To the Editor:
Obstructive sleep apnea (OSA) is common and associated with many adverse health outcomes (1–3). Continuous positive airway pressure (CPAP) virtually eliminates OSA, but adherence is variable (4). Thus, there has been great interest in interventions to improve CPAP usage, but success has been limited (4). Two recent studies suggest that the nonanatomic trait “low arousal threshold” (ArTH; waking up easily) predicts low CPAP adherence (5, 6). Importantly, the ArTH can be increased with sedatives such as eszopiclone (7) and thus may be a therapeutic target to increase CPAP usage. However, the two prior reports focused on distinct subsets of patients with OSA (post-stroke patients [6]), lacked in part objective adherence data (5), and did not assess the effect of interventions targeting the ArTH on CPAP usage (5, 6). To address these issues, we performed a secondary analysis of a randomized trial that showed that 2 weeks of eszopiclone 3 mg versus placebo at CPAP initiation improved objectively measured CPAP adherence over 6 months in a cohort of patients with general OSA (8). Thus, for the present study, we tested our hypotheses that 1) a low ArTH at baseline predicts low CPAP usage, and 2) the effect of eszopiclone on CPAP adherence is modified by the ArTH. Some of the results of the present study have been previously reported in the form of an abstract (9).
We obtained a deidentified subset of the original data set containing basic demographics, the Epworth sleepiness scores (ESS), OSA parameters, and CPAP adherence at 1 month for 162 subjects (2 subjects in the placebo group piloted study procedures before randomization of 160 subjects; we were unable to identify and remove these 2 nonrandomized subjects). Using a validated formula (10, 11), we estimated the ArTH for each subject based on age, sex, body mass index (BMI), apnea–hypopnea index (AHI, hypopneas defined by 3% desaturations or cortical arousals), oxygen saturation as measured by pulse oximetry (SpO2) nadir, and fraction of hypopneas and then dichotomized the ArTH into low versus high using a standard cutoff of 15 cm H2O (10). The BMI had originally been collected for each subject, but these data could not be retrieved for this secondary analysis. To allow calculation of the ArTH, we imputed individuals’ BMI using the mean BMI previously reported for the eszopiclone versus placebo group (mean, 30.3 vs. 30.4 kg/m2). Missing data for age (number missing = 3) and SpO2 nadir (number missing = 6) were minimal and thus not imputed. Outcomes were mean CPAP use per night (primary) and percentage of nights with >4 hours at 1 month (secondary). Using the Wilcoxon rank-sum test, we compared CPAP adherence in subjects 1) with a low versus high ArTH and 2) who received eszopiclone versus placebo stratified by the ArTH. To adjust for potential confounding by baseline characteristics (baseline AHI, ESS, CPAP level, sex, age, and residual AHI), we used median regression (backward selection based on Bayes’ information criterion) (6). We used median regression to assess for an interaction between ArTH and study drug (results from a two-way ANOVA were similar but the normality and homogeneous variance assumptions were violated despite log transformation). For the primary analysis, we assumed that subjects who discontinued CPAP within the first month had zero usage per night. In sensitivity analysis, we excluded such patients.
We included 153 of 162 subjects in whom we could estimate the ArTH (9 had missing data for age and/or SpO2 nadir). Subjects were mostly middle-aged (median, 44 yr [interquartile range (IQR), 36–50]) men (76%) with moderate OSA (AHI, 23/h [IQR, 13–43.5]) and a low ArTH (66%). The low-ArTH subgroup had less severe OSA than the high-ArTH subgroup (mean [SD] AHI, 18.3 [9.8] vs. 58.8/h [27.2]; P < 0.001); otherwise, groups were clinically similar: age (43.1 [9.6] vs. 44.6 [9.0] yr; P = 0.33), male sex (67.3% vs. 80.2%; P = 0.11), assignment to eszopiclone (50.5% vs. 50%; P = 0.95), subjective sleepiness (ESS, 12.0 [4.5] vs. 13.5 [4.3]; P = 0.048), residual AHI during titration (median 2.7/h [IQR, 0.9–6] vs. 3.4/h [2–11.6]; P = 0.04), and prescribed CPAP level (9.4 [2.1] vs. 10.6 [2.6] cm H2O; P = 0.003).
Patients with a low ArTH were more likely to discontinue CPAP during the first month than those with a high ArTH (36.6% vs. 17.3%; P = 0.02 using Fisher’s exact test). Eszopiclone did not significantly affect the percentage of patients who discontinued CPAP overall or within the ArTH subgroups (P ≥ 0.14 using Fisher’s exact test).
In patients with a low ArTH, median CPAP usage was 2.5 hours less than those with a high ArTH (1.3 h [IQR, 0–3.9] vs. 3.8 h [0.6 – 5.6]; P = 0.001; Figure 1A). Results were similar when adjusting for baseline characteristics (−2.1 h; adjusted P = 0.003; the final model included CPAP level, age, and residual AHI as covariates). Results were also similar in sensitivity analyses adjusting for baseline AHI as a covariate (−2.9 h; adjusted P = 0.04), or when excluding the subjects who discontinued CPAP by 1 month (−2.3 h; P = 0.04; Figure 2A). Moreover, the difference in median CPAP usage between the low- versus high-ArTH subgroups was numerically similar in women (−2.8 h; P = 0.09) and men (−2.3 h; P = 0.007), suggesting that sex did not substantially modify this relationship.
Figure 1.
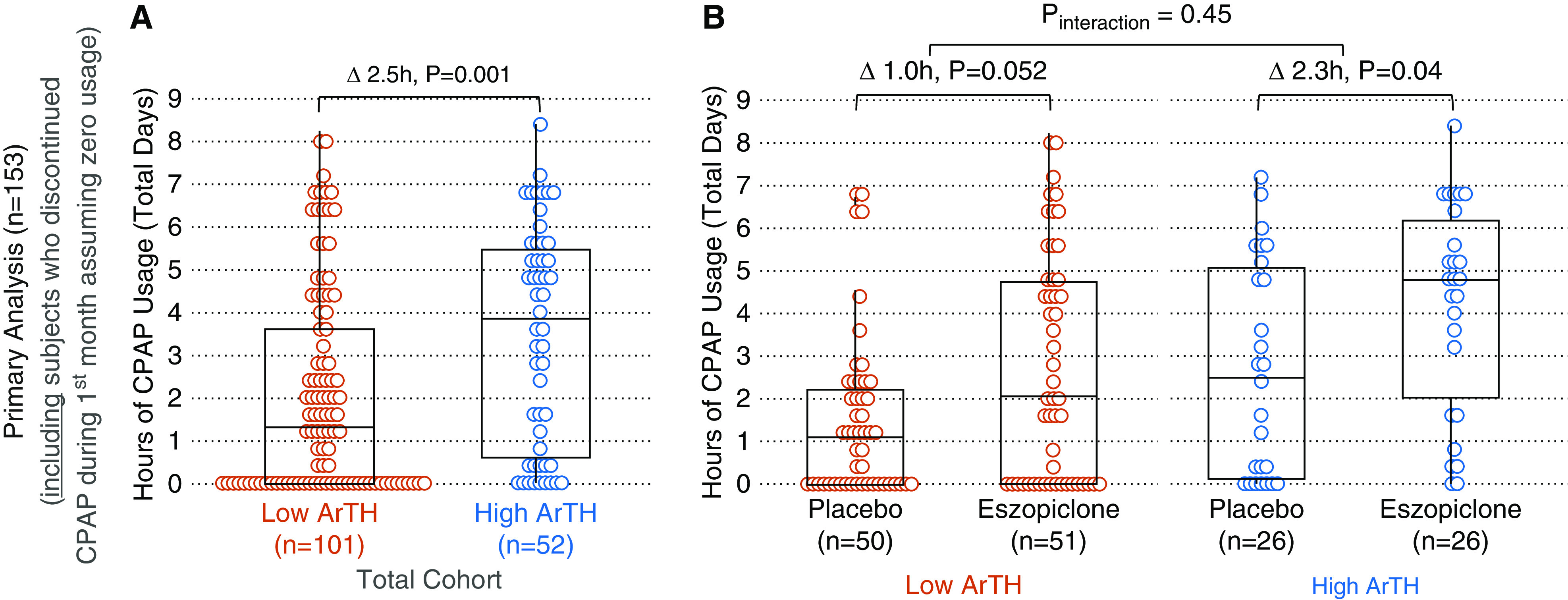
(A) Primary analysis: average hours of nightly continuous positive airway pressure (CPAP) use at 1 month. Boxplots show median (horizontal line), interquartile range (box), and range excluding outliers (vertical bars) for each (sub) group. Deltas (Δ) reflect differences in medians. (B) Subjects with a low arousal threshold (ArTH) who received eszopiclone had a similar CPAP usage as subjects with a high ArTH receiving placebo (P = 0.82) but had less CPAP usage than those with a high ArTH who received eszopiclone (P = 0.01). Reprinted from Reference 9.
Figure 2.
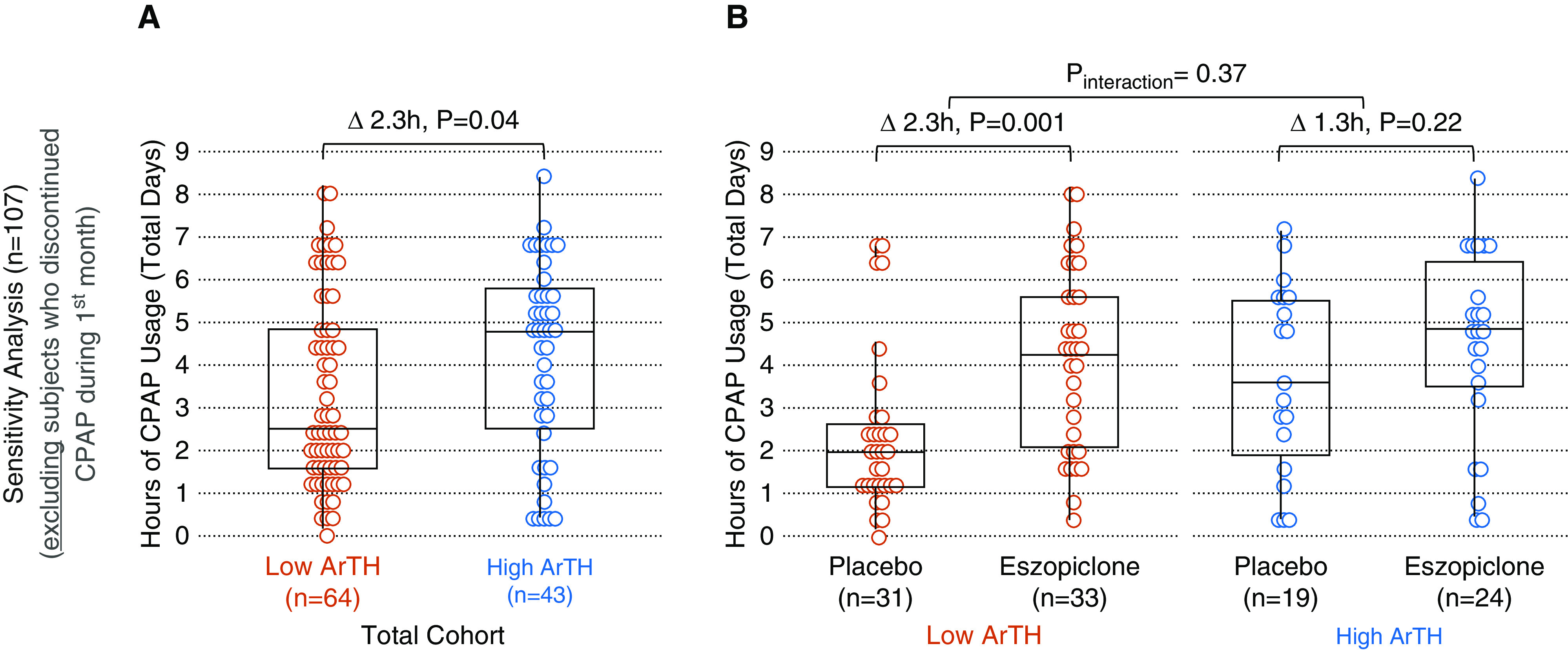
(A) Sensitivity analysis: average hours of nightly continuous positive airway pressure (CPAP) use at 1 month. (B) Subjects with a low arousal threshold (ArTH) who received eszopiclone had a similar CPAP usage as subjects with a high ArTH receiving placebo (P = 0.51) or eszopiclone (P = 0.40). Reprinted from Reference 9.
Overall, eszopiclone versus placebo increased median CPAP usage by 2.5 h (3.6 h [IQR, 0–5.5] vs. 1.1 h [IQR, 0–2.7]; P = 0.001). Among subjects who received eszopiclone, CPAP usage was less in those with a low ArTH than those with a high ArTH (2.1 h vs. 4.8 h; P = 0.01; Figure 1B), but usage was similar across ArTH strata when excluding subjects who discontinued CPAP during the first month (4.3 vs. 4.9 h; P = 0.40; Figure 2B).
There was no significant interaction between ArTH and study drug in primary (P = 0.45; adjusted P = 0.72; Figure 1B) or sensitivity (P = 0.37; Figure 2B) analyses.
Results were similar for percentage of nights with >4 hours as outcome (data not shown).
We note two major findings: First, in this cohort of patients with general OSA, a low ArTH at baseline was associated with substantially lower CPAP usage at 1 month than a high ArTH at baseline. This finding extends and validates results from two other studies that were performed in specific subsets of patients with OSA and/or used different methods to measure the ArTH and adherence (5, 6). Second, if a low ArTH causes low CPAP adherence, then one would expect that eszopiclone, which increases the ArTH, is only/more effective in patients with a low ArTH than those with a high ArTH. In this study, the effect of eszopiclone on CPAP use was not significantly modified by the ArTH, but considering that interaction tests have low power (12), we note the following: among patients who continued using CPAP for 1 month, eszopiclone increased CPAP usage substantially more in subjects with a low ArTH than those with a high ArTH (Figure 2B). Importantly, those with a low ArTH who received placebo had the lowest usage (2 h), whereas those with a low ArTH who received eszopiclone had a CPAP usage (4.3 h) similar to patients with a high ArTH (3.6 h with placebo; 4.9 h with eszopiclone; P ≥ 0.4). Thus, low-ArTH patients who persist using CPAP may particularly benefit from eszopiclone (which may also improve the AHI off CPAP) (7). However, eszopiclone appeared to improve adherence even in subjects with a high ArTH. The reason for this finding is unclear, but the difference in CPAP usage was largely driven by the higher percentage of patients who discontinued CPAP in the placebo group than in the eszopiclone group (27% vs. 8%; P = 0.14; see Figure 1B vs. 2B). Because CPAP can lower arousal threshold over time (13), in theory, the medication may be yielding improved adherence by diminishing this impact in patients with OSA with a high baseline arousal threshold. This study had several limitations. First, prior reports suggest that the effect of the ArTH on adherence is modified by the BMI, which could not be explored in our study. Moreover, to estimate the ArTH, we had to impute individual BMIs, which may have resulted in some misclassifications. But we do not think missing data substantially affected our findings because data were likely missing at random, BMI is a minor component of the ArTH formula, and results were similar when using another variant of the formula predicting low versus high ArTH based on three categorical variables (AHI, SpO2 nadir, and fraction hypopnea; data not shown) (7). Second, other factors may affect CPAP adherence (e.g., comorbidities, nasal congestion, claustrophobia, and psychosocial factors like a priori readiness to change behavior or partner support) (14–16), but we were unable to assess this. Third, we were unable to retrieve adherence data beyond 1 month precluding longitudinal analyses to assess the effect of the ArTH on long-term adherence and increase the power of interaction tests. But early adherence is a strong predictor of long-term adherence (17), and results from studies with longer follow-up were similar (5, 6). On the other hand, CPAP tends to lower the ArTH (13), perhaps explaining (in part) the “slowly declining” use seen in some patients (15).
In conclusion, we found that a low ArTH is associated with substantially reduced CPAP adherence and that eszopiclone improves CPAP usage overall, especially in subjects with a low ArTH who continued CPAP for 1 month. However, we emphasize that the presented results are based on exploratory post hoc analyses. Rigorous interventional trials assessing hard outcomes are needed to better understand the potential risks and benefits of targeting the ArTH to improve CPAP adherence and effectiveness.
Supplementary Material
Footnotes
C.N.S. received salary support from NHLBI (T32 grant HL134632 “Training the Next Generation in Respiratory Science”) and has been supported by the ATS ASPIRE fellowship during the conduct of the study. B.A.E. reports grants from the Heart Foundation of Australia during the conduct of the study, grants from the National Health and Medical Research Council, and grants from Apnimed. R.L.O. is funded by the NHLBI. A.M. is funded by the NHLBI. ResMed provided a philanthropic donation to University of California San Diego.
Author Contributions: All authors contributed substantially to the study conception and design (all), data acquisition (C.N.S. and C.J.L.), analysis (C.N.S.), or interpretation (all) of this study. C.N.S. drafted the manuscript, and all authors revised it critically for intellectual content. All authors gave final approval of this version to be submitted.
Originally Published in Press as DOI: 10.1164/rccm.202003-0502LE on July 16, 2020
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Jordan AS, McSharry DG, Malhotra A. Adult obstructive sleep apnoea. Lancet. 2014;383:736–747. doi: 10.1016/S0140-6736(13)60734-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benjafield AV, Ayas NT, Eastwood PR, Heinzer R, Ip MSM, Morrell MJ, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med. 2019;7:687–698. doi: 10.1016/S2213-2600(19)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mesarwi OA, Loomba R, Malhotra A. Obstructive sleep apnea, hypoxia, and nonalcoholic fatty liver disease. Am J Respir Crit Care Med. 2019;199:830–841. doi: 10.1164/rccm.201806-1109TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sawyer AM, Gooneratne NS, Marcus CL, Ofer D, Richards KC, Weaver TE. A systematic review of CPAP adherence across age groups: clinical and empiric insights for developing CPAP adherence interventions. Sleep Med Rev. 2011;15:343–356. doi: 10.1016/j.smrv.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zinchuk A, Edwards BA, Jeon S, Koo BB, Concato J, Sands S, et al. Prevalence, associated clinical features, and impact on continuous positive airway pressure use of a low respiratory arousal threshold among male United States veterans with obstructive sleep apnea. J Clin Sleep Med. 2018;14:809–817. doi: 10.5664/jcsm.7112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zinchuk AV, Redeker NS, Chu JH, Liang J, Stepnowsky C, Brandt CA, et al. Physiological traits and adherence to obstructive sleep apnea treatment in patients with stroke. Am J Respir Crit Care Med. 2020;201:1568–1572. doi: 10.1164/rccm.201911-2203LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eckert DJ, Owens RL, Kehlmann GB, Wellman A, Rahangdale S, Yim-Yeh S, et al. Eszopiclone increases the respiratory arousal threshold and lowers the apnoea/hypopnoea index in obstructive sleep apnoea patients with a low arousal threshold. Clin Sci (Lond) 2011;120:505–514. doi: 10.1042/CS20100588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lettieri CJ, Shah AA, Holley AB, Kelly WF, Chang AS, Roop SA CPAP Promotion and Prognosis-The Army Sleep Apnea Program Trial. Effects of a short course of eszopiclone on continuous positive airway pressure adherence: a randomized trial. Ann Intern Med. 2009;151:696–702. doi: 10.7326/0003-4819-151-10-200911170-00006. [DOI] [PubMed] [Google Scholar]
- 9.Schmickl CN, Lettieri CJ, Orr JE, Owens RL, Malhotra A. The arousal threshold predicts continuous positive airway pressure adherence in patients with obstructive sleep apnea: post hoc analysis of a randomized trial [abstract] Am J Respir Crit Care Med. 2020;201:A6440. doi: 10.1164/rccm.202003-0502LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edwards BA, Eckert DJ, McSharry DG, Sands SA, Desai A, Kehlmann G, et al. Clinical predictors of the respiratory arousal threshold in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2014;190:1293–1300. doi: 10.1164/rccm.201404-0718OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee RWW, Sutherland K, Sands SA, Edwards BA, Chan TO, S S Ng S, et al. Differences in respiratory arousal threshold in Caucasian and Chinese patients with obstructive sleep apnoea. Respirology. 2017;22:1015–1021. doi: 10.1111/resp.13022. [DOI] [PubMed] [Google Scholar]
- 12.Rothman KJ, Greenland S, Lash TL. Applications of stratified analysis methods. Philadelphia, PA: Lippincott Williams & Wilkins; 2008. Modern epidemiology; p. 299. [Google Scholar]
- 13.Eckert DJ, Younes MK. Arousal from sleep: implications for obstructive sleep apnea pathogenesis and treatment. J Appl Physiol (1985) 2014;116:302–313. doi: 10.1152/japplphysiol.00649.2013. [DOI] [PubMed] [Google Scholar]
- 14.Crawford MR, Espie CA, Bartlett DJ, Grunstein RR. Integrating psychology and medicine in CPAP adherence--new concepts? Sleep Med Rev. 2014;18:123–139. doi: 10.1016/j.smrv.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 15.Babbin SF, Velicer WF, Aloia MS, Kushida CA. Identifying longitudinal patterns for individuals and subgroups: an example with adherence to treatment for obstructive sleep apnea. Multivariate Behav Res. 2015;50:91–108. doi: 10.1080/00273171.2014.958211. [DOI] [PubMed] [Google Scholar]
- 16.Budhiraja R, Kushida CA, Nichols DA, Walsh JK, Simon RD, Gottlieb DJ, et al. Impact of randomization, clinic visits, and medical and psychiatric cormorbidities on continuous positive airway pressure adherence in obstructive sleep apnea. J Clin Sleep Med. 2016;12:333–341. doi: 10.5664/jcsm.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chai-Coetzer CL, Luo YM, Antic NA, Zhang XL, Chen BY, He QY, et al. Predictors of long-term adherence to continuous positive airway pressure therapy in patients with obstructive sleep apnea and cardiovascular disease in the SAVE study. Sleep (Basel) 2013;36:1929–1937. doi: 10.5665/sleep.3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


