Abstract
Chronic liver disease (CLD) is a growing cause of morbidity and mortality worldwide. The burden of CLD varies according to etiology and geographic location. We assessed the global burden of disability from the most important complications of CLD (cirrhosis and liver cancer [LC]) according to the most common etiologies between 2007 and 2017. We obtained years living with disability (YLD), years of life lost (YLL), and disability‐adjusted life‐years (DALYs) data from the Global Burden of Disease 2017 study. Between 2007 and 2017, LC DALYs decreased by 4.52% and cirrhosis DALYs decreased by 10.58%. Nevertheless, in 2017, CLD caused 62.16 million DALYs (33.4% LC and 66.5% cirrhosis), of which 96.8% came from YLL (34.1% LC and 65.9% cirrhosis) and 3.2% from YLD (11.6% LC and 88.4% cirrhosis). In 2017, Asia accounted for 66% of all DALYs globally. Central Asia, Africa regions, Southeast Asia, and Eastern Europe had the highest liver‐related DALYs (≥1,000 per 100,000), whereas the lowest rates (≤500 per 100,000) were seen in high‐income regions, such as Asia Pacific, North America, Western Europe, and Australasia. In 2007, hepatitis B virus caused the majority (47.5%) of liver‐related DALYs, followed by hepatitis C virus (23.7%), alcoholic liver disease (14.2%), and nonalcoholic fatty liver disease/nonalcoholic steatohepatitis (NAFLD/NASH) (6.4%). In 2017, these rates shifted to 45.7%, 24.1%, 4.8%, and 7.1%, respectively. Between 2007 and 2017, cirrhosis‐related DALYs due to NAFLD/NASH increased by 23.4%, whereas the increment was 37.5% for LC‐related DALYs due to NAFLD/NASH. Conclusion: DALYs due to viral hepatitis still account for the largest proportion of CLD‐related DALYs. Although DALYs from all other liver diseases have remained stable in the last decade, DALYs related to NAFLD/NASH are growing. National, regional, and global policies are needed to address the disability burden of NAFLD across the world.

Abbreviations
- A
Andean
- ALD
alcoholic liver disease
- Am
America
- C
Central
- CI
, confidence interval
- CLD
chronic liver disease
- COD
cause of death
- CVD
cardiovascular disease
- DALY
disability‐adjusted life‐year
- E
Eastern
- GBD
Global Burden of Disease
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HI
high income
- LC
liver cancer
- MENA
Middle East and North Africa
- N
North
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- Pac
Pacific
- S
Southern
- SE
Southeast
- sub‐Sah
sub‐Saharan
- T2DM
type 2 diabetes mellitus
- Trop
tropical
- UI
uncertainty interval
- W
Western
- WHO
World Health Organization
- YLD
years living with disability
- YLL
years of life lost
Chronic liver disease (CLD) is a major cause of morbidity and mortality around the globe.( 1 , 2 ) The mortality ranking of CLD has risen in recent years; it was the twelfth leading cause of death (COD) in 2016 and became the tenth leading COD in 2019.( 3 ) In addition to mortality, CLD is responsible for significant morbidity with impairment of patients’ quality of life.( 4 ) Viral hepatitis (either hepatitis B virus [HBV] or hepatitis C virus [HCV]), alcoholic liver disease (ALD), and nonalcoholic fatty liver disease/nonalcoholic steatohepatitis (NAFLD/NASH) are the leading causes of CLD. Over time, these types of CLD can lead to development of cirrhosis and its complications, including liver cancer (LC).( 5 )
Globally, the contribution of different etiologies to the burden of liver disease varies based on geographic location. In this context, despite a decrease in the incidence of HCV, complications of HCV‐related liver disease, such as hepatocellular carcinoma (HCC), seem to continue to rise.( 1 ) Furthermore, although complications of HBV may be preventable by vaccination, neonatal screening, and potentially antiviral therapy in some parts of the world, HBV remains a significant problem in other parts of the globe. In contrast, the rising epidemic of obesity is fueling the rapid increase in the burden of NAFLD.( 6 , 7 ) Although the burden of chronic diseases can be quantified in a number of ways, assessment of disability‐adjusted life‐years (DALYs) is an important surrogate. In this context, understanding the global burden of complications of CLD (cirrhosis and LC) according to etiology of CLD, geographic location, and trends over time will be important.( 8 )
Although there are adequate data on the prevalence and mortality associated with CLD, data on morbidity and DALYs related to CLD are not readily available. Therefore, the aim of the current study is to assess the global burden of disability caused by LC and cirrhosis by focusing on years living with disability (YLD), years of life lost (YLL), and DALYs between 2007 and 2017.
Materials and Methods
Data Sources
This study was based on data from the Global Burden of Disease (GBD) study,( 9 ) coordinated by the Institute for Health Metrics and Evaluation (IHME). As a continuous quality improvement, each annual GBD study re‐estimated the entire time series by including all known advances in data, modeling, estimation methods, and health knowledge, ensuring that each GBD contained the most up‐to‐date estimates. The GBD 2017 study was published in 2018, providing epidemiologic assessments of 359 diseases and injuries and 84 risk factors from 195 countries and territories. For this study, we obtained the publication estimates of incidental cases, deaths, and DALYs for LC and cirrhosis as well as five etiology groups (HBV, HCV, ALD, NAFLD, and other causes) for each 5‐year age group, sex, year, and location from GBD 2017.( 9 ) General methods for the GBD study have been published.( 10 , 11 ) Herein, we briefly present the GBD estimation process for LC and cirrhosis as well as their etiology for 2017.
Cardiovascular disease (CVD) included ischemic heart disease, stroke, hypertensive heart disease, rheumatic heart disease, aortic valve disease, cardiomyopathy, and myocarditis. Metabolic risks included high fasting plasma glucose, high low‐density lipoprotein cholesterol, high systolic blood pressure, high body mass index, low bone mineral density, and impaired kidney function.
GBD Estimation Framework
The GBD estimation process began with mortality. The compilation of data sources, i.e., the COD database, assembled various sources of incidence and mortality data through multiple processing steps for adjustments of age group and aggregated, implausible, and unspecified COD codes.( 12 ) The codes of the International Classification of Diseases, Ninth and Tenth revisions, that were used to identify LC and cirrhosis are shown in Supporting Table S1. In the COD database, LC and cirrhosis mortality were separately estimated by the COD ensemble model,( 10 , 13 , 14 ) which is an approach that incorporates a wide variety of individual models (linear mixed‐effects regression and spatiotemporal Gaussian process regression) and covariates to create a predictive model for CODs. All individual and ensemble models were evaluated using out‐of‐sample predictive validity tests and vetted by experts in each disease and then validated by specialists at IHME and their collaborators from around the world.( 10 , 15 ) The assessment for the quality of data in each country is available in Supporting Table S2.
DALYs were computed by the summation of YLL and YLD, which quantify the health loss due to specific diseases and injuries. We calculated YLL by multiplying the estimated number of deaths by age with a standard life expectancy at that age and YLD by multiplying prevalence by a disability weight ranging from 0 to 1, where 0 is a state of full health and 1 is death. LC and cirrhosis due to any cause have a disability weight of 0.451 (95% uncertainty interval [UI], 0.307‐0.600) and 0.178 (95% UI, 0.123‐0.250), respectively.( 12 )
Etiological Estimates for LC and Cirrhosis
Etiological estimates for LC and cirrhosis start with a systematic review to collect secondary data for the five etiologies: HBV, HCV, ALD, and NAFLD or NAFLD/NASH as well as other causes, such as hemochromatosis, autoimmune hepatitis, Wilson’s disease, cryptogenic, or unknown. Only LC and cirrhotic population‐based studies for the respective location were included. Importantly, cases due to NAFLD/NASH were defined only if NAFLD/NASH was listed as a specific etiology in the manuscript.( 10 ) The compilation of all studies through the systematic literature review was used as input for five independent disease modeling‐meta‐regression models,( 16 , 17 ) a Bayesian meta‐regression model, to estimate the proportion of LC and cirrhosis due to each etiology for each age, sex, location, and year. To integrate the etiological proportion model for these two causes, the modeled etiological proportions of LC were used as covariates for the cirrhosis etiological model. A complete list of predictive covariates used in the models can be found in Supporting Table S3.
Relevant metadata can be retrieved through the publicly available Data Input Sources Tool.( 18 )
For administrative and data analysis purposes, the world was split into 21 GBD regions and seven GBD super regions according to epidemiologic similarities and geographic proximity( 19 ) (Supporting Table S3). Flow charts for the COD database, input data and methodological summary for the models, and statistical codes are publicly available in compliance with the Guidelines for Accurate and Transparent Health Estimates Reporting developed by the World Health Organization (WHO).
Data Analysis
GBD estimates for a disease burden are reported with 95% UIs, including the true value of a parameter with 95% probability. UIs account for not only variance in parameter estimation but also uncertainty from data collection, model selection, and other sources of uncertainty under the parameter estimation process. Age‐standardized rates were computed by the direct method to the GBD population standard by 5‐year age groups.( 10 ) All rates reported here were age‐standardized rates per 100,000 population. Percentage change was based on the difference between the value in 2017 and in 2007 divided by the value in 2007; this was considered to be significant when the 95% UIs did not include zero.
The association of age‐standardized DALY rates for LC and cirrhosis combined due to NAFLD/NASH with age‐standardized DALY rates for type 2 diabetes mellitus (T2DM), CVD, and all‐cause death due to metabolic risks were determined using sociodemographic index‐adjusted partial Spearman correlation coefficients (ρ) with 95% confidence intervals (CIs).
Results
Results and findings of GBD 2017 can be explored interactively through the GBD Visualization Hub.( 20 ) Global estimates of YLL, YLD, and DALYs, respectively, are reported in Supporting Tables S4‐S9, with age‐standardized rates for LC and cirrhosis as reported for 21 GBD regions and 195 countries and territories as well as the percentage change in age‐standardized rates that occurred between 2007 and 2017.
In 2017, there was a global total of 2.50 billion DALYs, with 35.8% from YLD and 64.2% from YLL. There were 6.1 million worldwide incident cases of complications of CLD (referred to as liver related). Of these, 15.6% were from LC and 84.4% from cirrhosis. Additionally, there were 2.14 million liver‐related deaths (38.3% LC and 61.6% cirrhosis). Globally, LC was responsible for 20.54 million (95% UI, 19.68‐21.55) YLL, 0.23 million (95% UI, 0.16‐0.30) YLD, and 20.77 million (95% UI, 19.91‐21.81) DALYs, reflecting an age‐standardized rate of 250.73 (95% UI, 240.40‐262.99) YLL per 100,000 person‐years, 2.83 (95% UI, 2.02‐3.72) YLD, and 253.56 (95% UI, 243.16‐266.22) DALYs. The age‐standardized YLL and DALY rates for LC declined by −4.65% (95% UI, −7.96% to 0.06%) and −4.52% (95% UI, −7.80% to 0.17%), whereas the age‐standardized YLD rate inclined by 8.11% (95% UI, 3.87%‐13.71%), between 2007 and 2017 (Figs. 1 and 2; Supporting Figs. [Link], [Link], [Link]).
FIG. 1.
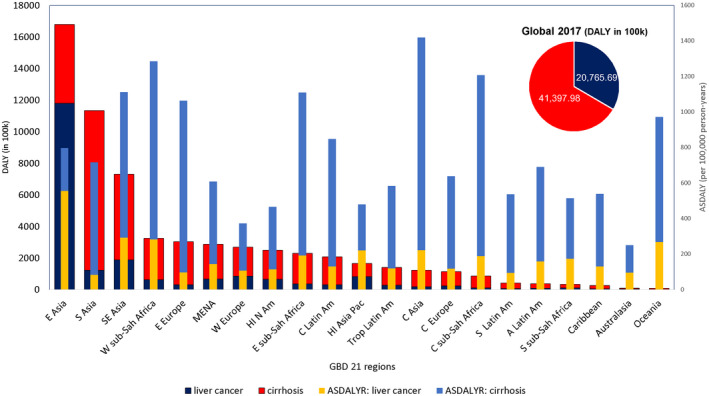
Global number of DALY and age‐standardized DALY rates due to LC and cirrhosis, according to GBD 2017.( 9 ) Abbreviation: ASDALYR, age‐standardized disability‐adjusted life‐year rate.
FIG. 2.
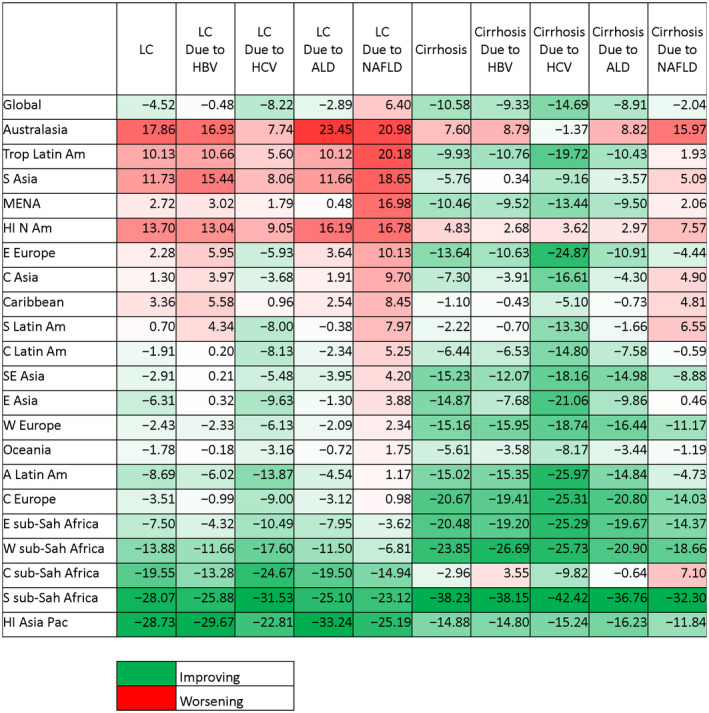
Percentage change in age‐standardized DALY rates for LC and cirrhosis from 2007 through 2017.
CLD Burden According to Geographic Distribution
In 2017, the highest liver‐related DALYs among 21 GBD regions were observed in Asia regions, accounting for 66% of global liver‐related DALYs, followed by western sub‐Saharan Africa (3.3 million), Eastern Europe (3.0 million), Western Europe (2.7 million), and high‐income North America (2.5 million). However, Central Asia, Africa regions, Southeast Asia, and Eastern Europe had the highest age‐standardized liver‐related DALY rates (1,064‐1,420 per 100,000) (Fig. 1). ALD was the dominant cause of liver‐related burden among Central Asia and Eastern Europe (≥34%), HBV in western sub‐Saharan Africa (48%), and hepatitis B and C among central sub‐Saharan Africa and Southeast Asia (≥30%) (Supporting Fig. S4). By contrast, high‐income regions, including Asia Pacific, North America, Western Europe, and Australasia, had the lowest age‐standardized DALY rates in 2017 (250‐480 per 100,000). The proportion of the liver‐related burden caused by NAFLD/NASH ranged from 5% in high‐income Asia to between 15% and 20% in the Andean region, tropical and central Latin America, and the Caribbean region. In all regions except East Asia, the age‐standardized DALYs due to cirrhosis were higher than LC (Fig. 1). In China in East Asia, LC DALYs were 2.4 times higher than cirrhosis DALYs. Similar findings were observed for liver‐related YLL. The highest age‐standardized liver‐related YLD (≥40 per 100,000) rates in 2017 were in high‐income Asia Pacific, Central Europe, and central and eastern Asia, whereas the lowest rates were in South and Southeast Asia, Oceania, and Australasia (≤15 per 100,000) (Supporting Fig. S1).
Changes in Burden of CLD From 2007 to 2017
The percentage change in age‐standardized LC DALYs from 2007 to 2017 differed substantially among GBD regions, with the highest increase in Australasia (20.98%; 95% UI, 8.64%‐34.58%), tropical Latin America (20.18%; 95% UI, 16.05%‐23.98%), South Asia (18.65%; 95% UI, 9.38%‐29.11%), and high‐income North America (16.78%; 95% UI, 12.73%‐21.05%) where the fastest incline in NAFLD among liver diseases was observed. LC DALYs caused by viral hepatitis and ALD improved in most regions. In contrast, the age‐standardized LC DALYs due to NAFLD/NASH increased globally (6.40%; 95% UI, 2.99%‐10.57%) (Fig. 2).
In contrast to LC, most regions experienced improving trends in age‐standardized cirrhosis DALYs, with the largest improvements in Africa regions and Central Europe (−20% to −38%), mostly driven by a decreasing trend in HBV. In contrast, only Australasia and high‐income North America experienced significant worsening trends (7.60%; 95% UI, −3.06 to 19.76 and 4.83%; 95% UI, 1.20‐8.54, respectively), and particularly Australia and United States in these regions had the largest increase in cirrhosis burden due to NAFLD/NASH. Compared to a decreasing trend for the other liver diseases, NAFLD/NASH‐cirrhosis showed increases in age‐standardized DALYs for 10 of 21 regions (Fig. 2).
The largest increases in YLD rates for LC were seen in East Asia (13.43%; 95% UI, 5.99%‐22.86%), tropical Latin America (11.59%; 95% UI, 8.53%‐15.37%), Australasia (19.96%; 95% UI, 5.27%‐34.76%), South Asia (12.95%; 95% UI, 4.39%‐23.59%), and high‐income North America (15.40%; 95% UI, 10.71%‐20.11%), whereas the largest decreases were found in Africa regions (−25% to −10%). Among liver diseases, the highest increases in the age‐standardized liver‐related YLD rates were due to NAFLD globally (Supporting Fig. S3).
Burden of LC by Etiologic Groups
In 2017, the leading global etiologies for LC DALYs were HBV (45.7%), HCV (24.1%), ALD (14.8%), and NAFLD/NASH (7.1%). HBV accounted for the largest proportion of LC DALYs in nine out of 21 GBD regions, ranging from 27.7% in the Caribbean to 58.7% in East Asia, whereas HCV was the leading cause of LC DALYs in nine out of 21 GBD regions (Fig. 3; Supporting Figs. S5 and S6).
FIG. 3.
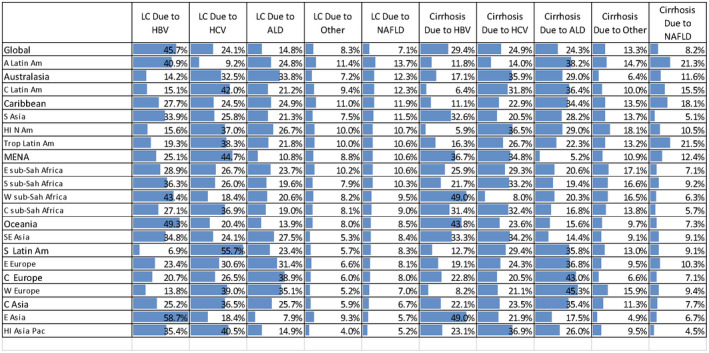
Contribution of different liver diseases on age‐standardized DALY rates for LC and cirrhosis, according to GBD 2017.( 9 )
From 2007 to 2017, the total number of YLL, YLD, and DALYs caused by NAFLD/NASH‐related LC increased by 37.28% (95% UI, 32.67%‐42.82%), 52.53% (95% UI, 47.45%‐58.88%), and 37.45% (95% UI, 32.83%‐42.95%), respectively. During the same period, age‐standardized NAFLD/NASH‐related LC YLL, YLD, and DALYs per 100,000 increased by 6.29% (95% UI, 2.88%‐10.47%), 16.14% (95% UI, 12.36%‐20.87%), and 6.40% (95% UI, 2.99%‐10.57%), respectively.
The highest number of DALYs due to NAFLD/NASH‐related LC was in most of the Asia regions, followed by high‐income North America, North Africa, and the Middle East. The highest age‐standardized NAFLD/NASH‐related LC DALYs were founded in East Asia, western sub‐Saharan Africa, and Southeast Asia (≥24 per 100,000); the lowest rates were seen in South Asia, Central Europe, southern Latin America, Eastern Europe, and Western Europe (≤10 per 100,000).
Burden of Cirrhosis by Etiologic Groups
In 2017, HBV, HCV, and ALD were responsible for 29.4%, 24.9%, and 24.3% of global cirrhosis DALYs, respectively. ALD was the largest proportion of cirrhosis DALYs in eight of 21 regions, HCV in eight regions, and HBV in five regions (Fig. 3; Supporting Figs. S5 and S6).
From 2007 to 2017, the total number of YLL, YLD, and DALYs caused by NAFLD/NASH‐related cirrhosis increased by 22.23% (95% UI, 16.56%‐27.24%), 54.61% (95% UI, 51.04%‐58.33%), and 23.35% (95% UI, 17.78%‐28.27%), respectively. Age‐standardized NAFLD/NASH‐related cirrhosis YLL and DALY rates decreased by −2.95% (−7.39% to 0.95%) and −2.04% (95% UI, −6.44% to 1.87), respectively, whereas age‐standardized YLD rates increased by 23.48% (95% UI, 20.75%‐26.38%).
In 2017, the highest number of NAFLD/NASH‐related cirrhosis DALYs was in most of the Asia regions, followed by Eastern Europe and central Latin America. In contrast, the highest age‐standardized NAFLD/NASH‐related cirrhosis DALY rates were found in the Andean region, central and tropical Latin America, Eastern Europe, and Central Asia (≥90 per 100,000), whereas the lowest rates were seen in Australasia, East Asia, and high‐income Asia Pacific (≤18 per 100,000).
Association of NAFLD With Diabetes, CVD, and Metabolic Risks in 195 countries and territories
The pattern of age‐standardized DALY rates for LC and cirrhosis combined due to NAFLD/NASH versus T2DM, CVD, and all‐cause death due to metabolic risks for 2017 are shown in Fig. 4A‐C.
FIG. 4.
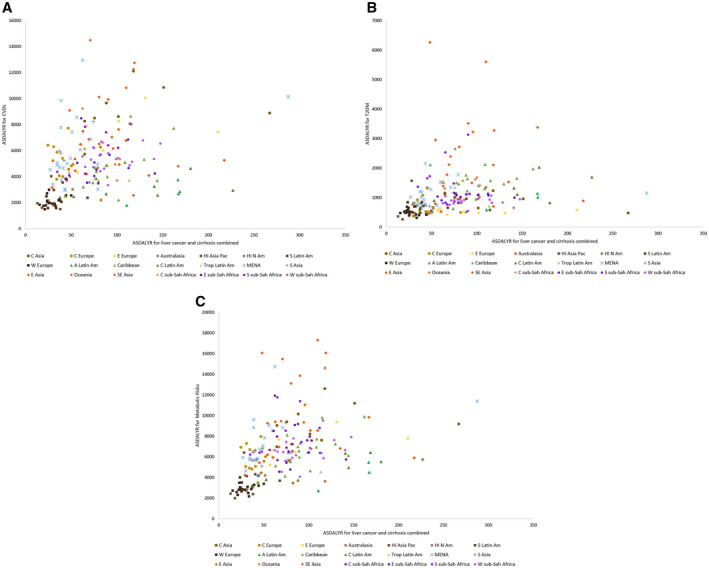
The pattern of age‐standardized DALY rates for LC and cirrhosis combined due to NAFLD/NASH versus (A) CVDs, (B) T2DM, and (C) metabolic risks for 195 countries and territories, according to GBD 2017.( 9 ) Abbreviation: ASDALYR, age‐standardized disability‐adjusted life‐year rate.
Globally, LC and cirrhosis combined due to NAFLD/NASH correlated with metabolic risks on the age‐standardized DALYs (ρ = 0.520; 95% CI, 0.410‐0.616), T2DM (ρ = 0.452; 95% CI, 0.333‐0.557), and CVD (ρ = 0.443; 95% CI, 0.322‐0.549).
Across seven GBD super regions, the highest correlation rate between NAFLD/NASH and metabolic risks was observed in Latin America and the Caribbean (ρ = 0.701; 95% CI, 0.466‐0.843), followed by sub‐Saharan Africa (ρ = 0.599; 95% CI, 0.373‐0.757), and high‐income (ρ = 0.498; 95% CI, 0.192‐0.716), and Central Europe, Eastern Europe, and Central Asia (ρ = 0.484; 95% CI, 0.143‐0.723) but not in South Asia (ρ = 0.80; 95% CI, −0.280 to 0.986), Southeast Asia, East Asia, and Oceania (ρ = 0.614; 95% CI, −0.284 to 0.456), and North Africa and the Middle East (ρ = 0.312; 95% CI, −0.139 to 0.655).
The highest correlation between NAFLD/NASH and T2DM was observed in sub‐Saharan Africa (ρ = 0.577; 95% CI, 0.344‐0.743), followed by Latin America and the Caribbean (ρ = 0.563; 95% CI, 0.267‐0.762), high‐income (ρ = 0.553; 95% CI, 0.264‐0.751), and Central Europe, Eastern Europe, and Central Asia (ρ = 0.460; 95% CI, 0.112‐0.707) but not in South Asia (ρ = 0.70; 95% CI, −0.477 to 0.978), Southeast Asia, East Asia, and Oceania (ρ = 0.132; 95% CI, −0.254 to 0.481), and North Africa and the Middle East (ρ = 0.04; 95% CI, −0.398 to 0.464).
The highest correlation between NAFLD/NASH and CVD was observed in Latin America and the Caribbean (ρ = 0.656; 95% CI, 0.399‐0.818), sub‐Saharan Africa (ρ = 0.530; 95% CI, 0.283‐0.711), high‐income (ρ = 0.452; 95% CI, 0.134‐0.685), Central Europe, Eastern Europe, and Central Asia (ρ = 0.368; 95% CI, 0.002‐0.678) but not in South Asia (ρ = 0.40; 95% CI, −0.745 to 0.948), North Africa and the Middle East (ρ = 0.111; 95% CI, −0.232 to 0.511), and Southeast Asia, East Asia, and Oceania (ρ = 0.053; 95% CI, −0.327 to 0.417).
Discussion
CLD is a growing cause of morbidity and mortality worldwide and currently is ranked among the top 10 causes of mortality in both the United States and the rest of the world.( 1 ) Cirrhosis and LC are two important complications of CLD and represent the drivers of liver‐related mortality and morbidity.( 21 ) In this context, in order to better comprehend the real burden of CLD, an assessment of CLD‐related YLL, YLD, and DALYs will be important.
Our data show there was a significant number of liver‐related deaths from cirrhosis and LC in 2017, which is in agreement with previous studies. In the United States, the incidence of LC has been increasing for the past 35 years and is expected to continue.( 22 , 23 , 24 ) Furthermore, the mortality rate of cirrhosis increased from 9.4 per 100,000 in 1999 to 11.5 per 100,000 in 2013.( 2 , 25 ) Expectedly, the uptrending incidence and prevalence rates of cirrhosis and LC are causing substantial mortality and morbidity, as reflected in YLL, YLD, and DALY rates.
Our study demonstrated that cirrhosis caused almost 41.4 million DALYs in 2017, corresponding to 510.65 per 100,000 population and that LC was responsible for 20.77 million DALYs, with a rate of 253.56 per 100,000 population. In 2017, Asia regions accounted for two thirds of all DALYs globally. The highest DALY rates were seen in Central Asia, Africa regions, Southeast Asia, and Eastern Europe, whereas the lowest DALY rates were observed in high‐income regions, such as Asia Pacific, North America, Western Europe, and Australasia. As expected, the underlying etiology differed based on region. For example, ALD was the leading cause of DALYs (34%) in Central Asia and Eastern Europe, while the leading cause in western sub‐Saharan Africa was HBV (48%). These findings are in agreement with reports demonstrating increased age‐standardized death rates in Asia and Eastern Europe, mainly driven by high ALD rates in those regions.( 26 , 27 ) In terms of regions in sub‐Saharan Africa, previous studies reported doubling of cirrhosis‐related deaths between 1980 and 2010, driven mainly by HBV and HCV and the struggles with access to treatment options for these conditions.( 28 ) On the contrary, high‐income regions had lower DALY rates, likely due to preventive measures and ease of access to health care when needed.
Our study showed that at the global level between 2007 and 2017 there was an 11.32% decrease in YLL and a 10.39% increase in YLD but overall a 10.58% decrease in DALYs due to cirrhosis. Per geographic distribution, the highest improvements were seen in Africa regions and Central Europe, with a 20% to 38% decrease in cirrhosis DALYs. This trend was driven by the decreasing effect of HBV in those areas. On the other hand, Australasia and high‐income North America demonstrated a worsening trend, with a 4.8% to 7.6% increase in cirrhosis DALYs that was driven by an increasing effect of NAFLD/NASH. Looking at the cirrhosis DALY burden in 2017, HBV was the leading etiology (29.4%), followed by HCV (24.9%) and ALD (24.3%). ALD burden was highest in Western Europe (45% of all DALYs), HCV was highest in high‐income North America (37% of all DALYs), and HBV was highest in western sub‐Saharan Africa (49% of all DALYs). Udompap et al.( 2 ) reported that ALD was responsible for almost 500,000 cirrhosis deaths worldwide in 2010, with a total of almost 15 million cirrhosis DALYs. These numbers represented 48% of cirrhosis deaths and 47% of cirrhosis DALYs at the global level in 2010. A report from the WHO in 2011 showed that Central Europe had the highest percentages of cirrhosis deaths and DALYs due to ALD, which is different from our results.( 29 ) However, it must be noted that after this finding the WHO set a goal to reduce ALD mortality to below 3.2 per 100,000 by 2020; our findings that Central Europe had the highest improvement in cirrhosis DALYs due to ALD (38% decrease between 2007 and 2017) suggest that this initiative by the WHO may be effective. On a cautionary note for Europe, it is worth recalling that the European region still has the highest levels of alcohol consumption globally and that there has been a change in consumption pattern, with drinking among the younger population and female individuals increasing and the average age of taking up drinking decreasing.( 30 ) On the other hand, similar to our findings, an excellent study by the GBD 2017 Cirrhosis Collaborators( 31 ) demonstrated that Western Europe and high‐income North America had low rates of cirrhosis mortality and DALYs, although the underlying etiologies were different. In Western Europe, the driver for cirrhosis DALYs was ALD whereas it was HCV in high‐income North America, and this finding was consistent with previous knowledge.( 32 ) It is well known that HCV has been the leading cause of cirrhosis, LC, and liver transplantation in the United States for decades.( 33 ) However, the contribution of HCV to CLD burden is anticipated to decrease in the United States not only because of the highly successful cure options we have now but also due to another growing problem, NAFLD/NASH, which is discussed in detail below.
In addition to cirrhosis, we covered the LC burden separately between 2007 and 2017. At the global level, YLL decreased by 4.65%, YLD increased by 8.11%, and DALYs decreased by 4.52%. The largest decreases in LC DALYs were seen in high‐income Asia Pacific (28.7%) and southern sub‐Saharan Africa (28%). On the contrary, the highest increases in LC DALYs were detected in Australasia (21%), tropical Latin America (20.2%), and South Asia (18.6%), all of which also represented the fastest growth rates of NAFLD among CLD. Globally in 2017, the leading causes of LC DALYs were HBV (45.7%), HCV (24.1%), ALD (14.8%), and NAFLD (7.1%). However, between 2007 and 2017, LC DALYs due to HBV, HCV, and ALD either were stable or improved whereas LC DALYs due to NAFLD/NASH increased globally. Previous reports demonstrated that in 2015, for both sexes, age‐standardized incidence and death rates for LC were the highest in the Asia Pacific region, followed by East Asia and sub‐Saharan Africa.( 34 ) At this point, our findings should be interpreted carefully because we report that the same regions had the highest improvement in LC DALYs between our study years. As one would expect, a possible explanation for this finding is that the main drivers of LC DALYs in those regions have been HBV and HCV, both of which are manageable. In Africa, for example, where NAFLD/NASH does not yet pose a significant threat, chronic viral hepatitis is the leading cause of HCC, and programs, such as improving HBV vaccination and national efforts to reduce HCV with direct‐acting antivirals, as in Egypt, play an important role in reducing LC DALYs.( 35 ) We point out that, similar to ALD, the WHO has adopted a global strategy to decrease the burden of viral hepatitis, with a target of 65% reduction in mortality related to HBV and HCV by 2030.( 36 ) In addition to WHO strategies, there have also been national efforts to decrease LC mortality in some Asian countries. For example, in an effort to reduce LC burden, China strictly reduced aflatoxin exposure and increased HBV vaccination rates, thus enjoying a 33% decrease in age‐standardized mortality rate for LC between 1990 and 2015.( 37 , 38 ) However, another Asian country, Mongolia, did not implement any prevention programs and suffered an incredible 171% increase in LC mortality in the same time period.( 39 ) On the other hand, high‐income regions, such as Australasia and North America, suffered a worsening trend in LC DALYs, mainly driven by the increased burden of NAFLD/NASH. It has been shown that high‐income countries, such as the United States, Canada, and Australia, suffered a greater than 20% increase in LC rates between 1990 and 2015.( 40 , 41 ) In addition to worsening trends in NAFLD/NASH, this increase was also attributed to increased ALD, the cohort effect of HCV by aging baby boomers, and for Australia, for example, mass migration from the Asia Pacific region.( 42 )
At this point, given how fast it has been growing, NAFLD/NASH merits special consideration. During the study years, cirrhosis DALYs due to NAFLD/NASH increased by 23.4%, from 2.78 million to 3.43 million, with the highest cirrhosis DALY rates due to NAFLD/NASH in Asia regions and Eastern Europe. More strikingly, LC DALYs due to NAFLD/NASH increased from 1.06 million to 1.46 million, representing a 37.5% increase. The highest LC DALY rates due to NAFLD/NASH were seen in Asia regions and high‐income North America. In the current study, we also analyzed the association between NAFLD/NASH, T2DM, CVD, and metabolic risk factors. Among various super regions, the highest correlations between NAFLD/NASH and metabolic risk factors were observed in Latin America, Caribbean, and sub‐Saharan Africa regions. This finding is expected as multiple studies have reported the changes in the most common causes of CLD and the growing impact of NAFLD/NASH in CLD burden.( 43 , 44 ) For Asia regions, for instance, due to the increase in metabolic diseases and NAFLD in the last decades, Vongsuvanh et al.( 45 ) used the term “sleeping tiger” to draw attention to dramatically increasing HCC incidence in this area. Recent studies using Markov modeling for future projections of NAFLD burden unfortunately report gloomy news. A study by Estes et al.( 46 ) focusing on data for the United States showed that the total NAFLD population by the year 2030 will increase by 21%, the number of NASH cases will increase by 63%, NAFLD/NASH‐related decompensated cirrhosis will increase by 180%, and NAFLD/NASH‐related HCC cases will increase by 146%. The anticipation in other parts of the world where the NAFLD/NASH burden has reached critical levels is not different. In a multinational study providing future projections by Markov modeling, it is expected that the NASH population by 2030 will increase by 48% in China and 43% to 49% in most parts of Europe, which will lead to an 86% increase in NASH‐related HCC in China, 93% increase in the United Kingdom, 107% increase in Germany, and 125% increase in France.( 47 ) We suggest that our findings of worsening DALYs due to NAFLD/NASH‐related cirrhosis and LC are the early signs of those projections and that NAFLD/NASH will increasingly contribute to the CLD burden globally, with an urgent need for effective interventions for the management of NAFLD/NASH.
The most important strength of the current study is that we used the data from GBD estimates. GBD estimates provide the only peer‐reviewed estimates of cause‐specific mortality available for each age, sex, year, and location throughout the world. However, these data also have a few limitations. Our analysis relied heavily on GBD estimates, so we share the limitations of those estimates. The accuracy of the GBD estimates was limited by the quality and availability of each country’s vital registration system and a large number of undefined cancer cases in their cancer registry data. For some locations without these data sources, such as the countries in sub‐Saharan Africa, GBD estimates heavily relied on the modeling process, predictive covariates, and trends from past time or trends from neighboring countries, resulting in some uncertainty. Additionally, the GBD framework of estimation tended to underestimate LC mortality in low‐income countries due to the lack of advanced diagnostic techniques. Also, estimates for LC and cirrhosis due to NAFLD/NASH must be interpreted with caution in that the age‐standardized prevalence of NAFLD and NASH that leads to LC or cirrhosis was 10.9%, which was lower than the global prevalence of 24%, most likely due to different adjustments for alcohol use.
In conclusion, CLD continues to be a growing health problem globally. Cirrhosis and LC have been causing significant morbidity and mortality, as reflected in disease‐specific DALY rates. Asia regions represent the majority of DALYs globally, whereas high‐income regions, such as Asia Pacific, North America, and Australasia, have relatively lower but worsening, trends. Based on etiology, DALYs secondary to HBV, HCV, and ALD have been improving and NAFLD/NASH is contributing more to the growing CLD burden worldwide, urgently necessitating the development of effective management options.
Supporting information
Fig S1
Fig S2
Fig S3
Fig S4
Fig S5
Fig S6
Table S1‐S9
Potential conflict of interest: Dr. Z. Younossi consults for Gilead, Intercept, NovoNordisk, BMS, AbbVie, Terns, and Viking. The other authors have nothing to report.
References
- 1. Younossi ZM, Stepanova M, Younossi Y, Golabi P, Mishra A, Rafiq N, et al. Epidemiology of chronic liver diseases in the USA in the past three decades. Gut 2020;69:564‐568. [DOI] [PubMed] [Google Scholar]
- 2. Udompap P, Kim D, Kim WR. Current and future burden of chronic nonmalignant liver disease. Clin Gastroenterol Hepatol 2015;13:2031‐2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J Hepatol 2019;70:151‐171. [DOI] [PubMed] [Google Scholar]
- 4. Younossi ZM, Golabi P, Henry L. A comprehensive review of patient‐reported outcomes in patients with chronic liver diseases. J Clin Gastroenterol 2019;53:331‐341. [DOI] [PubMed] [Google Scholar]
- 5. Stepanova M, Clement S, Wong R, Saab S, Ahmed A, Younossi ZM. Patients with diabetes and chronic liver disease are at increased risk for overall mortality: a population study from the United States. Clin Diabetes 2017;35:79‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 2015;148:547‐555. [DOI] [PubMed] [Google Scholar]
- 7. Younossi Z, Stepanova M, Ong JP, Jacobson IM, Bugianesi E, Duseja A, et al.; Global Nonalcoholic Steatohepatitis Council . Nonalcoholic steatohepatitis is the fastest growing cause of hepatocellular carcinoma in liver transplant candidates. Clin Gastroenterol Hepatol 2019;17:748‐755.e3. [DOI] [PubMed] [Google Scholar]
- 8. Mittal S, El‐Serag HB, Sada YH, Kanwal F, Duan Z, Temple S, et al. Hepatocellular carcinoma in the absence of cirrhosis in United States veterans is associated with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2016;14:124‐131.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Global Health Data Exchange . Global Burden of Disease Study 2017: GBD results tool. http://ghdx.healthdata.org/gbd‐results‐tool. Published 2018, regularly updated. Accessed August 2019.
- 10. GBD 2017 Causes of Death Collaborators . Global, regional, and national age‐sex‐specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1736‐1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. GBD 2016 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990‐2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017;390:1211‐1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. GBD 2017 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990‐2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1789‐1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Naghavi M, Makela S, Foreman K, O’Brien J, Pourmalek F, Lozano R. Algorithms for enhancing public health utility of national causes‐of‐death data. Popul Health Metr 2010;8:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Foreman KJ, Lozano R, Lopez AD, Murray CJ. Modeling causes of death: an integrated approach using CODEm. Popul Health Metr 2012;10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Institute for Health Metrics and Evaluation . The power of models. http://www.healthdata.org/acting‐data/power‐models. Published October 26, 2018. Accessed July 2019.
- 16. Flaxman AD, Vos T, Murray CJL, eds. An Integrative Metaregression Framework for Descriptive Epidemiology. Seattle, WA: University of Washington Press; 2015. [Google Scholar]
- 17. Mokdad AH, Ballestros K, Echko M, Glenn S, Olsen HE, Mullany E, et al.; US Burden of Disease Collaborators. The state of US health, 1990‐2016: burden of diseases, injuries, and risk factors among US states. JAMA 2018;319:1444‐1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Global Health Data Exchange . Global Burden of Disease Study 2017 (GBD 2017) data input sources tool. http://ghdx.healthdata.org/gbd‐2017/data‐input‐sources. Accessed June 2019.
- 19. Murray CJL, Ezzati M, Flaxman AD, Lim S, Lozano R, Michaud C, et al. GBD 2010: design, definitions, and metrics. Lancet 2012;380:2063‐2066. [DOI] [PubMed] [Google Scholar]
- 20. Institute for Health Metrics and Evaluation . GBD compare. http://www.healthdata.org/data‐visualization/gbd‐compare. Published November 8, 2018. Accessed June 2019.
- 21. Paik JM, Golabi P, Younossi Y, Mishra A, Younossi ZM. Changes in the global burden of chronic liver diseases from 2012 to 2017: the growing impact of nonalcoholic fatty liver disease. Hepatology 2020. doi: 10.1002/hep.31173. [DOI] [PubMed] [Google Scholar]
- 22. Petrick JL, Braunlin M, Laversanne M, Valery PC, Bray F, McGlynn KA. International trends in liver cancer incidence, overall and by histologic subtype, 1978‐2007. Int J Cancer 2016;139:1534‐1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Petrick JL, Kelly SP, Altekruse SF, McGlynn KA, Rosenberg PS. Future of hepatocellular carcinoma incidence in the United States forecast through 2030. J Clin Oncol 2016;34:1787‐1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Golabi P, Rhea L, Henry L, Younossi ZM. Hepatocellular carcinoma and non‐alcoholic fatty liver disease. Hepatol Int 2019;13:688‐694. [DOI] [PubMed] [Google Scholar]
- 25. Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2010. Natl Vital Stat Rep 2013;61:1‐117. [PubMed] [Google Scholar]
- 26. GBD 2016 Alcohol Collaborators . Alcohol use and burden for 195 countries and territories, 1990‐2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2018;392:1015‐1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liangpunsakul S, Haber P, McCaughan GW. Alcoholic liver disease in Asia, Europe, and North America. Gastroenterology 2016;150:1786‐1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vento S, Dzudzor B, Cainelli F, Tachi K. Liver cirrhosis in sub‐Saharan Africa: neglected, yet important. Lancet Glob Health 2018;6:e1060‐e1061. [DOI] [PubMed] [Google Scholar]
- 29. World Health Organization . Global status report on alcohol and health. http://www.who.int/substance_abuse/publications/global_alcohol_report/msbgsruprofiles.pdf. Published 2011. Accessed May 2019.
- 30. Pimpin L, Cortez‐Pinto H, Negro F, Corbould E, Lazarus JV, Webber L, et al. Burden of liver disease in Europe: epidemiology and analysis of risk factors to identify prevention policies. J Hepatol 2018;69:718‐735. [DOI] [PubMed] [Google Scholar]
- 31. GBD 2017 Cirrhosis Collaborators . The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol 2020;5:245‐266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zatoński WA, Sulkowska U, Mańczuk M, Rehm J, Boffetta P, Lowenfels AB, et al. Liver cirrhosis mortality in Europe, with special attention to Central and Eastern Europe. Eur Addict Res 2010;16:193‐201. [DOI] [PubMed] [Google Scholar]
- 33. Goldberg D, Ditah IC, Saeian K, Lalehzari M, Aronsohn A, Gorospe EC, et al. Changes in the prevalence of hepatitis C virus infection, nonalcoholic steatohepatitis, and alcoholic liver disease among patients with cirrhosis or liver failure on the waitlist for liver transplantation. Gastroenterology 2017;152:1090‐1099.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, Brenner H, et al.; Global Burden of Disease Cancer Collaboration . Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability‐adjusted life‐years for 32 cancer groups, 1990 to 2015: a systematic analysis for the Global Burden of Disease Study. JAMA Oncol 2017;3:524‐548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sayiner M, Golabi P, Younossi ZM. Disease burden of hepatocellular carcinoma: a global perspective. Dig Dis Sci 2019;64:910‐917. [DOI] [PubMed] [Google Scholar]
- 36. World Health Organization . Global health sector strategy on viral hepatitis 2016‐2021. http://www.who.int/hepatitis/strategy2016‐2021/ghss‐hep/en/. Published June 2016. Accessed February 2020.
- 37. Sun Z, Chen T, Thorgeirsson SS, Zhan Q, Chen J, Park J‐H, et al. Dramatic reduction of liver cancer incidence in young adults: 28 year follow‐up of etiological interventions in an endemic area of China. Carcinogenesis 2013;34:1800‐1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chen J‐G, Egner PA, Ng D, Jacobson LP, Muñoz A, Zhu Y‐R, et al. Reduced aflatoxin exposure presages decline in liver cancer mortality in an endemic region of China. Cancer Prev Res (Phila) 2013;6:1038‐1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chimed T, Sandagdorj T, Znaor A, Laversanne M, Tseveen B, Genden P, et al. Cancer incidence and cancer control in Mongolia: results from the National Cancer Registry 2008‐12. Int J Cancer 2017;140:302‐309. [DOI] [PubMed] [Google Scholar]
- 40. Akinyemiju T, Abera S, Ahmed M, Alam N, Alemayohu MA, Allen C, et al.; Global Burden of Disease Liver Cancer Collaboration . The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: results from the Global Burden of Disease Study 2015. JAMA Oncol 2017;3:1683‐1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol 2019;16:589‐604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Carville KS, MacLachlan JH, Thursfield V, Cowie BC. Hepatocellular carcinoma over three decades in Victoria, Australia: epidemiology, diagnosis and trends, 1984‐2013. Intern Med J 2018;48:835‐844. [DOI] [PubMed] [Google Scholar]
- 43. Younossi ZM, Otgonsuren M, Henry L, Venkatesan C, Mishra A, Erario M, et al. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology 2015;62:1723‐1730. [DOI] [PubMed] [Google Scholar]
- 44. Wong MCS, Huang JLW, George J, Huang J, Leung C, Eslam M, et al. The changing epidemiology of liver diseases in the Asia‐Pacific region. Nat Rev Gastroenterol Hepatol 2019;16:57‐73. [DOI] [PubMed] [Google Scholar]
- 45. Vongsuvanh R, van der Poorten D, George J. Non‐alcoholic fatty liver disease‐related hepatocellular carcinoma: a sleeping tiger in the Asia Pacific. Hepatol Int 2013;7(Suppl. 2):823‐832. [DOI] [PubMed] [Google Scholar]
- 46. Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018;67:123‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Estes C, Anstee QM, Arias‐Loste MT, Bantel H, Bellentani S, Caballeria J, et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016‐2030. J Hepatol 2018;69:896‐904. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Fig S3
Fig S4
Fig S5
Fig S6
Table S1‐S9


