Abstract
Oxidative stress, abnormal fatty acid metabolism, and impaired gut microbiota play a serious role in the pathology of autism. The use of dietary supplements to improve the core symptoms of autism is a common therapeutic strategy. The present study analyzed the effects of oral supplementation with Novavit, a multi-ingredient supplement, on ameliorating oxidative stress and impaired lipid metabolism in a propionic acid (PPA)-induced rodent model of autism. Male western albino rats were divided into three groups. The first group is the control, the second group was given an oral neurotoxic dose of PPA (250 mg/kg body weight/day) for 3 days and then received buffered saline until the end of the experiment. The third group received Novavit (70 mg/kg body weight/day for 30 days after the 3-day PPA treatment). Markers of oxidative stress and impaired fatty acid metabolism were measured in brain homogenates obtained from each group. Novavit modulation of the gut microbiota was also evaluated. While PPA induced significant increases in lipid peroxides and 5-lipoxygenase, together with significantly decreased glutathione, and cyclooxygenase 2, oral supplementation with Novavit ameliorated PPA-induced oxidative stress and impaired fatty acid metabolism. Our results showed that the presence of multivitamins, coenzyme Q10, minerals, and colostrum, the major components of Novavit, protects against PPA-induced neurotoxicity.
Keywords: autism, fatty acids, oxidative stress, gut microbiota, Novavit.
1. Introduction
Autism is a neurodevelopmental disorder characterized by abnormal social interactions with sensory dysfunction and repetitive and stereotyped behaviors. Prevalence estimates are 1–2 diagnoses per 100 individuals. Nutritional interventions for autism treatment aim to stimulate social interaction and other autistic phenotypic features. These may be managed by caregivers and professionals, with parents often functioning as cotherapists [1].
Several biomarker studies have reported significant abnormalities in antioxidant status and fatty acid metabolism in autistic children [2,3,4,5]. Autistic patients from Saudi Arabia have much lower levels of glutathione (GSH) and polyunsaturated fatty acid and elevated lipid peroxides, saturated fatty acids, prostaglandins, and 8-isoprostane compared to the age-matched controls [6,7]. A previous study showed that children with autism from Egypt had lower plasma levels of polyunsaturated fatty acids, except linoleic acid, compared to the healthy controls [8].
Prostaglandins, leukotrienes, and thromboxanes are metabolites produced by cyclooxygenases (COXs) and 5-lipoxygenase (ALOX5) that mediate the inflammatory response [9,10]. Each of these lipid mediators influence neural development, aging, and neurodegeneration [11].
Cytoplasmic phospholipase A2 alpha (cPLA2α) is an enzyme that hydrolyzes phospholipids to mobilize arachidonic acid (AA) for eicosanoid production, a major class of neuroinflammatory signaling molecules. In addition, cPLA2α also regulates the composition of membrane phospholipids to allow for proper membrane structure and function [12]. An important contributor to membrane structure and function is a phosphatidylinositol (PI) species which contains AA at both the sn-1 and sn-2 positions [13]. In addition to an important role in innate immune function, PI may also act as a short-lived acceptor for incorporation of AA into various cellular phospholipid classes [14]. Owing to the high AA content, PI is also a major source for AA release via cPLA2α in activated immune cells [13,14,15,16]. The importance of phospholipids in normal brain function suggests that membrane lipid replacement may be an effective treatment strategy to repair phospholipids in membranes of organelles, cells, and organs [17].
Animal models are typically used to evaluate pathological mechanisms of disease and to suggest possible treatment strategies that target affected metabolic pathways. Although autism is a human disorder, rodent models can contribute to the understanding of the etiology of autism and to evaluate therapeutic agents [18]. Previous studies [7,19] proposed that brain infusion or oral administration of propionic acid (PPA) to rat pups could induce many of the biochemical characteristics observed in individuals with autism. Moreover, histopathological changes, such as neuronal loss, hyaline bodies, and astrogliosis, together with several behavioral traits such as hyperactivity, impaired social interaction, reduced exploratory activity, and increased repetitive behaviors, have been recorded [19,20,21].
It is well accepted that the composition of microbiota regulates the levels of short-chain fatty acids (SCFAs), including acetic acid, PPA, and valeric acid. Wang et al. [22] reported that the levels of these SCFAs and ammonia in stool were considerably higher in children with autism when compared with healthy controls. Additionally, Shaw [23] reported higher concentrations of urinary 3-(3-hydroxyphenyl)-3-hydroxypropaonic acid in children with autism spectrum disorder (ASD) compared with controls. Song et al. [24] suggested that the source of this compound might be multiple species of anaerobic bacteria of the Clostridium genus. Recently, the involvement of microbiota in ASD pathogenesis and the possibility to use as a target to treat this disorder were greatly encouraged [25]. This information supports the use of PPA for induction of autism in a rodent model.
As autism etiology is multifactorial and the disorder is characterized by complex pathophysiology, the use of complex supplements might be successful as a treatment strategy. Vitamin B6 is widely used to decrease behavioral problems observed in autism [26], but the mechanism is not fully understood. Vitamin B6 plays a critical role in the synthesis of many neurotransmitters, such as gamma aminobutyric acid, serotonin, dopamine, and noradrenalin, and vitamin B6 supplementation can enhance many neurotransmitter systems affected in autism [27,28].
Ali et al. [29] produced a developmental vitamin D (DVD)-deficiency model of autism. This DVD-deficiency model may prove relevant for investigation of possible therapeutic strategies to reverse the abnormality of direct regulation of the fetal/placental immune response during pregnancy and improve impaired steroid biosynthesis, which are two accepted mechanisms relating DVD to autistic phenotypes. Although DVD may only represent one environmental contributor to autism, the ability to intervene safely and effectively during pregnancy might help to avoid the development of autistic phenotypes.
Similar results were observed with respect to vitamins E, C, and A and folic acid. Multiple studies correlated micronutrient deficiencies with development of autism [3,30]. Adams et al. [31] recommended vitamin/mineral supplementation as a valuable nutritional intervention strategy for children with autism. Supplementation resulted in remarkable improvements in GSH levels, oxidative stress, sulfation, methylation, ATP, NADH, and NADPH.
Coenzyme Q10 (CoQ10), a lipid-soluble benzoquinone, exerts intracellular antioxidant activity that preserves membrane phospholipids and mitochondrial proteins from free radical-induced oxidative damage [32]. CoQ10 supports mitochondrial functions such as electron transport, which is critical for ATP production [33]. Previous studies have shown that autistic children with CoQ10-restricted diet either due to sensory sensitivities or due to therapeutic measures suffer from mitochondrial dysfunction [34,35,36] and that CoQ10 supplementation in mice is usually accompanied by remarkable improvement in behavior [37]. Our most recent work showed that insufficient serum CoQ10 might play a role in autism pathophysiology [38].
Novavit is a nutritional supplement that acts as an antiaging and detoxification agent. This unique cellular food component contains a large quantity of embryonic predifferentiated duck stem cells as well as several minerals and vitamins to support health. Furthermore, Novavit contains many amino acids such as arginine, proline, lysine, carnitine, cysteine, and inositol. In addition, it contains CoQ10, folic acid, and a variety of essential vitamins such as vitamins B, E, D, and C that function with amino acids to enhance collagen synthesis and improve scar healing. Table 1 demonstrates the ingredients of Novavit (https://www.novavitcomplexusa.com/).
Table 1.
Ingredients of Novavit complex
| Compound | |
|---|---|
| Vitamins |
|
| Water-soluble vitamins: | |
| |
|
|
| Minerals | The natural clay’s basic chemical structure is MgO·Al2O3·5SiO2·nH2O with high content of magnesium, silica, potassium, calcium, phosphates, iron oxide, aluminum, manganese, and titanium. This clay has special benefits such as detoxifying action, remineralizing action, and is extremely absorbent |
| Other | Embryonic predifferentiated duck stem cells |
In addition to the discussion of neurological effects and disorders related to PPA and food supplement deficiency, it is important to highlight the critical role that the gut microbiota play in human metabolism and health [39]. Intestinal bacterial composition is important in digestion, protection against invading pathogens, and regulating immunity and metabolism of host cells [39]. Any disturbance in this composition can lead to the development of diseases and may trigger an autoimmune response. Diet also has an impact on the gut microbial composition, mainly noted on the following day [40], where a high number of certain bacterial species will dominate and suppress other strains [41].
We hypothesized that the use of Novavit as a complex nutritional supplement could minimize oxidative stress and neuroinflammation induced by PPA by modulating antioxidant defense. In addition, Novavit suppressed the growth of the Clostridia species, thus altering the impaired gut microbiota. We analyzed lipid peroxidation and antioxidant markers in addition to PLA2, COX2, ALOX5, phospholipids, leukotrienes, and prostaglandins in brain homogenates of PPA-treated rats as a rodent model of autism and characterized the gut microbial composition alteration prior and following Novavit dosage.
2. Methods
2.1. Animals
Twenty-one young male western albino rats (80–120 g) were obtained from King Saud University Riyadh. Rats were randomly allocated to the following groups. Group I, the control group, was given phosphate-buffered saline (PBS). Group II was given oral buffered PPA (250 mg/kg body weight/day for 3 days) followed by PBS solution until the end of the study. Group III was given Novavit complex (70 mg/kg body weight/day for 30 days after the 3-day PPA treatment), a product of Novavit, Inc., USA [6]. All animals were reared in a controlled temperature environment and were given access to food and water under standard laboratory conditions.
Ethical approval: The research related to animal use has been complied with all the relevant national regulations and institutional policies for the care and use of animals. All protocols were approved by the ethics committee of King Saud University.
2.2. Sample collection
2.2.1. Brain tissue
After sacrifice, the brain tissue was collected, washed, and homogenized with distilled water (1/10 by volume/weight ratio). After homogenization, the tissue was centrifuged at 3,500 rpm for 15 min. The clear supernatant obtained was used for the following assays.
2.3. Biochemical analyses
2.3.1. Spectrophotometric analysis
The method of Ruiz-Larrea et al. [42] was used for lipid oxidation, which is estimated by the formation of thiobarbituric acid reactive substances.
The method of Jagota and Dani [43] was used for vitamin C analysis.
The method of Beutler and Yeh [44] was used to assay GSH using 5,5′-dithiobis 2-nitrobenzoic acid with sulfhydryl compounds to produce a relatively stable yellow color.
2.3.2. ELISA
Sandwich ELISA principle was used to estimate phospholipase A2 and COX2. Kits from LSBio (Lifespan BioScience, Inc., North America) were used with a detection range of 3.12–200 and 0.156–10 ng/mL, respectively.
Competitive ELISAs were used for estimation of leukotriene B4 and prostaglandin E2 (PGE2). Kits were purchased from Cayman Chemical Company (Ann Arbor, MI, USA), with the assay range from 3.9–500 and 7.8–1,000 pg/mL, respectively.
2.4. Microbiological analyses
2.4.1. Collection and preparation of fecal samples
Fecal samples were collected from all animal groups before and after treatment and were stored at −80°C. One gram of fecal matter was homogenized using a sonicator for 30 s in 10 mL of 0.1 M pH 7.2 PBS. The solutions were then centrifuged at 4,500 rpm for 3 min at 4°C. One milliliter of the fecal supernatant was then serially diluted in 9 mL of sterile PBS solution four times [45].
2.4.2. Bacterial enumeration and culturing
Nutrient agar (Oxoid) plates, MacConkey plates, blood agar plates, and plates containing cycloserine–cefoxitin fructose agar (CCFA) medium selective for Clostridia were used to grow bacteria using 100 µL of each of the prepared dilutions for every group of animals. Anaerobic jars containing 5% CO2 at 37°C were used for CCFA medium selective for Clostridia, with a 3-day incubation period. All other culture media were incubated at 37°C under aerobic conditions for 18–24 h. The experiment was repeated twice. The average number of bacteria per plate was recorded. Gram staining and biochemical tests were used to identify the bacterial strains.
2.5. Statistical analysis
Results are expressed as mean ± SD. All statistical comparisons between the groups were performed using one-way analysis of variance (ANOVA) tests with Dunnett’s test for multiple comparisons. Adjusted P values were also calculated by the Bonferroni test. Significance was assigned at the level of P < 0.05. Receiver-operating characteristic (ROC) curve and area under the curve (AUC), the degrees of sensitivity and specificity, and the cutoff values were evaluated using Pearson’s correlations.
3. Results
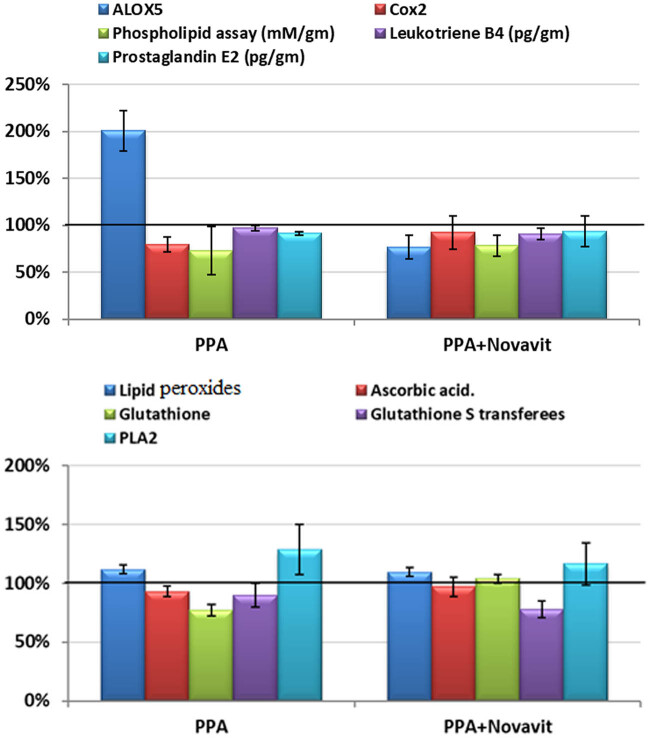
Table 2 and Figure 1 show mean ± SD and the percentage change in the measured parameters in the three studied groups. The PPA treatment significantly increased lipid peroxides by 11.72%, and ALOX5 level by 100.62%, compared to the control. In contrast, PPA-treated rats expressed GSH and COX2 at much lower levels than control rats, showing 22.65% and 20.14% decreases, respectively. Ascorbic acid, leukotriene B4, and PGE2 levels did not change significantly with PPA treatment. Treatment with Novavit reversed PPA-induced changes in GSH, PLA2, ALOX5, and COX2, as summarized in Figure 1 and Table 2.
Table 2.
Mean ± SD of the measured parameters in the three studied groups
| Parameter | Group | N | Min. | Max. | Mean ± SD | Percentage change | P valuea | P valueb | P valuec |
|---|---|---|---|---|---|---|---|---|---|
| Lipid peroxides | Control | 7 | 0.47 | 0.52 | 0.50 ± 0.02 | 100 | 0.001 | ||
| PPA | 7 | 0.52 | 0.59 | 0.56 ± 0.02 | 11.72 | 0.001 | 0.001 | ||
| PPA + Novavit | 7 | 0.51 | 0.57 | 0.55 ± 0.02 | 9.5 | 0.001 | 0.002 | ||
| Ascorbic acid | Control | 7 | 23.94 | 28.42 | 25.83 ± 1.40 | 100 | 0.126 | ||
| PPA | 7 | 23 | 26 | 24.02 ± 1.08 | −7.01% | 0.081 | 0.136 | ||
| PPA + Novavit | 7 | 22.89 | 29.21 | 25.07 ± 2.08 | −2.93% | 0.58 | 1 | ||
| Glutathione | Control | 7 | 88.88 | 97.92 | 93.15 ± 3.27 | 100 | 0.001 | ||
| PPA | 7 | 65.7 | 75.7 | 72.06 ± 3.50 | −22.60% | 0.001 | 0.001 | ||
| PPA + Novavit | 7 | 93.23 | 101.93 | 96.61 ± 3.49 | 3.71% | 0.132 | 0.138 | ||
| PLA2 | Control | 7 | 1160 | 2091.9 | 1,441.00 ± 317.85 | 100 | 0.102 | ||
| PPA | 7 | 1106 | 2290.1 | 1,852.28 ± 393.77 | 28.54% | 0.064 | 0.107 | ||
| PPA + Novavit | 7 | 1120.1 | 1891.3 | 1,677.42 ± 296.25 | 16.41% | 0.342 | 0.623 | ||
| ALOX5 | Control | 7 | 226.55 | 365.09 | 293.88 ± 46.28 | 100 | 0.001 | ||
| PPA | 7 | 406.68 | 795.32 | 589.59 ± 126.69 | 100.62 | 0.001 | 0.001 | ||
| PPA + Novavit | 7 | 192.51 | 271.27 | 225.08 ± 27.98 | −23.41% | 0.21 | 0.369 | ||
| COX2 | Control | 7 | 73.98 | 92.33 | 81.66 ± 6.50 | 100 | 0.011 | ||
| PPA | 7 | 54.96 | 72.6 | 65.21 ± 5.24 | −20.14% | 0.006 | 0.01 | ||
| PPA + Novavit | 7 | 60.42 | 95.63 | 75.55 ± 13.39 | −7.48% | 0.368 | 0.677 | ||
| Phospholipid (mM/g) | Control | 7 | 13.08 | 21.62 | 16.76 ± 2.80 | 100 | 0.01 | ||
| PPA | 7 | 7.35 | 16.13 | 12.21 ± 3.17 | −27.14% | 0.008 | 0.013 | ||
| PPA + Novavit | 7 | 11.75 | 15.77 | 13.10 ± 1.52 | −21.82% | 0.031 | 0.05 | ||
| Leukotriene B4 (pg/g) | Control | 7 | 386.04 | 413.65 | 404.55 ± 9.39 | 100 | 0.001 | ||
| PPA | 7 | 380.18 | 416.17 | 391.82 ± 12.40 | −3.15% | 0.239 | 0.424 | ||
| PPA + Novavit | 7 | 329.89 | 393.74 | 366.63 ± 21.83 | −9.37% | 0.001 | 0.001 | ||
| PGE2 (pg/g) | Control | 7 | 238.99 | 381.8 | 267.42 ± 51.26 | 100 | 0.511 | ||
| PPA | 7 | 239.91 | 253.47 | 244.75 ± 4.45 | −8.48 | 0.443 | 0.833 | ||
| PPA + Novavit | 7 | 213.57 | 332.73 | 249.45 ± 40.75 | −6.72 | 0.587 | 1 |
a P value between each group and the control group using Dunnett’s test as multiple comparisons.
b P value between each group and the control group using Bonferroni test as multiple comparisons.
c P value between all groups using one-way ANOVA.
Figure 1.
Percentage change of all parameters in all groups compared to control.
Table 3 summarizes the Pearson’s correlation coefficients between the measured variables. Lipid peroxides negatively correlated with GSH expression (P < 0.003) and positively correlated with ALOX5 (P < 0.049). GSH negatively correlated with ALOX5 (P < 0.001) and positively correlated with COX2 (P < 0.001). ALOX5 negatively correlated with COX2 (P < 0.029).
Table 3.
Correlations between the measured variables
| Parameters | R (Person correlation) | Sig. | Significance |
|---|---|---|---|
| Lipid peroxides with glutathione | −0.442** | 0.003 | Nb |
| Lipid peroxides with PLA2 | 0.554** | 0.001 | Pa |
| Lipid peroxides with ALOX5 | 0.306* | 0.049 | Pa |
| Ascorbic acid with phospholipid assay (mM/g brain tissue) | 0.457** | 0.002 | Pa |
| Glutathione with PLA2 | −0.476** | 0.001 | Nb |
| Glutathione with ALOX5 | −0.804** | 0.001 | Nb |
| Glutathione with COX2 | 0.488** | 0.001 | Pa |
| PLA2 with ALOX5 | 0.345* | 0.025 | Pa |
| ALOX5 with COX2 | −0.338* | 0.029 | Nb |
*Correlation is significant at the 0.05 level.
**Correlation is significant at the 0.01 level.
aPositive correlation.
bNegative correlation.
Table 4 presents the cutoff values, AUC, sensitivity, and specificity of each of the measured markers for the PPA-treated group and the PPA-treated group supplemented with Novavit. Most of the measured variables showed reasonable AUCs, specificity, and sensitivity as a marker of PPA neurotoxicity and/or the therapeutic effect of Novavit.
Table 4.
ROC analysis of all variables in all groups
| Parameter | Group | Area under the curve | Cutoff value | Sensitivity (%) | Specificity (%) | p value |
|---|---|---|---|---|---|---|
| Lipid peroxides | PPA | 0.990 | 0.515 | 100.0 | 85.7 | 0.002 |
| PPA + Novavit | 0.959 | 0.515 | 85.7 | 85.7 | 0.004 | |
| Ascorbic acid | PPA | 0.837 | 24.865 | 85.7 | 85.7 | 0.035 |
| PPA + Novavit | 0.673 | 24.605 | 57.1 | 85.7 | 0.277 | |
| Glutathione | PPA | 1.000 | 82.290 | 100.0 | 100.0 | 0.002 |
| PPA + Novavit | 0.796 | 93.192 | 100.0 | 71.4 | 0.064 | |
| PLA2 | PPA | 0.755 | 1527.55 | 85.7 | 85.7 | 0.110 |
| PPA + Novavit | 0.735 | 1507.70 | 85.7 | 85.7 | 0.142 | |
| ALOX5 | PPA | 1.000 | 385.89 | 100.0 | 100.0 | 0.002 |
| PPA + Novavit | 0.918 | 275.305 | 100.0 | 71.4 | 0.009 | |
| COX2 | PPA | 1.000 | 73.29 | 100.0 | 100.0 | 0.002 |
| PPA + Novavit | 0.633 | 71.58 | 57.1 | 100.0 | 0.406 | |
| Phospholipid assay (mM/g brain tissue) | PPA | 0.878 | 15.56 | 85.7 | 71.4 | 0.018 |
| PPA + Novavit | 0.878 | 15.10 | 85.7 | 71.4 | 0.018 | |
| Leukotriene B4 (pg/g brain tissue) | PPA | 0.796 | 397.435 | 85.7 | 85.7 | 0.064 |
| PPA + Novavit | 0.959 | 396.854 | 100.0 | 85.7 | 0.004 | |
| PGE2 (pg/g brain tissue) | PPA | 0.684 | 244.765 | 71.4 | 71.4 | 0.250 |
| PPA + Novavit | 0.673 | 243.030 | 57.1 | 85.7 | 0.277 |
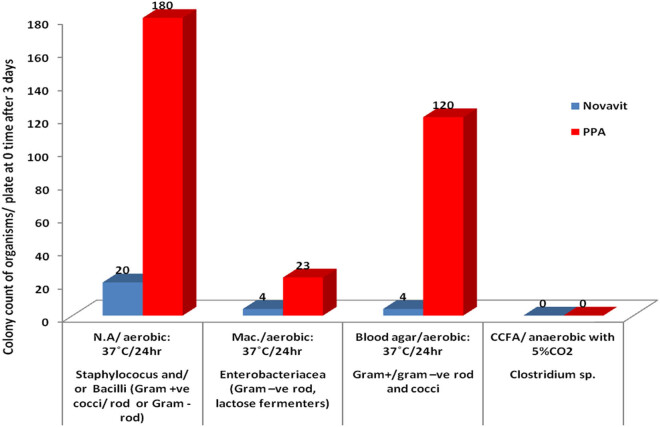
Fecal bacterial analysis from each of the animal groups in the study was performed and tabulated as an average of the bacterial count per plate. Data were compared between the groups before and after treatment with Novavit following PPA administration.
3.1. Microbial data
The microbial profile of all groups (Table 5 and Figures 2, 3) mainly consisted of Staphylococcus aureus, identified as a bacterium with a grape-like structure when observed under a microscope following gram staining. Very few enteric bacteria (gram-negative rod) were found during the study period following PPA and Novavit administration. Moreover, Novavit intake greatly decreased Clostridium sp.
Table 5.
(a) Estimating colony count/plate after 3 days of PPA dose followed by Novavit intake. Day 0 following treatment with Novavit. (b) Estimated colony count/plate following PPA treatment (1 week). (c) Estimated colony count/plate at 3 weeks
| (a) | ||||
|---|---|---|---|---|
| Isolated organisms | Media and incubation conditions | Novavit | PPA | Control (pretreatment) |
| Staphylococcus and/or bacilli (gram-positive cocci/rod and or gram-negative rod) | N.A./aerobic: 37°C/24 h | 20 | 180 | 300 |
| Enterobacteriaceae (gram-negative rod, lactose fermenters) | Mac/aerobic: 37°C/24 h | 4 | 23 | 0 |
| Gram-positive/gram-negative rod and cocci | Blood agar/aerobic: 37°C/24 h | 4 | 120 | 100 |
| Clostridium sp. | CCFA/anaerobic 37°C with 5% CO2 | 0 | 0 | 0 |
| (b) | |||
|---|---|---|---|
| Isolated organisms | Media and incubation conditions | Novavit | PPA |
| Staphylococcus and/or bacilli (gram-positive cocci/rod or gram-negative rod) | N.A./aerobic: 37°C/24 h | 0 | 30 |
| Enterobacteriacea (gram-negative rod, lactose fermenters) | Mac./aerobic: 37°C/24 h | 0 | 3 |
| Gram-positive/gram-negative rod and cocci | Blood agar/aerobic: 37°C/24 h | 0 | 16 |
| Clostridium sp. | CCFA/anaerobic 37°C with 5% CO2 | 0 | 0 |
| (c) | |||
|---|---|---|---|
| Isolated organisms | Media and incubation conditions | Novavit | PPA |
| Staphylococcus aureus (gram-positive cocci grape-like structure) | N.A/aerobic: 37°C/24 h | >300 | 20 |
| Enterobacteriaceae (gram-negative rod lactose fermenters) | Mac./aerobic: 37°C/24 h | 0 | 1 |
| Gram-positive cocci or bacilli | Blood agar/aerobic: 37°C/24 h | 200 | 10 |
| Clostridium sp. | CCFA/anaerobic 37°C with 5% CO2 | 0 | 0 |
Figure 2.
Average bacterial plate count following PPA treatment (3 days).
Figure 3.
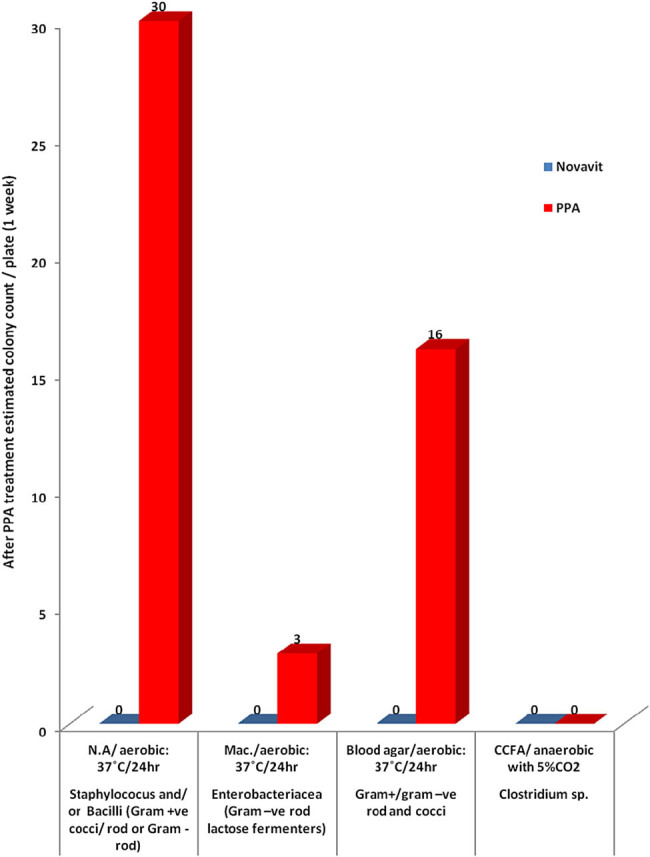
Bacterial plate count (1 week of treatment).
4. Discussion
Nutritional deficiencies may play a chief role in autism because many patients are selective eaters with high sensitivity toward many foods, which might lead to inadequate intake of nutrients [46]. Thus, nutritional interventions can significantly help these patients [47,48,49]. Adams [50] reported that vitamins, minerals, amino acids, and specialty supplements are key components in the biomedical approach for the treatment of autism. The use of complex supplements could be beneficial in treating this disorder.
The results summarized in Table 2 demonstrate significant irregularities in biomarkers of oxidative stress following PPA administration. While Novavit was ineffective in reducing lipid peroxides, it potently increased vitamin C levels. Additionally, Novavit ameliorated decreased GSH levels in PPA-treated rats (P < 0.001) to levels not significantly different from the control group (P < 0.580). The recorded increase in GSH and vitamin C in Novavit-treated rats is likely due to vitamins B, C, D, and E contained in Novavit. Vitamin D is effective in increasing GSH levels in the brain through calcitriol upregulation of γ-glutamyl transpeptidase, the rate-limiting enzyme in GSH synthesis [51,52,53]. A recent review by Jia et al. [54] reported improvement in GSH levels in autistic patients treated with vitamin D. Moreover, the remarkable increase in GSH levels in Novavit-treated rat brains can be attributed to CoQ10 treatment (30 or 60 mg CoQ10/day for 100 days), an important component of the electron transport chain known to reduce GSH peroxidase enzymatic activity and improve gastrointestinal (GI) problems and sleep disorders in children with autism [55].
Both glycine and glutamic acid, components of the tripeptide GSH, are readily available in the diet of most individuals. In contrast, cysteine is not readily available, making it the rate-limiting amino acid for GSH intracellular synthesis. In free amino acid form, cysteine is toxic and is broken down in the GI tract and the blood. Colostrum, the first milk secretion, is rich in cystine, a stable form of cysteine that can cross the blood–brain barrier (BBB). Based on this, colostrum is a unique component of Novavit that can explain the significant increases in GSH in Novavit-treated rats [56].
The brain is highly susceptible to lipid peroxidation due to the abundance of polyunsaturated fatty acids, high aerobic metabolism, and low levels of antioxidant enzymes [57]. Lipid peroxidation in the brain following administration of PPA may be responsible for PPA-induced neurotoxic effects [58]. Activation of phospholipase A2 as a phospholipid-hydrolyzing enzyme can contribute to depletion of brain phospholipids, potentially contributing to PPA-induced neurotoxicity [58]. Data summarized in Table 1 and Figure 1 demonstrate the activation of PLA2 with concomitant depletion of brain phospholipids in the PPA-treated groups. Moreover, Novavit reduced PLA2 activity, resulting in phospholipid replenishment.
The unexpected decreases in COX-2 and PGE2 in the PPA-treated rats (P < 0.005) (Table 2 and Figure 1) may be related to alteration in the gut microbiota (Table 5 and Figures 2, 3). COX2 plays a critical role in the adaptive cytoprotection response in GI mucosal cells. When the GI tract is inflamed in response to toxins resulting from pathogenic bacterial overgrowth, large amounts of prostaglandins (PGs) are produced at the sites of injury by rapidly induced COX2. This process typically aids in the healing process of the injured gut. Under these conditions, inhibition of COX2 should be avoided in patients who are vulnerable to GI inflammation (e.g., autistic patients) [59]. Moreover, enhanced BBB leakage also correlates with impaired markers of brain inflammation [60]. Animal models of autism support increased BBB permeability as a characteristic feature related to glutamate excitotoxicity as an etiological mechanism [61]. BBB disruption can affect influx and efflux of proteins and ions. This may contribute to the unexpected decrease in PLA2 and PGs in brains of PPA-treated animals [62,63]. This hypothesis agrees with the previous work of Qasem et al. [64,65] in which PLA2 and PG were markedly higher in the plasma of autistic patients compared to the controls. Novavit treatment increased COX2 to levels similar to the controls.
Data summarized in Table 2 and Figure 1 demonstrate that in spite of a twofold increase in ALOX5 in PPA-treated rat pups, leukotriene B4 levels did not significantly change. Leukotriene B4 is an enzymatic hydration product of AA that can be conjugated and excreted. Novavit was effective in counteracting the effects of PPA as a neurotoxic mediator through a significant decrease in ALOX5 to levels comparable to controls. Leukotriene B4 was significantly lower in the Novavit-treated group compared to the controls.
Table 2 shows the Pearson’s correlations between the measured parameters. Positive correlations were found among PLA2, ALOX5, and lipid peroxides, with a concomitant negative correlation with GSH. These results confirmed the contribution of oxidative stress, impaired lipid metabolism, and neuroinflammation as neurotoxic mechanisms of PPA to the rodent model of autism and the therapeutic efficacy of Novavit as a multisupplement.
Table 4 summarizes the AUCs for all measured markers of PPA neurotoxicity and Novavit therapeutic potency. With few exceptions, most of the measured variables showed high AUCs with satisfactory specificity and sensitivity, which supports their use as valid markers.
Similarly, Novavit intake had a remarkable effect on the gut bacterial composition. Our study demonstrated that following the administration of Novavit, a mixture of vitamins B, E C, and D, and folic acid, the gut bacterial composition and the total bacterial count were altered with abundance of gram-positive bacteria (mainly S. aureus) on day 1, followed by an absence of bacterial growth (total bacterial number was (0) at week 1), then a subsequent reemergence of gram-positive bacteria. PPA administration resulted in increased bacteria number per plate on day 1 and throughout the experiment compared to Novavit-induced bacterial count, in agreement with the findings of Wu et al. [40]. However, both PPA and Novavit negatively impacted Clostridium sp., as this bacterium was absent in both treatment groups (Table 2 and Figures 2, 3).
Previous studies of germ-free and conventional rodents, and of human volunteers, reported that the gut microflora can synthesize vitamins such as vitamin K and vitamin B including biotin, pyridoxine, riboflavin, thiamine, cobalamin, folates, nicotinic acid, and pantothenic acid [66]. These vitamins are important for bacterial and mammalian metabolism. Magnúsdóttir et al. [67] explored the genomes of 256 common gut bacteria for the presence of eight vitamin B (including biotin, cobalamin, folate, pyridoxine, riboflavin, and thiamin) producers and found that each vitamin is produced by a different phylum, for instance, riboflavin was found to be mainly synthesized by the phylum Bacteroidetes, whereas Firmicutes had the ability to produce vitamin B. Previous studies of vitamin A and D deficiencies [68,69,70] revealed that some vitamins can interfere with the microbial gut composition and result in alteration of gut bacterial composition at the phyla levels in response to vitamin intake. Mandal et al. [70] noted that vitamin D intake increases the ratios of Actinobacteria/Proteobacteria, Proteobacteria/Firmicutes, whereas higher intake of vitamin E caused a decrease in the ratio of Proteobacteria/Actinobacteria and Firmicutes. At the genus level, Mandal et al. [70] observed that with fat-soluble vitamin intake, Staphylococcus was most abundant after vitamin E intake and lower with vitamin D intake, which agreed with the present data, where Staphylococcus aureus was the most abundant species encountered at the beginning and end of the experiments following Novavit. This is consistent with increased abundance of GSH as the main sulfur source for bacterial growth [71]. Furthermore, the presence of colostrum in Novavit, which is rich in antimicrobial peptides [72], is of nutritive importance, regulates GI disorders, and alters the gut microbiota, resulting in abundance of certain types of bacteria [73,74,75]. Interestingly, the presence of duck embryonic stem cells as a unique component of Novavit may increase its nutritional value and therapeutic efficacy. Because cell therapy is one of the most exciting fields in translational medicine, the effect of duck embryonic stem cells in Novavit can be related to its potency in downregulating certain proinflammatory cytokines, cytokine receptors, and cell cycle transcripts and upregulating signal transduction, cell adhesion, and cytoskeletal protein transcripts [76]. Moreover, under appropriate conducive conditions, populations of stem cells change to cells with features of neuronal-like tissues, which might explain the recorded potency of Novavit in the present study [76].
The finding of the current study is of great support to our previous work in which vitamin D, CoQ10, vitamin B12, carnitine, and bioflavonoids (as major components of bee pollen) demonstrate significant potency in ameliorating PPA-induced persistent autistic features in the rodent model [77,78,79,80,81,82,83,84].
5. Conclusion
This study showed that Novavit, a multi-ingredient supplement usually given to modify risk factors associated with aging and disease, ameliorates PPA-induced neurotoxic in a rat model of autism. As only some products sold to consumers are validated scientifically, this study confirmed that multivitamins, CoQ10, minerals, and colostrum, components of Novavit, contribute to its potential use for the treatment of neurotoxicity and as prerequisite in translational medicine.
6. Limitations
There are two major limitations in this study that could be addressed in future research. First is the relatively small sample size, and second, the inability to measure the effectiveness of Novavit as a complex supplement in improving induced autistic behavior in the PPA-induced rodent model. Further studies using a larger number of subjects are required to determine whether the effect of substrate intake on gut microbiota is related to each substrate, nutrient alone, or food intake rich in these substrates.
Acknowledgment
This research project was supported by a grant from the research center of the Center for Female Scientific and Medical Colleges at King Saud University. The authors thank the Deanship of Scientific Research and RSSU at King Saud University for their technical support.
Footnotes
Conflict of interest: The authors state no conflict of interest.
References
- [1].Pasco G. The value of early intervention for children with autism. Paediatrics Child Health. 2018;28(8):364–7.; Pasco G. The value of early intervention for children with autism. Paediatrics Child Health. 2018;28(8):364–7. [Google Scholar]
- [2].Clark-Taylor T, Clark-Taylor BE. Is autism a disorder of fatty acid metabolism? Possible dysfunction of mitochondrial beta-oxidation by long chain acyl-CoA dehydrogenase. Med Hypotheses. 2004;62(6):970–5. [DOI] [PubMed]; Clark-Taylor T, Clark-Taylor BE. Is autism a disorder of fatty acid metabolism? Possible dysfunction of mitochondrial beta-oxidation by long chain acyl-CoA dehydrogenase. Med Hypotheses. 2004;62(6):970–5. doi: 10.1016/j.mehy.2004.01.011. [DOI] [PubMed] [Google Scholar]
- [3].Al-Gadani Y, El-Ansary A, Attas O, Al-Ayadhi L. Metabolic biomarkers related to oxidative stress and antioxidant status in Saudi autistic children. Clin Biochem. 2009;42(10–11):1032–40. [DOI] [PubMed]; Al-Gadani Y, El-Ansary A, Attas O, Al-Ayadhi L. Metabolic biomarkers related to oxidative stress and antioxidant status in Saudi autistic children. Clin Biochem. 2009;42(10–11):1032–40. doi: 10.1016/j.clinbiochem.2009.03.011. [DOI] [PubMed] [Google Scholar]
- [4].Yaffe H, Buxdorf K, Shapira I, Ein-Gedi S, Zvi MM, Fridman E, et al. LogSpin: a simple, economical and fast method for RNA isolation from infected or healthy plants and other eukaryotic tissues. BMC Res Notes. 2012;5(1):45. [DOI] [PMC free article] [PubMed]; Yaffe H, Buxdorf K, Shapira I, Ein-Gedi S, Zvi MM, Fridman E. et al. LogSpin: a simple, economical and fast method for RNA isolation from infected or healthy plants and other eukaryotic tissues. BMC Res Notes. 2012;5(1):45. doi: 10.1186/1756-0500-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Frye RE. Biomarker of abnormal energy metabolism in children with autism spectrum disorder. North Am J Med Sci. 2012;5:141–7.; Frye RE. Biomarker of abnormal energy metabolism in children with autism spectrum disorder. North Am J Med Sci. 2012;5:141–7. [Google Scholar]
- [6].El-Ansary AK, Bacha AG, Al-Ayahdi LY. Plasma fatty acids as diagnostic markers in autistic patients from Saudi Arabia. Lipids Health Dis. 2011;10:62. 10.1186/1476-511X-10-62.58. [DOI] [PMC free article] [PubMed]; El-Ansary AK, Bacha AG, Al-Ayahdi LY. Plasma fatty acids as diagnostic markers in autistic patients from Saudi Arabia. Lipids Health Dis. 2011;10:62. doi: 10.1186/1476-511X-10-62.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].El-Ansary A, Al-Ayadhi L. Lipid mediators in plasma of autism spectrum disorders. Lipids Health Dis. 2012;11:160. [DOI] [PMC free article] [PubMed]; El-Ansary A, Al-Ayadhi L. Lipid mediators in plasma of autism spectrum disorders. Lipids Health Dis. 2012;11:160. doi: 10.1186/1476-511X-11-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Mostafa GA, El-Gamal HA, El-Wakkad ASE, El-Shorbagy OE, Hamza MM. Polyunsaturated fatty acids, carnitine and lactate as biological markers of brain energy in autistic children. Int J Child Neuropsychiatry. 2005;2(2):179–88.; Mostafa GA, El-Gamal HA, El-Wakkad ASE, El-Shorbagy OE, Hamza MM. Polyunsaturated fatty acids, carnitine and lactate as biological markers of brain energy in autistic children. Int J Child Neuropsychiatry. 2005;2(2):179–88. [Google Scholar]
- [9].Tassoni D, Kaur G, Weisinger RS, Sinclair AJ. The role of eicosanoids in the brain. Asia-Pac J Clin Nutr. 2008;17:220–8. [PubMed]; Tassoni D, Kaur G, Weisinger RS, Sinclair AJ. The role of eicosanoids in the brain. Asia-Pac J Clin Nutr. 2008;17:220–8. [PubMed] [Google Scholar]
- [10].Tapiero H, Ba GN, Couvreur P, Tew KD. Polyunsaturated fatty acids (PUFA) and eicosanoids in human health and pathologies. Biomed Pharmacother. 2002;56:215–22. [DOI] [PubMed]; Tapiero H, Ba GN, Couvreur P, Tew KD. Polyunsaturated fatty acids (PUFA) and eicosanoids in human health and pathologies. Biomed Pharmacother. 2002;56:215–22. doi: 10.1016/s0753-3322(02)00193-2. [DOI] [PubMed] [Google Scholar]
- [11].Janssen CI, Kiliaan AJ. Long-chain polyunsaturated fatty acids (LCPUFA) from genesis to senescence: the influence of LCPUFA on neural development, aging, and neurodegeneration. Prog Lipid Res. 2014;53:1–17. [DOI] [PubMed]; Janssen CI, Kiliaan AJ. Long-chain polyunsaturated fatty acids (LCPUFA) from genesis to senescence: the influence of LCPUFA on neural development, aging, and neurodegeneration. Prog Lipid Res. 2014;53:1–17. doi: 10.1016/j.plipres.2013.10.002. [DOI] [PubMed] [Google Scholar]
- [12].Gil-de-Gómez L, Astudillo AM, Meana C, Rubio JM, Guijas C, Balboa MA, et al. A phosphatidylinositol species acutely generated by activated macrophages regulates innate immune responses. J Immunol. 2013;190(10):5169–77. [DOI] [PubMed]; Gil-de-Gómez L, Astudillo AM, Meana C, Rubio JM, Guijas C, Balboa MA. et al. A phosphatidylinositol species acutely generated by activated macrophages regulates innate immune responses. J Immunol. 2013;190(10):5169–77. doi: 10.4049/jimmunol.1203494. [DOI] [PubMed] [Google Scholar]
- [13].Balgoma D, Montero O, Balboa MA, Balsinde J. Calcium‐independent phospholipase A2‐mediated formation of 1,2‐diarachidonoyl‐glycerophosphoinositol in monocytes. FEBS J. 2008;275(24):6180–91. [DOI] [PubMed]; Balgoma D, Montero O, Balboa MA, Balsinde J. Calcium‐independent phospholipase A2‐mediated formation of 1,2‐diarachidonoyl‐glycerophosphoinositol in monocytes. FEBS J. 2008;275(24):6180–91. doi: 10.1111/j.1742-4658.2008.06742.x. [DOI] [PubMed] [Google Scholar]
- [14].Balgoma D, Astudillo AM, Pérez-Chacón G, Montero O, Balboa MA, Balsinde J. Markers of monocyte activation revealed by lipidomic profiling of arachidonic acid-containing phospholipids. J Immunoly. 2010;184(7):3857–65. [DOI] [PubMed]; Balgoma D, Astudillo AM, Pérez-Chacón G, Montero O, Balboa MA, Balsinde J. Markers of monocyte activation revealed by lipidomic profiling of arachidonic acid-containing phospholipids. J Immunoly. 2010;184(7):3857–65. doi: 10.4049/jimmunol.0902883. [DOI] [PubMed] [Google Scholar]
- [15].Gil-de-Gómez L, Astudillo AM, Guijas C, Magrioti V, Kokotos G, Balboa MA, et al. Cytosolic group IVA and calcium-independent group VIA phospholipase A2s act on distinct phospholipid pools in zymosan-stimulated mouse peritoneal macrophages. J Immunol. 2014;192(2):752–62. [DOI] [PubMed]; Gil-de-Gómez L, Astudillo AM, Guijas C, Magrioti V, Kokotos G, Balboa MA. et al. Cytosolic group IVA and calcium-independent group VIA phospholipase A2s act on distinct phospholipid pools in zymosan-stimulated mouse peritoneal macrophages. J Immunol. 2014;192(2):752–62. doi: 10.4049/jimmunol.1302267. [DOI] [PubMed] [Google Scholar]
- [16].Astudillo AM, Balboa MA, Balsinde J. Selectivity of phospholipid hydrolysis by phospholipase A(2) enzymes in activated cells leading to polyunsaturated fatty acid mobilization. Biochim Biophys Acta Mol Cell Biol Lipids. 2019;1864(6):772–83. [DOI] [PubMed]; Astudillo AM, Balboa MA, Balsinde J. Selectivity of phospholipid hydrolysis by phospholipase A(2) enzymes in activated cells leading to polyunsaturated fatty acid mobilization. Biochim Biophys Acta Mol Cell Biol Lipids. 2019;1864(6):772–83. doi: 10.1016/j.bbalip.2018.07.002. [DOI] [PubMed] [Google Scholar]
- [17].Nicolson GL, Ash ME. Membrane Lipid Replacement for chronic illnesses, aging and cancer using oral glycerolphospholipid formulations with fructooligosaccharides to restore phospholipid function in cellular membranes, organelles, cells and tissues. Biochim Biophys Acta Biomembr. 2017;1859(9 Pt B):1704–24. [DOI] [PubMed]; Nicolson GL, Ash ME. Membrane Lipid Replacement for chronic illnesses, aging and cancer using oral glycerolphospholipid formulations with fructooligosaccharides to restore phospholipid function in cellular membranes, organelles, cells and tissues. Biochim Biophys Acta Biomembr. 2017;1859(9 Pt B):1704–24. doi: 10.1016/j.bbamem.2017.04.013. [DOI] [PubMed] [Google Scholar]
- [18].Erdogan H, Antar V, Kaya AH, Firat L, Kubilay T, Tasdemiroglu E. Animal Models of Autism Spectrum Disorder. J Neurol Stroke. 2017;6(4):00209.; Erdogan H, Antar V, Kaya AH, Firat L, Kubilay T, Tasdemiroglu E. Animal Models of Autism Spectrum Disorder. J Neurol Stroke. 2017;6(4):00209. [Google Scholar]
- [19].MacFabe DF, Cain DP, Rodriguez-Capote K, Franklin AE, Hoffman JE, Boon F, et al. Neurobiological effects of intraventricular propionic acid in rats: possible role of short chain fatty acids on the pathogenesis and characteristics of autism spectrum disorders. Behav Brain Res. 2007;176:149–69. [DOI] [PubMed]; MacFabe DF, Cain DP, Rodriguez-Capote K, Franklin AE, Hoffman JE, Boon F. et al. Neurobiological effects of intraventricular propionic acid in rats: possible role of short chain fatty acids on the pathogenesis and characteristics of autism spectrum disorders. Behav Brain Res. 2007;176:149–69. doi: 10.1016/j.bbr.2006.07.025. [DOI] [PubMed] [Google Scholar]
- [20].Khalil SR, Abd-Elhakim YM, Selim ME, Al-Ayadhi LY. Apitoxin protects rat pups brain from propionic acid-induced oxidative stress: The expression pattern of Bcl-2 and Caspase-3 apoptotic genes. Neurotoxicology. 2015;49:121–31. 10.1016/j.neuro.2015.05.011. [DOI] [PubMed]; Khalil SR, Abd-Elhakim YM, Selim ME, Al-Ayadhi LY. Apitoxin protects rat pups brain from propionic acid-induced oxidative stress: The expression pattern of Bcl-2 and Caspase-3 apoptotic genes. Neurotoxicology. 2015;49:121–31. doi: 10.1016/j.neuro.2015.05.011. [DOI] [PubMed] [Google Scholar]
- [21].Al-Daghistani HI, D Shquir ATWA, Al-kharabsha MU, AL-Latif SM, Asympatomatic colonization of Staphylococcus aureus with intermediate resistance to vancomycin harboring VANB resistance gene. Asian J Pharm Clin Res. 2017;10(5):349–356.; Al-Daghistani HI, D Shquir ATWA, Al-kharabsha MU, AL-Latif SM. Asympatomatic colonization of Staphylococcus aureus with intermediate resistance to vancomycin harboring VANB resistance gene. Asian J Pharm Clin Res. 2017;10(5):349–356. [Google Scholar]
- [22].Wang L, Christophersen CT, Sorich MJ, Gerber JP, Angley MT, Conlon MA. Elevated fecal short chain fatty acid and ammonia concentrations in children with autism spectrum disorder. Dig Dis Sci. 2012;57(8):2096–102. [DOI] [PubMed]; Wang L, Christophersen CT, Sorich MJ, Gerber JP, Angley MT, Conlon MA. Elevated fecal short chain fatty acid and ammonia concentrations in children with autism spectrum disorder. Dig Dis Sci. 2012;57(8):2096–102. doi: 10.1007/s10620-012-2167-7. [DOI] [PubMed] [Google Scholar]
- [23].Shaw W. Increased urinary excretion of a 3-(3-hydroxyphenyl)-3-hydroxypropionic acid (HPHPA), an abnormal phenylalanine metabolite of Clostridia spp. in the gastrointestinal tract, in urine samples from patients with autism and schizophrenia. Nutr Neurosci. 2010 Jun;13(3):135-43. [DOI] [PubMed]; Shaw W. Increased urinary excretion of a 3-(3-hydroxyphenyl)-3-hydroxypropionic acid (HPHPA), an abnormal phenylalanine metabolite of Clostridia spp. in the gastrointestinal tract, in urine samples from patients with autism and schizophrenia. Nutr Neurosci. 2010 Jun;13(3):135, 43. doi: 10.1179/147683010X12611460763968. [DOI] [PubMed] [Google Scholar]
- [24].Song Y, Liu C, Finegold SM. Real-time PCR quantitation of clostridia in feces of autistic children. Appl Env Microbiol. 2004;70(11):6459–65. [DOI] [PMC free article] [PubMed]; Song Y, Liu C, Finegold SM. Real-time PCR quantitation of clostridia in feces of autistic children. Appl Env Microbiol. 2004;70(11):6459–65. doi: 10.1128/AEM.70.11.6459-6465.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].El-Ansary A, Bhat RS. Targeting gut microbiota as a possible therapeutic intervention in autism. In: Undurti ND, Neophytos P, Tatyana E-K, editor. Autism 360°. San Diego, United States: Academic Press, ch. 17; 2020. p. 301–327.; El-Ansary A, Bhat RS. Undurti ND, Neophytos P, Tatyana E-K. Autism 360°. San Diego, United States: Academic Press; 2020. Targeting gut microbiota as a possible therapeutic intervention in autism; pp. p. 301–327. editor . ch. 17. [Google Scholar]
- [26].Sato K. Why is vitamin B6 effective in alleviating the symptoms of autism? Med Hypotheses. 2018;115:103–106. [DOI] [PubMed]; Sato K. Why is vitamin B6 effective in alleviating the symptoms of autism? Med Hypotheses. 2018;115:103–106. doi: 10.1016/j.mehy.2018.04.007. [DOI] [PubMed] [Google Scholar]
- [27].Cellini B, Montioli R, Oppici E, Astegno A, Voltattorni CB. The chaperone role of the pyridoxal 5′-phosphate and its implications for rare diseases involving B6-dependent enzymes. Clin Biochem. 2014;47(3):158–65. [DOI] [PubMed]; Cellini B, Montioli R, Oppici E, Astegno A, Voltattorni CB. The chaperone role of the pyridoxal 5′-phosphate and its implications for rare diseases involving B6-dependent enzymes. Clin Biochem. 2014;47(3):158–65. doi: 10.1016/j.clinbiochem.2013.11.021. [DOI] [PubMed] [Google Scholar]
- [28].Clayton PT. B6-responsive disorders: a model of vitamin dependency. J Inherit Metab Dis. 2006;29(2–3):317–26. [DOI] [PubMed]; Clayton PT. B6-responsive disorders: a model of vitamin dependency. J Inherit Metab Dis. 2006;29(2–3):317–26. doi: 10.1007/s10545-005-0243-2. [DOI] [PubMed] [Google Scholar]
- [29].Ali A, Cui X, Eyles D. Developmental vitamin D deficiency and autism: Putative pathogenic mechanisms. J Steroid Biochem Mol Biol. 2018;175:108–118. [DOI] [PubMed]; Ali A, Cui X, Eyles D. Developmental vitamin D deficiency and autism: Putative pathogenic mechanisms. J Steroid Biochem Mol Biol. 2018;175:108–118. doi: 10.1016/j.jsbmb.2016.12.018. [DOI] [PubMed] [Google Scholar]
- [30].Altamimi M. Could autism be associated with nutritional status in the palestinian population? The outcomes of the palestinian micronutrient survey. Nutr Metab Insights. 2018;11:1178638818773078. 10.1177/1178638818773078. [DOI] [PMC free article] [PubMed]; Altamimi M. Could autism be associated with nutritional status in the palestinian population? The outcomes of the palestinian micronutrient survey. Nutr Metab Insights. 2018;11:1178638818773078. doi: 10.1177/1178638818773078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Adams JB, Audhya T, McDonough-Means S, Rubin RA, Quig D, Geis E, et al. Effect of a vitamin/mineral supplement on children and adults with autism. BMC Pediatr. 2011;11:111. [DOI] [PMC free article] [PubMed]; Adams JB, Audhya T, McDonough-Means S, Rubin RA, Quig D, Geis E. et al. Effect of a vitamin/mineral supplement on children and adults with autism. BMC Pediatr. 2011;11:111. doi: 10.1186/1471-2431-11-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Littarru GP, Bruge F, Tiano L. Biochemistry of Coenzyme Q10. Antioxid Androl. Cham: Springer; 2017. p. 34–23.; Littarru GP, Bruge F, Tiano L. Antioxid Androl. Cham: Springer; 2017. Biochemistry of Coenzyme Q10; p. p. 34.p. 23. [Google Scholar]
- [33].Cornelius N, Wardman JH, Hargreaves IP, et al. Evidence of oxidative stress and mitochondrial dysfunction in spinocerebellar ataxia type 2 (SCA2) patient fibroblasts: effect of coenzyme Q10 supplementation on these parameters. Mitochondrion. 2017;34:103–114. [DOI] [PubMed]; Cornelius N, Wardman JH, Hargreaves IP. et al. Evidence of oxidative stress and mitochondrial dysfunction in spinocerebellar ataxia type 2 (SCA2) patient fibroblasts: effect of coenzyme Q10 supplementation on these parameters. Mitochondrion. 2017;34:103–114. doi: 10.1016/j.mito.2017.03.001. [DOI] [PubMed] [Google Scholar]
- [34].Elham M, Mohammad-Ali G, Mohammad-Reza A, Sahar SN, Wesam K, Reza A. Mitochondrial dysfunction in autistic children and oral coenzyme Q10 supplementation treatment. Autism Open Access. 2016;6(4):1000189. 10.4172/2165-7890.1000189. [DOI]; Elham M, Mohammad-Ali G, Mohammad-Reza A, Sahar SN, Wesam K, Reza A. Mitochondrial dysfunction in autistic children and oral coenzyme Q10 supplementation treatment. Autism Open Access. 2016;6(4):1000189. doi: 10.4172/2165-7890.1000189. [DOI] [Google Scholar]
- [35].Elham M. Coenzyme-Q10 deficiency and stress oxidative in children with autism spectrum disorders; a poster presentation on the 17th international conference neurology and neuroscience. J Neurol Neurorehabil Res. 2017;2(3):54.; Elham M. Coenzyme-Q10 deficiency and stress oxidative in children with autism spectrum disorders; a poster presentation on the 17th international conference neurology and neuroscience. J Neurol Neurorehabil Res. 2017;2(3):54. [Google Scholar]
- [36].Mousavinejad E, Ghaffari MA, Riahi F, Hajmohammadi M, Tiznobeyk Z, Mousavinejad M. Coenzyme Q10 supplementation reduces oxidative stress and decreases antioxidant enzyme activity in children with autism spectrum disorders. Psychiatry Res. 2018;265:62–9. [DOI] [PubMed]; Mousavinejad E, Ghaffari MA, Riahi F, Hajmohammadi M, Tiznobeyk Z, Mousavinejad M. Coenzyme Q10 supplementation reduces oxidative stress and decreases antioxidant enzyme activity in children with autism spectrum disorders. Psychiatry Res. 2018;265:62–9. doi: 10.1016/j.psychres.2018.03.061. [DOI] [PubMed] [Google Scholar]
- [37].Bhardwaj M, Kumar A. Neuroprotective mechanism of coenzyme Q10 (CoQ10) against PTZ induced kindling and associated cognitive dysfunction: possible role of microglia inhibition. Pharmacol Rep. 2016;68(6):1301–11. [DOI] [PubMed]; Bhardwaj M, Kumar A. Neuroprotective mechanism of coenzyme Q10 (CoQ10) against PTZ induced kindling and associated cognitive dysfunction: possible role of microglia inhibition. Pharmacol Rep. 2016;68(6):1301–11. doi: 10.1016/j.pharep.2016.07.005. [DOI] [PubMed] [Google Scholar]
- [38].Khemakhem AM, Frye RE, El-Ansary A, Al-Ayadhi L, Bacha AB. Novel biomarkers of metabolic dysfunction is autism spectrum disorder: potential for biological diagnostic markers. Metab Brain Dis. 2017;32(6):1–15. [DOI] [PubMed]; Khemakhem AM, Frye RE, El-Ansary A, Al-Ayadhi L, Bacha AB. Novel biomarkers of metabolic dysfunction is autism spectrum disorder: potential for biological diagnostic markers. Metab Brain Dis. 2017;32(6):1–15. doi: 10.1007/s11011-017-0085-2. [DOI] [PubMed] [Google Scholar]
- [39].Rowland I, Gibson G, Heinken A, Scott K, Swann J, Thiele I, et al. Gut microbiota functions: metabolism of nutrients and other food components. Eur J Nutr. 2017;57(1):1–24. [DOI] [PMC free article] [PubMed]; Rowland I, Gibson G, Heinken A, Scott K, Swann J, Thiele I. et al. Gut microbiota functions: metabolism of nutrients and other food components. Eur J Nutr. 2017;57(1):1–24. doi: 10.1007/s00394-017-1445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Wu GD, Chen J, Hoffmann C, Bittinger K, Chen Y-Y, Keilbaugh SA, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105–8. [DOI] [PMC free article] [PubMed]; Wu GD, Chen J, Hoffmann C, Bittinger K, Chen Y-Y, Keilbaugh SA. et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105–8. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Singh RK, Chang HW, Yan D, Lee KM, Ucmak D, Wong K, et al. Influence of diet on the gut microbiome and implications for human health. J Transl Med. 2017;15(1):73. [DOI] [PMC free article] [PubMed]; Singh RK, Chang HW, Yan D, Lee KM, Ucmak D, Wong K. et al. Influence of diet on the gut microbiome and implications for human health. J Transl Med. 2017;15(1):73. doi: 10.1186/s12967-017-1175-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ruiz-Larrea MB, Leal AM, Liza M, Lacort M, de Groot H. Antioxidant effects of estradiol and 2-hydroxyestradiol on iron-induced lipid peroxidation of rat liver microsomes. Steroids. 1994;59(6):383–8. [DOI] [PubMed]; Ruiz-Larrea MB, Leal AM, Liza M, Lacort M, de Groot H. Antioxidant effects of estradiol and 2-hydroxyestradiol on iron-induced lipid peroxidation of rat liver microsomes. Steroids. 1994;59(6):383–8. doi: 10.1016/0039-128x(94)90006-x. [DOI] [PubMed] [Google Scholar]
- [43].Jagota SK, Dani HM. A new colorimetric technique for the estimation of vitamin C using Folin phenol reagent. Anal Biochem. 1982;127(1):178–82. [DOI] [PubMed]; Jagota SK, Dani HM. A new colorimetric technique for the estimation of vitamin C using Folin phenol reagent. Anal Biochem. 1982;127(1):178–82. doi: 10.1016/0003-2697(82)90162-2. [DOI] [PubMed] [Google Scholar]
- [44].Beutler E, Yeh MK. Erythrocyte glutathione reductase. Blood. 1963;21(5):573–85. [PubMed]; Beutler E, Yeh MK. Erythrocyte glutathione reductase. Blood. 1963;21(5):573–85. [PubMed] [Google Scholar]
- [45].Zhichao Z, Xichun P, Saoting L, Ning Z, Yong W, Hua W. Isolation and identification of quercetin degrading bacteria from human fecal microbes. PLOS One. 2014;9(3):e90531. 10.1371/journal.pone.0090531. [DOI] [PMC free article] [PubMed]; Zhichao Z, Xichun P, Saoting L, Ning Z, Yong W, Hua W. Isolation and identification of quercetin degrading bacteria from human fecal microbes. PLOS One. 2014;9(3):e90531. doi: 10.1371/journal.pone.0090531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Feucht S, Ogata B, Lucas B. Nutrition concerns of children with autism spectrum disorders. Nutrition Focus. 2010;25(4):1–13.; Feucht S, Ogata B, Lucas B. Nutrition concerns of children with autism spectrum disorders. Nutrition Focus. 2010;25(4):1–13. [Google Scholar]
- [47].Adams JB, Holloway C. Pilot study of a moderate dose multivitamin/mineral supplement for children with autistic spectrum disorder. J Altern Complem Med. 2004;10:1033–9. [DOI] [PubMed]; Adams JB, Holloway C. Pilot study of a moderate dose multivitamin/mineral supplement for children with autistic spectrum disorder. J Altern Complem Med. 2004;10:1033–9. doi: 10.1089/acm.2004.10.1033. [DOI] [PubMed] [Google Scholar]
- [48].Rossignol DA. Novel and emerging treatments for autism spectrum disorders: a novel systematic review. Ann Clin Psychiatry. 2009;21:213–36. [PubMed]; Rossignol DA. Novel and emerging treatments for autism spectrum disorders: a novel systematic review. Ann Clin Psychiatry. 2009;21:213–36. [PubMed] [Google Scholar]
- [49].Mousain-Bosc M, Roche M, Polge A, Pradal-Prat D, Rapin J, Bali JP. Improvement of neurobehavioral disorders in children supplemented with magnesium-vitamin B6. Magnes Res. 2006;19:46–52. [PubMed]; Mousain-Bosc M, Roche M, Polge A, Pradal-Prat D, Rapin J, Bali JP. Improvement of neurobehavioral disorders in children supplemented with magnesium-vitamin B6. Magnes Res. 2006;19:46–52. [PubMed] [Google Scholar]
- [50].Adams JB. Summary of biomedical treatments for autism. San Diego, CA: ARI Publ. 2007;40.; Adams JB. ARI Publ. San Diego, CA: 2007. Summary of biomedical treatments for autism; p. 40. [Google Scholar]
- [51].Garcion E, Thanh XD, Bled F, Teissier E, Dehouck MP, Rigault F, et al. 1,25-Dihydroxyvitamin D3 regulates gamma 1 transpeptidase activity in rat brain. Neurosci Lett. 1996;216(3):183–6. [DOI] [PubMed]; Garcion E, Thanh XD, Bled F, Teissier E, Dehouck MP, Rigault F. et al. 1,25-Dihydroxyvitamin D3 regulates gamma 1 transpeptidase activity in rat brain. Neurosci Lett. 1996;216(3):183–6. doi: 10.1016/0304-3940(96)87802-5. [DOI] [PubMed] [Google Scholar]
- [52].Garcion E, Wion-Barbot N, Montero-Menei CN, Berger F, Wion D. New clues about vitamin D functions in the nervous system. Trends Endocrinol Metab. 2002;13(3):100–5. [DOI] [PubMed]; Garcion E, Wion-Barbot N, Montero-Menei CN, Berger F, Wion D. New clues about vitamin D functions in the nervous system. Trends Endocrinol Metab. 2002;13(3):100–5. doi: 10.1016/s1043-2760(01)00547-1. [DOI] [PubMed] [Google Scholar]
- [53].Baas D, Prüfer K, Ittel ME, Kuchler-Bopp S, Labourdette G, Sarliève LL, et al. Rat oligodendrocytes express the vitamin D(3) receptor and respond to 1,25-dihydroxyvitamin D(3). Glia. 2000;31(1):59–68. [DOI] [PubMed]; Baas D, Prüfer K, Ittel ME, Kuchler-Bopp S, Labourdette G, Sarliève LL. et al. Rat oligodendrocytes express the vitamin D(3) receptor and respond to 1,25-dihydroxyvitamin D(3) Glia. 2000;31(1):59–68. doi: 10.1002/(sici)1098-1136(200007)31:1<59::aid-glia60>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- [54].Jia F, Shan L, Wang B, Li H, Feng J, Xu Z, Saad K. Fluctuations in clinical symptoms with changes in serum 25 (OH) vitamin D levels in autistic children: three cases report. Nutr Neurosci. 2018;10:1–4. [DOI] [PubMed]; Jia F, Shan L, Wang B, Li H, Feng J, Xu Z, Saad K. Fluctuations in clinical symptoms with changes in serum 25 (OH) vitamin D levels in autistic children: three cases report. Nutr Neurosci. 2018;10:1–4. doi: 10.1080/1028415X.2018.1458421. [DOI] [PubMed] [Google Scholar]
- [55].Mousavinejad E, Ghaffari MA, Riahi F, Hajmohammadi M, Tiznobeyk Z, Mousavinejad M. Coenzyme Q10 supplementation reduces oxidative stress and decreases antioxidant enzyme activity in children with autism spectrum disorders. Psychiat Res. 2018;265:62–9. [DOI] [PubMed]; Mousavinejad E, Ghaffari MA, Riahi F, Hajmohammadi M, Tiznobeyk Z, Mousavinejad M. Coenzyme Q10 supplementation reduces oxidative stress and decreases antioxidant enzyme activity in children with autism spectrum disorders. Psychiat Res. 2018;265:62–9. doi: 10.1016/j.psychres.2018.03.061. [DOI] [PubMed] [Google Scholar]
- [56].Blum JW, Baumrucker CR. Colostral and milk insulin-like growth factors and related substances: mammary gland and neonatal (intestinal and systemic) targets. Domest Anim Endocrinol. 2002;23(1-2):101–10. [DOI] [PubMed]; Blum JW, Baumrucker CR. Colostral and milk insulin-like growth factors and related substances: mammary gland and neonatal (intestinal and systemic) targets. Domest Anim Endocrinol. 2002;23(1-2):101–10. doi: 10.1016/s0739-7240(02)00149-2. [DOI] [PubMed] [Google Scholar]
- [57].Wang X, Michaelis EK. Selective neuronal vulnerability to oxidative stress in the brain. Front Aging Neurosci. 2010;2:12. [DOI] [PMC free article] [PubMed]; Wang X, Michaelis EK. Selective neuronal vulnerability to oxidative stress in the brain. Front Aging Neurosci. 2010;2:12. doi: 10.3389/fnagi.2010.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].El-Ansary A, Cannell JJ, Bjørklund G, Bhat RS, Al Dbass AM, Alfawaz HA, et al. In the search for reliable biomarkers for the early diagnosis of autism spectrum disorder: the role of vitamin D. Metab Brain Dis. 2018;33(3):917–31. [DOI] [PubMed]; El-Ansary A, Cannell JJ, Bjørklund G, Bhat RS, Al Dbass AM, Alfawaz HA. et al. In the search for reliable biomarkers for the early diagnosis of autism spectrum disorder: the role of vitamin D. Metab Brain Dis. 2018;33(3):917–31. doi: 10.1007/s11011-018-0199-1. [DOI] [PubMed] [Google Scholar]
- [59].Parente L. Pros and cons of selective inhibition of cyclooxygenase-2 versus dual lipoxygenase/cyclooxygenase inhibition: is two better than one? J Rheumatol. 2001;28(11):2375–82. [PubMed]; Parente L. Pros and cons of selective inhibition of cyclooxygenase-2 versus dual lipoxygenase/cyclooxygenase inhibition: is two better than one? J Rheumatol. 2001;28(11):2375–82. [PubMed] [Google Scholar]
- [60].Bataveljic D, Milosevic M, Radenovic L, Andjus P. Novel molecular biomarkers at the blood-brain barrier in ALS. Biomed Res Int. 2014;2014:907545. 10.1155/2014/9807545. [DOI] [PMC free article] [PubMed]; Bataveljic D, Milosevic M, Radenovic L, Andjus P. Novel molecular biomarkers at the blood-brain barrier in ALS. Biomed Res Int. 2014;2014:907545. doi: 10.1155/2014/9807545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Kumar H, Sharma B. Memantine ameliorates autistic behavior, biochemistry & blood brain barrier impairments in rats. Brain Res Bull. 2016;124:27–39. [DOI] [PubMed]; Kumar H, Sharma B. Memantine ameliorates autistic behavior, biochemistry & blood brain barrier impairments in rats. Brain Res Bull. 2016;124:27–39. doi: 10.1016/j.brainresbull.2016.03.013. [DOI] [PubMed] [Google Scholar]
- [62].Kumar H, Sharma B. Minocycline ameliorates prenatal valproic acid induced autistic behaviour, biochemistry and blood brain barrier impairments in rats. Brain Res. 2016;1630:83–97. [DOI] [PubMed]; Kumar H, Sharma B. Minocycline ameliorates prenatal valproic acid induced autistic behaviour, biochemistry and blood brain barrier impairments in rats. Brain Res. 2016;1630:83–97. doi: 10.1016/j.brainres.2015.10.052. [DOI] [PubMed] [Google Scholar]
- [63].Goasdoué K, Miller SM, Colditz PB, Björkman ST. Review: the blood-brain barrier; protecting the developing fetal brain. Placenta. 2017;54:111–6. [DOI] [PubMed]; Goasdoué K, Miller SM, Colditz PB, Björkman ST. Review: the blood-brain barrier; protecting the developing fetal brain. Placenta. 2017;54:111–6. doi: 10.1016/j.placenta.2016.12.005. [DOI] [PubMed] [Google Scholar]
- [64].Qasem H, Al-Ayadhi L, Al Dera H, El-Ansary A. Increase of cytosolic phospholipase A2 as hydrolytic enzyme of phospholipids and autism cognitive, social and sensory dysfunction severity. Lipids Health Dis. 2016;16(1):117. [DOI] [PMC free article] [PubMed]; Qasem H, Al-Ayadhi L, Al Dera H, El-Ansary A. Increase of cytosolic phospholipase A2 as hydrolytic enzyme of phospholipids and autism cognitive, social and sensory dysfunction severity. Lipids Health Dis. 2016;16(1):117. doi: 10.1186/s12944-016-0391-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Qasem H, Al-Ayadhi L, Bjørklund G, Chirumbolo S, El-Ansary A. Impaired lipid metabolism markers to assess the risk of neuroinflammation in autism spectrum disorder. Metab Brain Dis. 2018 Aug;33(4):1141–53. [DOI] [PubMed]; Qasem H, Al-Ayadhi L, Bjørklund G, Chirumbolo S, El-Ansary A. Impaired lipid metabolism markers to assess the risk of neuroinflammation in autism spectrum disorder. Metab Brain Dis. 2018 Aug;33(4):1141–53. doi: 10.1007/s11011-018-0206-6. [DOI] [PubMed] [Google Scholar]
- [66].Hill MJ. Intestinal flora and endogenous vitamin synthesis. Eur J Cancer Prev. 1997;(Suppl 6):S43–5. [DOI] [PubMed]; Hill MJ. Intestinal flora and endogenous vitamin synthesis. Eur J Cancer Prev. 1997;(Suppl 6)):S43–5. doi: 10.1097/00008469-199703001-00009. [DOI] [PubMed] [Google Scholar]
- [67].Magnúsdóttir S, Ravcheev D, de Crécy-Lagard V, Thiele I. Systematic genome assessment of B-vitamin biosynthesis suggests co-operation among gut microbes. Front Genet. 2015;6:148. [DOI] [PMC free article] [PubMed]; Magnúsdóttir S, Ravcheev D, de Crécy-Lagard V, Thiele I. Systematic genome assessment of B-vitamin biosynthesis suggests co-operation among gut microbes. Front Genet. 2015;6:148. doi: 10.3389/fgene.2015.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Mora J, Iwata M, von Andrian U. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat Rev Immunol. 2008;8:685–98. [DOI] [PMC free article] [PubMed]; Mora J, Iwata M, von Andrian U. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat Rev Immunol. 2008;8:685–98. doi: 10.1038/nri2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Aranow C. Vitamin D, and the immune system. J Investig Med. 2011;59:881–6. [DOI] [PMC free article] [PubMed]; Aranow C. Vitamin D, and the immune system. J Investig Med. 2011;59:881–6. doi: 10.231/JIM.0b013e31821b8755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Mandal S, Godfrey KM, McDonald D, Treuren WV, Bjørnholt JV, Midtvedt T, et al. Fat and vitamin intakes during pregnancy have stronger relations with a pro-inflammatory maternal microbiota than does carbohydrate intake. Microbiome. 2016;4(1):55. [DOI] [PMC free article] [PubMed]; Mandal S, Godfrey KM, McDonald D, Treuren WV, Bjørnholt JV, Midtvedt T. et al. Fat and vitamin intakes during pregnancy have stronger relations with a pro-inflammatory maternal microbiota than does carbohydrate intake. Microbiome. 2016;4(1):55. doi: 10.1186/s40168-016-0200-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Lithgow JK, Hayhurst EJ, Cohen G, Aharonowitz Y, Simon J. Foster role of a cysteine synthase in Staphylococcus aureus. J Bacteriol. 2004;186(6):1579–90. [DOI] [PMC free article] [PubMed]; Lithgow JK, Hayhurst EJ, Cohen G, Aharonowitz Y, Simon J. Foster role of a cysteine synthase in Staphylococcus aureus. J Bacteriol. 2004;186(6):1579–90. doi: 10.1128/JB.186.6.1579-1590.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Drago L, Toscano M, De Grandi R, Grossi E, Padovani EM, Peroni DG. Microbiota network and mathematic microbe mutualism in colostrum and mature milk collected in two different geographic areas: Italy versus Burundi. ISME J. 2017;11:875–84. [DOI] [PMC free article] [PubMed]; Drago L, Toscano M, De Grandi R, Grossi E, Padovani EM, Peroni DG. Microbiota network and mathematic microbe mutualism in colostrum and mature milk collected in two different geographic areas: Italy versus Burundi. ISME J. 2017;11:875–84. doi: 10.1038/ismej.2016.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Amoudruz P, Holmlund U, Schollin J, Sverremark-Ekström E, Montgomery SM. Maternal country of birth and previous pregnancies are associated with breast milk characteristics. Pediatr Allergy Immunol. 2009;20:19–29. [DOI] [PubMed]; Amoudruz P, Holmlund U, Schollin J, Sverremark-Ekström E, Montgomery SM. Maternal country of birth and previous pregnancies are associated with breast milk characteristics. Pediatr Allergy Immunol. 2009;20:19–29. doi: 10.1111/j.1399-3038.2008.00754.x. [DOI] [PubMed] [Google Scholar]
- [74].Peroni DG, Pescollderungg L, Piacentini GL, Rigotti E, Maselli M, Watschinger K, et al. Immune regulatory cytokines in the milk of lactating women from farming and urban environments. Pediatr Allergy Immunol. 2010;21:977–82. [DOI] [PubMed]; Peroni DG, Pescollderungg L, Piacentini GL, Rigotti E, Maselli M, Watschinger K. et al. Immune regulatory cytokines in the milk of lactating women from farming and urban environments. Pediatr Allergy Immunol. 2010;21:977–82. doi: 10.1111/j.1399-3038.2010.00995.x. [DOI] [PubMed] [Google Scholar]
- [75].Sohn K, Kalanetra KM, Mills DA, Underwood MA. Buccal administration of human colostrum: impact on the oral microbiota of premature infants. J Perinatol. 2016;36:106–11. [DOI] [PubMed]; Sohn K, Kalanetra KM, Mills DA, Underwood MA. Buccal administration of human colostrum: impact on the oral microbiota of premature infants. J Perinatol. 2016;36:106–11. doi: 10.1038/jp.2015.157. [DOI] [PubMed] [Google Scholar]
- [76].Boquest AC, Shahdadfar A, Frønsdal K, et al. Isolation and transcription profiling of purified uncultured human stromal stem cells: alteration of gene expression after in vitro cell culture. Mol Biol Cell. 2005;16(3):1131–41. 10.1091/mbc.e04-10-0949. [DOI] [PMC free article] [PubMed]; Boquest AC, Shahdadfar A, Frønsdal K. et al. Isolation and transcription profiling of purified uncultured human stromal stem cells: alteration of gene expression after in vitro cell culture. Mol Biol Cell. 2005;16(3):1131–41. doi: 10.1091/mbc.e04-10-0949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Alfawaz HA, Bhat RS, Al-Ayadhi L, El-Ansary AK. Protective and restorative potency of Vitamin D on persistent biochemical autistic features induced in propionic acid-intoxicated rat pups. BMC Complement Altern Med. 2014;25(14):416. [DOI] [PMC free article] [PubMed]; Alfawaz HA, Bhat RS, Al-Ayadhi L, El-Ansary AK. Protective and restorative potency of Vitamin D on persistent biochemical autistic features induced in propionic acid-intoxicated rat pups. BMC Complement Altern Med. 2014;25(14):416. doi: 10.1186/1472-6882-14-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].El-Ansary A, Al-Ghamdi M, Bhat RS, Al-Daihan S, Al-Ayadhi L. Potency of pre-post treatment of coenzyme Q10 and melatonin supplement in ameliorating the impaired fatty acid profile in rodent model of autism. Food Nutr Res. 2016;60:28127. [DOI] [PMC free article] [PubMed]; El-Ansary A, Al-Ghamdi M, Bhat RS, Al-Daihan S, Al-Ayadhi L. Potency of pre-post treatment of coenzyme Q10 and melatonin supplement in ameliorating the impaired fatty acid profile in rodent model of autism. Food Nutr Res. 2016;60:28127. doi: 10.3402/fnr.v60.28127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Alfawaz H, Al-Onazi M, Bukhari SI, Binobead M, Othman N, Algahtani N, et al. The Independent and combined effects of omega-3 and vitamin B12 in ameliorating propionic acid induced biochemical features in juvenile rats as rodent model of autism. J Mol Neurosci. 2018;66(3):403–13. [DOI] [PubMed]; Alfawaz H, Al-Onazi M, Bukhari SI, Binobead M, Othman N, Algahtani N. et al. The Independent and combined effects of omega-3 and vitamin B12 in ameliorating propionic acid induced biochemical features in juvenile rats as rodent model of autism. J Mol Neurosci. 2018;66(3):403–13. doi: 10.1007/s12031-018-1186-z. [DOI] [PubMed] [Google Scholar]
- [80].Alfawaz H, Bhat RS, Al-Mutairi M, Alnakhli OM, Al-Dbass A, AlOnazi M, et al. Comparative study on the independent and combined effects of omega-3 and vitamin B12 on phospholipids and phospholipase A2 as phospholipid hydrolyzing enzymes in PPA-treated rats as a model for autistictraits. Lipids Health Dis. 2018;17(1):205. [DOI] [PMC free article] [PubMed]; Alfawaz H, Bhat RS, Al-Mutairi M, Alnakhli OM, Al-Dbass A, AlOnazi M. et al. Comparative study on the independent and combined effects of omega-3 and vitamin B12 on phospholipids and phospholipase A2 as phospholipid hydrolyzing enzymes in PPA-treated rats as a model for autistictraits. Lipids Health Dis. 2018;17(1):205. doi: 10.1186/s12944-018-0850-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].El-Ansary A, Shaker G, Siddiqi NJ, Al-Ayadhi LY. Possible ameliorative effects of antioxidants on propionic acid/clindamycin – induced neurotoxicity in Syrian hamsters. Gut Pathog. 2013 Nov 4;5(1):32. [DOI] [PMC free article] [PubMed]; El-Ansary A, Shaker G, Siddiqi NJ, Al-Ayadhi LY. Possible ameliorative effects of antioxidants on propionic acid/clindamycin – induced neurotoxicity in Syrian hamsters. Gut Pathog. 2013 Nov 4;5(1):32. doi: 10.1186/1757-4749-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].El-Ansary A, Shaker GH, El-Gezeery AR, Al-Ayadhi L. The neurotoxic effect of clindamycin – induced gut bacterial imbalance and orally administered propionic acid on DNA damage assessed by the comet assay: protective potency of carnosine and carnitine. Gut Pathog. 2013 Apr 12;5(1):9. [DOI] [PMC free article] [PubMed]; El-Ansary A, Shaker GH, El-Gezeery AR, Al-Ayadhi L. The neurotoxic effect of clindamycin – induced gut bacterial imbalance and orally administered propionic acid on DNA damage assessed by the comet assay: protective potency of carnosine and carnitine. Gut Pathog. 2013 Apr 12;5(1):9. doi: 10.1186/1757-4749-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].El-Ansary A, Al-Salem HS, Asma A, Al-Dbass A. Glutamate excitotoxicity induced by orally administered propionic acid, a short chain fatty acid can be ameliorated by bee pollen. Lipids Health Dis. 2017 May 22;16(1):96. [DOI] [PMC free article] [PubMed]; El-Ansary A, Al-Salem HS, Asma A, Al-Dbass A. Glutamate excitotoxicity induced by orally administered propionic acid, a short chain fatty acid can be ameliorated by bee pollen. Lipids Health Dis. 2017 May 22;16(1):96. doi: 10.1186/s12944-017-0485-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Aabed K, Bhat RS, Al-Dbass A, Moubayed N, Algahtani N, Merghani NM, et al. Bee pollen and propolis improve neuroinflammation and dysbiosis induced by propionic acid, a short chain fatty acid in a rodent modelof autism. Lipids Health Dis. 2019 Nov 16;18(1):200. [DOI] [PMC free article] [PubMed]; Aabed K, Bhat RS, Al-Dbass A, Moubayed N, Algahtani N, Merghani NM. et al. Bee pollen and propolis improve neuroinflammation and dysbiosis induced by propionic acid, a short chain fatty acid in a rodent modelof autism. Lipids Health Dis. 2019 Nov 16;18(1):200. doi: 10.1186/s12944-019-1150-0. [DOI] [PMC free article] [PubMed] [Google Scholar]