Abstract
Background
Nivolumab is known to demonstrate superior overall survival compared with docetaxel in pretreated non‐small cell lung cancer (NSCLC) patients. Programmed death‐ligand 1 (PD‐L1) expression is reported to predict the outcome of treatment by nivolumab in lung cancer patients. However, the significance of the morphological characteristics of chest computed tomography (CT) as predictors of nivolumab efficacy for advanced NSCLC patients remains unknown.
Methods
We performed a multicenter retrospective trial from April 2013 to March 2017, to assess the significance of CT morphological characteristics as predictors of nivolumab efficacy for advanced NSCLC patients. A total of 78 NSCLC patients pretreated with nivolumab were enrolled. A chest radiologist used chest CT to assess the following morphological characteristics of each patient's main tumor and intrathoracic status prior to nivolumab treatment; interstitial septal thickening, peritumoral ground‐glass opacity, spiculated margin, air bronchogram, cavity or necrosis, adjacent organ invasion, bulky lymph node, and accumulation of small lymph nodes. Logistic regression and Cox proportional hazards regression models were used to analyze outcomes.
Results
A total of 60 (77%) patients were male and 72 (92%) had a performance status (PS) of 0 or 1. The objective response rates of male patients and heavy smokers were significantly higher than those of female patients and light or never smokers, respectively. Multivariate analysis identified light or never smoking, poor PS, histological type of squamous cell carcinoma, and interstitial septal thickening as independent negative predictors of progression free survival (PFS).
Conclusions
Interstitial septal thickening was a significant and independent predictor of PFS in NSCLC patients treated with nivolumab.
Key points
Significant findings of the study
Interstitial septal thickening is an independent predictor of progression free survival in non‐small lung cancer patients treated with nivolumab.
What this study adds
The current study reveals the significance of morphological characteristics obtained via chest computed tomography as a predictor of nivolumab efficacy for advanced non‐small cell lung cancer patients.
Keywords: Computed tomography, interstitial septal thickening, nivolumab, non‐small cell lung cancer, predictive biomarker
Interstitial septal thickening is an independent predictor of progression‐free survival in non‐small lung cancer patients treated with nivolumab.
Introduction
Programmed cell death (PD)‐1 immune checkpoint inhibitors have emerged as a promising treatment option for multiple cancer types. PD‐1 is a receptor expressed on the surface of activated T cells. 1 Programmed death‐ligand 1(PD‐L1) is a ligand of PD‐1 that inhibits T cell activation and promotes tumor immune escape. 2 , 3 Nivolumab, a fully humanized immunoglobulin G4 PD‐1 antibody, binds with high affinity to PD‐1, and activates T cell effector function. 4
Treatment using nivolumab has been shown to lead to superior overall survival in comparison to docetaxel in pretreated patients with non‐small cell lung cancer (NSCLC), 5 , 6 and is administered in patients with previously treated NSCLC. However, less than 20% of these patients achieve a durable response with nivolumab treatment. Therefore, patient selection is crucial in order to optimize the survival benefit of nivolumab. An association between PD‐L1 expression and nivolumab efficacy has been reported; however, some patients with negative PD‐L1 expression tumors have been reported to have achieved treatment efficacy. 5 , 7 The ability of PD‐L1 expression to predict the outcome of nivolumab treatment in lung cancer patients is still controversial. Tumor mutation burden has been reported to be a prognostic biomarker of nivolumab in advanced NSCLC. 8 In addition, some clinical biomarkers, such as neutrophil‐to‐lymphocyte ratio 9 or immune related adverse events, 10 are reported to be predictive biomarkers for nivolumab response.
Chest computed tomography (CT) is an essential investigation method for the diagnosis of lung cancer, and for assessing lung cancer response to chemotherapy. 11 However, the predictive value of CT morphological characteristics prior to treatment with nivolumab is unclear. Here, we performed a retrospective analysis to investigate whether the therapeutic response to nivolumab could be predicted by the morphological characteristics on CT images.
Methods
Patients
We reviewed the medical records of patients with recurrent or advanced NSCLC who were treated with nivolumab and were followed‐up for at least three months at Gunma Prefectural Cancer institute, Ibaraki Central Hospital, Fukushima Medical University Hospital, Tochigi Cancer Center Hospital or Aizu Medical Center Hospital between April 2013 and March 2017. We identified a total of 115 pretreated advanced NSCLC patients who had been treated with nivolumab. Of these patients, 78 had undergone chest CT imaging before initiation of nivolumab treatment. CT data was delivered using the raw Digital Imaging and Communications in Medicine (DICOM) format. Tumor response was assessed by CT according to the Response Evaluation Criteria in Solid Tumor, version 1.1. 12 Objective response rate (ORR) was defined as the proportion of patients whose best response was either complete response (CR) or partial response (PR). Disease control rate (DCR) was defined as the proportion of patients whose best responses were CR, PR or stable disease (SD) against the total number of subjects. Progression‐free‐survival (PFS) was defined as the duration between initiation of treatment and clinical or radiographic progression, or death from any cause. Overall survival (OS) was defined as the duration between first‐line treatment initiation and death. Nivolumab was administered intravenously at a dose of 3 mg/kg every two weeks. The present study was performed according to the protocols approved by the institutional review boards of each hospital; informed consent was waived for retrospective review of patient records and images. The study is registered with the University Hospital Medical Information Network Clinical Trial Registry in Japan (UMIN000026294).
CT scan
CT was performed as a part of a routine examination for the evaluation of lung cancer and was performed using 5 mm and 2 mm collimations. Most CT images were photographed using both mediastinal (level, 40 HU; width, 400 HU) and lung (level, −600 HU; width, 1600 HU) window settings; however, some patients' data could only be analyzed at the mediastinal setting.
Morphological characteristics
Morphological characteristics were selected from General Rule for Clinical and Pathological Record of Lung Cancer, eighth edition. 13 Most patients had advanced disease, and measurement of solitary lesions therefore was difficult. The CT scan had a 5 mm slice thickness, and some CT images were photographed in the mediastinal setting; we considered measurability, and selected the following target morphological characteristics: peritumoral interstitial septal thickening, peritumoral ground‐glass opacity, spiculated margin, air bronchogram, cavity or necrosis, adjacent organ invasion (Fig 1), bulky lymph node (≥ 2.5 cm), and accumulation of small lymph nodes. A chest radiologist reviewed the aforesaid characteristics on all patients' CT images, and was blinded to the patients' clinical histories and data.
Figure 1.
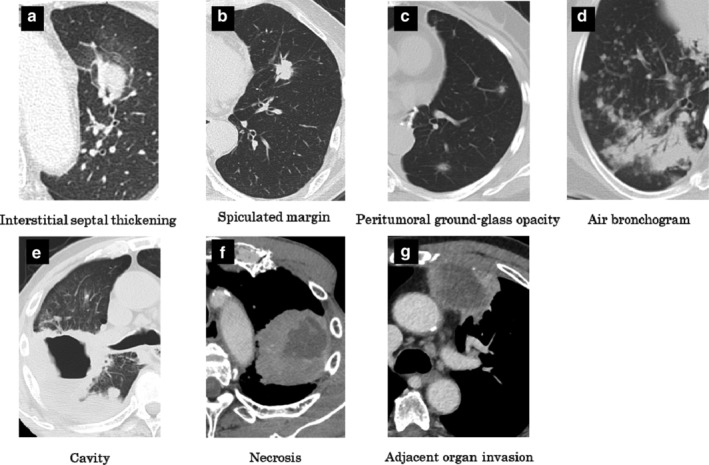
Representative graphics of morphological characteristics of lung cancer assessed by computer tomography scan. (a) Interstitial septal thickening: the surrounding bronchial vascular bundle of the left upper lobe lesion presents interstitial septal thickening. (b) Spiculated margin: the fluffy shadow projects from the tumor, reaching the interlobar pleura. (c) Peritumoral ground‐glass opacity: tumors are surrounded by a region like ground‐glass. (d) Air bronchogram: in the infiltrative shadow, radiolucent shadows of the bronchus are observed. (e) Cavity: the right lower lobe lesion was a cavitary lesion. (f) Necrosis: low‐density area in the left upper lobe tumor suggests the presence of necrotic materials. (g) Adjacent organ invasion: left upper lobe tumor invades the chest wall.
Statistical analysis
Statistical analysis with a Chi‐squared test was used to evaluate the clinical parameters associated with nivolumab efficacy. Kaplan‐Meier analysis of PFS was performed on these clinical characteristics, with differences between each pair of groups being assessed using the log‐rank test. The hazard ratio (HR) and 95% confidence interval (CI) were calculated using the univariate Cox proportional hazard model. Two‐sided P‐values of <0.05 were considered statistically significant. A multivariate Cox proportional hazards model was developed for clinically relevant factors and covariates that were determined to be statistically significant in the univariate analysis. The database was locked on 12 January 2018. All statistical analyses were performed using the STATA/SE version 14 statistical software package (StatCorp., College Station, TX, USA).
Results
Patient demographics
The study population consisted of 78 patients (60 men and 18 women; median age, 65; range, 32–84 years), whose characteristics are summarized in Table 1. Among all patients, 92% had a PS of 0 or 1 and 62% had adenocarcinoma. The ORR and DCR were 17.9% and 48.7%, respectively. The Kaplan‐Meier curves for PFS and OS are shown in Fig 2. The ORR of nine patients were not evaluated. At the time of the database lock, 61 PFS and 27 OS events had occurred.
Table 1.
Patient characteristics
| Items | Median (range) or number |
|---|---|
| Age | 65 (32–84) |
| Male/female | 60/18 |
| ECOG performance status 0 or 1/2/3 | 72/5/1 |
| Histology (adeno/squamous/others) | 48/18/12 |
| Clinical stage IIB/IIIA and B/IV | 1/14/51 |
| Smoking (heavy/light or never) | 56/22 |
| EGFR mutation/ALK translocation | 9/0 |
| Treatment line second/third or more | 44/34 |
| CT morphological characteristics | |
| Interstitial septal thickening | 17 |
| Peritumoral ground‐glass opacity | 41 |
| Spiculated margin | 16 |
| Air bronchogram | 8 |
| Cavity or necrosis | 16 |
| Adjacent organ invasion | 11 |
| Bulky lymph node (≥2.5 cm) | 11 |
| Accumulation of small lymph nodes | 4 |
N = 78.
Clinical stage was classified using TNM version 7.
Heavy smoker, Brinkman index ≥400; Light or never smoker, Brinkman index <400.
Figure 2.
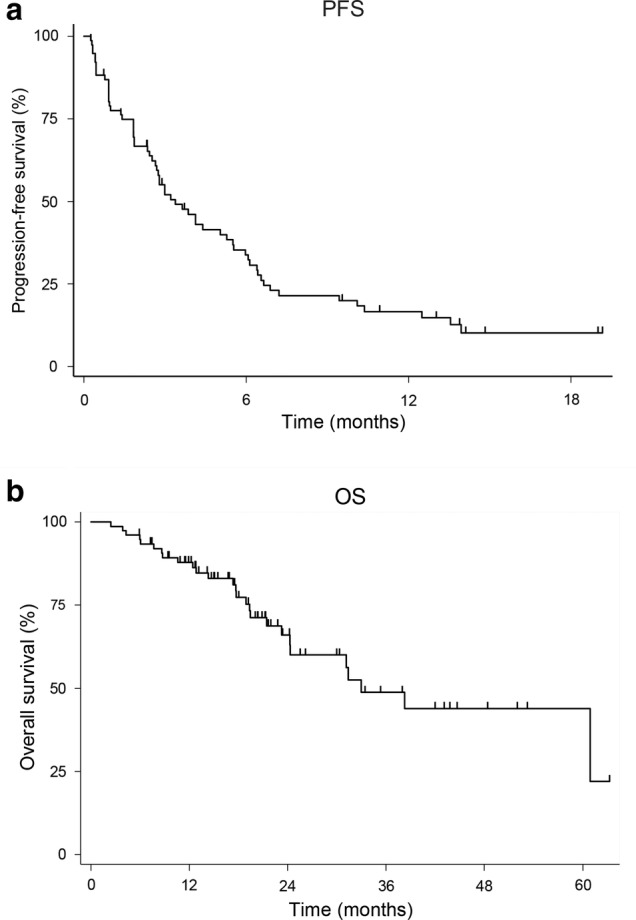
Progression‐free survival and overall survival among the study patients. Kaplan–Meier curves for progression‐free survival (a) and overall survival (b) are shown.
The patients' characteristics of squamous and nonsquamous cell carcinoma were as follows. The median age of patients with squamous cell carcinoma was 70 years and that of patients with nonsquamous cell carcinoma was 65.5 years (data not shown); however, the difference did not reach statistical significance (P = 0.097). A total of 94% of the patients with squamous cell carcinoma were heavy smokers, compared with 60% of those with nonsquamous cell carcinoma (P = 0.015). However, the proportions of gender and PS were not significantly different between the patients with squamous and nonsquamous cell carcinoma.
Efficacy
The details of the ORR of nivolumab based upon the clinical parameters are shown in Table 2. The ORRs of the male patients and heavy smokers were significantly higher than those of the female patients and light or never smokers, respectively.
Table 2.
Difference of the response to nivolumab according to the clinical parameters and CT morphological characteristics
| Parameters | Response to nivolumab, N | P‐value | |
|---|---|---|---|
| Age | ≥75 (n = 11) | 2 | 0.85 |
| <75 (n = 58) | 12 | ||
| Gender | Male (n = 52) | 14 | < 0.05 |
| Female (n = 17) | 0 | ||
| Smoking | Heavy smoker (n = 49) | 14 | < 0.05 |
| Light or never smoker (n = 20) | 0 | ||
| ECOG performance status | 0,1 (n = 64) | 13 | 0.987 |
| 2 (n = 5) | 1 | ||
| EGFR mutation | Positive (n = 8) | 1 | 0.547 |
| Negative (n = 60) | 13 | ||
| Histology | Squamous cell carcinoma (n = 16) | 4 | 0.286 |
| Others (n = 53) | 10 | ||
| Interstitial septal thickening | Positive (n = 16) | 1 | 0.093 |
| Negative (n = 50) | 13 | ||
| Peritumoral ground‐glass opacity | Positive (n = 37) | 5 | 0.099 |
| Negative (n = 30) | 9 | ||
| Spiculated margin | Positive (n = 13) | 1 | 0.21 |
| Negative (n = 56) | 13 | ||
| Air bronchogram | Positive (n = 6) | 1 | 0.817 |
| Negative (n = 63) | 13 | ||
| Cavity or necrosis | Positive (n = 14) | 3 | 0.906 |
| Negative (n = 55) | 11 | ||
| Adjacent organ invasion | Positive (n = 8) | 1 | 0.56 |
| Negative (n = 61) | 13 | ||
| Bulky lymph node (≥2.5 cm) | Positive (n = 10) | 2 | 0.98 |
| Negative (n = 59) | 12 | ||
| Accumulation of small lymph nodes | Positive (n = 3) | 1 | 0.566 |
| Negative (n = 66) | 13 |
Objective response rate could be evaluated in 69 of 78 subjects.
Heavy smoker, Brinkman index ≥400; Light or never smoker, Brinkman index <400.
Survival analysis
Univariate Cox proportional hazard analysis revealed that poorer ECOG PS, squamous cell carcinoma, and interstitial septal thickening were predictors of shorter PFS in the patients treated with nivolumab (Table 3). Furthermore, multivariate Cox analysis demonstrated that interstitial septal thickening was a predictor of shorter PFS, independent of other clinical features (Table 4).
Table 3.
Univariate Cox proportional hazard analysis predicting shorter progression‐free survival in patients with non‐small cell lung cancer treated with nivolumab
| Variate | HR (95% CI) | P‐value |
|---|---|---|
| Age ≥ 75 | 1.12 (0.57–2.22) | 0.740 |
| Male gender | 1.20 (0.64–2.21) | 0.565 |
| Heavy smoker | 0.73 (0.42–1.29) | 0.268 |
| ECOG performance status ≥2 | 5.44 (2.10–14.1) | 0.000 |
| EGFR‐mutant | 0.84 (0.36–1.96) | 0.691 |
| Squamous cell carcinoma | 2.03 (1.12–3.68) | 0.019 |
| Interstitial septal thickening | 2.15 (1.13–4.09) | 0.019 |
| Peritumoral ground‐glass opacity | 1.35 (0.80–2.26) | 0.256 |
| Spiculated margin | 0.94 (0.51–1.75) | 0.857 |
| Air bronchogram | 0.66 (0.26–1.66) | 0.375 |
| Cavity or necrosis | 1.33 (0.67–2.65) | 0.410 |
| Adjacent organ invasion | 2.38 (0.97–5.82) | 0.057 |
| Bulky lymph node (≥2.5 cm) | 1.82 (0.96–3.44) | 0.065 |
| Accumulation of small lymph nodes | 0.67 (0.21–2.16) | 0.505 |
CI, confidence interval; HR, hazard ratio,
Table 4.
Multivariate Cox proportional hazard analysis predicting shorter progression‐free survival in patients with non‐small cell lung cancer treated with nivolumab
| Variate | HR (95%CI) | P‐value |
|---|---|---|
| Age ≥75 | 0.84 (0.41–1.72) | 0.631 |
| Male gender | 1.61 (0.74–3.50) | 0.278 |
| Heavy smoker | 0.36 (0.18–0.75) | 0.006 |
| ECOG performance status ≥2 | 8.55 (3.09–23.7) | 0.000 |
| EGFR‐mutant | 0.76 (0.26–2.15) | 0.606 |
| Squamous cell carcinoma | 2.41 (1.24–2.15) | 0.011 |
| Interstitial septal thickening | 2.48 (1.24–4.94) | 0.010 |
CI, confidence interval; HR, hazard ratio.
The univariate Cox proportional hazard analysis revealed that poorer ECOG PS and interstitial septal thickening were associated with shorter OS in the patients treated with nivolumab (Table 5). The multivariate Cox analysis demonstrated that smoking status, ECOG PS and interstitial septal thickening were predictors of shorter OS, independent of other clinical features (Table 6).
Table 5.
Univariate Cox proportional hazard analysis predicting shorter overall survival in patients with non‐small cell lung cancer treated with nivolumab
| Variate | HR (95% CI) | P‐value |
|---|---|---|
| Age ≥75 | 0.57 (0.17–1.92) | 0.370 |
| Male gender | 1.27 (0.47–3.38) | 0.638 |
| Heavy smoker | 0.77 (0.73–4.17) | 0.207 |
| ECOG performance status ≥2 | 8.11 (3.17–20.7) | 0.000 |
| EGFR‐mutant | 0.44 (0.57–3.67) | 0.436 |
| Squamous cell carcinoma | 1.13 (0.45–2.83) | 0.787 |
| Interstitial septal thickening | 2.15 (1.13–4.09) | 0.019 |
| Peritumoral ground‐glass opacity | 1.13 (0.51–2.50) | 0.757 |
| Spiculated margin | 1.00 (0.38–2.68) | 0.986 |
| Air bronchogram | 0.42 (0.10–1.79) | 0.241 |
| Cavity or necrosis | 1.47 (0.59–3.66) | 0.412 |
| Adjacent organ invasion | 1.47 (0.59–3.66) | 0.207 |
| Bulky lymph node (≥2.5 cm) | 1.75 (0.73–4.18) | 0.938 |
| Accumulation of small lymph nodes | 1.08 (0.14–8.16) | 0.938 |
CI, confidence interval; HR, hazard ratio.
Table 6.
Multivariate Cox proportional hazard analysis predicting shorter overall survival in patients with non‐small cell lung cancer treated with nivolumab
| Variate | HR (95% CI) | P‐value |
|---|---|---|
| Age ≥75 | 0.70 (0.19–2.46) | 0.579 |
| Male gender | 1.67 (0.54–5.21) | 0.375 |
| Heavy smoker | 0.30 (0.10–0.86) | 0.025 |
| ECOG performance status ≥2 | 17.9 (5.17–62.0) | 0.000 |
| EGFR‐mutant | 0.26 (0.05–1.28) | 0.099 |
| Squamous cell carcinoma | 0.43 (0.15–1.30) | 0.135 |
| Interstitial septal thickening | 3.44 (1.19–9.33) | 0.099 |
CI, confidence interval; HR, hazard ratio.
Discussion
In the present study, smoking status, PS and presence of interstitial septal thickening around the tumor were found to be related to poor PFS. To the best of our knowledge, the present study is the first to demonstrate a possible association between CT morphological characteristics and nivolumab efficacy in a real‐world setting. A previous study reported an association between the morphological characteristics of stage I lung adenocarcinoma and prognosis. 14 Another study reported the relationship between CT morphological characteristics at diagnosis and EGFR tyrosine kinase inhibitor (TKI). 15 Recently deep learning of serial CT imaging was reported to predict the treatment response of patients with locally advanced NSCLC. 16 The combination of deep learning technology and CT imaging may bring another biomarker to predict the efficacy of nivolumab in NSCLC in the future. However, few studies have discussed the relationship between morphological characteristics detected using CT in advanced NSCLC and therapeutic efficacy of treatment for lung cancer.
The CT patterns of interstitial septal thickening around tumors contain pulmonary lymphangitic carcinomatosis. 17 Lung cancer with pulmonary lymphangitic carcinomatosis rarely responds to chemotherapy and has a poor prognosis. 18 The presence of peritumoral interstitial thickening on preoperative CT appears to predict pathological lymphovascular invasion and recurrence, 19 and pulmonary lymphangitic carcinomatosis suggests retrograde lymphatic spread of tumor cells from mediastinal and hilar lymph node metastases into the pulmonary lymphatics. 20 This may cause poor response to nivolumab in cases of NSCLC with pulmonary lymphangitic carcinomatosis.
Performance status, smoking status, EGFR status and metastatic site have been reported to be predictive clinical parameters for response to nivolumab. 21 , 22 , 23 Consistent with the results of a previous study, poorer PS was associated with shorter PFS and OS during nivolumab treatment in the present study. However, one of these studies 21 reported that EGFR status is a negative predictor of PFS, which is inconsistent with our analysis. The reason for this may be partly due to the current study's relatively small number of subjects. Since the ORR is comparable to those from other clinical trials that assessed nivolumab, the efficacy of nivolumab in the present study seemed to be identical to those trials. 5 , 6 PD‐L1 expression has been reported to be related with the response to nivolumab, 7 and there might be an association between CT morphological characteristics and PD‐L1 expression. However, we could not investigate this association because the measurement of PD‐L1 expression was not mandatory in clinical settings during our study period.
In the current analysis, the PFS of patients with squamous cell carcinoma was significantly shorter than that of the patients with nonsquamous cell carcinoma. This is in contrast to previous studies, which reported the PFS of squamous cell carcinoma patients to be significantly longer than that of patients with adenocarcinoma. 24 Regarding heavy smokers, there were significantly more in the patients with squamous cell carcinoma than in those with nonsquamous cell carcinoma in the present study. One possible reason for this is that the squamous cell carcinoma patients in our analysis tended to be older than the nonsquamous cell carcinoma patients, although this difference did not reach statistical significance (P = 0.097). Another reason is that PD‐L1 expression in these two groups might have been different.
There are some limitations to the present study. First, the study was retrospective in nature, so information bias cannot be excluded. Second, our multivariate analysis did not include significant potential confounding factors, such as tumor mutation burden, which is a predictive marker of efficacy of nivolumab. 25 Analysis for tumor mutation burden was not performed in the present study. Third, CT imaging settings were not unified among the institutes that participated in the study. Moreover, we did not elucidate the precise mechanisms governing the relationship between radiological characteristics, interstitial septal thickening, and the outcome of patients with NSCLC after nivolumab treatment. Finally, overall survival data were immature. Further pathological and prospective studies are needed to determine the predictive value of morphological characteristics detected using CT in NSCLC treated with nivolumab.
In conclusion, NSCLC with interstitial septal thickening may respond poorly to nivolumab.
Disclosure
Dr Minemura reports speaker honoraria from Ono Pharmaceutical Co., Ltd. and Nippon Boehringer Ingelheim Co., Ltd., Eli Lilly Japan K.K. and Chugai Pharmaceutical Co., Ltd. outside the submitted work.
Dr Kaira has received research grants and a speaker honorarium from Ono Pharmaceutical Company, Boehringer Ingelheim, Chugai Pharmaceutical, Taiho Pharmaceutical, Eli Lilly Japan, and AstraZeneca. Dr Yokouchi reports research funding from Bristol‐Myers Squibb. Dr Kaburagi has received research grants from Ono Pharmaceutical Company. Dr Suzuki reports personal fees and research funding from Ono Pharmaceutical Co., Ltd., Bristol‐Myers Squibb. and research funding from Ono Pharmaceutical Co., Ltd. and Bristol‐Myers Squibb. The rest of the authors have no affiliations with, or involvement in, any organization or entity with any financial interest.
Acknowledgments
This work was supported by the funding of Boehringer Ingelheim.
We thank Katsuhiko Murakami of Fukushima Medical University Hospital for his effort in collecting CT data.
References
- 1. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012; 12: 252–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD‐1 and its ligands in tolerance and immunity. Annu Rev Immunol 2008; 26: 677–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Velcheti V, Schalper KA, Carvajal DE et al Programmed death ligand‐1 expression in non‐small cell lung cancer. Lab Invest 2014; 94: 107–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang C, Thudium KB, Han M et al In vitro characterization of the anti‐PD‐1 antibody nivolumab, BMS‐936558, and in vivo toxicology in non‐human primates. Cancer Immunol Res 2014; 2: 846–56. [DOI] [PubMed] [Google Scholar]
- 5. Brahmer J, Reckamp KL, Baas P et al Nivolumab versus docetaxel in advanced squamous‐cell non‐small‐cell lung cancer. N Engl J Med 2015; 373: 123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Borghaei H, Paz‐Ares L, Horn L et al Nivolumab versus docetaxel in advanced nonsquamous non‐small‐cell lung cancer. N Engl J Med 2015; 373: 1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Taube JM, Klein A, Brahmer JR et al Association of PD‐1, PD‐1 ligands, and other features of the tumor immune microenvironment with response to anti‐PD‐1 therapy. Clin Cancer Res 2014; 20: 5064–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carbone DP, Reck M, Paz‐Ares L et al First‐line nivolumab in stage IV or recurrent non‐small‐cell lung cancer. N Engl J Med 2017; 376: 2415–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Park W, Kwon D, Saravia D et al Developing a predictive model for clinical outcomes of advanced non‐small cell lung cancer patients treated with nivolumab. Clin Lung Cancer 2018; 19: 280–8.e4. [DOI] [PubMed] [Google Scholar]
- 10. Haratani K, Hayashi H, Chiba Y et al Association of immune‐related adverse events with nivolumab efficacy in non‐small‐cell lung cancer. JAMA Oncol 2018; 4: 374–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pass HI, Ball D, Scagliotti GV. IASLC Thoracic Oncology, 2nd edn Elsevier, Philadelphia: 2018. [Google Scholar]
- 12. Eisenhauer EA, Therasse P, Bogaerts J et al New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–47. [DOI] [PubMed] [Google Scholar]
- 13. The Japan Lung Cancer Society . General Rule for Clinical and Pathological Record of Lung Cancer, 8th edn Kanehara, Tokyo: 2017. [Google Scholar]
- 14. Ma J, Yang YL, Wang Y, Zhang XW, Gu XS, Wang ZC. Relationship between computed tomography morphology and prognosis of patients with stage I non‐small cell lung cancer. Onco Targets Ther 2017; 10: 2249–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Choi CM, Kim MY, Lee JC, Kim HJ. Advanced lung adenocarcinoma harboring a mutation of the epidermal growth factor receptor: CT findings after tyrosine kinase inhibitor therapy. Radiology 2014; 270: 574–82. [DOI] [PubMed] [Google Scholar]
- 16. Xu Y, Hosny A, Zeleznik R et al Deep learning predicts lung cancer treatment response from serial medical imaging. Clin Cancer Res 2019; 25: 3266–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Oikonomou A, Prassopoulos P. Mimics in chest disease: Interstitial opacities. Insights Imaging 2013; 4: 9–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bruce DM, Heys SD, Eremin O. Lymphangitis carcinomatosa: A literature review. J R Coll Surg Edinb 1996; 41: 7–13. [PubMed] [Google Scholar]
- 19. Koo HJ, Xu H, Choi CM et al Preoperative CT predicting recurrence of surgically resected adenocarcinoma of the lung. Medicine (Baltimore) 2016; 95: e2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chandler GN, Telling M. Lymphangitis carcinomatosa. Br Med J 1952; 2: 639–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fujimoto D, Yoshioka H, Kataoka Y et al Efficacy and safety of nivolumab in previously treated patients with non‐small cell lung cancer: A multicenter retrospective cohort study. Lung Cancer 2018; 119: 14–20. [DOI] [PubMed] [Google Scholar]
- 22. Calles A, Liao X, Sholl LM et al Expression of PD‐1 and its ligands, PD‐L1 and PD‐L2, in smokers and never smokers with KRAS‐mutant lung cancer. J Thorac Oncol 2015; 10: 1726–35. [DOI] [PubMed] [Google Scholar]
- 23. Tamiya M, Tamiya A, Inoue T et al Metastatic site as a predictor of nivolumab efficacy in patients with advanced non‐small cell lung cancer: A retrospective multicenter trial. PLOS One 2018; 13: e0192227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wu YL, Lu S, Cheng Y et al Nivolumab versus docetaxel in a predominantly Chinese patient population with previously treated advanced non‐small cell lung cancer: CheckMate 078 randomized phase III clinical trial. J Thorac Oncol 2019; 14: 867–75. [DOI] [PubMed] [Google Scholar]
- 25. Hellmann MD, Ciuleanu TE, Pluzanski A et al Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med 2018; 378: 2093–104. [DOI] [PMC free article] [PubMed] [Google Scholar]


