Abstract
Diabetes is associated with liver disease and risk of hepatocellular carcinoma. In this study, we evaluated the association between liver fibrosis measured by transient elastography and four glucose metabolism measures in the Cameron County Hispanic Cohort, a population‐based, randomly selected cohort of Mexican American Hispanics with high rates of diabetes and liver cancer. We measured liver fibrosis (a risk factor for hepatocellular carcinoma) in 774 well‐characterized cohort participants using transient elastography. We evaluated the association of liver fibrosis with glycated hemoglobin (HbA1c), fasting blood glucose, insulin, and insulin resistance using multivariable linear regression models. In multivariable models, log‐transformed HbA1c had the strongest association with liver fibrosis (β = 0.37, 95% confidence interval [CI] 0.04‐0.69, P = 0.038), after controlling for waist circumference, aspartate aminotransferase, alanine aminotransferase, liver fat, and other known confounders. The association was statistically significant among women (β = 0.33, 95% CI 0.10‐0.56, P = 0.009) and similar but nonsignificant among men (β = 0.41, 95% CI −0.17 to 0.98, P = 0.593). Waist circumference, platelet count, aspartate transaminase, and liver steatosis were each associated with liver stiffness. Conclusions: Elevated HbA1c is associated with liver fibrosis, a key risk factor for HCC, particularly among women. Our results indicate that Mexican Americans with uncontrolled HbA1c may benefit from routine screening by liver elastography to identify individuals at risk of liver disease progression.
Abbreviations
- ALT
alanine aminotransferase
- APRI
AST‐to‐platelet ratio index
- AST
aspartate aminotransferase
- BARD
BMI, AST to ALT ratio, diabetes mellitus
- BMI
body mass index
- CAP
controlled attenuation parameter
- CCHC
Cameron County Hispanic Cohort
- CI
confidence interval
- FIB‐4
Fibrosis‐4 score
- HbA1c
glycated hemoglobin
- HBsAg
hepatitis B virus surface antigen
- HCC
hepatocellular carcinoma
- HCV‐Ab
hepatitis C virus antibody
- HOMA‐IR
Homeostatic Model Assessment of Insulin Resistance
- NAFLD
nonalcoholic fatty liver disease
- TE
transient elastography
The incidence of cirrhosis and hepatocellular carcinoma (HCC) is increasing in the United States among Hispanic populations, with nonalcoholic fatty liver disease (NAFLD) being the fastest‐growing underlying etiology.( 1 , 2 , 3 , 4 ) However, few studies have examined the clinical and demographic correlates of liver disease (fibrosis) preceding cirrhosis and HCC in Hispanic populations, as the gold standard of liver disease staging—liver biopsy—is inappropriate for screening at the population level.
Liver ultrasound, which is used routinely in clinical practice to reduce unnecessary biopsies, is not able to distinguish between simple fatty liver, which is not necessarily progressive, and liver fibrosis, which is usually progressive.( 5 , 6 , 7 ) Recent introduction of noninvasive, low‐cost elastographic methods, particularly transient elastography (TE), has permitted the study of otherwise asymptomatic liver fibrosis in population‐based settings.( 8 , 9 , 10 , 11 ) FibroScan is a low cost, high‐throughput, noninvasive method using transient wave elastography that provides two measures with proven reliability: liver fibrosis (stiffness) in kilopascals and intrahepatic steatosis (fat) using the controlled attenuation parameter (CAP) in decibels/meter.( 12 ) Elevated liver stiffness measured by elastography, like histologically confirmed liver fibrosis, has been associated with the risk of HCC.( 13 ) It is now important to identify clinical characteristics that are associated with liver fibrosis to further inform HCC risk prediction.
An association between diabetes and liver fibrosis has been documented in various studies, including some that use TE‐based definitions of fibrosis.( 8 , 14 , 15 , 16 , 17 , 18 ) However, the cutoff points for dichotomizing liver fibrosis differ, as did the definitions of diabetes, and the association has not been assessed in minority populations. In Hispanic populations with a significant burden of both diabetes and liver disease, an understanding of the relationship between standard metrics of glucose metabolism and noninvasive measures of liver fibrosis may aid risk stratification and prevention efforts.
In this study we evaluate the relationship between four glucose metabolism measures (glycated hemoglobin [HbA1c], fasting blood glucose, insulin, and insulin resistance) and liver fibrosis in a population‐based sample of Mexican Americans, after controlling for known predictors of liver fibrosis.
Methods
Setting and Participants
The Cameron County Hispanic Cohort (CCHC) is a population‐based sample of Mexican‐American individuals recruited from randomly selected households using a two‐stage sampling method based on US Census tracts and blocks (for more details see Fisher‐Hoch et al( 19 )). The CCHC was initiated in 2004; however, TE was not introduced until 2015, when FibroScan (Echosens, Waltham, MA) was approved for use by the US Food and Drug Administration. The current study is a cross‐sectional analysis of participants recruited between 2015 and 2019. Participants gave informed consent to participate, and all protocols were approved by the University of Texas Health Science Center at Houston Committee for the Protection of Human Subjects.
Participants underwent a comprehensive clinical examination, including a comprehensive interview of health and social history, family history of cancer and cardiovascular disease, demographic details, anthropometrics including weight, height and waist circumference, and a 10‐hour fasting blood draw. Blood samples were immediately sent to a Clinical Laboratory Improvement Amendments–accredited laboratory for a comprehensive metabolic panel including plasma glucose, complete blood count, lipid panel, and HbA1c measurement. Additional samples were separated into red blood cells, buffy coat, and plasma and stored at −80°C within 30 minutes of the blood draw. The stored plasma samples were used to assay fasting insulin, hepatitis C virus antibody (HCV‐Ab), and hepatitis B virus surface antigen (HBsAg). Fasting insulin was measured consistently using Mercodia enzyme‐linked immunosorbent assay kits (Uppsala, Sweden), and insulin resistance was calculated using the homeostatic model of insulin resistance (HOMA‐IR).( 20 ) The following definitions were used for traits relevant to liver disease. Heavy drinking was defined as self‐reported >21 drinks/week for men and >14 drinks/week for women( 21 ); obesity was defined as body mass index (BMI) ≥ 30 kg/m2; elevated waist circumference was defined as >102 cm for men and >88 cm for women. A combination of self‐reported diabetes status, fasting glucose levels, and/or use of glucose‐lowering medication were considered, and we used the American Diabetes Association's definition of diabetes status to group individuals as “No Diabetes,” “Impaired fasting glucose,” or “Diabetes.”( 22 )
Liver Fibrosis Measured by TE
After the clinical exam, trained operators routinely measured liver fibrosis and CAP on all visits using vibration‐controlled TE (either FibroScan 502 Touch or FibroScan 530 Compact, Echosens) with automatic probe selection (medium or XL) until a total of 10 valid measures were made. The primary outcome variable is median liver fibrosis in kilopascals. We excluded participants who had less than 10 valid fibrosis readings or whose liver fibrosis interquartile range‐to‐median ratio was greater than 30% (among those with liver fibrosis median >7.1 kPa), in accordance with a published quality control procedure.( 23 ) We also captured 10 measurements of CAP as a quantitative measure of liver steatosis; the median of 10 CAP measurements was recorded for analysis. Interrater variability for liver stiffness measured in kPa was estimated by calculating the intraclass correlation coefficient in a subset (n = 121) of participants with two assessments on the same day. There are no studies that have identified cutoff points for liver fibrosis using FibroScan in a nonclinical Hispanic population. Because an incorrect choice of cutoff can obscure true associations, we elected to use all the continuous values of liver stiffness to identify associations with clinical and demographic variables.
Statistical Methods
All statistical analyses were conducted using design‐based linear regression accounting for the two‐stage sampling of the CCHC using the survey package( 24 ) in R 3.5.1.( 25 ) We developed separate multivariable models of log‐transformed liver fibrosis on each of the four measures of glucose metabolism (log‐transformed) controlling for waist circumference (10‐cm unit scale), CAP (10‐unit scale), age, sex, place of birth (United States vs. other), current use of diabetes medication, heavy drinking, log‐transformed aspartate transaminase (AST) and alanine transaminase (ALT), and log‐transformed platelet count. The measures of glucose metabolism were Z‐standardized to enable head‐to‐head comparisons of each measure. Finally, we identified the measure of glucose metabolism with the largest statistically significant adjusted association with liver fibrosis for our final regression model. We then stratified the final model by sex.
Sensitivity Analysis
Most studies rely on kilopascal cutoff points for staging of fibrosis, which have been validated primarily in European and Asian clinical populations. The published cutoffs for “significant” (F2‐F4) fibrosis range from 5.8 to 11 kPa, but have never been validated in nonclinical, general populations or in Hispanic populations in the United States.( 12 ) Although there is no established cutoff for significant fibrosis among nonclinical populations, we explored the use of a “significant fibrosis” cutoff of 7.1 kPa, as recommended by the manufacturer,( 26 ) to determine whether our results were robust with a dichotomous parameterization of liver fibrosis. Second, we refit the final linear models adjusting for HCV‐Ab and HBsAg positivity in the smaller subset with results for these assays, to determine whether the small burden of viral hepatitis affects the association between glucose control measures and liver fibrosis. Finally, it is known that among clinical populations with cirrhosis (histological fibrosis score F4, kPA > 25), measures of HbA1c are known to vary and thus be unreliable.( 27 ) For this reason, we excluded participants (n = 13) with possible cirrhosis (kPa ≥ 21.0) and re‐estimated the multivariable model.
Results
Characteristics of the study population stratified by sex are found in Table 1.
Table 1.
Descriptive Statistics of the Analytic Data Set by Sex
| Categorical | ||||
|---|---|---|---|---|
| Female | Male | |||
| Count | Percentage § | Count | Percentage § | |
| Age (years) | ||||
| <40 | 90 | 18.3 | 52 | 20.4 |
| 40‐64 | 310 | 56.8 | 141 | 51.2 |
| 65+ | 119 | 24.9 | 61 | 28.5 |
| Born in Mexico | ||||
| No | 141 | 33.5 | 103 | 41.2 |
| Yes | 377 | 66.5 | 152 | 58.8 |
| Health Insurance* | ||||
| No | 280 | 50.1 | 123 | 41.0 |
| Yes | 237 | 49.9 | 131 | 59.0 |
| BMI (kg/m2) | ||||
| <20 | 4 | 0.6 | 0 | 0 |
| 20 to <25 | 83 | 16.3 | 28 | 8.1 |
| 25 to <30 | 164 | 33.0 | 100 | 38.5 |
| 30 to <35 | 229 | 42.3 | 110 | 45.1 |
| ≥35 | 38 | 7.8 | 16 | 8.5 |
| Diabetes † | ||||
| No | 370 | 74.6 | 173 | 72.0 |
| Yes | 142 | 25.4 | 79 | 28.0 |
| Heavy drinking ‡ | ||||
| No | 516 | 99.8 | 243 | 96.2 |
| Yes | 3 | 0.2 | 12 | 3.8 |
| HCV‐Ab positive | 7 | 1.0 | 4 | 1.3 |
| HBsAg positive | 8 | 2.0 | 4 | 11.7 |
| Continuous Variables | ||||
|---|---|---|---|---|
| Female | Male | |||
| Median § | 95 CI § | Median § | 95 CI § | |
| Age (continuous) | 55 | 52, 57 | 53 | 50, 58 |
| BMI (kg/m2) | 30.0 | 29.2, 30.9 | 30.4 | 29.6, 31.6 |
| Liver fibrosis (kPa) | 4.5 | 4.3, 4.7 | 4.8 | 4.4, 5.3 |
| APRI | 0.2 | 0.22, 0.24 | 0.36 | 0.24, 0.28 |
| NAFLD fibrosis score | −1.8 | −1.9, −1.7 | −1.5 | −1.7, −1.2 |
| CAP (dB/m) | 277 | 270, 288 | 306 | 278, 319 |
| Fasting glucose (mg/dL) | 93 | 92, 94 | 100 | 96, 104 |
| HbA1c (%) | 5.8 | 5.7, 5.9 | 5.9 | 5.8, 6.0 |
| Fasting insulin (pmol/L) | 9.3 | 8.7, 10.8 | 10.5 | 6.9, 13.7 |
| HOMA‐IR || | 2.4 | 2.1, 2.8 | 2.8 | 2.1, 3.8 |
| Waist circumference (cm) | 100 | 98, 102 | 105 | 102, 107 |
| Platelet count (×103/uL) | 253.8 | 248, 265 | 231.5 | 215, 244 |
| AST (U/L) | 19 | 18, 20 | 21 | 19, 23 |
| ALT (U/L) | 25 | 24, 27 | 32 | 28, 37.9 |
| Triglycerides (mg/dL) | 128 | 118, 138 | 141.5 | 124.7, 157.5 |
| Total cholesterol (mg/dL) | 187 | 182, 192 | 175.8 | 168, 186 |
| HDL (mg/dL) | 51 | 49, 53 | 41 | 38, 46 |
| LDL (mg/dL) | 107 | 102, 109 | 104 | 96, 110.3 |
Any health insurance, including private and public (Medicare/Medicaid) coverage.
According to the American Diabetes Association 2010 guidelines.
Defined as >14 drinks/week for female participants and >21 drinks/week for male participants.
All statistics are calculated using design‐based analysis accounting for complex sampling design.
Homeostasis model of insulin resistance, calculated using the formula glucose (mmol/L)* insulin (mU/L)/22.5.
Abbreviations: APRI, AST‐to‐platelet ratio index; BMI, Body Mass Index; CAP, controlled attenuation parameter; HBsAg, Heptatis B Surface Antigen; HCV‐Ab, Hepatitis C Virus Antibody; HDL, high‐density lipoprotein; kPa, kilopascals; LDL, low‐density lipoprotein; NAFLD, non‐alcoholic fatty liver disease.
After removing 13 participants with uninterpretable TE results, our final data set included 774 participants with valid liver stiffness and CAP measurement. For the majority (504, 65%), the medium probe was automatically selected during the examination, including for a number of obese and morbidly obese participants (Supporting Table S1). The ICC for liver stiffness indicated good inter‐observer agreement (ICC 0.94, 95% CI 0.92‐0.96). Liver stiffness measured by TE had modest correlations with blood‐based markers of liver fibrosis, including statistically significant associations with the NAFLD fibrosis score (r = 0.15, P < 0.001) and Fibrosis‐4 score (FIB‐4) (r = 0.19, P < 0.001) but no statistically significant association with the BARD (for “BMI, AST to ALT ratio, diabetes mellitus”) score (r = 0.04, P value = 0.2) (Table 2).
Table 2.
Correlation Between Liver Stiffness and Blood‐Based Markers of Liver Fibrosis
| Blood‐Based Liver Fibrosis Scores | Correlation With Liver Stiffness (kPa) Measured by TE (r)* | P Value |
|---|---|---|
| BARD score | 0.04 | 0.2 |
| NAFLD Fibrosis Score | 0.15 | <0.001 |
| FIB‐4 | 0.19 | <0.001 |
| APRI | 0.32 | <0.001 |
Spearman correlation coefficient.
Abbreviations: APRI, AST‐to‐platelet ratio index; TE, transient elastography.
In univariable analysis of the association between measures of glucose metabolism (HbA1c, fasting glucose, fasting insulin, and insulin resistance) and measures of liver fibrosis (TE, FIB‐4, AST‐to‐platelet ratio index [APRI], NAFLD fibrosis score, and BARD score), all four measures of glucose metabolism had significant associations with liver stiffness measured by TE (Table 3). The correlations were stronger between measures of glucose metabolism and BARD score and NAFLD fibrosis score, as these scores include diabetes in their calculations.
Table 3.
Correlation Between Measures of Glucose Control With Liver Stiffness Measured by TE and Blood‐Based Markers of Liver Fibrosis
| TE | BARD* | NAFLD Fibrosis Score* | FIB4 | APRI | |
|---|---|---|---|---|---|
| r † (P value) | r (P value) | r (P value) | r (P value) | r (P value) | |
| HbA1c | 0.18 (<0.001) | −0.10 (0.004) | 0.45 (<0.001) | 0.11 (0.003) | 0.02 (0.587) |
| Fasting glucose | 0.22 (<0.001) | −0.14 (<0.001) | 0.41 (<0.001) | 0.12 (0.001) | 0.07 (0.071) |
| Fasting insulin | 0.27 (<0.001) | −0.11 (0.005) | 0.11 (0.007) | 0.01 (0.843) | 0.09 (0.028) |
| HOMA‐IR | 0.31 (<0.001) | −0.15 (<0.001) | 0.23 (<0.001) | 0.04 (0.371) | 0.10 (0.018) |
Includes diabetes in definition of score.
Spearman correlation coefficient.
Abbreviations: APRI, AST‐to‐platelet ratio index; HOMA‐IR, homeostasis model of insulin resistance.
In multivariable models of the association between glucose metabolism and liver stiffness, the only measure of glucose control statistically significantly associated with liver stiffness was HbA1c (β, log‐transformed and centered = 0.07, 95% confidence interval [CI] 0.01‐0.14], P = 0.039) after controlling for waist circumference, CAP, current use of diabetes medication, platelet count, levels of AST, ALT, country of birth (United States vs. other), and heavy alcohol use (Supporting Table S2). We therefore focused on the association of HbA1c with liver fibrosis, as it was the largest adjusted association in multivariable models. Waist circumference, platelets, AST, and CAP measures were statistically significantly associated with liver fibrosis in each model. Serum triglycerides, high‐density lipoprotein, and low‐density lipoprotein were not associated with liver stiffness and did not improve the multivariable model fit. The final multivariable models, overall and by sex, of the association between log‐transformed liver stiffness (kPa) and log‐transformed HbA1c is given in Table 4. Overall, log‐transformed HbA1c had a statistically significant adjusted association with liver stiffness (β = 0.37, 95% CI 0.04‐0.69). Because both HbA1c and liver fibrosis are log‐transformed, this result can be interpreted as follows: a 10% increase in HbA1c is associated with a 4% increase in liver stiffness (kPa), given by (1.1)0.37 = 1.04. For comparison, for the association with waist circumference, which is scaled in 10‐unit increments (β = 0.06 for a 10‐cm increase in waist circumference), we expect a 7% increase in liver stiffness for every 10‐cm increase in waist circumference, given by exp(0.06) = 1.06. When stratifying by sex, there was a statistically significant association between liver stiffness and HbA1c among female participants (β = 0.33, 95% CI 0.10‐0.56, P = 0.009), but not male participants (β = 0.41, 95% CI −0.17 to 0.98, P = 0.593) (Table 4).
Table 4.
Multivariable Association Between HbA1C and Liver Stiffness in the Cameron County Hispanic Cohort
| Variable | Model 1: Overall (n = 744) | Model 2: Male Participants (n = 225) | Model 3: Female Participants (n = 519) |
|---|---|---|---|
| β (95% CI)* | β (95% CI) | β (95% CI) | |
| HbA1c (% mmol/mol) | 0.37 (0.04‐0.69) | 0.41 (−0.17 to 0.98) | 0.33 (0.10‐0.56) |
| Waist circumference (cm) | 0.06 (0.03‐0.08) | 0.03 (−0.03 to 0.09) | 0.07 (0.04‐0.10) |
| Diabetes medication† | −0.02 (−0.15 to 0.11) | −0.11 (−0.34 to 0.13) | 0.04 (−0.10 to 0.18) |
| Platelet count (×103/uL) | −0.19 (−0.35 to 0.03) | −0.18 (−0.47 to 0.11) | −0.18 (−0.41 to 0.04) |
| AST (U/L) | 0.24 (0.11‐0.38) | 0.26 (−0.01 to 0.52) | 0.22 (0.05‐0.40) |
| ALT (U/L) | 0.01 (−0.02 to 0.03) | 0.01 (−0.01 to 0.03) | −0.01 (−0.18 to 0.16) |
| Born in United States | 0.04 (−0.02 to 0.11) | 0.01 (−0.11 to 0.13) | 0.08 (0.0004‐0.17) |
| CAP (dB/M) | 0.01 (−0.01 to 0.02) | 0.01 (0.0003‐0.03) | 0.01 (0.007‐0.02) |
| Age | −0.008 (−0.03 to 0.02) | −0.005 (−0.04 to 0.03) | −0.006 (−0.04 to 0.02) |
| Male | −0.04 (−0.14 to 0.06) | — | — |
| Heavy drinking‡ | −0.09 (−0.32 to 0.14) | −0.10 (−0.29 to 0.09) | −0.05 (−0.37 to 0.26) |
Estimated in survey‐based multivariable logistic regression.
Current use of diabetes medication.
Defined as >14 drinks/week for female participants and >21 drinks/week for male participants.
Abbreviations: CAP, controlled attenuation parameter.
Waist circumference and HbA1c were the strongest predictors of liver stiffness. We compared the median liver stiffness and prevalence of kPa > 7.1 across four categories defined by waist circumference and HbA1c. We found that the median liver stiffness in kPa and prevalence of liver stiffness >7.1 kPa was greatest among those with both elevated waist circumference and elevated HbA1c compared to those with only one risk factor (Fig. 1). We also attempted to adjust for TE probe type (XL vs. medium) in our models but found that problematic collinearity with waist circumference caused a reversal of coefficient directions; instead, we stratified our models by probe type. We found that the coefficient estimates for HbA1c were similar for the XL probe (n = 266; β = 0.33, P = 0.056) and the medium probe (n = 504; β = 0.37, P = 0.136). In a further exploratory analysis, we stratified the model restricted to female participants by age (<50 and ≥50) as a proxy for menopausal age to further assess the sex differences between HbA1c and liver stiffness, and HbA1c was more strongly associated with liver stiffness in females younger than 50 (β = 0.46, 95% CI 0.14‐0.78, P = 0.010) and was weaker and not significantly associated with liver stiffness among those 50 and older (β = 0.27, 95% CI −0.02 to 0.56, P = 0.081) (Supporting Table S3).
Fig. 1.
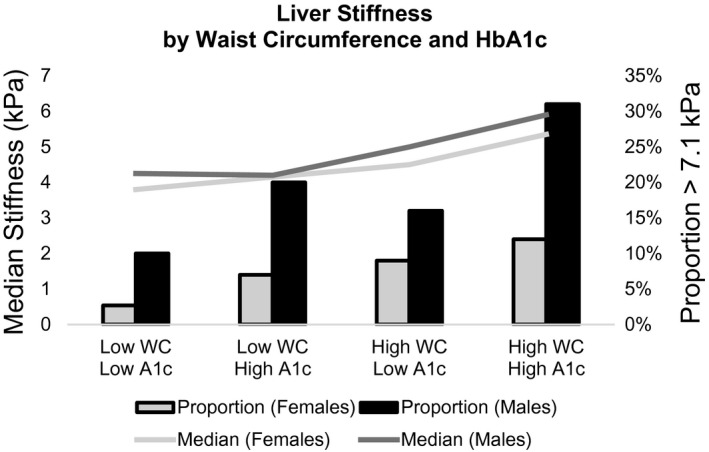
Liver stiffness by waist circumference and HbA1c (A1c). Abbreviations: kPa, kilopascals; WC, waist circumference.
SENSITIVITY ANALYSES
The association between HbA1c and liver fibrosis was similar and borderline statistically significant (β = 0.32, 95% CI −0.02 to 0.67, P = 0.0778) when removing those with possible cirrhosis (remaining n = 761). None of the participants with possible cirrhosis had evidence of anemia, suggesting that no participants had decompensated cirrhosis. The results were similar and statistically significant after adjusting for HCV‐Ab and HBsAg in a smaller subset of participants. We re‐estimated the multivariable HbA1c model with a dichotomous liver fibrosis outcome and found that log‐transformed HbA1c was not statistically significantly associated with dichotomous liver fibrosis (odds ratio = 1.64, 95% CI −0.22 to 3.59, P = 0.096) after adjustment for waist circumference and all other covariates.
Discussion
Elevated glucose and hyperinsulinemia are among the potential biological mechanisms suggested in liver fibrosis progression.( 28 , 29 ) In this study we tested the association between glucose metabolism measures and liver fibrosis in a large Mexican‐American study population accounting for other well‐known risk factors for chronic liver disease (central obesity, alcohol abuse, viral hepatitis infections). Of the four glucose metabolism measures evaluated, HbA1c was the only measure statistically significantly associated with liver stiffness, controlling for known confounders. Although blood glucose levels are implicated in liver fibrosis progression, fasting blood glucose was not statistically significantly associated with liver fibrosis in this population. Similarly, we did not identify associations between fasting insulin or insulin resistance and liver fibrosis at the population level. Because HbA1c can be measured on nonfasting individuals and is more stable over time, we consider this the most promising measure for improving liver disease risk stratification. Moreover, this association between HbA1c and liver stiffness is independent of the association between waist circumference and liver stiffness.
Previous studies have shown that liver enzymes, central adiposity, and the CAP measure of hepatic fat are associated with liver fibrosis,( 8 , 12 , 30 , 31 ) which we corroborated in this study, particularly for women. Among men, neither central adiposity nor liver enzymes were significantly associated with liver stiffness. This population of men is known to have a high burden of undiagnosed liver disease( 32 , 33 ) and the well‐studied risk factors of obesity and diabetes may not be as predictive among Hispanic men as in other populations. As we showed in Supporting Fig. S1 and Supporting Table S2, men have higher liver stiffness levels, on average, but our model inferences based on male participants is limited, and more studies of NAFLD in men are needed. Other work in a European population has shown an association between blood lipids and liver fibrosis,( 8 ) for which we did not find evidence in this Mexican‐American population. Similarly, we did not identify associations between alcohol consumption and liver fibrosis in this representative sample of Mexican Americans, suggesting that alcohol consumption is a minor contributor to the outsized burden of liver disease in south Texas.
A limitation in our study is the lack of a validated FibroScan cutoff for staging of fibrosis in nonclinical Hispanic populations, which led us to evaluate associations with continuous values of liver stiffness. Although liver biopsy and magnetic resonance spectroscopy are considered the gold standards for the diagnosis of NAFLD, these are invasive and/or expensive tests that are not feasible in this community‐dwelling cohort.( 34 , 35 , 36 ) However, TE is an objective measure of liver stiffness that has been validated against histologically assessed liver fibrosis and is positively associated with increasing fibrosis severity based on blood‐based markers of liver fibrosis.( 11 ) Additionally, our interoperator reliability metrics suggest strong concordance between operators in obtaining kilopascal and CAP measures. Relative strengths of the study include its population‐based recruitment of a US minority group that has a growing burden of liver disease but limited representation in the liver literature. This cohort is drawn from a population with high prevalence of diabetes (28%) and a high incidence of reported HCC (14.6 of 100,000) compared with 8.3 of 100,000 nationally.( 37 )
In a population with limited access to health care, screening using simple measures such as HbA1c and TE, which are affordable and portable, are important for the prevention of liver disease progression and liver cancer.( 38 ) HbA1c is particularly promising as an aid to liver disease risk prediction because it is a consistent measure of glucose control whether or not an individual is fasting. Additionally, a recent study showed that increased HbA1c was associated with the risk of liver fibrosis progression to HCC,( 39 ) suggesting that HbA1c may aid in risk stratification throughout the spectrum of liver disease progression.
In conclusion, elevated HbA1c —which is a routine measure in many clinical settings—is an important metric for triggering further screening of hepatic fibrosis and susceptibility to liver cancer, particularly among Mexican Americans. Our results urge a coordinated approach to the prevention and control of liver disease and liver cancer alongside diabetes in Mexican‐American communities. In south Texas, where the burdens of liver cancer, obesity, and diabetes are all high, health promotion programs should be tailored to use these simple tools to screen for advancing liver disease at a stage where it might be prevented.
Supporting information
Supplementary Material
Acknowledgments
The authors would like to thank our cohort team, particularly Rocío Uribe and her team, who not only recruited and interviewed the participants, but also performed the ultrasound and elastography studies under expert supervision; Marcela Morris and Hugo Soriano and their teams for laboratory and data support; Norma Pérez‐Olazarán and Christina Villarreal for the administrative support; Valley Baptist Medical Center, Brownsville, Texas, for providing us space for our Center for Clinical and Translational Science Clinical Research Unit; and the community of Brownsville and the participants who so willingly participated in this study in their city.
Supported by the Center for Clinical and Translational Sciences, National Institutes of Health (NIH) (UL1 TR000371); a Predoctoral Fellowship from the University of Texas School of Public Health Cancer Education and Career Development Program/National Cancer Institute/NIH (R25 CA057712); NIH (C06 RR020547); National Institute of Environmental Health Sciences (5P42ES010337); National Center for Advancing Translational Sciences (5UL1TR001442); National Institute of Diabetes and Digestive and Kidney Diseases (R01DK106419 and P30DK120515); and Department of Defense Prostate Cancer Research Program (CA170674P2).
Potential conflict of interest: Dr. Loomba serves as a consultant or advisory board member for Anylam/Regeneron, Arrowhead Pharmaceuticals, AstraZeneca, Bird Rock Bio, Boehringer Ingelheim, Bristol‐Myer Squibb, Celgene, Cirius, CohBar, Conatus, Eli Lilly, Galmed, Gemphire, Gilead, Glympse bio, GNI, GRI Bio, Inipharm, Intercept, Ionis, Janssen Inc., Merck, Metacrine, Inc., NGM Biopharmaceuticals, Novartis, Novo Nordisk, Pfizer, Prometheus, Promethera, Sanofi, Siemens, and Viking Therapeutics. In addition, his institution has received grant support from Allergan, Boehringer‐Ingelheim, Bristol‐Myers Squibb, Cirius, Eli Lilly and Company, Galectin Therapeutics, Galmed Pharmaceuticals, GE, Genfit, Gilead, Intercept, Grail, Janssen, Madrigal Pharmaceuticals, Merck, NGM Biopharmaceuticals, NuSirt, Pfizer, pH Pharma, Prometheus, and Siemens. He is also co‐founder of Liponexus, Inc.
References
Author names in bold designate shared co‐first authorship.
- 1. Wong RJ, Cheung R, Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology 2014;59:2188‐2195. [DOI] [PubMed] [Google Scholar]
- 2. Adams LA, Lymp JF, St. Sauver J, Sanderson SO, Lindor KD, Feldstein A, et al. The natural history of nonalcoholic fatty liver disease: a population‐based cohort study. Gastroenterology 2005;129:113‐121. [DOI] [PubMed] [Google Scholar]
- 3. Seeff LB. Natural history of chronic hepatitis C. Hepatology 2002;36:s35‐s46. [DOI] [PubMed] [Google Scholar]
- 4. Steele CB. Vital signs: trends in incidence of cancers associated with overweight and obesity—United States, 2005‐2014. MMWR Morb Mortal Wkly Rep 2017;66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yin M, Venkatesh SK. Ultrasound or MR elastography of liver: which one shall I use? Abdom Radiol (NY) 2018;43:1546‐1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Loomba R, Lim JK, Patton H, El‐Serag HB. AGA clinical practice update on screening and surveillance for hepatocellular carcinoma in patients with nonalcoholic fatty liver disease: expert review. Gastroenterology 2020;158:1822‐1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hsu C, Caussy C, Imajo K, Chen J, Singh S, Kaulback K, et al. Magnetic resonance vs transient elastography analysis of patients with non‐alcoholic fatty liver disease: a systematic review and pooled analysis of individual participants. Clin Gastroenterol Hepatol 2018;17:630‐637.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Caballeria L, Pera G, Arteaga I, Rodriguez L, Aluma A, Morillas RM, et al. High prevalence of liver fibrosis among European adults with unknown liver disease. A population‐based study. Clin Gastroenterol Hepatol 2018;16:1138‐1145.e5. [DOI] [PubMed] [Google Scholar]
- 9. Conti F, Vukotic R, Foschi FG, Domenicali M, Giacomoni P, Savini S, et al. Transient elastography in healthy subjects and factors influencing liver stiffness in non‐alcoholic fatty liver disease: an Italian community‐based population study. Dig Liver Dis 2016;48:1357‐1363. [DOI] [PubMed] [Google Scholar]
- 10. Huh JH, Kim KJ, Kim SU, Han SH, Han K‐H, Cha B‐S, et al. Obesity is more closely related with hepatic steatosis and fibrosis measured by transient elastography than metabolic health status. Metabolism 2017;66:23‐31. [DOI] [PubMed] [Google Scholar]
- 11. Siddiqui MS, Vuppalanchi R, Van Natta ML, Hallinan E, Kowdley KV, Abdelmalek M, et al. Vibration‐controlled transient elastography to assess fibrosis and steatosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2019;17:156‐163.e152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mikolasevic I, Orlic L, Franjic N, Hauser G, Stimac D, Milic S. Transient elastography (FibroScan®) with controlled attenuation parameter in the assessment of liver steatosis and fibrosis in patients with nonalcoholic fatty liver disease—Where do we stand? World J Gastroenterol 2016;22:7236‐7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Izumi T, Sho T, Morikawa K, et al. Assessing the risk of hepatocellular carcinoma by combining liver stiffness and the controlled attenuation parameter. Hepatol Res 2019;49:1207‐1217. [DOI] [PubMed] [Google Scholar]
- 14. Xiao G, Zhu S, Xiao X, Yan L, Yang J, Wu G. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: a meta‐analysis. Hepatology 2017;66:1486‐1501. [DOI] [PubMed] [Google Scholar]
- 15. Koehler EM, Plompen EPC, Schouten JNL, Hansen BE, Darwish Murad S, Taimr P, et al. Presence of diabetes mellitus and steatosis is associated with liver stiffness in a general population: the Rotterdam study. Hepatology 2016;63:138‐147. [DOI] [PubMed] [Google Scholar]
- 16. Lee HW, Kim BK, Kim SU, Park JY, Kim DY, Ahn SH, et al. Prevalence and predictors of significant fibrosis among subjects with transient elastography‐defined nonalcoholic fatty liver disease. Dig Dis Sci 2017;62:2150‐2158. [DOI] [PubMed] [Google Scholar]
- 17. Goh GB, Pagadala MR, Dasarathy J, Unalp‐Arida A, Sargent R, Hawkins C, et al. Clinical spectrum of non‐alcoholic fatty liver disease in diabetic and non‐diabetic patients. BBA Clin 2015;3:141‐145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Doycheva I, Cui J, Nguyen P, Costa EA, Hooker J, Hofflich H, et al. Non‐invasive screening of diabetics in primary care for NAFLD and advanced fibrosis by MRI and MRE. Aliment Pharmacol Ther 2016;43:83‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fisher‐Hoch SP, Rentfro AR, Salinas JJ, Perez A, Brown HS, Reininger BM, et al. Socioeconomic status and prevalence of obesity and diabetes in a Mexican American community, Cameron County, Texas, 2004‐2007. Prev Chronic Dis 2010;7:A53. [PMC free article] [PubMed] [Google Scholar]
- 20. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412‐419. [DOI] [PubMed] [Google Scholar]
- 21. Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non‐alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology 2012;142:1592‐1609. [DOI] [PubMed] [Google Scholar]
- 22. American Diabetes Association . Standards of medical care in diabetes—2010. Diabetes Care 2010;33:S11‐S61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Boursier J, Zarski J‐P, de Ledinghen V, Rousselet M‐C, Sturm N, Lebail B, et al. Determination of reliability criteria for liver stiffness evaluation by transient elastography. Hepatology 2013;57:1182‐1191. [DOI] [PubMed] [Google Scholar]
- 24. Lumley T. Analysis of complex survey samples. J Stat Softw 2004;1. [Google Scholar]
- 25. Core Team R. R: A Language and Environment for Statistical Computing. Vienna, Austria: Foundation for Statistical Computing; 2013. [Google Scholar]
- 26. FibroScan® and Liver Disease . 2018. https://www.uhn.ca/PatientsFamilies/Health_information/Health_Topics/Documents/FibroScan_Liver_Disease.pdf. Accessed April 6, 2020.
- 27. Nadelson J, Satapathy SK, Nair S. Glycated hemoglobin levels in patients with decompensated cirrhosis. Int J Endocrinol 2016;2016:8390210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Paradis V, Perlemuter G, Bonvoust F, et al. High glucose and hyperinsulinemia stimulate connective tissue growth factor expression: a potential mechanism involved in progression to fibrosis in nonalcoholic steatohepatitis. Hepatology 2001;34(Pt 1):738‐744. [DOI] [PubMed] [Google Scholar]
- 29. Li X, Jiao Y, Xing Y, Gao P. Diabetes mellitus and risk of hepatic fibrosis/cirrhosis. Biomed Res Int 2019;2019:5308308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Harman DJ, Ryder SD, James MW, Wilkes EA, Card TR, Aithal GP, et al. Obesity and type 2 diabetes are important risk factors underlying previously undiagnosed cirrhosis in general practice: a cross‐sectional study using transient elastography. Aliment Pharmacol Ther 2018;47:504‐515. [DOI] [PubMed] [Google Scholar]
- 31. Lallukka S, Sadevirta S, Kallio MT, Luukkonen PK, Zhou Y, Hakkarainen A, et al. Predictors of liver fat and stiffness in non‐alcoholic fatty liver disease (NAFLD)—an 11‐year prospective study. Sci Rep 2017;7:14561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Watt GP, Lee M, Pan J‐J, Fallon MB, Loomba R, Beretta L, et al. High prevalence of hepatic fibrosis, measured by elastography, in a population‐based study of Mexican Americans. Clin Gastroenterol Hepatol 2019;17:968‐975.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pan J‐J. Burden of nonalcoholic fatty liver disease and advanced fibrosis in a Texas Hispanic community cohort. World J Hepatol 2015;7:1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Balakrishnan M, Loomba R. The role of noninvasive tests for differentiating NASH from NAFL and diagnosing advanced fibrosis among patients with NAFLD. J Clin Gastroenterol 2020;54:107‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Caussy C, Reeder SB, Sirlin CB, Loomba R. Non‐invasive, quantitative assessment of liver fat by MRI‐PDFF as an endpoint in NASH trials. Hepatology 2018;68:763‐772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Castera L, Friedrich‐Rust M, Loomba R. Noninvasive assessment of liver disease in patients with nonalcoholic fatty liver disease. Gastroenterology 2019;156:1264‐1281.e1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Carrion AF, Ghanta R, Carrasquillo O, Martin P. Chronic liver disease in the Hispanic population of the United States. Clin Gastroenterol Hepatol 2011;9:834‐841; quiz e109‐e110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Donadon V, Balbi M, Valent F, Avogaro A. Glycated hemoglobin and antidiabetic strategies as risk factors for hepatocellular carcinoma. World J Gastroenterol 2010;16:3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Daijo K, Nakahara T, Inagaki Y, Nanba M, Nishida Y, Uchikawa S, et al. Risk factors for histological progression of nonalcoholic steatohepatitis analyzed from repeated biopsy cases. J Gastroenterol Hepatol 2020;35:1412‐1419. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


