Abstract
We report a preliminary experience of adjuvant therapy with Hemoperfusion (HP) in patients with Severe Acute Respiratory Syndrome-CoronaVirus 2 (SARS-CoV2) pneumonia. Currently, there are no approved treatments for CoronaVirus Disease 19 (COVID-19); however, therapeutic strategies based on the preclinical evidence include supportive measures, such as oxygen supplementation, antiviral, and anticoagulant agents. Despite these treatments, 10% of patients worsen and develop severe acute respiratory distress syndrome (ARDS). Since the pathogenic mechanism of ARDS is an uncontrolled inflammatory state, we speculate that removing inflammation effectors from blood may contrast tissue injury and improve clinical outcome. In a scenario of dramatic medical emergency, we conducted an observational study on 9 consecutive patients hospitalized in COVID Intensive Care Unit, where 5 of 9 consecutive patients were treated with HP, due to the emergency overload made it impossible to deliver blood purification in the other 4 patients. COVID-19 was diagnosed through the identification of virus sequences by reverse transcription-PCR on respiratory specimens. All patients had severe pneumonia requiring continuous positive airway pressure. HP was started in all patients 6–7 days after hospital admission. The treated patients (T) received 2 consecutive sessions of HP using CytoSorb cartridge. Our results show a better clinical course of T compared to control patients (C), in fact all T except 1 survived, and only 2 of them were intubated, while all C required intubation and died. Lymphocytopenia worsened in C but not in T. C-reactive protein decreased in both patients, but to a greater extent in T. IL-6, IL-8, and TNF-α decreased after HP, IL-10 did not change. Respiratory function remained stable and did not worsen in T compared to C. The limited sample size and observational study design preclude a sound statement about the potential effectiveness of HP in COVID-19 patients, but our experience suggests a potential therapeutic role of adjuvant CytoSorb HP in the early course of COVID-19 pneumonia. A randomized clinical trial is ongoing.
Keywords: Blood purification, COVID-19, Critical illness cytokines, Hemoperfusion, Case report
Introduction
In December 2019, a new strain of the Coronavirus family (2019-nCo) was identified as the cause of a severe respiratory distress that rapidly spread from its native site, Wuhan, worldwide. Italy was the first country after China to be heavily invaded and has cumulated 209,328 cases of swab-documented infection and 28,710 cases of death. Symptoms might range from fever, cough, nasal discharge, and anosmia/ageusia to primary severe acute respiratory distress syndrome (ARDS), which might often lead to multiorgan failure, all contributing to the high mortality [1]. Currently, there are no approved treatments for CoronaVirus Disease 19 (COVID-19); however, therapeutic strategies based on the available preclinical evidence include supportive measures, as oxygen supplementation, antiviral agents [2, 3], anticoagulant agents [4], and hydroxychloroquine [2, 3].
Although frank evidence is missing, antimicrobial therapy is frequently commenced in these patients. Despite these treatments, approximately 10% of patients worsen and develop ARDS requiring continuous positive airway pressure or invasive mechanical ventilation, up to extra-corporeal membrane oxygenation (ECMO) [2]. The bulk of cases needing intensive care has challenged the Italian healthcare system and has stimulated attempts of supportive therapies. One of the suggested mechanisms responsible for the development of ARDS is an excessive and uncontrolled inflammatory state [5]. A rational approach to contrast inflammation caused tissue injury, while waiting for a vaccine or an effective new antiviral drug is to attenuate the harm of virus-induced inflammation. At least 2 mechanisms are identified to explain the potential harmful results of the host inflammatory response: (1) cytotoxic effect of cytokines [6] and (2) a compromised immune response induced by prolonged release of inflammatory mediators [7].
Several pro- and anti-inflammatory cytokines (IL-1 − IL-2 − IL-6 − IL-8 − IL-10 − TNF-α − MCP-1 − IFN-γ) are involved in COVID-19 [2], and it is reasonable to speculate that their removal from blood might limit organ damage. Hemoperfusion (HP) is a technique developed to adsorb molecules in the middle molecular weight range (up to 55 kDa), having a weight cut-off much higher than the cut-off of conventional high-flux hemodiafilters [8]. Studies in vitro and in vivo [9] have shown that HP is highly effective in clearing blood from a number of cytokines that are candidate culprits for 2019-nCo-induced inflammation. Here, we describe the preliminary clinical experience of 9 critically ill patients with Severe Acute Respiratory Syndrome-CoronaVirus 2 (SARS-CoV2) pneumonia and negative prognostic factors treated with and without HP using CytoSorb cartridge of the pathology before the occurrence of renal failure, in adjunction to standard antiviral and supportive therapies.
Case Report
We report a case series of 9 consecutive COVID-patients admitted to our COVID Intensive Care Unit (ICU). Five of them were treated with HP using CytoSorb (treatment patient), due to the heavy emergency overload it was impossible to deliver blood purification in the other 4 patients (control patient), who were also considered as potential candidates by the attending medical team. COVID-19 was diagnosed through the identification of virus unique RNA sequences by reverse transcription-PCR on respiratory specimens. All patients had pneumonia and respiratory failure requiring continuous positive airway pressure. They were hospitalized in the first days of explosive spreading of COVID-19, when healthcare personnel and technical equipment were dramatically challenged by the unexpectedly high-crowd of patients needing intensive care, and no evidence-based treatment was available. Consequently, different antibacterial prophylaxes, antiviral, and anti-inflammatory therapies including steroids were delivered (Table 1). No vasopressor drugs were required. The decision of starting HP as a possible treatment aimed to abate the cytokine storm was based on the consensus of the medical team, also being the authors of this article. A written informed consent was obtained for all patients. Eligibility criteria, besides a confirmed SARS-CoV2 pneumonia were PaO2/FiO2 <200 mm Hg, c-reactive protein (CRP) level >10 mg/dL, and Lymphocyte count <1,500/mmc. HP was performed using multiFiltrate machine (Fresenius Medical Care, Bad Homburg, Germany) and CytoSorb cartridge (CytoSorbents Corporation, Monmouth Junction, NJ, USA; Aferetica, Bologna, Italy). It consists of biocompatible polystyrene divinylbenzene copolymer beads with a huge adsorption capability linked to a surface area of >40,000 m2 for a 300 mL cartridge of resin that attracts several key cytokines through hydrophobic interactions, ionic attraction, hydrogen bonding, and van der Waals interactions [8]. HP was delivered to each patient for 4 h sessions in 2 consecutive days; blood flow rates were set between 100 and 120, and unfractioned heparin was used as anticoagulation to maintain activated clotting time post-cartridge around 180–200 s. HP treatment was started in all patients 6–7 days after hospital admission, and no patients met criteria for the invasive mechanical ventilation at HP start. No further blood purification therapies were performed in addition to HP. Serum cytokine's levels were measured before and after HP by ELISA, whereas CRP, lymphocyte count, and respiratory parameters were monitored in all patients every day. Data are expressed as means and standard error, and no statistical analyses were performed due to the limited number of patients. Over the observational period, we studied 9 consecutive patients, in whom the clinical condition showed the similar pattern of rapid worsening.
Table 1.
Drug therapy administered to patients with COVID-19
| Therapy | All patients, n = 9 | T, n = 5 | C, n = 4 |
|---|---|---|---|
| Antiviral therapy, n (%) | 5 (55.5) | 2 (40) | 3 (75) |
| Antibiotic therapy, n (%) | 7 (77.7) | 5 (100) | 2 (50) |
| Tocilizumab, n (%) | 1 (11.1) | 0 | 1 (25) |
| Hydroxychloroquine, n (%) | 7 (77.7) | 4 (80) | 3 (75) |
| Steroids, n (%) | 8 (88.8) | 4 (80) | 4 (100) |
Data are expressed as number/total (percentage). COVID-19, CoronaVirus Disease 19; T, treatment patient; C, control patient.
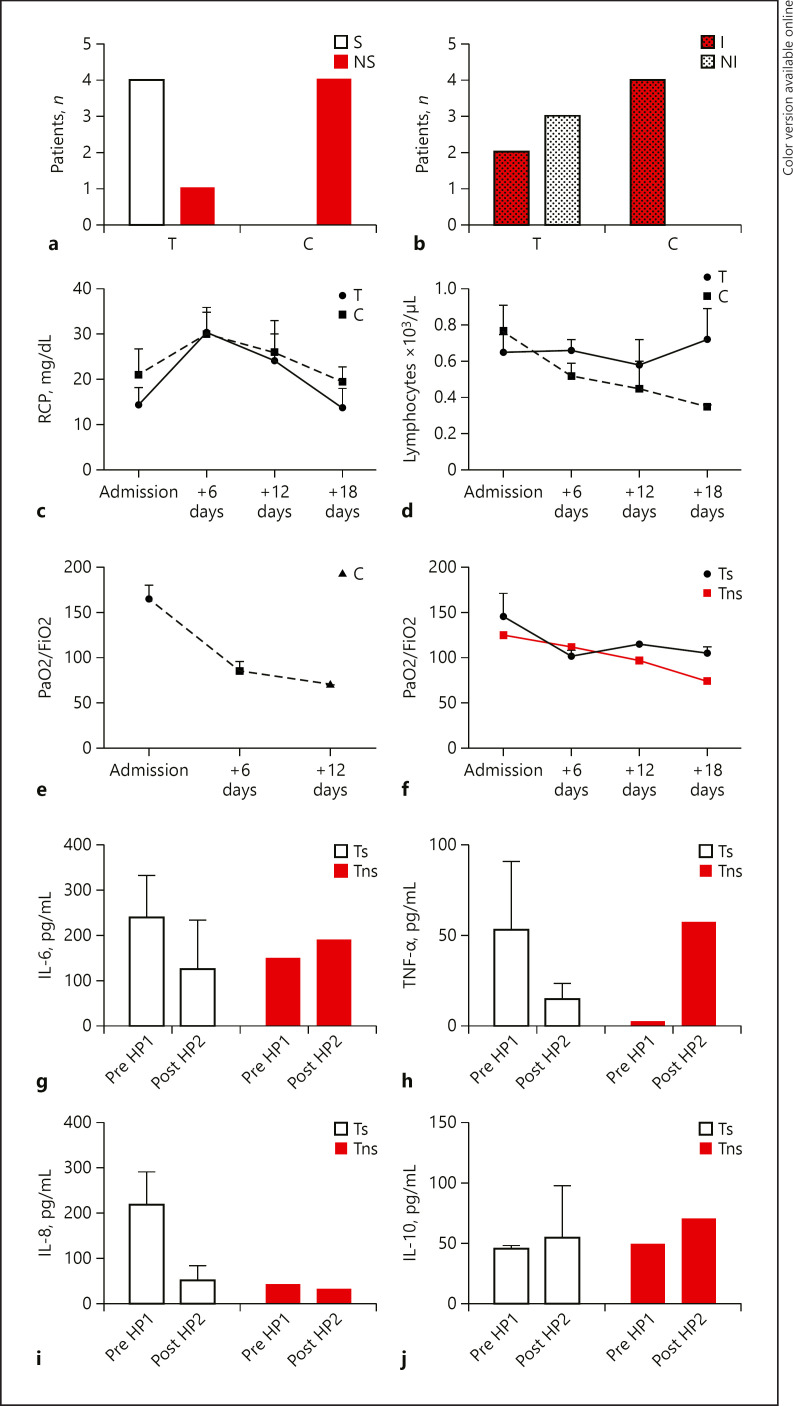
The demographic data are summarized in Table 2. CT scan of all patients showed severe pneumonia. Age, BMI, comorbidities, respiratory parameters (PaO2/FiO2 and SpO2%), blood arterial pressure, CRP, blood lymphocyte count, and days from onset of illness to hospital admission were similar between the 2 groups of patients. Overall, the clinical course of treated patients was less severe than that patients who did not receive HP. In fact, all treated patients except 1 survived and only 2 of them needed to be intubated, while all the not treated patients required intubation and eventually died (Fig. 1a, b). Currently, after 2 months, all treated survived patients are discharged from the hospital and in good clinical conditions.
Table 2.
Demographic and clinical characteristics of patients with COVID-19
| Variables | All patients, n = 9 | T, n = 5 | C, n = 4 |
|---|---|---|---|
| Age, years (m ± SEM) | 61.3±2.42 | 57.8±3.39 | 65.7±2.0 |
| BMI (m ± SEM) | 26.49±1.86 | 25.79±1.41 | 27.42±4.41 |
| Male, % | 89 | 100 | 75 |
| Female, % | 11 | 0 | 25 |
| Comorbidity | |||
| Any comorbidity, n (%) | 5 (55.5) | 3 (60) | 2 (50) |
| Diabetes | 0 | 0 | 0 |
| Hypertension, n (%) | 3 (33.3) | 2 (40) | 1 (25) |
| Cardiovascular disease, n (%) | 1 (11.1) | 1 (20) | 0 |
| Chronic pulmonary disease | 0 | 0 | 0 |
| CKD | 0 | 0 | 0 |
| Current smoking (Patients n and %) | 1 (11.1) | 0 | 1 (25) |
| Lymphocytes nr, × 103/µL (m ± SEM) | 0.71±0.08 | 0.65±0.10 | 0.77±0.14 |
| Oxygen saturation, % (m ± SEM) | 90.12±2.1 | 92.42±1.2 | 87.25±4.38 |
| PaO2/FiO2, mm Hg % (m ± SEM) | 216.4±14.39 | 259.8±52.32 | 164.78±14.9 |
| CRP, mg/dL (m ± SEM) | 18.43±3.13 | 16.37±3.6 | 21.01±5.76 |
| Blood arterial pressure, mm Hg (m ± SEM) | 75±5 | 74±6 | 80±4 |
| Signs and symptoms, n (%) | |||
| Fever | 9 (100) | 5 (100) | 4 (100) |
| Cough | 6 (66.6) | 4 (80) | 2 (100) |
| Myalgia and/or arthralgia | 3 (33.3) | 3 (60) | 0 |
| Headache | 3 (33.3) | 3 (60) | 0 |
| Diarrhoea | 2 (22.2) | 2 (33.3) | 0 |
| Dyspnoea | 9 (100) | 5 (100) | 4 (100) |
| Anosmia and/or ageusia | 1 (11.1) | 1 (20) | 0 |
| Cold | 2 (22.2) | 2 (33.3) | 0 |
| Sore throat (Patients n and %) | 4 (44.4) | 3 (60) | 1 (25) |
| Days from onset of illness to hospital admission (m ± SEM) | 6.4±0.7 | 7±0.9 | 5.7±1.1 |
Data are expressed as means and standard error or number/total percentage. COVID-19, CoronaVirus Disease 19; T, treatment patient; C, control patient.
Fig. 1.
Outcome, laboratory data, and respiratory parameters of patients with COVID-19. Data are expressed as means and standard errors. a Number of patients with COVID-19 treated with CytoSorb HP (T) and not treated with CytoSorb HP (C) survivors (S), ns. b Number of patients with COVID-19 treated with CytoSorb HP (T) and not treated with CytoSorb HP (C) I, NI. c CRP serum levels of patients with COVID-19 T and C at hospital admission (admission), and after 6, 12, 18 days. d Lymphocyte's count of patients with COVID-19 treated with CytoSorb HP (T) and not treated with CytoSorb HP (C) at hospital admission (admission), and after 6, 12, 18 days. e PaO2/FiO2 ratio of patients with COVID-19 not treated with CytoSorb HP (C) at hospital admission (admission) and after 6, 12 days. PaO2 was measured in mm Hg and FiO2 measured as fraction of inspired oxygen. f PaO2/FiO2 ratio in COVID-19 patients treated with CytoSorb HP (T) s, ns at hospital admission (admission) and after 6, 12,18 days. PaO2 was measured in mm Hg and FIO2 measured as fraction of inspired oxygen. g IL-6 serum levels of survivors (Ts) and not survivor (Tns) treated patients with COVID-19 pre-HP1 and post-HP2. h TNFα serum levels of survivors (Ts) and not survivor (Tns) treated patients with COVID-19 pre-HP 1, and post-HP 2. i IL-8 serum levels of survivors (Ts) and not survivor (Tns) treated patients with COVID-19 pre-HP 1, and post-HP2. j IL-10 serum levels of survivors (Ts) and not survivor (Tns) treated patients with COVID-19 pre-HP 1), and post-HP 2). NI, not incubated; Pre-HP 1, before the first HP session; Post-HP 2, after the second HP session; ns, not survivor; s, survivors; I, intubated; T, treated patient; C, control patient; COVID-19, CoronaVirus Disease 19.
CRP decreased in both groups, but to a greater extent after HP (Fig. 1c). Lymphocytopenia worsened in control patient (C) but not in treated patient (T) after HP (Fig. 1d). Procalcitonin increased in 2 of the not treated patients.
Respiratory function remained stable and did not worsen after HP in T compared to C, as shown by the PaO2/FiO2 ratio (Fig. 1e, f). After HP in T SpO2% was steadily 92–94%, instead in C SpO2% decreased to 87–83%. No complications occurred during HP treatment (clotting, vascular access problems, and bleeding).
Performing an observation analysis in T on the serum cytokine levels, in all survived patients (n = 4) HP reduced pro-inflammatory cytokines, as IL-6, TNF-α, and IL-8 (Fig. 1g–i). Notably, a striking effect was observed on IL-6 levels that at the end of the second session were decreased by a 40% than before the first treatment. Serum levels of IL-8 and TNF-α were lowered within normal range, starting from a 7- and 2-fold higher than normal level, respectively. HP did not modify IL-2 and IL-1 levels that showed normal baseline values and were not increased by viral infection (IL-2 range min–max 3.4–21 pg/mL and IL-1 range min-max 0.7–3.9 pg/mL). In all patients, IL-10 did not change after HP (Fig. 1j).
Discussion and Conclusion
We reported our preliminary clinical experience of CytoSorb HP treatments in patients with SARS-CoV2 pneumonia. We treated 5 out of 9 consecutive patients in a condition of life-threatening medical emergency admitted in ICU. HP treatment was delivered only to 5 patients because the dramatic overload in ICU made it impossible to devote personnel and machinery to treat the other ones. Baseline clinical characteristics of all patients were similarly severe and were rapidly worsening. Our experience showed potential positive results with HP, saving 4 out of 5 patients whereas all non-treated patients died. We observed in T an improvement in the clinical course, associated with a prompt reduction in IL-6 levels and a visible decrease of IL-8 and TNF-α. Furthermore, HP induced a CRP decrease, associated with an improvement in PaO2/FiO2, SpO2, and a rise of peripheral lymphocytes count. In addition, in T only 2 patients needed to be intubated, while all C patients required intubation.
Admittedly, these preliminary observations do not represent a sound demonstration that HP is a life-saving treatment for SARS-CoV2 pneumonia. However, the possible role of HP in an early phase is suggested not only by better survival in the treated patients but also by the remodulation of cytokine levels associated with the treatment. Interestingly, adsorption was effective in spite that the treatment lasted 4 h rather than the 12 h usually recommended. This observation confirms results of in vitro study showing that the bulk of cytokine removal occurs in the first hours of treatment [10]. The precipitous fall of IL-6 levels, a powerful effector of inflammation, points to clearance of IL-6 by the cartridge as the mechanism causing clinical improvement, but we found also a reset of blood IL-8 and TNF-α levels that suggests a possible reprogramming of cytokine spectrum and consequently maybe immune response, whose relevance cannot be defined by our data.
Our experience suggests a potentially beneficial role of adjuvant CytoSorb HP in the early course of COVID-19 pneumonia, as shown by observations of improved respiratory function and eventually lower mortality in the treated patients. The limited sample size and retrospective design preclude a definitive statement about the potential effectiveness of this treatment in COVID-19 patients, and these observations require evaluation in a randomized clinical trial which is ongoing.
Statement of Ethics
The research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. Subjects (or their parents or guardians) have given their written informed consent to publish their cases.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
No funding sources were received for this study.
Author Contributions
Teresa Rampino contributed to the conception of the work and wrote the manuscript. Marilena Gregorini contributed to statistical analysis of the data, interpretation of the data and to critically revision of the manuscript. Luciano Perotti contributed to the conception of the work. Fiorenza Ferrari contributed to the conception of the work and to the final revision of the manuscript. Eleonora Francesca Pattonieri contributed to the acquisition and critically revision of the data. Maria Antonietta Grignano contributed to the data collection, figure creation, and data analysis. Mauro Valente contributed to the data collection. Alberto Garrone contributed to the data collection. Tefik Islam contributed to the data collection. Carmelo Libetta contributed to critically and final revision of the manuscript. Vincenzo Sepe contributed to critically and final revision of the manuscript. Riccardo Albertini contributed to the biochemical analysis. Raffaele Bruno contributed to critically and final revision of the manuscript. Mirko Belliato contributed to the conception of the work.
References
- 1.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 Feb;395((10223)):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Felsenstein S, Herbert JA, McNamara PS, Hedrich CM. COVID-19: immunology and treatment options. Clin Immunol. 2020 Jun;215:108448. doi: 10.1016/j.clim.2020.108448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li L, Li R, Wu Z. Yang X, Zhao M, Liu J, et al. Therapeutic strategies for critically ill patients with COVID-19. Ann Intensive Care. 2020;10((1)):45. doi: 10.1186/s13613-020-00661-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kollias A, Kyriakoulis KJ, Dimakakos E, Poulakou G, Stergiou GS, Syrigos K. Thromboembolic risk and anticoagulant therapy in COVID-19 patients: emerging evidence and call for action. Br J Haematol. 2020;189((5)):846–7. doi: 10.1111/bjh.16727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tisoncik JR, Korth MJ, Simmons CP, Farrar J, Martin TR, Katze MG. Into the eye of the cytokine storm. Microbiol Mol Biol Rev. 2012 Mar;76((1)):16–32. doi: 10.1128/MMBR.05015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ronco C, Tetta C, Mariano F, Wratten ML, Bonello M, Bordoni V, et al. Interpreting the mechanisms of continuous renal replacement therapy in sepsis: the peak concentration hypothesis. Artif Organs. 2003 Sep;27((9)):792–801. doi: 10.1046/j.1525-1594.2003.07289.x. [DOI] [PubMed] [Google Scholar]
- 7.Hotchkiss RS, Coopersmith CM, McDunn JE, Ferguson TA. The sepsis seesaw: tilting toward immunosuppression. Nat Med. 2009 May;15((5)):496–7. doi: 10.1038/nm0509-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rimmelé T, Kellum JA. Clinical review: blood purification for sepsis. Crit Care. 2011;15((1)):205. doi: 10.1186/cc9411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brouwer WP, Duran S, Kuijper M, Ince C. Hemoadsorption with CytoSorb shows a decreased observed versus expected 28-day all-cause mortality in ICU patients with septic shock: a propensity-score-weighted retrospective study. Crit Care. 2019 Sep;23((1)):317. doi: 10.1186/s13054-019-2588-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harm S, Schildböck C, Hartmann J. Cytokine removal in extracorporeal blood purification: an in vitro study. Blood Purif. 2020;49((1–2)):33–43. doi: 10.1159/000502680. [DOI] [PubMed] [Google Scholar]