Abstract
Background
Esophageal cancer is a common gastrointestinal malignancy in China. We evaluated the efficacy and safety of adding Apatinib to concurrent chemoradiotherapy in patients with locally advanced esophageal squamous cell carcinoma.
Material/Methods
In this single-center retrospective study, we compared short-term efficacy, long-term efficacy, and adverse events between patients who received Apatinib and concurrent chemoradiotherapy (Apatinib group), and those who received only concurrent chemoradiotherapy (CCRT group).
Results
Sixty-five patients with stage II and III esophageal squamous cell carcinoma were enrolled (31 in the Apatinib group, 34 in the CCRT group). After treatment, the therapy response rate (the sum of the complete and partial remission rates) was significantly higher in the Apatinib group than in the CCRT group (P=0.045); the complete remission rate was particularly higher in the Apatinib group. Median progression-free survival in the Apatinib group (12 months) was higher than that of the CCRT group (7 months), and the 1- and 2-year progression-free survival rates were significantly higher in the Apatinib group than in the CCRT group (47.0% vs. 30.3% and 20.2% vs. 12.1%, respectively; P=0.040). The main adverse effects of Apatinib treatment were elevated blood pressure, proteinuria, hand-foot syndrome, fatigue, and oral mucositis, all of which were level 1–2. Cox multivariate regression analysis indicated T stage and short-term efficacy were independent prognostic factors for overall and progression-free survival.
Conclusions
For patients with locally advanced esophageal squamous cell carcinoma, combining Apatinib with concurrent chemoradiotherapy can improve patient survival and significantly prolong progression-free survival, with tolerable adverse reactions.
MeSH Keywords: Chemoradiotherapy, Drug-Related Side Effects and Adverse Reactions, Esophageal Neoplasms
Background
Esophageal cancer is one of the most common gastrointestinal malignant cancers in China. According to a 2019 report from the National Cancer Center, China’s cases of esophageal cancer account for 43% of the global annual new cases and 37% of the annual global deaths due to this disease [1]. Among these cases, 86.3% were esophageal squamous cell carcinoma, and more than 70% of those patients had either advanced esophageal cancer or a complication of heart and lung disease as the primary diagnosis, preventing surgical treatment [2]. Radiation therapy is one of the main treatments for esophageal cancer. Although radiotherapy technology has dramatically improved in recent years, and concurrent chemoradiotherapy has shown good efficacy, the 5-year survival rate of locally advanced esophageal squamous cell carcinoma is still only 23% to 34% [3]. Apatinib is a new anti-angiogenesis small molecule drug, which can selectively inhibit the activity of VEGFR-2 tyrosine kinase and block the signal transmission of VEGF and its receptor, thus inhibiting tumor angiogenesis and tumor growth [4]. Previously, through in vitro assays, we found that Apatinib can induce apoptosis and cell cycle arrest in esophageal cancer cells Kyse-150; moreover, Apatinib increased the radiosensitivity of these cells and showed a synergistic effect when combined with X-ray radiation [5]. Li et al. consistently found that Apatinib was effective as a second- or further-line treatment for advanced esophageal cancer [4]. However, for patients with nonoperative locally advanced esophageal squamous cell carcinoma, the safety and efficacy of combining Apatinib with concurrent chemoradiotherapy are still unclear. Therefore, we aimed to investigate the prognosis of patients with nonoperative locally advanced esophageal squamous cell carcinoma and examined whether the combination of Apatinib and concurrent chemoradiotherapy could improve patient prognosis and survival.
Material and Methods
Clinical data
We retrospectively analyzed the records of patients admitted to the radiotherapy department of our hospital from May 2016 to April 2019. All patients had been confirmed to have esophageal squamous cell carcinoma by histopathology. The inclusion criteria were (1) age ≥18 years old; (2) Karnofsky Performance Score ≥80 points; (3) patients refused surgery or did not undergo surgery for other reasons; (4) patients received concurrent chemoradiotherapy as the treatment method, and the dose of radiotherapy was ≥50 Gy; (5) complete clinical and follow-up information was available; (6) there were no complications with serious internal disease and/or a second primary tumor. The exclusion criteria were (1) active hemorrhage or severe coagulation dysfunction; (2) severe uncontrolled hypertension; (3) severe cardiopulmonary diseases or abnormal liver and kidney function.
The age, sex, history of hypertension and diabetes, esophageal cancer location and type, TNM stage, tumor markers, treatment method (with or without Apatinib treatment), adverse reactions, and follow-up information of the enrolled patients were recorded. According to the treatment method received, the enrolled patients were divided into 2 groups: (1) concurrent chemoradiotherapy combined with Apatinib treatment (Apatinib group) and (2) concurrent chemoradiotherapy only (CCRT group) (Figure 1). All patients signed an informed consent form. The protocol of this study followed the principles of the Declaration of Helsinki and was approved by the Ethics Committee of our hospital.
Figure 1.
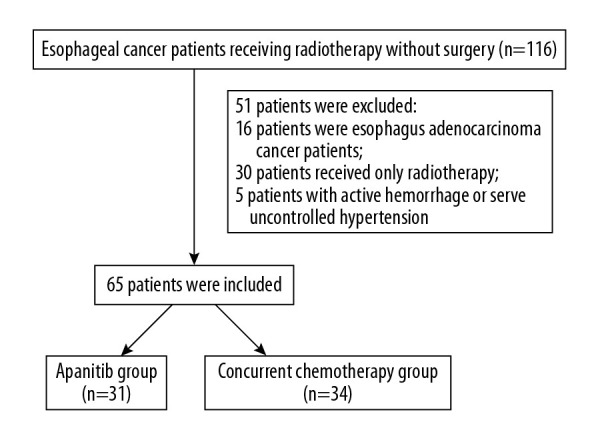
Flowchart of the study.
Treatment methods
The 3 treatment methods that were administered in this study were as follows:
Radiotherapy: The 6-MV X-ray conformal radiotherapy and intensity-modulated radiotherapy from Siemens (Germany) were used. Computed tomography (CT) simulation was used to design the specific radiotherapy plan of patients. The gross tumor volume (GTV) included the esophagus with the thickened wall as shown by CT, esophageal film, and esophagoscopy and swollen lymph nodes with a short diameter ≥1 cm. The clinical target volume (CTV) was 0.5 cm to 0.8 cm extended from the GTV on the front, back, left, and right, and 2.5 cm to 3.0 cm extended on the top and bottom, plus the corresponding lymphatic drainage area. The planning target volume (PTV) was 0.5 cm extended from the CTV on all sides. The planning gross tumor volume (PGTV) was 0.5 cm to 1 cm extended from the primary GTV on the front, back, left and right, and 1 cm extended on the top and bottom, plus the 0.5 cm to 1 cm extended from the lymph node GTV on all sides. The target area was adjusted smaller for patients older than 75 years. The prescription dose for PGTV was 60 Gy to 66 Gy, 2 Gy per time, 1 time per day, 5 days per week. The prescription dose for PTV was 50 Gy, 2 Gy per time, 1 time per day, 5 days per week, divided regularly. The maximum dose delivered to the spinal cord was <45 Gy; the average dose to the lung <13 Gy; lung V20 <28%; and heart V50 <45%.
Chemotherapy: The combination chemotherapy of liposomal paclitaxel and cisplatin was used. The liposomal paclitaxel (Nanjing Lvye Sike Pharmaceutical Co, China) was given via IV infusion of 135 mg/m2 on the first day; then, cisplatin (Jiangsu Haosen Pharmaceutical Co, China) was given via IV infusion of 20 mg/m2 from day 1 to day 4 (Figure 2). One treatment cycle was 21 days, and the patients received 2 chemotherapy treatment cycles.
Apatinib treatment: All patients in the Apatinib group received Apatinib treatment (Jiangsu Hengrui Pharmaceutical Co, China) at a dose of 500 mg once per day. Apatinib was taken orally 30 min after breakfast. The treatment continued until the disease progressed or unacceptable adverse reactions occurred. According to the severity of the adverse reactions, the drug was either suspended or the dose was adjusted to 250 mg once per day (Figure 2).
Figure 2.
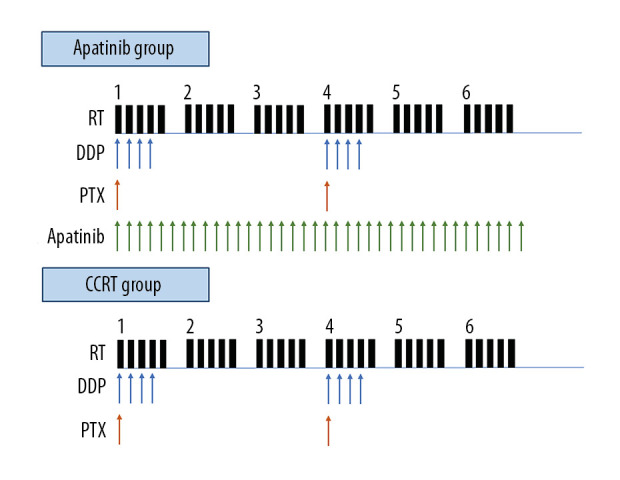
Schematic of the treatment plan for the chemoradiotherapy plus Apatinib (Apatinib) group and the concurrent chemoradiotherapy only (CCRT) group.
Evaluation criteria of short-term efficacy and acute adverse reactions
To evaluate treatment efficacy in the first and third months, all patients received an esophageal X-ray after swallowing barium, chest and abdomen CT, and other relevant examinations. According to WHO standards, treatment efficacy can be divided into complete remission, partial remission, stable disease, and disease progression. Complete remission and partial remission were considered clinically effective. The acute adverse reactions of radiotherapy were evaluated according to the early radiation reaction standard of the American Radiation Oncology Group, and the adverse reactions of chemotherapy were assessed according to the acute and subacute toxicity index scale of anti-cancer drugs established by the WHO.
Follow-up
Patients were followed up every 3 months in the first 2 years after treatment and every 6 months after 2 years. Follow-up included medical history, physical examination, laboratory examination, electrocardiogram, abdominal ultrasound, esophageal barium meal, and chest CT. The overall survival (OS), progression-free survival (PFS), and local control rate were used as evaluation indexes. The follow-up ended on April 30, 2020, with a median follow-up time of 30 months (12–48 months). Two cases were lost to follow-up (2.99%).
Statistical methods
SPSS 26.0 software was used to perform the statistical analysis. Normally distributed continuous variables were expressed as mean±standard deviation, and non-normally distributed continuous variables were expressed as median (P50) and interquartile boundary values (P25, P75). Categorical variables were expressed as frequency (%). For comparison between groups, continuous variables were tested using the unpaired t test or Mann-Whitney nonparametric test, and categorical variables were tested using the Pearson chi-square test or Fisher exact test. Survival analysis was conducted using the Kaplan-Meier method and log-rank test. Multivariate analysis was performed using the Cox proportional hazards model. A nomogram was drawn by R software (version 3.4.3, http://www.R-project.org). P<0.05 was considered statistically significant.
Results
Clinical data
A total of 65 patients were enrolled in this study, of which 31 were in the Apatinib group and 34 were in the CCRT group. The comparison of clinical data between the 2 groups is shown in Table 1.
Table 1.
Baseline characteristics of study participants.
| Apatinib group (n=31) | CCRT group (n=34) | P | |
|---|---|---|---|
| Male (%) | 21 (77.4%) | 24 (73.5%) | 0.716 |
| Age (years) | 70.5±6.7 | 69.5±8.2 | 0.516 |
| Hypertension (%) | 15 (48.4%) | 12 (35.3%) | 0.285 |
| Diabetes (%) | 3 (10.0%) | 3 (8.8%) | 0.872 |
| Tumor location | 0.389 | ||
| Neck, upper thoracic part | 11 (35.5%) | 7 (20.6%) | |
| Middle thoracic part | 14 (45.2%) | 20 (58.8%) | |
| Lower thoracic part | 6 (19.4%) | 7 (20.6%) | |
| Tumor type | 0.618 | ||
| Medullary type | 23 (74.2%) | 27 (79.4%) | |
| Ulcerative type, constrictive type | 8 (25.8%) | 7 (20.6%) | |
| T staging | 0.509 | ||
| T1–2 | 15 (48.4%) | 12 (35.3%) | |
| T3 | 9 (29.0%) | 14 (41.2%) | |
| T4 | 7 (22.6%) | 8 (23.5%) | |
| N staging | 0.643 | ||
| N0 | 1 (3.2%) | 2 (5.9%) | |
| N1 | 15 (48.4%) | 19 (55.9%) | |
| N2 | 15 (48.4%) | 13 (38.2%) | |
| TNM staging | 0.571 | ||
| Stage II | 14 (45.2%) | 13 (38.2%) | |
| Stage III | 17 (54.8%) | 21 (61.8%) | |
| CEA | 2.7 (1.5, 4.0) | 2.7 (1.9, 3.9) | 0.927 |
| SCC | 1.5 (0.8, 2.5) | 1.8 (1.2, 3.5) | 0.184 |
| Radiotherapy dose (Gy) | 56.3±5.8 | 57.5±5.4 | 0.405 |
Apatinib group – chemoradiotherapy plus Apatinib group; CCRT group – concurrent chemoradiotherapy only group; CEA – carcinoembryonic antigen; SCC – squamous cell carcinoma antigen.
Short-term efficacy evaluation
After treatment, the percentage of patients who achieved complete remission and partial remission in the Apatinib group were 22.6% and 61.3%, respectively, and the combined response rate was 83.9%; the complete remission and partial remission rates in the CCRT group were 2.9% and 70.6%, respectively, and the combined response rate was 73.5%. There was a significant difference in response rate between the 2 groups (P=0.045).
Survival analysis
The median OS of all patients was 16 months, and the median PFS was 9 months. The 1-year and 3-year OS rates for all patients were 67.7% and 20.0%, respectively, and the 1-year and 3-year PFS rates were 40.0% and 6.2%, respectively. In the Apatinib group, the median OS and 1-year and 3-year OS rates were 20 months and 71.0% and 24.1%, respectively; and in the CCRT group, these values were 16 months and 61.8% and 12.4%, respectively; the differences between the 2 groups were not significantly different (χ2=0.711, P=0.399, Figure 3). Moreover, the median PFS and 1-year and 2-year PFS rates in the Apatinib group were 12 months and 47.0% and 20.2%, respectively; and 7 months and 30.3% and 12.1%, respectively, in the CCRT group. These differences between the 2 groups were significantly different (χ2=4.225, P=0.040, Figure 4).
Figure 3.
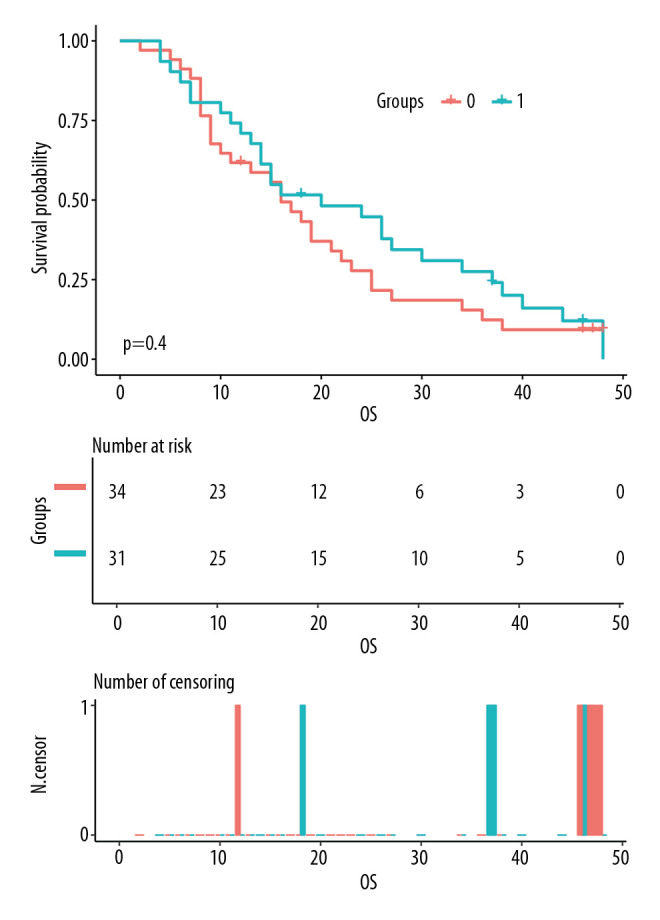
Comparison of overall survival (OS) between the chemoradiotherapy plus Apatinib (Apatinib) group and the concurrent chemoradiotherapy only (CCRT) group. Group 1: Apatinib group; Group 0: CCRT group.
Figure 4.
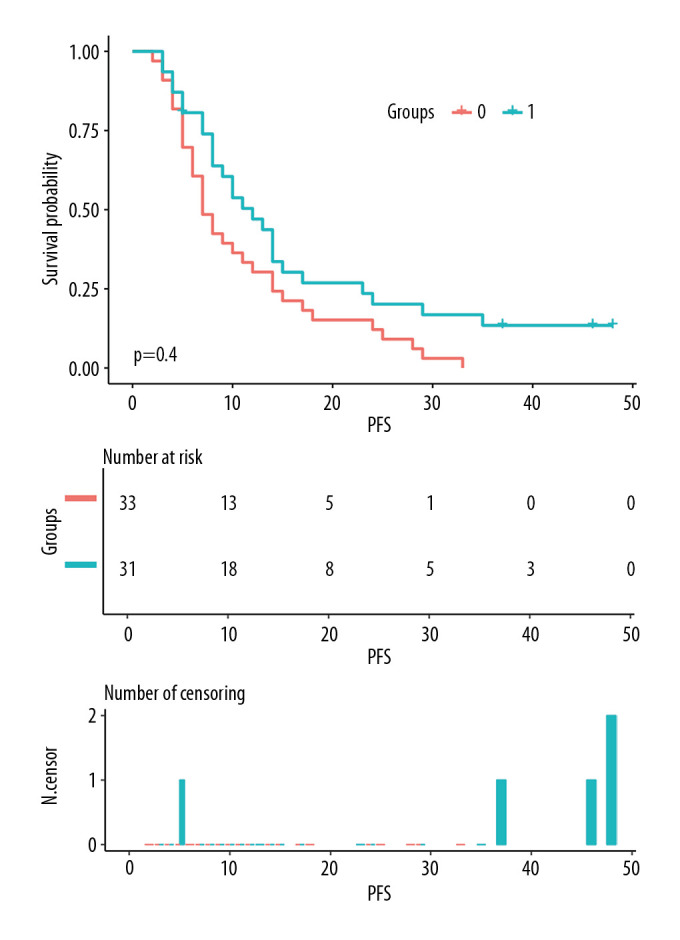
Comparison of progression-free survival (PFS) between the chemoradiotherapy plus Apatinib (Apatinib) group and the concurrent chemoradiotherapy only (CCRT) group. Group 1: Apatinib group; Group 0: CCRT group.
Comparison of adverse reactions between the 2 groups
The main adverse reactions of Apatinib were increased blood pressure (25.8%), proteinuria (9.7%), fatigue (16.1%), hand-foot syndrome (3.2%), and oral mucositis (3.2%), all of which were level 1 to 2 adverse reactions. The incidences of radiation esophagitis, radiation pneumonia, and bone marrow suppression in the 2 groups are compared in Table 2.
Table 2.
Comparing the incidences of radiation esophagitis, radiation pneumonia, and bone marrow suppression between the 2 groups.
| Adverse reactions | Apatinib group (31 cases) | CCRT group (34 cases) | χ2 | P |
|---|---|---|---|---|
| Bone marrow suppression III–IV degree | 11 (35.5%) | 13 (38.2%) | 1.323 | 0.989 |
| Radiation esophagitis 3–4 degree | 14 (45.1%) | 24 (70.5%) | 4.638 | 0.071 |
| Radiation pneumonia 2–3 degree | 16 (51.6%) | 22 (64.7%) | 6.076 | 0.056 |
Apatinib group – chemoradiotherapy plus Apatinib group; CCRT group – concurrent chemoradiotherapy only group.
Analysis of prognostic factors
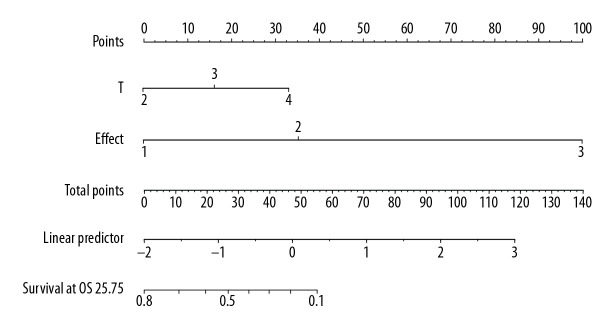
Univariate analysis showed that T stage, N stage, TNM stage, short-term efficacy, and CEA (>4.3 ng/ml) were the influencing factors of OS time (χ2=9.53–65.50, P<0.05), while sex, age, treatment group, tumor location, pathological type, and SCC (>1.5 ng/ml) had no significant effect on patient OS time (χ2=0.000–1.304, P>0.05). However, treatment group, T stage, N stage, TNM stage, short-term efficacy, and CEA (>4.3 ng/ml) were the influencing factors for PFS time (χ2=4.225–53.690, P<0.05). Cox multivariate regression analysis showed that T stage and short-term efficacy were independent prognostic factors for OS time and PFS time (Table 3). Based on this result, we constructed the nomogram for predicting OS (Figure 5) and PFS (Figure 6) of patients with nonoperative esophageal squamous cell carcinoma. The analysis showed that earlier T stage and better short-term efficacy could predict longer OS and PFS.
Table 3.
Cox multivariate regression analysis of the prognostic factors for nonoperative esophageal squamous cell carcinoma patients.
| Overall survival | Progression-free survival | |||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| T staging | ||||
| T1–2 | 1 (Reference) | 0.006 | 1 (Reference) | 0.045 |
| T3 | 2.108 (1.119, 3.973) | 0.224 | 1.644 (1.001, 2.999) | 0.035 |
| T4 | 3.275 (1.529, 7.013) | 0.002 | 2.385 (1.147, 4.960) | 0.020 |
| Short-term efficacy | ||||
| CR | 1 (Reference) | 0.000 | 1 (Reference) | 0.000 |
| PR | 3.654 (1.256, 10.629) | 0.017 | 3.539 (1.327, 9.439) | 0.012 |
| SD | 51.519 (13.744, 193.116) | 0.000 | 31.080 (9.147, 105.598) | 0.000 |
CR – complete remission; HR – hazards ratio; PR – partial remission; SD – stable disease.
Figure 5.
The nomogram predicting the overall survival time of patients with locally advanced esophageal squamous cell carcinoma.
Figure 6.
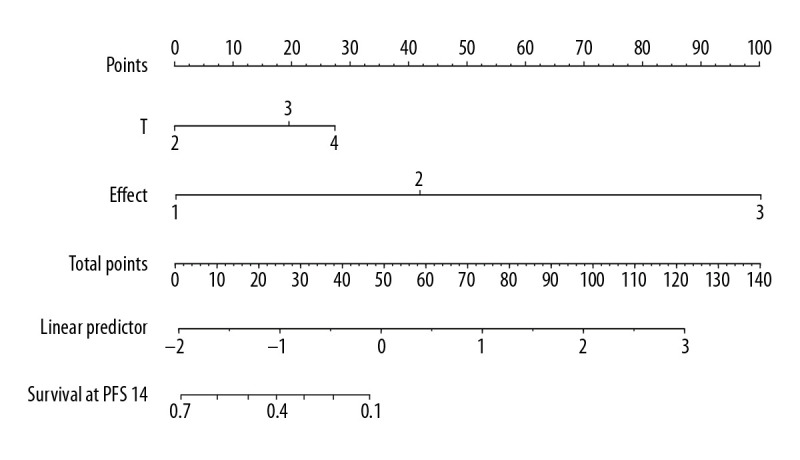
The nomogram predicting the progression-free survival time of patients with locally advanced esophageal squamous cell carcinoma.
Discussion
The RTOG85-01 trial showed that in patients with T1-3N0-1M0 esophageal cancer, the 5-year survival rate of cisplatin/5-fluorouracil chemotherapy plus 50.4 Gy radiotherapy was significantly higher than 64 Gy radiotherapy alone [6]. This result demonstrated the efficacy of concurrent radiotherapy and chemotherapy in the nonsurgical treatment of locally advanced esophageal cancer. However, even with concurrent therapies, the local recurrence rate is still as high as 45% [6,7]. A multicenter phase II clinical trial (JCOG9516), which included 60 patients with clinical T4 and/or distant lymph node metastasis (M1 Lym) thoracic esophageal squamous cell carcinoma, found that the complete remission rate of concurrent chemoradiotherapy was only 15%, and the 2-year survival rate was 31.5% [8]. Our present study also included patients with clinical T4 esophageal squamous cell carcinoma, and we found that the median OS of the CCRT group was only 16 months, and the 2-year OS rate was only 12.4%. Therefore, for the Southeast Asia countries with a high incidence of esophageal squamous cell carcinoma, improvement of the therapeutic effect, reduction of recurrence and metastasis, and improvement of prognosis are the major goals of esophageal cancer research.
In recent years, the development of molecular targeted therapy has provided new treatment strategies for esophageal cancer. Studies have shown that patients with esophageal cancer with a high expression of VEGF had 1.82 times higher risk of death, indicating that anti-VEGF therapy may improve the efficacy and prognosis of esophageal cancer [9]. Bevacizumab was the first drug approved by the FDA for anti-tumor angiogenesis therapy and has been widely used in the treatment of colorectal cancer, non-small-cell lung cancer, glioma, cervical cancer, and ovarian cancer [10]; however, most patients with squamous cell carcinoma cannot benefit from it [11]. Apatinib is a new small molecule tyrosine kinase inhibitor. It can selectively suppress VEGFR-2 tyrosine kinase activity, block its downstream signal transduction, and effectively inhibit tumor angiogenesis. The phase III clinical study conducted by Li et al. [12] (registration number: NCT01512745) showed that, in patients with advanced gastric cancer or adenocarcinoma of the gastroesophageal junction that failed second-line standard chemotherapy or above, the median OS and median PFS of the Apatinib group were both significantly longer than those of the placebo group (6.5 months vs. 4.7 months, HR: 0.709, 95% CI: 0.537–0.937, P=0.0149; 2.6 months vs. 1.8 months, HR: 0.444, 95% CI: 0.331–0.595, P<0.001, respectively). Moreover, Apatinib showed good efficacy and safety in the treatment of various tumors such as lung cancer [13], liver cancer [14], and breast cancer [15]. However, Apatinib has been rarely used in esophageal cancer treatment, especially esophageal squamous cell carcinoma, and there is a lack of medical evidence about whether Apatinib can improve the efficacy of concurrent chemoradiotherapy in patients with locally advanced esophageal squamous cell carcinoma.
Through in vitro studies, we found that Apatinib could induce apoptosis in esophageal squamous carcinoma cell lines KYSE-150 and ECA-109, thus inhibiting cell proliferation, migration, clone formation, and the growth of xenografted esophageal tumors in nude mice [16]. Moreover, we found that Apatinib had a synergistic effect with X-ray for killing esophageal squamous carcinoma cells through the mechanisms of increasing cell radiosensitivity, inhibiting cell proliferation, promoting apoptosis, causing DNA double-strand break, and cell cycle arrest [5]. In the present retrospective study, we found that the median OS of the patients treated with Apatinib and concurrent chemoradiotherapy was better than that of the CCRT group, although the difference was not significant (P>0.05); and the median PFS of the Apatinib group was significantly better than that of the control group (P<0.05), suggesting that Apatinib can improve the survival of patients with locally advanced esophageal squamous cell carcinoma. Moreover, the use of Apatinib increased the incidence of related adverse reactions, including increased blood pressure, proteinuria, hand-foot syndrome, fatigue, and oral mucositis, which was similar to the previously reported phase II/III clinical studies [4,12]. However, we did not observe the increased occurrence of bone marrow suppression, radiation esophagitis, or radiation pneumonia in the present study. All adverse reactions were manageable and tolerable in the present study. In addition, previous studies [4] found that patients with grade 3/4 toxicities had a longer PFS than those without grade 3/4 toxicities, and some toxicities were predictive factors for the efficacy of Apatinib treatment.
Univariate analysis in the present study indicated that the combined use of Apatinib could improve the prognosis of patients with locally advanced esophageal squamous cell carcinoma, but it was not a prognostic factor in Cox multivariate regression analysis, and the only independent influencing factors were short-term efficacy and T stage. This result was consistent with the observations in our in vitro experiments [5], indicating that Apatinib has only a synergistic or sensitizing effect on concurrent chemoradiotherapy. Regardless of the treatment plan used in the present study, short-term efficacy was positively correlated with prognosis. Furthermore, we found that T stage was an independent prognostic factor for locally advanced esophageal squamous cell carcinoma. The reason might be that, with the increase of T stage, the tumor load and depth of lesion infiltration gradually increased, with apparent tumor invasion by the T4 stage. Therefore, patients with higher a T stage of esophageal cancer might have a poorer response [17].
This study had several limitations. First, the study was a single-center retrospective study, which could have caused the bias in the selection of patients receiving Apatinib treatment. Second this study had a relatively small sample size. Third, the WHO criteria that we used were not as practical as, for instance, the response evaluation criteria in solid tumors (RECIST). Although these limitations existed in our research, we still observed the benefits of Apatinib treatment in patients with esophageal squamous cell carcinoma. However, a large sample and randomized multicenter prospective clinical trial is needed to confirm our results.
Conclusions
In summary, for patients with locally advanced esophageal squamous cell carcinoma, the combined use of Apatinib and concurrent chemoradiotherapy can improve patient survival and significantly prolong PFS, with tolerable adverse reactions.
Footnotes
Conflict of interest
None.
Source of support: This study was supported by the Jiangsu Province “333 Project” Second Level Talent Scientific Research Funding Project (No. BRA2019025), Changzhou High Level Health Talent Project (No. 2016C2BJ007), and Key Project of Science and Technology Development Fund of Nanjing Medical University (No. 2017NJMUZD039)
References
- 1.Sun K, Zheng R, Zhang S, et al. Report of cancer incidence and mortality in different areas of China, 2015. Chin J Cancer. 2019;28:1–11. [Google Scholar]
- 2.Wang X, Wang L, Chen J, et al. Prognostic analysis of definitive three-dimensional radiotherapy for non-surgically resectable esophageal squamous cell carcinoma: A multi-center retrospective study (3JECROG R-01) Chin J Radiat Oncol. 2018;27:959–64. [Google Scholar]
- 3.Welsh J, Settle SH, Amini A, et al. Failure patterns in patients with esophageal cancer treated with definitive chemoradiation. Cancer. 2012;118:2632–40. doi: 10.1002/cncr.26586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yanwei L, Feng H, Ren P, et al. Safety and efficacy of Apatinib monotherapy for unresectable, metastatic esophageal cancer: A single-arm, open-label, phase II study. Oncologist. 2020 doi: 10.1634/theoncologist.2020-0310. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu L, Sun F, Sun Z, et al. Apatinib enhances the radiosensitivity of the esophageal cancer cell line KYSE-150 by inducing apoptosis and cell cycle redistribution. Oncol Lett. 2019;17:1609–16. doi: 10.3892/ol.2018.9803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper JS, Guo MD, Herskovic A, et al. Chemoradiotherapy of locally advanced esophageal cancer: Long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA. 1999;281:1623–27. doi: 10.1001/jama.281.17.1623. [DOI] [PubMed] [Google Scholar]
- 7.Minsky BD, Pajak TF, Ginsberg RJ, et al. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: High-dose versus standard-dose radiation therapy. J Clin Oncol. 2002;20:1167–74. doi: 10.1200/JCO.2002.20.5.1167. [DOI] [PubMed] [Google Scholar]
- 8.Ishida K, Ando N, Yamamoto S, et al. Phase II study of cisplatin and 5-fluorouracil with concurrent radiotherapy in advanced squamous cell carcinoma of the esophagus: A Japan Esophageal Oncology Group (JEOG)/Japan Clinical Oncology Group trial (JCOG9516) Jpn J Clin Oncol. 2004;34:615–19. doi: 10.1093/jjco/hyh107. [DOI] [PubMed] [Google Scholar]
- 9.Wang J, Yu JP, Wang JL, et al. [Pathologic response and changes of serum VEGF during chemoradiotherapy may predict prognosis in non-surgical patients with esophageal carcinoma]. Zhonghua Zhong Liu Za Zhi. 2016;38:589–95. doi: 10.3760/cma.j.issn.0253-3766.2016.08.005. [in Chinese] [DOI] [PubMed] [Google Scholar]
- 10.Teleanu RI, Chircov C, Grumezescu AM, Teleanu DM. Tumor angiogenesis and anti-angiogenic strategies for cancer treatment. J Clin Med. 2019;9(1):84. doi: 10.3390/jcm9010084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manzo A, Montanino A, Carillio G, et al. Angiogenesis inhibitors in NSCLC. Int J Mol Sci. 2017;18:2021. doi: 10.3390/ijms18102021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li J, Qin S, Xu J, et al. Randomized, double-blind, placebo-controlled phase III trial of Apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J Clin Oncol. 2016;34:1448–54. doi: 10.1200/JCO.2015.63.5995. [DOI] [PubMed] [Google Scholar]
- 13.Tang J, Li XY, Liang JB, et al. Apatinib plus chemotherapy shows clinical activity in advanced NSCLC: A retrospective study. Oncol Res. 2019;27:635–41. doi: 10.3727/096504018X15288447760357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liao J, Jin H, Li S, et al. Apatinib potentiates irradiation effect via suppressing PI3K/AKT signaling pathway in hepatocellular carcinoma. J Exp Clin Cancer Res. 2019;38:454. doi: 10.1186/s13046-019-1419-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu J, Liu Q, Li Y, et al. Efficacy and safety of camrelizumab combined with apatinib in advanced triple-negative breast cancer: An open-label phase II trial. J Immunother Cancer. 2020;8(1):e000696. doi: 10.1136/jitc-2020-000696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng Y, Zhou MY, Sun F, et al. [The inhibition effects of apatinib on cell proliferation, migration and apoptosis in esophageal carcinoma via Ras/Raf/MEK/ERK and JAK2/STAT3 pathways]. Zhonghua Zhong Liu Za Zhi. 2019;41:263–75. doi: 10.3760/cma.j.issn.0253-3766.2019.04.005. [in Chinese] [DOI] [PubMed] [Google Scholar]
- 17.Sun NN, Liu C, Ge XL, Wang J. Dynamic contrast-enhanced MRI for advanced esophageal cancer response assessment after concurrent chemoradiotherapy. Diagn Interv Radiol. 2018;24:195–202. doi: 10.5152/dir.2018.17369. [DOI] [PMC free article] [PubMed] [Google Scholar]