Abstract
Background:
Flow cytometric immunophenotpying (FCI) of cerebrospinal fluid (CSF) and other paucicellular fluids has been demonstrated to have increased sensitivity in detection of lymphoma and leukemia when compared to cytomorphology [(1) de Graaf et al., Cytometry Part B 2011, 80B:271–281; (2) Szamosi et al., CLSI Document H56-A—Body Fluid Analysis for Cellular Composition; Approved Guideline, Wayne, PA: Clinical and Laboratory Standards Institute, 2006; (3) Kraan et al., Flow Cytometric Immunophenotyping of Cerebrospinal Fluid. Current Protocols in Cytometry, Hoboken, NJ: Wiley, 2008]. However, low cellularity has been an historical problem with these samples. Several studies indicate that immediate addition of a stabilization media (e.g., RPMI with fetal calf serum (FCS)) to CSF improves the cell yield for FCI [(1) de Graaf et al.]. Such stabilization medias can, however, significantly increase cost.
Methods:
We compared FCI results in CSF stabilized with RPMI 1640 (without additional additives) to results obtained using non-stabilized CSF. Samples were processed according to published CLSI guidelines [(2) Szamosi et al.].
Results:
About 98/105 (93%) CSF specimens stabilized with RPMI had adequate numbers of viable cells (>100) for performing FCI. About 65/217 (30%) CSF specimens without stabilization had adequate numbers of viable cells for analysis (70% either quantity not sufficient (QNS) or specimen viability below analytical limits).
Conclusions:
Utilizing RMPI without FCS as a stabilization media results in increased cell yield and improved FCI results. We have found FCS is not required to achieve high quality results in FCI of paucicellular CSF specimens.
Keywords: stabilization media, cerebrospinal fluid, leukemia, lymphoma
INTRODUCTION
Flow cytometry immunophenotyping (FCI) of paucicellular specimens such as cerebrospinal fluid (CSF) has historically been challenging due to the primary lack of sufficient cells for adequate testing purposes as a result of rapid cell loss post-specimen collection. Several flow cytometric protocols designed to increase cellular yield have been reported, including immediate stabilization of cells in Transfix™ reagent, RPMI 1640 cell culture media with fetal calf serum (FCS), and phosphate buffered saline (PBS) with FCS at the time of specimen collection (1–6). Transfix, however, requires exact volume to reagent ratios and may alter intensity of surface antigen staining. In addition, these media increase the cost of FCI.
We have used RPMI-1640 without FCS for stabilization of fine-needle aspirate specimens during transport to the laboratory for a number of years with very good success in retaining both viability and adequate cellularity. CSF specimens, however, were not stabilized and were transported unaltered to the laboratory. In approximately one-third of cases few if any cells were detected in these specimens and “Quantity not sufficient” or “Viability below analytical limits” results were reported. In many cases, these specimens are collected from pediatric patients with possible CNS involvement with leukemia. Therefore, optimal FCI is vital due to the potentially serious consequences if the correct diagnosis is missed. We report on a comparison of cell recovery and FCI results in CSF alone versus CSF stabilized in RPMI 1640 in a series of paucicellular CSF specimens.
MATERIALS AND METHODS
CSF Samples
Three hundred twenty-two CSF specimens from patients with documented or suspected leukemia or lymphoma were submitted for FCI.
Non-Stabilized CSF
Two hundred seventeen consecutive CSF specimens received in the laboratory were held at room temperature until processing. The time of collection to arrival in our laboratory was in most cases less than 2 h if collected before the lab was closed. Otherwise, specimens were held refrigerated (4° C) overnight for an average of 10 h. Samples were then sent to the lab and processed within an hour of arrival. If the specimen was not routed to the Body Fluid bench or Cytology first it was transported as is with no buffers or additives to the flow cytometry lab. Upon arrival in the flow cytometry lab the CSF specimen was transferred into a 15-ml conical tube. An equal volume of PBS was then added and the specimen was pelleted by centrifugation (400g, RT, 5 min), and then all but 0.5ml of the supernatant was removed. A 0.25 ml aliquot of the re-suspended cells was then used to set up a single-tube 8-color cocktail for leukemia or lymphoma evaluation based on patient history (see Figs. 1 and 2).
Fig. 1.
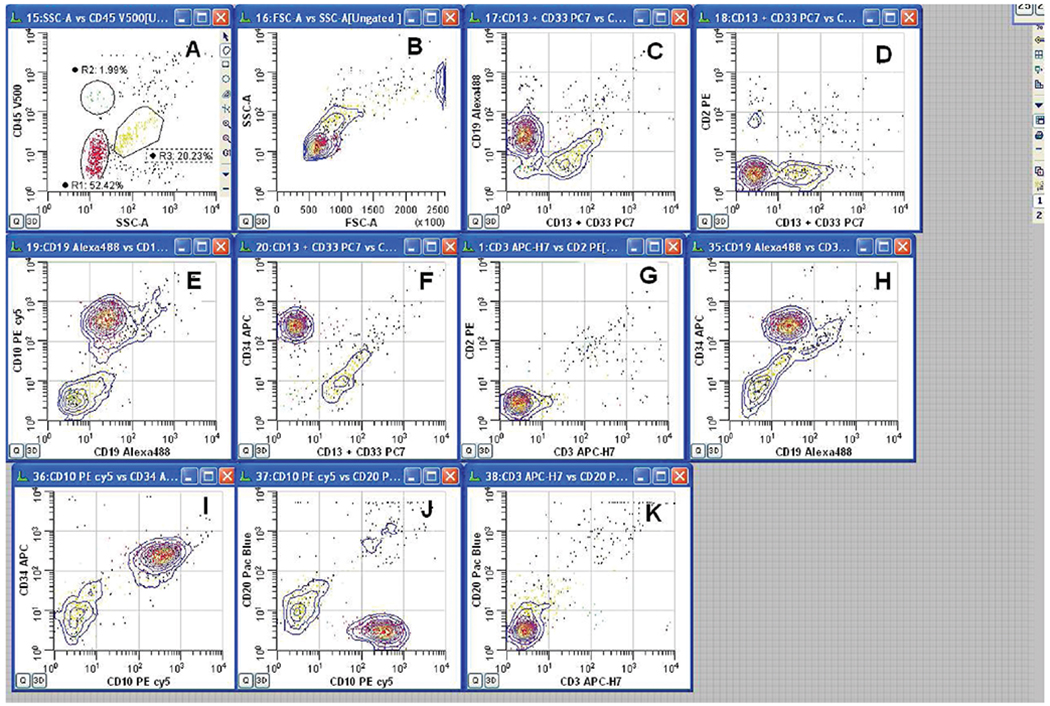
8-Color CSF leukemia panel using RPMI 1640. R1 (red) = leukemic blast population, R2 (green) = lymphocytes, R4 (gold) = granulocytes. A, B: Ungated data showing analysis gates—all other plots are ungated. C, D: Blasts are negative for CD13+Cd33 and CD2 but positive for CD19; (E–H): blasts are positive for CD19, CD10, and CD34 and negative for CD13+ CD33, CD3, and CD2. (I–K): Blasts are positive for CD10 and CD34 and negative for CD20 and CD3.
Summary of Analysis: R1-gated leukemic blast population expresses Pre-B markers CD19, CD10, and CD34. Patient previously diagnosed with acute Pre-B leukemia. Central nervous system (CNS) involvement confirmed with identification of same phenotypic population in CSF specimen submitted for flow cytometry analysis.
- CD19 Alexa 488 (ABD Serotec, clone LT19, cat. no. MCA1940A488)
- CD2 PE (Becton-Dickinson, clone S5.2 cat. no. 340701)
- CD10 PE-cy5 (Beckman Coulter, clone ALB1 cat. no. A07761)
- CD13 PC7 (Beckman Coulter, clone IMM103.44, cat. no. A46528
- CD33 PC7 (Beckman Coulter, clone D3HL60.251, cat. no. A54824
- CD34 APC, Becton-Dickinson, clone 8G12 cat. no. 340667)
- CD3 APC-H7, (BD, clone SK7 cat. no. 641406)
- CD20 PB (ABD Serotec clone 2H7 cat. no. MCA1710PB)
- CD45 V500 (Becton-Dickinson, clone HI30 cat. no. 560777)
- Separate tube for viability assay: 7-AAD, Molecular Probes, no. AI310.
Fig. 2.

8-Color CSF lymphoma panel using RPMI 1640. R1 (red) = CD19+ B lymphocyte population, R2 (green) = CD3+ T lymphocyte population. Top row plots (A,B): ungated data showing analysis gates. Plot (C), Forward vs. Side-scatter plot shows T cells as small to intermediate in size and B cells intermediate to large in size. Middle row plots (D–F): CD19+B cells are positive for Lambda light chain and negative for Kappa light chain; bottom row plots (G–J): CD3+ T cells are positive for CD8 and CD7, and there are separate populations of T cells that are CD8+CD4− and CD8−CD4+.
Summary of analysis: R1-gated population expresses pan B-cell marker CD19 and co-expresses Lambda light chain with little or no expression of Kappa light chain. T cell markers CD3 and CD7 appear normal as do T cell subsets CD4 and CD8. Patient previously diagnosed with follicular center-cell lymphoma confirmed by flow studies of peripheral blood. CNS involvement confirmed with identification of same phenotypic population in CSF specimen submitted for flow cytometry analysis.
- Kappa FITC (Becton-Dickinson, clone TB28-2, cat. no. 643774)
- Lambda PE (Becton-Dickinson, clone 1-155-2, cat. no. 642924)
- CD4 Pac Blue (Becton-Dickinson, clone SK3, cat. no. 558116)
- CD19 PC7 (Beckman Coulter, clone J3-119, cat. no. IM3628U)
- CD8 APC, (Becton-Dickinson, clone SK1, cat. no. 340659)
- CD3 APC-H7, (BD, clone SK7, cat. no. 641406)
- CD7 PerCP-cy5.5, (Becton-Dickinson, clone MT701, cat. no. 561602
Stabilized CSF
One hundred five consecutive CSF specimens, instead of being aliquoted into 15-ml conical tubes with matched volumes of plain PBS, were instead matched with an equal volume of RPMI 1640 (no FCS) (Cellgro, Mediatech, Manassas, VA), and held at room temperature for an average of < 1 h until processing. At the time of processing the sample in the vial with RPMI media was spun once (400g, RT, 5 min), and then all but 0.5 ml of the supernatant was removed. A 0.25 ml aliquot of the re-suspended cells in RPMI 1640 with no additives was then used to set up a single-tube 8-color cocktail for leukemia or lymphoma evaluation based upon patient history (Figs. 1 and 2).
RESULTS
This brief communication reports the results on the use of a stabilization process for CSF and its subsequent use with flow cytometric analysis. Collected CSF samples (N = 105) were immediately placed in RPMI 1640 without FCS and held at room temperature prior to their standard processing for an 8-color panel assessment. The non-stabilized CSF samples (N = 217) were held at room temperature without addition of any other material and then processed for the same flow panel as used for the stabilized samples. The information provided in this report offers a technical modification that appears to have good value for the clinical flow cytometry laboratory.
The standard approach of using non-stabilized CSF for flow cytometric studies yielded 65/217 (30%) of cases not having an adequate number of viable cells with the remaining 152/217 (70%) having either quantity not sufficient or specimen viability below analytical limits for study. However, when the CSF was stabilized as described above 98/105 (94%) of samples yielded adequate numbers of viable cells that were suitable for flow cytometric analysis.
DISCUSSION
Adding RMPI 1640 as a stabilization media results in increased cell yield and improved flow cytometry testing results. Previous studies have indicated that RPMI 1640 with FCS is a useful stabilizer of CSF specimens. However, the addition of FCS increases cost significantly (see Table 1). We have found that RPMI 1640 without FCS is an excellent stabilizer of CSF that results in dramatically improved cell recovery and viability and thus improved quality of flow cytometry testing.
Table 1.
Cost/Test of RPMI 1640 vs RPMI 1640 with FCS vs Transfix™
| Source | Reagent | Cat. no. | Size | No. of tests | Price | Cost/test |
|---|---|---|---|---|---|---|
| Mediatech | RPMI 1640 | 10-040-CV | 10 × 500 ml | 330 | 87.59a | $0.26 |
| Lonza | Fetal calf serum | 14-503F | 500 ml | 300 | $334.00b | $2.23 |
| MBL International | Transfix | TF-B1-1 | 1 ml | 1 | $12.00c | $12.00 |
Mediatech, Product Pricelist, February, 2013.
Lonza, Product Pricelist, February 2013.
Price quote from MBL, February 15, 2013.
LITERATURE CITED
- 1.de Graaf MT, de Jongste AHC, Kraan J, Boonstra JG, Sillevis Smitt PAE, Gratama JW. Flow cytometric characterization of cerebrospinal fluid cells. Cytometry Part B 2011;80B:271–281. [DOI] [PubMed] [Google Scholar]
- 2.Szamosi MA, Bautista JM, et al. CLSI Document H56-A—Body Fluid Analysis for Cellular Composition; Approved Guideline. Wayne, PA, USA: Clinical and Laboratory Standards Institute; 2006. [Google Scholar]
- 3.Kraan J, Gratama J, Haioun C, Orfao A, Planquet A, Porwit A, Quijano S, Stetler-Stevenson S, Subira D Wilson W. Flow cytometric immunophenotyping of cerebrospinal fluid Current Protocols in Cytometry 2008; Hoboken, NJ: Wiley. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Craig F, Ohori NP Gorrill TS, Swerdlow S. Flow cytometric immunophenotyping of cerebrospinal fluid. Am J Clin Pathol 2011;135:22–34. [DOI] [PubMed] [Google Scholar]
- 5.Stacchini A, Demurtas A, Aliberti S. Flow cytometric detection of liposomal cytarabine in cerebrospinal fluid of patients treated with intrathecal chemotherapy. Cytometry Part B 2012;82B:280–282. [DOI] [PubMed] [Google Scholar]
- 6.Lanza F Issue Highlights—January 2013. Cytometry Part B 2013; 84B:1–4. [Google Scholar]


