Abstract
Dedifferentiated adipose cells (DAs) and adipose-derived stem cells (ADSCs) are two of the primary types of stem cells derived from adipose tissue, which have been reported to possess similar characteristics, but also exhibit unique phenotypic and functional advantages. However, several reports have described inconsistent results regarding their differences in multilineage differentiation function. Moreover, to the best of our knowledge, there are no studies assessing their myogenic ability, or the differences in the transcriptome between the two cell types derived from lipoaspirates via tumescent liposuction from the same donors. The aim of the present study was to compare the properties and expression profiles of these cell types. Subcutaneous adipose tissue of three female patients (aged 23–30 years) with a physiological BMI (19.1–23.9 kg/m2) were obtained during tumescent liposuction of the abdomen or the thigh. The stromal vascular fraction and mature adipocytes were obtained via collagenase digestion, and ADSCs and DAs were cultured successively. To determine the differences between DAs and ADSCs after 6–7 passages, cell proliferation assays, phenotypic assessment, differentiation assays and high-throughput RNA sequencing (seq) were used. Similar cell morphologies, proliferation dynamics, surface markers and transcriptome expression profiles were observed between the DAs and ADSCs. Whilst there were notable individual differences in the osteogenic, lipogenic, chondrogenic and myogenic abilities of the DAs and ADSCs, it was difficult to determine their differentiation potential based only on the cell source. Interestingly, the myogenic ability was relatively stronger in cells with relatively weaker lipogenic ability. Only 186 differentially expressed genes between the two groups were identified using RNAseq. Several of these genes were involved in biological functions such as transcription regulation, protein translation regulation, cytokine interactions and energy metabolism regulation. The results of the present study suggested a similar functional potential of DAs and ADSCs from young donors undergoing tumescent liposuction operation in regeneration areas and the balance of the differentiative ability of the same cell populations. These data may provide a foundation for further clinical administration of stem cells derived from adipose tissues in therapy.
Keywords: dedifferentiated adipose cells, adipose-derived stem cells, differentiation, mRNA expression profiles, tumescent liposuction
Introduction
Tumescent liposuction is a commonly used and matured technique for plastic and aesthetic surgery in body contouring and fat grafting (1). Thus, it is relatively easy and safe to obtain a large quantity of abandoned fat tissues in the clinical practice. In heathy young individuals with a medium BMI, due to the improved elasticity of their skin, they are more suited for body shaping and fat filling via liposuction (1). As adipose tissue is difficult to preserve for long periods of time, traditionally, the redundant adipose tissue is discarded (2). However, these discarded tissues contain a substantial quantity of stem cells that may be used for tissue repair and regeneration via autologous transplantation, especially when the donors get older (3). Elderly patients are the primary target population that experience various diseases, and additionally, several studies have reported that the viability and differentiation capacities of adipose-derived stem cells (ADSCs) decrease significantly with age (3–6). In the present study, the adipose tissue of young healthy individuals undergoing tumescent liposuction was used in order to provide an experimental basis for clinical transformation of stem cells.
In adipose tissue, ADSCs and dedifferentiated adipose cells (DAs) are the two major stem cell groups that can be isolated, cultured and amplified in vitro (7). Since Zuk et al (8) reported their potential in 2001, ADSCs have been widely used in experimental and clinical research (9,10). At present, collagenase digestion combined with centrifugation is used to separate the stromal vascular fraction (SVF) from adipose tissues. SVF consists of multiple cell groups with a complex of cell components, primarily including a certain number of ADSCs, fibroblasts, endothelial cells, vascular smooth muscle cells and macrophages (11). As the endothelial cells in SVF disappear relatively quickly following subculturing, ADSCs are the main types of cell that can be successfully maintained. Heo et al (12) revealed that ADSCs and bone marrow-derived mesenchymal stem cells exhibited similar proliferation rates, clonal formation rates, immunophenotypes and differentiation potentials in vitro. Although ADSCs are of mesodermal origin, they can also be differentiated under the correct conditions into cells of ectodermal or endodermal origin (13). Kornicka et al (4) reported that the growth kinetics of ADSCs were positively correlated with donor age. The number of both apoptotic and senescent cells increases with age. While the osteogenic differentiation potential of ADSCs decreases with donor age, the adipogenic differentiation potential appears to remain constant throughout the entire ageing process (4). Compared with seeking efficient biotechnological solutions that may rejuvenate ADSCs in vitro, isolation and storage of an individual's stem cells from lipoaspirates when the donors are still young and healthy may be an alternative option.
In 1986, Sugihara et al (14) reported that mature adipocytes (MAs) isolated from fat tissue can be dedifferentiated into fibroblast-like cells using an in vitro dedifferentiation strategy, known as ‘ceiling culture’. DAs have been found to possess similar functions as ADSCs, but also have certain advantages. DAs are highly homogeneous cell populations (high purity), as well as possess a multilineage potential for differentiation into various cell types under appropriate inducing conditions in vitro and in vivo (15). Kishimoto et al (16) cultured DAs and ADSCs from human buccal fat pad, and found that DAs showed increased osteoblastic differentiation capacity compared with ADSCs. Watson et al (17) also reported an increased ability of DAs to redifferentiate and transdifferentiate into adipocytes and osteoblasts compared with ADSCs in an obese diabetic donor. However, comparisons between these two types of cells have not resulted in consistent results. For example, Saler et al (18) reported that DAs responded more favorably to the addition of the adipogenic medium compared with ADSCs. While the osteoblastic differentiation capacity of DAs and ADSCs seem to be similar, there were small differences in the induction time. Additionally, ADSCs have been reported to produce greater amounts of acidic mucopolysaccharides compared with DAs during chondrogenic differentiation; however, in this study, donors were aged 60–70 years old with a BMI of 22.5–26.5 kg/m2 (18). Thus, these inconsistent results may be associated with the age of the donors, BMI, site and isolation and culture methods, amongst other factors (19).
Together, the current body of literature suggests that the characteristics and potential of ADSCs and DAs are similar with small differences. However, to the best of our knowledge, there are no studies comparing these two cell types when obtained from the same young individual following tumescent liposuction, and in particular with regards to their potential use in tissue engineering and differential gene expression. Moreover, which type of cell is more suited for application in transplantation to regenerate fat, bone, cartilage and muscle remains unknown. To further improve the understanding of the characteristics of stem cells from adipose tissue sources, and to determine the most appropriate use of these tissue resources, three pairs of human DAs and ADSCs derived from the same donors were comprehensively compared to further examine the mechanisms of tissue transformation. In addition, the present study aimed to provide an experimental basis for the use of these stem cells in regenerative medicine, and identify the best use case for each type.
Materials and methods
Ethical approval and sample collection
The present study was approved by and performed in accordance with the guidelines and study protocols of the Peking University Third Hospital Medical Science Research Ethics Committee (approval no. 2014020). Samples of subcutaneous adipose tissue (~20 ml from each person) were collected from three females with written informed consent during liposuction surgery performed at Peking University Third Hospital between January and May 2018. The procedure of tumescent liposuction was as follows: A large amount of tumescent solution containing adrenaline, lidocaine, sodium bicarbonate and normal saline was rapidly injected into the subcutaneous fat tissue of the liposuction site. Adipose tissues were then sucked out with tissue fluid into a sterile container using negative pressure suction. None of the patients had diabetes or other severe systemic illness. The characteristics of the subjects are presented in Table I.
Table I.
Characteristics of patients enrolled in this study.
| Patient no. | Age, years | BMI | Biopsy site |
|---|---|---|---|
| 1 | 30 | 23.9 | Abdomen |
| 2 | 24 | 20.4 | Thigh |
| 3 | 23 | 19.1 | Thigh |
Isolation and culture of DAs and ADSCs
In total, ~20 ml granular adipose tissue without visible blood vessels and connective tissue per person was centrifuged with PBS once with 250 × g at room temperature (RT) for 5 min. Then, 1.67X volume 0.14% collagenase I (w/v in DMEM hyperglycemia medium) was added to the granular adipose tissue and shaken at 37°C with 120 rpm at RT for 50 min. After digestion, loose connective tissue was further discarded via filtration through a 425-µm mesh (Beijing Solarbio Science & Technology Co., Ltd.) and centrifugated with 250 × g at RT for 5 min, the floating uppermost layer (containing MAs), as well as the sedimentation at the bottom of the centrifuge tube (containing SVFs) were collected. ADSCs were extracted from SVFs obtained above and amplified through conventional adhesive culture methods (20) in complete culture medium to obtain ADSCs (Cyagen Biosciences, Inc.).
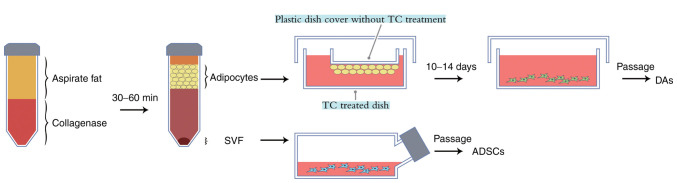
Preparation of DAs was based on a modified procedure based on previous studies (8,15). Briefly, MAs were placed in a tissue culture (TC) treated dish and covered with a plastic dish cover without TC treatment, such that floating fat cells could attach to it (Fig. 1). A small quantity of DMEM/F12 medium (HyClone; Cytiva) supplemented with 10% FBS (Biological Industries) was added to the dish. The medium was changed every 3–5 days to prevent drying up and a plastic tube was used to prevent the upper cover drifting into the medium. After 8–14 days, multiple DAs could be seen growing on the bottom surface of the TC treated dish. Amplification of DAs was performed using the same culture medium and method as ADSCs. A total of 6–7 passages of cells was used as this resulted in a homogeneous culture of both cell types.
Figure 1.
Schematic diagram of the one-step method used for isolation and culture of ADSCs and DAs from adipose tissues. Following collagenase digestion and centrifugation the fat cell layer was transferred a TC-treated dish and a TC untreated dish cover was placed in it, so that the floating fat cells could attach. After 10–14 days, spindle cells grew on the bottom dish treated with TC, which were then passaged regularly to acquire the DAs, and ADSCs were obtained via the conventional culture of SVF. TC, tissue culture; ADSCs, adipose-derived stem cells; DAs, dedifferentiated adipose cells; SVF, stromal vascular fraction.
Cell proliferation assay
A Cell Counting Kit-8 assay (Dojindo Molecular Technologies, Inc.) was used to assess cell proliferation according to the manufacturer's instructions. Cells were trypsinized and seeded in 96-well tissue culture plates (1,500 cells/well). After 4, 24, 48, 72, 96, 120 or 144 h, CCK-8 solution (10 µl/well) was added to the cells and incubated at 37°C for 1.5 h. The absorbance at 450 nm was measured using a microplate reader. The optical density (OD) values represented the survival/proliferation of cells.
Immunophenotypic assessment using flow cytometry
DAs and ADSCs from the same samples were digested when they reached 80% confluence and were resuspended in 90 µl PBS (1×105 cells per sample). Then, cell aliquots were incubated with primary antibodies (1:10) in the dark for 15–20 min at RT. Subsequently, the cell suspension was centrifuged at 300 × g for 5 min at RT, followed by removal of the supernatant and resuspension of the sediment in 200 µl PBS. The following antibodies were used at 1:20 dilution: Anti-CD90 (cat. no. E-AB-F1167D), anti-CD44 (cat. no. E-AB-F1038D), anti-CD31 (cat. no. E-AB-F1050C), anti-CD34 (cat. no. E-AB-F1143C), anti- CD45 (cat. no. E-AB-F1137C) and their isotype controls (Elabscience, Inc.), anti-CD105 (cat. no. 12-1057-41) and its isotype control (eBioscience, Inc.). Positive cells were counted and compared with the signal of the corresponding immunoglobulin isotypes. Samples were analyzed on a BD FACS Aria II (BD Biosciences) and data were analyzed using FlowJo version 10.0 (FlowJo, LLC).
Multi-lineage differentiation assay
Differentiation assays for adipocytes, osteoblasts, chondrocytes and skeletal muscle cells (SkMCs) were performed on the three pairs of stem cells.
Assays for adipogenic differentiation potential were performed according to the manufacturer's protocol (Cyagen Biosciences, Inc.). Briefly, 4.5×104 cells were seeded into 6-well plates. When the cells reached 80% confluence, the medium was replaced with adipogenic differentiation medium A. After using medium A for 3 days, the medium was replaced with medium B for 24 h. After using medium A and B alternately three times (12 days of culture), cells were stained with Oil Red O as follows: Cells were fixed with 4% paraformaldehyde at RT for 20 min and then dyed with Oil Red O for 15 min at RT. Induced fat cells contained orange-red oil droplets. After washing with distilled water, the cells were observed under a light microscope with magnification ×100. Then, 2 ml isopropanol was added to each well for 1 h at RT. When the Oil Red O was dissolved in the isopropanol solution, the absorbance was detected on the spectrophotometer at 500 nm. Then, total proteins in each well were extracted with a RIPA lysis buffer (Applygen Technologies Inc.) and quantified using a bicinchoninic acid protein assay kit (CW Biosciences).
For osteogenic differentiation assays, 4.5×104 cells were seeded into 6-well plates. When the cells reached 60% confluence, the medium was replaced with osteogenic differentiation medium (Cyagen Biosciences, Inc.). Osteogenic media were changed every 3 days. After 12 days of culture, Alizarin red S staining was performed. Cells were fixed with 70% ethanol at RT for 45 min, and then stained with Alizarin red S for 20 min at RT. After washing the floating color with distilled water, the cells were visualized under a light microscope with magnification of 100 times. Then, 10% cetylpyridinium chloride (w/v in distilled water) was added into each well to wash the dye for 1 h at RT. The OD value was measured at 550 nm, and the protein in each well was extracted and quantified.
For chondrogenic differentiation, 2.5×105 cells per pellet in 15-cm3 conical tubes were used. Cells were maintained at 37°C with 5% CO2 in the chondrogenic differentiation medium (Cyagen Biosciences, Inc.) for 25 days. The pellets were fixed with 4% paraformaldehyde at RT for 24 h, then underwent dehydration, embedding, sectioning at 5 µm, staining with alcian blue at RT for 30 min and observed under a light microscope magnification ×40.
For SkMC differentiation, cells were seeded into 24-well plates. When confluence reached 90–100%, cells were incubated with myogenic induction solution (DMEM/F12 supplemented with 10% FBS, 5% equine serum (Gibco; Thermo Fisher Scientific, Inc.), 0.1 µmol/l dexamethasone and 50 µmol/l hydrocortisone) for 10 days. Then, cells were fixed in 4% paraformaldehyde at RT for 15 min, permeabilized in 0.2% Triton X-100, blocked with 10% normal goat serum (Beyotime Institute of Biotechnology) for 20 min at RT. Then incubated with a rabbit anti-desmin antibody (1:50; cat. no. ab32362; Abcam) overnight at 4°C followed by Cy3 goat anti-rabbit IgG antibody (1:500; Beyotime Institute of Biotechnology). After staining the nuclei with DAPI for 3 min at RT, the samples were examined using a High Content Imaging system (Operetta CLS; PerkinElmer, Inc.).
Comparison of the gene expression profiles between DAs and ADSCs using RNA sequencing (seq)
Cell samples were harvested using trypsin digestion. The total RNA was extracted using TRIzol® (cat. no. 15596026; Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions. RNA degradation and contamination was monitored on 1% agarose gels. RNA purity was checked using the NanoPhotometer® spectrophotometer (Implen GmbH). RNAseq was performed by BerryGenomics company. Briefly, sequencing libraries were generated using NEBNext® Ultra™ RNA Library Prep Kit for Illumina® (NEB) following manufacturer's recommendations and index codes were added to attribute sequences to each sample. The library preparations were sequenced on an Illumina NovaSeq platform and 150 bp paired-end reads were generated. After removing reads containing poly-N, adapters and low-quality reads from the raw data, clean high-quality data obtained were used for the subsequent analysis. EdgeR package (version 3.3.3) was used for the differential expression analysis (21). A |log2(fold change)|>1.00 and P<0.05 were used as criteria to classify differentially expressed genes (DEGs). Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis were used in the Database for Annotation, Visualization and Integrated Discovery platform (https://david.ncifcrf.gov/). The data obtained have been deposited in the Gene Expression Omnibus archive (accession no. GSE141708).
Statistical analysis
Data are presented as the mean ± standard deviation. Statistical analysis was performed using SPSS version 16.0 (SPSS, Inc.). A two-sided t-test was used for intergroup comparisons. P<0.05 was considered to indicate a statistically significant difference.
Results
Isolation and morphological characteristics of ADSCs and DAs
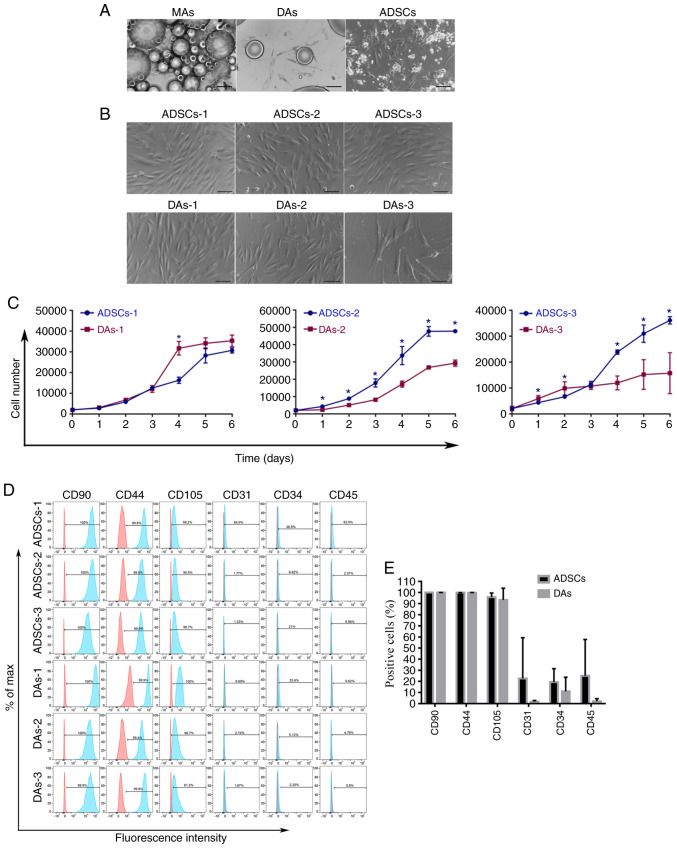
ADSCs and MAs were extracted from the same depot site. Primary ADSCs adhered to the plate after 24–48 h, after which the culture medium was changed and cells were passaged regularly. Most primary DAs, which exhibited a spindle shape, adhered to the bottom of the plates after 7–14 days. During digestion, it was found that the primary DAs adhered to the dish firmly and thus required longer digestion periods, then cells were passaged regularly the same as ADSCs. Fig. 2A presents the newly inoculated MAs, the primary DAs and the primary ADSCs. Fig. 2B demonstrates cells after passages 6 or 7. Both cell types were spindle-shaped, and DAs-3 were significantly larger compared with the other cells.
Figure 2.
Basic characteristics of ADSCs and DAs. (A) Newly inoculated adipocytes, primary DAs, and primary ADSCs are shown successively from left to right. Scale bar, 200 µm. (B) ADSCs and DAs of 6–7 passages had similar spindle shapes without lipid droplets. Scale bar, 100 µm. (C) Cell proliferation curves showed notably individual differences in growth speed, while no definite patterns of growth speed were observed between the two types of cells. *P<0.05. (D) FACS results of three pairs of cells. The red histogram represents isotype control, and the blue histograms represents the detected markers. (E) Summary of the immunophenotype of six cell types demonstrated no significant differences in the expression of the six markers between the two groups. MA, mature adipocytes; ADSCs, adipose-derived stem cells; DAs, dedifferentiated adipose cells; FACS, fluorescent activated cell sorting.
Cell proliferation curves and characterization of surface markers. The growth curves of each cell group are presented in Fig. 2C. There were notable individual differences in the growth speed of the cells. There was no definite trend in growth speed between the two types of cells from the same individual source, DAs-1 grew faster compared with ADSCs-1, ADSCs-2 grew faster than DAs-2 while ADSCs-3 grew at first slower and then faster compared with DAs-3. In general, compared with the first and the second pair of cells, ADSCs-3 and DAs-3 proliferated slowly, and the growth curve of ADSCs-3 exhibited no obvious downward trend at the detection point of 6 days, although the initial cell density was the same in all cells.
FACS results are shown in Fig. 2D and Table II. The intensity and percentage of CD90, CD44 and CD105 expressing cells were similar. Although there were markedly higher percentages of CD31, CD34 and CD45 expressing cells in ADSCs compared with DAs, the intensity of expression was low. There were no significant differences in the expression of the six markers between the two groups (Fig. 2E).
Table II.
Comparison of the surface antigens of DAs and ADSCs from three patients.
| Antigen | ADSCsa | DAsa |
|---|---|---|
| CD90 | 100±0% | 99.97±0.06% |
| CD44 | 99.83±0.06% | 99.73±0.29% |
| CD105 | 95.8±3.76% | 93.3±10.50% |
| CD31 | 22.63±36.61% | 1.77±1.03% |
| CD34 | 19.37±12.02% | 11.02±12.70% |
| CD45 | 24.94±32.75% | 2.06±2.34% |
Data are presented as the mean percentage of cells expressing each antigen. ADSCs, adipose-derived stem cells; DAs, dedifferentiated adipose cells.
Multilineage differentiation ability
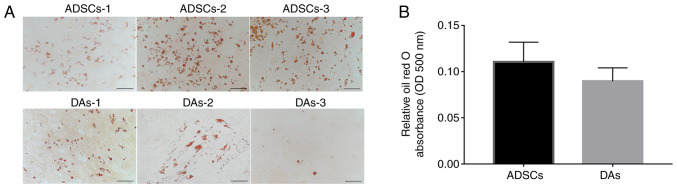
With regards to lipogenic differentiation, 12 days after induction, each case of cells showed notable lipid droplet formation (Fig. 3A). The average ratio of OD500 per µg protein indicated that ADSCs (0.11±0.02) accumulated relatively more dye compared with the DAs (0.09±0.01), but the difference was not significant (Fig. 3B).
Figure 3.
Adipogenic capacity of ADSCs and DAs. (A) Morphologies of formed lipid droplets were identified using staining with Oil Red O solution. Scale bar, 100 µm. (B) Semi-quantitative analysis using an absorbance wavelength of 500 nm per µg protein. Data are presented as the mean ± SD. ADSCs, adipose-derived stem cells; DAs, dedifferentiated adipose cells; OD, optical density.
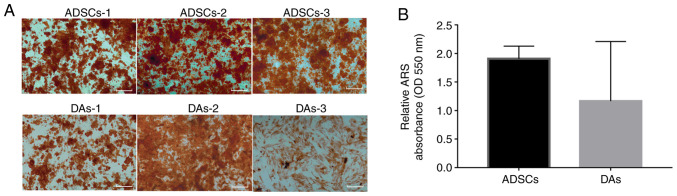
Osteogenic differentiation examination revealed that 12 days after incubation in osteogenic-inducing medium, calcium nodules could be seen in each case of cells and mineralization of the cells was confirmed via Alizarin Red S staining (Fig. 4A). The average ratio of OD550 per µg protein indicated that ADSCs (1.91±0.22) accumulated more dye compared with the DAs (1.16±1.04), but the difference was not significant (Fig. 4B).
Figure 4.
Osteogenic capacity of ADSCs and DAs. (A) Morphologies of cells stained using ARS. Scale bar, 500 µm. (B) Semi-quantitative analysis using an absorbance wavelength of 550 nm per µg protein. Data are presented as the mean ± SD. ADSCs, adipose-derived stem cells; DAs, dedifferentiated adipose cells; OD, optical density; ARS, Alizarin red S.
With regards to chondrogenic differentiation, only 3/6 cases (Fig. 5) formed typical cell spheres when harvested, which were also stained with alcian blue. In the other three cases, only scattered small particles were formed, which were too small to be further stained and identified (ADSCs1, ADSCs2 and DAs3).
Figure 5.
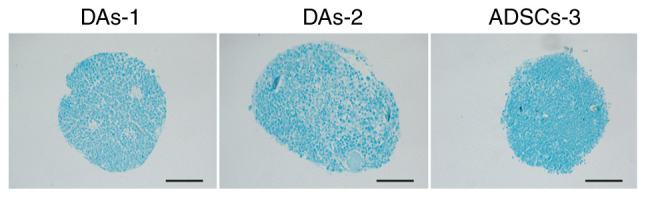
Chondrogenic differentiation. Morphologies of cells stained using alcian blue. Scale bar, 1,000 µm. ADSCs, adipose-derived stem cells; DAs, dedifferentiated adipose cells.
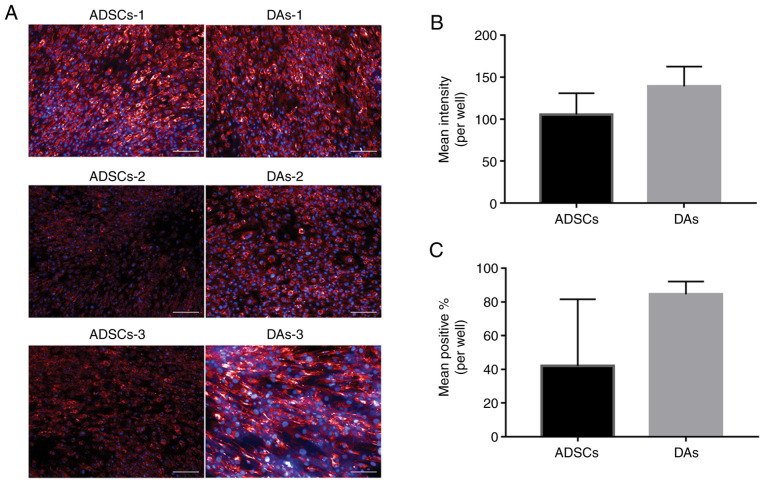
Myogenic differentiation results are presented in Fig. 6. The expression of desmin positive cells following myogenic induction was measured, and the differences of positive signal intensity of each cell line were notable (Fig. 6A). In terms of the average values, ADSCs (105.46±25.49) showed fewer total signals compared with the DAs (139.12±23.57), and the percentage of positively stained cells also exhibited the same trend in ADSCs (ADSCs, 41.95±39.65%; DAs, 84.52±7.57%), but there were no significant differences between the groups (Fig. 6B and C). Interestingly, the myogenic ability was relatively stronger in four cases (ADSCs-1, DAs-1, DAs-2 and DAs3), which exhibited a relatively weaker lipogenic ability compared with the other two cases (ADSCs-1 and ADSCs-2).
Figure 6.
Myogenic capacity of ADSCs and DAs. (A) Desmin immunofluorescence staining was used to assess myogenic differentiation. The cytoplasm of the positive cells were dyed red, and the blue signals represent DAPI-counterstained nuclei. Scale bar, 200 µm. (B) Semi-quantitative analysis showing the mean intensity per well of positive signals in the cytoplasm. (C) Semi-quantitative analysis showing the mean percentage of positive cells. Data are presented as the mean ± SD. ADSCs, adipose-derived stem cells; DAs, dedifferentiated adipose cells.
Comparison of gene expression profiles between DAs and ADSCs
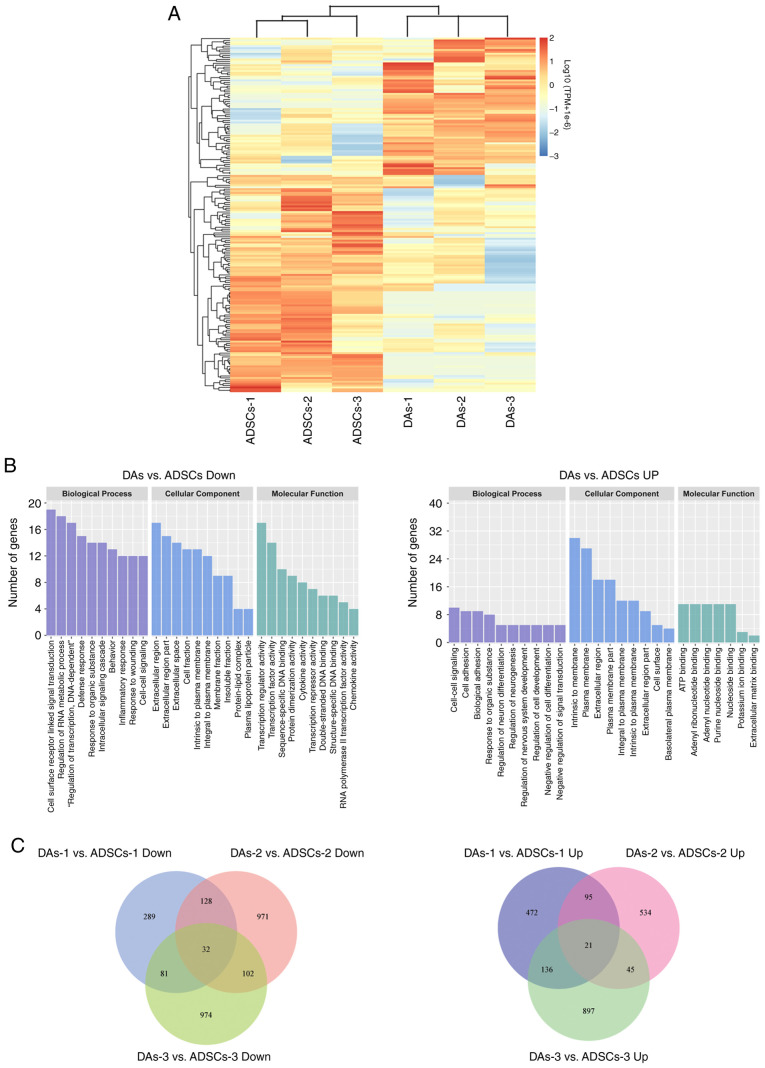
To further compare the gene expression profiles of DAs and ADSCs, the current study adopted second-generation seq technology to detect the three pairs of cells. P<0.05 and |log2(fold change)|>1.00 were used as the criteria to screen the DEGs between groups and between each pair of samples. Only 186 DEGs were identified between the two groups, with 112 downregulated and 74 upregulated in DAs vs. ADSCs (Fig. 7A).
Figure 7.
Diagrams summarizing the DEGs in ADSCs and DAs. (A) Heatmap of 186 DEGs. The colors in the heat map represent gene expression levels [log10(TPM+1×10−6)]. Red represents higher expression; blue represents lower expression. The brackets represent cluster analysis. (B) GO enrichment bar plots. The clusters with the largest number of DEGs were shown as two histograms (only top 10 if >10 clusters). (C) Venn diagrams summarizing the DEGs in each pair of ADSCs and DAs. DEG, differentially expressed gene; GO, gene ontology; ADSCs, adipose-derived stem cell; DAs, dedifferentiated adipose cell.
Based on GO and KEGG analysis, the top 10 clusters containing the highest number of gene counts were plotted (Fig. 7B). In the downregulated DEGs in DAs vs. ADSCs, the most abundant GO-biological processes were ‘cell surface receptor linked signal transmission’, ‘regulation of RNA metabolic process’ and ‘regulation of transcription, DNA dependent’. The most abundant GO-cellular component was ‘extracellular region’. The most enriched GO-molecular function was ‘primarily transcription regulator activity’, ‘transcription factor activity’ and ‘sequence specific DNA binding’. ‘Cytokine-cytokine receptor interactions’ was the most abundant KEGG pathway, with a total of eight genes: C-C motif chemokine ligand 3 (CCL3), IL6, IL7, C-C motif chemokine receptor 1 (CCR1), C-X-C motif chemokine ligand 2 (CXCL2), CX3CL1, CCL5 and TNF superfamily member 18.
In the upregulated DEGs in DAs, the most abundant GO-biological process was primarily ‘cell-cell signaling’, ‘cell adhesion’ and ‘biological adhesion’. The most enriched GO-cellular component was primarily ‘intrinsic to membrane’, ‘plasma membrane’ and ‘extracellular region’, while the most enriched GO-molecular function was primarily ‘ATP binding’. The most abundant KEGG pathway was ‘pathways in cancer’, with only six genes: Fibroblast growth factor 18 (FGF18), WNT16, integrin subunit α 6, MET, hedgehog interacting protein (HHIP) and MDS1 and EVI1 complex locus (MECOM).
When comparing the data of each pair of cells, several DEGs were identified, but the number of common DEGs across all three cases was low. Venn diagrams (Fig. 7C) showing the numbers of downregulated or upregulated DEGs in DAs was 32 (FOSB, CCND2, DUSP1, RGS16, CXCL2, HES4, FOS, ATF3, ZFP36, DUSP8, GALNT13, RASD1, ITIH5, F11R, FGD5, FZD3, BCL2A1, CCR1, G0S2, FAM84B, KLF4, CCDC102B, GADD45G, ALDH8A1, CX3CL1, CCR7, USP2, HTR2B, ZNF853, CCL11, TDRD1 and TULP2) and 21 (FGF18, IGF2BP1, SLC38A4, SCN9A, ELOVL2, LYPD1, ALDH1A1, HHIP, NEFM, PLD5, MYH1, RIMS1, IL7R, MECOM, ST6GALNAC3, NOXO1, ADRA2C, NLGN1, CAMK4, COL6A6 and GREB1L), respectively.
According to the RNAseq results (GEO database, accession number GSE141708), the relative mRNA expression levels of perilipin 1, peroxisome proliferator activated receptor γ, C/EBPA (CCAAT enhancer binding protein α) and fatty acid binding protein 4 involved in fat metabolism or RUNX family transcription factor 2 (RUNX2), SP7, activating transcription factor 4 (ATF4) and bone γ-carboxyglutamate protein (BGLAP) involved in osteogenic regulation showed only individual differences among the three pairs of samples in this experiment, but no significant relationship with adipogenic or osteogenic differences. The expression of bone morphogenetic protein 6 (BMP6) in DAs-1, DAs-2 and ADSCs-3 with relatively stronger chondrogenic abilities was significantly higher compared with the corresponding ADSCs-1, ADSCs-2 and DAs-3.
Discussion
In young adults, adipose tissues can be easily obtained via safe and minimally invasive tumescent liposuction. However, as individuals age, the likelihood of suffering from a disease where stem cell therapy is required, such as osteoarthritis, refractory wounds, various tissue defects and other systemic diseases, increases (1,4). ADSCs and DAs are the primary types of stem cells derived from SVF and MAs, respectively, in adipose tissues, and they possess a wide range of therapeutic potential in the field of regenerative medicine. However, aging may attenuate their regenerative potential and metabolic functions (3–6,22). The purpose of the present study was to provide a reference for future autotransplantation by studying the differentiation and gene expression differences of these two types of cells from the same young donors. In the present study, a modified DA culture method was adopted. ADSCs and DAs from the same individual were obtained using a one-step method, and 6–7 generations of cells were used to compare their characteristics. The results indicated that there were notable individual differences in the multilineage differentiation abilities for DAs and ADSCs, and this was not directly determined by the source of the cells. For the same individuals, DAs and ADSCs had their own advantages for different applications, including osteogenic, lipogenic, chondrogenic or myogenic repair and regeneration, and future stem cell autotransplantation should be a personalized treatment. Thus, this study may improve the understanding of the clinical therapeutic potential of adipose tissues.
ADSCs are defined as mesenchymal cells within adipose tissue with multipotent differentiation and self-renewal capacity (23). Since the initial discovery of ADSCs, their molecular profiles has been the subject of debate. This has been primarily due to the use of different ADSC purification and culture protocols, as well as the differing use of sub-total vs. whole SVF (24). To date no markers have been reported to be exclusively expressed in ADSCs (25). In general, the culture of SVF cells on plastic surfaces yields an adherent subpopulation of ADSCs. SVF separated from adipose tissues with collagenase digestion contains a variety of cell types, including B and T lymphocytes, endothelial cells, fibroblasts, macrophages, pericytes and preadipocytes (25). ADSCs are a relatively homogenous population lacking hematopoietic lineage markers after culturing and expanding (26). In fact, the therapeutic efficacy of ADSCs and SVF are very similar when used for autotransplantation in various disease conditions, such as orthopedic, inflammatory, degenerative tissue or organ and autoimmune diseases, in a clinical trial (26). Our previous study revealed that ADSCs and SVF significantly improved the wound healing processes, and found that both SVF and ADSCs improved the function of endotheliocytes and fibroblasts, regulated gene expression and jointly promoted skin healing. However, there were no significant differences in the effect or mechanisms between SVF and ADSCs (20). This may be due to the fact that ADSCs are the primary biological functional cell type in SVF. According to the minimum criteria for identifying MSCs described by the International Federation of Adipose Therapeutics and Sciences and the International Society for Cell Therapy, ADSCs express high levels of CD90, CD105 and CD44, whilst remaining negative for CD31 (endothelial marker), CD34 (a well-known stem cell marker for both hematopoietic and endothelial lineages) and CD45 (known as a leukocyte common antigen) (27). In the present study, all cells expressed high levels of CD90, CD44 and CD105, while the intensity and individual differences in the percentage of cells expressing CD31, CD34 and CD45 were notably lower. Moreover, although ADSCs-1 contained a high proportion of CD31, CD34 and CD45 expressing cells, the expression intensity was too weak, and there was no significant difference in the differentiation function between the ADSCs-1 and other cases of cells. These findings suggested that ADSCs mixed with a small number of hematopoietic and endothelial lineages did not significantly affect their differentiation abilities.
MAs are another abundant cell group present in adipose tissues. When MAs are cultured in vitro, due to the state of ischemia and hypoxia, they gradually remove lipid droplets and changes in their morphology are visible; becoming spindle cells without lipid droplets and fibroblast-like (15). In the present study, these cells were referred to as DAs. The mechanism of adipocyte dedifferentiation has not been fully elucidated. Jumabay and Bostrom (15) suggested that there are two means of dedifferentiation for MAs: By removing lipid droplets or asymmetric division to form offspring adipocytes and adipose free fibroblast-like cells. Maurizi et al (28) also showed that the dedifferentiation of adipocytes was not due to gradual lipolysis, but instead due to the secretion and excretion of lipid droplets. These authors suggested that ceiling culture, as a microenvironment stimulus, could induce human adipocytes to reprogram and secrete lipid droplets in large quantities to obtain new phenotypes adapted to the novel environment. In the present study, two simple cell culture materials, non-TC treated and TC treated were used, creating a similar niche of hypoxia and low nutrition to the ceiling culture method, and thus obtained DAs simply and successfully. Furthermore, it was demonstrated that lipid droplets appeared to be secreted from the surrounding fibroblast-like cells.
According to previous studies, comparisons of the differentiation abilities between DAs and ADSCs are not exactly the same. For instance, it has been reported that the lipogenic, osteogenic and chondrogenic abilities of DAs are higher compared with those of ADSCs (16,17,29), and that the osteoblastic differentiation capacity of DAs and ADSCs appear to be similar, while the chondrogenic differentiation capacity of DAs seems to be weaker (18). It was hypothesized that these differences may be associated with the age, BMI, source of donors, cell generations, culture medium and the detection time points of differentiation induction. To the best of our knowledge, no SkM differentiation ability comparisons have been previously reported between DAs and ADSCs.
For the first time, two types of stem cells obtained from liposuction were compared in healthy young women in the present study. Interestingly, with the decrease of the donors' BMI, the proliferation activity of the corresponding DAs decreased compared with ADSCs. This tendency was consistent with clinical experience, that is, the survival rate of fat transplantation in obese patients is relatively higher compared with lean individuals, which may be associated with the higher activity of MAs, although the specific mechanism is yet to be elucidated (30). Due to the limited number of cases in the present study, this result may also be caused by factors such as different donor site locations, thus further experiments are required to clarify this hypothesis.
In plastic surgery, several clinical applications have been suggested regarding ADSCs. In addition to promoting fat graft survival, preclinical and clinical studies have demonstrated the efficacy of ADSCs in muscle, tendon, bone and cartilage regeneration (31). Similarly, DAs can transdifferentiate into several mature cell types, including adipocytes, chondrocytes, osteoblasts and skeletal myocytes (32–34). The present study aimed to establish a more suitable means of seeding cells for different application directions of adipose tissue with rich sources by evaluating their differentiation ability into various lines. Thus, four-lineage induced differentiation experiments and semi-quantitative analysis were performed. Although there were significant differences between each pair of cases, there was no significant tendency between the DA and ADSC groups overall. Together, on average, the osteogenic and lipogenic abilities of ADSCs used in the present study were slightly stronger compared with that of DAs, and the myogenic abilities of DAs were slightly higher compared with that of ADSCs.
Although the majority of previous studies have revealed that DAs are more favorable than ADSCs for lipogenesis, and our unpublished data has suggested that DAs of earlier generations do exhibit significantly higher fat induction ability compared with ADSCs. The results of the present study demonstrated that ADSCs and DAs exhibited similar lipogenic abilities, which may be associated with the higher cell generations used in the present study. It was hypothesized that the long-term growth in the same medium makes the two types of cells become homogenous in their differentiation abilities.
The semi-quantitative results of osteogenesis and chondrogenesis also demonstrated the lack of difference in the differentiation ability between the two groups. Sasahara et al (7) reported a general trend toward decreased CpG methylation and that increased trimethylation levels of histone H3 at lysine 4 existed in the DAs, compared with the ADSCs in an epigenetic survey of the promoters of four osteogenic regulatory genes (RUNX2, SP7, ATF4 and BGLAP). Moreover, these authors speculated that these genes were more likely to be highly expressed in DAs, and that may underlie the improved osteogenic ability of DAs compared with ADSCs (7). In the present study, analysis of the differences in the expression of these four genes in the three pairs of samples and their corresponding osteogenic ability yielded inconsistent results, suggesting that the differences in the osteogenic capacity between cells in each case may be the result of the interaction of multiple genes. Interestingly, the expression of BMP6 in cells with relatively stronger chondrogenic abilities in the present study was significantly higher compared with that of the other cells, suggesting that the basic expression of BMP6 in cells may be associated with chondrogenic differentiation, which was consistent with several previous studies (35,36).
In terms of myogenic differentiation, it has been reported that ADSCs possess spontaneous myogenic differentiation capacity, although the efficiency is very low (37). In the present study, a baseline level of expression of MYH and desmin (DES) in ADSCs and DAs was observed, specific to each gene (GEO database accession number GSE141708); however, the expression trends were not consistent. It is worth noting that the skeletal marker MYH1 was one of the upregulated genes in DAs common across the three patients, although the expression level was low. Cells with relatively strong myogenic ability had relatively weak lipogenic ability; suggesting a potential opposing effects of genes involved in these two processes. It has been reported that microRNAs directly enhance mitochondrial translation during muscle differentiation (38). Additionally, differing CpG methylation and trimethylation of histone H3 profiles exist in ADSCs and DAs (22). Therefore, a deeper understanding of the regulatory mechanisms beyond transcriptomic differences may assist in elucidating the differences of genes in cell differentiation.
The sequencing results of the present study identified a limited number of DEGs between the two groups of cells, similar to previous studies. Perrini et al (39) (GEO accession no. GSE37324) compared genome-wide mRNA expression profiles of subcutaneous adipose tissue-derived stem cells ASCSVF and ASCCeiling from the same fat depot, and reported that both principal component analysis and hierarchical cluster analysis indicated that ASCCeiling and ASCSVF shared a similar pattern of closely related genes. By contrast to the present study, their specimens were obtained from open-abdominal surgery (nine men and six women; average age, 68 years; BMI, 27.06±1.5 kg/m2), and they used the fourth generation cells. Another project (GEO no. GSE47869) of microarray analysis without detailed case information in the GEO database, revealed that the global mRNA expression profiles of human DAs were very similar to those of ADSCs, but the work has not been published as of yet. GEO2R, an online tool of the GEO database (40), was used to analyze the data of GSE37324 by comparing Sc-ASCCeiling (DAs) and Sc-ASCSVF (ADSCs), using a criteria of P<0.05 and |log2(fold change)|>1.00 to screen the DEGs, only 141 named genes were identified, of which 83 were upregulated by ADSCs (no consistent genes with the present study), and 58 were upregulated in DAs (seven of which were also upregulated in the DAs in the present study: BEX1, ENPP1, SCUBE3, HHIP, IL7R, FOXD1 and PODXL; however, there were two genes that were downregulated in the DAs in the present study: EGR1 and IL6, that were upregulated in GSE37324). Similarly, using GEO2R to analyze the data of GSE47869 by comparing ADSCs and DAs with the same screening standards, only 16 DEGs named genes were identified, and there were no common DEGs between the present study and GSE37324.
In the DEGs screened in the present study, GO analysis suggested that cytokines may serve a more important role in the biological function of ADSCs than they do in DAs, and that there may be more significant differences in the transcription, protein translation regulation and energy metabolism levels between the two types of cells after multiple passages. Additionally, although more DEGs can be obtained by comparing the data of each pair of cells, there were only a few common DEGs identified in the differential genes of each pair of samples, suggesting that several factors may influence the gene expression differences of the two types of cells from the same depot. Thus, it is difficult to explain this based only on the sources of the cells or other individual difference. The results indicated that DAs and ADSCs possessed similar mRNA expression profiles and differentiation abilities on average, and DAs and ADSCs may have their own advantages for individuals in different applications in repair and regeneration.
There are some limitations in the present study. First, samples of subcutaneous adipose tissue were collected from three healthy females with a medium BMI. The number of patients was small, and therefore the results may not be generalizable. However, the existing data indicated that there were significant individual differences between the two types of cells. In addition, most studies have reported that obesity and age can significantly affect the differentiation ability of adipose derived stem cells, and whether there is an opposite effect for DAs has only been the subject of relatively few studies (41,42). As suggested in the present study, obesity may enhance the proliferation of DAs. However, additional data are required to verify this hypothesis. Only transcriptomic differences were studied between the two types of cells, while the mechanistic processes of the differentiation abilities of adult stem cells derived from adipose tissue requires additional study on the role of epigenetic regulatory factors, such as post-translational histone modifications and non-coding RNAs. Further study on the differentiation potential and mechanisms involved in the two types of cells may assist in improved utilizing of adipose tissue for regenerative medicine.
In conclusion, DAs and ADSCs from liposuction of adipose tissues in young individuals, can both be easily isolated and amplified in vitro, as well as exhibit similar morphologies, proliferation dynamics, surface markers and transcriptome expression profiles. There were notable individual differences in osteogenic, lipogenic, chondrogenic and myogenic abilities, and it was difficult to determine whether the differences in differentiation potential was due to cell source or other factors. Thus, DAs and ADSCs from young donors have similar application potential in general, while for individuals, DAs and ADSCs may have their own advantages based on the specific repair and regeneration applications.
Acknowledgements
Not applicable.
Funding
This work was supported by the Interdisciplinary Medicine Seed Fund of Peking University (grant no. BMU2018MX001) and the National Nature Science Foundation of China (grant no. 81501681).
Authors' contributions
FN designed the study, analyzed the data and wrote the manuscript. HB designed the experiments and revised the manuscript. CZ collected the specimen and performed the experiments. PD performed the experiments and analyzed the data. All authors read and approved the final manuscript.
Availability of data and materials
The RNAseq data have been deposited at the Gene Expression Omnibus (accession number GSE141708). The other datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The present study was approved by Peking University Third Hospital Medical Science Research Ethics Committee (approval no. 2014020). Samples of subcutaneous adipose tissue were collected from three females with written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Wu S, Coombs DM, Gurunian R. Liposuction: Concepts, safety, and techniques in body-contouring surgery. Cleve Clin J Med. 2020;87:367–375. doi: 10.3949/ccjm.87a.19097. [DOI] [PubMed] [Google Scholar]
- 2.Svalgaard JD, Juul S, Vester-Glovinski PV, Haastrup EK, Ballesteros OR, Lynggaard CD, Jensen AK, Fischer-Nielsen A, Herly M, Munthe-Fog L. Lipoaspirate storage time and temperature: Effects on stromal vascular fraction quality and cell composition. Cells Tissues Organs. 2020;209:54–63. doi: 10.1159/000507825. [DOI] [PubMed] [Google Scholar]
- 3.Choudhery MS, Badowski M, Muise A, Pierce J, Harris DT. Donor age negatively impacts adipose tissue-derived mesenchymal stem cell expansion and differentiation. J Transl Med. 2014;12:8. doi: 10.1186/1479-5876-12-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kornicka K, Marycz K, Tomaszewski KA, Marędziak M, Śmieszek A. The effect of age on osteogenic and adipogenic differentiation potential of human adipose derived stromal stem cells (hASCs) and the impact of stress factors in the course of the differentiation process. Oxid Med Cell Longev. 2015;2015:309169. doi: 10.1155/2015/309169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nordberg RC, Zhang J, Griffith EH, Frank MW, Starly B, Loboa EG. Electrical cell-substrate impedance spectroscopy can monitor age-grouped human adipose stem cell variability during osteogenic differentiation. Stem Cells Transl Med. 2017;6:502–511. doi: 10.5966/sctm.2015-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu M, Kohan E, Bradley J, Hedrick M, Benhaim P, Zuk P. The effect of age on osteogenic, adipogenic and proliferative potential of female adipose-derived stem cells. J Tissue Eng Regen Med. 2009;3:290–301. doi: 10.1002/term.165. [DOI] [PubMed] [Google Scholar]
- 7.Sasahara Y, Kubota Y, Kosaka K, Adachi N, Yamaji Y, Nagano H, Akita S, Kuroda M, Tanaka T, Bujo H, et al. Adipose-derived stem cells and ceiling culture-derived preadipocytes cultured from subcutaneous fat tissue differ in their epigenetic characteristics and osteogenic potential. Plast Reconstr Surg. 2019;144:644–655. doi: 10.1097/PRS.0000000000005913. [DOI] [PubMed] [Google Scholar]
- 8.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.e02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kunze KN, Burnett RA, Wright-Chisem J, Frank RM, Chahla J. Adipose-derived mesenchymal stem cell treatments and available formulations. Curr Rev Musculoskelet Med. 2020;13:264–280. doi: 10.1007/s12178-020-09624-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsubosaka M, Matsumoto T, Sobajima S, Matsushita T, Iwaguro H, Kuroda R. The influence of adipose-derived stromal vascular fraction cells on the treatment of knee osteoarthritis. BMC Musculoskelet Disord. 2020;21:207. doi: 10.1186/s12891-020-03231-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramakrishnan VM, Boyd NL. The adipose stromal vascular fraction as a complex cellular source for tissue engineering applications. Tissue Eng Part B Rev. 2018;24:289–299. doi: 10.1089/ten.teb.2017.0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heo JS, Choi Y, Kim HS, Kim HO. Comparison of molecular profiles of human mesenchymal stem cells derived from bone marrow, umbilical cord blood, placenta and adipose tissue. Int J Mol Med. 2016;37:115–125. doi: 10.3892/ijmm.2015.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naderi N, Combellack EJ, Griffin M, Sedaghati T, Javed M, Findlay MW, Wallace CG, Mosahebi A, Butler PE, Seifalian AM, et al. The regenerative role of adipose-derived stem cells (ADSC) in plastic and reconstructive surgery. Int Wound J. 2017;14:112–124. doi: 10.1111/iwj.12569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sugihara H, Yonemitsu N, Miyabara S, Yun K. Primary cultures of unilocular fat cells: Characteristics of growth in vitro and changes in differentiation properties. Differentiation. 1986;31:42–49. doi: 10.1111/j.1432-0436.1986.tb00381.x. [DOI] [PubMed] [Google Scholar]
- 15.Jumabay M, Boström KI. Dedifferentiated fat cells: A cell source for regenerative medicine. World J Stem Cells. 2015;7:1202–1214. doi: 10.4252/wjsc.v7.i10.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kishimoto N, Momota Y, Hashimoto Y, Tatsumi S, Ando K, Omasa T, Kotani J. The osteoblastic differentiation ability of human dedifferentiated fat cells is higher than that of adipose stem cells from the buccal fat pad. Clin Oral Investig. 2014;18:1893–1901. doi: 10.1007/s00784-013-1166-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watson JE, Patel NA, Carter G, Moor A, Patel R, Ghansah T, Mathur A, Murr MM, Bickford P, Gould LJ, et al. Comparison of markers and functional attributes of human adipose-derived stem cells and dedifferentiated adipocyte cells from subcutaneous fat of an obese diabetic donor. Adv Wound Care (New Rochelle) 2014;3:219–228. doi: 10.1089/wound.2013.0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saler M, Caliogna L, Botta L, Benazzo F, Riva F, Gastaldi G. hASC and DFAT, multipotent stem cells for regenerative medicine: A comparison of their potential differentiation in vitro. Int J Mol Sci. 2017;18:18. doi: 10.3390/ijms18122699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reumann MK, Linnemann C, Aspera-Werz RH, Arnold S, Held M, Seeliger C, Nussler AK, Ehnert S. Donor site location is critical for proliferation, stem cell capacity, and osteogenic differentiation of adipose mesenchymal stem/stromal cells: Implications for bone tissue engineering. Int J Mol Sci. 2018;19:1868. doi: 10.3390/ijms19071868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bi H, Li H, Zhang C, Mao Y, Nie F, Xing Y, Sha W, Wang X, Irwin DM, Tan H. Stromal vascular fraction promotes migration of fibroblasts and angiogenesis through regulation of extracellular matrix in the skin wound healing process. Stem Cell Res Ther. 2019;10:302. doi: 10.1186/s13287-019-1415-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robinson MD, McCarthy DJ, Smyth GK. edgeR: A bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tansriratanawong K, Tabei I, Ishikawa H, Ohyama A, Toyomura J, Sato S. Characterization and comparative DNA methylation profiling of four adipogenic genes in adipose-derived stem cells and dedifferentiated fat cells from aging subjects. Hum Cell. 2020;33:974–989. doi: 10.1007/s13577-020-00379-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shukla L, Yuan Y, Shayan R, Greening DW, Karnezis T. Fat therapeutics: The clinical capacity of adipose-derived stem cells and exosomes for human disease and tissue regeneration. Front Pharmacol. 2020;11:158. doi: 10.3389/fphar.2020.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baer PC. Adipose-derived mesenchymal stromal/stem cells: An update on their phenotype in vivo and in vitro. World J Stem Cells. 2014;6:256–265. doi: 10.4252/wjsc.v6.i3.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brooks AES, Iminitoff M, Williams E, Damani T, Jackson-Patel V, Fan V, James J, Dunbar PR, Feisst V, Sheppard HM. Ex vivo human adipose tissue derived mesenchymal stromal cells (ASC) are a heterogeneous population that demonstrate rapid culture-induced changes. Front Pharmacol. 2020;10:1695. doi: 10.3389/fphar.2019.01695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kilinc MO, Santidrian A, Minev I, Toth R, Draganov D, Nguyen D, Lander E, Berman M, Minev B, Szalay AA. The ratio of ADSCs to HSC-progenitors in adipose tissue derived SVF may provide the key to predict the outcome of stem-cell therapy. Clin Transl Med. 2018;7:5. doi: 10.1186/s40169-018-0183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bourin P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, March KL, Redl H, Rubin JP, Yoshimura K, Gimble JM. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT) Cytotherapy. 2013;15:641–648. doi: 10.1016/j.jcyt.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maurizi G, Poloni A, Mattiucci D, Santi S, Maurizi A, Izzi V, Giuliani A, Mancini S, Zingaretti MC, Perugini J, et al. Human white adipocytes convert into ‘rainbow’ adipocytes in vitro. J Cell Physiol. 2017;232:2887–2899. doi: 10.1002/jcp.25743. [DOI] [PubMed] [Google Scholar]
- 29.Shen JF, Sugawara A, Yamashita J, Ogura H, Sato S. Dedifferentiated fat cells: An alternative source of adult multipotent cells from the adipose tissues. Int J Oral Sci. 2011;3:117–124. doi: 10.4248/IJOS11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bougaret L, Delort L, Billard H, Lequeux C, Goncalves-Mendes N, Mojallal A, Damour O, Vasson MP, Caldefie-Chezet F. Supernatants of adipocytes from obese versus normal weight women and breast cancer cells: In vitro impact on angiogenesis. J Cell Physiol. 2017;232:1808–1816. doi: 10.1002/jcp.25701. [DOI] [PubMed] [Google Scholar]
- 31.Torres-Torrillas M, Rubio M, Damia E, Cuervo B, Del Romero A, Peláez P, Chicharro D, Miguel L, Sopena JJ. Adipose-derived mesenchymal stem cells: A promising tool in the treatment of musculoskeletal diseases. Int J Mol Sci. 2019;20:20. doi: 10.3390/ijms20123105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kazama T, Fujie M, Endo T, Kano K. Mature adipocyte-derived dedifferentiated fat cells can transdifferentiate into skeletal myocytes in vitro. Biochem Biophys Res Commun. 2008;377:780–785. doi: 10.1016/j.bbrc.2008.10.046. [DOI] [PubMed] [Google Scholar]
- 33.Matsumoto T, Kano K, Kondo D, Fukuda N, Iribe Y, Tanaka N, Matsubara Y, Sakuma T, Satomi A, Otaki M, et al. Mature adipocyte-derived dedifferentiated fat cells exhibit multilineage potential. J Cell Physiol. 2008;215:210–222. doi: 10.1002/jcp.21304. [DOI] [PubMed] [Google Scholar]
- 34.Oki Y, Watanabe S, Endo T, Kano K. Mature adipocyte-derived dedifferentiated fat cells can trans-differentiate into osteoblasts in vitro and in vivo only by all-trans retinoic acid. Cell Struct Funct. 2008;33:211–222. doi: 10.1247/csf.08038. [DOI] [PubMed] [Google Scholar]
- 35.Diekman BO, Estes BT, Guilak F. The effects of BMP6 overexpression on adipose stem cell chondrogenesis: Interactions with dexamethasone and exogenous growth factors. J Biomed Mater Res A. 2010;93:994–1003. doi: 10.1002/jbm.a.32589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jeon HJ, Yoon KA, An ES, Kang TW, Sim YB, Ahn J, Choi EK, Lee S, Seo KW, Kim YB, et al. Therapeutic effects of human umbilical cord blood-derived mesenchymal stem cells combined with cartilage acellular matrix mediated via bone morphogenic protein 6 in a rabbit model of articular cruciate ligament transection. Stem Cell Rev Rep. 2020;16:596–611. doi: 10.1007/s12015-020-09958-9. [DOI] [PubMed] [Google Scholar]
- 37.Sung SE, Hwang M, Kim AY, Lee EM, Lee EJ, Hwang SK, Kim SY, Kim HK, Jeong KS. MyoD overexpressed equine adipose-derived stem cells enhanced myogenic differentiation potential. Cell Transplant. 2016;25:2017–2026. doi: 10.3727/096368916X691015. [DOI] [PubMed] [Google Scholar]
- 38.Zhang X, Zuo X, Yang B, Li Z, Xue Y, Zhou Y, Huang J, Zhao X, Zhou J, Yan Y, et al. MicroRNA directly enhances mitochondrial translation during muscle differentiation. Cell. 2014;158:607–619. doi: 10.1016/j.cell.2014.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perrini S, Ficarella R, Picardi E, Cignarelli A, Barbaro M, Nigro P, Peschechera A, Palumbo O, Carella M, De Fazio M, et al. Differences in gene expression and cytokine release profiles highlight the heterogeneity of distinct subsets of adipose tissue-derived stem cells in the subcutaneous and visceral adipose tissue in humans. PLoS One. 2013;8:e57892. doi: 10.1371/journal.pone.0057892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barrett T, Wilhite SE, Ledoux P, Evangelista C, Kim IF, Tomashevsky M, Marshall KA, Phillippy KH, Sherman PM, Holko M, et al. NCBI GEO: Archive for functional genomics data sets-update. Nucleic Acids Res. 2013;41(D1):D991–D995. doi: 10.1093/nar/gks1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Conley SM, Hickson LJ, Kellogg TA, McKenzie T, Heimbach JK, Taner T, Tang H, Jordan KL, Saadiq IM, Woollard JR, et al. Human obesity induces dysfunction and early senescence in adipose tissue-derived mesenchymal stromal/stem cells. Front Cell Dev Biol. 2020;8:197. doi: 10.3389/fcell.2020.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prieto González EA. Heterogeneity in adipose stem cells. Adv Exp Med Biol. 2019;1123:119–150. doi: 10.1007/978-3-030-11096-3_8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The RNAseq data have been deposited at the Gene Expression Omnibus (accession number GSE141708). The other datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.