Abstract
Prohibitin (PHB), an evolutionarily conserved mitochondrial inner membrane protein, is highly expressed in cells that require strong mitochondrial function. Recently, we demonstrated that the deletion of Phb in spermatocytes results in impaired mitochondrial function. In addition, PHB expression in the mitochondrial sheath of human sperm has a significantly negative correlation with mitochondrial reactive oxygen species levels, but a positive one with mitochondrial membrane potential and sperm motility. These results suggest that mitochondrial PHB expression plays a role in sperm motility. However, the mechanism of PHB-mediated regulation of sperm motility remains unknown. Here, we demonstrate for the first time that PHB interacts with protein kinase B (AKT) and exists in a complex with phospho-PHB (pT258) and phospho-AKT in the mitochondrial sheath of murine sperm, as determined using colocalization and coimmunoprecipitation assays. After blocking AKT activity using wortmannin (a phosphatidylinositol 3-kinase [PI3K] inhibitor), murine sperm have significantly (P < 0.05) decreased levels of phospho-PHB (pT258) and the total and progressive motility. Furthermore, significantly (P < 0.05) lower levels of phospho-PI3K P85 subunit α+γ (pY199 and pY467) and phospho-AKT (pS473; pT308) are found in sperm from infertile asthenospermic and oligoasthenospermic men compared with normospermic subjects, which suggest a reduced activity of the PI3K/AKT pathway in these infertile subjects. Importantly, these sperm from infertile subjects also have a significantly (P < 0.05) lower level of phospho-PHB (pT258). Collectively, our findings suggest that the interaction of PHB with AKT in the mitochondrial sheath is critical for sperm motility, where PHB phosphorylation (pT258) level and PI3K/AKT activity are key regulatory factors.
Keywords: male infertility, prohibitin (PHB), protein kinase B (AKT), sperm motility
INTRODUCTION
Prohibitin (PHB) is an evolutionarily conserved inner membrane mitochondrial protein. It has been shown that PHB is highly expressed in cells that have a demand for a strong mitochondrial function, where it plays key roles in mitochondrial respiratory chain subunit assembly, mitochondrial biogenesis, and mitophagy.1,2 Silencing of the PHB gene (Phb) in murine endothelial cells reduces mitochondrial membrane potential (MMP) and respiratory complex I activity.3 Our recent findings have shown that the deletion of Phb in spermatocytes reduces MMP and respiratory complex subunits expression levels.4 Furthermore, sperm with poor motility and/or low concentrations from infertile men have a reduced expression level of PHB that is negatively correlated with mitochondrial reactive oxygen species (mROS) level, but positively correlated with MMP and sperm motility.5,6 These findings suggest that PHB expression levels are critical for germ cell mitochondrial integrity, and could be used as an indicator of human sperm quality in the in vitro fertilization (IVF) clinic.4,5,6
Mitochondria are helically arranged around the mid-piece axoneme of mature sperm, where they provide the major source of ROS and play key roles in ATP production and the maintenance of sperm motility.7 Previous researches have shown that protein kinase B (AKT) and phospho-AKT proteins are expressed in the mitochondrial sheath of sperm from fertile men.8 Using a phosphatidylinositol 3-kinase (PI3K) inhibitor, wortmannin, AKT activity has been proven to be critical for mROS generation and sperm motility.8 The similarity of functional roles in mROS generation and sperm motility5,6,8 suggests that PHB expression levels might to some extent have association with AKT activity in the mitochondria of mammalian sperm. In somatic cells, AKT phosphorylates PHB and the resulting phospho-PHB modulates cellular function by enhancing the activity of the PI3K/AKT signaling pathway.9,10 However, the interaction of PHB and AKT in sperm mitochondria remains undetermined. The goal of the present study was therefore to determine if PHB interacts with AKT in sperm mitochondria to coordinately modulate sperm motility. Using colocalization and coimmunoprecipitation assays, we investigated for the first time the colocalization of PHB with AKT in the mitochondria of mature mouse sperm. After blocking AKT activity using wortmannin in these sperm, we further examined the cause-and-effect relationship of PI3K/AKT activity and PHB phosphorylation and their impact upon sperm motility. Finally, we also evaluated the levels of PI3K/AKT activity and PHB phosphorylation in sperm from infertile asthenospermic (A) and oligoasthenospermic (OA) men compared with those from normospermic (N) subjects.
Our results demonstrate that PHB interacts with AKT in a complex also containing phospho-PHB (pT258) and phospho-AKT in the mitochondrial sheath of murine sperm. Inhibition of AKT activity in these sperm reduces the level of PHB phosphorylation (pT258) as well as the total and progressive motility. Importantly, the phosphorylation levels of PI3K/AKT pathway and PHB proteins are significantly reduced in sperm with poor motility from infertile men compared with those from normospermic males. Collectively, our observations provide new evidence that PI3K/AKT activity impacts the levels of PHB phosphorylation and sperm motility.
MATERIALS AND METHODS
Chemicals
All chemicals were purchased from Sigma Chemical Co. (St. Louis, MO, USA) unless otherwise stated. Phosphate-buffered saline (PBS) solution was purchased from Ambion Inc. (Austin, TX, USA). MitoTracker mitochondria Red CMXRos probe (M7512) was purchased from Molecular Probes Inc. (Eugene, OR, USA). Normal goat serum (NGS), mouse immunoglobulin G (IgG), rabbit IgG, and VECTASHIELD Mounting Medium with 4',6-diamidino-2-phenylindole (DAPI; H1200) were from Vector Laboratories, Inc. (Burlingame, CA, USA). The PI3K inhibitor (wortmannin; #9951) was from Cell Signaling Technology, Inc. (Danvers, MA, USA). Protein A/G PLUS-Agarose (sc-2003) was from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). 3-([3-Cholamidopropyl] dimethylammonio)-1-propane sulfonate (CHAPS), urea, thiourea, dithiothreitol (DTT), and acrylamide were purchased from Amersham Biosciences (Uppsala, Sweden). All buffers were prepared using Milli-Q water (Millipore, Bedford, MA, USA).
Animals
Mature C57BL/6J male mice were purchased from the Animal Center of the Chinese Academy of Sciences (Shanghai, China). The mice were maintained at 20°C–25°C with a 12 h light/dark cycle (specific pathogen free [SPF]; certificate No. SYXK [Shanghai] 2009-0082). Food and water were freely available. All animal experiments were conducted according to a protocol approved by the Institutional Animal Care and Use Committee at Fudan University (Shanghai, China).
Human sperm collection and preparation
The project was approved by the Ethics Committee of the Fudan University for Investigations in Humans. After informed consent had been obtained, semen samples were collected according to the 2010 World Health Organization (WHO) criteria11 from male subjects between 30 and 40 years of age who were attempting intracytoplasmic sperm injection (ICSI) or IVF at the Shanghai Ji'Ai Genetics and IVF Institute of Fudan University (Shanghai, China). Immediately after liquefaction at 37°C for 30 min, the collected samples were subjected to sperm concentration, motility, and morphology analyses by computer-assisted sperm analysis (CASA). Parameter settings of the CASA system were as follows: 25 frames per s, 4–40 μm2 for head area, curvilinear velocity (VCL) >18 μm s−1 to classify a spermatozoon as motile, and a minimum of 1000 spermatozoa examined. According to the 2010 WHO criteria, subjects were diagnosed as normospermic (N; n = 33), asthenospermic (A; n = 36), and oligoasthenospermic (OA; n = 32) subjects as shown in Table 1. The controls were the subjects with normal semen characteristics (N). Semen samples were washed twice with PBS, followed by centrifugation (5417R, Eppendorf, Hamburg, Germany) at 14 000g for 15 min at 4°C, then processed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and western blotting for analysis of sperm protein expression.
Table 1.
Analysis of the semen parameters of the subjects included in this study (mean ± standard error of mean [s.e.m.])
| Subject parameter | N (n=33) | A (n=36) | OA (n=32) |
|---|---|---|---|
| Sperm count (×106) | 191.4±12.6 | 125.1±12.1 | 16.2±1.9 |
| Sperm concentration (106 ml−1) | 64.6±3.3 | 36.9±2.6 | 5.5±0.5 |
| Total motility (%) | 70.6±1.3 | 22.1±1.7 | 4.9±0.6 |
| Progressive motility (%) | 61.6±1.4 | 17.9±1.5 | 3.4±0.6 |
| Age (year) | 31.2±0.9 | 31.9±1.0 | 30.2±0.8 |
N: normospermia; A: asthenospermia; OA: oligoasthenospermia
Mouse sperm collection and treatments
Mouse caudal epididymides from sexually mature males (12–16 weeks old) were excised, several slits were cut using iridectomy scissors, and the tissues were transferred to wells of a cell culture plate containing 2 ml of Biggers, Whitten and Whittingham (BWW) medium pre-warmed at 37°C.12 After incubation for 5 min, the caudal epididymal sperm were collected and adjusted to 5 × 106 – 10 × 106 sperm ml−1 for the following assays.
To determine the mitochondrial membrane potential of collected sperm, the samples were incubated with a pre-warmed staining solution of 250 nmol l−1 MitoTracker mitochondria Red CMXRos probe (MITO; Molecular Probes) at 37°C for 10 min.5 After washed twice with pre-warmed fresh PBS, sperm were then attached to poly-L-lysine-coated slides and dried at room temperature (RT) for the immunofluorescence double staining.
To block AKT activity in collected mouse sperm, the samples were treated for 3 h in a 5% CO2 incubator at 37°C with different concentrations of PI3K inhibitor wortmannin (Cell Signaling Technology) at 0, 1, 5, 10, and 20 μmol l−1, respectively. The viability of treated sperm was then evaluated using MitoTracker mitochondria Red CMXRos probe (MITO) as described above.5 The level of sperm motility and phosphorylation levels of AKT and PHB were examined as described below.
Mouse sperm motility analysis
After treatment with wortmannin, sperm suspensions were loaded into rectangular 100 μm tubes (HTR1099, VitroCom Inc., Mt. Lks. NJ, USA). Sperm concentration and motility parameters were assessed using the CASA system (HTM-TOX IVOS, Hamilton-Thorne, Beverly, MA, USA) with standard instrument settings.13
Immunofluorescence
The colocalization of PHB with AKT, AKT pT308, AKT pS473, or colocalization of PHB pT258 with AKT pT308, were assessed in sperm mitochondrial sheath labeled by a MMP probe (MITO), as previously reported.5 Briefly, slides of sperm treated with MITO were fixed in cold acetone for 15 min and permeabilized with 1% Triton in PBS for 15 min. After blocking for 1 h at RT in PBS with 5% NGS and 5% bovine serum albumin (BSA), the slides were incubated at 4°C overnight with primary antibodies or rabbit and mouse IgG isotypes diluted in PBS containing 0.5% Tween 20 and 1% NGS (antibody diluent). The following day, the slides were washed three times for 5 min in PBS containing 0.2% Tween 20 and 1% NGS. Slides were then treated for 2 h at RT with Alexa Fluor 647-conjugated secondary antibodies for PHB or PHB pT258 staining, or with biotin-conjugated secondary antibody for AKT, AKT pT308, or AKT pS473 staining. After three washes, the slides were incubated for 1 h at RT with Alexa Fluor 488-conjugated streptavidin in antibody diluent. Then the slides were washed three times for 5 min with the PBS and mounted using VECTASHIELD Mounting Medium with DAPI (Vector Laboratories). The negative controls were run with rabbit and mouse IgG isotypes, instead of specific primary antibodies. Images were captured using a confocal Leica TCS SP8 scanning microscope (Leica Microsystems, Mannheim, Germany). The colocalization analysis used the line scan function in LAS AF Lite software (version: 2.6.0 build 7266, Leica Microsystems).
The following primary and secondary antibodies were used: rabbit anti-PHB pT258 (1:100, GTX55299, GeneTex, Irvine, CA, USA), mouse anti-AKT pT308 (1:25, 200-301-269S, Rockland Immunochemicals, Philadelphia, PA, USA), mouse anti-PHB (1:5, MA5-12858, Thermo Fisher Scientific, Waltham, MA, USA); rabbit anti-AKT (1:25, #4060), rabbit anti-AKT pT308 (1:100, #9275), and rabbit anti-AKT pS473 (1:100, #9272) purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA); Alexa Fluor 647 goat anti-mouse IgG (1:500, A32728) and Alexa Fluor 647 goat anti-rabbit IgG (1:500, A32733) purchased from Molecular Probes Inc. (Eugene, OR, USA); and biotin-conjugated goat anti-rabbit IgG (1:500, 111-067-003), goat anti-mouse IgG (1:500, 115-067-003), and Alexa Fluor 488-conjugated streptavidin (1:1000, 016-540-084) purchased from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA, USA).
Coimmunoprecipitation assay
The interaction of PHB with AKT was assessed using a coimmunoprecipitation (co-IP) assay with mouse caudal epididymal sperm after incubated in BWW for 3 h in a 5% CO2 incubator at 37°C. Briefly, collected sperm were homogenized in 1 ml of cold lysis buffer (150 mmol l−1 NaCl, 200 mmol l−1 Tris-HCl, 1 mmol l−1 ethylene diamine tetraacetie acid [EDTA] pH 8.0, 1% Triton X-100, 0.5% NP-40, 1 × proteinase inhibitor cocktail, 1 × phosphatase inhibitor cocktail) for 40 min on ice before centrifugation (14 000g, 15 min, 4°C). Total protein concentrations of the lysates were quantified using the Bradford method (Pierce Biotechnology, Rockford, IL, USA). Lysate samples with 1 mg of total protein were gently rotated at 4°C overnight while incubated with 5 μg of PHB antibody (ab75766, Abcam, Cambridge, MA, USA) or 5 μg rabbit IgG. The PHB- or IgG-targeted immune-complex was collected after incubation with 50 μl of protein A+G agarose beads (Santa Cruz Biotechnology) via gently shaking at 4°C for 4 h before centrifugation at 1500g for 2 min at 4°C. The precipitate was washed three times with ice-cold washing buffer, resuspended in 4× protein loading buffer, and boiled for 10 min to dissociate the immune-complex from the beads. The supernatant was collected by centrifugation and subjected to standard SDS-PAGE and immunoblot procedures as previously reported.5,6
SDS-PAGE and western blotting
The phosphorylation levels of PHB and the PI3K/AKT pathway in mouse sperm after treatment with wortmannin, and in human sperm with reduced motility from infertile males, were measured using western blotting analysis as previously described.5,6 The following primary antibodies were used: rabbit anti-PHB pT258 (1:500, GTX55299, GeneTex); rabbit anti-PI3 Kinase p85 (1:500, #4292), rabbit anti-AKT (1:500, #4060), rabbit anti-AKT pT308 (1:500, #9275) and rabbit anti-AKT pS473 (1:500, #9272) purchased from Cell Signaling Technology; rabbit anti-PI3K p85 alpha+gamma (Phospho Y199+Y467; 1:500, ab196001) and rabbit anti-PHB (1:500, ab75766) from Abcam; mouse anti-α-tubulin (1:1000, 66031-1-Ig) and mouse anti-β-actin (1:1000, 60008-1-Ig) from Proteintech Group, Inc. (Rosemont, IL, USA). To block the interference of IgG heavy and light chains on IP lysate blotting, a specific IP secondary antibody (the VeriBlot for IP secondary antibody [horseradish peroxidase, HRP; 1:40, ab131366]) purchased from Abcam was applied to detect the bound protein in the IP lysate. Images were captured using a Fujifilm LAS-4000 Mini Luminescent Imaging Analyzer (Fuji Film, Tokyo, Japan). The band intensities were quantified with ImageJ Software (National Institutes of Health, Bethesda, MA, USA).
Statistical analyses
All results are presented as mean ± standard error of mean (s.e.m.) except for human sperm protein expression results presented as median with interquartile range. The results of mouse sperm were analyzed using one-way analysis of variance (ANOVA) followed by the Newman–Keuls posttest, while that of human sperm were examined using the Kruskal–Wallis test followed by the Dunn's posttest (Prism software version 5.0, GraphPad, San Diego, CA, USA). P < 0.05 (two-sided probability) was considered statistically significant. Each experiment was repeated at least three times.
RESULTS
PHB and phospho-PHB (pT258) colocalize with AKT and phospho-AKT in the mitochondrial sheath of murine sperm
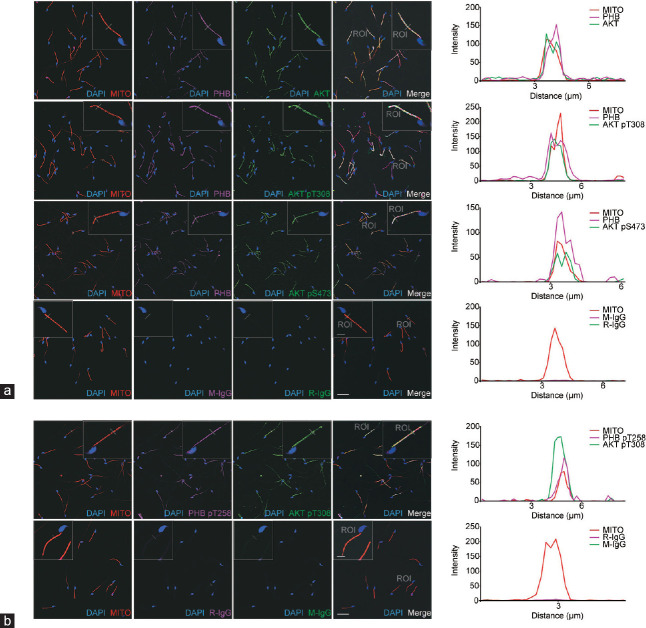
To assess the involvement of PHB in the PI3K/AKT pathway in mouse cauda epididymal sperm, the colocalization of PHB and AKT proteins in the mitochondrial sheath was examined using confocal immunofluorescence double-staining analysis. The negative controls used rabbit and mouse IgG isotypes, instead of specific primary antibodies. The mitochondrial sheath was labeled with the MitoTracker® mitochondrial membrane potential red probe (Figure 1a and 1b), first column), and PHB and phospho-PHB (pT258) were detected by the magenta fluorescence (second column), and AKT and phospho-AKT (pT308; pS473) by green fluorescence (third column). The light-yellow fluorescence indicated the regions in which all of the probes overlapped (fourth column). Sperm nuclei were counterstained with DAPI. Consequently, in the merged images (fourth column), PHB and phospho-PHB (pT258; magenta) colocalized with AKT and phospho-AKT (pT308; pS473; green) in the mitochondrial sheath (red), where was the typical long rod-shaped mid-piece (light yellow) of sperm. This colocalization was further demonstrated using the “Line Scan” application of LAS AF Lite software, as shown in the last column.
Figure 1.
PHB and phospho-PHB (pT258) colocalize with AKT and phospho-AKT in the mitochondrial sheath of mouse mature sperm. Colocalization of (a) PHB (magenta) or (b) phospho-PHB (pT258; magenta) with AKT (green) or phospho-AKT (pT308, pS473; green), and the mitochondrial sheath (red) of mouse cauda epididymal sperm, was determined using a mitochondrial membrane potential probe (MITO) and confocal fluorescence microscopy. The inserts showed the image amplified for “Line Scan” colocalization analysis. The plot profiles of MITO (red), PHB (magenta) or phospho-PHB (pT258; magenta), AKT (green) or phospho-AKT (pT308, pS473; green) colocalization along the region of interest (ROI) were constructed and analyzed using LAS AF Lite software, as shown in the last column on the right. The negative controls (the last rows of a and b) were run with rabbit (R-IgG) and mouse (M-IgG) IgG isotypes, instead of specific primary antibodies. Scale bars = 20 μm. For amplified image (insert), scale bars = 5 μm. PHB: prohibitin; AKT: protein kinase B; DAPI: 4',6-diamidino-2-phenylindole.
PHB interacts with AKT in a complex with phospho-PHB (pT258) and phospho-AKT (pT308) in murine sperm
To determine if there is an interaction of PHB with AKT in mouse cauda epididymal sperm, co-IP analysis was performed. As shown in Figure 2, after co-IP with PHB antibody, PHB was found to bind with AKT in a complex that also contained phospho-PHB (pT258) and phospho-AKT (pT308).
Figure 2.
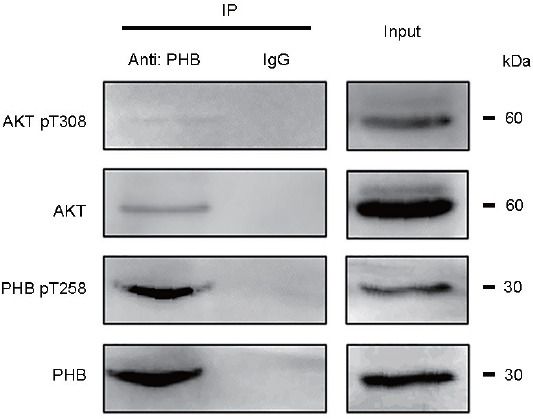
PHB interacts with AKT and exists in a complex with phospho-PHB (pT258) and phospho-AKT (pT308) in mouse mature sperm. The interaction of PHB with AKT in mouse cauda epididymal sperm was determined using coimmunoprecipitation analysis. A total lysate indicated as input was used as a positive control while a lysate with IgG treatment was used as a negative control. After co-IP with a PHB antibody, PHB was found to bind with AKT in a complex, in which phospho-PHB (pT258) and phospho-AKT (pT308) were present as well. PHB: prohibitin; AKT: protein kinase B; IP: immunoprecipitation.
AKT activity impacts PHB phosphorylation (pT258) and the total and progressive motility of murine sperm
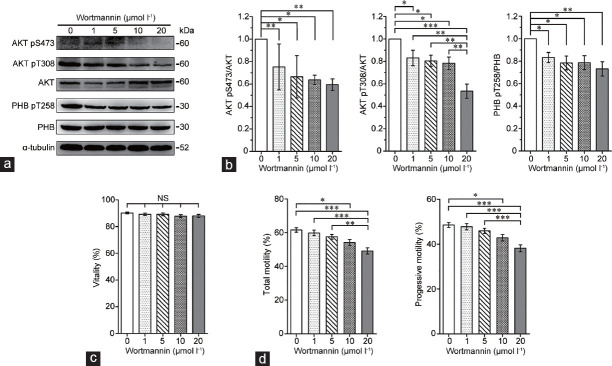
To elucidate the cause-and-effect relationship between the activity of the PI3K/AKT pathway and PHB phosphorylation, the phosphorylation levels of AKT and PHB and the resulting impact on sperm motility were examined after blocking AKT activity (using wortmannin) in mouse cauda epididymal sperm. As shown in Figure 3a and 3b, the levels of phospho-AKT (pS473; pT308) significantly (P < 0.05) decreased after wortmannin exposure without impact on sperm viability (Figure 3c). More importantly, the levels of phospho-PHB (pT258) and the total and progressive motility were also significantly (P < 0.05) decreased, especially in the presence of 10 μmol l−1 and 20 μmol l−1 wortmannin (Figure 3a, 3b, and d).
Figure 3.
AKT activity impacts the levels of PHB phosphorylation (pT258) as well as the total and progressive motility of mouse mature sperm. (a) After wortmannin (a phosphatidylinositol 3-kinase inhibitor) treatment for 3 h, the phosphorylation levels of AKT (pS473; pT308) and PHB (pT258) in mouse cauda epididymal sperm were analyzed by western blotting. (b) Alpha-tubulin was used as a loading control and densitometric analysis used the relative ratios of AKT pS473 versus AKT, AKT pT308 versus AKT, or PHB pT258 versus PHB. Fifty micrograms of protein were loaded in each lane. (c) Vitality was evaluated via MitoTracker mitochondria Red CMXRos probe staining, and (d) the total and progressive sperm motility were determined by CASA. Mean ± s.e.m., and *P < 0.05; **P < 0.01; ***P < 0.001 determined by one-way ANOVA analysis with the Newman–Keuls posttest. PHB: prohibitin; AKT: protein kinase B; NS: not significant; CASA: computer-assisted sperm analysis; s.e.m.: standard error of mean; ANOVA: analysis of variance.
Decreased PI3K/AKT activity and PHB phosphorylation in sperm from asthenospermic and oligoasthenospermic men
To test if sperm with poor motility from infertile human subjects have impaired PI3K/AKT activity along with decreased PHB phosphorylation, the phosphorylation levels of PI3K, AKT and PHB proteins in sperm from A and OA subjects were examined with a comparison to normospermic (N) subjects (Table 1). As shown in Figure 4, the phosphorylation levels of the PI3K P85 subunit α+γ (pY199 and pY467) and AKT (pS473; pT308) proteins were significantly (P < 0.05) lower in sperm from A and OA subjects compared with those from normospermic (N) subjects. Importantly, the phosphorylated PHB (pT258) protein was also significantly (P < 0.05) decreased in the sperm with reduced motility from A and OA subjects.
Figure 4.
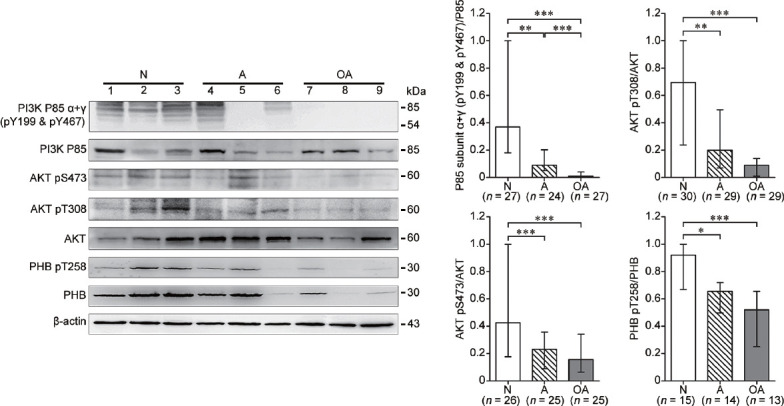
Human sperm with reduced motility from infertile subjects exhibit a significant decrease in PI3K/AKT activity and PHB phosphorylation. The phosphorylation levels of PI3K P85 subunit α+γ (pY199 and pY467), AKT (pS473; pT308), and PHB (pT258) in human sperm from normospermic (N), asthenospermic (A) and oligoasthenospermic (OA) subjects were analyzed by western blotting. Beta-actin was used as the loading control and densitometric analysis used the relative ratios of P85 subunit α+γ (pY199 and pY467) versus P85, AKT pS473 versus AKT, AKT pT308 versus AKT, or PHB pT258 versus PHB. Fifty micrograms of protein were loaded in each lane. The detected number (n) in each cohort was indicated in each histogram. Median with interquartile range, and *P < 0.05; **P < 0.01; ***P < 0.001 determined by the Kruskal–Wallis test followed by the Dunn's posttest. PHB: prohibitin; AKT: protein kinase B; PI3K: phosphatidylinositol 3-kinase.
In summary, PHB interacts with AKT and exists in a complex with phospho-PHB (pT258) and phospho-AKT in the mitochondrial sheath of murine sperm. After blocking AKT activity, murine sperm have significantly decreased levels of PHB phosphorylation (pT258) and the total and progressive motility. Additionally, both PI3K/AKT activity and PHB phosphorylation (pT258) are significantly reduced in sperm with poor motility from infertile men compared with those from normospermic males.
DISCUSSION
It is known that the mitochondrial sheath of sperm performs important functional roles in ATP production and the maintenance of sperm motility.7 Similar to the report in somatic cells,2 our recent findings have shown that the deletion of Phb in spermatocytes affects ATP production associated with the reduced expression levels of respiratory complex subunits.4 We therefore propose that a more pronounced decrease in ATP levels in sperm from asthenospermic patients14 may be associated with the reduced mitochondrial PHB expression in sperm from asthenospermic patients reported in our previous study,5,6 and ultimately account for impaired sperm motility. The present study shows that PHB is expressed in the mitochondrial sheath of murine mature sperm. This expression pattern is similar to our previous report in human sperm whose PHB is expressed in the mitochondrial sheath and is associated with mROS generation and sperm motility.5,6 Taken together, these results extend our previous findings that PHB in the sperm mitochondrial sheath may play a role in the maintenance of sperm motility.5,6
The PI3K/AKT activity has been demonstrated to be critical for sperm motility.8 This function is similar to that for PHB in sperm as described above, suggesting that PI3K/AKT activity may have possible association with PHB in the mitochondria of mammalian sperm. In the present study, using colocalization and co-IP assays, we have demonstrated for the first time the formation of a complex of PHB with AKT also containing phospho-PHB (PHB pT258) and phospho-AKT in the mitochondrial sheath of murine sperm. A previous study has reported that AKT phosphorylates PHB via their interaction with each other in somatic cells.9 Therefore, as reported for somatic cells,10 AKT may interact with PHB to induce PHB phosphorylation (PHB pT258) in murine sperm, and phosphorylated PHB may in turn enhance the activity of the PI3K/AKT pathway and account for the presence of phospho-AKT. Additionally, the finding of AKT and phospho-AKT localization in mature sperm is consistent with previous reports by Koppers et al.8 and Vadnais et al.15 showing that mature human or mouse sperm have phospho-AKT and/or AKT proteins in the mitochondrial sheath, in which AKT activity is critical for mROS generation and/or sperm motility. Collectively, the present study shows that PHB interacts with AKT and exists in a complex with phospho-PHB and phospho-AKT in the mitochondrial sheath of sperm. These results suggest that impaired interaction of PHB with AKT may impact PI3K/AKT activity and PHB phosphorylation, and ultimately affects sperm motility.
Wortmannin is a highly selective PI3K inhibitor that has been used to demonstrate mitochondrial AKT activity critical for mROS generation and sperm motility,8 although it is also a potent inhibitor of mammalian polo-like kinases.16 In the current study, by inhibiting AKT activity using wortmannin, we have determined the relationship between PHB and AKT in mature mouse sperm. As shown in (Figure 3), wortmannin treatment significantly decreases the total and progressive sperm motility, consistent with the report by Vadnais et al.15 Importantly, PHB phosphorylation (pT258) levels in mature sperm are significantly decreased after wortmannin treatment. These results show that AKT activity impacts the level of PHB phosphorylation (pT258) and sperm motility, in which the interactive AKT activity and PHB phosphorylation in mitochondrial sheath may ultimately play key roles in modulating sperm motility.
On the other hand, the current study also shows significantly lower levels of phospho-PI3K P85 subunit α+γ (pY199 and pY467) and phospho-AKT (pS473; pT308) in sperm from infertile men compared with those from normospermic subjects. This result is consistent with a previous report indicating that PI3K/AKT activity is important to human sperm motility.8 Previously, we have shown that PHB is involved in loss of human sperm motility associated with increased generation of mROS at the mitochondrial complex I.6 Interestingly, the present study also shows that phospho-PHB (pT258) levels are significantly decreased in sperm with reduced motility from infertile subjects. Collectively, these results suggest that impaired interaction of AKT with PHB in these infertile males may account for loss of sperm motility.
It should be noted that this reduced interaction of AKT and PHB may also account for the decreased expression of PHB in the mitochondria of sperm from infertile subjects in our previous studies.5,6 This interpretation is supported by recent reports indicating that the level of PHB localized in mitochondria of somatic cells is impacted by AKT-mediated PHB phosphorylation.17,18 Based on these findings, we propose that decreased PI3K/AKT activity in sperm from infertile men may reduce the levels of PHB and subsequent phospho-PHB in the mitochondrial sheath of sperm. Decreased levels of PHB and phospho-PHB may in turn result in impaired mitochondrial function, leading to increased mROS levels and decreased MMP, and ultimately the loss of sperm motility. Our previous findings that loss of human sperm motility is associated with decreased PHB expression and impaired mitochondrial function, such as decreased MMP and increased mROS generation at the mitochondrial complex I,5,6 may also be explained by the significantly lower levels of PI3K/AKT activity and phospho-PHB shown in the present study.
CONCLUSION
The current study demonstrates for the first time that PHB interacts with AKT and exists in a complex with phospho-PHB (pT258) and phospho-AKT in the mitochondrial sheath of murine sperm. Significantly lower levels of PHB phosphorylation (pT258) and sperm motility are observed after blocking AKT activity. In addition, decreased PI3K/AKT activity and PHB phosphorylation (pT258) are detected in sperm with reduced motility from infertile men. These findings suggest that sperm motility is regulated by the phosphorylation of PHB and the activity of the PI3K/AKT pathway in the mitochondrial sheath.
AUTHOR CONTRIBUTIONS
HC planned the project and supervised the overall work. XHL, RRC, GWC, HJS, and HC designed the experiments. XHL, RRC, GWC, LFZ, WJTT, and HC conducted the experiments. XHL, RRC, GWC, and HC analyzed the data. XHL, RRC, PAMD, WSO, and HC wrote the paper. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declared no competing interests.
ACKNOWLEDGMENTS
We gratefully acknowledge Dr. Liming Wei at the Institute of Biomedical Sciences, Shanghai Medical College, Fudan University, and Dr. Zimei Ni at the Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences for their help and advice regarding the western blotting and CASA experiments. This project was funded by grants from the National Natural Science Foundation of China (No. 81270738) and the Major State Basic Research Development Program of China (No. 2014CB943104).
REFERENCES
- 1.Merkwirth C, Langer T. Prohibitin function within mitochondria: essential roles for cell proliferation and cristae morphogenesis. Biochim Biophys Acta. 2009;1793:27–32. doi: 10.1016/j.bbamcr.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 2.Hernando-Rodriguez B, Artal-Sanz M. Mitochondrial quality control mechanisms and the PHB (prohibitin) complex. Cells. 2018;7:238. doi: 10.3390/cells7120238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schleicher M, Shepherd BR, Suarez Y, Fernandez-Hernando C, Yu J, et al. Prohibitin-1 maintains the angiogenic capacity of endothelial cells by regulating mitochondrial function and senescence. J Cell Biol. 2008;180:101–12. doi: 10.1083/jcb.200706072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang LF, Tan-Tai WJ, Li XH, Liu MF, Shi HJ, et al. PHB regulates meiotic recombination via JAK2-mediated histone modifications in spermatogenesis. Nucleic Acids Res. 2020;48:4780–96. doi: 10.1093/nar/gkaa203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang MJ, Ou JX, Chen GW, Wu JP, Shi HJ, et al. Does prohibitin expression regulate sperm mitochondrial membrane potential, sperm motility, and male fertility? Antioxid Redox Signal. 2012;17:513–9. doi: 10.1089/ars.2012.4514. [DOI] [PubMed] [Google Scholar]
- 6.Chai RR, Chen GW, Shi HJ, O WS, Martin-DeLeon PA, et al. Prohibitin involvement in the generation of mitochondrial superoxide at complex I in human sperm. J Cell Mol Med. 2017;21:121–9. doi: 10.1111/jcmm.12945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jansen RP, Burton GJ. Mitochondrial dysfunction in reproduction. Mitochondrion. 2004;4:577–600. doi: 10.1016/j.mito.2004.07.038. [DOI] [PubMed] [Google Scholar]
- 8.Koppers AJ, Mitchell LA, Wang P, Lin MJ, Aitken RJ. Phosphoinositide 3-kinase signalling pathway involvement in a truncated apoptotic cascade associated with motility loss and oxidative DNA damage in human spermatozoa. Biochem J. 2011;436:687–98. doi: 10.1042/BJ20110114. [DOI] [PubMed] [Google Scholar]
- 9.Han EK, Mcgonigal T, Butler C, Giranda VL, Luo Y. Characterization of Akt overexpression in MiaPaCa-2 cells: prohibitin is an Akt substrate both in vitro and in cells. Anticancer Res. 2008;28:957–63. [PubMed] [Google Scholar]
- 10.Ande SR, Mishra S. Prohibitin interacts with phosphatidylinositol 3,4,5-triphosphate (PIP3) and modulates insulin signaling. Biochem Biophys Res Commun. 2009;390:1023–8. doi: 10.1016/j.bbrc.2009.10.101. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen. 5th ed. Cambridge: Cambridge University Press; 2010. [Google Scholar]
- 12.Chen H, Griffiths G, Galileo DS, Martin-DeLeon PA. Epididymal SPAM1 is a marker for sperm maturation in the mouse. Biol Reprod. 2006;74:923–30. doi: 10.1095/biolreprod.105.048587. [DOI] [PubMed] [Google Scholar]
- 13.Zhou YC, Wu F, Zhang M, Xiong ZQ, Yin QQ, et al. EMC10 governs male fertility via maintaining sperm ion balance. J Mol Cell Biol. 2018;10:503–14. doi: 10.1093/jmcb/mjy024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grishchenko VI, Parashchuk I, Smaglii N. The adenosine phosphate content in human sperm and their motility. Urol Nefrol (Mosk) 1989;6:48–50. Article in Russian. [PubMed] [Google Scholar]
- 15.Vadnais ML, Aghajanian HK, Lin A, Gerton GL. Signaling in sperm: toward a molecular understanding of the acquisition of sperm motility in the mouse epididymis. Biol Reprod. 2013;89:127. doi: 10.1095/biolreprod.113.110163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu YS, Shreder KR, Gai WZ, Corral S, Ferris DK, et al. Wortmannin, a widely used phosphoinositide 3-kinase inhibitor, also potently inhibits mammalian polo-like kinase. Chem Biol. 2005;12:99–107. doi: 10.1016/j.chembiol.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 17.Jiang L, Dong P, Zhang Z, Li C, Li Y, et al. Akt phosphorylates Prohibitin 1 to mediate its mitochondrial localization and promote proliferation of bladder cancer cells. Cell Death Dis. 2015;6:e1660. doi: 10.1038/cddis.2015.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng JY, Yang JB, Liu Y, Xu M, Huang YY, et al. Profiling and targeting of cellular mitochondrial bioenergetics: inhibition of human gastric cancer cell growth by carnosine. Acta Pharmacol Sin. 2019;40:938–48. doi: 10.1038/s41401-018-0182-8. [DOI] [PMC free article] [PubMed] [Google Scholar]