Abstract
Assisted reproductive technologies involving the use of spermatozoa and eggs for in vitro fertilization (IVF) have come as the solution for many infertile couples to become parents. However, in some cases, the use of ejaculated spermatozoa delivers poor IVF performance. Some studies have suggested the use of testicular spermatozoa in severe male infertility cases, but no guidelines regarding their utilization are currently available. In the present study, we found the mRNA protamine 1/protamine 2 (P1/P2) ratio to be a valuable biomarker of poor sperm function that could be used as a diagnostic key for the identification of cases that would benefit from the use of testicular spermatozoa. A total of 23 couples undergoing egg donation cycles with at least one previous cycle failure were studied. All couples underwent two consecutive intracytoplasmic sperm injection (ICSI) cycles with either ejaculated or testicular spermatozoa (TESA). The sperm mRNA P1/P2 ratio, fertilization rate, blastocyst rate, and pregnancy and live birth rate were compared. Results showed improved ICSI and clinical outcomes in cycles with testicular spermatozoa in men with altered mRNA P1/P2 ratios. TESA cycles presented significantly higher rates of fertilization (mean ± standard deviation: 76.1% ± 15.1% vs 65.5% ± 18.8%), blastocyst formation (55.0% ± 20.3% vs 30.8% ± 23.8%), and good morphological quality blastocyst (28.9% ± 22.9% vs 13.5% ± 17.9%) and also improvements on pregnancy (60.9% vs 0%) and healthy birth rates (56.5% vs 0%) than EJACULATE cycles. The results described here suggest that in patients with previous IVF/ICSI failures and aberrant mRNA protamine ratios, the use of testicular spermatozoa may be a good alternative to improve clinical outcomes.
Keywords: DNA damage, male infertility, mRNA protamine ratio, sperm chromatin, testicular spermatozoa
INTRODUCTION
For successful mammalian fertilization and adequate embryo development, fusion of spermatozoon and oocyte must be followed by a cascade of reactions including DNA repair, oocyte metabolic activation, and microtubule assembly for the formation of the mitotic spindle.1,2 Fertilization failure or defective preimplantation embryo development may be due to compromised quality of either the oocyte or the spermatozoon.3
With regard to sperm quality, some studies have shown, on the one hand, that embryo development and implantation depend in part on sperm DNA integrity,4 and on the other hand, that production of competent spermatozoa requires correct replacement of DNA-binding histones by sperm-specific nucleoproteins called protamines.5 For this reason, these parameters have been proposed as suitable biomarkers for assessing male fertility potential.6 Sperm DNA fragmentation is a more popular biomarker than sperm nucleoprotein analysis. The analysis of sperm DNA integrity has been incorporated slowly by in vitro fertilization (IVF) centers worldwide as part of their standard spermiogram.7 The study of protamines is, however, hardly considered as a routine parameter for sperm quality assessment.
Sperm protamines are small arginine-rich nuclear proteins that allow denser packaging of DNA in the spermatozoon than do histones. The main role of this kind of proteins is preserving DNA integrity in the sperm head by preventing harmful attacks from exogenous or endogenous agents.8,9 A regulatory role for sperm chromatin structure has also been proposed.10,11 Human spermatozoa express two types of protamines: P1 and P2, and both of them are essential for sperm function. The relative proportion of P1 to P2 is regulated at approximately 1:1 ratio at both mRNA and protein levels.12,13 Alterations in this protamine ratio are rare in fertile men, but common in infertile men5 and have been related to poor sperm quality, increased DNA damage, and decreased fertility.14,15 Some authors have proposed that the analysis of protamine content in sperm could be used as a biomarker for male infertility diagnosis in the clinical setting.13,16
Ejaculated spermatozoa that have completed their maturation during passage through the male reproductive tract usually have better fertilization potential than testicular spermatozoa.17 However, the use of spermatozoa isolated from testicular tissue has been proposed in some severe male factor infertility cases. Studies have reported improved embryo and pregnancy rates18,19 and no negative effects on the health of the offspring from the use of testicular spermatozoa compared with those from the use of ejaculated spermatozoa.20,21 These results can be related to the fact that testicular spermatozoa exhibit lower levels of DNA damage.22 The explanation behind these findings may be that spermatozoa are susceptible to damage during their transit through the male reproductive tract.23 Currently, no guidelines or protocols have been proposed regarding the utilization of testicular spermatozoa in severe male factor infertility cases where ejaculated spermatozoa are available but have shown poor IVF or intracytoplasmic sperm injection (ICSI) performance. Some studies have suggested the use of testicular spermatozoa in cases of elevated sperm DNA fragmentation (SDF).22,24,25 However, the number of studies addressing this issue is limited.
There is a need to look for new biomarkers of poor sperm function that could serve as diagnostic keys for the identification of cases that would benefit from the use of testicular instead of ejaculated spermatozoa. In the present study, we propose that the mRNA P1/P2 ratio could be used as a valuable indicator of sperm maturity and fertilization ability identifying specific cases that could benefit from the use of testicular spermatozoa in assisted reproductive technology (ART). Here, we evaluate whether the use of testicular spermatozoa improves ART outcomes in patients with previously failed ART egg donation cycles with ejaculated spermatozoa with altered mRNA P1/P2 ratios.
PATIENTS AND METHODS
Patient selection
This unicentric, observational, and retrospective study included the review of records of patients seeking treatment at the IVF center, IVF-Spain (Alicante, Spain), between January 2014 and February 2016.
The study group comprised a total of 23 subfertile couples undergoing egg donation for assisted reproduction treatment. All couples included in the study had (i) infertility duration >1 year; (ii) no female factor infertility with no uterine pathology; (iii) experienced at least one previous egg donation cycle at our center with poor or null blastocyst rate and no pregnancy; (iv) presented no evidence of subclinical genital infections or leukocytospermia; and (v) presented an abnormal sperm mRNA P1/P2 ratio. A comprehensive sperm quality assessment (including mRNA P1/P2 ratio) is performed at our center when a poor or null blastocyst rate is observed and no pregnancy is achieved. The study was approved by the IVF-Spain Institutional Review Board. Signed informed consent for the use of gametes in research was obtained from all patients.
Study design
All couples were subjected to two consecutive ICSI cycles using, in the first one, ejaculated spermatozoa (EJACULATE-cycle group) and, in the second, testicular spermatozoa (TESA-cycle group). Preimplantation development and clinical outcomes of both types of cycle were compared.
Semen sample collection and preparation
Ejaculates were obtained by on-site masturbation after 1–2 days of sexual abstinence. After liquefaction, specimens were processed and assessed for semen volume, sperm count, motility, vitality, morphology, and leukocytes following the current World Health Organization laboratory manual guidelines.26 The sperm mRNA P1/P2 ratio was analyzed in all cases. For this purpose, 1 ml aliquots of ejaculated sperm samples were preserved in RNAlater™ solution (Ambion, Heppenheim, Germany) and stored in liquid nitrogen until further processing.
Protamine mRNA ratio analysis
The protamine mRNA ratio was evaluated by quantitative reverse transcription polymerase chain reaction (RT-qPCR) following the protocol previously described by Rogenhofer et al.3 Briefly, RNA extraction was conducted with Rneasy Mini Kit and Rneasy Plus Micro Kit (Qiagen, Hilden, Germany), cDNA synthesis was performed with Omniscript™ according to the manufacturer's protocol (Qiagen), and protamine 1 and 2 gene expression analysis was performed by real-time RT-qPCR using iQ™ SYBR Green SuperMix and iCycler (BioRad, Munich, Germany).
Testicular sperm retrieval: TESA
Retrievals were performed by standard testicular sperm aspiration (TESA) under local anesthesia. The extracted testicular tissue was flushed into a Petri dish containing culture medium (MHM® with Gentamicin, Irvine Scientific, Santa Ana, CA, USA). Subsequently, to ensure seminiferous wall breakdown, cellular content loss, and sperm extraction, the seminiferous tubules were mechanically minced with fine gauge needles attached to syringes. The presence of flagellated spermatozoa was defined as successful retrieval for sperm injections.
Assisted reproductive technology procedures
Controlled ovarian stimulation of donors was performed with an antagonist protocol. Ovarian stimulation was initiated with 150–300 U day−1 recombinant follicle-stimulating hormone (rec-FSH; Puregon®; Merck and Co., Inc., Whitehouse Station, NJ, USA/Elonva®; Merck and Co., Inc.) or u-FSH (Fostipur; IBSA, Lodi, Italy) 5 days after stopping contraceptive pills, and gonadotropin-releasing hormone (GnRH) antagonist (ganirelix; Orgalutran®; Merck and Co., Inc.) for pituitary suppression was introduced according to a multiple-dose protocol (0.25 mg day−1) when the leading follicle of 15 mm or estradiol concentrations of 800 pg ml−1 were reached. Daily dosages were adjusted, according to individual ovarian responses, as monitored by vaginal ultrasound scans and serum concentrations of estradiol and progesterone from the third/fifth day of stimulation. Triggering was performed with 0.4 mg of leuprolide acetate subcutaneous (Procrin®; Abbot Laboratories, Madrid, Spain), when at least 1 follicle >20 mm was present. Oocytes were retrieved by transvaginal ultrasound-guided aspiration at the 36th h following leuprolide acetate.
All mature oocytes (metaphase II) were inseminated via ICSI 4 h after ovum pick-up. Fertilization was assessed after 16–18 h postinsemination and it was considered normal when two clearly distinct pronuclei containing nucleoli were present. Embryos were cultured routinely up to the blastocyst stage. Transfers of the best morphologically available blastocysts were performed in all cases. Embryo selection for transfer was based on blastocyst maturity, trophectoderm, and inner cell mass (ICM) differentiation, according to Gardner and Schoolcraft.27 Embryo transfer was guided by transvaginal ultrasound.
Preimplantation development outcomes
Regarding preimplantation embryo development, the main outcome measures were: (i) fertilization rate (FERT), defined as the percentage of oocytes fertilized of the microinjected oocytes; (ii) blastocyst formation rate (BT), defined as the percentage of embryos that reached the blastocyst stage; and (iii) good-quality blastocyst rate (GQBT), defined as the percentage of blastocysts classified as A-B quality according to Gardner criteria of the total number of blastocysts (27).
Clinical outcomes
The following clinical outcome measures per cycle were studied in both groups (EJACULATE-cycle and TESA-cycle groups): (i) beta-human chorionic gonadotropin (β-hCG)-positive pregnancy rate (βR), determined by a β-hCG positive result; (ii) clinical pregnancy rate (CLI), determined by the visualization of a gestational sac with an embryo showing cardiac activity on ultrasound at weeks 5–7; and (iii) live birth rate (LB), determined by the live birth of a healthy baby.
Statistical analyses
Data were analyzed using the IBM SPSS Statistics version 20 software (IBM Corporation, Armonk, NY, USA). Comparisons of fertilization, blastocyst formation, and good-quality blastocyst rates between EJACULATE-cycle and TESA-cycle groups were performed with paired Student's t-test or Wilcoxon signed-rank test as appropriate; P <0.05 was considered statistically significant.
RESULTS
A total of 23 patients from our egg donation program who had experienced at least one previous cycle failure (with poor or null blastocyst rate observed, and no pregnancy achieved) at our center were included in this study. The age (mean ± standard deviation [s.d.]) of male and female partners was 46.9 ± 6.6 years and 43.6 ± 4.3 years, respectively.
All patients underwent a first cycle where ejaculated spermatozoa were used for fertilization. All first cycles resulted in a negative result, with either no transfer or implantation failure. Then, a second cycle using testicular spermatozoa obtained through TESA was performed in all 23 cases.
Comprehensive sperm analysis including sperm protamine gene expression evaluation (mRNA P1/P2 ratio) confirmed altered transcription levels in all ejaculated samples (Table 1). The normal relative proportion of P1 to P2 in human spermatozoa is regulated at approximately a 1:1 ratio.16 mRNA P1/P2 ratio reference range for normality is established from 0.85 to 1.30. mRNA P1/P2 ratios either below 0.85 or above 1.30 have been associated with male infertility.4 In our study, overall, 16 out of 23 samples presented an altered mRNA protamine ratio below the lower end range (<0.85) and 7 out of 23 presented an mRNA P1/P2 ratio above the upper end range (>1.30).
Table 1.
Age, conventional sperm parameter diagnosis, and mRNA protamine 1/protamine 2 ratio in the group of patients studied
| Patient ID | Age (year) | WHO conventional sperm parameter diagnosis | mRNA P1/P2 ratio |
|---|---|---|---|
| 1 | 49 | N | 0.58 |
| 2 | 40 | N | 0.64 |
| 3 | 54 | OAT | 0.22 |
| 4 | 49 | N | 0.37 |
| 5 | 42 | N | 2.05 |
| 6 | 42 | OA | 2.41 |
| 7 | 41 | OA | 0.61 |
| 8 | 41 | A | 0.08 |
| 9 | 40 | OA | 0.82 |
| 10 | 51 | OA | 1.69 |
| 11 | 44 | OA | 1.50 |
| 12 | 44 | N | 0.34 |
| 13 | 68 | O | 0.36 |
| 14 | 54 | A | 0.50 |
| 15 | 51 | O | 0.25 |
| 16 | 49 | OA | 0.30 |
| 17 | 46 | OAT | 0.25 |
| 18 | 41 | N | 4.70 |
| 19 | 48 | OAT | 0.80 |
| 20 | 41 | N | 1.37 |
| 21 | 53 | A | 1.40 |
| 22 | 43 | T | 0.68 |
| 23 | 48 | N | 0.75 |
A: asthenozoospermia; N: normozoospermia; T: teratozoospermia; O: oligozoospermia; OA: oligoasthenozoospermia; OAT: oligoasthenoteratozoospermia; P1/P2: protamine 1/protamine 2
The presence of these mRNA P1/P2 ratio alterations together with failure in the first cycle with ejaculated spermatozoa directed the consideration of the performance of TESA. TESA was performed in all 23 patients included in the study. The sperm retrieval success rate after the procedure was 100%.
When comparing the influence of the use of ejaculated versus testicular spermatozoa in preimplantation embryo development parameters, there was a significant improvement in fertilization rates when testicular spermatozoa were used compared with ejaculated spermatozoa (paired Student's t-test, P = 0.018; Figure 1).
Figure 1.
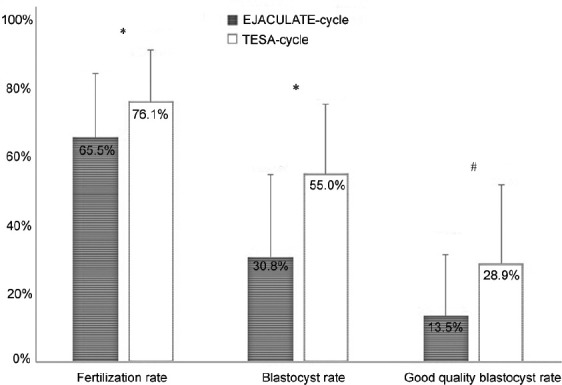
Preimplantation embryo development rates in cycles using ejaculated spermatozoa (EJACULATE cycle) and testicular sperm cells (TESA cycle). Significantly higher fertilization, blastocyst, and good-quality blastocyst rates were obtained in the TESA-cycle group compared with the EJACULATE-cycle group. Significant differences between EJACULATE-cycle and TESA-cycle groups are highlighted with an asterisk (*) with paired Student‘s t-test/a pound (#) with Wilcoxon signed-rank test, P< 0.05. Error bars represent the standard deviation.
The use of testicular spermatozoa was also associated with a significantly higher blastocyst formation rate (paired Student's t-test, P < 0.001). Interestingly, not only the frequency of blastocysts, produced as a result of the fertilization of oocytes with testicular sperm, was significantly higher, but the morphological quality of the blastocysts produced was also significantly better, with more than 2-fold good-quality blastocysts in the TESA-cycle group compared to the EJACULATE-cycle group (Wilcoxon signed-rank test, P = 0.018; Figure 1).
When comparing the influence of the use of ejaculated versus testicular spermatozoa on ART clinical outcomes, a higher β-hCG positive pregnancy rate was observed in the TESA cycles compared to the EJACULATE cycles (87.0% vs 43.5%). A significantly higher clinical pregnancy and live birth rate was also confirmed in the TESA-cycle group (60.9% vs 0%; and 56.5% vs 0%).
DISCUSSION
In this study, we provide the first evidence for the successful use of testicular spermatozoa for ICSI in cases of patients with an abnormal sperm mRNA P1/P2 ratio. We demonstrated a significant increase of ICSI outcomes with testicular spermatozoa regarding fertilization and good-quality blastocyst formation as well as an improvement in pregnancy and live birth rates. Our findings are in line with data from a number of previous original studies that have reported the effectiveness of ICSI with testicular compared with ejaculated spermatozoa in men with different causes of infertility. Kahraman et al.28 assessed the efficacy of ICSI with testicular and ejaculated spermatozoa in 24 couples with immotile spermatozoa. In their findings, they reported similar fertilization rates between sperm sources (53.5% vs 54.5%), but an increase in clinical pregnancy (57.1% vs 20%) and ongoing pregnancy rates (6 ongoing vs 0 ongoing pregnancy) in the testicular spermatozoa group. Moreover, Ben-Ami et al.29 and Weissman et al.30 in their small studies involving patients with cryptozoospermia (n = 17) and severe oligoteratoasthenozoospermia (n = 4) whose partners failed to conceive after repeated ICSI cycles with ejaculated spermatozoa, showed that the use of testicular spermatozoa was associated with higher delivery rates than those with ejaculated spermatozoa. In another study, Greco et al.22 analyzed the effect of the use of testicular spermatozoa in 18 couples whose male partners had ≥15% of ejaculated spermatozoa with damaged DNA and who had undergone at least two unsuccessful ICSI attempts with ejaculated spermatozoa. Although they found similar fertilization rates (74.9% vs 70.8%) and embryo morphology score (51.1% vs 47.6%) for the treatment attempts with testicular and ejaculated spermatozoa, respectively, their results showed significantly higher implantation rates (20.7% vs 1.8%) in ICSI with testicular spermatozoa. Finally, data from Esteves et al.25 suggest that the use of testicular spermatozoa are associated with improved ICSI outcomes in men with oligozoospermia and persistent high sperm DNA fragmentation levels. No increased risk of congenital malformations at birth has been detected either when comparing the possible adverse effects on the health of the offspring depending on the origin of the sperm (testicular vs ejaculated sperm).20,21
Although the etiology of recurrent development of poor-quality embryos or unexplained repeated assisted reproduction failures in ICSI is still unclear, the possibility of the existence of a paternal factor such as the presence of increased levels of DNA damage, defects in chromatin packaging, or both should be taken into consideration. Several origins for DNA fragmentation have been proposed: (i) oxidative stress, (ii) defective apoptosis, and (iii) abnormal chromatin packaging.31 This third origin for the presence of DNA damage can result in both DNA fragmentation and abnormal protamine content. During the replacement of histones by protamines, endogenous nuclease activity is required to create nicks to provide relief of torsional stress, in order to facilitate chromatin arrangement.32,33 Alteration in the control of this process could result in the presence of chromatin packaging anomalies and unrepaired DNA nicks.31 In addition, protamine deficiency can increase DNA susceptibility to external stress resulting in higher risk of sperm DNA damage.16,34,35,36,37
Sperm chromatin stability depends on the quantity of disulfide bonds acquired in the process of sperm maturation during epididymal transit.35,37,38,39 Spermatozoa with incomplete chromatin condensation are prone to increased DNA damage, as they are more susceptible to posttesticular assault.31 This most likely represents the underlying reason that it has been postulated, in some individuals that DNA fragmentation levels in testicular spermatozoa may be lower than those in ejaculated spermatozoa.19,24,25 The presence of DNA strand breaks resulting from the long sperm journey from production to ejaculation can be prevented by removing them before their entrance into the epididymis.31 TESA or testicular sperm extraction (TESE), therefore, would permit the selection of less damaged spermatozoa.30 A number of studies have shown that these gametes have the potential to develop into healthy adults. Ogura et al.40 injected round spermatids, the first haploid cell type resulting from meiosis, into mouse oocytes and reported that pups developed normally.
On the other hand, sperm chromatin packaging has been described to fulfill additional functions beyond DNA protection, such as the regulation of gene expression.10 In human sperm chromatin, only a small portion of the paternal DNA remains bound to histones;41 the vast majority of sperm DNA is coiled into toroids by protamines.42 Among these three types of structures, histone-bound DNA and matrix attachment regions (MARS) are inherited by the embryo and are most likely required for proper development. The histone-bound regions, primarily located at gene promoters in gene families important for embryo development, are the first to be expressed after fertilization.37,43,44 Then, 2–4 h after fertilization, protamines are completely replaced by histones so that the paternal chromatin has the same accessible chromatin as all other somatic cells.45
Other layers of epigenetic information consist of methylation of DNA regions. In the case of male gametes, most DNA methylation modifications observed during spermiogenesis occur mainly in spermatogonia and spermatocytes in early meiotic phase.46 DNA methylation modifications are almost exclusively completed by the end of the pachytene spermatocyte stages. This may also be the cause why testicular spermatozoa have been successful in producing healthy offspring.
The altered sperm mRNA P1/P2 ratio observed in the group of patients selected in this study may reflect an abnormal sperm chromatin packaging. As in our study, Simon and colleagues reported that an aberrant P1/P2 ratio was associated with low fertilization rate and poor embryo quality.47 A recent study reported that poor sperm protamination is associated with the development of low-quality embryos after in vitro fertilization.48 Correct chromatin structure, based on adequate histone retention and precise protamine incorporation, serves as a protection layer and also a regulatory mark that contributes to sperm function and embryo development. Altered protamine-bound sperm chromatin domain organization may compromise in vitro fertilization treatment results, causing altered expression of genes required in the early phases of development. The absence of protamine “marks” in the chromatin, achieved by the use of testicular spermatozoa, may help these cells skip the first phase of development where correct protamine localization is essential, providing better results when this altered pattern of protamination is absent. Hence, not only infertile patients with high levels of DNA-damaged spermatozoa but also patients with altered protamine expression ratio may benefit from the use of testicular spermatozoa in their ART treatments.
Despite the rapid development of molecular biology techniques, the evaluation of semen quality in most IVF centers worldwide is still limited to the analysis of standard parameters such as sperm concentration, motility, and morphology.7 However, when assessing only these routine parameters, it is not possible to detect alterations in sperm chromatin organization, such as aberrant protamination and DNA damage, which are essential parameters for correct sperm function.49,50
In summary, the results described here are very encouraging and suggest that the use of testicular spermatozoa from patients with previous failures after ICSI with ejaculated spermatozoa with aberrant mRNA protamine ratio, could significantly improve clinical outcomes. These observations suggest that owing to the critical roles played by protamines in sperm maturation and function, alterations in protamine expression could be a significant cause of unexplained male infertility and, for this reason, protamine mRNA ratio represents an excellent marker to analyze sperm quality in addition to standard semen parameters. Basic research studies directed to understand the role of protamines in sperm function and embryo development are needed. In addition, further nonselection studies are desirable to confirm clinical utility of this parameter.
AUTHOR CONTRIBUTIONS
JS and ME participated in the study design and data analysis and drafted the manuscript. LG was involved in sample processing and manuscript preparation. AL and KS were involved in sample processing. JA participated in the design and supervision of the study. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declared no competing interests.
ACKNOWLEDGMENTS
The authors thank all the patients for consenting to participate in this study. Furthermore, the technical assistance of Barbara Fröhlich and Mareike Buch-Heberling is gratefully acknowledged. KS was supported by a Research Grant from the University Medical Center Giessen and Marburg (UKGM, project 29/2015GI). This research did not receive any other specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
REFERENCES
- 1.Barroso G, Valdespin C, Vega E, Kershenovich R, Avila R, et al. Developmental sperm contributions: fertilization and beyond. Fertil Steril. 2009;92:835–48. doi: 10.1016/j.fertnstert.2009.06.030. [DOI] [PubMed] [Google Scholar]
- 2.Kashir J, Heindryckx B, Jones C, de Sutter P, Parrington J, et al. Oocyte activation, phospholipase C zeta and human infertility. Hum Reprod Update. 2010;16:690–703. doi: 10.1093/humupd/dmq018. [DOI] [PubMed] [Google Scholar]
- 3.Rogenhofer N, Dansranjavin T, Schorsch M, Spiess A, Wang H, et al. The sperm protamine mRNA ratio as a clinical parameter to estimate the fertilizing potential of men taking part in an ART programme. Hum Reprod. 2013;28:969–78. doi: 10.1093/humrep/des471. [DOI] [PubMed] [Google Scholar]
- 4.Ahmadi A, Ng SC. Fertilizing ability of DNA-damaged spermatozoa. J Exp Zool. 1999;284:696–704. doi: 10.1002/(sici)1097-010x(19991101)284:6<696::aid-jez11>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 5.Steger K, Cavalcanti MCO, Schuppe HC. Prognostic markers for competent human spermatozoa: fertilizing capacity and contribution to the embryo. Int J Androl. 2011;36:513–27. doi: 10.1111/j.1365-2605.2010.01129.x. [DOI] [PubMed] [Google Scholar]
- 6.Spanò M, Bonde JP, Hjøllund HI, Kolstad HA, Cordelli E, et al. Sperm chromatin damage impairs human fertility. Fertil Steril. 2000;73:43–50. doi: 10.1016/s0015-0282(99)00462-8. [DOI] [PubMed] [Google Scholar]
- 7.Majzoub A, Agarwal A, Cho CL, Esteves SC. Sperm DNA fragmentation testing: a cross sectional survey on current practices of fertility specialists. Transl Androl Urol. 2017;6:S710–9. doi: 10.21037/tau.2017.06.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuretake S, Kimura Y, Hoshi K, Yanagimachi R. Fertilization and development of mouse oocytes injected with isolated sperm heads. Biol Reprod. 1996;55:789–95. doi: 10.1095/biolreprod55.4.789. [DOI] [PubMed] [Google Scholar]
- 9.Sotolongo B, Lino E, Ward WS. Ability of hamster spermatozoa to digest their own DNA. Biol Reprod. 2003;69:2029–35. doi: 10.1095/biolreprod.103.020594. [DOI] [PubMed] [Google Scholar]
- 10.Castillo J, Estanyol JM, Ballescá JL, Oliva R. Human sperm chromatin epigenetic potential: genomics, proteomics, and male infertility. Asian J Androl. 2015;17:601–9. doi: 10.4103/1008-682X.153302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ward WS. Function of sperm chromatin structural elements in fertilization and development. Mol Hum Reprod. 2009;16:30–6. doi: 10.1093/molehr/gap080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steger K, Wilhelm J, Konrad L, Stalf T, Greb R, et al. Both protamine-1 to protamine-2 mRNA ratio and Bcl2 mRNA content in testicular spermatids and ejaculated spermatozoa discriminate between fertile and infertile men. Hum Reprod. 2008;23:11–6. doi: 10.1093/humrep/dem363. [DOI] [PubMed] [Google Scholar]
- 13.Torregrosa N, Domínguez-Fandos D, Camejo MI, Shirley CR, Meistrich ML, et al. Protamine 2 precursors, protamine 1/protamine 2 ratio, DNA integrity and other sperm parameters in infertile patients. Hum Reprod. 2006;21:2084–9. doi: 10.1093/humrep/del114. [DOI] [PubMed] [Google Scholar]
- 14.Aoki VW, Liu L, Carrell DT. Identification and evaluation of a novel sperm protamine abnormality in a population of infertile males. Hum Reprod. 2005;20:1298–306. doi: 10.1093/humrep/deh798. [DOI] [PubMed] [Google Scholar]
- 15.Chevaillier P, Mauro N, Feneux D, Jouannet P, David G. Anomalous protein complement of sperm nuclei in some infertile men. Lancet. 1987;2:806–7. doi: 10.1016/s0140-6736(87)92547-5. [DOI] [PubMed] [Google Scholar]
- 16.Aoki VW, Moskovtsev SI, Willis J, Liu L, Mullen JB, et al. DNA integrity is compromised in protamine-deficient human sperm. J Androl. 2005;26:741–8. doi: 10.2164/jandrol.05063. [DOI] [PubMed] [Google Scholar]
- 17.Stalf T, Mehnert C, Hajimohammad A, Manolopoulos K, Shen Y, et al. Influence of motility and vitality in intracytoplasmic sperm injection with ejaculated and testicular sperm. Andrologia. 2005;37:125–30. doi: 10.1111/j.1439-0272.2005.00665.x. [DOI] [PubMed] [Google Scholar]
- 18.Kang YN, Hsiao YW, Chen CY, Wu CC. Testicular sperm is superior to ejaculated sperm for ICSI in cryptozoospermia: an update systematic review and meta-analysis. Sci Rep. 2018;8:7874. doi: 10.1038/s41598-018-26280-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehta A, Bolyakov A, Schlegel PN, Paduch DA. Higher pregnancy rates using testicular sperm in men with severe oligospermia. Fertil Steril. 2015;104:1382–7. doi: 10.1016/j.fertnstert.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 20.Wennerholm UB, Bergh C, Hamberger L, Westlander G, Wikland M, et al. Obstetric outcome of pregnancies following ICSI, classified according to sperm origin and quality. Hum Reprod. 2000;15:1189–94. doi: 10.1093/humrep/15.5.1189. [DOI] [PubMed] [Google Scholar]
- 21.Woldringh GH, Besselink DE, Tillema AH, Hendriks JC, Kremer JA. Karyotyping, congenital anomalies and follow-up of children after intracytoplasmic sperm injection with non-ejaculated sperm: a systematic review. Hum Reprod Update. 2010;16:12–9. doi: 10.1093/humupd/dmp030. [DOI] [PubMed] [Google Scholar]
- 22.Greco E, Scarselli F, Iacobelli M, Rienzi L, Ubaldi F, et al. Efficient treatment of infertility due to sperm DNA damage by ICSI with testicular spermatozoa. Hum Reprod. 2005;20:226–30. doi: 10.1093/humrep/deh590. [DOI] [PubMed] [Google Scholar]
- 23.Suganuma R, Yanagimachi R, Meistrich ML. Decline in fertility of mouse sperm with abnormal chromatin during epididymal passage as revealed by ICSI. Hum Reprod. 2005;20:3101–8. doi: 10.1093/humrep/dei169. [DOI] [PubMed] [Google Scholar]
- 24.Moskovtsev SI, Jarvi K, Mullen JBM, Cadesky KI, Hannam T, et al. Testicular spermatozoa have statistically significantly lower DNA damage compared with ejaculated spermatozoa in patients with unsuccessful oral antioxidant treatment. Fertil Steril. 2010;93:1142–6. doi: 10.1016/j.fertnstert.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 25.Esteves SC, Sánchez-Martín F, Sánchez-Martín P, Schneider DT, Gosálvez J. Comparison of reproductive outcome in oligozoospermic men with high sperm DNA fragmentation undergoing intracytoplasmic sperm injection with ejaculated and testicular sperm. Fertil Steril. 2015;104:1398–405. doi: 10.1016/j.fertnstert.2015.08.028. [DOI] [PubMed] [Google Scholar]
- 26.World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen. 5th ed. Geneva: WHO Press; 2010. [Google Scholar]
- 27.Gardner DK, Schoolcraft WB. In Vitro Culture of Human Blastocyst. In: Jansen R, Mortimer D, editors. Towards Reproductive Certainty: Fertility and Genetics Beyond 1999. The Plenary Proceedings of the 11th World Congress. Carnforth: The Parthenon Publishing Group Limited; 1999. pp. 377–88. [Google Scholar]
- 28.Kahraman S, Tasdemir M, Tasdemir I, Vicdan K, Özgür S, et al. Pregnancies achieved with testicular and ejaculated spermatozoa in combination with intracytoplasmic sperm injection in men with totally or initially immotile spermatozoa in the ejaculate. Hum Reprod. 1996;11:1343–6. doi: 10.1093/oxfordjournals.humrep.a019384. [DOI] [PubMed] [Google Scholar]
- 29.Ben-Ami I, Raziel A, Strassburger D, Komarovsky D, Ron-El R, et al. Intracytoplasmic sperm injection outcome of ejaculated versus extracted testicular spermatozoa in cryptozoospermic men. Fertil Steril. 2013;99:1867–71. doi: 10.1016/j.fertnstert.2013.02.025. [DOI] [PubMed] [Google Scholar]
- 30.Weissman A, Horowitz E, Ravhon A, Nahum H, Golan A, et al. Pregnancies and live births following ICSI with testicular spermatozoa after repeated implantation failure using ejaculated spermatozoa. Reprod Biomed Online. 2008;17:605–9. doi: 10.1016/s1472-6483(10)60306-9. [DOI] [PubMed] [Google Scholar]
- 31.Sakkas D, Alvarez JG. Sperm DNA fragmentation: mechanisms of origin, impact on reproductive outcome, and analysis. Fertil Steril. 2010;93:1027–36. doi: 10.1016/j.fertnstert.2009.10.046. [DOI] [PubMed] [Google Scholar]
- 32.McPherson SM, Longo FJ. Localization of DNase I-hypersensitive regions during rat spermatogenesis: stage-dependent patterns and unique sensitivity of elongating spermatids. Mol Reprod Dev. 1992;31:268–79. doi: 10.1002/mrd.1080310408. [DOI] [PubMed] [Google Scholar]
- 33.McPherson S, Longo FJ. Chromatin structure-function alterations during mammalian spermatogenesis: DNA nicking and repair in elongating spermatids. Eur J Histochem. 1993;37:109–28. [PubMed] [Google Scholar]
- 34.Ni K, Steger K, Yang H, Wang H, Hu K, et al. Sperm protamine mRNA ratio and DNA fragmentation index represent reliable clinical biomarkers for men with varicocele after microsurgical varicocele ligation. J Urol. 2014;192:170–6. doi: 10.1016/j.juro.2014.02.046. [DOI] [PubMed] [Google Scholar]
- 35.Oliva R. Protamines and male infertility. Hum Reprod Update. 2006;12:417–35. doi: 10.1093/humupd/dml009. [DOI] [PubMed] [Google Scholar]
- 36.Ni K, Spiess AN, Schuppe HC, Steger K. The impact of sperm protamine deficiency and sperm DNA damage on human male fertility: a systematic review and meta-analysis. Andrology. 2016;4:789–99. doi: 10.1111/andr.12216. [DOI] [PubMed] [Google Scholar]
- 37.Castillo J, Simon L, de Mateo S, Lewis S, Oliva R. Protamine/DNA ratios and DNA damage in native and density gradient centrifuged sperm from infertile patients. J Androl. 2011;32:324–32. doi: 10.2164/jandrol.110.011015. [DOI] [PubMed] [Google Scholar]
- 38.Kosower NS, Katayose H, Yanagimachi R. Thiol-disulfide status and acridine orange fluorescence of mammalian sperm nuclei. J Androl. 1992;13:342–8. [PubMed] [Google Scholar]
- 39.Saowaros W, Panyim S. The formation of disulfide bonds in human protamines during sperm maturation. Experientia. 1979;35:191–2. doi: 10.1007/BF01920608. [DOI] [PubMed] [Google Scholar]
- 40.Ogura A, Matsuda J, Yanagimachi R. Birth of normal young after electrofusion of mouse oocytes with round spermatids. Proc Natl Acad Sci U S A. 1994;91:7460–2. doi: 10.1073/pnas.91.16.7460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hammoud SS, Nix DA, Zhang H, Purwar J, Carrell DT, et al. Distinctive chromatin in human sperm packages genes for embryo development. Nature. 2009;460:473–8. doi: 10.1038/nature08162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hud NV, Downing KH, Balhorn R. A constant radius of curvature model for the organization of DNA in toroidal condensates. Proc Natl Acad Sci U S A. 1995;92:3581–5. doi: 10.1073/pnas.92.8.3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van der Heijden GW, Ramos L, Baart EB, van den Berg IM, Derijck AA, et al. Sperm-derived histones contribute to zygotic chromatin in humans. BMC Dev Biol. 2008;8:34. doi: 10.1186/1471-213X-8-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carrell DT. Epigenetics of the male gamete. Fertil Steril. 2012;97:267–74. doi: 10.1016/j.fertnstert.2011.12.036. [DOI] [PubMed] [Google Scholar]
- 45.Ajduk A, Yamauchi Y, Ward MA. Sperm chromatin remodeling after intracytoplasmic sperm injection differs from that of in vitro fertilization. Biol Reprod. 2006;75:442–51. doi: 10.1095/biolreprod.106.053223. [DOI] [PubMed] [Google Scholar]
- 46.Oakes CC, La Salle S, Smiraglia DJ, Robaire B, Trasler JM. A unique configuration of genome-wide DNA methylation patterns in the testis. Proc Natl Acad Sci U S A. 2007;104:228–33. doi: 10.1073/pnas.0607521104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simon L, Castillo J, Oliva R, Lewis SE. Relationships between human sperm protamines, DNA damage and assisted reproduction outcomes. Reprod Biomed Online. 2011;23:724–34. doi: 10.1016/j.rbmo.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 48.Marchiani S, Tamburrino L, Benini F, Fanfani L, Dolce R, et al. Chromatin protamination and CatSper expression in spermatozoa predict clinical outcomes after Assisted Reproduction Programs. Sci Rep. 2017;7:15122. doi: 10.1038/s41598-017-15351-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bianchi PG, Manicardi GC, Urner F, Campana A, Sakkas D. Chromatin packaging and morphology in ejaculated human spermatozoa: evidence of hidden anomalies in normal spermatozoa. Mol Hum Reprod. 1996;2:139–44. doi: 10.1093/molehr/2.3.139. [DOI] [PubMed] [Google Scholar]
- 50.Sakkas D, Urner F, Bizzaro D, Manicardi G, Bianchi PG, et al. Sperm nuclear DNA damage and altered chromatin structure: effect on fertilization and embryo development. Hum Reprod. 1998;13(Suppl 4):11–9. doi: 10.1093/humrep/13.suppl_4.11. [DOI] [PubMed] [Google Scholar]


