Abstract
Purpose:
This study assessed the safety and tolerability of therapeutic immunization against the human papillomavirus (HPV) viral oncoproteins E6 and E7 in patients with cervical cancer after chemoradiation.
Methods and Materials:
MEDI0457 (INO-3112) is a DNA-based vaccine targeting E6 and E7 of HPV-16/18 that is coinjected with an IL-12 plasmid followed by electroporation with the CELLECTRA 5P device. At 2 to 4 weeks after chemoradiation, patients with newly diagnosed stage IB1-IVA (cohort 1) or persistent/recurrent (cohort 2) cervical cancers were treated with 4 immunizations of MEDI0457 every 4 weeks. The primary endpoints were incidence of adverse events and injection site reactions. Immune responses against HPV antigens were measured by ELISpot for interferon-γ (IFNγ), enzyme-linked immunosorbent assay for antibody responses and multiplexed immunofluorescence for immune cells in cervical biopsy specimens.
Results:
Ten patients (cohort 1, n = 7; cohort 2, n = 3) with HPV16 (n = 7) or HPV18 (n = 3) cervical cancers received MEDI0457 after chemoradiation. Treatment-related adverse events were all grade 1, primarily related to the injection site. Eight of 10 patients had detectable cellular or humoral immune responses against HPV antigens after chemoradiation and vaccination: 6 of 10 patients generated anti-HPV antibody responses and 6 of 10 patients generated IFNγ-producing T cell responses. At the completion of chemoradiation and vaccination, cervical biopsy specimens had detectable CD8+ T cells and decreased PD-1+CD8+, PD-L1+CD8+, and PD-L1+CD68+ subpopulations. All patients cleared detectable HPV DNA in cervical biopsies by completion of chemoradiation and vaccination.
Conclusions:
Adjuvant MEDI0457 is safe and well tolerated after chemoradiation for locally advanced or recurrent cervical cancers, supporting further investigation into combining tumor-specific vaccines with radiation therapy.
Introduction
Persistent human papillomavirus (HPV) infection, primarily by the HPV16 and HPV18 subtypes, often leads to cervical cancer, which afflicts upward of 500,000 women per year worldwide.1 Many patients present with locally advanced disease and experience relatively high recurrence and poor survival rates after chemoradiation: approximately 50% and 70% at 5 years, respectively.2,3 Harnessing immune responses against non-self, tumor antigens may improve the treatment of locally advanced cervical cancers by specifically targeting cancer cells. Unlike the majority of prophylactic HPV vaccines,4 no therapeutic vaccines are approved that are effective against existing preinvasive or invasive lesions.5-8 Because HPV cancers express the viral oncogenes E6 and E7, these proteins can also serve as non-self antigens that can be incorporated into therapeutic vaccines against HPV cancers.9 Furthermore, cervical cancers are unlikely to lose expression of E6 or E7 to escape effective anti-HPV immune responses because E6 and E7 are necessary for cancer cell proliferation and survival.10,11
Recently, MEDI0457 (previously INO-3112), a DNA-based immunotherapy for HPV cancers, was developed. It combines plasmids encoding modified, nononcogenic E6 and E7 viral oncoproteins of HPV16 and HPV18 (VGX-3100) with a plasmid encoding IL-12 (INO-9012); it is delivered intramuscularly and combined with electroporation using CELLECTRA (Inovio Pharmaceuticals, San Diego, CA), a constant current device.12-14 MEDI0457 is a therapeutic vaccine and is not cross-reactive against prophylactic HPV vaccines such as Gardasil and Cervarix, which generate immune responses against the HPV capsid protein L1, which is often lost during cervical carcinogenesis. Once injected into skeletal muscle, these plasmids are taken up and the E6, E7, and IL-12 genes are expressed by host cells, a process that is enhanced by electroporation.15,16 The protein products of these plasmids are primarily expressed by skeletal muscle and other non—antigen-presenting host cells. The E6 and E7 proteins are then released from host cells and endocytosed by dendritic and other antigen-presenting cells, which process the proteins into molecular fragments, or epitopes, and are cross presented on human leukocyte antigen molecules. Alternatively, host dendritic cells or other antigen-presenting cells acquire the plasmids and directly express, process, and present the E6E7 antigenic epitopes to T cells. The HPV DNA vaccine is coupled with an IL-12 expression plasmid, which has previously been shown to increase the immunogenicity of other DNA vaccines with minimal toxicity.17-19 In a phase 2 trial of cervical intraepithelial neoplasia (CIN) 2/3 lesions, VGX-3100, a DNA vaccine similar to MEDI0457 but without IL-12 plasmid, caused regression in approximately 50% of lesions.8
However, incorporation of therapeutic HPV immunization strategies into the treatment of patients with cervical cancers undergoing chemoradiotherapy remains largely unexplored. Here, we assessed the feasibility of incorporating MEDI0457 immediately after chemoradiation or radiation therapy (RT) for locally advanced (cohort 1) and persistent/recurrent (cohort 2) cervical cancer, respectively. This study reports the safety and tolerability of this treatment as well as the local and systemic immune responses against HPV antigens generated by MEDI0457 treatment after chemoradiation for cervical cancer.
Methods and Materials
Study design
HPV-004 was a phase 1/2a, open-label study to evaluate the safety, tolerability, and immunogenicity of MEDI0457 delivered intramuscularly by electroporation (EP) in female patients with biopsy-proven, stage IB-IVB, inoperable, invasive cervical carcinoma associated with HPV16 or HPV18 subtypes (Fig. E1; available online at https://doi.org/10.1016/j.ijrobp.2020.02.031). The study was conducted in accordance with the ethical principles described in the Declaration of Helsinki and with Good Clinical Practice guidelines as denoted in the International Council for Harmonization E6 requirements. Informed consent was obtained from all patients, and patients were assigned a unique identifier to maintain confidentiality.
Patients were stratified into 2 cohorts. In cohort 1 (n = 7), patients with newly diagnosed cancers were treated with chemoradiation with curative intent. In cohort 2, patients with persistent or recurrent cervical cancer were treated with chemoradiation or radiation alone (n = 3). After chemoradiation, patients received MEDI0457 (6 mg of VGX-3100 and 1 mg of INO-9012) immediately followed by EP with the CELLECTRA 5P device given every 4 weeks for a total of 4 doses. This dosing strategy is based on Morrow et al,20 who demonstrated that 4 vaccinations generated greater cellular and humoral immune responses compared with 3 vaccinations used in patients with preinvasive CIN2/3.8,12
Patients were followed for at least 6 months after the last vaccination. Tumor biopsy specimens for immunohistochemical analyses and cervical cytology for HPV testing using ThinPrep (Hologic, Marlborough, MA) were collected, in general, before chemoradiation, before vaccination, and 1 month after completing vaccination or 6 months after completing vaccination. Positron emission tomography (PET)/computed tomography (CT) imaging was performed within 4 weeks before vaccination, 3 to 4 months after vaccination, or in accordance with institutional practices.
The primary objective of this study was to evaluate the safety and tolerability of immunotherapy with MEDI0457 when delivered intramuscularly followed by EP with the CELLECTRA 5P device in women treated with chemoradiation for newly diagnosed, inoperable cervical cancer or persistent and/or recurrent cervical cancer associated with HPV16 and/or HPV18. Secondary objectives were to evaluate the cellular and humoral immune responses to HPV 16/18 E6/E7 and treatment response as measured by clinical examination and PET/CT imaging after chemoradiation and DNA vaccination. Safety endpoints were assessed per National Cancer Institute Common Terminology Criteria for Adverse Events version 4 criteria. Analysis of safety endpoints included all patients who received at least 1 immunization or EP as treated. Analysis of immune responses used a modified intention to treat population in which all patients received at least 1 immunization and EP as assigned. Treatment response was assessed using investigator-reported Response Evaluation Criteria in Solid Tumors v1.1 response. Progression-free survival was reported from the start of the study immunization.
Patients
Between June 6, 2014 and September 7, 2017, 10 patients were enrolled at 2 centers. Female patients were included in the study if they were between 18 and 70 years of age with histologic diagnosis of squamous cell carcinoma, adenocarcinoma, or adenosquamous cell carcinoma of the cervix containing HPV16 or HPV18 DNA. Only patients with stage IB-IVB inoperable invasive cervical carcinoma (cohort 1) or persistent and/or recurrent cervical cancer (cohort 2) with a life expectancy of at least 12 months were enrolled. Other inclusion criteria included a negative pretreatment pregnancy test, Eastern Cooperative Oncology Group performance status of ≤1, and laboratory or clinical findings grade ≥1 in severity at screening. Patients were excluded if they had a history of a therapeutic HPV vaccination (patients with prophylactic HPV vaccinations could be included); had positive HIV, hepatitis B, or hepatitis C serology; had undergone major surgery within 4 weeks of vaccination; or were taking topical or systemic steroids or immunosuppressants within 4 weeks of vaccination.
Chemoradiation
Chemoradiation must have been completed within 10 weeks of initiation. RT planning included standard immobilization and CT simulation. Radiation was delivered using a combination of external beam RT (EBRT) and intracavitary techniques. EBRT fields included the pelvis (n = 4) ± paraortic nodal chain (n = 3) to a total dose of 45 Gy in 25 fractions delivered using intensity modulated RT or 3-dimensional conformal techniques. Parametrial and nodal boosts (55-65 Gy), as determined by the disease stage, were delivered after pelvic irradiation or, for the latter, in combination with simultaneous integrated boost concurrently with pelvic field irradiation. Intracavitary or interstitial brachytherapy was delivered using high-dose-rate techniques to deliver 79 to 86 Gy (mean 82.5 Gy; low-dose-rate equivalent) to point A using 5 to 7.2 Gy per high-dose-rate fraction. Weekly cisplatin chemotherapy (40 mg/m2) was administered on day 1 of EBRT and given during weeks 1 to 5 of standard EBRT and during the parametrial boost.
Statistics
Statistical comparisons were performed using JMP (SAS, version 14). We used hematologic toxicity as the primary endpoint for safety and tolerability. Assuming a grade ≥3 hematologic toxicity rate of 23%,3 a sample size of 10 patients provides a 92% chance of observing at least 1 grade 3 or 4 hematologic toxicity during vaccination. If 0 adverse events (AEs) among 10 patients were observed, it was estimated with 95% confidence that the true proportion of such events would be <31%. Continuous patient variables were reported as the mean and range, and categorical patient variables were reported in aggregate. ELISpot, anti-HPV antibody immune responses, were analyzed by calculating the mean responses along with associated standard deviation. Multiplex immunofluorescence images were analyzed by calculating the mean cell population across all samples at each time point along with the standard deviation and standard error of the mean. Significance was assessed using a 2-tailed Student t test. Additional methods used in this manuscript are contained in Methods E1 (available online at https://doi.org/10.1016/j.ijrobp.2020.02.031).
Results
Patient and treatment characteristics
Of 44 patients screened, 10 were enrolled across 2 sites, including 7 patients in cohort 1 and 3 patients in cohort 2, with 23 screen failures due to HPV 16 or 18 negative status. Patient characteristics are given in Table 1. All patients received EBRT; 8 of 10 received subsequent brachytherapy and 9 of 10 received RT with cisplatin. All radiation was completed within 56 days from initiation. All patients in cohort 1 received 4 of 4 planned immunizations with MEDI0457; in cohort 2, 1 patient received 4 of 4 planned immunizations, 1 patient received 3 of 4 planned immunizations, and 1 patient received 2 of 4 planned immunizations (Table E1; available online at https://doi.org/10.1016/j.ijrobp.2020.02.031). Of the 8 patients who received 4 planned immunizations, 6 patients received successful EP after each immunization (5 patients in cohort 1 and 1 patient in cohort 2). Seven patients completed all study treatments and follow-up visits, including 6 (86%) in cohort 1 and 1 (33%) in cohort 2. Three patients discontinued study treatment or the observational period: 1 owing to fatal intestinal perforation and disseminated intravascular coagulation, 1 to disease progression, and 1 to changes in insurance carriers during the observational period.
Table 1.
Patient characteristics
| Characteristics | All patients | Cohort 1 | Cohort 2 |
|---|---|---|---|
| Median age (range), y | 51.5 (26-60) | 52 (29-60) | 44 (26-50) |
| Race | |||
| White | 6 | 5 | 1 |
| Black | 2 | 1 | 1 |
| Other | 2 | 1 | 1 |
| Ethnicity | |||
| Hispanic or Latino | 3 | 2 | 1 |
| Non-Hispanic or Latino | 7 | 5 | 2 |
| Median BMI (range), kg/m2 | 26.9 (18.7-47.9) | 24.3 (18.7-47.9) | 32.1 (27.0-37.3) |
| FIGO clinical stage | |||
| IB1 | 3 | 2 | 1 |
| IB2 | 2 | 2 | 0 |
| IIB | 2 | 2 | 0 |
| IIIB | 2 | 1 | 1 |
| IVA | 1 | 0 | 1 |
| Lymph node status | |||
| Positive | 4 | 2 | 2 |
| Negative | 5 | 4 | 1 |
| Missing | 1 | 1 | 0 |
| Cell type | |||
| Squamous | 4 | 3 | 1 |
| Adenocarcinoma | 6 | 4 | 2 |
| Tumor grade | |||
| 1 | 4 | 4 | 0 |
| 2 | 3 | 2 | 1 |
| 3 | 3 | 1 | 2 |
| Median tumor diameter, (range), cm | 5.4 (3.8-6.4) | 5.6 (3.8-6.4) | 5.2 (4.5-6.0) |
| HPV status | |||
| HPV16 | 7 | 5 | 2 |
| HPV18 | 3 | 2 | 1 |
| HPV16 and HPV18 | 0 | 0 | 0 |
Abbreviations: BMI = body mass index; FIGO = Federation of Gynecology and Obstetrics; HPV = human papillomavirus.
Adverse events
MEDI0457 was well tolerated when administered intramuscularly followed by EP with CELLECTRA. Treatment-related AEs occurred in 8 patients (Table 2; cohort 1, 5 of 7 patients; cohort 2, 3 of 3 patients). The only treatment-related AEs reported in more than 1 patient were injection site bruising (n = 2) and injection site pain (n = 2). AEs related to the injection site were all grade 1. Serious AEs (SAEs) were reported in 5 patients (cohort 1, 3 patients; cohort 2, 2 patients), and none of these SAEs were assessed as treatment related. All patients experienced at least 1 treatment-emergent AE (TEAE) during the study (cohort 1, 98 TEAEs; cohort 2, 34 TEAEs). Treatment-related TEAEs occurred in 8 patients (cohort 1, 5 patients; cohort 2, 3 patients). The majority of TEAEs were grade 1 or 2, but TEAEs grade ≥3 were reported in 4 patients (cohort 1, 2 patients; cohort 2, 2 patients). In cohort 1, grade ≥3 TEAEs were abdominal pain (grade 3) and pneumonia (grade 3). In cohort 2, grade ≥3 TEAEs were pathologic fracture (grade 3), anemia (grade 3), intestinal perforation (grade 5), and disseminated intravascular coagulation (grade 5). The patient who experienced intestinal perforation and disseminated intravascular coagulation discontinued the study early and died of this AE. The grade 5 toxicity occurred after chemoradiation and 3 doses of INO 3112. The patient experience intestinal perforation possibly attributed to progression of intra-abdominal metastases requiring total colectomy and developed disseminated intravascular coagulation postoperatively. All grade ≥3 TEAEs were considered unrelated; no other deaths were reported, and no other patients discontinued the study because of an AE.
Table 2.
Adverse events
| Adverse events | Total patients | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 |
|---|---|---|---|---|---|---|
| Treatment-related (injection site) | 8 | 8 | 0 | 0 | 0 | 0 |
| Any serious adverse events* | 5 | 0 | 1 | 3 | 0 | 1 |
| DIC | 1 | 0 | 0 | 0 | 0 | 1 |
| Abdominal pain | 1 | 0 | 0 | 1 | 0 | 0 |
| Intestinal perforation | 1 | 0 | 0 | 0 | 0 | 1 |
| Endometritis | 1 | 0 | 1 | 0 | 0 | 0 |
| Pneumonia | 1 | 0 | 0 | 1 | 0 | 0 |
| Pathologic fracture | 1 | 0 | 0 | 1 | 0 | 0 |
Abbreviation: DIC = disseminated intravascular coagulation.
Before starting study treatment, 1 patient developed grade 3 febrile neutropenia and 1 patient developed grade 3 diarrhea. These patients were not included in the analysis.
Immune responses
Immune responses after chemoradiation and MEDI0457 immunization were assessed by HPV 16/18 E6/E7 IFNγ production in peripheral blood mononuclear cells (PBMC) as measured by ELISpot, antibody responses as measured by enzyme-linked immunosorbent assay (ELISA), and tumor-infiltrating immune cells as measured by multiplexed immunofluorescence. Before chemoradiation, cohort 1 had significantly higher lymphocytes compared with cohort 2 (Fig E2, available online at https://doi.org/10.1016/j.ijrobp.2020.02.031; P = .008).
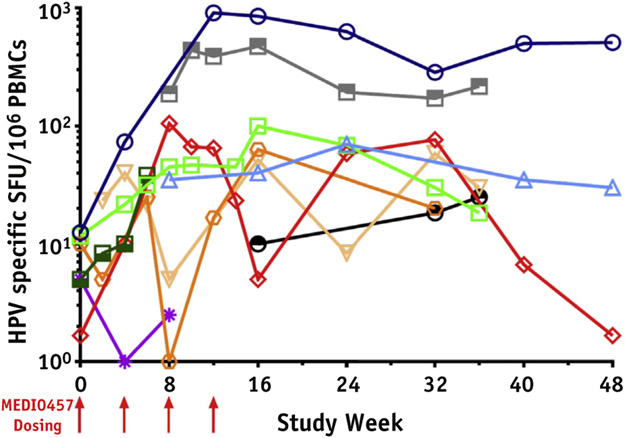
Overall, 8 patients had detectable cellular or humoral immune responses after chemoradiation and MEDI0457 immunization (Table 3; Fig. 1; Fig. E3 and Table E3, available online at https://doi.org/10.1016/j.ijrobp.2020.02.031). On a per-patient basis, 6 patients showed increased IFNγ responses over baseline against HPV16 antigens (all 6 patients showed increased responses to E6, and 4 of 6 patients showed increased responses to E7), and 5 patients showed increased IFNγ responses over baseline against HPV18 antigens (all 5 patients showed increased responses to E6, and 4 of 5 patients showed increased responses to E7). In cohort 1, 4 of 7 patients exhibited IFNγ-producing spots exceeding 100 SFU/106 PBMC, whereas no patients produced similar responses in cohort 2. IFNγ ELISpot responses in patients who discontinued treatment because of progression of disease or SAEs (patients 46-429 and 46-430) are shown in Figure E4 (available online at https://doi.org/10.1016/j.ijrobp.2020.02.031). Anti-HPV responses were numerically greater in cohort 1 (23.3 SFU/106 PBMC to 369 SFU/106 PBMC) compared with cohort 2 (6.7 SFU/106 PBMC to 63.3 SFU/106 PBMC). Unlike cohort 2, cellular responses in cohort 1 persisted through week 48. In cohort 1, T cell responses to E7 were numerically greater than T cell responses to E6 for both HPV16 and HPV18 subtypes. Overall cellular immune responses were assessed using ELISpot PBMC responses against nonspecific CEF (CMV, EBV, and influenza) peptides (Table E2; available online at https://doi.org/10.1016/j.ijrobp.2020.02.031).
Table 3.
Cellular immune responses against HPV16 and HPV18 E6 and E7 antigens
| Total ELISpot (SFU/106 PBMC) | Total (N = 10) | Cohort 1 (N = 7) | Cohort 2 (N = 3) |
|---|---|---|---|
| Baseline mean (n) | 7.6 (6) | 8.6 (3) | 6.7 (3) |
| SD | 4.4 | 6.0 | 2.9 |
| Week 4 mean (n) | 25.8 (6) | 36.3 (4) | 5.0 (2) |
| SD | 27.0 | 27.6 | 7.1 |
| Mean increase from baseline (n) | 16.8 (5) | 26.4 (3) | 2.5 (2) |
| SD increase from baseline | 24.9 | 29.8 | 3.5 |
| Week 8 mean (n) | 54.2 (7) | 75.3 (5) | 1.3 (2) |
| SD | 69.1 | 72.1 | 1.8 |
| Mean increase from baseline (n) | 34.2 (4) | 68.3 (2) | 0.0 (2) |
| SD increase from baseline | 48.7 | 49.5 | 0.0 |
| Week 12 mean (n) | 298.5 (5) | 369.0 (4) | 16.7 (1) |
| SD | 371.8 | 388.8 | NA |
| Mean increase from baseline (n) | 266.7 (4) | 353.3 (3) | 6.7 (1) |
| SD increase from baseline | 422.8 | 472.3 | NA |
| Week 16 mean (n) | 199.4 (8) | 218.8 (7) | 63.3 (1) |
| SD | 304.7 | 323.7 | NA |
| Mean increase from baseline (n) | 246.0 (4) | 310.3 (3) | 53.3 (1) |
| SD increase from baseline | 397.0 | 460.0 | NA |
| Week 24 mean (n) | 171.7 (6) | 171.7 (6) | ND |
| SD | 233.5 | 233.5 | |
| Mean increase from baseline (n) | 244.2 (3) | 244.2 (3) | |
| SD increase from baseline | 324.8 | 324.8 | |
| Week 32 mean (n) | 94.3 (7) | 106.7 (6) | 20.0 (1) |
| SD | 99.5 | 102.9 | NA |
| Mean increase from baseline (n) | 94.0 (4) | 121.9 (3) | 10 (1) |
| SD increase from baseline | 122.5 | 133.4 | NA |
| Week 48 mean (n) | 180.6 (3) | 180.6 (3) | ND |
| SD | 285.7 | 285.7 | |
| Mean increase from baseline (n) | 248.8 (2) | 248.8 (2) | |
| SD increase from baseline | 351.8 | 351.8 |
Abbreviations: CI = confidence interval; NA = not applicable; ND = not done; SD = standard deviation.
The (n) for mean increase from baseline at each time point represents only the patients who also had corresponding baseline samples available. Therefore, the number of patient samples analyzed at the indicated time point may differ from the number of patients in whom the mean increase from baseline could be assessed.
Fig. 1.
MEDI0457 and chemoradiation is associated with cellular immune responses against HPV 16 or HPV 18 E6/E7 antigens in the majority of patients with cervical cancer treated with chemoradiation. Log scale plots on IFNγ ELISpot assays of PBMC taken at various time points after MEDI0457 immunizations. On day 0, patients received MEDI0457 followed by EP (arrows) beginning 2 to 4 weeks after chemoradiation. MEDI0457 was given every 4 weeks for a total of 4 injections. PBMCs were collected beginning on day 0 and at weeks 2, 4, 6, 8, 10, 12, 14, 16, 24, 32, 36, 40, and 48. PBMCs were assayed for IFNγ production in response to E6E7 peptides by ELISpot. Data represent the combined ELISpot results for HPV16 and HPV18 E6 and E7 peptide stimulations (represented as SFU/106 PBMCs). Each line/color represents a patient. Half-filled symbols (n = 3) represent HPV18 detected at screening, open symbols (n = 7) represent HPV16 detected at screening. Abbreviations: ELISpot = enzyme-linked immunosorbent spot; EP = electroporation; HPV = human papilloma virus; IFNγ = interferon gamma; PBMCs = peripheral blood mononuclear cell; SFU = spot forming units.
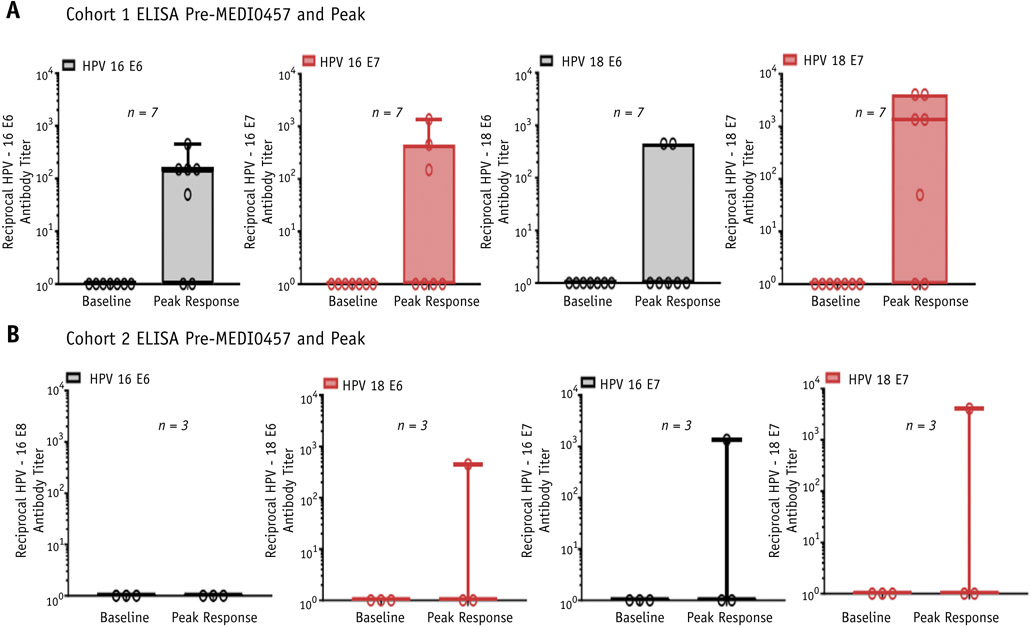
Six of 10 patients exhibited de novo sero-responses to HPV16 antigens, and 6 of 10 patients exhibited de novo sero-responses to HPV18 antigens (Fig. 2; Fig. E5, available online at https://doi.org/10.1016/j.ijrobp.2020.02.031). For HPV16 antigens, vaccination and chemoradiation was associated with antibody responses against E6 in 5 of 10 patients and against E7 in 4 of 10 patients. For HPV18 antigens, vaccination and chemoradiation was associated with antibody responses against E6 in 3 of 10 patients and against E7 in 6 of 10 patients. Antibody titers against the E6 and E7 antigens of both HPV 16 and HPV18 increased at most time points after immunization in cohort 1 and increased in at least 1 time point after vaccination in cohort 2. Patients 46-429 and 46-430 had no positive ELISA titers to any of the 4 HPV antigens tested.
Fig. 2.
MEDI0457 and chemoradiation is associated with humoral immune responses against HPV16 or HPV17 E6/E7 antigens in the majority of patients with cervical cancer treated with chemoradiation. (A) Antibody responses against HPV16 E6 antigen (far left panel), HPV18 E6 antigen (center left panel), HPV16 E7 antigen (center right panel), and HPV18 E7 antigen (far left panel) in patients treated for newly diagnosed, locally advanced cervical cancer (cohort 1). (B) Antibody responses against HPV16 or HPV18 E6/E7 antigen in patients treated for recurrent or persistent cervical cancer (cohort 2). Antibody titers were measured using ELISA assays. Abbreviations: ELISA = enzyme-linked immunosorbent assay; HPV = human papilloma virus.
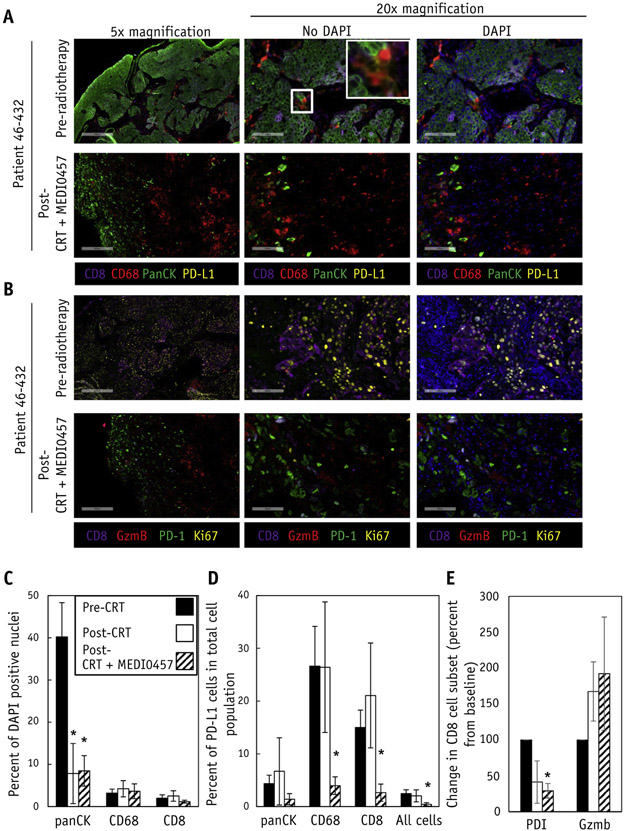
Local immune responses were assessed using multiplexed immunofluorescence of cervical biopsies taken before chemoradiation (pre-CRT), after chemoradiation (post-CRT), and at the completion of MEDI0457 immunizations (post-CRT + MEDI0457; Fig. 3A, 3B). To assess local immune responses and potential immune suppression, we assessed the expression of the checkpoint receptor PD-L1 on panCK+ tumor cells, CD68+ macrophages, and CD8+ T cells in serial biopsy specimens. We chose CD8+ T cells and CD68+ macrophages because these cell populations have been associated with antitumor and protumor immune responses in several cancers, including cervical cancers.21,22 Post-CRT and post-CRT + MEDI0457 cervical specimens were associated with decreased epithelial cells, consistent with the tumor regression observed in these patients after chemoradiation (Fig. 3C). The immunosuppressive marker PD-L1 was detectable on panCK+ tumor cells and CD68+ macrophages at pre-CRT and post-CRT biopsies, consistent with the immunosuppressive role of these cell populations in cancer. We also detected PD-L1 expression on CD8+ T cells, which has previously been shown to be a marker of immunosuppression and negatively correlated with outcomes in patients with melanoma.23,24 The receptor of PD-L1, PD-1, and the cytotoxic T cell marker granzyme B were also assessed on CD8+ T cells (Fig. 3B). Compared with pre-CRT and post-CRT time points, post-CRT + MEDI0457 biopsies were associated with decreased PD-L1+CD8+, PD-1+CD8+, and PD-L1+CD68+ subpopulations (Fig. 3A, 3B, 3D, and 3E). The decrease in PD-1 subpopulations in T cells and PD-L1 subpopulations in T cells and macrophages was not due to an overall loss of these cell populations because the percentages of CD8+ and CD68+ cells remained stable at pre-CRT, post-CRT, and post-CRT + MEDI0457 time points (Fig. 3C). Furthermore, granzyme B, a marker for cytolytic T cell activity, was detected in both CD8+ and CD8− cells of pre-CRT, post-CRT, and post-CRT + MEDI0457 cervical specimens (Fig. 3B).
Fig. 3.
Chemoradiation and MEDI0457 is associated with decreased PD-L1 expressing T cells and macrophages at the treated tumor site. (A) Multiplexed immunofluorescence for PD-L1 expression on CD8+ T cells, CD68+ macrophages, and panCK+ epithelial cells in cervical biopsies taken before chemoradiation (pre-CRT; n = 6), after chemoradiation (post-CRT; n = 5), and after chemoradiation and MEDI0457 (post-CRT + MEDI0457; n = 4). Inset in middle panels demonstrated PD-L1 costaining with CD8 or CD68. (B) Multiplexed immunofluorescence for CD8+ T cells (purple), Granzyme B (red), PD-1 (green), and the proliferative marker Ki-67 (yellow) from cervical biopsy specimens taken before chemoradiation and after chemoradiation and MEDI0457. Scale bar represents 400 μM for 5× magnification and 100 μM for 20× magnification. Post-CRT + MEDI0457 biopsies were taking at 63 and 122 days after the first MEDI0457 dose. (C) Percentages of panCK+ cells decrease whereas CD8+ T cells or CD68+ macrophages remain unchanged in pre-CRT, post-CRT, and post-CRT + MEDI0457 specimens. (D) Compared with pre-CRT and post-CRT time points, PD-L1+CD8+ T cells and PD-L1+CD68+ macrophages are decreased at post-CRT + MEDI0457 time points whereas PD-L1+PanCK+ epithelial cells remain unchanged. (E) CD8+PD-1+ cells are decreased whereas CD8+Granzyme B+ cells are increased after chemoradiation and MEDI0457. Calculated as percent change from prechemoradiation baseline. (C-E) represent quantitation of all available patient samples in the study. The asterisks (*) represents P < .05 as determined by Student t test. Error bars represent standard error of the mean. Abbreviations: CRT = chemoradiotherapy; DAPI = 4′,6-diamidino-2-phenylindole; PD-1 = programmed cell death protein 1; PD-L1 = programmed death-ligand 1; Vac = vaccine.
Outcomes
All cohort 1 patients remain alive with no evidence of disease clinically or by PET/CT (Fig. E6; available online at https://doi.org/10.1016/j.ijrobp.2020.02.031). Of the cohort 2 patients, 1 died, 1 had persistent disease, and 1 remains free of disease. The estimated progression-free survival (PFS) at 12 months was 88.9% overall, 100% in cohort 1, and 50% in cohort 2. Median PFS time was not reached in either cohort or in the combined population. Of the 8 patients with available assessment of target lesions, 7 achieved a complete response (6 of 7 in cohort 1 and 1 of 3 in cohort 2), and 1 (cohort 1) achieved partial response after completion of the immunization series. PET/CT imaging demonstrated decreased or stable hypermetabolic activity after chemoradiation and MEDI0457, as shown in Figure. E6 (available online at https://doi.org/10.1016/j.ijrobp.2020.02.031).
Clearance of HPV-positive cancer cells was assessed using ThinPrep testing for HPV PCR and RNAScope LSx in situ hybridization of cervical biopsies. Before chemoradiation, ThinPrep testing for HPV16 or HPV18 was positive in 6 patients (HPV16, 4 in cohort 1 and 1 in cohort 2; HPV18, 1 in cohort 1). One patient with undetectable HPV DNA by ThinPrep tested positive for HPV18 by PCR. At the completion of radiation, 3 of 6 patients has persistent HPV DNA by ThinPrep. All patients cleared detectable HPV DNA at week 16 after immunizations with available data at the 3 given time points. RNAScope in situ hybridization was assessed for 6 patients with available prechemoradiation, postchemoradiation, and postvaccination (Fig. E7; available online at https://doi.org/10.1016/j.ijrobp.2020.02.031). Prechemoradiation HPV16 RNA was detectable in 4 patients, and HPV18 RNA was detectable in 2 patients. Five of 6 patients had cleared HPV RNA by in situ hybridization at the completion of the immunization series.
Discussion
Here, we report the safety, tolerability, and immune responses associated with HPV DNA immunotherapy with MEDI0457 after chemoradiation in patients with newly diagnosed stage IB-IVB or persistent/recurrent cervical cancers. MEDI0457 was well tolerated, with only grade 1 treatment-related AEs. MEDI0457 and chemoradiation was associated with increased HPV 16/18 E6/E7-specific T cell responses and humoral responses. Of note, T cell and antibody responses were numerically greater in patients treated for newly diagnosed cervical cancers compared with patients treated for recurrent or persistent disease. Furthermore, at the completion of MEDI0457 and chemoradiation, cervical biopsy specimens displayed decreased immunosuppressive cell populations. The 12-month PFS was 88.9% overall, with 100% and 50% PFS in patients with newly diagnosed disease and recurrent/persistent disease, respectively. In contrast to Aggarwal et al,5 who waited 2 months after radiation for oropharyngeal cancers before initiating MEDI0457, we observed that immunization within 2 weeks after chemoradiation was still associated with detectable immune responses. Thus, immunotherapy using MEDI0457 with chemoradiation is well tolerated and is associated with detectable tumor-specific immune responses.
DNA vaccines have several advantages over other vaccine platforms such as live-attenuated viruses and recombinant protein—based vaccines. Unlike viral vector—based constructs, DNA vaccines can be used for repeated administration because the efficacy of plasmid vectors is not influenced by pre-existing neutralizing antibodies.25 Furthermore, the ability to engineer specific targeting of multiple antigenic components allows the inclusion of specific antigens, adjuvants, or targeting sequences. Thus, DNA immunization strategies can be easily incorporated with chemoradiation approaches for HPV-associated cancers.
Because EP increases the immunogenicity of MEDI0457, patients who received only MEDI0457 or EP may generate diminished immune responses against HPV. To this end, 5 of 7 patients in cohort 1 received all doses MEDI0457 and EP, whereas only 2 of 7 patients received MEDI0457 without EP in 1 dose. Yet, missing EP in only 1 of 4 vaccine time points was still sufficient to stimulate immune responses against HPV antigens, as evidenced by the efficacy of DNA vaccines in preinvasive disease using only 3 vaccine time points.8,12
Persistent HPV DNA in cervical specimens has been associated with recurrence after RT.26-28 Even at 12 to 24 months of follow-up, persistent HPV DNA was detected in 18.6% to 56.7% of patients with locally advanced cervical cancer after RT. Similarly, at 1-month postchemoradiation and before vaccination, 3 of 6 patients in our study had persistent HPV DNA as detected by ThinPrep testing in our cohort. All patients cleared HPV DNA by ThinPrep testing after vaccination, which supports the hypothesis that immunotherapy improves treatment efficacy after chemoradiation for cervical cancer.
Our findings strengthen the feasibility that immunotherapies can elicit de novo or boost existing immune responses after chemoradiation regimens that employ larger elective nodal irradiation fields. In preclinical models, the immunostimulatory effects of high-dose radiation29,30 may be limited with the addition of elective nodal radiation fields.31 Lymphopenia has been observed in patients treated for cervical cancers, head and neck cancers, and other cancers that incorporate elective nodal irradiation.32-35 Patients with cervical cancer treated with pelvic radiation to 50 Gy demonstrated impaired immune responses in tumor draining lymph nodes that were associated with increased regulatory CD4+ T cells, a marker of immune suppression, and a loss of Th1 and Tc1 polarization, markers of antitumor CD4+ and CD8+ T cell responses.36 In our study, immunization against HPV antigens occurred at sites distant from the elective nodal radiation fields, which likely bypassed the potential adverse effect of radiation fields on antitumor immune responses and generated robust anti-HPV immune responses in a majority of patients. Thus, immunization against cancer antigens is feasible even when using potentially immunosuppressive radiation fields encompassing the tumor-draining lymphatics.
In our study, patients with invasive cervical cancer mounted lower HPV-specific immune responses compared with previous studies immunizing patients with preinvasive CIN2/3 disease.8 Namely, as measured in IFNγ ELISpot assays, patients with invasive cervical cancer had an average of 298.5 SFU/106 PBMCs compared with >500 SFU/106 PBMCs for patients with CIN2/3 disease at completion of vaccination. It is tempting to speculate that this diminished immune response in patients with invasive cancer was due to a combination of immune exhaustion in patients with cancer, lingering immune suppression after chemoradiation, and/or the selection of patients with diminished immune responses against HPV antigens making them prone to develop cervical cancer.
The effect of RT or chemoradiotherapy on immune responses against HPV antigens in head and neck and cervical cancer has been mixed. Some studies have demonstrated enhanced anti-HPV immune responses after RT,37,38 whereas others have demonstrated suppressed immune responses after RT in both head and neck and cervical cancers.36,39-41 The decreased immune responses to HPV antigens parallels the global lymphopenia observed in both head and neck and cervical cancers after RT.32,35,42,43 The conflicting immunostimulatory and immunosuppressive effects described after RT may be due to differences in treatment techniques, target volumes, or fraction sizes. To this end, Battaglia et al demonstrated that pelvic radiation doses under 40 Gy enhanced immune responses whereas pelvic radiation doses of 50 Gy, the treatment regimen used in this work, were associated with suppressed immune responses in the tumor-draining lymph nodes of patients with cervical cancer.36 Regardless, vaccination after chemoradiation may rescue suppressed or even enhance existing immune responses against HPV antigens. It is interesting to speculate that the decrease in CD8+PD-1+, CD8+PD-L1+, and CD68+PD-L1+ cells followed chemoradiation and vaccination reflects a decreased local immunosuppression and increased probability for tumor regression.
Our observations are consistent with the hypothesis that patients with newly diagnosed cancers will likely respond better to immunotherapies than patients with recurrent or persistent disease, possibly owing to immune suppression or immune exhaustion. MEDI0457 and chemoradiation was associated with numerically greater immune responses in patients with newly diagnosed cervical cancers compared with patients with persistent or recurrent disease. Although our studies enrolled a limited number of patients with recurrent or persistent disease, we observed that these patients displayed decreased immune responses to HPV antigens compared with patients with newly diagnosed cancers. In addition, patients in cohort 2 had fewer absolute lymphocytes before the start of chemoradiation, supporting the notion that they were more immunosuppressed than patients in cohort 1. Similarly, Mohme et al demonstrated that patients with recurrent glioblastomas displayed features of greater immune exhaustion compared with patients with newly diagnosed glioblastomas.44 In preclinical models, Predina et al observed an increase in immunosuppressive macrophages and regulatory T cells at the tumor site and draining lymph nodes in animals with recurrent cancers compared with animals with primary cancers.45 Thus, both immune exhaustion and immunosuppressive microenvironments may regulate immune responses in patients with recurrent cancers differently than in patients with newly diagnosed disease.
Nevertheless, there are several limitations to this study. This study enrolled a relatively small number of patients, and treatment efficacy needs to be confirmed with larger phase 2 trials. Second, the antigen dose and timing of MEDI0457 as well as the use of an IL-12 adjuvant was based on studies of preinvasive disease, and it remains unclear whether this schedule requires modification when treating invasive disease. Third, we immunized patients within 2 to 4 weeks after chemoradiation, and it remains unclear whether a longer recovery interval after chemoradiation or vaccination before chemoradiation would improve anti-HPV immune responses. Finally, immune responses against HPV antigens were likely a consequence of vaccination and chemoradiation. Because of the natural history of HPV infection, HPV-mediated carcinogenesis, and the effect of chemoradiation on immune responses, the efficacy and changes in immune responses mediated by vaccination after chemoradiation cannot be clearly assessed without a chemoradiation-only cohort. In fact, more than half of patients with cervical or head and neck cancer have detectable immune responses against E6 or E7 at diagnosis.46-50 However, because we only assessed immune responses after chemoradiation, we cannot comment on the extent of existing immune responses against HPV before treatment. Furthermore, Masterson et al demonstrated that increased CD8+ T cell responses against E6 or E7 after chemoradiation alone was associated with improved disease-free survival.51 Conversely, patients with unchanged or decreased immune responses after chemoradiation may benefit from strategies, such as vaccines, that boost anti-HPV immunity. Because this work primarily addresses the safety and tolerability of the combination of chemoradiation and MEDI0457 vaccine, the contribution of MEDI0457 to inducing anti-HPV immune responses after chemoradiation is hypothesis-generating.
Conclusions
We report an initial experience of incorporating therapeutic HPV vaccinations after chemoradiation for patients with advanced cervical cancers. There were no serious AEs associated with MEDI0457 immunization. Furthermore, MEDI0457 after chemoradiation was associated with both cellular and humoral immune responses to HPV antigens and was associated with decreased immunosuppressive markers in the tumor microenvironment. Consequently, the findings presented here support further study of incorporating HPV vaccination strategies into the curative treatment of patients with locally advanced cervical cancers. Future trials will be needed to address whether chemoradiation alone boosts pre-existing immunity to HPV antigens or whether MEDI0457 immunization after chemoradiation is sufficiently immunogenic to induce potent antitumor immune responses.
Supplementary Material
Acknowledgments—
We thank Helen Snyder (Cell IDx) for assistance with immunofluorescence experiments.
Research support was provided by Inovio Pharmaceuticals, Inc and AstraZeneca; the Burroughs Wellcome Career Award for Medical Scientists 1010964 (M.T.S.); and the National Institutes of Health/National Institute of Dental and Craniofacial Research R01DE027445-01 (M.T.S.). This work was supported in part by a grant for the National Institutes of Health R01DE027445-01 (M.T.S) and the Virginia and D.K. Ludwig Fund for Cancer Research (R.R.W and M.T.S).
Footnotes
Disclosures: M.T.E. and R.K. are employees of and own stock in AstraZeneca. J.B., K.K., M.M., J.S., and A.S. are employees of Inovio Pharmaceuticals and own shares or have been awarded stock options in the company. M.B. owns stock in Inovio Pharmaceuticals. D.G. reports grants from Inovio Pharmaceuticals, Inc, and grants from AstraZeneca, during the conduct of the study. S.J. reports other from Inovio, during the conduct of the study. G.F. reports other from Corcept Therapeutics, AbbVie, Genentech, Tesaro, Syndax, Forty Seven, Iovance, Syros, Astex, Marck, Wolters Kluwer, and Sermonix and personal fees from Vaniam Group, during the conduct of the study.
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
Supplementary material for this article can be found at https://doi.org/10.1016/j.ijrobp.2020.02.031.
Presented at American Society of Clinical Oncology 2018, Chicago IL.
References
- 1.Crosbie EJ, Einstein MH, Franceschi S, et al. Human papillomavirus and cervical cancer. Lancet 2013;382:889–899. [DOI] [PubMed] [Google Scholar]
- 2.Morris M, Eifel PJ, Lu J, et al. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. New Engl J Med 1999;340: 1137–1143. [DOI] [PubMed] [Google Scholar]
- 3.Rose PG, Bundy BN, Watkins EB, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. New Engl J Med 1999;340:1144–1153. [DOI] [PubMed] [Google Scholar]
- 4.Arbyn M, Xu L, Simoens C, et al. Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochrane Database Syst Rev 2018;5:CD009069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aggarwal C, Cohen RB, Morrow MP, et al. Immunotherapy targeting HPV16/18 generates potent immune responses in hpv-associated head and neck cancer. Clin Cancer Res 2019;25:110–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brun JL, Dalstein V, Leveque J, et al. Regression of high-grade cervical intraepithelial neoplasia with TG4001 targeted immunotherapy. Am J Obstet Gynecol 2011;204:169.e1–169.e8. [DOI] [PubMed] [Google Scholar]
- 7.Kenter GG, Welters MJ, Valentijn AR, et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. New Engl J Med 2009;361:1838–1847. [DOI] [PubMed] [Google Scholar]
- 8.Trimble CL, Morrow MP, Kraynyak KA, et al. Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasia 2/3: A randomised, double-blind, placebo-controlled phase 2b trial. Lancet 2015;386: 2078–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ressing ME, Sette A, Brandt RM, et al. Human CTL epitopes encoded by human papillomavirus type 16 E6 and E7 identified through in vivo and in vitro immunogenicity studies of HLA-A*0201-binding peptides. J Immunol 1995;154:5934–5943. [PubMed] [Google Scholar]
- 10.Butz K, Ristriani T, Hengstermann A, et al. Sirna targeting of the viral E6 oncogene efficiently kills human papillomavirus-positive cancer cells. Oncogene 2003;22:5938–5945. [DOI] [PubMed] [Google Scholar]
- 11.Sima N, Wang W, Kong D, et al. RNA interference against HPV16 E7 oncogene leads to viral E6 and E7 suppression in cervical cancer cells and apoptosis via upregulation of RB and P53. Apoptosis 2008;13: 273–281. [DOI] [PubMed] [Google Scholar]
- 12.Bagarazzi ML, Yan J, Morrow MP, et al. Immunotherapy against HPV16/18 generates potent TH1 and cytotoxic cellular immune responses. Sci Transl Med 2012;4:155ra138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan J, Harris K, Khan AS, et al. Cellular immunity induced by a novel HPV18 DNA vaccine encoding an E6/E7 fusion consensus protein in mice and rhesus macaques. Vaccine 2008;26:5210–5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yan J, Reichenbach DK, Corbitt N, et al. Induction of antitumor immunity in vivo following delivery of a novel HPV-16 DNA vaccine encoding an E6/E7 fusion antigen. Vaccine 2009;27:431–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Broderick KE, Humeau LM. Electroporation-enhanced delivery of nucleic acid vaccines. Expert Rev Vaccines 2015;14:195–204. [DOI] [PubMed] [Google Scholar]
- 16.Cheng MA, Farmer E, Huang C, et al. Therapeutic DNA vaccines for human papillomavirus and associated diseases. Hum Gene Ther 2018; 29:971–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chattergoon MA, Saulino V, Shames JP, et al. Co-immunization with plasmid IL-12 generates a strong T-cell memory response in mice. Vaccine 2004;22:1744–1750. [DOI] [PubMed] [Google Scholar]
- 18.Kalams SA, Parker SD, Elizaga M, et al. Safety and comparative immunogenicity of an HIV-1 DNA vaccine in combination with plasmid interleukin 12 and impact of intramuscular electroporation for delivery. J Infect Dis 2013;208:818–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim JJ, Ayyavoo V, Bagarazzi ML, et al. In vivo engineering of a cellular immune response by coadministration of IL-12 expression vector with a DNA immunogen. J Immunol 1997;158:816–826. [PubMed] [Google Scholar]
- 20.Morrow MP, Kraynyak KA, Sylvester AJ, et al. Augmentation of cellular and humoral immune responses to HPV16 and HPV18 E6 and E7 antigens by VGX-3100. Mol Ther Oncolytics 2016;3:16025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peranzoni E, Lemoine J, Vimeux L, et al. Macrophages impede CD8 T cells from reaching tumor cells and limit the efficacy of anti-PD-1 treatment. Proc Natl Acad Sci U S A 2018;115:E4041–E4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piras F, Colombari R, Minerba L, et al. The predictive value of CD8, CD4, CD68, and human leukocyte antigen-d-related cells in the prognosis of cutaneous malignant melanoma with vertical growth phase. Cancer 2005;104:1246–1254. [DOI] [PubMed] [Google Scholar]
- 23.Brochez L, Meireson A, Chevolet I, et al. Challenging PD-l1 expressing cytotoxic T cells as a predictor for response to immunotherapy in melanoma. Nat Commun 2018;9:2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacquelot N, Roberti MP, Enot DP, et al. Predictors of responses to immune checkpoint blockade in advanced melanoma. Nat Commun 2017;8:592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chattergoon M, Boyer J, Weiner DB. Genetic immunization: A new era in vaccines and immune therapeutics. FASEB J 1997;11:753–763. [DOI] [PubMed] [Google Scholar]
- 26.Datta NR, Kumar P, Singh S, et al. Does pretreatment human papillomavirus (HPV) titers predict radiation response and survival outcomes in cancer cervix?—A pilot study. Gynecol Oncol 2006;103:100–105. [DOI] [PubMed] [Google Scholar]
- 27.Nagai Y, Toma T, Moromizato H, et al. Persistence of human papillomavirus infection as a predictor for recurrence in carcinoma of the cervix after radiotherapy. Am J Obstet Gynecol 2004;191:1907–1913. [DOI] [PubMed] [Google Scholar]
- 28.Song YJ, Kim JY, Lee SK, et al. Persistent human papillomavirus DNA is associated with local recurrence after radiotherapy of uterine cervical cancer. Int J Cancer 2011;129:896–902. [DOI] [PubMed] [Google Scholar]
- 29.Lee Y, Auh SL, Wang Y, et al. Therapeutic effects of ablative radiation on local tumor require CD8+ t cells: Changing strategies for cancer treatment. Blood 2009;114:589–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang B, Bowerman NA, Salama JK, et al. Induced sensitization of tumor stroma leads to eradication of established cancer by T cells. J Exp Med 2007;204:49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marciscano AE, Ghasemzadeh A, Nirschl TR, et al. Elective nodal irradiation attenuates the combinatorial efficacy of stereotactic radiation therapy and immunotherapy. Clin Cancer Res 2018;24:5058–5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gray WC, Chretien PB, Suter CM, et al. Effects of radiation therapy on T-lymphocyte subpopulations in patients with head and neck cancer. Otolaryngol Head Neck Surg 1985;93:650–660. [DOI] [PubMed] [Google Scholar]
- 33.Stratton JA, Byfield PE, Byfield JE, et al. A comparison of the acute effects of radiation therapy, including or excluding the thymus, on the lymphocyte subpopulations of cancer patients. J Clin Invest 1975;56:88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang C, Liao Z, Gomez D, et al. Lymphopenia association with gross tumor volume and lung V5 and its effects on non-small cell lung cancer patient outcomes. Int J Radiat Oncol Biol Phys 2014; 89:1084–1091. [DOI] [PubMed] [Google Scholar]
- 35.Verastegui EL, Morales RB, Barrera-Franco JL, et al. Long-term immune dysfunction after radiotherapy to the head and neck area. Int Immunopharmacol 2003;3:1093–1104. [DOI] [PubMed] [Google Scholar]
- 36.Battaglia A, Buzzonetti A, Martinelli E, et al. Selective changes in the immune profile of tumor-draining lymph nodes after different neoadjuvant chemoradiation regimens for locally advanced cervical cancer. Int J Radiat Oncol Biol Phys 2010;76:1546–1553. [DOI] [PubMed] [Google Scholar]
- 37.Delgado FG, Martinez E, Cespedes MA, et al. Increase of human papillomavirus-16 e7-specific T helper type 1 response in peripheral blood of cervical cancer patients after radiotherapy. Immunology 2009; 126:523–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fattorossi A, Battaglia A, Ferrandina G, et al. Neoadjuvant therapy changes the lymphocyte composition of tumor-draining lymph nodes in cervical carcinoma. Cancer 2004;100:1418–1428. [DOI] [PubMed] [Google Scholar]
- 39.Al-Taei S, Banner R, Powell N, et al. Decreased HPV-specific T cell responses and accumulation of immunosuppressive influences in oropharyngeal cancer patients following radical therapy. Cancer Immunol Immunother 2013;62:1821–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parikh F, Duluc D, Imai N, et al. Chemoradiotherapy-induced upregulation of PD-1 antagonizes immunity to HPV-related oropharyngeal cancer. Cancer Res 2014;74:7205–7216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qinfeng S, Depu W, Xiaofeng Y, et al. In situ observation of the effects of local irradiation on cytotoxic and regulatory T lymphocytes in cervical cancer tissue. Radiat Res 2013;179:584–589. [DOI] [PubMed] [Google Scholar]
- 42.Cho O, Chun M, Chang SJ, et al. Prognostic value of severe lymphopenia during pelvic concurrent chemoradiotherapy in cervical cancer. Anticancer Res 2016;36:3541–3547. [PubMed] [Google Scholar]
- 43.van Meir H, Nout RA, Welters MJ, et al. Impact of (chemo)radiotherapy on immune cell composition and function in cervical cancer patients. Oncoimmunology 2017;6:e1267095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mohme M, Schliffke S, Maire CL, et al. Immunophenotyping of newly diagnosed and recurrent glioblastoma defines distinct immune exhaustion profiles in peripheral and tumor-infiltrating lymphocytes. Clin Cancer Res 2018;24:4187–4200. [DOI] [PubMed] [Google Scholar]
- 45.Predina J, Eruslanov E, Judy B, et al. Changes in the local tumor microenvironment in recurrent cancers may explain the failure of vaccines after surgery. Proc Natl Acad Sci U S A 2013;110:E415–E424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anderson KS, Dahlstrom KR, Cheng JN, et al. HPV16 antibodies as risk factors for oropharyngeal cancer and their association with tumor HPV and smoking status. Oral Oncol 2015;51:662–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fakhry C, Qualliotine JR, Zhang Z, et al. Serum antibodies to HPV16 early proteins warrant investigation as potential biomarkers for risk stratification and recurrence of HPV-associated oropharyngeal cancer. Cancer Prev Res (Phila) 2016;9:135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hanna GJ, Sridharan V, Margalit DN, et al. Salivary and serum hpv antibody levels before and after definitive treatment in patients with oropharyngeal squamous cell carcinoma. Cancer Biomark 2017;19:129–136. [DOI] [PubMed] [Google Scholar]
- 49.Lang Kuhs KA, Kreimer AR, Trivedi S, et al. Human papillomavirus 16 E6 antibodies are sensitive for human papillomavirus-driven oropharyngeal cancer and are associated with recurrence. Cancer 2017;123:4382–4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spector ME, Sacco AG, Bellile E, et al. E6 and E7 antibody levels are potential biomarkers of recurrence in patients with advanced-stage human papillomavirus-positive oropharyngeal squamous cell carcinoma. Clin Cancer Res 2017;23:2723–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Masterson L, Lechner M, Loewenbein S, et al. CD8(+) t cell response to human papillomavirus 16 E7 is able to predict survival outcome in oropharyngeal cancer. Eur J Cancer 2016;67:141–151. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.