Abstract
The KAI1/CD82 tetraspanin is a widely expressed cell surface molecule thought to organize diverse cellular signaling processes. KAI1/CD82 suppresses metastasis but not tumorigenicity, establishing it as one of a class of metastasis suppressor genes. In order to further assess its functions, we have characterized the phenotypic properties of Kai1/Cd82 deleted mice, including viability, fertility, lymphocyte composition, blood chemistry and tissue histopathology, and of their wild-type and heterozygote littermates. Interestingly, Kai1/Cd82−/− showed no obvious genotype associated defects in any of these processes and displayed no genotype associated histopathologic abnormalities after 12 or 18 months of life. Expression profiles of non-immortal, wild-type and Kai1/Cd82−/− mouse embryo fibroblast (MEFs) indicated distinct sex-specific and genotype-specific profiles. These data identify 191 and 1,271 differentially expressed transcripts (by twofold at P < 0.01) based on Kai1/CD82 genotype status in female and male MEFs, respectively. Differentially expressed genes in male MEFs were surprisingly enriched for cell division related processes, suggesting that Kai1/Cd82 may functionally affect these processes. This suggests that Kai/Cd82 has an unappreciated role in the early establishment of proliferation and division when challenged with a new environment that might play a role in adaptability to new metastatic sites.
Keywords: metastasis suppressor, gene knockout, CD82
INTRODUCTION
Cancer progression, specifically the process of metastasis, is the critical step in determining whether many cancers become fatal. Many gene products have been implicated in growth, maintenance, and suppression of cancer, yet few function specifically to suppress metastasis without affecting the overall growth of the cancer. These genes collectively called metastasis suppressors are defined by their ability to functionally impede metastatic spread but have no effect on the growth or maintenance of the primary lesion in orthotopic tumor models. One of the first genes identified that met this definition was the KAI1 gene. KAI1 was originally identified for its ability to suppress prostate cancer cell metastasis. Specifically, KAI1 impeded lung metastasis formation when introduced into the highly metastatic AT6.1 Dunning rat prostate cancer cell line, whereas no effect was noted in primary tumor formation [1]. Since this seminal discovery, KAI1 has been implicated in the metastatic spread of numerous cancer types. Reduced or lack of KAI1 expression in cancers is often linked with poor prognosis and its elevated expression is associated with a less severe clinical course [2–13].
KAI1, also known as CD82, is a member of the tetraspanin family of membrane spanning cell surface molecules. Tetraspanins play a wide variety of roles in cell adhesion, migration, and in the organization of effective cell signaling. Much research has been undertaken to understand the role of tetraspanins and specifically KAI1 in these processes. Thus, Kai1/Cd82 is hypothesized to function as an adaptor molecule involved in a wide variety of processes. KAI1/CD82 is known to interact with a wide variety of molecules including integrins, other tetraspanins as well as cell surface receptor tyrosine kinases such as c-met and epidermal growth factor receptor [14,15]. Deregulation of tetraspanins and integrins are common findings in cancer cells further implicating Kai1 in these processes. Kai1 has also been shown to functionally interact with DARC, the Duffy antigen receptor for chemokines, and this interaction has also been suggested as key to its function [16,17]. The role of Kai1 as a metastasis suppressor has been expertly reviewed [14,18,19].
KAI1/CD82 is highly expressed in immune cells of the blood where its expression is integral to viral infection of T cells [20,21]. The C33 monoclonal antibody to KAI1/CD82 was able to block virus-induced syncytium formation in T cells. These studies pointed to the necessity of the molecule to allow syncytium formation and selective viral uptake [20,21]. The role of KAI1/CD82 in epithelial cells is much less clear. KAI1/CD82 mRNA is expressed in most tissues, unlike some other tetraspanins which exhibit more restricted tissue expression [22,23]. In our previous study, we developed the first mouse specific Cd82 antibody and confirmed Kai1/Cd82 protein expression in specific cell types in most mouse organs. We found varying levels of Kai/Cd82 expression in all tissues with the exception of smooth muscle [24]. Murine and human KAI1/CD82 are fairly conserved at the amino acid level principally differing only in the number of n-linked glycosylation sites, further suggesting that the murine model is appropriate for study of the normal functions of KAI1/CD82.
The effect of germline deletion has been examined for several tetraspanins but has not been reported for Kai1/Cd82. These murine germline deletion experiments have uncovered several unexpected functions of some tetraspanins. Most notably germline deletion of CD9 results in the inability of sperm egg fusion with CD9 required on egg plasma membrane [25–27]. However, more commonly germline deletion of tetraspanins in mice results in few grossly detectable defects noted in vivo. Deletion of CD37, CD81, CD151, CD63, and TSSC6 all result in viable mice having only subtle or no altered phenotypes [28–32]. Notable phenotypes were not seen for CD37 or Tssc6 mice, while increased brain size was noted as the only major phenotype alteration in CD81 null animals [31]. CD151 null mice, although outwardly phenotypically normal, exhibit an increased propensity to bleed and demonstrate a failure in wound healing [33]. CD151 mice also exhibit an increased resistance to metastasis [34]. CD63 mice exhibit abnormal water balance due to kidney dysfunction [28]. Several of these models exhibit increased proliferation of lymphocytes upon stimulation in vitro [35,36]. The lack of appreciable phenotypes in tetraspanin knockouts is perhaps unsurprising given the high level of protein conservation among the tetraspanins. The redundancy of tetraspanin function was further noted by the ability of CD81 to rescue the CD9 null sperm egg fusion phenotype [37]. In order to further examine the function of Kai1 we deleted it in the mouse and performed an initial characterization of its gross phenotypes and effect on viability.
METHODS
Targeting Vector
A mouse BAC (Life Technologies, Foster City, CA) containing murine Kai1 was recombined into PLMJ235. Sal1 and EcoRV flanked 5′ homology and EcoRV and Not1 flanked 3′ homology arms were generated by PCR ligation to PBSK and used to create a loxP-neo cassette via ligation to PLMJ237. The cassette was used to recombine with PLMJ235 genomic DNA plasmid. The final recombined plasmid was linearized and used to target ES cells. The recombination strategy for building the targeting vector utilized has been expertly detailed [38].
Mice
ES cells were screened by Southern blotting. Targeted cells were introduced to pseudo-pregnant mice. Resultant chimeric offspring were bred and Kai1/Cd82-targeted strains were identified. Two independent strains of mice were backcrossed to the C57BL/6 strain using the NCI speed congenic service, SAIC Frederick MD. Southern blotting, Northern blotting and immunoblotting and immunohistochemistry with mouse anti-Kai1 all confirmed absence of Kai1/Cd82 in knockout mice.
Genotyping and Sex Determination
Probes were constructed by polymerase chain reaction at both the 5′ and 3′ end; primers for probes were 32623-F-ctcttgggctgtttatcctgcaa and 33040-R-tcaagttgtgcccaaagttgcc for 5′ and 43367-F-ggtcatgtgagaggcaggatca and 44134-R-cagtccccagaaaagtcaccag for 3′. These were cloned in vector PCR-4-TOPO (Life Technology). Plasmids were isolated, confirmed by sequence and inserts purified following EcoR1 digestion. Five microgram genomic DNA was digested with Xho1 and Kpn1 and separated on 1% gels. Radio-labeled random primed probes were prepared and hybridized as previously described [39]. Sex of mouse embryo fibroblasts (MEFs) were determined using PCR and primers targeting Sry and Tsh essentially as described [40].
Pathology
Mice at 6–8 wk, 12 and 18 months of age were evaluated for hematology, serum biochemistry, and histologic abnormalities of all major organs without prior knowledge of genotypes by board certified veterinary pathologists (R.M.S. and J.D.W.). Gross necropsies and blood analyses were performed at the NCI-Frederick (Frederick, MD) contract facilities. Blood collection (<0.2 ml) was performed by mandibular bleeding. For the complete blood count (CBC) analysis, whole blood was collected in Microvette EDTA collection tubes (Sarstedt, Inc., Newton, NC) and 20 μl was analyzed using a Hemavet 950 Multispecies Hematology Analyzer (Drew Scientific, Waterbury, CT). For blood chemistry analysis, blood was collected in serum separator tubes (Becton Dickinson, Palo Alto, CA) and allowed to sit at room temperature for 30 min. Tubes were centrifuged at 2,000 rpm for 10 min. The chemistry analysis was done using 100 μl of serum on the VetScan (Abaxis, Union City, CA) and/or 40–50 μl of serum on the Vitros analyzer (Ortho Clinical Diagnostics Raritan, NJ). Immunohistochemistry and immunoblotting were performed on murine tissues using rabbit anti-Kai1 antibodies as previously described by our group [24].
Gene Expression
Embryonic day 12.5 embryos were collected, extensively rinsed in PBS, and cells were harvested in 100 mm dishes for in vitro culture. When wells became confluent, cells were harvested, counted, and replated 1:4 into 60 mm dishes. Three dishes were used for RNA isolation and the remaining dish was used for future growth studies, as well as genotyping and sex determination. Selected MEFs were serially passaged until immortal and are available for future study. Total RNAs were used to evaluate gene expression on Affymetrix GeneChip® Mouse Genome 430 2.0 Array (Affymetrix, Inc., Santa Clara, CA).
Expression Analysis
Expression Console software Ver 1.1 (Affymetrix, Inc.) was used to determine the signal values according to the MAS5 algorithm. Total intensity normalization was applied by normalizing each of the arrays to trimmed mean signal value of 500. All the statistical calculations were done on logarithmic values of signals to the base 2. Global expression profiles were examined by multidimensional scaling (MDS) using 1-correlation as distance metric. About 25,000 transcripts that were detected at least in half of the arrays were included for this analysis. The 3D graph of arrays from this analysis indicated that male Kai1/Cd82−/− MEFs (16 cases) inappropriately clustered together with male wild type, though male to female MEFs were well segregated as expected. Examination of Cd82 expression levels of MEF (16 cases) indicated similar values to those of wild-type MEFs. The Xist gene expressions of all male MEFs were much lower than female MEFs, rationalizing the dissimilarities observed in MDS. Global expression profiles between WT and Kai1/Cd82−/− male MEFs were found to be significantly different at global test P = 0.001 after excluding MEF (16 cases; BRB ArrayTools software ver. 4). Differentially expressed genes between WT and Kai1/Cd82−/− MEFs were determined by two-sample t-tests at two-tailed P-value < 0.01. There were 1271 transcripts up- or down-regulated by twofold at this P-value threshold and having a geometric average signal value >100. Similar comparison of female MEFs identified 191 transcripts up- or down-regulated by twofold at P < 0.01 where the global test P-value = 0.01. Gene ontology analysis of 1,271 transcripts by Expression Analysis Systematic Explorer (EASE) software identified enrichment for down-regulated immune response and cell proliferation pathway genes. Similar analysis of 191 transcripts altered by twofold in female MEFs indicated enrichment for up-regulated morphogenesis and cell migration-related genes.
RESULTS
We isolated the murine Kai1 locus in a bacterial artificial chromosome (BAC) following a PCR screen. This BAC was used to create a gene targeting vector with the goal to remove the second exon of the Kai1/Cd82 gene. In the mouse, this exon contains the translation initiation codon and we predicted that its elimination would result in a null allele. We followed an established recombination vector creation strategy in use at our institution [38]. Our vector was designed to be multifunctional and could result in subsequent Cre recombinase driven deletion (Figure 1A and 1B). We introduced linearized vector to ES cells and screened for recombinants. Of the initial 37 clones analyzed, 12 contained the expected bands following Southern blot analysis using both 5′ and 3′ specific probes. In addition, we amplified across the entire locus and sequenced the recombined locus to confirm the integration. Upon this sequencing we noted that an internal recombination within the targeting vector had removed an internal loxP site (and exon 2). This unintended loss will affect the utility of the resultant mouse strain for use in promoter specific Cre driven recombination of this locus. The resultant recombinant mice described in this article therefore represent a conventional gene deletion for exon 2 of the Kai1/Cd82 locus.
Figure 1.
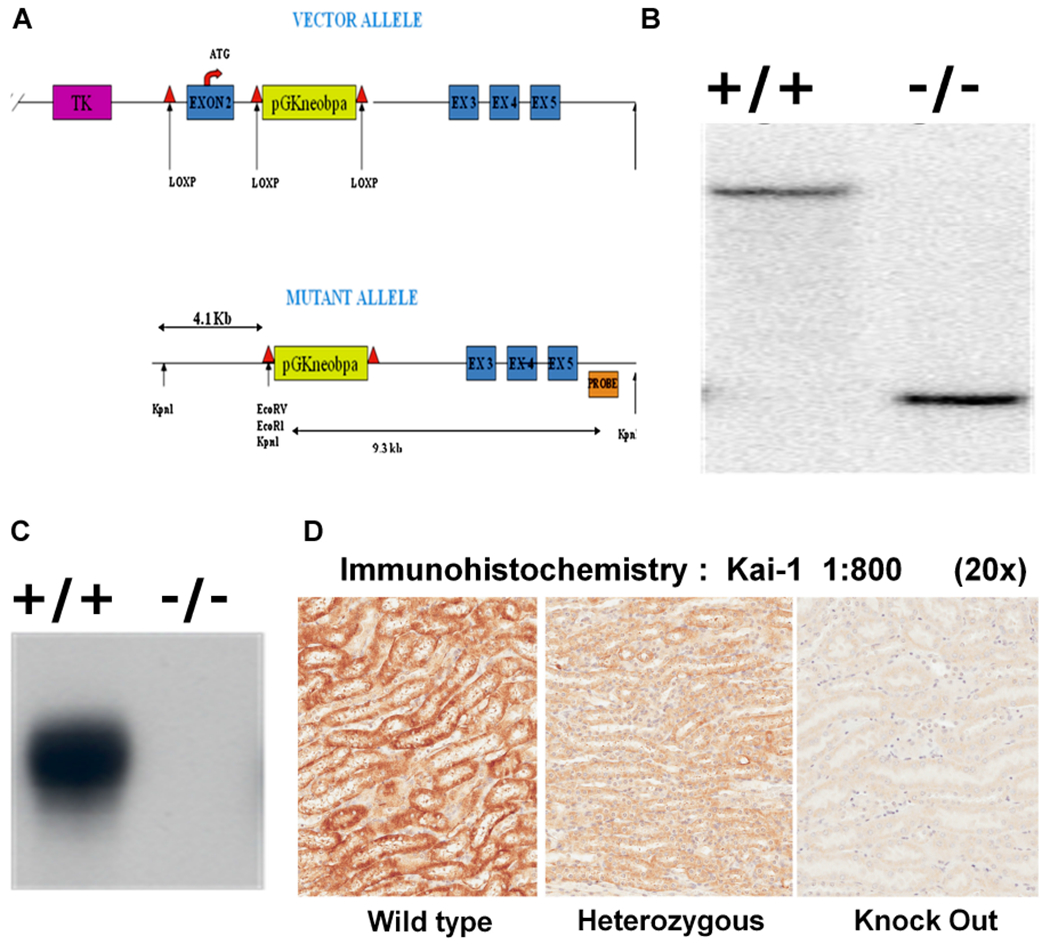
(A) Knock-out recombination strategy. (B) Southern blotting of wild-type (+/+) and homozygous deleted (−/−) Kai1/Cd82 mice. (C) Kai1/Cd82 immunoblot in proteins isolated from wild-type (+/+) and homozygous deleted (−/−) Kai1/Cd82 mouse spleens. (D) Immunohistochemical detection of Kai1/Cd82 in kidney sections from wild-type (+/+), heterozygous (+/−), and knockout (−/−) mice.
We crossed resultant chimeric mice in order to generate Kai1-targeted mice and created two independent lines of Kai1-targeted mice. These initial crosses resulted in normal litter sizes and offspring with apparent normal Mendelian ratios of targeted and non-targeted alleles and a roughly equal distribution of female and male offspring (data not shown). We examined these offspring for Kai1/Cd82 mRNA message and protein. First, we utilized RT-PCR assays to examine the expression of Kai1 message in mice that had been genotyped as +/+ and −/− by Southern blotting. Assays utilizing RNA isolated from the spleen, an organ with abundant Kai1 expression, confirmed a lack of message in −/− mice (data not shown). To confirm that the lack of message results in mice null for Kai1 protein, we examined the expression of Kai1 in the spleens from wild-type (+/+) and suspected null (−/−) mice. Wild-type mice expressed abundant Kai1 protein, whereas −/− mice exhibited no detectable protein (Figure 1C). We further examined Kai1 expression using immunohistochemistry and confirmed the lack of Kai1 in null mice (Figure 1D). These data confirmed that our targeted strategy resulted in efficient removal of Kai1/Cd82 exon 2 and that this removal results in a locus incapable of producing normal Kai1/Cd82 protein.
As a first pass to determine if Kai1/Cd82 loss contributed to any noticeable phenoptyic changes in mice, we examined a limited number of these mixed background mice at approximately 6 months of age. Specifically, we performed a detailed histopathologic analysis of three mice for each sex and genotype at this age. In general, there were no obvious genotype-specific differences. However, histopathologic examination identified several pancreatic abnormalities in two male −/− and in one male heterozygous mouse. The pancreases from these mice contained cystic, hyperplastic foci, suggestive of early islet cell adenoma (data not shown).
To follow up these observations, we designed a phenotypic study with greater statistical and longitudinal power. Furthermore, we performed extensive background crossing to C57BL/6 mice in order to produce near isogenic animals. These backcrosses were supported by genotypic speed congenics, which selected animals with the highest percentage of C57BL/6 background for successive breeding. Following these speed congenic background crosses, we obtained mice with the Kai1/Cd82 null allele that were confirmed to be comprised of >99% C57BL/6 background (data not shown). Using these mice we performed more extensive histopathologic analyses at 12 and 18 months of age, as well as performed CBC and blood chemistry analyses.
The results of breeding on the C57BL/6 background supported our initial breeding results in mixed background mice. These breedings resulted in 31 broods with 260 males and 206 females born. These included 100 wild-type, 240 heterozygote, and 122 homozygous knock-out mice. Near Mendelian ratios of offspring indicated that Kai1/Cd82 did not affect viability (Table 1). Furthermore, we examined whether Kai1 null animals were fertile. Eighteen matings of −/− animals from two independent knockout strains produced 112 offspring resulting in an average litter size of 6.22. These data were entirely consistent with data obtained from with 16 matings of +/+ animals which produced 98 offspring with an average litter of 6.12. These data are also in agreement with average litter size of 6.2 reported for this strain of mice [41]. Taken together these data are consistent with there being no effect of Kai1 on viability or fertility.
Table 1.
Breedings Resulted in Near Mendelian Ratios of Offspring Indicating That Kai1/Cd82 Did Not Affect Viability
| Ratio | |
|---|---|
| Female hets | 0.507 |
| Female KO | 0.275 |
| Female WT | 0.217 |
| Male hets | 0.525 |
| Male KO | 0.259 |
| Male WT | 0.216 |
No genotypic-related histopathologic abnormalities were observed in the pancreas of these mice, in contrast to proliferative changes noted in small numbers of mixed background mice. As expected from older animals, we detected numerous lesions in 12 and 18-month-old mice including benign neoplasms and some malignancies (Table 2). Specifically, we noted 37 neoplasms among all aged mice. However, none of these occurred with unexpected frequency compared to conventional C57BL/6 mice, and therefore were not associated with any specific genotype.
Table 2.
Neoplasms in 12- and 18-Month-Old Kai1/Cd82 Null Mice and Littermates
| Tumor | 12 Months | Genotype (WT/het/KO) | 18 Months | Genotype WT/het/KO |
|---|---|---|---|---|
| Lymphoma | 2 | 0/1/1 | 16 | 5/7/4 |
| Pulmonary adenoma/adenocarcinoma | 1 | 0/1/0 | 4 | 1/3/0 |
| Harderian gland adenoma | 0 | 2 | 0/1/1 | |
| Histiocytic sarcoma | 1 | 0/0/1 | 4 | 0/1/3 |
| Anaplastic round cell tumor (sarcoma) | 0 | 1 | 1/0/0 | |
| Pheochromocytoma | 0 | 1 | 0/0/1 | |
| Adrenocortical adenoma | 0 | 1 | 1/0/0 | |
| Hepatocellular carcinoma | 0 | 2 | 0/0/2 | |
| Intestinal polyp | 0 | 2 | 1/1/0 |
We performed analysis of blood chemistry (Table 3) and blood composition (Table 4) in littermates from Kai1/Cd82+/− matings. While some of the values were statistically different none of these apparent abnormalities in blood composition relating to genotype fell outside the normal range for this strain of mouse.
Table 3.
Blood Chemistry Analysis of Male and Female Wild-Type (WT) and Cd82−/− (KO) Mice
| Male |
Female |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal range |
Normal range |
WT M vs. F (P-value) | KO M vs. F (P-value) | WT vs. KO M & F (P-value) | ||||||||||
| Assay | Units | WTO | KO | Low | High | P-value | WT | KO | Low | High | P-value | |||
| ALB | g/dl | 3.4 | 3.5 | 2.1 | 2.46 | 0.572 | 4.1 | 3.6 | 2.72 | 2.32 | 0.082 | 0.013 | 0.151 | 0.836 |
| ALP | U/L | 82 | 83 | 125 | 176 | 0.943 | 155 | 129 | 153 | 210 | 0.276 | 0.037 | 0.022 | 0.880 |
| ALT | U/L | 57 | 51 | 45 | 93 | 0.430 | 37.3 | 40.6 | 41 | 76 | 0.728 | 0.091 | 0.166 | 0.383 |
| AMY | U/L | 1,034 | 1,050 | 0.818 | 1,116 | 974 | 0.495 | 0.679 | 0.472 | 0.609 | ||||
| TBIL | mg/dl | 0.3 | 0.3 | 0.1 | 0.29 | 1.000 | 0.3 | 0.3 | 0.1 | 0.32 | 0.688 | 0.511 | 0.710 | 0.925 |
| BUN | mg/dl | 23.5 | 21.6 | 17.5 | 23.2 | 0.067 | 23.0 | 19.4 | 13.7 | 20.4 | 0.251 | 0.575 | 0.106 | 0.027 |
| Ca++ | mg/dl | 9.9 | 9.8 | 9.85 | 10.54 | 0.743 | 10.0 | 9.8 | 9.94 | 10.55 | 0.560 | 0.444 | 0.985 | 0.531 |
| PHOS | mg/dl | 8.3 | 8.3 | 10 | 12.37 | 0.935 | 8.7 | 8.3 | 8.51 | 12.23 | 0.714 | 0.524 | 0.973 | 0.779 |
| CRE | mg/dl | 0.4 | 0.2 | 0.24 | 0.32 | 0.020 | 0.3 | 0.4 | 0.22 | 0.31 | 0.049 | 0.604 | 0.003 | 0.634 |
| GLU | mg/dl | 148 | 146 | 139.3 | 184.3 | 0.911 | 152 | 132 | 139.2 | 194.1 | 0.465 | 0.859 | 0.519 | 0.520 |
| Na+ | mmol/L | 159 | 157 | 149.4 | 153.4 | 0.247 | 162 | 154 | 147.7 | 151.2 | 0.025 | 0.125 | 0.002 | 0.010 |
| K+ | mmol/L | 6.6 | 6.7 | 5.54 | 7 | 0.490 | 6.8 | 6.2 | 5.78 | 6.61 | 0.173 | 0.802 | 0.014 | 0.410 |
| TP | g/dl | 6.0 | 6.1 | 4.92 | 5.5 | 0.688 | 6.4 | 6.1 | 5.1 | 5.57 | 0.241 | 0.171 | 0.890 | 0.678 |
| GLOB | g/dl | 2.7 | 2.6 | 0.802 | 2.3 | 2.4 | 0.581 | 0.058 | 0.081 | 0.736 | ||||
| CLORIDE | mmol/L | 116 | 112 | 110.8 | 116.9 | 0.149 | 117 | 114 | 112.1 | 116.3 | 0.532 | 0.286 | 0.611 | 0.103 |
| LIPASE | U/L | 904 | 948 | 0.301 | 1,385 | 1,145 | 0.143 | 0.003 | 0.004 | 0.974 | ||||
| CHOL. | mg/dl | 106 | 103 | 100 | 116 | 0.673 | 106 | 87 | 88 | 110 | 0.340 | 0.865 | 0.124 | 0.214 |
| TRIGLY | mg/dl | 130 | 128 | 0.884 | 80 | 109 | 0.137 | 0.009 | 0.171 | 0.752 | ||||
| AST | U/L | 107 | 101 | 65 | 142 | 0.738 | 73 | 102 | 57 | 199 | 0.387 | 0.221 | 0.941 | 0.884 |
| IRON | mg/dl | 148 | 167 | 145.1 | 215.7 | 0.286 | 156 | 164 | 146.2 | 223.3 | 0.693 | 0.686 | 0.890 | 0.230 |
ALB, albumin; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AMY, amylase; TBIL, total bilirubin; BUN, blood urea nitrogen; PHOS, phosphorus; CRE, creatinine; GLU, glucose; TP, total protein; GLOB, globulin; CHOL., cholesterol; TRYGLY, tryglycerides; AST, aspartate aminotransferase.
Values of pooled samples from eight mice each of male WT, KO and female KO and of four female WT mice.
P-values < 0.05 are indicated in bold face.
Table 4.
Blood Composition (CBC) Analysis of Male and Female Wild-Type (WT) and Cd82−/− (KO) Mice
| Male |
Female |
Normal range |
WT M vs. F (P-value) | KO M vs. F (P-value) | WT vs. KO M & F (P-value) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Assay | WT | KO | P-value | WT | KO | P-value | Low | High | |||
| Leukocytes | |||||||||||
| WBC | 9.00 | 8.47 | 0.506 | 5.48 | 5.74 | 0.755 | 1.8 | 10.7 | 0.003 | 0.0004 | 0.141 |
| NE | 2.46 | 2.49 | 0.931 | 1.07 | 1.48 | 0.123 | 0.1 | 2.4 | 0.001 | 0.004 | 0.646 |
| LY | 6.21 | 5.60 | 0.261 | 4.20 | 4.02 | 0.802 | 0.9 | 9.3 | 0.019 | 0.003 | 0.059 |
| MO | 0.274 | 0.309 | 0.471 | 0.175 | 0.218 | 0.376 | 0 | 0.4 | 0.037 | 0.074 | 0.704 |
| EO | 0.044 | 0.054 | 0.567 | 0.027 | 0.031 | 0.748 | 0 | 0.2 | 0.335 | 0.186 | 0.810 |
| BA | 0.008 | 0.014 | 0.260 | 0.003 | 0.008 | 0.326 | 0 | 0.2 | 0.193 | 0.353 | 0.234 |
| Leukocytes (%) | |||||||||||
| NE | 27.43 | 28.58 | 0.708 | 20.69 | 26.60 | 0.150 | 6.6 | 38.9 | 0.055 | 0.557 | 0.415 |
| LY | 68.84 | 67.19 | 0.619 | 75.31 | 68.53 | 0.163 | 55.8 | 91.6 | 0.103 | 0.723 | 0.308 |
| MO | 3.13 | 3.51 | 0.362 | 3.28 | 4.03 | 0.418 | 0 | 7.5 | 0.789 | 0.411 | 0.143 |
| EO | 0.50 | 0.57 | 0.746 | 0.62 | 0.65 | 0.927 | 0 | 3.9 | 0.642 | 0.738 | 0.653 |
| BA | 0.09 | 0.15 | 0.290 | 0.11 | 0.19 | 0.524 | 0 | 2 | 0.835 | 0.684 | 0.196 |
| Erythrocytes | |||||||||||
| RBC | 10.11 | 10.48 | 0.148 | 9.83 | 9.94 | 0.712 | 6.36 | 9.42 | 0.377 | 0.039 | 0.412 |
| Hb | 14.33 | 14.55 | 0.571 | 14.30 | 14.43 | 0.736 | 11 | 15.1 | 0.961 | 0.720 | 0.530 |
| HCT | 41.68 | 42.74 | 0.535 | 42.17 | 41.71 | 0.796 | 35.1 | 45.4 | 0.815 | 0.507 | 0.746 |
| MCV | 41.14 | 40.69 | 0.603 | 42.87 | 41.94 | 0.269 | 45.4 | 60.3 | 0.108 | 0.105 | 0.678 |
| MCH | 14.16 | 13.89 | 0.085 | 14.55 | 14.54 | 0.961 | 14.1 | 19.3 | 0.126 | 0.0004 | 0.818 |
| MCHC | 34.48 | 34.22 | 0.657 | 34.02 | 34.71 | 0.310 | 30.2 | 34.2 | 0.489 | 0.410 | 0.765 |
| RDW | 18.30 | 18.36 | 0.803 | 18.10 | 17.88 | 0.511 | 12.4 | 27 | 0.595 | 0.046 | 0.481 |
| Thrombocytes | |||||||||||
| PLT | 980 | 969 | 0.840 | 771 | 879 | 0.148 | 592 | 2,972 | 0.008 | 0.140 | 0.961 |
| MPV | 4.17 | 4.24 | 0.635 | 4.17 | 4.26 | 0.615 | 5 | 20 | 0.991 | 0.852 | 0.444 |
WBC, white blood cells; NE, neutrophils; LY, lymphocytes; MO, monocytes; EO, eosinophils; BA, basophils; RBC, red blood cells; Hb, hemoglobin; HCT, hematocrit; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; RDW, red cell distribution width; PLT, platelets; MPV, mean platelet volume.
Average values of eight mice each of male WT, KO and female KO and of four female WT mice.
P-values < 0.05 are indicated in bold face.
Because we noted no obvious Kai1/Cd82 genotype knockout effects on mouse physiology and longevity, we performed a gene expression array analysis designed to test whether Kai1/Cd82 had any effect on gene expression. Rather than examine an organ or tissue from adult mice we instead chose to examine non-immortalized cells grown from Day 12.5 mouse embryos. Specifically, we chose to examine these cells at the point where they first begin to grow in culture to simulate an adaptive challenge. We harvested Day 12.5 embryos and established them in culture. At the first passage these cells were harvested in triplicate for gene expression array, genotype, and sex determination. We performed Affymetrix gene expression analysis on the resultant samples.
Unsupervised MDS analysis showed that gene expression from MEFs was tightly linked to the sex of the derived cells as well as to the Kai1/Cd82 genotype. Specifically, total gene expression in MDS showed four distinct groupings of samples based on sex and genotype (Figure 2A). These initial findings suggested that there will be many significant sex-associated transcripts as well as those whose differential expression is attributable to Kai1/Cd82. As expected we identified several well known and established sex-associated transcripts in comparison of male to female mice, including Xist, which likely drive the unsupervised clustering of these samples (Supplementary Tables S1 and S2). To determine the effect of Kai1/Cd82, we also performed differential gene expression analysis of males and females separately and according to genotype. Many genes were identified that were associated with Kai1/Cd82 genotype (presence or absence) in both male and female mice and some were only sex dependent (Supplementary Tables S3 and S4). The top differentially expressed genes in male and female knockout mice are provided in Tables 5 and 6.
Figure 2.
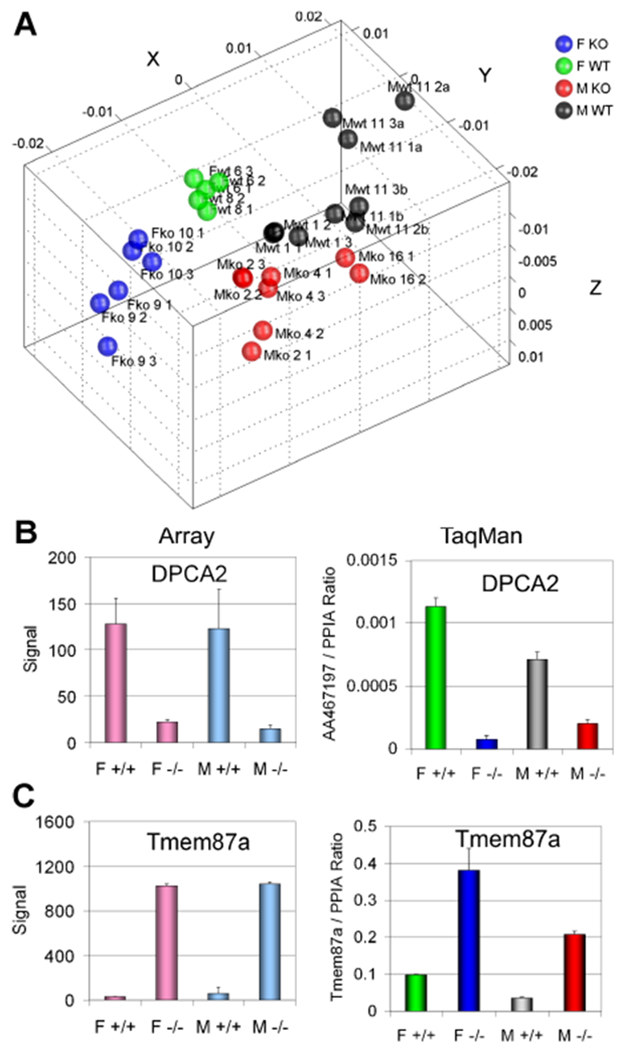
(A) Gene expression of passage 1 cultures of Day 12.5. WT and null mouse embryos identify genes systemically regulated by Kai1/Cd82. Global effect of gene expression demonstrated using unsupervised multidimensional scaling analysis. F KO, female knockout (−/−); F WT, female wild type; M KO, male knockout (−/−); M WT, male wild-type mice. (B,C) Tmem87a and DPCA2 gene expressions that are affected by Cd82/Kai1 genotype. F = female, M = male, +/+ = WT, −/− = KO. Left: Microarray. Right: TaqMan validation.
Table 5.
Differentially Expressed Genes Up- and Down-Regulated by Kai1/Cd82−/− in Male MEFs
| Affymetrix Id | Gene | Description | M KO signal | M WT signal | F KO signal | F WT signal | Male KO/WT | P-value | Female KO/WT | P-value |
|---|---|---|---|---|---|---|---|---|---|---|
| Male | ||||||||||
| 1421058_at | Adh7 | Alcohol dehydrogenase 7, mu or sigma | 11 | 184 | 13 | 22 | 0.0609 | 6E–06 | 0.5882 | 0.2169 |
| 1460248_at | Cpxm2 | Carboxypeptidase X 2 (M14 family) | 51 | 488 | 39 | 39 | 0.1045 | 5E–10 | 1.0055 | 0.9806 |
| 1419728_at | Cxcl5 | Chemokine (C-X-C motif) ligand 5 | 7 | 231 | 5 | 5 | 0.0302 | 3E–06 | 1.1236 | 0.8662 |
| 1418283_at | Cldn4 | Claudin 4 | 170 | 20 | 283 | 144 | 8.5366 | 0.0023 | 1.9665 | 0.0001 |
| 1453092_at | Crct1 | Cysteine-rich C-terminal 1 | 1,711 | 196 | 2,332 | 1,247 | 8.7516 | 1E–09 | 1.8697 | 2E–08 |
| 1435494_s_at | Dsp | Desmoplakin | 109 | 16 | 247 | 47 | 6.7231 | 4E–05 | 5.2668 | 8E–06 |
| 1439746_at | Dusp27 | Dual specificity phosphatase 27 (putative) | 233 | 24 | 246 | 184 | 9.6682 | 0.0015 | 1.3407 | 0.021 |
| 1422947_at | Hist1h3a | Histone cluster 1, H3a | 233 | 15 | 314 | 118 | 16.013 | 5E–06 | 2.6684 | 1E–06 |
| 1428014_at | Hist1h4h | Histone cluster 1, H4h | 241 | 27 | 309 | 135 | 8.9152 | 1E–05 | 2.3 | 3E–05 |
| 1449499_at | Hoxa7 | Homeo box A7 | 270 | 16 | 245 | 232 | 16.514 | 2E–09 | 1.0549 | 0.5373 |
| 1457666_s_at | Ifi202b | Interferon-activated gene 202B | 1 | 3,269 | 3 | 1 | 0.0004 | 7E–11 | 2.2985 | 0.1337 |
| 1451567_a_at | Ifi203 | Interferon-activated gene 203 | 82 | 1,184 | 116 | 182 | 0.0689 | 3E–10 | 0.6393 | 0.0131 |
| 1426278_at | Ifi27 | Interferon, alpha-inducible protein 27 | 115 | 1,337 | 191 | 200 | 0.0864 | 2E–08 | 0.9537 | 0.5953 |
| 1427300_at | Lhx8 | LIM homeobox protein 8 | 37 | 514 | 68 | 38 | 0.0729 | 4E–08 | 1.7899 | 0.0573 |
| 1425828_at | Nkx6-1 | NK6 homeobox 1 | 125 | 19 | 157 | 108 | 6.4826 | 1E–05 | 1.4504 | 0.0832 |
| 1434046_at | AA467197 | Dresden Prostate Cancer Associated 2 | 14 | 220 | 22 | 128 | 0.0644 | 2E–06 | 0.1748 | 2E–05 |
| 1457088_at | Pldn | Pallidin | 324 | 35 | 342 | 42 | 9.3682 | 1E–09 | 8.0977 | 7E–09 |
| 1421917_at | Pdgfra | Platelet-derived growth factor receptor, alpha | 121 | 1,293 | 69 | 112 | 0.0936 | 6E–10 | 0.6186 | 0.0164 |
| 1417466_at | Rgs5 | Regulator of G-protein signaling 5 | 882 | 118 | 1,277 | 702 | 7.4665 | 4E–09 | 1.8201 | 0.0007 |
| 1449340_at | Sostdc1 | Sclerostin domain containing 1 | 31 | 334 | 47 | 36 | 0.0919 | 1E–05 | 1.3274 | 0.1462 |
| 1418105_at | Stmn4 | Stathmin-like 4 | 278 | 22 | 344 | 251 | 12.612 | 0.0003 | 1.3722 | 0.0015 |
| 1442140_at | Tnn | Tenascin N | 92 | 1,855 | 46 | 43 | 0.0495 | 2E–10 | 1.0673 | 0.9125 |
| 1424454_at | Tmem87a | Transmembrane protein 87A | 1,041 | 19 | 1,025 | 29 | 54.054 | 4E–06 | 35.777 | 2E–09 |
| 1429947_a_at | Zbp1 | Z-DNA binding protein 1 | 29 | 390 | 81 | 70 | 0.0736 | 7E–05 | 1.163 | 0.5703 |
M, male; F, female; WT, Cd82+/+; KO, Cd82−/−; KO/WT, Cd82−/−/Cd82+/+ signal ratio.
Signal values are geometric mean values.
Table 6.
Differentially Expressed Genes Up- and Down-Regulated by Kai1/Cd82−/− in Female MEFs
| Affymetrix Id | Gene | Description | M KO signal | M WT signal | F KO signal | F WT signal | Male KO/WT | P-value | Female KO/WT | P-value |
|---|---|---|---|---|---|---|---|---|---|---|
| 1458560_at | Aspm | Abnormal spindle-like, microcephaly associated | 85 | 223 | 63 | 150 | 0.3821 | 0.0038 | 0.4178 | 0.0003 |
| 1448595_a_at | Bex1 | Brain expressed gene 1 | 982 | 305 | 3,461 | 850 | 3.2185 | 3E–09 | 4.0696 | 4E–06 |
| 1449434_at | Car3 | Carbonic anhydrase 3 | 61 | 10 | 166 | 37 | 6.0038 | 0.0019 | 4.5467 | 2E–05 |
| 1418710_at | Cd59a | CD59a antigen | 295 | 690 | 390 | 1,305 | 0.4274 | 5E–06 | 0.2987 | 2E–10 |
| 1454112_a_at | Cep27 | centrosomal protein 27 | 121 | 325 | 114 | 303 | 0.3716 | 7E–08 | 0.3765 | 7E–06 |
| 1448734_at | Cp | Ceruloplasmin | 76 | 38 | 347 | 64 | 2.0054 | 0.0348 | 5.3877 | 2E–06 |
| 1424131_at | Col6a3 | Collagen, type VI, alpha 3 | 3,119 | 10,666 | 1,471 | 3,923 | 0.2924 | 1E–07 | 0.3749 | 1E–09 |
| 1435494_s_at | Dsp | Desmoplakin | 109 | 16 | 247 | 47 | 6.7231 | 4E–05 | 5.2668 | 8E–06 |
| 1452406_x_at | Erdr1 | Erythroid differentiation regulator 1 | 411 | 1,191 | 237 | 1,270 | 0.3448 | 0.0432 | 0.1868 | 0.0004 |
| 1445191_at | Exdl1 | Exonuclease 3′–5′ domain-like 1 | 131 | 34 | 176 | 27 | 3.8183 | 3E–05 | 6.5177 | 0.0004 |
| 1456655_at | Ext1 | Exostosin-1 | 1,510 | 2,182 | 59 | 1190 | 0.6917 | 0.0552 | 0.0495 | 3E–08 |
| 1416200_at | Il33 | Interleukin 33 | 130 | 45 | 363 | 83 | 2.9143 | 0.0002 | 4.3525 | 3E–06 |
| 1425045_at | Jmjd7 | jmjC domain-containing protein 7 | 62 | 206 | 79 | 252 | 0.3013 | 1E–07 | 0.3119 | 1E–06 |
| 1417595_at | Meox1 | Mesenchyme homeobox 1 | 92 | 366 | 56 | 143 | 0.2522 | 0.0001 | 0.3911 | 0.0005 |
| 1434046_at | AA467197 | Dresden prostate cancer associated 2 | 14 | 220 | 22 | 128 | 0.0644 | 2E–06 | 0.1748 | 2E–05 |
| 1419271_at | Pax6 | Paired box gene 6 | 34 | 21 | 122 | 26 | 1.5808 | 0.361 | 4.7538 | 0.0002 |
| 1457088_at | Pldn | Pallidin | 324 | 35 | 342 | 42 | 9.3682 | 1E–09 | 8.0977 | 7E–09 |
| 1449586_at | Pkp1 | Plakophilin 1 | 141 | 264 | 77 | 258 | 0.5355 | 0.0015 | 0.2994 | 0.0081 |
| 1434365_a_at | Scyl3 | Protein-associating with the carboxyl-terminal | 93 | 217 | 52 | 142 | 0.4284 | 0.0002 | 0.3639 | 0.0002 |
| 1444061_at | A030004J04Rik | RIKEN cDNA A030004J04 gene | 98 | 45 | 328 | 77 | 2.1624 | 0.0101 | 4.2384 | 4E–07 |
| 1422198_a_at | Shmt1 | Serine hydroxymethyltransferase 1 (soluble) | 59 | 234 | 32 | 101 | 0.2538 | 0.0027 | 0.318 | 0.0029 |
| 1454211_a_at | Shroom3 | Shroom family member 3 | 93 | 30 | 203 | 25 | 3.067 | 0.0224 | 8.2233 | 0.0027 |
| 1424454_at | Tmem87a | Transmembrane protein 87A | 1,041 | 19 | 1,025 | 29 | 54.054 | 4E–06 | 35.777 | 2E–09 |
M, male; F, female; WT, Cd82+/+; KO, Cd82−/−; KO/WT, Cd82−/−/Cd82+/+ signal ratio.
Signal values are geometric mean values.
Of these differentially expressed transcripts we noted a marked up-regulation of the Tmem87A transcript in both male and female Kai1/Cd82 null cells. This uncharacterized transcript putatively encodes a transmembrane protein of unknown function (Figure 2B). Interestingly, we also noted the loss of the DPCA2 transcript in Kai1/Cd82 null cells. This transcript encodes the Dresden Prostate Cancer Associated 2 transcript. This transcript is normally considered a prostate-specific transcript, but was detected in both male and female MEFs in our experiment. Loss of DPCA2 was noted in both male and female Kai1/Cd82−/− MEFS (Figure 2C). Real-time quantitative PCR confirmed the differential expressions of these transcripts (Figure 2).
Because we examined these MEF cultures at a point where they were still establishing growth in culture and prior to immortalization we were interested in seeing if Kai1/Cd82 genotype affected any global processes. Therefore, we examined the differentially expressed genes related to genotype using Gene Ontology analysis. As might be expected based on known Kai1/Cd82 function, we found that gene ontologies related to immune response and cell adhesion were among the four most highly over-represented gene ontologies (Table 7). Surprisingly, we also noted that genes associated with cell proliferation and mitosis were also among the top four ontologies (Table 7). Finally, we examined the expression of known metastasis associated genes in Kai1/Cd82 wild-type and null cell (Figure 3 and Table 8) and noted differentially expressed genes in this list.
Table 7.
Gene Ontology Analysis Selected Ontology Terms at Top EASE Scores
| Gene category | No. of genes | EASE score | List of genes |
|---|---|---|---|
| GO Biological Process: immune response | 71 | 5.56 | Up: Cxcl12, Rnf128, Cd55, Ccl17, Selp, Cd40 |
| E–14 | Down: Psmb9, C2, Serping1, Irf8, C1qb, Ccl6, C1qa, Hells, Rgs1, Rac2, Ccl9, Gbp2, Fcer1g, Gbp3, Ptx3, Cxcl16, Sfpi1, Tnfsf11, Tlr2, Cxcl1, C3ar1, Cd93, Ccr1, Was, Mx2, Cxcl5, Slc11a1, Ccl2, Ifi202b, Ccl4, Trem2, Tlr7, Ptprc, C5ar1, Hfe, Tlr3, Dock2, Ly86, Psmb8, Cybb, Oasl1, Oas1g, Oas2, Gbp6, H2-K1, Oas3, H2-L, Lst1, Ncf1, Ifi205, Fcgr2b, S1pr3, Lyz2, Ncf2, Cxcr4, Ifi203, Ifit3, C1qc, Itgb2, Psmb9, Ifit1, Tyrobp, H2-D1, Adam33, Mx1, Cfp, Oasl2 | ||
| GO Biological Process: cell proliferation | 91 | 1.35 | Up: Fabp3, Prkar1b, Crip2, Cxcl12, Cdkn1c, Foxg1, Pgf, Tgfb2, 2810003C17Rik, Pitx2, 1434378_a_at, 1443620_at, Ccng2, Cdkn2a, Grpr, Hoxb4 |
| E–10 | Down: Uhrf1, Mcm5, Mcm7, Ccnb1-rs1, Rad21, Coro1a, Cdc45l, Gas1, Cdc6, Psip1, Nek2, Pycard, Tacc3, Gmnn, Rac2, Mybl2, Ccna2, Rad51, Prim1, Clec11a, Cxcl1, Egfl6, Pola1, Ect2, Plk4, Csf1r, Ccnb1, Jag1, Pdgfra, Cdc25b, Igfbp4, Cenph, Mad2l1, Cables1, Ccnf, Ccne2, Dock2, Incenp, Hdgfrp3, Prc1, Ncapd2, Bub1, Aurkb, Cdt1, Rbl1, Rfc4, Brca1, Ncf1, Cdc7, Mki67, Pold3, Pole2, Smc4, Smc2, Bcl11b, Mcm4, S1pr3, Mcm6, Figf, Top2a, Hgf, Bub1b, Plk1, Cxcr4, Cd68, Evi2a, Rad54l, Gsg2, Ifrd2, Adam33, Chtf18, Rfc5, Rpa2, Cdc25c, Smc6 | ||
| GO Biological Process: mitotic cell cycle | 39 | 2.72 | Up: Foxg1, Ccng2, Cdkn2a |
| E–08 | Down: Mcm5, Mcm7, Ccnb1-rs1, Rad21, Coro1a, Cdc45l, Gas1, Nek2, Ccna2, Prim1, Pola1, Ccnb1, Cdc25b, Cenph, Mad2l1, Cables1, Ccnf, Ccne2, Incenp, Ncapd2, Bub1, Cdt1, Rfc4, Pold3, Pole2, Smc4, Smc2, Mcm4, Mcm5, Mcm6, Top2a, Bub1b, Plk1, Chtf18, Rfc5, Rpa2, Cdc25c | ||
| GO Biological Process: cell adhesion | 51 | 5.80 | Up: Mcam, Cxcl12, Itga7, Selp, Col19a1, Itga3, Col10a1, Nell2, Itgbl1, Jup, Lama5, Aplp1, Cldn1, Cpxm1, Col2a1, Arvcf, Chodl |
| E–05 | Down: Cd34, Cd82, Adam8, Sirpa, Rac2, Omd, Clec11a, Cpxm2, Wisp2, Cd93, Clec4n, Col5a3, Pcdha1, Clec4d, Cd36, Plxnc1, Tpbg, Mfap4, Icam1, Col6a3, Pcdha6, Lama4, Lpxn, Cd72, Col6a2, Tspan11, Col8a2, Emilin2, Gpnmb, Clstn2, Col6a1, Col15a1, Acan, Itgb2, Emr1 |
Figure 3.
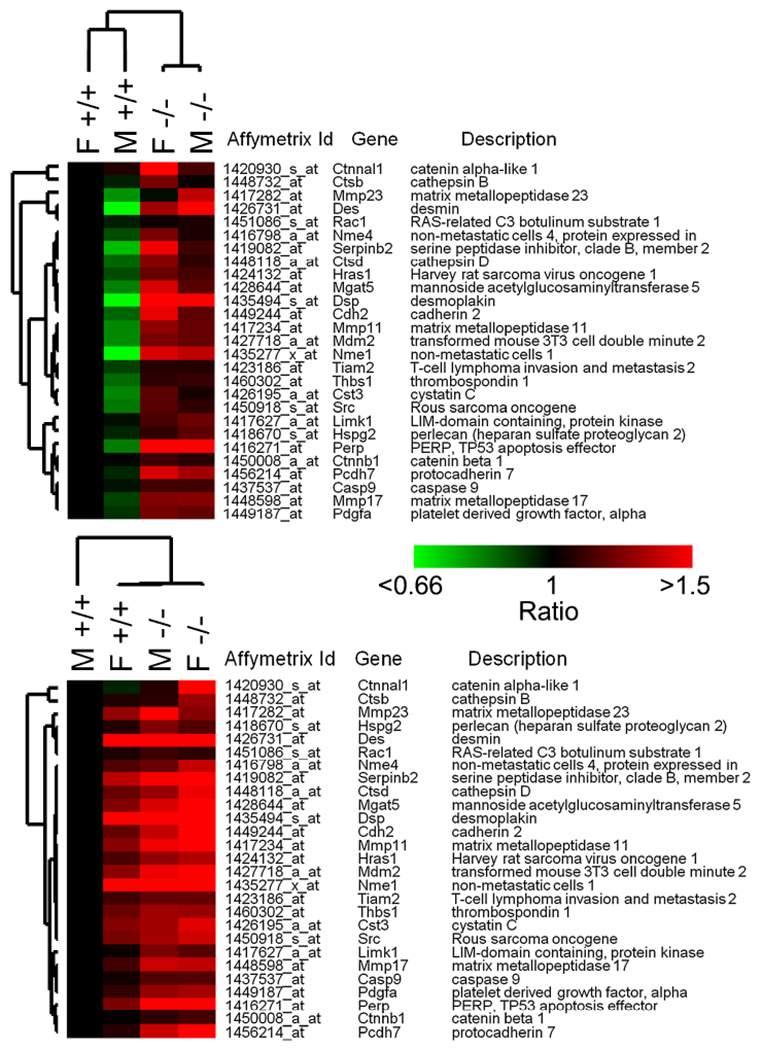
Metastasis-related genes differentially expressed in MEFs derived from wild-type and knock-out Kai1/Cd82 mice. Red is up-regulated, Green is down-regulated. Top: reference is male Cd82+/+. Bottom: reference is female Cd82+/+.
Table 8.
Metastasis-Related Genes Differentially Regulated Between Wild-Type and Kai1/Cd82−/− MEFs by Twofold at P < 0.01
| Affymetrix Id | Gene | Description | M KO signal | M WT signal | F KO signal | F WT signal | KO/WT Male | P-value | KO/WT Female | P-value |
|---|---|---|---|---|---|---|---|---|---|---|
| 1419872_at | Csf1r | Colony-stimulating factor 1 receptor | 524 | 2,160 | 1,353 | 919 | 0.243 | 2E–08 | 1.472 | 4E–06 |
| 1416298_at | Mmp9 | Matrix metallopeptidase 9 | 147 | 604 | 110 | 144 | 0.243 | 4E–06 | 0.760 | 0.1135 |
| 1416401_at | Cd82 | CD82 antigen | 192 | 640 | 234 | 466 | 0.299 | 2E–08 | 0.502 | 1E–06 |
| 1451866_a_at | Hgf | Hepatocyte growth factor | 44 | 136 | 37 | 56 | 0.324 | 3E–05 | 0.664 | 0.0535 |
| 1420798_s_at | Pcdha1 | Protocadherin alpha 1 | 55 | 145 | 88 | 55 | 0.376 | 0.0001 | 1.587 | 0.016 |
| 1424341_s_at | Pcdha6 | Protocadherin alpha 6 | 42 | 103 | 57 | 42 | 0.405 | 9E–05 | 1.343 | 0.0479 |
| 1433930_at | Hpse | Heparanase | 56 | 115 | 45 | 53 | 0.484 | 0.0006 | 0.843 | 0.5041 |
| 1450040_at | Timp2 | Tissue inhibitor of metalloproteinase 2 | 5,720 | 2,757 | 6,422 | 4,914 | 2.074 | 8E–07 | 1.307 | 0.0009 |
| 1435277_x_at | Nme1 | Non-metastatic cells 1 | 9,273 | 4,221 | 9,701 | 6,782 | 2.197 | 0.0022 | 1.430 | 0.0506 |
| 1425092_at | Cdh10 | Cadherin 10 | 1,178 | 510 | 881 | 1,085 | 2.310 | 2E–07 | 0.812 | 0.013 |
| 1449153_at | Mmp12 | Matrix metallopeptidase 12 | 566 | 213 | 1,001 | 602 | 2.654 | 3E–06 | 1.663 | 8E–05 |
| 1420558_at | Selp | Selectin, platelet | 142 | 48 | 179 | 118 | 2.987 | 7E–07 | 1.522 | 0.0162 |
| 1419675_at | Ngf | Nerve growth factor | 4,689 | 1,450 | 4,026 | 4,006 | 3.234 | 6E–10 | 1.005 | 0.9273 |
| 1426731_at | Des | Desmin | 776 | 239 | 513 | 394 | 3.247 | 2E–07 | 1.301 | 0.0391 |
| 1421997_s_at | Itga3 | Integrin alpha 3 | 105 | 28 | 217 | 180 | 3.701 | 0.0021 | 1.202 | 0.1766 |
| 1435494_s_at | Dsp | Desmoplakin | 109 | 16 | 247 | 47 | 6.723 | 4E–05 | 5.267 | 8E–06 |
| 1416271_at | Perp | PERP, TP53 apoptosis effector | 849 | 455 | 1,436 | 557 | 1.864 | 6E–06 | 2.579 | 0.0002 |
M KO, male Cd82−/−; M WT, male Cd82+/+; F KO, female Cd82−/−; F WT, female Cd82+/+; KO/WT, ratio of Cd82−/− to Cd82+/+ signals.
All of them are altered by twofold in male MEFs except for Perp that is 1.86-fold. In female MEFs, Dsp, Perp are altered by twofold other than Kai1 down-regulation.
DISCUSSION
We successfully deleted the Kai1/Cd82 gene in the germline of mice. These mice were viable and exhibited no genotype-associated lesions or physiological abnormalities affecting health or growth at advanced age (18 months). The Kai1/Cd82 null mice, unlike other tetraspanin-deleted models, did not have any observed effect on reproductive viability. These mice showed no increased or decreased propensity to neoplasia and exhibited blood chemistries and hematologic parameters in the normal range for mice on the C57BL/6 background.
Although these mice were outwardly phenotypically normal, subtle defects may exist for which we did not test. Several tetraspanins exhibit alterations in lymphocyte proliferation following stimulation. We did not test this in Kai1/Cd82 mice but it is possible that this process is also affected in Kai1/Cd82 mice. Given the well-known role of CD82 in immune functions, several detailed examinations of altered immune activities could be examined in future studies utilizing these mice.
The Cd151 tetraspanin is known to affect wound healing in mice. Specifically, mice with deleted Cd151 have decreased wound healing function [33]. Furthermore, this defect may be related to angiogenesis [42]. Although we do not present the data in this article, preliminary experiments did not reveal an overtly obvious defect in wound healing in Kai1/Cd82 null mice (Riss, Risinger, and Barrett, unpublished data). Some emerging evidence suggests a reciprocal function of CD151 and CD82. Data on cancers suggest loss of Kai1/Cd82 contributes to metastasis but similar data suggest gain or retention of CD151 is associated with advanced and metastatic disease and that Cd151 actively promotes invasion and motility [43–45]. Cd151 null mice are also less prone to metastasis than wild-type mice [34]. Recently, interdependence of Cd151 and Kai1/Cd82 were also demonstrated on the vitro motility and invasion of cancer cells [46]. Interestingly, our lab has also determined that CD151 and CD82 physically interact in yeast 2-hybrid studies (Risinger and Barrett, unpublished). The reciprocal roles of Cd151 and Kai1/Cd82 will require future studies to unravel their specific functions in cells when both are expressed or when one member is preferentially expressed.
We examined the global mRNA expression of Kai1/Cd82 mice and their wild-type littermate MEFs in cell culture. We performed an unbiased assessment of gene expression in passage 1 MEFs at the initial phases of cell growth in culture when these cells are challenged with a new environment. We hoped to uncover novel functions of Kai1 which could be related through downstream affects on gene expression. Data from these studies clearly showed sex and genotype effects on global expression. In fact both these were globally significant suggesting a profound effect of Kai1/Cd82 on the transcriptome of mice. The lists of differentially expressed genes identified several interesting genes. These included a dramatic up-regulation of TMEM87a, Desmoplakin and Palladin in Kai1/Cd82 null cells. TMEM87a is an unstudied gene whose protein sequence suggests a protein in a transmembrane location. This gene was the most highly up-regulated transcript in response to Kai1 loss in MEFs. Message levels of desmoplakin an integral component of desmosome mediated cell attachments was also significantly increased in Kai1 null cells. The palladin gene suspected of association with actin cytoskelelon was also up-regulated. These findings suggest that Kai1 influences cell attachments and cell shape.
CONCLUSION
Early work defining metastasis suppressors noted the ability of certain human chromosomes to suppress metastasis in orthotopic cancer models without affecting tumor formation [47–50]. Several different human chromosomes including 7, 10, 11, and 17 were shown to possess this property. Kai1/Cd82 was originally identified as the metastasis suppressor from chromosome 11. The human KAI1 gene was able to effectively suppress the formation of lung metastasis in the Rat AT6 cell line without affecting tumorigenicity. These studies have resulted in focusing future metastasis suppressor research on metastatic processes such as motility, invasion, etc. rather than those related to central tumor processes which might include apoptosis or proliferation. However, the results of our ontology analysis on the differentially expressed genes identified in the comparison of wild-type to Kai1/Cd82 null mice showed both expected and unexpected results. Unsurprisingly, based on known Kai1/Cd82 functions, we noted cell adhesion and immune function as ontologies over-represented. However, we were surprised to see that mitosis and cell proliferation were the other two top ontologies. This suggests that Kai/Cd82 has an unappreciated role in the early establishment of proliferation and division processes when cells are challenged with a new environment, in our case cell culture; in vivo this might be adaptability to new metastatic sites.
In summary, we report the generation of Kai1/Cd82 null mice. Although these mice were outwardly normal they are likely to yield further valuable information in other studies related to metastasis and normal biology regarding immune function.
Supplementary Material
Abbreviations:
- MEF
mouse embryo fibroblast
- CBC
complete blood count
- MDS
multidimensional scaling
- EASE
Expression Analysis Systematic Explorer
- BAC
bacterial artificial chromosome
Footnotes
Additional supporting information may be found in the online version of this article.
REFERENCES
- 1.Dong JT, Lamb PW, Rinker-Schaeffer CW, et al. KAI1, a metastasis suppressor gene for prostate cancer on human chromosome 11p11.2. Science 1995;268:884–886. [DOI] [PubMed] [Google Scholar]
- 2.Protzel C, Kakies C, Kleist B, Poetsch M, Giebel J. Down-regulation of the metastasis suppressor protein KAI1/CD82 correlates with occurrence of metastasis, prognosis and presence of HPV DNA in human penile squamous cell carcinoma. Virchows Arch 2008;452:369–375. [DOI] [PubMed] [Google Scholar]
- 3.Lee HS, Lee HK, Kim HS, Yang HK, Kim WH. Tumour suppressor gene expression correlates with gastric cancer prognosis. J Pathol 2003;200:39–46. [DOI] [PubMed] [Google Scholar]
- 4.Muneyuki T, Watanabe M, Yamanaka M, Shiraishi T, Isaji S. KAI1/CD82 expression as a prognosic factor in sporadic colorectal cancer. Anticancer Res 2001;21:3581–3587. [PubMed] [Google Scholar]
- 5.Schindl M, Birner P, Breitenecker G, Oberhuber G. Down-regulation of KAI1 metastasis suppressor protein is associated with a dismal prognosis in epithelial ovarian cancer. Gynecol Oncol 2001;83:244–248. [DOI] [PubMed] [Google Scholar]
- 6.Schindl M, Birner P, Bachtiary B, Breitenecker G, Selzer E, Oberhuber G. The impact of expression of the metastasis suppressor protein KAI1 on prognosis in invasive squamous cell cervical cancer. Anticancer Res 2000;20:4551–4555. [PubMed] [Google Scholar]
- 7.Uchida S, Shimada Y, Watanabe G, et al. Motility-related protein (MRP-1/CD9) and KAI1/CD82 expression inversely correlate with lymph node metastasis in oesophageal squamous cell carcinoma. Br J Cancer 1999;79:1168–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang CI, Kohno N, Ogawa E, Adachi M, Taki T, Miyake M. Correlation of reduction in MRP-1/CD9 and KAI1/CD82 expression with recurrences in breast cancer patients. Am J Pathol 1998;153:973–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adachi M, Taki T, Ieki Y, Huang CL, Higashiyama M, Miyake M. Correlation of KAI1/CD82 gene expression with good prognosis in patients with non-small cell lung cancer. Cancer Res 1996;56:1751–1755. [PubMed] [Google Scholar]
- 10.Berditchevski F, Odintsova E. Tetraspanins as regulators of protein trafficking. Traffic 2007;8:89–96. [DOI] [PubMed] [Google Scholar]
- 11.Hemler ME. Tetraspanin functions and associated microdomains. Nat Rev Mol Cell Biol 2005;6:801–811. [DOI] [PubMed] [Google Scholar]
- 12.Liu WM, Zhang XA. KAI1/CD82, a tumor metastasis suppressor. Cancer Lett 2006;240:183–194. [DOI] [PubMed] [Google Scholar]
- 13.Levy S, Shoham T. The tetraspanin web modulates immune-signalling complexes. Nat Rev Immunol 2005;5:136–148. [DOI] [PubMed] [Google Scholar]
- 14.Miranti CK. Controlling cell surface dynamics and signaling: How CD82/KAI1 suppresses metastasis. Cell Signal 2009; 21:196–211. [DOI] [PubMed] [Google Scholar]
- 15.Sridhar SC, Miranti CK. Tetraspanin KAI1/CD82 suppresses invasion by inhibiting integrin-dependent crosstalk with c-Met receptor and Src kinases. Oncogene 2006;25: 2367–2378. [DOI] [PubMed] [Google Scholar]
- 16.Iiizumi M, Bandyopadhyay S, Watabe K. Interaction of Duffy antigen receptor for chemokines and KA I1: A critical step in metastasis suppression. Cancer Res 2007;67:1411–1414. [DOI] [PubMed] [Google Scholar]
- 17.Bandyopadhyay S, Zhan R, Chaudhuri A, et al. Interaction of KAI1 on tumor cells with DARC on vascular endothelium leads to metastasis suppression. Nat Med 2006;12:933–938. [DOI] [PubMed] [Google Scholar]
- 18.Horak CE, Lee JH, Marshall JC, Shreeve SM, Steeg PS. The role of metastasis suppressor genes in metastatic dormancy. APMIS 2008;116:586–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tonoli H, Barrett JC. CD82 metastasis suppressor gene: A potential target for new therapeutics? Trends Mol Med 2005;1 1:563–570. [DOI] [PubMed] [Google Scholar]
- 20.Imai T, Yoshie O. C33 antigen and M38 antigen recognized by monoclonal antibodies inhibitory to syncytium formation by human T cell leukemia virus type 1 are both members of the transmembrane 4 superfamily and associate with each other and with CD4 or CD8 in T cells. J Immunol 1993; 151:6470–6481. [PubMed] [Google Scholar]
- 21.Fukudome K, Furuse M, Imai T, et al. Identification of membrane antigen C33 recognized by monoclonal antibodies inhibitory to human T-cell leukemia virus type 1 (HTLV-1)-induced syncytium formation: Altered glycosylation of C33 antigen in HTLV-1-positive T cells. J Virol 1992;66:1394–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang C, Taki T, Adachi M, et al. MRP-1/CD9 and KAI1/CD82 expression in normal and various cancer tissues. Int J Oncol 1997;11:1045–1051. [DOI] [PubMed] [Google Scholar]
- 23.Nagira M, Imai T, Ishikawa I, Uwabe KI, Yoshie O. Mouse homologue of C33 antigen (CD82), a member of the transmembrane 4 superfamily: Complementary DNA, genomic structure, and expression. Cell Immunol 1994;157:144–157. [DOI] [PubMed] [Google Scholar]
- 24.Custer MC, Risinger JI, Hoover S, Simpson RM, Patterson T, Barrett JC. Characterization of an antibody that can detect the Kai1/CD82 murine metastasis suppressor. Prostate 2006; 66:567–577. [DOI] [PubMed] [Google Scholar]
- 25.Miyado K, Yamada G, Yamada S, et al. Requirement of CD9 on the egg plasma membrane for fertilization. Science 2000;287:321–324. [DOI] [PubMed] [Google Scholar]
- 26.Kaji K, Oda S, Shikano T, et al. The gamete fusion process is defective in eggs of Cd9-deficient mice. Nat Genet 2000; 24:279–282. [DOI] [PubMed] [Google Scholar]
- 27.Le Naour F, Rubinstein E, Jasmin C, Prenant M, Boucheix C. Severely reduced female fertility in CD9-deficient mice. Science 2000;287:319–321. [DOI] [PubMed] [Google Scholar]
- 28.Schroder J, Lullmann-Rauch R, Himmerkus N, et al. Deficiency of the tetraspanin CD63 associated with kidney pathology but normal lysosomal function. Mol Cell Biol 2009;29: 10831094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wright MD, Geary SM, Fitter S, et al. Characterization of mice lacking the tetraspanin superfamily member CD151. Mol Cell Biol 2004;24:5978–5988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knobeloch KP, Wright MD, Ochsenbein AF, et al. Targeted inactivation of the tetraspanin CD37 impairs T-cell-dependent B-cell response under suboptimal costimulatory conditions. Mol Cell Biol 2000;20:5363–5369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Geisert EE Jr., Williams RW, Geisert GR, et al. Increased brain size and glial cell number in CD81-null mice. J Comp Neurol 2002;453:22–32. [DOI] [PubMed] [Google Scholar]
- 32.Tarrant JM, Groom J, Metcalf D, et al. The absence of Tssc6, a member of the tetraspanin superfamily, does not affect lymphoid development but enhances in vitro T-cell proliferative responses. Mol Cell Biol 2002;22:5006–5018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cowin AJ, Adams D, Geary SM, Wright MD, Jones JC, Ashman LK. Wound healing is defective in mice lacking tetraspanin CD151. J Invest Dermatol 2006;126:680–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takeda Y, Li Q, Kazarov AR, et al. Diminished metastasis in tetraspanin CD151-knockout mice. Blood 118:464–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Spriel AB, Puls KL, Sofi M, et al. A regulatory role for CD37 in T cell proliferation. J Immunol 2004;172:2953–2961. [DOI] [PubMed] [Google Scholar]
- 36.Kelic S, Levy S, Suarez C, Weinstein DE. CD81 regulates neuron-induced astrocyte cell-cycle exit. Mol Cell Neurosci 2001;17:551–560. [DOI] [PubMed] [Google Scholar]
- 37.Kaji K, Oda S, Miyazaki S, Kudo A. Infertility of CD9-deficient mouse eggs is reversed by mouse CD9, human CD9, or mouse CD81; polyadenylated mRNA injection developed for molecular analysis of sperm-egg fusion. Dev Biol 2002;247:327–334. [DOI] [PubMed] [Google Scholar]
- 38.Tessarollo L, Palko ME, Akagi K, Coppola V. Gene targeting in mouse embryonic stem cells. Methods Mol Biol 2009; 530:141–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Risinger JI, Terry LA, Boyd J. Use of representational difference analysis for the identification of mdm2 oncogene amplification in diethylstilbestrol-induced murine uterine adenocarcinomas. Mol Carcinog 1994;11:13–18. [DOI] [PubMed] [Google Scholar]
- 40.Lavrovsky Y, Song CS, Chatterjee B, Roy AK. A rapid and reliable PCR-based assay for gene transmission and sex determination in newborn transgenic mice. Transgenic Res 1998;7:319–320. [DOI] [PubMed] [Google Scholar]
- 41.Nagasawa H, Miyamoto M, Fujimoto M. [Reproductivity in inbred strains of mice and project for their efficient production (author’s transl)]. Jikken Dobutsu 1973;22:119–126. [DOI] [PubMed] [Google Scholar]
- 42.Takeda Y, Kazarov AR, Butterfield CE, et al. Deletion of tetraspanin Cd151 results in decreased pathologic angiogenesis in vivo and in vitro. Blood 2007;109:1524–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ke AW, Shi GM, Zhou J, et al. Role of overexpression of CD151 and/or c-Met in predicting prognosis of hepatocellular carcinoma. Hepatology 2009;49:491–503. [DOI] [PubMed] [Google Scholar]
- 44.Kohno M, Hasegawa H, Miyake M, Yamamoto T, Fujita S. CD151 enhances cell motility and metastasis of cancer cells in the presence of focal adhesion kinase. Int J Cancer 2002;97:336–343. [DOI] [PubMed] [Google Scholar]
- 45.Tokuhara T, Hasegawa H, Hattori N, et al. Clinical significance of CD151 gene expression in non-small cell lung cancer. Clin Cancer Res 2001;7:4109–4114. [PubMed] [Google Scholar]
- 46.Bari R, Zhang YH, Zhang F, et al. Transmembrane interactions are needed for KAI1/CD82-mediated suppression of cancer invasion and metastasis. Am J Pathol 2009;174: 647–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hosoki S, Ota S, Ichikawa Y, et al. Suppression of metastasis of rat prostate cancer by introduction of human chromosome 13. Asian J Androl 2002;4:131–136. [PubMed] [Google Scholar]
- 48.Nihei N, Ichikawa T, Kawana Y, et al. Localization of metastasis suppressor gene(s) for rat prostatic cancer to the long arm of human chromosome 10. Genes Chromosomes Cancer 1995;14:112–119. [DOI] [PubMed] [Google Scholar]
- 49.Rinker-Schaeffer CW, Hawkins AL, Ru N, et al. Differential suppression of mammary and prostate cancer metastasis by human chromosomes 17 and 11. Cancer Res 1994;54: 6249–6256. [PubMed] [Google Scholar]
- 50.Ichikawa T, Ichikawa Y, Dong J, et al. Localization of metastasis suppressor gene(s) for prostatic cancer to the short arm of human chromosome 11. Cancer Res 1992;52:3486–3490. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


