylplatinum(II) 2,6-Bis(N -Alkylbenzimidazol-2′-Yl)Pyridine Amphiphiles. J. Am. Chem. Soc. 2018, 140 (24), 7637–7646. 10.1021/jacs.8b03628. [DOI] [PubMed] [Google Scholar]
- Bäumer N.; Kartha K. K.; Allampally N. K.; Yagai S.; Albuquerque R. Q.; Fernández G. Exploiting Coordination Isomerism for Controlled Self-Assembly. Angew. Chem., Int. Ed. 2019, 58 (44), 15626–15630. 10.1002/anie.201908002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan P.; Li Y.; Li L.; Deng J.; Liu M. Multiresponsive Chiroptical Switch of an Azobenzene-Containing Lipid: Solvent, Temperature, and Photoregulated Supramolecular Chirality. J. Phys. Chem. B 2011, 115 (13), 3322–3329. 10.1021/jp110636b. [DOI] [PubMed] [Google Scholar]
- Anetai H.; Takeda T.; Hoshino N.; Araki Y.; Wada T.; Yamamoto S.; Mitsuishi M.; Tsuchida H.; Ogoshi T.; Akutagawa T. Circular Polarized Luminescence of Hydrogen-Bonded Molecular Assemblies of Chiral Pyrene Derivatives. J. Phys. Chem. C 2018, 122 (11), 6323–6331. 10.1021/acs.jpcc.7b12747. [DOI] [Google Scholar]
- Hifsudheen M.; Mishra R. K.; Vedhanarayanan B.; Praveen V. K.; Ajayaghosh A. The Helix to Super-Helix Transition in the Self-Assembly of π-Systems: Superseding of Molecular Chirality at Hierarchical Level. Angew. Chem., Int. Ed. 2017, 56 (41), 12634–12638. 10.1002/anie.201707392. [DOI] [PubMed] [Google Scholar]
- Lin Y.; Penna M.; Thomas M. R.; Wojciechowski J. P.; Leonardo V.; Wang Y.; Pashuck E. T.; Yarovsky I.; Stevens M. M. Residue-Specific Solvation-Directed Thermodynamic and Kinetic Control over Peptide Self-Assembly with 1D/2D Structure Selection. ACS Nano 2019, 13 (2), 1900–1909. 10.1021/acsnano.8b08117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmans A. R. A.; Vekemans J. A. J. M.; Havinga E. E.; Meijer E. W. Sergeants-and-Soldiers Principle in Chiral Columnar Stacks of Disc-Shaped Molecules with C3 Symmetry. Angew. Chem., Int. Ed. Engl. 1997, 36 (23), 2648–2651. 10.1002/anie.199726481. [DOI] [Google Scholar]
- Isare B.; Linares M.; Zargarian L. L.; Fermandjian S.; Miura M.; Motohashi S.; Vanthuyne N.; Lazzaroni R.; Bouteiller L. Chirality in Dynamic Supramolecular Nanotubes Induced by a Chiral Solvent. Chem. - Eur. J. 2010, 16 (1), 173–177. 10.1002/chem.200902399. [DOI] [PubMed] [Google Scholar]
- Ghosh S.; Li X.-Q.; Stepanenko V.; Würthner F. Control of H- and J-Type π Stacking by Peripheral Alkyl Chains and Self-Sorting Phenomena in Perylene Bisimide Homo- and Heteroaggregates. Chem. - Eur. J. 2008, 14 (36), 11343–11357. 10.1002/chem.200801454. [DOI] [PubMed] [Google Scholar]
- Ogi S.; Stepanenko V.; Thein J.; Würthner F. Impact of Alkyl Spacer Length on Aggregation Pathways in Kinetically Controlled Supramolecular Polymerization. J. Am. Chem. Soc. 2016, 138 (2), 670–678. 10.1021/jacs.5b11674. [DOI] [PubMed] [Google Scholar]
- Stepanenko V.; Li X.-Q.; Gershberg J.; Würthner F. Evidence for Kinetic Nucleation in Helical Nanofiber Formation Directed by Chiral Solvent for a Perylene Bisimide Organogelator. Chem. - Eur. J. 2013, 19 (13), 4176–4183. 10.1002/chem.201204146. [DOI] [PubMed] [Google Scholar]
- Xu H.; Ghijsens E.; George S. J.; Wolffs M.; Tomović Ž.; Schenning A. P. H. J.; De Feyter S. Chiral Induction and Amplification in Supramolecular Systems at the Liquid-Solid Interface. ChemPhysChem 2013, 14 (8), 1583–1590. 10.1002/cphc.201300212. [DOI] [PubMed] [Google Scholar]
- Li F.; Li X.; Wang Y.; Zhang X. Trismaleimide Dendrimers: Helix-to-Superhelix Supramolecular Transition Accompanied by White-Light Emission. Angew. Chem., Int. Ed. 2019, 58 (50), 17994–18002. 10.1002/anie.201908837. [DOI] [PubMed] [Google Scholar]
- George S. J.; Tomović Ž.; Schenning A. P. H. J.; Meijer E. W. Insight into the Chiral Induction in Supramolecular Stacks through Preferential Chiral Solvation. Chem. Commun. 2011, 47 (12), 3451–3453. 10.1039/c0cc04617e. [DOI] [PubMed] [Google Scholar]
- Shen Z.; Jiang Y.; Wang T.; Liu M. Symmetry Breaking in the Supramolecular Gels of an Achiral Gelator Exclusively Driven by π-π Stacking. J. Am. Chem. Soc. 2015, 137 (51), 16109–16115. 10.1021/jacs.5b10496. [DOI] [PubMed] [Google Scholar]
- Ślęczkowski M. L.; Mabesoone M. F. J.; Ślęczkowski P.; Palmans A. R. A.; Meijer E. W. Competition between Chiral Solvents and Chiral Monomers in the Helical Bias of Supramolecular Polymers. Nat. Chem. 2020, 10.1038/s41557-020-00583-0. [DOI] [PubMed] [Google Scholar]
- Greenfield J. L.; Evans E. W.; Di Nuzzo D.; Di Antonio M.; Friend R. H.; Nitschke J. R. Unraveling Mechanisms of Chiral Induction in Double-Helical Metallopolymers. J. Am. Chem. Soc. 2018, 140 (32), 10344–10353. 10.1021/jacs.8b06195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue S.; Xing P.; Zhang J.; Zeng Y.; Zhao Y. Diverse Role of Solvents in Controlling Supramolecular Chirality. Chem. - Eur. J. 2019, 25 (31), 7426–7437. 10.1002/chem.201900714. [DOI] [PubMed] [Google Scholar]
- Chapados C.; M. Leblanc R. Aggregation of Chlorophylls in Monolayers. Biophys. Chem. 1983, 17 (3), 211–244. 10.1016/0301-4622(83)87006-9. [DOI] [PubMed] [Google Scholar]
- Agostiano A.; Monica M. D.; Palazzo G.; Trotta M. Chlorophyll a Auto-Aggregation in Water Rich Region. Biophys. Chem. 1993, 47 (2), 193–202. 10.1016/0301-4622(93)85036-H. [DOI] [Google Scholar]
- Liu C.; Jin Q.; Lv K.; Zhang L.; Liu M. Water Tuned the Helical Nanostructures and Supramolecular Chirality in Organogels. Chem. Commun. 2014, 50 (28), 3702–3705. 10.1039/c4cc00311j. [DOI] [PubMed] [Google Scholar]
- Johnson R. S.; Yamazaki T.; Kovalenko A.; Fenniri H. Molecular Basis for Water-Promoted Supramolecular Chirality Inversion in Helical Rosette Nanotubes. J. Am. Chem. Soc. 2007, 129 (17), 5735–5743. 10.1021/ja0706192. [DOI] [PubMed] [Google Scholar]
- Francisco K. R.; Dreiss C. A.; Bouteiller L.; Sabadini E. Tuning the Viscoelastic Properties of Bis(Urea)-Based Supramolecular Polymer Solutions by Adding Cosolutes. Langmuir 2012, 28 (41), 14531–14539. 10.1021/la3025606. [DOI] [PubMed] [Google Scholar]
- Ogasawara M.; Lin X.; Kurata H.; Ouchi H.; Yamauchi M.; Ohba T.; Kajitani T.; Fukushima T.; Numata M.; Nogami R.; Adhikari B.; Yagai S. Water-Induced Self-Assembly of an Amphiphilic Perylene Bisimide Dyad into Vesicles, Fibers, Coils, and Rings. Mater. Chem. Front. 2018, 2 (1), 171–179. 10.1039/C7QM00494J. [DOI] [Google Scholar]
- Dong S.; Leng J.; Feng Y.; Liu M.; Stackhouse C. J.; Schönhals A.; Chiappisi L.; Gao L.; Chen W.; Shang J.; Jin L.; Qi Z.; Schalley C. A. Structural Water as an Essential Comonomer in Supramolecular Polymerization. Sci. Adv. 2017, 3 (11), eaao0900. 10.1126/sciadv.aao0900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Zee N. J.; Adelizzi B.; Mabesoone M. F. J.; Meng X.; Aloi A.; Zha R. H.; Lutz M.; Filot I. A. W.; Palmans A. R. A.; Meijer E. W. Potential Enthalpic Energy of Water in Oils Exploited to Control Supramolecular Structure. Nature 2018, 558 (7708), 100–103. 10.1038/s41586-018-0169-0. [DOI] [PubMed] [Google Scholar]
- Adelizzi B.; Filot I. A. W.; Palmans A. R. A.; Meijer E. W. Unravelling the Pathway Complexity in Conformationally Flexible N-Centered Triarylamine Trisamides. Chem. - Eur. J. 2017, 23 (25), 6103–6110. 10.1002/chem.201603938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabesoone M. F. J.; ter Huurne G. M.; Palmans A. R. A.; Meijer E. W. How Water in Aliphatic Solvents Directs the Interference of Chemical Reactivity in a Supramolecular System. J. Am. Chem. Soc. 2020, 142 (28), 12400–12408. 10.1021/jacs.0c04962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkata Rao K.; Miyajima D.; Nihonyanagi A.; Aida T. Thermally Bisignate Supramolecular Polymerization. Nat. Chem. 2017, 9 (11), 1133–1139. 10.1038/nchem.2812. [DOI] [PubMed] [Google Scholar]
- Rao K. V.; Mabesoone M. F. J.; Miyajima D.; Nihonyanagi A.; Meijer E. W.; Aida T. Distinct Pathways in “Thermally Bisignate Supramolecular Polymerization”: Spectroscopic and Computational Studies. J. Am. Chem. Soc. 2020, 142 (1), 598–605. 10.1021/jacs.9b12044. [DOI] [PubMed] [Google Scholar]
- Krieg E.; Bastings M. M. C.; Besenius P.; Rybtchinski B. Supramolecular Polymers in Aqueous Media. Chem. Rev. 2016, 116 (4), 2414–2477. 10.1021/acs.chemrev.5b00369. [DOI] [PubMed] [Google Scholar]
- Casellas N. M.; Pujals S.; Bochicchio D.; Pavan G. M.; Torres T.; Albertazzi L.; García-Iglesias M. From Isodesmic to Highly Cooperative: Reverting the Supramolecular Polymerization Mechanism in Water by Fine Monomer Design. Chem. Commun. 2018, 54 (33), 4112–4115. 10.1039/C8CC01259H. [DOI] [PubMed] [Google Scholar]
- Rest C.; Philips D. S.; Dünnebacke T.; Sutar P.; Sampedro A.; Droste J.; Stepanenko V.; Hansen M. R.; Albuquerque R. Q.; Fernández G. Tuning Aqueous Supramolecular Polymerization by an Acid-Responsive Conformational Switch. Chem. - Eur. J. 2020, 26 (44), 10005–10013. 10.1002/chem.202001566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillissen M. A. J.; Koenigs M. M. E.; Spiering J. J. H.; Vekemans J. A. J. M.; Palmans A. R. A.; Voets I. K.; Meijer E. W. Triple Helix Formation in Amphiphilic Discotics: Demystifying Solvent Effects in Supramolecular Self-Assembly. J. Am. Chem. Soc. 2014, 136 (1), 336–343. 10.1021/ja4104183. [DOI] [PubMed] [Google Scholar]
- ter Huurne G. M.; Gillissen M. A. J.; Palmans A. R. A.; Voets I. K.; Meijer E. W. The Coil-to-Globule Transition of Single-Chain Polymeric Nanoparticles with a Chiral Internal Secondary Structure. Macromolecules 2015, 48 (12), 3949–3956. 10.1021/acs.macromol.5b00604. [DOI] [Google Scholar]
- Yang C.; Li W.; Wu C. Laser Light-Scattering Study of Solution Dynamics of Water/Cycloether Mixtures. J. Phys. Chem. B 2004, 108 (31), 11866–11870. 10.1021/jp048356a. [DOI] [Google Scholar]
- Cohen E.; Weissman H.; Pinkas I.; Shimoni E.; Rehak P.; Král P.; Rybtchinski B. Controlled Self-Assembly of Photofunctional Supramolecular Nanotubes. ACS Nano 2018, 12 (1), 317–326. 10.1021/acsnano.7b06376. [DOI] [PubMed] [Google Scholar]
- Lafleur R. P. M.; Lou X.; Pavan G. M.; Palmans A. R. A.; Meijer E. W. Consequences of a Cosolvent on the Structure and Molecular Dynamics of Supramolecular Polymers in Water. Chem. Sci. 2018, 9 (29), 6199–6209. 10.1039/C8SC02257G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Q.; Xiao X.-S.; To W.-P.; Lu W.; Chen Y.; Low K.-H.; Che C.-M. Counteranion- and Solvent-Mediated Chirality Transfer in the Supramolecular Polymerization of Luminescent Platinum(II) Complexes. Angew. Chem., Int. Ed. 2018, 57 (52), 17189–17193. 10.1002/anie.201811943. [DOI] [PubMed] [Google Scholar]
- Görl D.; Würthner F. Entropically Driven Self-Assembly of Bolaamphiphilic Perylene Dyes in Water. Angew. Chem., Int. Ed. 2016, 55 (39), 12094–12098. 10.1002/anie.201606917. [DOI] [PubMed] [Google Scholar]
- Grande V.; Soberats B.; Herbst S.; Stepanenko V.; Würthner F. Hydrogen-Bonded Perylene Bisimide J-Aggregate Aqua Material. Chem. Sci. 2018, 9 (34), 6904–6911. 10.1039/C8SC02409J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syamala P. P. N.; Soberats B.; Görl D.; Gekle S.; Würthner F. Thermodynamic Insights into the Entropically Driven Self-Assembly of Amphiphilic Dyes in Water. Chem. Sci. 2019, 10 (40), 9358–9366. 10.1039/C9SC03103K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syamala P. P. N.; Würthner F. Modulation of the Self-assembly of π-Amphiphiles in Water from Enthalpy- to Entropy-Driven by Enwrapping Substituents. Chem. - Eur. J. 2020, 26 (38), 8426–8434. 10.1002/chem.202000995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenmakers S. M. C.; Leenders C. M. A.; Lafleur R. P. M.; Lou X.; Meijer E. W.; Pavan G. M.; Palmans A. R. A. Impact of the Water-Compatible Periphery on the Dynamic and Structural Properties of Benzene-1,3,5-Tricarboxamide Based Amphiphiles. Chem. Commun. 2018, 54 (79), 11128–11131. 10.1039/C8CC04818E. [DOI] [PubMed] [Google Scholar]
- Ahlers P.; Götz C.; Riebe S.; Zirbes M.; Jochem M.; Spitzer D.; Voskuhl J.; Basché T.; Besenius P. Structure and Luminescence Properties of Supramolecular Polymers of Amphiphilic Aromatic Thioether–Peptide Conjugates in Water. Polym. Chem. 2019, 10 (23), 3163–3169. 10.1039/C8PY01712C. [DOI] [Google Scholar]
- Vantomme G.; Meijer E. W. The Construction of Supramolecular Systems. Science 2019, 363 (6434), 1396–1397. 10.1126/science.aav4677. [DOI] [PubMed] [Google Scholar]