Abstract
Objective
Metabolic syndrome (MetS) has been associated with hypercoagulative status. However, previous studies evaluating the association between MetS and incidence of venous thromboembolism (VTE) after total joint arthroplasty (TJA) showed inconsistent results. We performed a meta-analysis to evaluate the influence of MetS on the risk of VTE following TJA.
Methods
Cohort studies were identified by the search of PubMed, Embase, and the Cochrane’s Library databases. A random-effect model was used if considerable heterogeneity was detected; otherwise, a fixed-effect model was used. Subgroup analyses according to the category of VTE, definition of MetS, category of procedure, and follow-up durations were performed.
Results
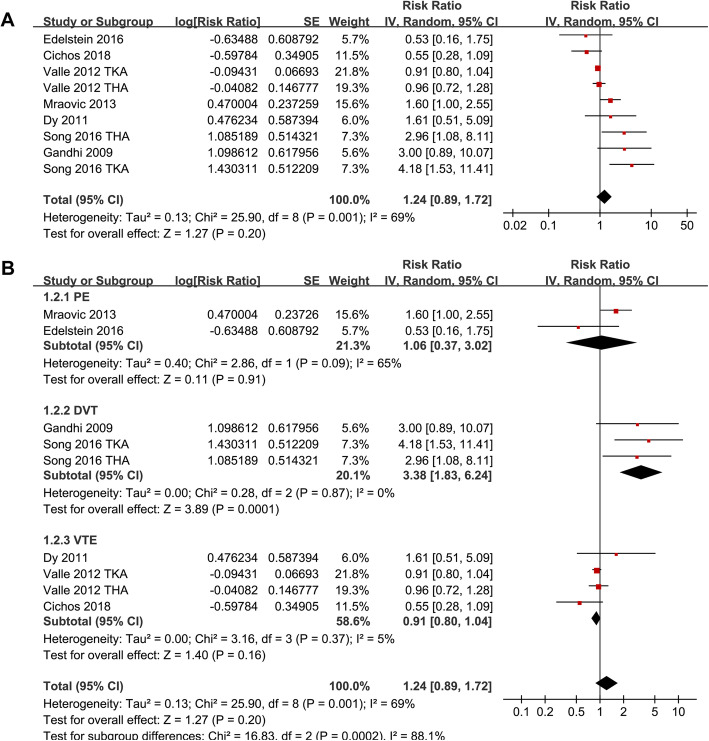
Seven cohort studies with 1,341,457 patients that underwent TJA were included, with 118,060 MetS patients (8.8%) at baseline. With a follow-up duration up to 3 months after surgery, 9788 patients had VTE. Pooled results with a random-effect model showed that MetS was not associated with increased overall VTE after TJA (adjusted risk ratio [RR] = 1.24, 95% confidence interval [CI] 0.89 ~ 1.72, p = 0.20; I2 = 69%). The results were not significantly affected by the diagnostic criteria of MetS, category of the procedure, and follow-up durations. Subgroup analyses showed that MetS was not associated with an increased the risk of pulmonary embolism ([PE], RR 1.06, 95% CI 0.37 ~ 3.02, p = 0.91), but an increased risk of deep vein thrombosis (DVT) after TJA (RR 3.38, 95% CI 1.83 ~ 6.24, p < 0.001).
Conclusions
Current evidence from observational studies suggests MetS might be associated with an increased risk of DVT but not PE after TJA.
Keywords: Metabolic syndrome, Total knee arthroplasty, Total hip arthroplasty, Venous thromboembolism, Meta-analysis
Introduction
Patients undergoing total joint arthroplasty (TJA), including total knee arthroplasty (TKA) and total hip arthroplasty (THA), are at a higher risk for the development of venous thromboembolism (VTE) [1–3]. Despite the routine use of prophylactic measures against thrombosis in these patients, the incidences of VTE, including pulmonary embolism (PE) and deep vein thrombosis (DVT) remains high [4]. A previous systematic review including 44,844 cases from 47 studies showed that in patients receiving recommended prophylaxis, the pooled rates of symptomatic postoperative VTE before hospital discharge were 1.09% for patients undergoing TKA and 0.53% for those undergoing THA [5]. Moreover, VTE has become an important cause of morbidity and mortality in patients after TJA [6]. Identification of risk factors for VTE in patients that received TJA is clinically important [6].
Metabolic syndrome (MetS) refers to a cluster of metabolic abnormalities including abdominal adiposity, insulin resistance, hyperglycemia, hypertension, and dyslipidemia [7, 8]. Accumulating evidence suggests that patients MetS are characterized by hypercoagulative status [9–13]. Indeed, MetS has been associated with a 2-fold increase in arterial thrombotic diseases, such as coronary artery disease and stroke [14]. Moreover, a previous individual-patient data-based meta-analysis showed that MetS is associated with an increased risk of unprovoked VTE in general population [15]. Since MetS is prevalent in patients undergoing TJA, accumulating studies have evaluated the association between MetS and risk of VTE in these patients [16–22]. However, the results of these studies are inconsistent. Some of them suggested that MetS may be a risk factor for VTE after TJA [16, 19, 21], while other studies did not [17, 18, 20, 22]. Therefore, we aimed to perform a meta-analysis to evaluate the association between MetS and risk of VTE in patients after TJA. The influences of categories of VTE, definitions of MetS, and types of procedures on the association were also analyzed.
Methods
The meta-analysis was designed and performed in accordance with the MOOSE (Meta-analysis of Observational Studies in Epidemiology) [23] and Cochrane’s Handbook [24] guidelines.
Literature search
Electronic databases of PubMed, Embase, and the Cochrane’s Library were systematically searched using the combination of the following terms: [1] “metabolic syndrome” OR “insulin resistance syndrome” OR “syndrome X” [2]; “hip” OR “knee”; and [3] “arthroplasty” OR “replacement” OR “surgery” OR “operation”. We used this extensive search strategy to avoid missing potential studies. The search was limited to human studies published in English or Chinese. The reference lists of original and review articles were also analyzed manually. The final literature search was performed on April 2, 2020.
Study selection
Studies were included if they met the following criteria: [1] published as full-length article, [2] designed as cohort studies, [3] included patients that received TKA or THA, [4] MetS was identified as exposure of interest at baseline, [5] documented the incidence of any VTE events (overall VTE, PE, or DVT) in patients with and without MetS, and [6] reported the adjusted risk ratios (RRs, at least adjusted for age and sex) and their corresponding 95% confidence intervals (CIs) for the incidence of VTE in patients with and without MetS. Definitions of MetS were consistent with what was applied in the original studies. Reviews, editorials, preclinical studies, and non-cohort studies were excluded.
Data extracting and quality evaluation
Literature search, data extraction, and study quality assessment were independently performed by two authors according to the predefined inclusion criteria. If inconsistencies occurred, discussion with the corresponding author was suggested to resolve the disagreement. The following data were extracted: [1] name of the first author, publication year, study location, and study design [2]; characteristics and numbers of patients that received TKA or THA, criteria for the diagnosis of MetS, prevalence of MetS, and follow-up period; and [3] number of cases with any VTE events during follow-up, VTE prophylactic strategies, methods for the validation of the outcome, and variables adjusted. The quality of each study was evaluated using the Newcastle-Ottawa Scale (NOS) [25]. This scale ranges from 1 to 9 stars and judges the quality of each study regarding three aspects: the selection of the study groups, the comparability of the groups, and the ascertainment of the outcome of interest.
Statistical analyses
The association between MetS and VTE in patients after TKA or THA was measured by RRs. To stabilize its variance and normalized the distribution, RR data and its corresponding stand error (SE) from each study was logarithmically transformed [24]. The Cochrane’s Q test was performed to evaluate the heterogeneity among the include cohort studies [24, 26], and the I2 statistic was also calculated. A significant heterogeneity was considered if I2 > 50%. A random effect model was used to pool the results if significant heterogeneity was detected; otherwise, a fixed-effect model was used. Sensitivity analysis by omitting one study at a time was performed to evaluate the stability of the results [24]. To evaluate the influences of categories of VTE, definitions of MetS, types of procedures, and follow-up durations on the association, subgroup analyses were performed. Potential publication bias was assessed by visual inspection of the symmetry of the funnel plots, complemented with the Egger regression test [27]. P < 0.05 was considered as statistically significant. The RevMan (Version 5.1; Cochrane Collaboration, Oxford, UK) and STATA software were used for the statistics.
Results
Literature search
The flowchart of the database search was shown in Fig. 1. Briefly, 788 studies were obtained from the database search, and 768 of them were excluded primarily due to the irrelevance to the purpose of the study. For the remaining 20 potential relevant studies that underwent full-text review, 13 were further excluded for the reasons listed in Fig. 1. Finally, seven cohort studies were included [16–22].
Fig. 1.
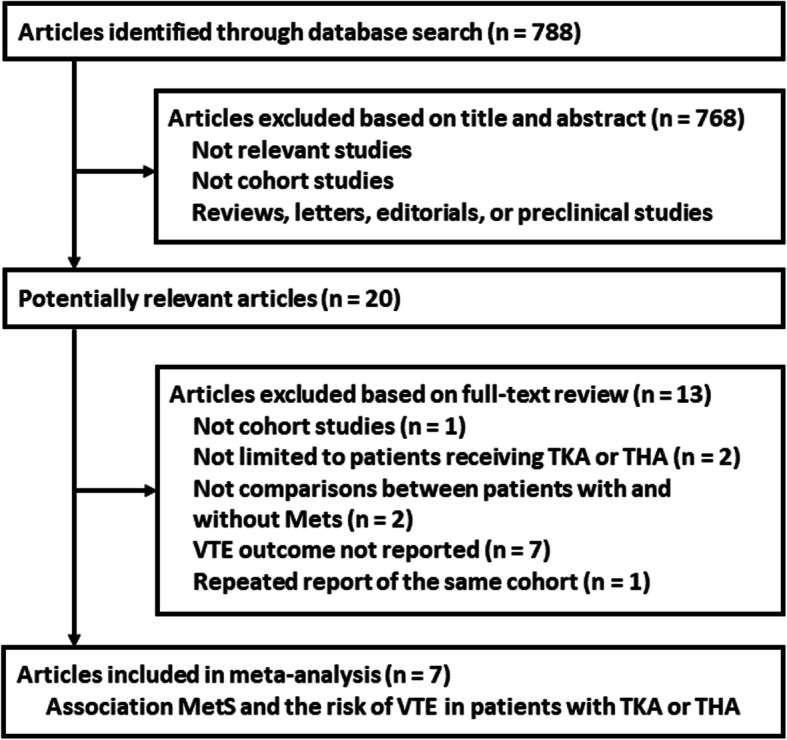
The flowchart of database search and study inclusion
Study characteristics and quality
Characteristics of the included studies were summarized in Table 1. Overall, seven cohort studies with 1,341,457 patients that underwent TJA were included [16–22]. These studies were performed in the USA, Canada, and China and published between 2009 and 2018. One of them was a prospective cohort study [16], and the others were retrospective cohorts [17–22]. Since two studies reported the incidence of VTE events in patients received TKA and THA separately, these datasets were independently included. Therefore, nine datasets were available for the meta-analysis, and 118,060 patients (8.8%) were diagnosed as MetS at baseline according to the World Health Organization (WHO) [16, 20, 21] or the National Cholesterol Education Program Expert Panel and Adult Treatment Panel III (NCEP-ATP III) criteria [17–19, 22]. The follow-up duration varied between within hospitalization and 3 months after surgeries, and 9788 patients had VTE during follow-up. Strategies for VTE prophylaxis were reported in three studies [16, 19, 21], and anticoagulants of warfarin, rivaroxaban, or low-molecular-weight heparin were used. The methods for VTE assessment were also reported in three studies [16, 19, 21], which involved the application of Doppler ultrasonography, venography, and CT and/or lung VQ scan. Potential confounding factors, including age, sex, body mass index (BMI), smoking, and comorbidities, were adjusted to a varying degree in the included studies. The qualities of the included follow-up studies were generally good, with the NOS ranging from 6 ~ 8 (Table 2).
Table 1.
Characteristics of the included cohort studies
| Study | Country | Design | Patient characteristics | Sample size | Mean age years | Male (%) | MetS diagnosis | MetS at baseline, n (%) | Follow-up duration | Prophylactic methods | Outcomes reported | Outcome validation | Variables adjusted |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gandhi 2009 [16] | Canada | PC | Patients received unilateral TKA | 1460 | 66.5 | 35.9 | WHO | 135 (9.2) | 3 months | LMWH | Symptomatic DVT (65) | DUS in systematic patients | Age, sex, education, BMI, and CCI |
| Dy 2011 [17] | The US | RC | Patients received TKA or THA | 16317 | 64.8 | 39.9 | NCEP-ATP III | 1093 (6.7) | 3 months | NR | VTE (148) | ICD-9 codes | Age, sex, procedure type, and BMI |
| Valle 2012-TKA [18] | The US | RC | Patients received TKA | 806672 | 66.9 | 36.1 | NCEP-ATP III | 82852 (10.3) | In-hospital | NR | VTE (6476) | ICD-9 codes | Age, sex, race, admission type, comorbidities, and hospital level |
| Valle 2012-THA [18] | The US | RC | Patients received THA | 406265 | 65.6 | 42.5 | NCEP-ATP III | 24269 (6.0) | In-hospital | NR | VTE (1986) | ICD-9 codes | Age, sex, race, admission type, comorbidities, and hospital level |
| Mraovic 2013 [19] | The US | RC | Patients received TKA or THA | 7282 | 70.2 | 44.0 | NCEP-ATP III | 958 (13.2) | In-hospital | Warfarin to achieve INR: 1.5 ~ 2.0 | Symptomatic PE (107) | CT and/or lung VQ scan in systematic patients | Age, sex, procedure type, BMI, and comorbidities |
| Song 2016-TKA [21] | China | RC | Patients received TKA | 560 | 67.2 | 18.0 | WHO | 45 (8.0) | 1 month | Rivaroxaban or LMWH | Symptomatic DVT (25) | Routine venography | Age, sex, comorbidities, and smoking |
| Song 2016-THA [21] | China | RC | Patients received THA | 993 | 63.4 | 37.3 | WHO | 34 (3.4) | 1 month | Rivaroxaban or LMWH | Symptomatic DVT (53) | Routine venography | Age, sex, comorbidities, and smoking |
| Edelstein 2016 [20] | The US | RC | Patients received TKA or THA | 1462 | NR | 36.5 | WHO | 237 (16.2) | 1 month | NR | PE (33) | Medical insurance data | Age, sex, BMI, and comorbidities |
| Cichos 2018 [22] | The US | RC | Patients with hip fracture received THA | 100446 | 77.6 | 31.2 | NCEP-ATP III | 8437 (8.4) | In-hospital | NR | VTE (904) | ICD-9 codes | Age, sex, race, payer status and comorbidities |
MetS metabolic syndrome, TKA total knee arthroplasty, THA total hip arthroplasty, US United States, PC prospective cohort, RC retrospective cohort, WHO World Health Organization, NCEP-ATP III the National Cholesterol Education Program Expert Panel and Adult Treatment Panel III, PE pulmonary embolism, DVT deep vein thrombosis, VTE venous thromboembolism, DUS Doppler ultrasonography, ICD-9 the 9th revision of International Classification of Diseases, CT computed tomography, VQ ventilation/perfusion, BMI body mass index, CCI Charlson Comorbidity Index, NR not reported, INR international normalized ratio, LMWH low-molecular-weight heparin
Table 2.
Details of the study quality evaluation via the Newcastle-Ottawa Scale
| Study | Representativeness of the exposed cohort | Selection of the non-exposed cohort | Ascertainment of exposure | Outcome not present at baseline | Control for age and sex | Control for other confounding factors | Assessment of outcome | Enough long follow-up duration | Adequacy of follow-up of cohorts | Total |
|---|---|---|---|---|---|---|---|---|---|---|
| Gandhi 2009 [16] | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 8 |
| Dy 2011 [17] | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 6 |
| Valle 2012-TKA [18] | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 6 |
| Valle 2012-THA [18] | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 6 |
| Mraovic 2013 [19] | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 6 |
| Song 2016-TKA [21] | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Song 2016-THA [21] | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Edelstein 2016 [20] | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 7 |
| Cichos 2018 [22] | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 6 |
This Newcastle-Ottawa Scale ranges from 1 to 9 stars and judges the quality of each study regarding the nine domains as listed in the table, with higher scores indicating better study quality
Association between MetS and VTE after TJA
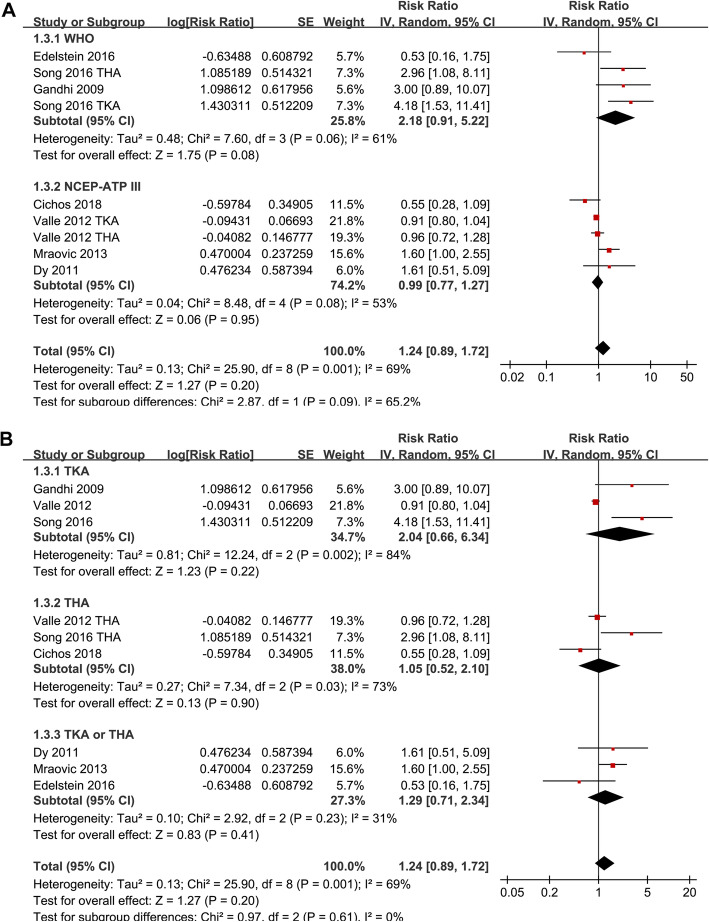
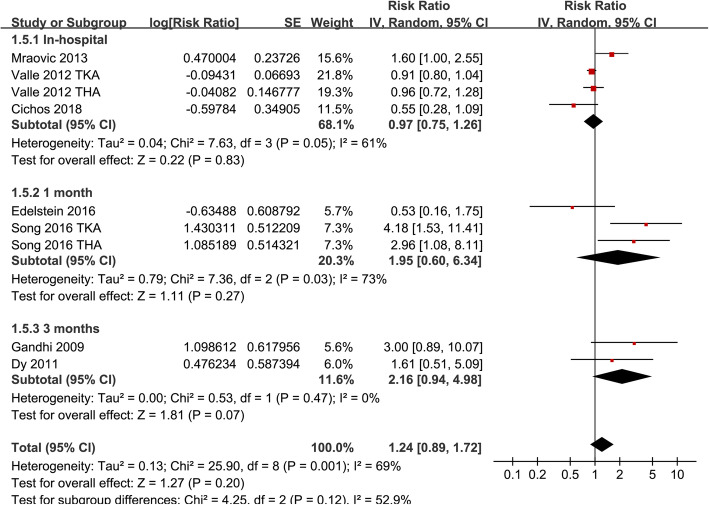
Significant heterogeneity was detected among the studies that evaluated the association between MetS and VTE risk after TJA (P for Cochrane’s Q test = 0.001, I2 = 69%). Pooled results with a random-effect model showed that MetS was not associated with increased overall VTE after TJA (adjusted RR = 1.24, 95% CI 0.89 ~ 1.72, p = 0.20; I2 = 69%; Fig. 2a). Sensitivity analysis by excluding one study at a time did not significantly affect the results (Table 3). Subgroup analyses showed that MetS was not associated with an increased the risk of PE (RR 1.06, 95% CI 0.37 ~ 3.02, p = 0.91) or VTE (RR 0.91, 95% CI 0.80 ~ 1.04, p = 0.16), but with an increased risk of DVT after TJA (RR 3.38, 95% CI 1.83 ~ 6.24, p < 0.001; p for subgroup difference < 0.001; Fig. 2b). Further studies showed that MetS was not significantly associated with an increased risk of VTE after TJA in studies with MetS diagnosed with WHO (RR = 2.18, 95% CI 0.91 ~ 5.22, p = 0.08) or NCEP-ATP III criteria (RR = 0.99, 95% CI 0.77 ~ 1.27, p = 0.95; Fig. 3a), in studies of patients received TKA (RR = 2.04, 95% CI 0.66 ~ 6.34, p = 0.22), THA (RR = 1.05, 95% CI 0.52 ~ 2.10, p = 0.90), or both (RR = 1.29, 95% CI 0.71 ~ 2.34, p = 0.41; Fig. 3b), and in studies of follow-up within hospitalization (RR = 0.97, 95% CI 0.75 ~ 1.26, p = 0.83), 1 month (RR = 1.95, 95% CI 0.60 ~ 6.34, p = 0.27), and 3 months (RR = 2.16, 95% CI 0.94 ~ 4.98, p = 0.07; Fig. 4) after JTA.
Fig. 2.
Meta-analysis for the association between MetS and the incidence of VTE after TKA or THA. a The main meta-analysis for the overall incidence of VTE. b subgroup Analysis according to the categories of VTE events
Table 3.
Results of sensitivity analysis
| Studies omitted | RR | 95% CI | I2 | P for effect |
|---|---|---|---|---|
| Gandhi 2009 [16] | 1.17 | 0.84 to 1.62 | 69% | 0.35 |
| Dy 2011 [17] | 1.22 | 0.86 to 1.72 | 72% | 0.26 |
| Valle 2012-TKA [18] | 1.40 | 0.89 to 2.22 | 68% | 0.15 |
| Valle 2012-THA [18] | 1.38 | 0.87 to 2.17 | 73% | 0.17 |
| Mraovic 2013 [19] | 1.18 | 0.82 to 1.68 | 67% | 0.37 |
| Song 2016-TKA [21] | 1.10 | 0.82 to 1.48 | 61% | 0.53 |
| Song 2016-THA [21] | 1.14 | 0.83 to 1.58 | 67% | 0.41 |
| Edelstein 2016 [20] | 1.31 | 0.93 to 1.84 | 72% | 0.13 |
| Cichos 2018 [22] | 1.37 | 0.95 to 1.98 | 70% | 0.10 |
RR risk ratio, CI confidence interval
Fig. 3.
Subgroup analyses the association between MetS and the incidence of VTE after TKA or THA. a Subgroup analysis according to the diagnostic criteria of MetS. b Subgroup analysis according to the types of surgical procedures
Fig. 4.
Subgroup analyses the association between MetS and the incidence of VTE after TKA or THA according to the follow-up durations
Publication bias
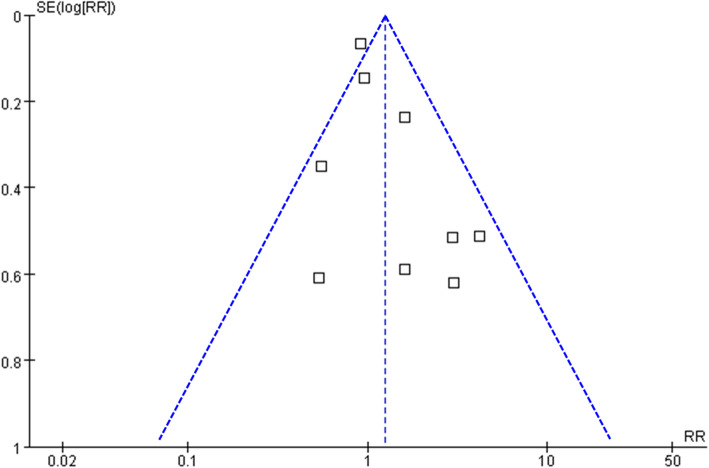
The funnel plots for the association between MetS and VTE after TKA or THA were shown in Fig. 5. The plots were symmetrical on visual inspection, suggesting low risks of publication biases. Results of Egger’s regression tests also showed similar results (p = 0.389).
Fig. 5.
Funnel plots for the meta-analyses of the association between MetS and the incidence of VTE after TKA or THA
Discussion
In this meta-analysis of cohort study, we found that MetS at baseline was not associated with an increased risk of overall VTE events in patients after TJA. Subgroup analysis showed that MetS was independently associated with an increased risk of DVT, but not PE in patients after TJA. Moreover, the results were not significantly affected by the diagnostic criteria of MetS, the types of surgical procedures, and the follow-up durations. Taken together, current evidence from observational studies suggests that MetS might be associated with an increased risk of DVT, but not PE in patients after TJA.
To the best of our knowledge, our study is the first meta-analysis to evaluate the potential association between MetS and VTE risk in patients after TJA. Although we did not show that MetS was independently associated with an increased incidence of overall VTE, subgroup analysis suggests that MetS was associated with an increased risk of DVT but not PE in these patients. The potential reasons for the findings remain unknown. Generally, PE was resulted by thrombi from the lower extremities of patients with DVT. Due to the routine use of prophylactic measures against VTE in patients after TJA, the incidence of PE in these patients is lower than the incidence of DVT [28]. In our study, the pooled incidence of DVT was 4.7% and compared to 1.6% for PE. It could be hypothesized that potential differences in patient characteristics or diagnostic strategies may be responsible for the observed different associations between MetS with DVT and PE. However, limited datasets included in the subgroup analysis prevented further analysis. Moreover, we found that the results were not significantly affected by the diagnostic criteria of MetS or the types of surgical procedures. Previous studies showed that patients after TKA seem to have a higher incidence of VTE events than those after THA [5, 28]. Although the overall meta-analysis did not show a significant association between MetS and VTE after TJA, subgroup analysis suggested that MetS was associated with increased DVT after TJA. Since only three datasets were included in the subgroup analysis of DVT events, the findings should be validated in large-scale prospective studies. A previous case-control study showed that patients with uncontrolled MetS had a significantly higher incidence of VTE after TJA compared to patients without MetS, while those with controlled MetS had a similar risk of VTE compared to patients without MetS [29], and the VTE events occurred were mainly DVT. These findings may suggest the importance of MetS control for the reducing the risk of DVT after TJA.
The potential pathophysiological mechanisms underlying the association between MetS and increased risk of DVT after TJA may be multifactorial. Patients with MetS are characterized by a persistent activated chronic inflammatory response. Since inflammation is a major activator of coagulation, these patients are generally at hypercoagulative status, thereby vulnerable to venous thrombosis [10, 11]. Moreover, it has been shown that patients with MetS have impaired spontaneous thrombolytic activity as reflected by an impaired expression of tissue-type plasminogen activator [30], which may also be involved in their vulnerability to thrombotic events. Besides, patients with MetS tend to be overnutrition and are more likely to be exposed to high-fat diet. Previous studies showed that high-fat diet maintains high endogenous thrombin potential, which is associated with venous and arterial thrombosis independently of obesity and insulin resistance [31]. However, the correlation of nutritional status and VTE risk could be complex, since recent studies showed that the poor nutrition status was also associated with a high risk of developing DVT, particularly for those who underwent major surgeries [32, 33]. Further studies are needed to elucidate the mechanisms underlying the association between MetS and DVT.
Our study has limitations, which should be considered when interpreting the results. Firstly, as a meta-analysis of observational studies, although we combined RR data after adjustment of potential confounding factors, we could not exclude other residual factors that may confound the association between MetS and DVT after TJA, such as the comorbidities of the patients and concurrent medications [34]. Secondly, due to the limited number of the included studies, the result of the subgroup analysis should be interpreted with caution. In addition, most of the included studies were retrospective, which may be limited by the recall bias compared to prospective studies. Moreover, the lengths of follow-up varied among the included studies. However, subgroup analysis showed that MetS was not associated with increased VTE risk after TJA in studies of follow-up within hospitalization, 1 month, and 3 months after JTA. Besides, the long-term (> 3 months) association between MetS and VTE risk after TJA remains to be determined in future studies. Finally, a causative relationship between MetS and increased DVT after TJA should not be retrieved from our results since it is a meta-analysis of observational studies.
Conclusions
In conclusion, our meta-analysis showed that current evidence from observational studies suggests MetS might increase the risk of DVT but not PE in patients that received TJA. Although these findings should be validated in prospective studies, the results of this meta-analysis may suggest the importance of MetS control for reducing the risk of DVT after TJA.
Acknowledgements
Not applicable.
Abbreviations
- MetS
Metabolic syndrome
- VTE
Venous thromboembolism
- DVT
Deep vein thrombosis
- PE
Pulmonary embolism
- TJA
Total joint arthroplasty
- TKA
Total knee arthroplasty
- THA
Total hip arthroplasty
- WHO
World Health Organization
- NCEP-ATP III
Revised National Cholesterol Education Program’s Adults Treatment Panel III
- MOOSE
Meta-analysis of Observational Studies in Epidemiology
- RRs
Risk ratios
- SE
Stand error
Authors’ contributions
YY and JT designed the study. YY and ZL performed the database search, literature review, data extraction, and study quality evaluation. YY and HL performed the statistical analyses, data interpretation, and drafted the manuscript. JT critically revised the manuscript. The authors reviewed the manuscript and approved its submission.
Funding
Not received.
Availability of data and materials
The raw data used in our study are included in the manuscript with tables, figures, and its supplementary information files. All the authors agreed that the data could be shared if required by the researchers.
Ethics approval and consent to participate
All analyses were based on previous published studies; thus, no ethical approval and patient consent are required. All previous published studies were approved by the ethics committee respectively.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Trivedi NN, Fitzgerald SJ, Schmaier AH, Wera GD. Venous thromboembolism chemoprophylaxis in total hip and knee arthroplasty: a critical analysis review. JBJS Rev. 2019;7(1):e2. doi: 10.2106/JBJS.RVW.18.00010. [DOI] [PubMed] [Google Scholar]
- 2.Mula V, Parikh S, Suresh S, Bottle A, Loeffler M, Alam M. Venous thromboembolism rates after hip and knee arthroplasty and hip fractures. BMC Musculoskelet Disord. 2020;21(1):95. doi: 10.1186/s12891-020-3100-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Warren JA, Sundaram K, Kamath AF, Molloy RM, Krebs VE, Mont MA, et al. Venous thromboembolism rates did not decrease in lower extremity revision total joint arthroplasty from 2008 to 2016. J Arthroplasty. 2019;34(11):2774–2779. doi: 10.1016/j.arth.2019.05.012. [DOI] [PubMed] [Google Scholar]
- 4.Lieberman JR, Heckmann N. Venous thromboembolism prophylaxis in total hip arthroplasty and total knee arthroplasty patients: from guidelines to practice. J Am Acad Orthop Surg. 2017;25(12):789–798. doi: 10.5435/JAAOS-D-15-00760. [DOI] [PubMed] [Google Scholar]
- 5.Januel JM, Chen G, Ruffieux C, Quan H, Douketis JD, Crowther MA, et al. Symptomatic in-hospital deep vein thrombosis and pulmonary embolism following hip and knee arthroplasty among patients receiving recommended prophylaxis: a systematic review. JAMA. 2012;307(3):294–303. doi: 10.1001/jama.2011.2029. [DOI] [PubMed] [Google Scholar]
- 6.Shahi A, Bradbury TL, Guild GN, 3rd, Saleh UH, Ghanem E, Oliashirazi A. What are the incidence and risk factors of in-hospital mortality after venous thromboembolism events in total hip and knee arthroplasty patients? Arthroplast Today. 2018;4(3):343–347. doi: 10.1016/j.artd.2018.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weihe P, Weihrauch-Bluher S. Metabolic syndrome in children and adolescents: diagnostic criteria, therapeutic options and perspectives. Curr Obes Rep. 2019;8(4):472–479. doi: 10.1007/s13679-019-00357-x. [DOI] [PubMed] [Google Scholar]
- 8.Engin A. The definition and prevalence of obesity and metabolic syndrome. Adv Exp Med Biol. 2017;960:1–17. doi: 10.1007/978-3-319-48382-5_1. [DOI] [PubMed] [Google Scholar]
- 9.Santilli F, Vazzana N, Liani R, Guagnano MT, Davi G. Platelet activation in obesity and metabolic syndrome. Obes Rev. 2012;13(1):27–42. doi: 10.1111/j.1467-789X.2011.00930.x. [DOI] [PubMed] [Google Scholar]
- 10.Mao X, Ait-Aissa K, Lagrange J, Youcef G, Louis H. Hypertension, hypercoagulability and the metabolic syndrome: a cluster of risk factors for cardiovascular disease. Biomed Mater Eng. 2012;22(1-3):35–48. doi: 10.3233/BME-2012-0688. [DOI] [PubMed] [Google Scholar]
- 11.Morange PE, Alessi MC. Thrombosis in central obesity and metabolic syndrome: mechanisms and epidemiology. Thromb Haemost. 2013;110(4):669–680. doi: 10.1160/TH13-01-0075. [DOI] [PubMed] [Google Scholar]
- 12.Gil JS, Drager LF, Guerra-Riccio GM, Mostarda C, Irigoyen MC, Costa-Hong V, et al. The impact of metabolic syndrome on metabolic, pro-inflammatory and prothrombotic markers according to the presence of high blood pressure criterion. Clinics (Sao Paulo). 2013;68(12):1495–1501. doi: 10.6061/clinics/2013(12)04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Basurto L, Diaz A, Rodriguez A, Robledo A, Vega S, Garcia-Vega J, et al. Circulating levels of plasminogen activator inhibitor-1 are associated with metabolic syndrome rather than with menopause. Gynecol Endocrinol. 2019:1–4. [DOI] [PubMed]
- 14.Mottillo S, Filion KB, Genest J, Joseph L, Pilote L, Poirier P, et al. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol. 2010;56(14):1113–1132. doi: 10.1016/j.jacc.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 15.Ageno W, Di Minno MN, Ay C, Jang MJ, Hansen JB, Steffen LM, et al. Association between the metabolic syndrome, its individual components, and unprovoked venous thromboembolism: results of a patient-level meta-analysis. Arterioscler Thromb Vasc Biol. 2014;34(11):2478–2485. doi: 10.1161/ATVBAHA.114.304085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gandhi R, Razak F, Tso P, Davey JR, Mahomed NN. Metabolic syndrome and the incidence of symptomatic deep vein thrombosis following total knee arthroplasty. J Rheumatol. 2009;36(10):2298–301. [DOI] [PubMed]
- 17.Dy CJ, Wilkinson JD, Tamariz L, Scully SP. Influence of preoperative cardiovascular risk factor clusters on complications of total joint arthroplasty. Am J Orthop (Belle Mead NJ). 2011;40(11):560–565. [PubMed] [Google Scholar]
- 18.Gonzalez Della Valle A, Chiu YL, Ma Y, Mazumdar M, Memtsoudis SG. The metabolic syndrome in patients undergoing knee and hip arthroplasty: trends and in-hospital outcomes in the United States. J Arthroplasty. 2012;27(10):1743–1749. doi: 10.1016/j.arth.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 19.Mraovic B, Hipszer BR, Epstein RH, Parvizi J, Pequignot EC, Chervoneva I, et al. Metabolic syndrome increases risk for pulmonary embolism after hip and knee arthroplasty. Croat Med J. 2013;54(4):355–361. doi: 10.3325/cmj.2013.54.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edelstein AI, Suleiman LI, Alvarez AP, Sacotte RM, Qin CD, Beal MD, et al. The interaction of obesity and metabolic syndrome in determining risk of complication following total joint arthroplasty. J Arthroplasty. 2016;31(9 Suppl):192–196. doi: 10.1016/j.arth.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 21.Song K, Rong Z, Yao Y, Shen Y, Zheng M, Jiang Q. Metabolic syndrome and deep vein thrombosis after total knee and hip arthroplasty. J Arthroplasty. 2016;31(6):1322–1325. doi: 10.1016/j.arth.2015.12.021. [DOI] [PubMed] [Google Scholar]
- 22.Cichos KH, Churchill JL, Phillips SG, Watson SL, McGwin G, Jr, Ghanem ES, et al. Metabolic syndrome and hip fracture: epidemiology and perioperative outcomes. Injury. 2018;49(11):2036–2041. doi: 10.1016/j.injury.2018.09.012. [DOI] [PubMed] [Google Scholar]
- 23.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 24.Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration. 2011. [Google Scholar]
- 25.Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2010. [Google Scholar]
- 26.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 27.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sloan M, Sheth N, Lee GC. Is obesity associated with increased risk of deep vein thrombosis or pulmonary embolism after hip and knee arthroplasty? A large database study. Clin Orthop Relat Res. 2019;477(3):523–532. doi: 10.1097/CORR.0000000000000615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zmistowski B, Dizdarevic I, Jacovides CL, Radcliff KE, Mraovic B, Parvizi J. Patients with uncontrolled components of metabolic syndrome have increased risk of complications following total joint arthroplasty. J Arthroplasty. 2013;28(6):904–907. doi: 10.1016/j.arth.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 30.Xie W, Zhai Z, Yang Y, Kuang T, Wang C. Free fatty acids inhibit TM-EPCR expression through JNK pathway: an implication for the development of the prothrombotic state in metabolic syndrome. J Thromb Thrombolysis. 2012;34(4):468–474. doi: 10.1007/s11239-012-0793-8. [DOI] [PubMed] [Google Scholar]
- 31.Sanchez C, Poggi M, Morange PE, Defoort C, Martin JC, Tanguy S, et al. Diet modulates endogenous thrombin generation, a biological estimate of thrombosis risk, independently of the metabolic status. Arterioscler Thromb Vasc Biol. 2012;32(10):2394–2404. doi: 10.1161/ATVBAHA.112.250332. [DOI] [PubMed] [Google Scholar]
- 32.e S, Yamato Y, Hasegawa T, Yoshida G, Kobayashi S, Yasuda T, et al. Association between a prognostic nutritional index less than 50 and the risk of medical complications after adult spinal deformity surgery. J Neurosurg Spine. 2020:1–6. 10.3171/2020.1.SPINE191410. Online ahead of print. [DOI] [PubMed]
- 33.Iguchi T, Sugimachi K, Mano Y, Kono M, Kagawa M, Nakanoko T, et al. The preoperative prognostic nutritional index predicts the development of deep venous thrombosis after pancreatic surgery. Anticancer Res. 2020;40(4):2297–2301. doi: 10.21873/anticanres.14195. [DOI] [PubMed] [Google Scholar]
- 34.Landy DC, Bradley AT, King CA, Puri L. Stratifying venous thromboembolism risk in arthroplasty: do high-risk patients exist? J Arthroplasty. 2020;35(5):1390–6. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data used in our study are included in the manuscript with tables, figures, and its supplementary information files. All the authors agreed that the data could be shared if required by the researchers.