Abstract
Rationale: Noninvasive ventilation decreases the need for invasive mechanical ventilation and mortality among patients with chronic obstructive pulmonary disease but has not been well studied in asthma.
Objectives: To assess the association between noninvasive ventilation and subsequent need for invasive mechanical ventilation and in-hospital mortality among patients admitted with asthma exacerbation to the ICU.
Methods: We performed a retrospective cohort study using administrative data collected during 2010–2017 from 682 hospitals in the United States. Outcomes included receipt of invasive mechanical ventilation and in-hospital mortality. Generalized estimating equations, propensity-matched models, and marginal structural models were used to assess the association between noninvasive ventilation and outcomes.
Measurements and Main Results: The study population included 53,654 participants with asthma exacerbation. During the study period, 13,540 patients received noninvasive ventilation (25.2%; 95% confidence interval [CI], 24.9–25.6%), 14,498 underwent invasive mechanical ventilation (27.0%; 95% CI, 26.7–27.4%), and 1,291 died (2.4%; 95% CI, 2.3–2.5%). Among those receiving noninvasive ventilation, 3,013 patients (22.3%; 95% CI, 21.6–23.0%) required invasive mechanical ventilation after first receiving noninvasive ventilation, 136 of whom died (4.5%; 95% CI, 3.8–5.3%). Across all models, the use of noninvasive ventilation was associated with a lower odds of receiving invasive mechanical ventilation (adjusted generalized estimating equation odds ratio, 0.36; 95% CI, 0.32–0.40) and in-hospital mortality (odds ratio, 0.48; 95% CI 0.40–0.58). Those who received noninvasive ventilation before invasive mechanical ventilation were more likely to have comorbid pneumonia and severe sepsis.
Conclusions: Noninvasive ventilation use during asthma exacerbation was associated with improved outcomes but should be used cautiously with acute comorbid conditions.
Keywords: continuous positive airway pressure, critical care outcomes, respiratory insufficiency, retrospective studies, mechanical ventilators
At a Glance Commentary
Scientific Knowledge on the Subject
Noninvasive ventilation has been demonstrated to decrease rates of invasive mechanical ventilation and mortality among hospitalized patients with acute exacerbation of chronic obstructive lung disease. Although the pathophysiology of acute asthma exacerbations and chronic obstructive lung disease are similar, the impact of noninvasive ventilation on outcomes in acute asthma exacerbation has not been well studied.
What This Study Adds to the Field
In a nationally representative cohort of patients admitted to the ICU with acute asthma exacerbation, we found that the use of noninvasive ventilation decreased the odds of requiring invasive mechanical ventilation and in-hospital mortality. Findings were consistent across covariate-adjusted generalized estimating equations, propensity-matched analysis, and marginal structural models. These observations parallel the benefit of using noninvasive ventilation in acute exacerbations of chronic obstructive pulmonary disease and highlight the association between using noninvasive ventilation in hospitalized, critically ill patients with acute asthma exacerbation and improved outcomes.
Asthma exacerbations lead to an estimated 2 million emergency department visits and 500,000 hospitalizations in the United States each year (1, 2) accounting for a sizable burden of asthma-associated healthcare costs (3–6). Approximately 10% of patients hospitalized for asthma will be cared for in an ICU, and approximately 2–4% of all hospitalized patients will require endotracheal intubation and invasive mechanical ventilation (IMV) (6, 7). ICU admission and IMV have been associated with increased risk of mortality in these patients (6, 8, 9).
Multiple randomized controlled trials and systematic reviews have demonstrated that use of noninvasive ventilation (NIV) reduces the risk of IMV and mortality among patients admitted with an acute exacerbation of chronic obstructive pulmonary disease (COPD) (10). Despite having similar obstructive pathophysiology, it is unclear whether NIV has the same beneficial effects among patients with acute exacerbations of asthma. A 2012 Cochrane review evaluating five randomized controlled trials of NIV use in the setting of acute asthma exacerbations including 203 patients reported a small reduction in hospitalizations (11), increased emergency department discharges (11, 12), and improved spirometry (11, 13). Data were insufficient for the authors to evaluate the effects of NIV on need for IMV or mortality (14). A systematic review of 13 studies including small randomized controlled trials found insufficient evidence to support the use of NIV in acute asthma exacerbation (15). Retrospective studies have observed an increase in NIV use over time in asthma but inconsistent effects of NIV on mortality (9, 16, 17).
The objective of this study was to assess the association between NIV and subsequent need for IMV and in-hospital mortality among patients admitted with acute asthma exacerbation to the ICU. Some of these results have been previously reported in the form of an abstract (18).
Methods
We conducted a retrospective cohort study of patients admitted between January 2010 and March 2017 to the ICU with an acute asthma exacerbation. Data originated from the Premier Healthcare Database that included more than 700 hospitals in the United States representing approximately 80 million hospitalizations, or approximately 20% of national inpatient discharges. The database contained information included in the standard hospital discharge file including International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes from 2010 until 2015 then ICD-10-CM codes from 2016 through 2017, physician and hospital data, and billed medications and procedures for each patient by hospital day (19–22).
Patients admitted to the ICU 18 years or older who had a primary admission diagnosis of asthma exacerbation or status asthmaticus or primary admission diagnosis of respiratory failure or arrest with secondary diagnosis of asthma exacerbation or status asthmaticus were included in the study (Table E1 in online supplement). This method was adapted from the COPD literature (20, 21). Patients who had been transferred from outside hospitals, who had underlying diagnoses of lung diseases (cystic fibrosis, interstitial lung disease, hypersensitivity pneumonitis, or bronchiectasis), or who were pregnant were excluded. Patients with diagnoses of COPD or concomitant heart failure were also excluded (10, 23, 24). Patients with sleep apnea were included in the population because admission to the ICU with asthma exacerbation would typically supersede chronic disease management of underlying sleep apnea. This study was approved by the Colorado Multiple Institutional Review Board (#16-0505).
Information on age, sex, race/ethnicity, insurance status, and comorbidities was collected for each patient. Medical comorbidities were classified using ICD-9-CM and ICD-10-CM secondary diagnosis codes and used to create the Elixhauser Comorbidity Index (25). Acute comorbidities including sepsis, acute renal failure, and pneumonia were identified using methods described elsewhere (26–28). Data regarding medication administration and diagnostic testing were collected for each patient by abstracting daily pharmacy charges using methods described previously (19–22).
NIV and Outcome Measurement
NIV use was identified using daily billing charges specific to NIV use. The exposure period was admission through Hospital Day 2. As hourly data were not available, those who were billed for NIV and IMV on the same day, but who were no longer intubated on the subsequent day, were considered nonexposed as the use of postextubation NIV could not be excluded.
Outcomes of interest were receipt of IMV and in-hospital mortality. Receipt of IMV was identified using daily billing charges associated with mechanical ventilation. If a patient underwent IMV at any point during his or her hospitalization, he or she met criteria for this outcome. In-hospital mortality was identified using hospital discharge codes.
Analysis
Bivariate analyses were performed to compare frequencies of baseline characteristics between the NIV-exposed and not-exposed groups using chi-square tests, t tests, and Kruskal-Wallis tests as appropriate.
Three types of multivariate models were developed to assess the relationship between NIV exposure with the primary outcomes of IMV and in-hospital mortality. First, we used generalized estimating equations (GEEs) with an exchangeable correlation structure to account for the lack of independence between patients admitted at the same hospital. Models were adjusted for relevant patient characteristics, comorbidities, early treatments and testing, and hospital characteristics (Table E2).
Second, propensity scores were created using a logistic regression model to assess the probability of requiring NIV before Hospital Day 2 with the same baseline covariates used in the multivariate models. Participants were matched using greedy nearest neighbor matching with a caliper of 0.02. This method matches treated participants with the best matched control but does not match a treated participant if there is not a control participant less than the specified caliper (29).
The baseline characteristics of the matched cohort were evaluated in the same manner as the full cohort. The associations of NIV exposure and the primary outcomes were assessed using conditional logistic regression models with the matched cohort controlling for any remaining unbalanced covariates after matching. We also repeated the previous GEE models with the full cohort, adjusting for propensity scores. Third, marginal structural models (MSMs) using stabilized inverse probability of treatment weights were used to address time-varying confounding and time-varying exposures (30–32).
In a preplanned subgroup analysis, we compared the baseline covariates of all patients exposed to NIV who eventually received IMV with patients exposed to NIV only. We conducted a subgroup analysis stratifying the population by primary diagnosis of asthma to assess the impact of diagnosis code on outcomes.
Given the possibility of underlying sleep apnea influencing the likelihood of receiving NIV, we performed a sensitivity analysis excluding patients with comorbid sleep apnea. Furthermore, we performed a sensitivity analysis removing patients with severe sepsis because this diagnosis may have influenced outcomes.
E-values were calculated to determine the strength needed for unmeasured confounders to negate the observed odds ratio (OR) (33–35). Trends in NIV and IMV use over time were assessed using Cochrane-Armitage test for trend. All analyses were completed in SAS software version 9.4 (SAS Institute).
Results
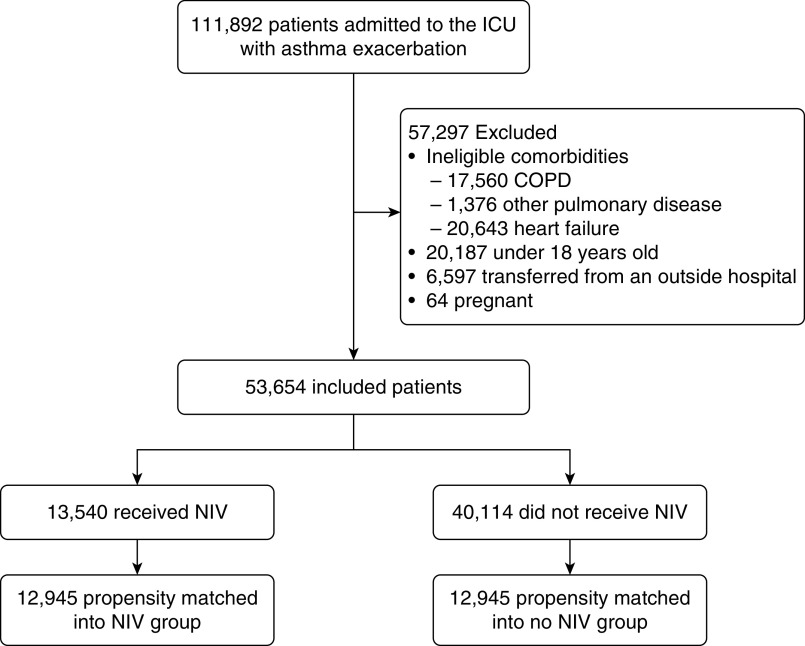
During the study period, 111,982 patients were admitted to the ICU for asthma exacerbation and 53,654 met inclusion criteria (Figure 1). A total of 682 hospitals contributed a median of 45 patients (interquartile range [IQR], 16–103 patients). Patient characteristics are described in Table 1. In general, comorbid conditions were similar between patients with NIV exposure compared with those without; however, participants receiving NIV were more likely to have a diagnosis of sleep apnea (26.7% vs. 15.3%).
Figure 1.
Patient selection criteria. COPD = chronic obstructive pulmonary disease; NIV = noninvasive ventilation.
Table 1.
Patient Characteristics
| Total Study Population |
Propensity-matched Population |
|||||
|---|---|---|---|---|---|---|
| Total Population (N = 53,654) | Noninvasive Ventilation (n = 13,540) | No Noninvasive Ventilation (n = 40,114) | Total Population (n = 25,890) | Noninvasive Ventilation (n = 12,945) | No Noninvasive Ventilation (n = 12,945) | |
| Sex, M* | 17,120 (31.9) | 4,566 (33.7) | 12,554 (31.3) | 8,764 (33.9) | 4,388 (33.9) | 4,367 (33.8) |
| Age, mean (SD), yr | 50.8 (17.2) | 50.9 (16.4) | 50.8 (17.4) | 50.6 (16.7) | 50.7 (16.5) | 50.4 (16.9) |
| Race/ethnicity† | ||||||
| White | 28,968 (54.1) | 6,929 (51.3) | 22,039 (55.1) | 13,299 (51.4) | 6,693 (51.7) | 6,606 (51.0) |
| Black | 15,027 (28.1) | 4,209 (31.2) | 10,818 (27.0) | 8,010 (31.0) | 3,981 (30.8) | 4,029 (31.1) |
| Hispanic | 1,279 (2.4) | 332 (2.5) | 947 (2.4) | 647 (2.5) | 318 (2.5) | 329 (2.5) |
| Other | 8,266 (15.4) | 2,025 (15.0) | 6,241 (15.6) | 3,934 (15.2) | 1,953 (15.1) | 1,981 (15.3) |
| Marital status‡ | ||||||
| Single | 31,986 (59.8) | 8,134 (60.3) | 23,852 (59.6) | 15,571 (60.1) | 7,779 (60.1) | 7,792 (60.2) |
| Married | 16,209 (30.3) | 3,873 (28.7) | 12,336 (30.9) | 7,428 (28.7) | 3,738 (28.9) | 3,690 (28.5) |
| Other | 5,295 (9.9) | 1,492 (11.1) | 3,803 (9.5) | 2,891 (11.2) | 1,428 (11.0) | 1,463 (11.3) |
| Primary insurance | ||||||
| Medicare | 18,502 (34.5) | 4,839 (35.7) | 13,663 (34.1) | 9,093 (35.1) | 4,572 (35.3) | 4,521 (34.9) |
| Medicaid | 12,955 (24.2) | 3,525 (26.0) | 9,430 (23.5) | 6,688 (25.8) | 3,346 (25.9) | 3,342 (25.8) |
| Private | 13,251 (24.7) | 3,030 (22.4) | 10,221 (25.5) | 5,904 (22.8) | 2,940 (22.7) | 2,964 (22.9) |
| Self-pay, charity, other | 8,946 (16.7) | 2,146 (15.9) | 6,800 (17.0) | 4,205 (16.2) | 2,087 (16.1) | 2,118 (16.4) |
| Admission year | ||||||
| 2010 | 6,838 (12.7) | 1,380 (20.2) | 5,458 (79.8) | 2,719 (10.5) | 1,355 (10.5) | 1,362 (10.5) |
| 2011 | 8,348 (15.6) | 1,778 (21.3) | 6,570 (78.7) | 3,451 (13.3) | 1,748 (13.5) | 1,703 (13.2) |
| 2012 | 9,561 (17.8) | 2,209 (23.1) | 7,352 (76.9) | 4,296 (16.6) | 2,139 (16.5) | 2,157 (16.7) |
| 2013 | 10,353 (19.3) | 2,509 (24.2) | 7,844 (75.8) | 4,840 (18.7) | 2,427 (18.7) | 2,413 (18.6) |
| 2014 | 10,301 (19.2) | 2,752 (26.7) | 7,549 (73.3) | 5,191 (20.1) | 2,608 (20.2) | 2,583 (20.0) |
| 2015 | 5,139 (9.6) | 1,893 (36.8) | 3,246 (63.2) | 3,459 (13.4) | 1,714 (13.2) | 1,745 (13.5) |
| 2016 | 2,503 (4.7) | 834 (33.3) | 1,669 (66.7) | 1,593 (6.2) | 777 (6.0) | 816 (6.3) |
| 2017 | 611 (1.1) | 185 (30.3) | 426 (69.7) | 343 (1.3) | 177 (1.4) | 166 (1.3) |
| Comorbidities | ||||||
| Hypertension | 27,372 (51.0) | 7,104 (52.5) | 20,268 (50.5) | 13,424 (51.8) | 6,714 (51.9) | 6,701 (51.8) |
| Diabetes | 13,735 (25.6) | 3,706 (27.4) | 10,029 (25.0) | 6,927 (26.8) | 3,473 (26.8) | 3,454 (26.7) |
| Obesity | 14,779 (27.6) | 4,448 (32.9) | 10,331 (25.8) | 8,095 (31.3) | 4,068 (31.4) | 4,027 (31.1) |
| Right heart failure | 2,067 (3.9) | 743 (5.5) | 1,324 (3.3) | 1,223 (4.7) | 688 (5.3) | 535 (4.1) |
| End-stage renal disease | 517 (1.0) | 156 (1.1) | 361 (0.9) | 268 (1.0) | 143 (1.1) | 125 (1.0) |
| Peripheral vascular disease | 1,082 (2.0) | 280 (2.1) | 802 (2.0) | 523 (2.0) | 257 (2.0) | 266 (2.1) |
| Neurologic disorders | 4,433 (8.3) | 1,016 (7.5) | 3,417 (8.5) | 1,965 (7.6) | 991 (7.7) | 974 (7.5) |
| Valvular disease | 1,380 (2.6) | 290 (2.1) | 1,090 (2.7) | 632 (2.4) | 275 (2.1) | 357 (2.8) |
| Psychoses | 3,872 (7.2) | 1,095 (8.1) | 2,777 (6.9) | 2,009 (7.8) | 1,050 (8.1) | 959 (7.4) |
| Sleep apnea | 9,729 (18.1) | 3,613 (26.7) | 6,116 (15.3) | 6,361 (24.6) | 3,177 (24.5) | 3,184 (24.6) |
| Pulmonary circulation disease | 2,153 (4.0) | 768 (5.7) | 1,385 (3.5) | 1,272 (4.9) | 711 (5.5) | 561 (4.3) |
| Cirrhosis | 548 (1.0) | 131 (1.0) | 417 (1.0) | 274 (1.1) | 122 (0.9) | 152 (1.2) |
| Tobacco use | 16,634 (31.0) | 4,785 (35.3) | 11,849 (29.5) | 9,024 (34.9) | 4,538 (35.1) | 4,486 (34.7) |
| Acute conditions | ||||||
| Pneumonia | 9,000 (16.8) | 2,595 (19.2) | 63,405 (16.0) | 4,868 (18.8) | 2,452 (18.9) | 2,416 (18.7) |
| Severe sepsis | 5,479 (10.2) | 1,562 (11.5) | 3,917 (9.8) | 3,056 (11.8) | 1,510 (11.7) | 1,546 (11.9) |
| Acute renal failure | 3,498 (6.5) | 975 (7.2) | 2,523 (6.3) | 1,881 (7.3) | 930 (7.2) | 951 (7.4) |
Data are shown as n (%) unless otherwise indicated.
n = 53,650.
n = 53,540.
n = 53,490.
Hospital characteristics and early treatments and tests are described in Table 2. The most common early therapies were short-acting bronchodilators and intravenous steroids. Despite the fact that a minority of patients had a diagnosis for pneumonia or severe sepsis, 75.4% (n = 40,478) received antibiotics.
Table 2.
Hospital and Treatment Characteristics
| Total Study Population [n (%)] |
Propensity-matched Population [n (%)] |
|||||
|---|---|---|---|---|---|---|
| Total Population (N = 53,654) | Noninvasive Ventilation (n = 13,540) | No Noninvasive Ventilation (n = 40,114) | Total Population (n = 25,450) | Noninvasive Ventilation (n = 12,725) | No Noninvasive Ventilation (n = 12,725) | |
| Hospital beds | ||||||
| <200 | 12,286 (22.9) | 2,887 (21.3) | 9,399 (23.4) | 5,506 (21.3) | 2,776 (21.4) | 2,730 (21.1) |
| 200–299 | 11,599 (21.6) | 2,849 (21.0) | 8,750 (21.8) | 5,493 (21.2) | 2,749 (21.2) | 2,744 (21.2) |
| 300–499 | 15,593 (29.1) | 4,219 (31.2) | 11,374 (28.4) | 7,977 (30.8) | 3,997 (30.9) | 3,980 (30.8) |
| ≥500 | 14,176 (26.4) | 3,585 (26.5) | 10,591 (26.4) | 6,914 (26.7) | 3,423 (26.4) | 3,491 (27.0) |
| Population served | ||||||
| Urban | 47,765 (89.0) | 12,080 (89.2) | 35,685 (89.0) | 23,130 (89.3) | 11,536 (89.1) | 11,594 (89.5) |
| Rural | 5,889 (11.0) | 1,460 (10.8) | 4,429 (11.0) | 2,760 (10.7) | 1,409 (10.9) | 1,351 (10.4) |
| Teaching status | ||||||
| Teaching | 20,358 (37.9) | 5,255 (38.8) | 15,103 (37.7) | 10,176 (39.3) | 5,044 (39.0) | 5,132 (39.6) |
| Nonteaching | 33,296 (62.1) | 8,285 (61.2) | 25,011 (62.4) | 15,714 (60.7) | 7,901 (61.0) | 7,813 (60.4) |
| Geographic region | ||||||
| Midwest | 11,590 (21.6) | 3,075 (22.7) | 8,515 (21.2) | 5,909 (22.8) | 2,939 (22.7) | 2,970 (22.9) |
| Northeast | 7,747 (14.4) | 1,812 (13.4) | 5,935 (14.8) | 3,537 (13.7) | 1,758 (13.6) | 1,779 (13.7) |
| South | 25,525 (47.1) | 6,145 (45.4) | 19,107 (47.6) | 11,714 (45.3) | 5,904 (45.6) | 5,810 (44.9) |
| West | 9,065 (16.9) | 2,508 (18.5) | 6,557 (16.4) | 4,730 (18.3) | 2,344 (18.1) | 2,386 (18.4) |
| Attending specialty | ||||||
| Critical care | 903 (1.7) | 302 (2.2) | 601 (1.5) | 585 (2.3) | 279 (2.2) | 306 (2.4) |
| Pulmonology | 4,037 (7.5) | 1,058 (7.8) | 2,979 (7.4) | 2,047 (7.9) | 1,016 (7.9) | 1,031 (8.0) |
| Internal medicine | 40,473 (75.4) | 10,122 (74.8) | 30,351 (75.7) | 19,333 (74.7) | 9,679 (74.8) | 9,654 (74.6) |
| Family medicine | 6,300 (11.7) | 1,518 (11.2) | 4,782 (11.9) | 2,864 (11.1) | 1,461 (11.3) | 1,403 (10.8) |
| Other | 1,941 (3.6) | 540 (4.0) | 1,401 (3.5) | 1,615 (4.1) | 510 (3.9) | 551 (4.3) |
| Early tests and treatments | ||||||
| Short-acting bronchodilators | 49,542 (92.3) | 12,740 (94.1) | 36,802 (91.7) | 24,305 (93.9) | 12,158 (93.9) | 12,147 (93.8) |
| Any steroids | 50,850 (94.8) | 13,091 (96.7) | 37,759 (94.1) | 25,000 (96.6) | 12,502 (96.6) | 12,498 (96.6) |
| Intravenous steroids | 49,200 (91.7) | 12,795 (94.5) | 36,405 (90.8) | 24,388 (94.2) | 12,218 (94.4) | 12,170 (94.0) |
| Oral steroids | 10,766 (20.1) | 2,527 (18.7) | 8,239 (20.5) | 4,853 (18.7) | 2,417 (18.7) | 2,436 (18.8) |
| Magnesium | 19,808 (36.9) | 6,058 (44.7) | 13,750 (34.3) | 11,582 (44.7) | 5,719 (44.2) | 5,863 (45.3) |
| Inhaled corticosteroid | 16,376 (30.5) | 4,430 (32.7) | 11,946 (29.8) | 8,323 (32.2) | 4,171 (32.2) | 4,152 (32.1) |
| Long-acting β2-agonist | 10,004 (18.7) | 2,713 (20.0) | 7,291 (18.2) | 5,079 (19.6) | 2,542 (19.6) | 2,537 (19.6) |
| Long-acting antimuscarinic | 3,422 (6.4) | 1,066 (7.9) | 2,365 (5.9) | 1,946 (7.5) | 970 (7.5) | 976 (7.5) |
| Leukotriene modifier | 14,791 (27.6) | 3,934 (29.1) | 10,857 (27.1) | 7,407 (28.6) | 3,705 (28.6) | 3,702 (28.6) |
| Methylxanthine bronchodilator (theophylline or aminophylline) | 2,961 (5.5) | 946 (7.0) | 2,015 (5.0) | 1,737 (6.7) | 866 (6.7) | 871 (6.7) |
| Antibiotics | 40,478 (75.4) | 10,452 (77.2) | 30,026 (74.9) | 19,929 (77.0) | 9,988 (77.2) | 9,941 (76.8) |
| Vasopressors | 2,043 (3.8) | 456 (3.4) | 1,587 (4.0) | 900 (3.5) | 449 (3.5) | 451 (3.5) |
| Opiates | 24,247 (45.2) | 6,363 (47.0) | 17,884 (44.6) | 12,211 (47.2) | 6,092 (47.1) | 6,119 (47.3) |
| Benzodiazepines | 23,326 (43.5) | 6,568 (48.5) | 16,758 (41.8) | 12,699 (49.1) | 6,301 (48.7) | 6,398 (49.4) |
| Sputum testing | 7,724 (14.4) | 1,889 (14.0) | 5,835 (14.6) | 3,765 (14.5) | 1,852 (14.3) | 1,913 (14.8) |
| Spirometry or peak flow | 6,766 (12.6) | 1,538 (11.4) | 5,228 (13.0) | 3,050 (11.8) | 1,507 (11.6) | 1,543 (11.9) |
| Arterial blood gas | 33,156 (61.8) | 11,398 (84.2) | 21,758 (54.2) | 21,755 (84.0) | 10,824 (83.6) | 10,931 (84.4) |
Twenty-five percent of patients (n = 13,540) received NIV. Among patients receiving NIV, 86.1% of patients (n = 11,656) required this modality on Day 1, and 54.5% of the population (n = 7,373) received NIV on Day 2. The median number of days patients received NIV was 2 (IQR, 1–4) days.
Among the 14,498 (27.0%) patients who received IMV, 3,013 (20.8%) received NIV before its initiation (Table 3). Patients received IMV a median of 4 (IQR, 2–6) days. Only 813 (5.6%) of those who underwent IMV were intubated after Hospital Day 3. Overall, 1,291 patients (2.4%) died during their admission. The median length of stay was 4 days (IQR, 2–6 d).
Table 3.
Outcomes among the Total Population and Stratified by Primary Diagnosis
| Entire Cohort [n (%)] |
Primary Diagnosis Respiratory Failure [n (%)] |
Primary Diagnosis Asthma Exacerbation [n (%)] |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Total (N = 53,654) | Noninvasive Ventilation (n = 13,540) | No Noninvasive Ventilation (n = 40,114) | Total (n = 17,387) | Noninvasive Ventilation (n = 6,457) | No Noninvasive Ventilation (n = 10,930) | Total (n = 37,361) | Noninvasive Ventilation (n = 7,510) | No Noninvasive Ventilation (n = 29,851) | |
| Invasive mechanical ventilation | 14,498 (27.0) | 3,013 (22.3) | 11,485 (28.6) | 8,829 (50.8) | 1,942 (30.1) | 6,887 (63.0) | 6,156 (16.6) | 1,175 (15.6) | 4,981 (16.7) |
| In-hospital mortality | 1,291 (2.4) | 223 (1.7) | 1,068 (2.7) | 860 (5.0) | 151 (2.3) | 709 (6.5) | 443 (1.2) | 75 (1.0) | 368 (1.2) |
| Propensity-matched cohort | n = 25,450 | n = 12,725 | n = 12,725 | n = 11,350 | n = 5,675 | n = 5,675 | n = 14,846 | n = 7,423 | n = 7,423 |
| Invasive mechanical ventilation | 8,197 (31.7) | 2,897 (22.4) | 5,300 (40.1) | 5,095 (44.9) | 1,808 (35.5) | 3,287 (64.5) | 3,111 (21.0) | 1,161 (15.6) | 1,950 (26.8) |
| In-hospital mortality | 605 (2.3) | 216 (1.7) | 389 (3.0) | 390 (3.4) | 137 (2.4) | 253 (4.5) | 197 (1.3) | 74 (1.0) | 123 (1.7) |
Trends in NIV, IMV, and Mortality during the Study Period
During the study period, the use of NIV increased from 18.5% (95% confidence interval [95% CI], 16.9–20.3%) in 2010 to 29.9% (26.2–33.9%) in 2017 (P < 0.0001, Figure 2A). The proportion of participants undergoing IMV per year was 28.5% (26.3–30.8%) in 2010 and 26.3% (22.8–30.1%) in the final year; however, this was not a statistically significant decrease over time (P = 0.29, Figure 2A). In-hospital mortality ranged from 2.9% (2.2–3.8%) in the beginning of 2010 to a peak of 4.7% (3.8–5.9%) in early 2015 before decreasing again to 2.9% (1.7–4.7%) at the end of the study period in 2017 (P = 0.0003, Figure 2A).
Figure 2.
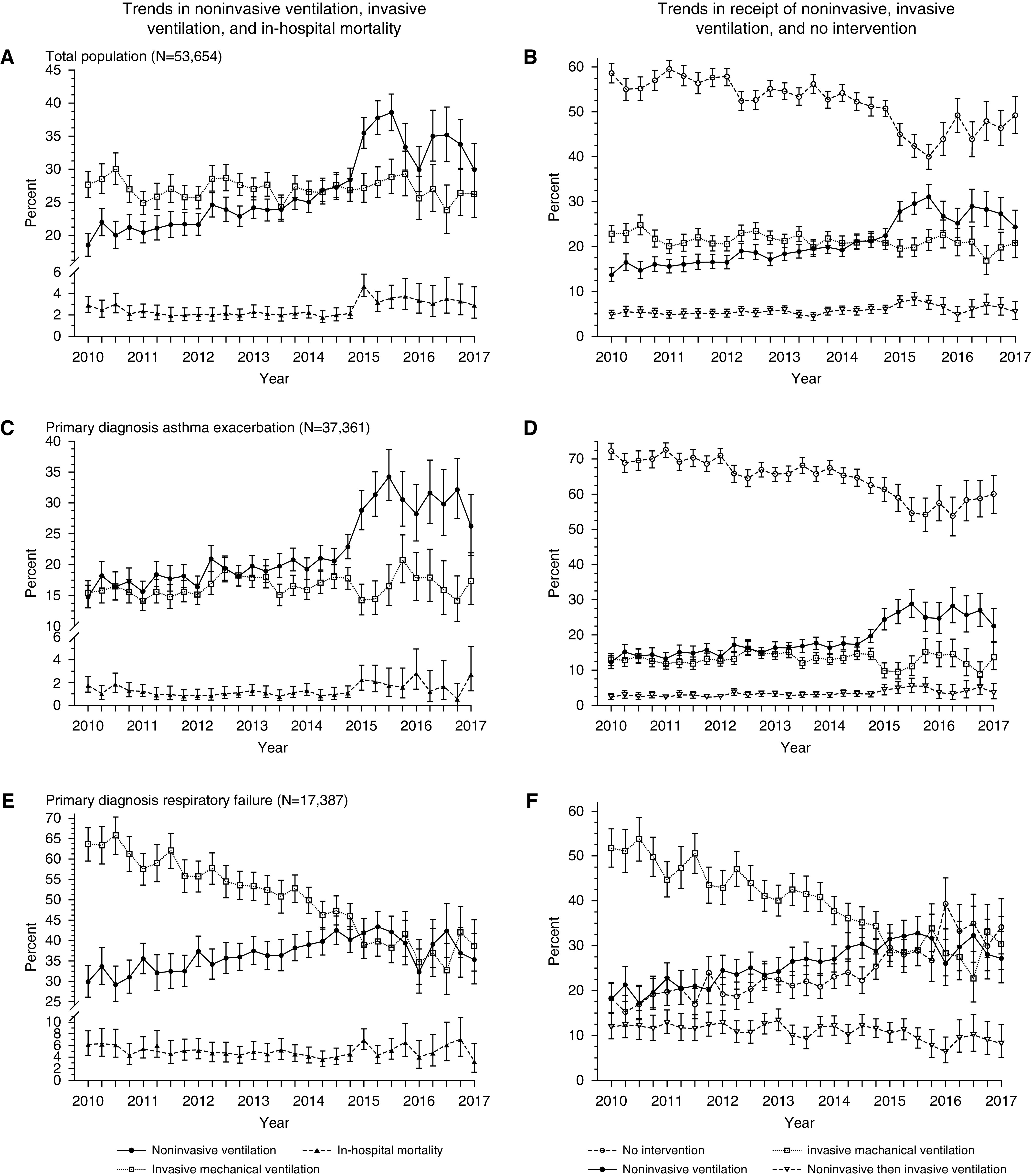
Trends in noninvasive ventilation (NIV), invasive mechanical ventilation (IMV), and in-hospital mortality over time. (A) Cochrane-Armitage test for trend: NIV P < 0.0001, IMV P = 0.61, in-hospital mortality P = 0.0003. (B) Cochrane-Armitage test for trend: no intervention P < 0.0001, NIV only P < 0.0001, IMV only P = 0.0007, and NIV followed by IMV P < 0.0001. (C) Cochrane-Armitage test for trend: NIV P < 0.0001, IMV P = 0.02, in-hospital mortality P = 0.09. (D) Cochrane-Armitage test for trend: no intervention P < 0.0001, NIV only P < 0.0001, IMV only P = 0.87, and NIV followed by IMV P < 0.0001. (E) Cochrane-Armitage test for trend: NIV P < 0.0001, IMV P < 0.0001, in-hospital mortality P = 0.33. (F) Cochrane-Armitage test for trend: no intervention P < 0.0001, NIV only P < 0.0001, IMV only P < 0.0001, and NIV followed by IMV P < 0.0001.
The proportion of patients receiving neither NIV nor IMV decreased during the study period (P < 0.0001, Figure 2B), and the proportion of patients receiving NIV increased (P < 0.0001). The proportion of patients who underwent IMV without prior NIV decreased over the study period (P = 0.0007), whereas the proportion of patients who received IMV after NIV increased (P < 0.0001, Figure 2B). The proportion of patients receiving IMV after NIV was 4.8% (3.9–5.9%) at the beginning of the study period, reached a peak of 8.2% (6.8–9.8%) in 2015, and then decreased to 5.5% (3.8–7.7%) by 2017 (P < 0.0001, Figure 2B). In-hospital mortality did not significantly change over time among those who only received IMV (P = 0.90) or among those who received IMV after NIV (P = 0.37).
Characteristics and Outcomes among Patients Failing NIV
Among the 13,298 patients exposed to NIV, 3,013 (22.3%; 21.6–23.0%) subsequently received IMV. Among the 14,498 patients who received IMV, 79.2% (78.6–79.9%) did not receive NIV before intubation. The majority of patients who underwent intubation after trial of NIV received NIV at least 1 day before intubation. When comparing these patients with the 10,527 who received NIV but not IMV, those who failed NIV were more likely to use tobacco or drugs and have comorbid conditions including pneumonia, severe sepsis, and acute renal failure (Tables E5–E7).
One hundred thirty-six participants (4.5%; 3.8–5.3%) who failed NIV died, compared with 87 participants (0.8%; 0.7–1.0%) who received NIV only. This mortality rate was higher among those who failed NIV than among the entire study population (2.4%; 2.3–2.5%) but remained lower than among those who only received IMV (7.6%; 7.2–8.1%).
Multivariate Analysis of the Entire Population
Results from multivariate models assessing the relationship between NIV and the primary outcomes are described in Figure 3. In all GEE models, NIV use was associated with a decreased odds of IMV (adjusted OR, 0.36; 95% CI, 0.32–0.40) and mortality (adjusted OR, 0.48; 95% CI, 0.40–0.58). The MSMs yielded similar results with OR 0.44 (0.41–0.47) for IMV and 0.76 (0.63–0.92) for mortality.
Figure 3.
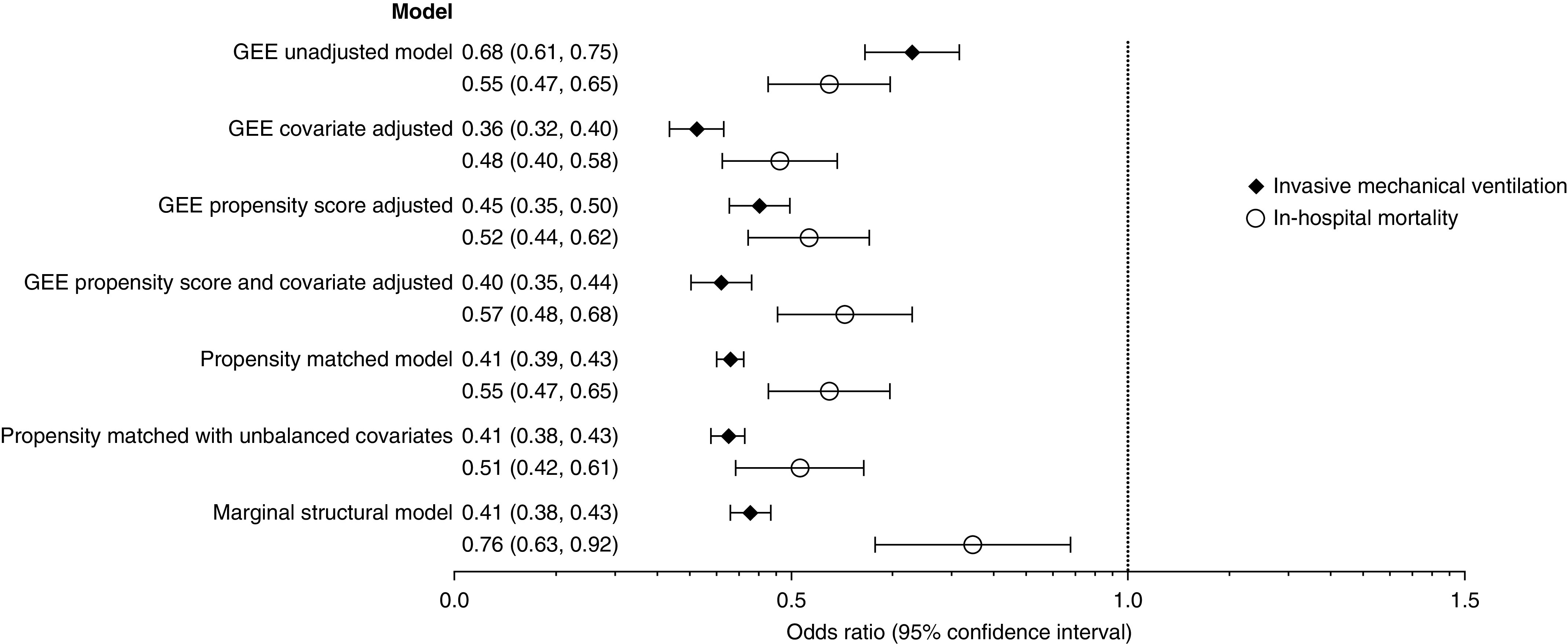
Associations between noninvasive ventilation and subsequent invasive mechanical ventilation and mortality among patients admitted with asthma exacerbation to the ICU. Generalized estimating equation (GEE) and marginal structural models N = 53,654. Propensity-matched models n = 25,450. GEE covariate adjusted included age, sex, race, marital status, primary insurance, admission year and quarter, primary admitting diagnosis of asthma, primary admitting diagnosis of respiratory failure, Elixhauser comorbidity score, hypertension, diabetes, hypothyroidism, obesity, right heart failure, valvular disease, psychoses, alcohol abuse, sleep apnea, weight loss, solid tumor, drug abuse, pulmonary circulation disease, tobacco use, pneumonia, severe sepsis, quartile of hospital beds, population served, teaching hospital, geographic region, attending specialty, and the following early treatments and tests: magnesium, inhaled corticosteroid, inhaled long-acting β-agonist, antibiotics, spirometry, and arterial blood gas. The propensity-matched model with unbalanced covariates included right heart failure, valvular disease, pulmonary circulation disease, solid tumor, psychosis, end-stage renal disease, cirrhosis, and arterial blood gas. Marginal structural model: time-varying confounder included in stabilized inverse probability of treatment weights included administration of magnesium, antibiotics, inhaled corticosteroid, spirometry or peak flow measurement, arterial blood gas, and exposure to noninvasive ventilation in the days prior in addition to baseline covariates included in the GEE covariate-adjusted model.
Propensity-matched Analysis
Of the 13,540 patients in the NIV treatment group, matches were identified for 95.6% (n = 12,945), for a total propensity-matched sample of 25,890. After matching, the only baseline covariates with statistically significant differences between the groups were right heart failure, valvular disease, pulmonary circulatory disease, solid tumor, and psychosis (Tables 1 and 2). Standardized differences were less than 0.1 for all variables after propensity matching. In a conditional logistic regression model that included adjustment for unbalanced covariates, NIV was associated with lower odds of IMV (OR, 0.41; 0.38–0.43) and mortality (OR, 0.51; 0.42–0.61).
Subgroup Analysis by Admission Diagnosis
In the preplanned analyses stratifying the sample according to primary admission diagnosis, 37,361 (69.6%) had a primary or admission diagnosis of asthma exacerbation, whereas 17,387 (32.4%) had a primary or admission diagnosis of respiratory failure with a secondary diagnosis of asthma exacerbation. There were 1,256 (3.4%) patients with both asthma exacerbation and respiratory failure as either admission or primary diagnosis. Patients with respiratory failure were more likely to receive IMV (50.8% vs. 16.0%) and suffer in-hospital mortality (5.0% vs. 1.1%) than those with primary diagnosis of asthma exacerbation (Table 3). NIV was associated with a decreased odds of IMV and in-hospital mortality in both the respiratory failure and asthma subgroups, although the magnitude was larger among the subgroup with a primary diagnosis of respiratory failure (Tables E8 and E9).
Among patients with a primary diagnosis of asthma exacerbation, the proportion of patients receiving NIV increased over the study period from 14.8% (13.0–16.7%) in 2010 to 26.2% (21.5–31.3%) in 2017 (P < 0.0001, Figure 2C). IMV use peaked in this group in 2015 (P = 0.02, Figure 2C). In-hospital mortality did not significantly change over the study period (P = 0.09). Among this subgroup, the majority of patients received neither NIV nor IMV, although this decreased from 72.2% (69.9–74.5%) in 2010 to 60.1% (54.5–65.4%) in 2017 (P < 0.0001, Figure 2D). The proportion of patients receiving NIV increased over time, although the proportion receiving IMV remained stable overall (Figure 2D).
Among the subgroup with a diagnosis of respiratory failure, the proportion of patients receiving NIV increased over time from 29.9% (26.1–34.0%) in 2010 to 35.4% (29.4–41.8%) in 2017 (P < 0.0001, Figure 2E). The use of IMV significantly decreased over time among patients with the admission diagnosis of respiratory failure from 63.7% (59.5–67.7%) in 2010 to 38.7% (32.5–45.1%) in 2017 (P < 0.0001, Figure 2E). In-hospital mortality did not change over time.
There was an increase in the proportion of patients who received neither NIV nor IMV over the study period (P < 0.0001, Figure 2F) and a decrease in patients who received IMV only (P < 0.0001) and NIV followed by IMV (P < 0.0001, Figure 2F). The overall decrease in interventions is secondary to the decrease in IMV as the use of NIV increased in this subgroup (P < 0.0001, Figure 2F).
Sensitivity Analyses
Among those without sleep apnea, the findings of the role of NIV were unchanged (Tables E10–E12). In the GEE model with propensity score and covariate adjustment, the OR for IMV and mortality among those receiving NIV were 0.33 (0.29–0.37) and 0.55 (0.46–0.67), respectively. Similarly, findings were unchanged overall when patients with severe sepsis were removed from the cohort (Tables E13–E15).
E-values were used in lieu of traditional sensitivity analyses to assess the degree of unmeasured confounding that would be required to nullify the observed ORs. E-values of the OR ranged from 1.79 to 3.05 for IMV and 2.00 to 4.08 for mortality. The E-values indicate that the observed ORs for IMV and mortality could be explained by an unmeasured confounder with a magnitude of greater than 1.79 for NIV and each outcome.
Discussion
In this multicenter cohort study, we determined that the use of NIV among patients with asthma exacerbation admitted to the ICU was associated with a reduced requirement for IMV and small but significantly lower odds of in-hospital mortality. These observations were consistent after accounting for demographic differences, comorbid conditions, and early therapies. The beneficial impact of NIV in asthma on receipt of IMV and in-hospital mortality was most robust among patients with a primary diagnosis of respiratory failure in conjunction with asthma, suggesting that the impact of use of this modality in the asthma setting may favor patients who have a more fulminant presentation.
In a clinical practice guideline, the American Thoracic Society and European Respiratory Society were unable to issue a recommendation on the use of NIV in acute asthma exacerbation (36). Our study is the first to find NIV beneficial in reducing subsequent IMV among patients with acute asthma exacerbation and provides evidence that begins to fill this gap in the literature.
To date, randomized controlled trials have been underpowered to assess the effects of NIV on the need for intubation and mortality in patients with asthma (11, 12). One small retrospective study examined the rates of IMV before and after the introduction of NIV at a hospital and demonstrated a reduction in IMV rates after the introduction of NIV (37). Stefan and colleagues observed a mortality reduction in asthma among patients who received NIV compared with those who received IMV (16). Although this study provided evidence to support NIV’s safety in asthmatics, it could not assess whether early NIV use influenced the risk of receiving IMV subsequently. Our investigation is the first to identify a reduction in mortality and risk for IMV among patients with asthma treated with early NIV. Moreover, our findings are consistent with clinical trials indicating reduced mortality and reduced IMV when NIV is used early in acute COPD exacerbations (10).
Other investigations have reported an increase in NIV use for acute respiratory failure in recent years, including its use for disorders in which no beneficial outcome has previously been demonstrated (9, 38–40). Although use of NIV in the acute asthma setting does not have a strong evidence base, the increased NIV use among patients with asthma exacerbations during the study period reflects this sentiment.
Despite the increase in NIV use over the study period, there was no statistically significant change in the rate of IMV use. This raises the concern that the increase in NIV reflects use in patients who would not have been intubated regardless of NIV use. In a planned subgroup analysis, patients admitted with a primary or admission diagnosis of respiratory failure and secondary admission diagnosis of asthma exacerbation appeared to represent a more critically ill subset of the population compared with those admitted with primary or admission diagnosis of asthma exacerbation. In this population, we found a striking decrease in IMV use over time, with a concomitant increase in NIV use. Although there are likely some patients who received NIV who would have never required IMV, this does not appear to be the case among this more critically ill subset of the population.
One prior investigation evaluated clinical differences between NIV failure and success in the asthma setting, demonstrating no difference in demographics or arterial blood gas measurements, but did not evaluate acute comorbid conditions (41). Another study reported that patients with asthma with comorbid pneumonia were more likely to fail NIV (16). In our investigation, patients who failed NIV were more likely to have acute comorbidities, like pneumonia, and had a higher rate of in-hospital mortality than the overall study population. This finding is consistent with prior studies evaluating NIV failure in COPD and other forms of respiratory failure (42, 43).
In our investigation, we are unable to assess the temporality of comorbid conditions within a unique hospital admission, given that these were identified using ICD-9-CM and ICD-10-CM codes, Regardless, our data suggest that in patients presenting with an acute asthma exacerbation and concurrent acute condition, NIV may not be appropriate initial management and should be used cautiously.
Our observations are not without limitations, the primary being its retrospective cohort design with its nonrandom allocation of treatment assignment. We attempted to address this by using propensity-matched analysis; however, this cannot completely eliminate all possible selection bias. Whereas the Elixhauser comorbidity index was used to adjust for baseline comorbidities, the Premier Database does not have information available to calculate Acute Physiology and Chronic Health Evaluation or Sequential Organ Failure Assessment scores that would have allowed for additional risk adjustment. E-values suggest that an unmeasured confounder would need to have an OR of approximately two to negate our observed results. We found that the majority of confounders had ORs less than two, making it less likely that an unmeasured confounder influenced our results. In addition to propensity-matched models, we used GEE models to account for lack of independence between individuals treated at the same hospital and MSMs to account for time-varying exposure and confounders. These models yielded similar ORs, emphasizing the robustness of these results.
No validation studies using ICD-9-CM codes to identify patients admitted with asthma exacerbation have been performed. We employed methods similar to those used in COPD exacerbation studies using administrative data that have been validated and found to have high specificity, but likely underestimate the number of acute exacerbations of COPD (44). We feel that we were able to identify true cases of asthma exacerbation as rates of IMV use and mortality in acute asthma in this study are consistent with those previously reported in patients with asthma requiring ICU admission (6). The use of billing codes to identify NIV and IMV may have led to some misclassification of exposure and outcome. Although we assume that if a participant was billed for NIV or IMV that they received this intervention, this cannot be verified.
Given the pervasiveness and increasing use of NIV in acute asthma exacerbation demonstrated in this study, it is unlikely that it will be feasible to perform a large randomized controlled trial to address this research question. It would, however, be beneficial to better understand potential benefits of NIV use and risk of NIV failure in those with severe disease in a randomized controlled trial. Until then, the judicious use of NIV with close monitoring in the sickest patients may be warranted. Overall, this study provides evidence that in patients admitted to the ICU with acute asthma exacerbation, the use of NIV may reduce the risk of intubation and in-hospital mortality.
Supplementary Material
Footnotes
F.H. is supported by the NIH (R01 HL146542-01). M.M. is supported by grants from the NIH/NHLBI (K24 HL089223). P.M.H. is supported by grants from the NHLBI, the Veterans Affairs Health Services Research and Development Service, and the University of Colorado School of Medicine. He has a research agreement with Bristol-Myers Squibb through the University of Colorado.
Author Contributions: M.D.A. developed research questions, study design, and analytic plan, completed data analysis, and was the primary author of the manuscript. F.H. aided in developing research questions, study design, data analysis plan, interpretation of data, and preparation of manuscript. F.Y. aided in developing analytic models, particularly the marginal structural models, interpretation of data, and revision of manuscript. G.K.G. reviewed the analytic plan and aided in developing statistical models, interpretation of results, and revision of manuscript. M.M. aided in developing research questions, study design, and preparation and editing of the manuscript. R.W.V. aided in developing research questions, study design, and preparation and editing of the manuscript. P.M.H. aided in developing research questions, study design, interpretation of results, and preparation and editing of the manuscript. T.H.K. aided in developing research questions, study design, and preparation and editing of the manuscript. E.L.B. aided in developing research questions, study design, preparation and editing of the manuscript, and interpretation of results. All authors have read and approve of the final manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201910-2021OC on July 14, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Akinbami LJ, Moorman JE, Bailey C, Zahran HS, King M, Johnson CA, et al. Trends in asthma prevalence, health care use, and mortality in the United States, 2001-2010 NCHS Data Brief 2012. 94):1–8. [PubMed] [Google Scholar]
- 2. Epidemiology and Statistics Unit, Research and Health Education Division, American Lung Association. Trends in asthma morbidity and mortality. September 2012 [accessed 2018 Feb 22]. Available from: https://www.lung.org/research/trends-in-lung-disease/asthma-trends-brief/trends-and-burden.
- 3.Rodrigo GJ, Rodrigo C, Hall JB. Acute asthma in adults: a review. Chest. 2004;125:1081–1102. doi: 10.1378/chest.125.3.1081. [DOI] [PubMed] [Google Scholar]
- 4.Bahadori K, Doyle-Waters MM, Marra C, Lynd L, Alasaly K, Swiston J, et al. Economic burden of asthma: a systematic review. BMC Pulm Med. 2009;9:24. doi: 10.1186/1471-2466-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dougherty RH, Fahy JV. Acute exacerbations of asthma: epidemiology, biology and the exacerbation-prone phenotype. Clin Exp Allergy. 2009;39:193–202. doi: 10.1111/j.1365-2222.2008.03157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pendergraft TB, Stanford RH, Beasley R, Stempel DA, Roberts C, McLaughlin T. Rates and characteristics of intensive care unit admissions and intubations among asthma-related hospitalizations. Ann Allergy Asthma Immunol. 2004;93:29–35. doi: 10.1016/S1081-1206(10)61444-5. [DOI] [PubMed] [Google Scholar]
- 7.Krishnan V, Diette GB, Rand CS, Bilderback AL, Merriman B, Hansel NN, et al. Mortality in patients hospitalized for asthma exacerbations in the United States. Am J Respir Crit Care Med. 2006;174:633–638. doi: 10.1164/rccm.200601-007OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leatherman J. Mechanical ventilation for severe asthma. Chest. 2015;147:1671–1680. doi: 10.1378/chest.14-1733. [DOI] [PubMed] [Google Scholar]
- 9.Nanchal R, Kumar G, Majumdar T, Taneja A, Patel J, Dagar G, et al. Utilization of mechanical ventilation for asthma exacerbations: analysis of a national database. Respir Care. 2014;59:644–653. doi: 10.4187/respcare.02505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Osadnik CR, Tee VS, Carson-Chahhoud KV, Picot J, Wedzicha JA, Smith BJ. Non-invasive ventilation for the management of acute hypercapnic respiratory failure due to exacerbation of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2017;7:CD004104. doi: 10.1002/14651858.CD004104.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soroksky A, Stav D, Shpirer I. A pilot prospective, randomized, placebo-controlled trial of bilevel positive airway pressure in acute asthmatic attack. Chest. 2003;123:1018–1025. doi: 10.1378/chest.123.4.1018. [DOI] [PubMed] [Google Scholar]
- 12.Gupta D, Nath A, Agarwal R, Behera D. A prospective randomized controlled trial on the efficacy of noninvasive ventilation in severe acute asthma. Respir Care. 2010;55:536–543. [PubMed] [Google Scholar]
- 13.Brandao DC, Lima VM, Filho VG, Silva TS, Campos TF, Dean E, et al. Reversal of bronchial obstruction with bi-level positive airway pressure and nebulization in patients with acute asthma. J Asthma. 2009;46:356–361. doi: 10.1080/02770900902718829. [DOI] [PubMed] [Google Scholar]
- 14.Lim WJ, Mohammed Akram R, Carson KV, Mysore S, Labiszewski NA, Wedzicha JA, et al. Non-invasive positive pressure ventilation for treatment of respiratory failure due to severe acute exacerbations of asthma. Cochrane Database Syst Rev. 2012;12:CD004360. doi: 10.1002/14651858.CD004360.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Green E, Jain P, Bernoth M. Noninvasive ventilation for acute exacerbations of asthma: A systematic review of the literature. Aust Crit Care. 2017;30:289–297. doi: 10.1016/j.aucc.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Stefan MS, Nathanson BH, Lagu T, Priya A, Pekow PS, Steingrub JS, et al. Outcomes of noninvasive and invasive ventilation in patients hospitalized with asthma exacerbation. Ann Am Thorac Soc. 2016;13:1096–1104. doi: 10.1513/AnnalsATS.201510-701OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bond KR, Horsley CA, Williams AB. Non-invasive ventilation use in status asthmaticus: 16 years of experience in a tertiary intensive care. Emerg Med Australas. 2018;30:187–192. doi: 10.1111/1742-6723.12876. [DOI] [PubMed] [Google Scholar]
- 18.Althoff M, Holguin F, Yang F, Grunwald GK, Moss M, Vandivier RW, et al. Noninvasive ventilation use in critically ill patients with acute asthma exacerbations [abstract] Am J Respir Crit Care Med. 2019;199:A7050. doi: 10.1164/rccm.201910-2021OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sottile PD, Kiser TH, Burnham EL, Ho PM, Allen RR, Vandivier RW, et al. Colorado Pulmonary Outcomes Research Group (CPOR) An observational study of the efficacy of cisatracurium compared with vecuronium in patients with or at risk for acute respiratory distress syndrome. Am J Respir Crit Care Med. 2018;197:897–904. doi: 10.1164/rccm.201706-1132OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindenauer PK, Pekow PS, Lahti MC, Lee Y, Benjamin EM, Rothberg MB. Association of corticosteroid dose and route of administration with risk of treatment failure in acute exacerbation of chronic obstructive pulmonary disease. JAMA. 2010;303:2359–2367. doi: 10.1001/jama.2010.796. [DOI] [PubMed] [Google Scholar]
- 21.Rothberg MB, Pekow PS, Lahti M, Brody O, Skiest DJ, Lindenauer PK. Antibiotic therapy and treatment failure in patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. JAMA. 2010;303:2035–2042. doi: 10.1001/jama.2010.672. [DOI] [PubMed] [Google Scholar]
- 22.Kelmenson DA, Held N, Allen RR, Quan D, Burnham EL, Clark BJ, et al. Outcomes of ICU patients with a discharge diagnosis of critical illness polyneuromyopathy: a propensity-matched analysis. Crit Care Med. 2017;45:2055–2060. doi: 10.1097/CCM.0000000000002763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gray A, Goodacre S, Newby DE, Masson M, Sampson F, Nicholl J 3CPO Trialists. Noninvasive ventilation in acute cardiogenic pulmonary edema. N Engl J Med. 2008;359:142–151. doi: 10.1056/NEJMoa0707992. [DOI] [PubMed] [Google Scholar]
- 24.Masip J, Roque M, Sánchez B, Fernández R, Subirana M, Expósito JA. Noninvasive ventilation in acute cardiogenic pulmonary edema: systematic review and meta-analysis. JAMA. 2005;294:3124–3130. doi: 10.1001/jama.294.24.3124. [DOI] [PubMed] [Google Scholar]
- 25.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Waikar SS, Wald R, Chertow GM, Curhan GC, Winkelmayer WC, Liangos O, et al. Validity of International Classification of Diseases, Ninth Revision, Clinical Modification codes for acute renal failure. J Am Soc Nephrol. 2006;17:1688–1694. doi: 10.1681/ASN.2006010073. [DOI] [PubMed] [Google Scholar]
- 28.Skull SA, Andrews RM, Byrnes GB, Campbell DA, Nolan TM, Brown GV, et al. ICD-10 codes are a valid tool for identification of pneumonia in hospitalized patients aged > or = 65 years. Epidemiol Infect. 2008;136:232–240. doi: 10.1017/S0950268807008564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Austin PC. A comparison of 12 algorithms for matching on the propensity score. Stat Med. 2014;33:1057–1069. doi: 10.1002/sim.6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11:550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 31.Hernán M, Robins J. Causal inference. Boca Raton, FL: Chapman & Hall/CRC; 2019. [Google Scholar]
- 32.Lavikainen P, Helin-Salmivaara A, Eerola M, Fang G, Hartikainen J, Huupponen R, et al. Statin adherence and risk of acute cardiovascular events among women: a cohort study accounting for time-dependent confounding affected by previous adherence. BMJ Open. 2016;6:e011306. doi: 10.1136/bmjopen-2016-011306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. 2017;167:268–274. doi: 10.7326/M16-2607. [DOI] [PubMed] [Google Scholar]
- 34.Mathur MB, Ding P, Riddell CA, VanderWeele TJ. Web site and R package for computing E-values. Epidemiology. 2018;29:e45–e47. doi: 10.1097/EDE.0000000000000864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haneuse S, VanderWeele TJ, Arterburn D. Using the E-value to assess the potential effect of unmeasured confounding in observational studies. JAMA. 2019;321:602–603. doi: 10.1001/jama.2018.21554. [DOI] [PubMed] [Google Scholar]
- 36.Rochwerg B, Brochard L, Elliott MW, Hess D, Hill NS, Nava S, et al. Official ERS/ATS clinical practice guidelines: noninvasive ventilation for acute respiratory failure. Eur Respir J. 2017;50:1602426. doi: 10.1183/13993003.02426-2016. [DOI] [PubMed] [Google Scholar]
- 37.Murase K, Tomii K, Chin K, Tsuboi T, Sakurai A, Tachikawa R, et al. The use of non-invasive ventilation for life-threatening asthma attacks: changes in the need for intubation. Respirology. 2010;15:714–720. doi: 10.1111/j.1440-1843.2010.01766.x. [DOI] [PubMed] [Google Scholar]
- 38.Chandra D, Stamm JA, Taylor B, Ramos RM, Satterwhite L, Krishnan JA, et al. Outcomes of noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease in the United States, 1998-2008. Am J Respir Crit Care Med. 2012;185:152–159. doi: 10.1164/rccm.201106-1094OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stefan MS, Shieh MS, Pekow PS, Rothberg MB, Steingrub JS, Lagu T, et al. Epidemiology and outcomes of acute respiratory failure in the United States, 2001 to 2009: a national survey. J Hosp Med. 2013;8:76–82. doi: 10.1002/jhm.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mehta AB, Douglas IS, Walkey AJ. Evidence-based utilization of noninvasive ventilation and patient outcomes. Ann Am Thorac Soc. 2017;14:1667–1673. doi: 10.1513/AnnalsATS.201703-208OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ganesh A, Shenoy S, Doshi V, Rishi M, Molnar J. Use of noninvasive ventilation in adult patients with acute asthma exacerbation. Am J Ther. 2015;22:431–434. doi: 10.1097/MJT.0000000000000184. [DOI] [PubMed] [Google Scholar]
- 42.Corrêa TD, Sanches PR, de Morais LC, Scarin FC, Silva E, Barbas CS. Performance of noninvasive ventilation in acute respiratory failure in critically ill patients: a prospective, observational, cohort study. BMC Pulm Med. 2015;15:144. doi: 10.1186/s12890-015-0139-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Phua J, Kong K, Lee KH, Shen L, Lim TK. Noninvasive ventilation in hypercapnic acute respiratory failure due to chronic obstructive pulmonary disease vs. other conditions: effectiveness and predictors of failure. Intensive Care Med. 2005;31:533–539. doi: 10.1007/s00134-005-2582-8. [DOI] [PubMed] [Google Scholar]
- 44.Stein BD, Bautista A, Schumock GT, Lee TA, Charbeneau JT, Lauderdale DS, et al. The validity of International Classification of Diseases, Ninth Revision, Clinical Modification diagnosis codes for identifying patients hospitalized for COPD exacerbations. Chest. 2012;141:87–93. doi: 10.1378/chest.11-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.