Abstract
Background
Exclusive breastfeeding rates in many high-income countries are considerably lower than the World Health Organization recommendations. Younger mothers are less likely than older mothers to exclusively breastfeed or to exclusively breastfeed for a long duration. This systematic review explores interventions to increase the rate of exclusive breastfeeding among young mothers in high-income countries.
Methods
A systematic search of the following databases was completed in August 2020: CINAHL, PubMed, MEDLINE, ProQuest, PsychInfo, Web of Science, Cochrane, Scopus and Embase. A manual search of the reference lists of all the included studies and published systematic reviews was also performed. The Cochrane Collaboration Risk of Bias Tool was used to assess the quality of the included studies. A random effects model meta-analyses was applied. Heterogeneity of outcomes between the studies was assessed using both the χ2 test and the I2 statistic.
Results
Of 955 records identified in the search, 392 duplicates were removed, and nine studies met the inclusion criteria. Seven studies were randomised controlled trial (RCTs) and two were quasi-experimental in design. Eight were conducted in the United States. The interventions included peer counselling, telephone support, massage, gift packs, financial incentive and antenatal education. Most studies included a combination of strategies, peer counselling being the most common. A meta-analysis of four of nine included studies did not detect a difference in rate of exclusive breastfeeding to 3 months postpartum (RR 1.44; 95% CI 0.82, 2.55; p = 0.204).
This review is limited by the relatively few studies which met the inclusion criteria and the small sample sizes of most included studies. High rates of attrition and formula supplementation among the participants made it difficult to detect a statistically significant effect. Consistency in follow up times would enable more studies to be included in a meta-analysis.
Conclusions
Peer counselling was the most promising strategy associated with higher rates of exclusive breastfeeding. However, further studies are needed to understand the breastfeeding experiences of young mothers. Young mothers should be targeted specifically in intervention studies.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13006-020-00340-6.
Keywords: Exclusive breastfeeding, Interventions, Young mothers, High income countries, Systematic review
Background
Increasing the rates of breastfeeding and in particular, exclusive breastfeeding (EBF) to 6 months, is a public health priority across the world [1]. The World Health Organization (WHO) defines ‘exclusive breastfeeding’ as an infant receiving only breast milk (whether that be directly from the breast, from a bottle or from a donor/wet nurse) [2]. This definition allows the infant to receive prescribed drops or syrups (vitamins, minerals, medicines) but nothing else [2]. The WHO recommends that infants be exclusively breastfed until 6 months of age and continue to be breastfed, in conjunction with solids, for up to 2 years and beyond if desired [3].
Although any amount of breastfeeding provides multiple health benefits for both the mother and infant [1, 4–11], EBF provides greater benefits than partial breastfeeding during the first 6 months of life, particularly in relation to preventing gastrointenstinal and respiratory infections [5, 12]. Kramer and Kakuma [5] conducted a systematic review of the literature and concluded that 6 months was the optimal duration for EBF and had significantly more health benefits than EBF to 3 or 4 months. Further, there is evidence to show that the longer the duration of EBF (up to 6 months), the greater the health benefits it may provide [13]. It is also established that supplementation affects the mother’s milk supply and thus EBF is associated with longer duration of any breastfeeding [14].
Although the benefits of EBF are well-established, globally only 40% of infants under the age of 6 months are exclusively breastfed [15]. In most high-income countries the proportion of babies exclusively breastfed may be significantly less than the global average [4]. The Centers for Disease Control and Prevention reported that 24.9% of babies in the US in 2018 were exclusively breastfed to 6 months [16]. In Australia, the National Infant Feeding Survey for 2010 reported only 15.4% of babies were exclusively breastfed for five completed months (to 5 months) and only 2.1% were exclusively breastfed for the recommended six completed months (to 6 months), despite high breastfeeding initiation rates of 90% [17]. Low rates of EBF to 6 months are reported in other high-income countries such as the United Kingdom (1%), Norway (7%), Denmark (17%) and the Netherlands (17%) [18].
Younger age has been found to be associated with poorer breastfeeding practices in a large number of studies. For example, Jones et al. [19] found that mothers in the US aged 30 years or older were more than twice as likely, compared with mothers 20 years or younger, to exclusively breastfeed to 6 months (18.0% vs 8.3%). Similarly, the 2010 Australian National Infant Feeding Survey reported that the proportion of mothers aged 24 years or younger who exclusively breastfed to 5 months (6.2%) was less than one-third that of mothers aged 35 years or older (19.2%) [17]. The Infant Feeding Survey, UK – 2010 reported that mothers aged 24 years or younger were less likely to exclusively breastfeed at each month of age to 6 months [20].
A broad range of interventions and programs has been implemented in various contexts to promote breastfeeding initiation, duration, and exclusivity, with varying degrees of success. Interventions have included strategies such as peer counselling, professional counselling, online support, phone support, antenatal breastfeeding education, multimedia approaches, motivational interviewing, breastfeeding-friendly hospital practices, breastfeeding-friendly workplaces, and parental leave policies [21–28]. Despite this extant research, young mothers do not appear to be well represented in these intervention studies. Most interventions do not target young mothers specifically and some even exclude an important sub-group of young mothers (adolescent mothers aged less than 18 years). However, given that young mothers exhibit particularly low rates of EBF in high-income countries [17, 19, 20, 29], they are an important population on which to focus.
The purpose of this systematic review and meta-analysis is to examine the range and effectiveness of interventions which have been designed to increase rates of EBF among young mothers in high-income countries. The specific focus on high-income countries allows the findings to directly inform the development and implementation of an intervention in a high-income country setting.
Methods
This review was conducted based on the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines (see Additional file 1 for complete PRISMA checklist) [30]. The protocol for this systematic review is registered with PROSPERO International Prospective Register of Systematic Reviews (2018: CRD42018083989) [31]. The Population Intervention Comparator Outcome Study Design (PICOS) criteria were used to devise the review question and search terms [32]. The PICOS table is presented in Table 1. A combination of MeSH terms and keywords was drafted and peer reviewed for comprehensiveness. The search strategy was pre-tested in the MEDLINE database (see Additional file 2) and subsequently adapted to the syntax and subject headings of all other databases.
Table 1.
Population Intervention Comparator Outcome Study Design (PICOS) table
| P – Population | Young mothers (mothers aged 24 years or less) in high-income countries; infants 0–6 months |
| I – Intervention | Any intervention |
| C – Comparator | Any comparator (most commonly, Usual Care) |
| O – Outcome | Increasing exclusive breastfeeding rates |
| S – Study Design | Randomized Controlled Trials and Quasi Experimental designs (prospective with a control group) |
Information sources
A systematic search of the following databases was conducted: MEDLINE (OVID), Scopus, Web of Science (ISI), PubMed, PsychInfo, ProQuest Central, Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library), Embase (OVID), and Cumulative Index to Nursing and Allied Health Literature (CINAHL) (EBSCO). Keywords used in the searches included: exclusive; breastfeeding; infant feeding; adolescents; young mothers and randomized controlled trial. Appropriate truncations and search functions (such as subject headings) were utilized and modified according to the database. Date limitations were not applied to the search. The primary search was conducted in February 2018 and the final search was completed in August 2020 to ensure that more recent studies would not be omitted. Additionally, a manual search of the reference lists of all the included studies and published systematic reviews was performed.
Eligibility criteria
Inclusion criteria were based on PICOS criteria (see Table 1; Additional file 3). Studies were included if they were conducted in a high-income country, were an RCT or had a quasi-experimental design, were published in a peer reviewed journal, were written in the English language, measured EBF and had a sample population of mothers with a mean or median age of less than 25 years. The mean or median age of the sample population was used to identify studies which had a large proportion of young mothers (i.e., younger than 25 years). If a mean or median age was not reported, the study was excluded. All studies which met the criteria except for age were reviewed for sub-group analysis by age. Studies with follow-up times of up to 6 months were included. The introduction of solids is recommended around 6 months of age and therefore EBF is rare and usually unnecessary after this time [3]. Quasi-experimental designs were deemed acceptable if they were prospective and had a control group, as randomization is not always possible or appropriate in breastfeeding research. The World Bank classification of countries was used to identify high-income countries [33]. No restriction on publication dates was applied as the review aimed to assess all published studies related to the aims.
Study selection
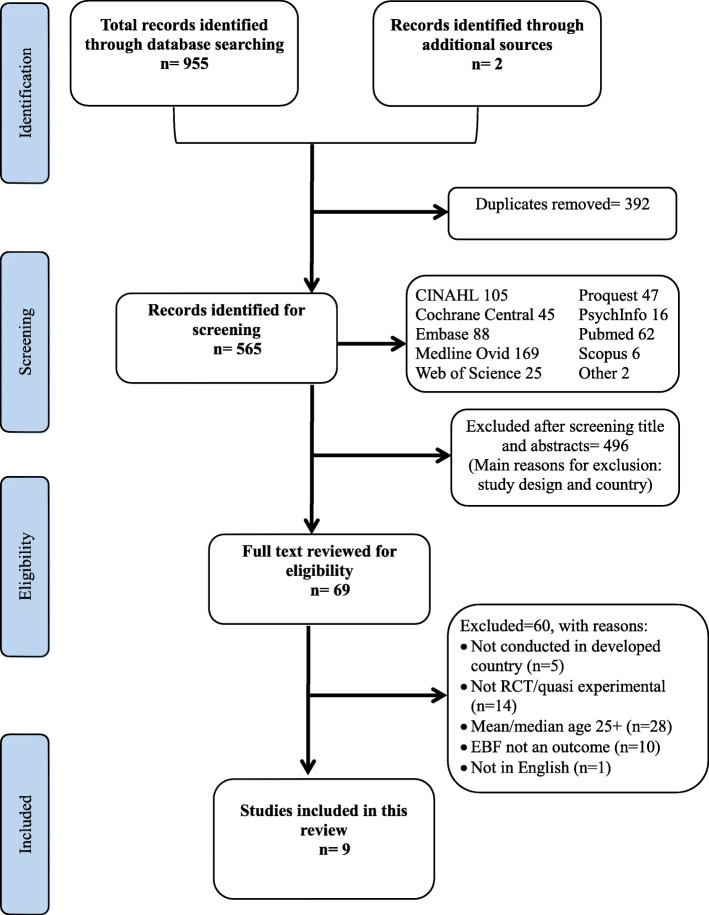
Studies identified through the electronic databases were exported to Endnote X8 for removing duplicates, screening, and selection [34]. Two reviewers (CB and AA) independently and in duplicate screened the studies against the inclusion criteria mentioned above. Full texts of the articles that met the inclusion criteria were independently assessed by two reviewers (CB and AA). In case of uncertainty regarding the eligibility and study selection, the study authors were contacted to seek additional information. A total of three contact attempts were made, and if no response was received, the articles were screened for eligibility based on the information available. Any disagreements were resolved through discussion with two further reviewers (GSK and DH). The reasons for excluding studies that did not meet the inclusion criteria were recorded (Additional file 4). The search strategy resulted in nine studies being included in this review. This process is summarized in the PRISMA flow diagram (Fig. 1).
Fig. 1.
PRISMA flowchart of study selection process
Data collection process and data items
A standardized data extraction form was developed and pilot-tested independently by two reviewers (CB and AA). Extracted data included first author, publication year, country, study design, sample size, sample characteristics, intervention description, comparison, and reported outcomes. Data extraction was conducted primarily by two reviewers (CB and AA) independently. GSK and DH provided feedback and resolution for any disagreements. In case of missing data and/or uncertainties, the study authors were contacted for further information with a maximum of three attempts. The extracted data are listed in Table 2.
Table 2.
Summary of included studies targeting young mothers in high-income countries to increase EBF
| Study, Country | Design | n | Sample characteristics | Intervention Description | Comparison | Reported Outcomes |
|---|---|---|---|---|---|---|
| Arlotti et al. (1998), USA [35] | Quasi experimental |
36 Exp: 18 Con: 18 |
Convenience sample: Prenatal and postpartum mothers who were enrolled in Women, Infants and Children (WIC) program. Low income population in North Florida. Mean age: 23.3 (SD 4.4) Age range: 15–36 |
Support from peer counsellors a few days after delivery, then at 2 weeks, 1 month, 2 months and 3 months postpartum via telephone, letter or in person at WIC office. | No counsellor/Usual care at same time intervals. |
Mean rates of EBFa, experimental VS control: • at 2 weeks, 53% vs 17% • at 1 month, 40% vs 27% • at 2 months, 33% vs 13% • at 3 months, 17% vs 6% |
| Bunik et al. (2010), USA [36] | RCT |
341 Exp: 161 Con: 180 |
Convenience sample of medically underserved (88% Hispanic/Latino) mothers, recruited from a subsidized hospital in Denver, Colorado. Median age: 22 Age range: 18+ (range not provided) |
Two weeks of daily telephone calls by trained bilingual nurses using a culturally informed script. Outcomes assessed by maternal report at 1, 3 and 6 months postpartum. |
Usual care | No mothers EBF. There was no significant difference in any BFb or predominantc BF between the groups. Participants in the experimental group, who planned to EBF were more likely to “predominantly” BF at 1, 3 and 6 months. This trend was not seen in the control group. |
| Chapman et al. (2013), USA [37] | RCT |
206 Exp: 103 Con: 103 |
206 pregnant, overweight/obese, low-income women were recruited. 154 met inclusion criteria at delivery. Pre-pregnancy BMI = 27.0+ Mostly Hispanic, singleton pregnancy, unemployed, high school education, vaginal birth. Median age (exp): 23 (IQR 21–28) Median age (con): 25 (IQR 22–31) Age range: not reported |
Specialized breastfeeding peer counselling (SBFPC) 3 x Prenatal, daily in hospital after delivery and up to 11 x postnatal sessions plus routine care. |
Brief breastfeeding discussions during routine prenatal visits at the clinic | Experimental group had higher rates of EBF at some time points but the difference was not significant. At 2 weeks experimental group had higher rates of any BF (93% vs 84%, P = 0.09) and ≥ 50% of feeds as breast milk (81% vs 67%, P = 0.08). |
| Di Meglio et al. (2010), USA [38] | RCT |
78 Exp: 38 Con: 40 |
Breastfeeding mothers with a healthy full-term infant recruited from hospitals in Rochester, New York Mean age (exp): 18.4 (SD 1.3) Mean age (con):18.2 (SD 1.4) Age range: Not reported but participants were < 20 years old. |
Telephone support at 2, 4, and 7 days and 2, 3, 4, and 5 weeks post discharge from the hospital; content based on WIC’s breastfeeding promotion efforts, gave telephone numbers. Facilitators: Adolescent peer counsellors trained by La Leche League | Usual care | There was no significant difference for any BF duration between the groups. 68% of experimental group and 75% of control group received supplements at the hospital prior to discharge. Out of the remaining participants, duration of EBF was significantly increased in the experimental group (median 35 days vs 10 days, P < 0.01). |
| Pugh et al. (2002), USA [39] | RCT |
41 Exp: 21 Con: 20 |
Low-income mothers receiving financial medical assistance support, recruited from hospitals in mid-Atlantic region. Predominantly African American and mostly single. Mean age (exp): 20.86 (SD 3.58) Mean age (con): 22.35 (SD 4.98) Age range: not reported |
Usual care plus supplementary visits from a community health nurse/peer counsellor team, daily during hospitalization and then at home during weeks 1, 2, and 4; peer counsellors provided support over the phone twice weekly through week 8 and weekly through month 6. Facilitators: Community health nurse and peer counsellor | Usual care included support from hospital nurses, telephone “warm line” and one visit by a lactation consultant if they birth on a weekday. |
More mothers in the experimental group EBF their infants however it wasn’t significant due to the small sample size. • 45% (n = 9) vs 25% (n = 5) at 3 months • 30% (n = 6) vs 15% (n = 3) at 6 months X2 = 1.29–1.75; P = 0.09–0.12 |
| Serano et al. (2010), Chile [40] | Quasi-experimental comparative panel design - RCT deemed not appropriate due to risk of contamination |
100 Exp: 35 Con: 65 |
Mothers with healthy newborns recruited from 3 health clinics in Santiago, Chile. Low-income community. Predominantly unemployed. Mean age: 24.3 (SD 5.9) Mean age (con): 24.08 (SD 5) Age range: 14–43 |
During 2nd well-child clinic visit, nurses provided video instruction on how to massage baby. Mothers also received a booklet and were encouraged to massage their baby for 10–15 min once a day from Day 15. | No treatment | At age 2 months, massage group infants weighed significantly more than control-group infants. There were no weight differences between the 2 groups at age 4 months. There were no differences between the 2 groups on the incidence of exclusive maternal breast-feeding at age 2 or 4 months. |
| Snell et al. (1992), USA [41] | RCT |
88 Exp: 50 Con: 38 |
Hispanic mothers recruited from Family Centered Perinatal Care Unit in California. Mean age (exp): 25.2 (18–43) Mean age (con): 24.3 (18–36) Age range: 18–43 |
The study period (12 weeks) was divided into 2-week blocks and randomly assigned to experimental (non-gift pack) and control (gift pack) groups. Mothers were then interviewed (by telephone) at 1 and 3 weeks old. | The control group received a gift pack including samples of formula. |
• EBF at 1 week: 80% vs 68% • EBF at 3 weeks: 68% vs 33% Supplementing or bottle feeding: • At 1 week: 20% vs 32% • At 3 weeks: 32% vs 66% |
| Wambach et al. (2011), USA [42] | RCT |
390 Exp: 128 Con1: 128 Con2: 134 |
Enrolled adolescent mothers in their second trimester from prenatal clinics and school settings. All first-time mothers. Predominantly African American, and single. Mean age: 17 (SD 0.9) Age range: 15–18 |
Co-delivered by lactation consultant (LC) and peer counsellor (PC). Two prenatal classes consisting of content from Breastfeeding Educated and Supportive Teen club (BEST) curriculum, in-hospital support and postpartum telephone calls at 4, 7, 11, 18 days and 4 weeks. Participants also received a free double electric pump. |
Con1 = attention control delivered by nurse and PC, included 2 prenatal classes, telephone support, and in-hospital PC visit (not on the topic of BF). Con2 = usual care |
• Median BF duration was significantly higher in experimental group: 177 (exp) vs 42 (con1) vs 61 (con2) days. P < 0.001 • No significant differences for EBF initiation or duration • High overall rates of supplementation. At 3 weeks postpartum 69% (exp), 70% (con1), 82% (con2) were supplementing with formula. |
| Washio et al. (2017), USA [43] | Randomized two-arm parallel group design |
36 Exp: 18 Con: 18 |
Puerto Rican mothers enrolled in a WIC program, who initiated breastfeeding. Mean age (exp): 24.1 (SD 4.7) Mean age (con): 23.0 (SD 4.6) Age range: not reported |
Standard breastfeeding services from WIC – onsite LC, bilingual PC, weekly peer support meetings, free breast pump, enhanced food package for BF mothers. Plus, monthly financial incentives (total = $270) if they could demonstrate BF or pumping. | Standard breastfeeding services from WIC |
Significantly higher rates of BF in experimental group vs control: • 89% vs 44% at 1 month • 89% vs 17% at 3 months • 72% vs 0% at 6 months • Mean duration of BF was 149 (exp) vs 49 (con) days. (P < 0.001) No significant difference in self-reported EBF rate. |
Notes & Abbreviations:
aEBF Exclusive breastfeeding
bBF Breastfeeding
cPredominant breastfeeding in this study was defined as feeding 4 oz. or less of formula per day
Exp Experimental/intervention group
Con Control group
PC Peer counsellor
LC Lactation consultant
WIC Women, Infants and Children Office
Quality assessment
The Cochrane Collaboration Risk of Bias Tool (RoBT) was used to assess the quality of the included studies (Additional file 5) [44]. The RoBT is a systematic process whereby studies can be measured against specific criteria. Each study was given a rating of high, low or unclear in the following domains: selection bias, performance bias, detection bias, attrition bias, and reporting bias. The RoBT also allows the assessors to report any other biases which may not fall into these five domains.
The RoBT was completed by two reviewers (CB and AA) independently. If there were disagreements, consensus was reached through discussion with two other reviewers (GSK and DH). Study authors were contacted in the event of insufficient details being available to confidently assess the methodological quality; and if a response was not received after three attempts, the study quality was assessed based on the available information.
Data synthesis
Due to the diverse range of interventions, the low number of included studies, and that many studies were mixed-mode interventions comprising several strategies, it was not possible to conduct a meta-analysis on a specific intervention. Thus, data were analyzed to compare the effectiveness in terms of EBF outcomes of ‘any intervention’ versus ‘no intervention’. The most commonly reported outcome measures were EBF rates to 1-, 3-, and 6-months after birth. The number of women EBF and the number of women not EBF were extracted at each of these timepoints. Papers not reporting any of these outcomes were excluded. The data extracted from the included articles and used in the meta-analyses are listed in Additional file 6.
The relative risk of EBF at each of the three timepoints was determined using random effects meta-analyses. Results were reported as forest plots displaying both the relative risk and 95% confidence interval for each individual study and the equivalent pooled results.
Heterogeneity of outcomes between the studies was assessed using both the χ2 test and the I2 statistic. P-values less than 0.05 from the χ2 test were interpreted as statistically significant evidence of heterogeneity. I2 statistics of 0–40% were considered ‘not important’, 30–60% ‘may represent moderate heterogeneity’, and 75–90% ‘considerable heterogeneity’ [45]. Sensitivity analyses were performed to exclude studies whose quality rating was ‘poor’ in order to evaluate the impact of study quality on the results. Neither sub-group analyses nor assessment of publication bias could be undertaken due to the low number of included studies (< 10) [46, 47]. Analyses were performed using the metafor package in R software [48].
Results
A total of 955 titles and abstracts were identified across all selected electronic databases. Two additional titles and abstracts were identified through a manual search of the reference lists of systematic reviews found in the database search. After removal of duplicates (n = 392), a total of 565 titles and abstracts were identified for further examination. The most common reasons for exclusion at this stage were the study design and country in which the study was conducted. Sixty-nine studies were identified as meeting the inclusion criteria for full text reading, and of these, nine studies were included in the systematic review and four in the meta-analyses. The 60 excluded studies and the reason for exclusion are presented in Additional file 4. All studies which met the criteria except for age were reviewed for sub-group analysis by age but none of them reported on this (possibly due to small numbers of younger mothers) and hence remained excluded. The inter-reader agreement for the entire search process was 100%. A PRISMA flow diagram was constructed showing the identification, screening, eligibility and included studies (Fig. 1).
Characteristics of included studies
Nine studies were included in this review [35–43]. Of these, eight studies were conducted in the USA [35–39, 41–43] and one in Chile [40]. Seven studies were RCTs [36–39, 41–43] and two were of a quasi-experimental study design [35, 40]. The publication dates ranged between 1992 and 2017. The studies had relatively small sample size, ranging from 36 to 390 participants. Six of the studies had 100 or fewer participants [35, 38–41, 43]. The follow up time ranged from 3 weeks to 6 months. The mean or median age of mothers ranged from 17 to 24 years. Seven studies [35, 38–43] reported a mean age and two reported a median age [36, 37]. Two studies had narrow age ranges of up to 5 years, three studies had a broad age range of 20 years or more, while the remaining four studies did not report the age range. In one study, all mothers had a pre-pregnancy body mass index of 27 or above [37].
The strategies implemented in the studies included prenatal breastfeeding education (n = 2) [37, 42], peer support (n = 5) [35, 37–39, 42], professional support (n = 4) [36, 39, 40, 42], financial incentives (n = 1) [43], gift pack (n = 1) [41], telephone support (n = 5) [35, 36, 38, 39, 42] and massage (n = 1) [40]. Five of the studies included a peer counselling component and four of these used a combination of peer counselling and telephone support. Control groups received usual care or no treatment.
Four studies were included in the meta-analyses. Three of these were peer counselling interventions [35, 37, 39] and one involved education and telephone support [42]. The common follow-up time points across the included studies were 1-month, 3-months, and 6-months, however, only the 3-month time point was common across all four studies. The one-month time point was included in two studies [35, 37] and the six-month timepoint was included in three studies [37, 39, 42].
Peer counselling
Peer counselling refers to support provided by a non-professional person from the community who has personal experience breastfeeding and a willingness to support others. Five studies included a peer counselling component [35, 37–39, 42]. Arlotti et al. [35] conducted a quasi-experimental study which included postnatal support from a peer counsellor a few days after birth, then at 2 weeks, 1 month, 2 months and 3 months postpartum. This support was delivered via telephone, letter or in person at the Women, Infants and Children (WIC) office.
In the RCT study by Chapman et al. [37], participants received three ‘specialized breastfeeding peer counselling’ sessions prenatally, daily visits in hospital after the birth, up to 11 postnatal sessions and routine care. Di Meglio et al. [38] conducted an RCT where the participants in the intervention group received telephone support from adolescent peer counsellors at 2, 4, and 7 days and 2, 3, 4, and 5 weeks after discharge from the hospital. The peer counsellors were trained by La Leche League and the content was based on WIC’s breastfeeding promotion materials.
In the RCT by Pugh et al. [39], the intervention group received usual care plus supplementary visits from a nurse and peer counsellor team. The visits were daily while the mothers were at the hospital, then at 1, 2, and 4 weeks postpartum when the mothers returned home. The peer counsellor provided telephone support twice weekly until 8 weeks postpartum followed by weekly telephone support until 6 months. Wambach et al. [42], included a lactation consultant and peer counsellor team who co-delivered two prenatal classes and provided telephone support over the first 4 weeks.
Telephone support
Five studies included a telephone support component [35, 36, 38, 39, 42]. Only one study by Bunik et al. [36] used telephone support as its sole strategy and this support was delivered via a nurse (i.e., professional support). Four studies had a combination of telephone and peer counselling [35, 38, 39, 42] and two of these also included professional support via a lactation consultant or nurse [39, 42].
Bunik et al. [36] conducted an RCT where the mothers received 2 weeks of daily telephone calls from a bilingual nurse using a culturally informed script. Wambach et al. [42] conducted an RCT where the intervention group received a combination of prenatal classes and telephone support co-delivered by a lactation consultant and peer counsellor. The mothers also received in-hospital support from the peer counsellor and lactation consultant. Telephone support was provided at 4, 7, 11, 18 days, and 4 weeks.
Prenatal education
Two studies included a prenatal education component [37, 42]. In the study by Chapman et al. [37], the education was delivered by peer counsellors who provided personalized breastfeeding education during three prenatal sessions. The peer counsellors were trained in La Leche League curricula [37]. In the study by Wambach et al. [42], a lactation consultant and peer counsellor co-delivered two prenatal classes based on Breastfeeding Educated and Supportive Teen club (BEST) curriculum. These studies also included a peer counselling element, with peer counselling provided in the home [37] or via telephone support [42].
Other interventions
The remaining studies used a number of other strategies including massage [40], gift pack [41], and financial incentive [43]. Serrano et al. [40] conducted a quasi-experimental study where the participants were provided video instruction and a booklet on how to massage their baby. The aim was to evaluate the effect of massage on infant weight gain and EBF.
Snell et al. [41] conducted an RCT where mothers were randomly assigned to receive a gift pack which contained formula samples or not to receive a gift pack. As it is standard practice for USA hospitals to give formula samples to mothers, the “non-gift pack” group was the intervention group and the gift pack group was the control.
Washio et al. [43] conducted an RCT where the mothers received standard breastfeeding services from WIC plus monthly financial incentives, totaling USD $270 if they could demonstrate breastfeeding or pumping.
Effectiveness of interventions on EBF
Overall there was modest to no effect with respect to increasing the EBF rates in the studies. Five studies reported no evidence of effect on rates of EBF [36, 37, 40, 42, 43]. These five studies included telephone support, peer counselling, massage, prenatal education, and financial incentives. Three studies reported a positive effect [35, 38, 41]. Two of these studies included a combination of peer counselling and telephone support in their intervention [35, 38]. Arlotti et al. [35], in their study involving peer counselling, reported a 36 percentage point difference in the mean rates of EBF to 2 weeks in the intervention versus usual care group (53% vs 17% respectively; n = 36). In the RCT by Di Meglio et al. [38], a statistically significantly longer duration of EBF was observed in the experimental group (telephone support from adolescent peer counsellors) compared to the usual care group (median 35 days vs 10 days, p = 0.01; n = 78). The study by Pugh et al. [39], which involved peer counselling, telephone support and a community health nurse, reported a non statistically significant positive effect of the intervention on rates of EBF (45% vs 25% to 3 months; n = 14; 30% vs 15% to 6 months; n = 9). Two studies, which used a combination of education and peer counselling [42] and financial incentives [43] found a statistically significant positive effect of the intervention for breastfeeding duration or breastfeeding rates but not for exclusive breastfeeding (Table 3).
Table 3.
Effectiveness of interventions in included studies
| Author, Year | N | Intervention | Results |
|---|---|---|---|
| Arlotti et al. (1998) [35] | 36 |
Peer counselling (telephone, letter and in person at office) |
Mean rates of EBF^, experimental VS control: • at 2 weeks, 53% vs 17% • at 1 month, 40% vs 27% • at 2 months, 33% vs 13% • at 3 months, 17% vs 6% |
| Bunik et al. (2010) [36] | 341 | Telephone support from a nurse | No mothers EBF |
| Chapman et al. (2013) [37] | 206 | Peer counselling including prenatal session (education), in-hospital and in-home postnatal sessions |
EBF rates, experimental VS control: • at 1 month, 17.6% vs 12.1% (p = 0.37) • at 2 months, 11.9% vs 11.1% (p = 0.88) • at 3 months, 5.0% vs 9.4% (p = 0.49) • at 4 months, 1.6% vs 4.8% (p = 0.62) • at 5 months, 1.6% vs 1.6% (p = 0.999) • at 6 months, 1.7% vs 0.0% (p = 0.49) |
| Di Meglio et al. (2010) [38] | 78 | Peer counselling via telephone support |
Duration of EBF, experimental vs control: Median 35 days vs 10 days, P < 0.01. |
| Pugh et al. (2002) [39] | 41 |
Nurse/peer counselling team In-hospital, in-home and telephone support |
Mean rates of EBF, experimental VS control: • at 3 months, 45% (n = 9) vs 25% (n = 5) • at 6 months, 30% (n = 6) vs 15% (n = 3) χ2 = 1.29–1.75; P = 0.09–0.12 |
| Serano et al. (2010) [40] | 100 | Video instruction for baby massage delivered by nurses |
Mean rates of EBF, experimental VS control: • at 2 months, 85.7% (n = 30) vs 81.54% (n = 53) • at 4 months, 71.4% (n = 25) vs 73.8% (n = 48) χ2 = 0.28–0.07; P = 0.595–0.795 |
| Snell et al. (1992) [41] | 88 | Gift pack vs non gift pack |
EBF rates, non-gift pack vs gift pack group: • at 1 week: 80% vs 68% (p > 0.05) • at 3 weeks: 68% vs 33% (p < 0.004) |
| Wambach et al. (2011) [42] | 390 |
Lactation consultant and peer counsellor team Prenatal education, in-hospital and telephone support |
EBF rates, experimental vs controls: • at 3 months: 32.14% vs 13.33% • at 6 months: 35.71% vs 22.22% |
|
Washio et al. (2017) [43] |
36 | Financial incentives | No significant difference in self-reported EBF rate. (figures not reported) |
Quality assessment
The seven studies with an RCT design demonstrated a low risk of bias for random sequence generation [36–39, 41–43] and five of these were also deemed to have a low risk of bias for allocation concealment (Fig. 2) [36–39, 43]. The two studies with a quasi-experimental design did not randomize their participants and therefore, had a potential high risk of bias for both random sequence generation and allocation concealment [35, 40]. Blinding of participants, personnel and outcomes was limited for six of the nine studies, with either a high or unclear risk of bias [35, 36, 39–42]. Blinding was poorly described in the reports. Five studies were deemed to have high or unclear risk of bias in relation to incomplete data and attrition rates [35, 37–40]. Four studies had had low attrition rates and thus a low risk of bias in this domain [36, 41–43]. Most of the studies provided thorough reporting of outcomes and, given that the majority reported no effect, the risk of bias was deemed to be low in the selective reporting domain. One study [38] noted that Hispanic teens were less likely to participate in their study than Caucasian and African American teens, hence this was recorded as an ‘unclear’ risk of participation bias (in ‘other’).
Fig. 2.
Quality assessment summary of studies included in the systematic review
Meta-analyses
Between two and four studies were included in the meta-analyses, based on reporting of EBF outcomes, common time points and dichotomous data [35, 37, 39, 42]. The χ2 test and the I2 statistic for each analysis demonstrated sufficient homogeneity to combine the studies.
Two studies, both involving peer counselling, provided data for the 1-month analysis [35, 37]. There was no statistically significant effect of intervention on the rate of EBF to 1 month (RR 1.44; 95% CI 0.77, 2.69; p = 0.248; Fig. 3), compared with usual care. There was no evidence of heterogeneity (I2 = 0%; p-value 0.933). A sensitivity analysis for study quality was not possible due to the low number of included studies.
Fig. 3.
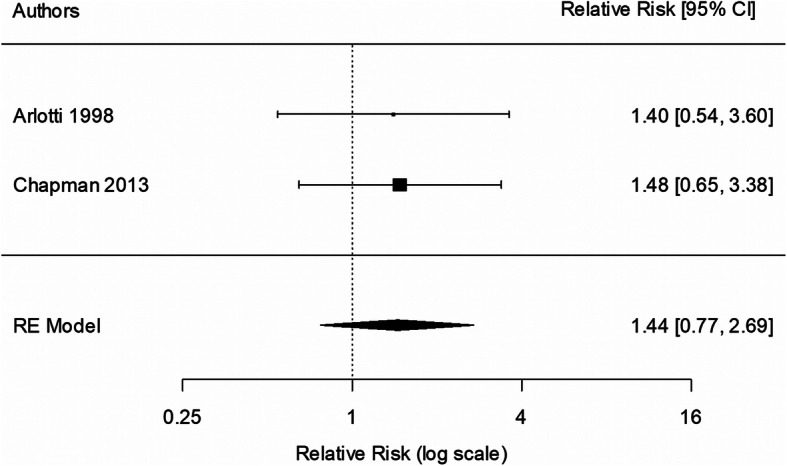
Meta-analysis of exclusive breastfeeding to 1 month
Four studies were included in the 3-month analysis [35, 37, 39, 42]. All four studies had a peer counselling element and three of the four studies [35, 39, 42] also included telephone support. Two studies had a prenatal education element [37, 42]. There was no statistically significant effect of intervention on the rate of EBF to 3 months (RR 1.44; 95% CI 0.82, 2.55; p = 0.204; Fig. 4), compared with usual care. There was no evidence of heterogeneity (I2 = 0%; p = 0.430). One study [35] was excluded (due to lower quality) for the sensitivity analysis. Sensitivity analysis indicated that the findings were unaffected by the study quality (RR 1.37, 95% CI 0.76, 2.46).
Fig. 4.
Meta-analysis of exclusive breastfeeding to 3 months
Three studies were included in the 6-month analysis [37, 39, 42]. All three studies included peer counselling, with two of the three studies [39, 42] including telephone support and two studies [37, 42] including prenatal education. There was no statistically significant effect of intervention on the rate of EBF to 6 months (RR 1.89; 95% CI 0.77,4.61; p = 0.164; Fig. 5), compared with usual care. There was no evidence of heterogeneity (I2 = 0%; p = 0.938). Exclusion of the lowest quality study from the analysis provided a RR of 2.10 (95% CI 0.66, 6.66).
Fig. 5.
Meta-analysis of exclusive breastfeeding to 6 months
Discussion
This review sought to examine the range and effectiveness of interventions designed to increase rates of EBF among young mothers in high-income countries. Due to the heterogeneity of the interventions and the multiple-strategy nature of most of the interventions, it was not possible to conclude which strategies were most effective. However, interventions which involved peer counselling either as the main strategy or as one component of the overall intervention appear to be the most successful in increasing rates of EBF among young mothers. Two of the three studies which showed a significant positive effect on EBF used peer counselling as the principal strategy [35, 38]. A further study, Pugh et al. [39], which showed a non-statistically significant positive effect, also used peer counselling as the primary strategy.
There were however, several variations among the studies involving peer counselling. Peer counselling was delivered via different formats (telephone and in-person support) and at various time points across interventions, hence the optimal timing and format of peer counselling for this age-group of mothers is uncertain. For example, Chapman et al. [37] included three prenatal sessions and a large number of in-hospital and in-home postnatal sessions, whereas Arlotti et al. [35] delivered only five postnatal sessions. In two interventions, peer counselling was coupled with professional support from a nurse [39] or lactation consultant [42]. In the study by Di Meglio et al. [38] all peer counselling sessions were delivered by telephone whereas in the study by Arlotti et al. [35] only some sessions were delivered by telephone. The variety of formats used to deliver the peer counselling suggests that the exact format may not be important.
Several systematic reviews have examined the effectiveness of peer support in improving breastfeeding practices. Shakya et al. [49] and Jolly et al. [50] examined the effects across developed versus developing countries and indicated that this mode of intervention appears to be effective in developing countries but not developed countries. A review of 12 studies examining interventions designed to promote EBF in high-income countries by Skouteris et al. [51] showed that interventions with long-duration postnatal support components were effective at increasing EBF to 6 months.
There were two studies which combined prenatal education and peer support, and the education sessions were delivered by trained peer counsellors. In Chapman et al. [37] the peer counsellors delivered personalized one-on-one breastfeeding education, and in Wambach et al. [42] the peer counsellors facilitated two prenatal group classes. However, these two studies were ineffective at increasing the rate of EBF in young mothers in the USA [37, 42].
When compared to other breastfeeding education interventions, the systematic review by Lumbiganon et al. [24] also found no statistically significant evidence of effect of prenatal breastfeeding education on the rate of EBF among studies conducted predominantly in developed countries (RR 1.06 to 3 months; RR 1.07 to 6 months; pooled analyses for Summary of findings). However, Haroon et al. [52] demonstrated a positive effect of breastfeeding education on rates of EBF in both developed and developing countries, although with a stronger effect in those studies conducted in developing countries (RR 1.31 vs 2.88).
With respect to other strategies, only the ‘gift pack’ intervention conducted by Snell et al. [41] demonstrated a statistically significant effect on EBF. This may be relevant for countries in which hospitals still commonly practice giving gifts of formula to new mothers, however, may not be applicable to countries which have ceased this practice. The USA is one such country which does not adhere to the World Health Organization’s International Code of Marketing of Breast-milk Substitutes as infant formula advertising is widespread and free samples are often distributed in hospitals [53].
Eight of the nine included studies in the current review were conducted in the USA and as such, the findings may not be generalizable to other countries. Furthermore, many of the participants were from low socioeconomic communities or specific ethnic communities (such as Hispanic) and may not be generalizable to other communities. Although the rates of EBF in young mothers is low in most high-income countries, there are significant differences between high-income countries in relation to societal attitudes toward breastfeeding and system supports. For example, the USA only recently legalized breastfeeding in public in all 50 states (societal attitudes) and has no paid parental leave (system support) [53]. Thus, the factors and interventions influencing mothers from the USA could differ from the factors and interventions affecting mothers from other high-income countries.
The meta-analysis combined data from four of the nine included studies [35, 37, 39, 42]. Different outcome measurements made it difficult to compare studies, with varying follow-up times (2 weeks to 6 months) and various time points for data collection. Further, the data were sometimes expressed as continuous data (duration) and at other times dichotomous data (rates) so these were not able to be combined.
Overall, studies were of moderate quality. There was a lack of blinding and allocation concealment in many studies [35, 36, 39–42]. Sample attrition was common and randomization was not always possible or appropriate. It is possible that the results of interventions with large age ranges may not be reflective of what works with solely younger populations. The ages of the mothers who continued to exclusively breastfeed were not reported.
Limitations
The findings of this systematic review and meta-analysis should be considered in light of several limitations. The review is limited by the relatively few studies which met the eligibility criteria. The sample sizes were small, with six of the nine studies having 100 or fewer participants. High rates of sample attrition and formula supplementation compounded this issue and made it difficult to detect a statistically significant effect. Because of the focus on high income countries in this review the results are not readily generalizable to low- and middle-income countries. Further, the included studies were mostly from the USA and may not be relevant to other high-income countries with different societal attitudes and system supports. As well, there was no exploration as to why mothers were not EBF or ceasing EBF early, as this was beyond the scope of the review.
As the eligibility for included studies was based on a mean or median age of less than 25 years, there were studies which had a wide age range. Without stratification it is not possible to determine whether the younger participants, specifically, found the intervention beneficial or not. This approach was necessary however as there were so few studies which included only mothers aged 24 years or younger and no studies were found which provided a sub-group analysis by age (possibly due to small numbers of younger mothers). The two studies [38, 42] which did, included only adolescent mothers (15–18 years) which was too narrow for the purpose of this review. Furthermore, the results of these two studies were not able to be combined in meta-analysis as the outcome measures were not compatible.
Recommendations
More RCTs are required to test the effectiveness of interventions aimed at promoting rates of EBF among young mothers in high-income countries. Studies should specifically target young mothers (24 years or younger) or report the results of younger participants separately for studies conducted with a large age range. Young mothers have a unique set of characteristics, needs and barriers [28]. As such, it is important to understand the specific needs of young mothers so that interventions and health promotion programs can be tailored to suit them. It is recommended that future studies take into account blinding and allocation concealment to reduce potential for bias and increase reliability. Consistency in relation to follow up times would be advantageous so that intervention effectiveness can be more easily compared and/or findings combined for meta-analysis.
Conclusions
Although this review included only a small number of studies, and the study populations differed in age range, there is an indication that peer counselling could be a promising intervention for improving rates of EBF among young mothers in high-income countries. This age group of mothers is understudied with respect to EBF promotion and support interventions. Intervention studies need to focus on young mothers or include sufficient numbers of young mothers to enable sub-group analysis by age.
Supplementary Information
Additional file 1: Appendix 1. PRISMA Checklist.
Additional file 2: Appendix 2. Search Strategy example using the MEDLINE database.
Additional file 3: Appendix 3. Inclusion and exclusion criteria. Full list of inclusion and exclusion criteria.
Additional file 4: Appendix 4. List of excluded studies and reasons for exclusion.
Additional file 5: Appendix 5. Assessment of risk of bias in included studies – full table.
Additional file 6: Appendix 6. Raw data extracted for meta-analyses of exclusive breastfeeding to 1, 3, and 6 months.
Acknowledgements
We thank Ms. Katrina Chaudhary for her guidance with the database search. We also thank the study authors who assisted with our requests for further information.
Abbreviations
- BF
Breastfeeding
- EBF
Exclusive breastfeeding
- RCT
Randomisd controlled trial
- RoBT
Risk of Bias Tool
- WHO
World Health Organization
- WIC
Women, Infants and Children office
Authors’ contributions
CB conceptualised, designed and conducted the study (review), drafted the initial manuscript, and made amendments based on feedback from the other authors. DH provided guidance throughout the study and reviewed and revised the manuscript. GSK provided guidance throughout the study and reviewed and revised the manuscript. PF provided guidance on the meta-analysis and reviewed and revised the manuscript. AA conceptualised the study (review), conducted the study selection, data extraction and quality assessment, and reviewed and revised the manuscript. All authors read and approved the final manuscript.
Funding
No funding was secured for this study. AA was supported by Australian National Health and Medical Research Council Grants (1033213, 1069861, 1134075).
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Christa Buckland, Email: christabuckland@outlook.com.
Debra Hector, Email: debra.hector@canceraustralia.gov.au.
Gregory S. Kolt, Email: g.kolt@westernsydney.edu.au
Paul Fahey, Email: p.fahey@westernsydney.edu.au.
Amit Arora, Email: a.arora@westernsydney.edu.au.
References
- 1.World Health Organization . Breastfeeding advocacy initiative: For the best start in life. 2015. [Google Scholar]
- 2.World Health Organization . Indicators for assessing infant and young child feeding practices. 2008. [Google Scholar]
- 3.World Health Organization . Global strategy on infant and young child feeding. 2002. [Google Scholar]
- 4.Victora CG, Bahl R, Barros AJ, Franca GV, Horton S, Krasevec J, et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet. 2016;387:475–490. doi: 10.1016/S0140-6736(15)01024-7. [DOI] [PubMed] [Google Scholar]
- 5.Kramer MS, Kakuma R. Optimal duration of exclusive breastfeeding. Cochrane Database Syst Rev. 2012;2012(8):CD003517. doi: 10.1002/14651858.CD003517.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Young J, Watson EL, Raven L. Responding to evidence: breastfeed baby if you can – the sixth public health recommendation to reduce the risk of sudden and unexpected death in infancy. Breastfeed Rev. 2012;20(1):7–16. [PubMed] [Google Scholar]
- 7.Heikkilä K, Kelly Y, Renfrew MJ, Sacker A, Quigley MA. Breastfeeding and educational achievement at age 5. Matern Child Nutr. 2014;10:92–101. doi: 10.1111/j.1740-8709.2012.00402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Academy of Pediatrics Policy statement: breastfeeding and the use of human milk. Pediatrics. 2012;129(3):e827–e841. doi: 10.1542/peds.2011-3552. [DOI] [PubMed] [Google Scholar]
- 9.Bartick M, Reinhold A. The burden of suboptimal breastfeeding in the United States: a pediatric cost analysis. Pediatrics. 2010;125(5):e1048–e1056. doi: 10.1542/peds.2009-1616. [DOI] [PubMed] [Google Scholar]
- 10.Smith JP, Thompson JF, Ellwood DA. Hospital system costs of artificial infant feeding: estimates for the Australian Capital Territory. Aust N Z J Public Health. 2002;26(2):543–551. doi: 10.1111/j.1467-842X.2002.tb00364.x. [DOI] [PubMed] [Google Scholar]
- 11.Chowdhury R, Sinha B, Sankar MJ, Taneja S, Bhandari N, Rollins N, et al. Breastfeeding and maternal health outcomes: a systematic review and meta-analysis. Acta Paediatr. 2015;104(467):96–113. doi: 10.1111/apa.13102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khan J, Vesel L, Bahl R, Martines JC. Timing of breastfeeding initiation and exclusivity of breastfeeding during the first month of life: effects on neonatal mortality and morbidity—a systematic review and meta-analysis. Matern Child Health J. 2015;19:468–479. doi: 10.1007/s10995-014-1526-8. [DOI] [PubMed] [Google Scholar]
- 13.Smith JP, Harvey PJ. Chronic disease and infant nutrition: is it significant to public health? Public Health Nutr. 2010;14(2):279–289. doi: 10.1017/S1368980010001953. [DOI] [PubMed] [Google Scholar]
- 14.Scott J, Binns C, Oddy W, Graham KI. Predictors of breastfeeding duration. Evidence from a cohort study. Paediatrics. 2006;117(4):e646–e655. doi: 10.1542/peds.2005-1991. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization . Global Breastfeeding Scorecard, 2017 tracking progress for breastfeeding policies and programmes. 2017. [Google Scholar]
- 16.Centers for Disease Control and Prevention . Breastfeeding report card: United States 2018. 2018. [Google Scholar]
- 17.Australian Institute of Health and Welfare . 2010 Australian National Infant Feeding Survey: Indicator results. (Cat no. PHE 156) 2011. [Google Scholar]
- 18.World Health Organization . Global Health Observatory data repository: Exclusive breastfeeding under 6 months Data by country. 2017. [Google Scholar]
- 19.Jones JR, Kogan MD, Singh GK, Dee DL, Grummer-Strawn LM. Factors associated with exclusive breastfeeding in the United States. Pediatrics. 2011;128(6):1117–1125. doi: 10.1542/peds.2011-0841. [DOI] [PubMed] [Google Scholar]
- 20.NHS Digital. Infant Feeding Survey – UK. 2010; Retrieved from https://digital.nhs.uk/data-and-information/publications/statistical/infant-feeding-survey/infant-feeding-survey-uk-2010.
- 21.Balogun OO, O’Sullivan EJ, McFadden A, Ota E, Gavine A, Garner CD, et al. Interventions for promoting the initiation of breastfeeding. Cochrane Database Syst Rev. 2016;11:CD001688. doi: 10.1002/14651858.CD001688.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bevan G, Brown M. Interventions in exclusive breastfeeding: a systematic review. Br J Nurs. 2014;23(2):86–89. doi: 10.12968/bjon.2014.23.2.86. [DOI] [PubMed] [Google Scholar]
- 23.Elliott-Rudder M, Pilotto L, McIntyre E, Ramanathan S. Motivational interviewing improves exclusive breastfeeding in an Australian randomised controlled trial. Acta Paediatr. 2014;103:e11–e16. doi: 10.1111/apa.12434. [DOI] [PubMed] [Google Scholar]
- 24.Lumbiganon P, Martis R, Laopaiboon M, Festin MR, Ho JJ, Hakimi M. Antenatal breastfeeding education for increasing breastfeeding duration. Cochrane Database Syst Rev. 2016;12(12):CD006425. doi: 10.1002/14651858.CD006425.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olaiya O, Dee D, Sharma A, Smith R. Maternity care practices and breastfeeding among adolescent mothers aged 12-19 years--United States, 2009-2011. MMWR Morb Mortal Wkly Rep. 2016;65(2):17–22. doi: 10.15585/mmwr.mm6502a1. [DOI] [PubMed] [Google Scholar]
- 26.Scott S, Pritchard C, Szatkowski L. The impact of breastfeeding peer support for mothers aged under 25: a time series analysis. Matern Child Nutr. 2017;13(1):e12241. doi: 10.1111/mcn.12241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Health Organization, UNICEF . Baby-friendly hospital initiative: Revised, updated and expanded for integrated care. 2009. [PubMed] [Google Scholar]
- 28.Mellin PS, Poplawski DT, Defreest N, Massler K, Gole A. Does skin-to-skin contact at birth really make a difference in exclusive breastfeeding rates at discharge? J Obstetr Gynecologic Neonatal Nurs. 2012;41(s1):S141–S142. doi: 10.1111/j.1552-6909.2012.01362_33.x. [DOI] [Google Scholar]
- 29.Centers for Disease Control and Prevention. National Center for Chronic Disease Prevention and Health Promotion, Division of Nutrition, Physical Activity, and Obesity. Data, Trend and Maps [online]. Available from https://www.cdc.gov/nccdphp/dnpao/data-trends-maps/index.html. Accessed 23 Nov 2020.
- 30.Moher D, Tetzlaff J, Altman DG, The PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buckland C, Kolt G, Hector D, Arora A. Interventions for increasing exclusive breastfeeding among young mothers in high-income countries: a systematic review of randomised controlled trials. PROSPERO 2018 CRD42018083989 Available from: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42018083989. Accessed 23 Nov 2020.
- 32.Costantino G, Montano N, Casazza G. When should we change our clinical practice based on the results of a clinical study? Searching for evidence: PICOS and PubMed. Intern Emerg Med. 2015;10(4):525–527. doi: 10.1007/s11739-015-1225-5. [DOI] [PubMed] [Google Scholar]
- 33.World Bank . World Bank Country and Lending Groups: High-income economies ($12,056 or more) 2018. [Google Scholar]
- 34.Clarivate Analytics . Endnote X8. 2016. [Google Scholar]
- 35.Arlotti J, Cottrell B, Lee S, Curtin J. Breastfeeding among low-income women with and without peer support. J Community Health Nurs. 1998;15(3):163–178. doi: 10.1207/s15327655jchn1503_4. [DOI] [PubMed] [Google Scholar]
- 36.Bunik M, Shobe P, O'Connor ME, Beaty B, Langendoerfer S, Krane L, et al. Are 2 weeks of daily breastfeeding support insufficient to overcome the influences of formula? Acad Pediatr. 2010;10:21–28. doi: 10.1016/j.acap.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 37.Chapman DJ, Morel KL, Bermúdez-Millán A, Young SM, Damio G, Pérez-Escamilla R. Breastfeeding education and support trial for overweight and obese women: a randomized trial. Pediatrics. 2013;131(1):e162–e170. doi: 10.1542/peds.2012-0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Di Meglio G, Mcdermott MP, Klein JD. A randomized controlled trial of telephone peer support's influence on breastfeeding duration in adolescent mothers. Breastfeed Med. 2010;5(1):41–47. doi: 10.1089/bfm.2009.0016. [DOI] [PubMed] [Google Scholar]
- 39.Pugh LC, Milligan RA, Frick KD, Spatz D, Bronner Y. Breastfeeding duration, costs, and benefits of a support program for low-income breastfeeding women. Birth. 2002;29(2):95–100. doi: 10.1046/j.1523-536X.2002.00169.x. [DOI] [PubMed] [Google Scholar]
- 40.Serrano MS, Doren FA, Wilson L. Teaching Chilean mothers to massage their full-term infants: effects on maternal breast-feeding and infant weight gain at age 2 and 4 months. J Perinat Neonatal Nurs. 2010;24(2):172–181. doi: 10.1097/JPN.0b013e3181db5377. [DOI] [PubMed] [Google Scholar]
- 41.Snell BJ, Krantz M, Keeton R, Delgado K, Peckham C. The association of formula samples given at hospital discharge with the early duration of breastfeeding. J Hum Lact. 1992;8(2):67–72. doi: 10.1177/089033449200800213. [DOI] [PubMed] [Google Scholar]
- 42.Wambach KA, Aaronson L, Breedlove G, Williams Domian E, Rojjanasrirat W, Yeh HW. A randomized controlled trial of breastfeeding support and education for adolescent mothers. West J Nurs Res. 2011;33(4):486–505. doi: 10.1177/0193945910380408. [DOI] [PubMed] [Google Scholar]
- 43.Washio Y, Humphreys M, Colchado E, Sierra-Ortiz M, Zhang A, Collins BN, et al. Incentive-based intervention to maintain breastfeeding among low-income Puerto Rican mothers. Pediatrics. 2017;139(3):e20163119. doi: 10.1542/peds.2016-3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Higgins JPT, Altman DG, Sterne JAC, (editors). Chapter 8: assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane handbook for systematic reviews of interventions vVersion 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
- 45.Deeks JJ, Higgins JP, Altman DG. Analysing data and undertaking meta-analyses. In: JPT H, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Chichester: The Cochrane Collaboration and John Wiley and Sons Ltd. 2008. pp. 243–296. [Google Scholar]
- 46.Borenstein M. Wiley InterScience. Chapter 10, in Introduction to meta-analysis. Chichester: Wiley; 2009. [Google Scholar]
- 47.Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. Br Med J. 2011;343:d4002. doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- 48.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna; 2013. http://www.R-project.org/.
- 49.Shakya P, Kunieda MK, Koyama M, Rai SS, Miyaguchi M, Dhakal S, et al. Effectiveness of community-based peer support for mothers to improve their breastfeeding practices: a systematic review and meta-analysis. PLoS One. 2017;12(5):e0177434. doi: 10.1371/journal.pone.0177434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jolly K, Ingram L, Khan KS, Deeks JJ, Freemantle N, MacArthur C. Systematic review of peer support for breastfeeding continuation: Meta-regression analysis of the effect of setting, intensity, and timing. BMJ (Clinical Research Ed.) 2012;344:d8287. doi: 10.1136/bmj.d8287. [DOI] [PubMed] [Google Scholar]
- 51.Skouteris H, Bailey C, Nagle C, Hauck Y, Bruce L, Morris H. Interventions designed to promote exclusive breastfeeding in high-income countries: a systematic review update. Breastfeed Med. 2017;12(10):64–614. doi: 10.1089/bfm.2017.0065. [DOI] [PubMed] [Google Scholar]
- 52.Haroon S, Das JK, Salam RA, Imdad A, Bhutta ZA. Breastfeeding promotion interventions and breastfeeding practices: a systematic review. BMC Public Health. 2013;13(Suppl 3):S20. doi: 10.1186/1471-2458-13-S3-S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.U.S. Department of Health and Human Services . Barriers to breastfeeding in the United States. Washington, D.C.: In The Surgeon General’s Call to Action to Support Breastfeeding; 2011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Appendix 1. PRISMA Checklist.
Additional file 2: Appendix 2. Search Strategy example using the MEDLINE database.
Additional file 3: Appendix 3. Inclusion and exclusion criteria. Full list of inclusion and exclusion criteria.
Additional file 4: Appendix 4. List of excluded studies and reasons for exclusion.
Additional file 5: Appendix 5. Assessment of risk of bias in included studies – full table.
Additional file 6: Appendix 6. Raw data extracted for meta-analyses of exclusive breastfeeding to 1, 3, and 6 months.
Data Availability Statement
Not applicable.