Abstract
Background:
The development of transcatheter aortic valve replacement (TAVR) has led to an improvement in morbidity–mortality in the treatment of severe aortic stenosis in patients at high surgical risk. However, the procedure is not free from life-threatening cardiovascular outcomes and conductive disturbances. The objective of our study was to analyze the prognostic impact of aortic valve calcium score on the occurrence of complications following the procedure.
Materials and Methods:
Patients who have benefited from TAVR with the implantation of new-generation Sapien 3 and Evolut R aortic valve prostheses between January 2017 and July 2018 with the prior realization of a cardiac computed tomography with measurement of the aortic valve calcium score were retrospectively analyzed. Primary endpoint was a composite of death, stroke, and myocardial infarction within a period of 1 month after TAVR. Relation between valvular calcium and conductive disturbances was secondarily analyzed over the same period, and occurrences of high-degree atrioventricular block (paroxysmal or permanent), new-onset left bundle branch block, and the need for permanent or transient cardiac stimulation were associated with the secondary endpoint.
Results:
Overall, 144 patients were included. The aortic valve calcium score was not significantly higher in patients who reached the primary endpoint (2936 ± 1235 vs. 3051 ± 1440, P = 0.93). Among the 106 patients analyzed after excluding subjects with a prior pacemaker or left bundle branch block, aortic valvular calcium score was not statistically associated with the occurrence of conduction disturbances (3210 ± 1436 vs. 2948 ± 1223, P = 0.31).
Conclusion:
Our results suggest that the measurement of aortic valve calcium score has no prognostic value regarding mortality, cardiovascular events, or conductive disturbances after TAVR using the new generation of valves.
Keywords: Aortic stenosis, aortic valve calcium score, cardiac computed tomography, transcatheter aortic valve replacement
INTRODUCTION
Percutaneous aortic valve replacement (TAVR) is one of the most remarkable advances in the field of interventional cardiology in the past decade. It is now a recognized alternative to surgical valvular replacement for inoperable or high-risk surgical patients,[1] with prospects of widening the indications based on the most recent studies.[2] Despite a marked decrease in morbidity–mortality compared to standard surgical treatment in this fragile population,[3] this invasive procedure remains potentially at risk of serious and fatal complications.[4] Cardiovascular events and high-degree conductive disorders are the most common. According to the France TAVI registry,[5] mortality, myocardial infarction, and stroke rates at 30 days are 5.4%, 0.2%, and 2%, respectively. The occurrence of high-degree intracardiac conductive disorders also increases post-TAVR morbidity–mortality, with pacemaker implantation rates ranging from 4% to 31% depending on the studies and valve model used.[6]
Identification of anatomical factors predicting these complications has been the subject of numerous studies.[7] Among these, the calcium aortic valve score, a simple measure during the routine preoperative scanner, could affect the rate of postoperative complications.[8] However, the growing experience of the operators and the development of new-generation valves make this association uncertain.[9] The objective of this study was to determine the predictive value of the aortic valvular calcium score on mortality and the occurrence of cardiovascular outcomes following a procedure of TAVR with new-generation valves.
MATERIALS AND METHODS
This single-center observational and retrospective study was carried out in the cardiology department of the University Hospital of Poitiers in France, following the recommendations of good clinical practice and in accordance with the Declaration of Helsinki. All patients who received percutaneous aortic valve replacement in our department between January 1, 2017, and July 20, 2018, were screened. Valvular replacement was indicated in symptomatic patients with severe aortic stenosis defined by an aortic area <1 cm2 or 0.6 cm2/m2. The choice of a percutaneous technique was decided collegially by the heart team. As a routine procedure, a cardiac computed tomography (CT) was systematically realized as part of the preoperative assessment. Patients who did not perform this scan at our center or for whom the sequence for measuring the calcium score had not been performed were excluded from the study. Other exclusion factors were a valve-in-valve procedure in patients already carrying a valvular prosthesis and in percutaneous approaches other than femoral or supra-aortic. To analyze the impact of aortic valvular calcium score on the occurrence of conductive disturbances, patients with prior pacemaker, defibrillator, or left bundle branch block were excluded secondarily. For each patient included, past medical history, clinical characteristics, and preoperative data such as age, sex, cardiovascular risk factors, STS, and EuroScore risk scores were collected. In common practice, all patients had received preoperative electrocardiogram and cardiac transthoracic echography. They were also systematically reviewed at 1 month for a follow-up consultation with an electrocardiogram and a cardiac echography. The two valve models used for percutaneous aortic valve replacement were the Edwards Sapien 3 and Medtronic CoreValve Evolut R.
The realization of a cardiac and aortic scanner as part of the preoperative assessment for percutaneous aortic valve replacement has become systematic and unavoidable by the wealth of information it provides to practitioners. For all patients included in this study, cardiac CT was performed on a 320 multidetector scanner (Aquilion One, Canon medical systems, Tokyo, Japan) before the TAVR procedure. The calcium score was measured retrospectively using the department's software according to the Agatston method described for coronary calcifications[10] (semiautomatic measurement, the area selected by the operator operator as in Figure 1) and for which good reproducibility has been demonstrated.[11] The default threshold for calcifications was 130 Hounsfield units (HUs). Measurement of aortic calcium score included calcifications of the sinotubular junction, aortic annular, valve leaflets, left ventricle outflow tract with exclusion of coronary, and tubular aorta calcifications.[12]
Figure 1.
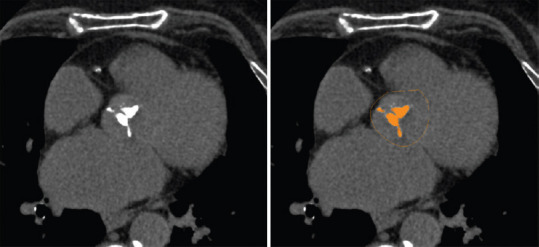
Measurement of the aortic valve calcium score with Agatston's method
The primary endpoint was a composite endpoint associating the occurrence of all-cause death or major cardiovascular events 1 month after procedure: cardiovascular death, myocardial infarction, and stroke. Then, the occurrence of a high-degree atrioventricular block (paroxysmal or permanent), new-onset left bundle branch block, and the need for permanent or transient cardiac stimulation at 1 month were associated with a composite secondary endpoint. Finally, postprocedural moderate or severe aortic regurgitations were also analyzed at 1 month.
The R software was used for the statistical analysis of the study. The data were presented as mean ± standard deviation for continuous variables and as numbers with percentages for qualitative variables. The groups were compared using the Wilcoxon–Mann–Whitney U-test for continuous variables and the Chi-square test for categorical variables. We considered P < 0.05 for statistical significance.
RESULTS
Three hundred and twelve patients benefited from a TAVR procedure over the observation period in our department [Figure 2]. Among these, a CT scan was performed at our center for 242 and with calcium scoring for 152. Six patients were already carrying a biological aortic prosthesis and two had severe aortic regurgitation. A total of 144 patients were included (73 men, aged 84 ± 5.5 years). Seventy patients (48%) benefited from the implantation of an Edwards Sapien 3 valve and 74 patients (52%) of a CoreValve Evolut R valve. The mean aortic valvular calcium score for all patients was 3045 ± 1436 HU. Other demographic data and clinical characteristics of patients are summarized in Table 1.
Figure 2.
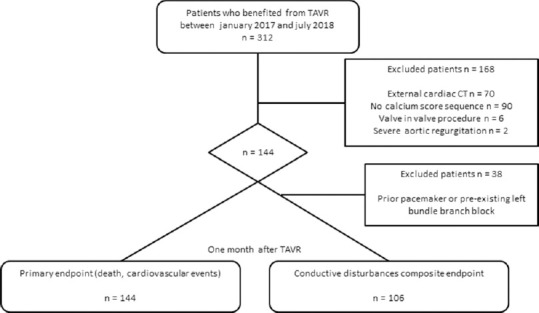
Study flow chart
Table 1.
Population characteristics
| Age (years) | 84±5.5 |
| Male (%) | 73 (51) |
| STS (%) | 5.8±2.9 |
| Euroscore 1 (%) | 20±11 |
| Hypertension (%) | 117 (82) |
| Diabete (%) | 39 (27) |
| Dyslipidemia (%) | 50 (35) |
| Heart failure with reduced ejection fraction (%) | 29 (20) |
| Severe chronic kidney failure (%) | 14 (10) |
| Prior myocardial infarction (%) | 15 (10) |
| Prior stroke (%) | 17 (12) |
| Prior permanent pacemaker or defibrillator (%) | 21 (15) |
| Preexisting left bundle branch block (%) | 19 (13) |
| Preexisting first degree atrioventricular block (%) | 29 (20) |
| Atrial fibrillation (%) | 54 (38) |
| LVEF (%) | 60±14 |
| Low-flow low-gradient aortic stenosis (%) | 17 (12) |
| Aortic valve mean gradient (mmHg) | 51±17 |
| Aortic valve calcium score | 3045±1436 |
| Symptoms | |
| NYHA III/IV dyspnea (%) | 114 (79) |
| Angina pectoris (%) | 33 (23) |
| Syncope (%) | 4 (3) |
| Corevalve EvolutR (%) | 74 (52) |
| Sapien 3 (%) | 70 (48) |
STS=Society of Thoracic Surgeons, LVEF=Left ventricular ejection fraction, NYHA=New York Heart Association
Primary endpoint occurred in 7 (4.9%) patients. Three patients (2.1%) died: one from a cardiac tamponade, one from a stroke, and one from cardiogenic shock with fatal multi-visceral failure. Then, stroke and myocardial infarction, respectively, occurred in 4 (2.8%) and 1 (0.7%) patients. All complications occurred within 5 days after valve implantation. In univariate analysis, the aortic valve calcium score was not significantly higher in patients who died or had a major cardiovascular event after implantation (2936 ± 1235 vs. 3051 ± 1440, P = 0.93). The pejorative prognostic impact of other factors was studied at the same time: age, risk scores, impaired left ventricular ejection fraction, or prior myocardial infarction were not statistically associated with mortality or major cardiovascular events [Table 2].
Table 2.
Predictive factors of death and cardiovascular outcomes one month after transcatheter aortic valve replacement in univariate analysis
| Outcomes (n=7) | Any outcomes (n=137) | P | |
|---|---|---|---|
| Aortic valve calcium score | 2936±1235 | 3051±1440 | 0.93 |
| Age (years) | 87±4.9 | 84±5.5 | 0.29 |
| STS (%) | 6.4±2.8 | 5.8±4 | 0.34 |
| Euroscore 1 (%) | 24±18 | 19±11 | 0.75 |
| LVEF (%) | 68±7 | 59±14 | 0.1 |
| Aortic valve mean gradient (mmHg) | 49±11 | 50±17 | 0.76 |
| Hypertension | 5 (71) | 112 (82) | 0.49 |
| Diabete | 2 (29) | 37 (27) | 0.93 |
| Prior myocardial infarction (%) | 2 (29) | 13 (9) | 0.28 |
| HFrEF (%) | 0 | 29 (21) | 0.17 |
| Atrial fibrillation (%) | 3 (43) | 51 (37) | 0.42 |
STS=Society of Thoracic Surgeons, LVEF=Left ventricular ejection fraction, HFrEF=Heart failure with reduced ejection fraction
Twenty-one patients were already carrying a pacemaker and 17 patients had prior left bundle branch block before TAVR. They were excluded from the analysis of the secondary endpoint. Among the 106 patients, 31 (29%) benefited from the implantation of a pacemaker after the procedure and 16 patients (15%) required transient cardiac pacing. Thirty-two patients (30%) had persistent new-onset left bundle branch block after surgery. The aortic valvular calcium score was not statistically different among patients with or without postimplantation conductive disturbances (3210 ± 1436 vs. 2948 ± 1223, P = 0.31) [Figure 3]. Regarding the influence of valve model [Table 3], the risk of conduction disturbances appeared to be more important after the implantation of EvolutR valves compared to Sapien 3 valves without reaching the threshold of significance (47% vs. 65%, P = 0.07).
Figure 3.
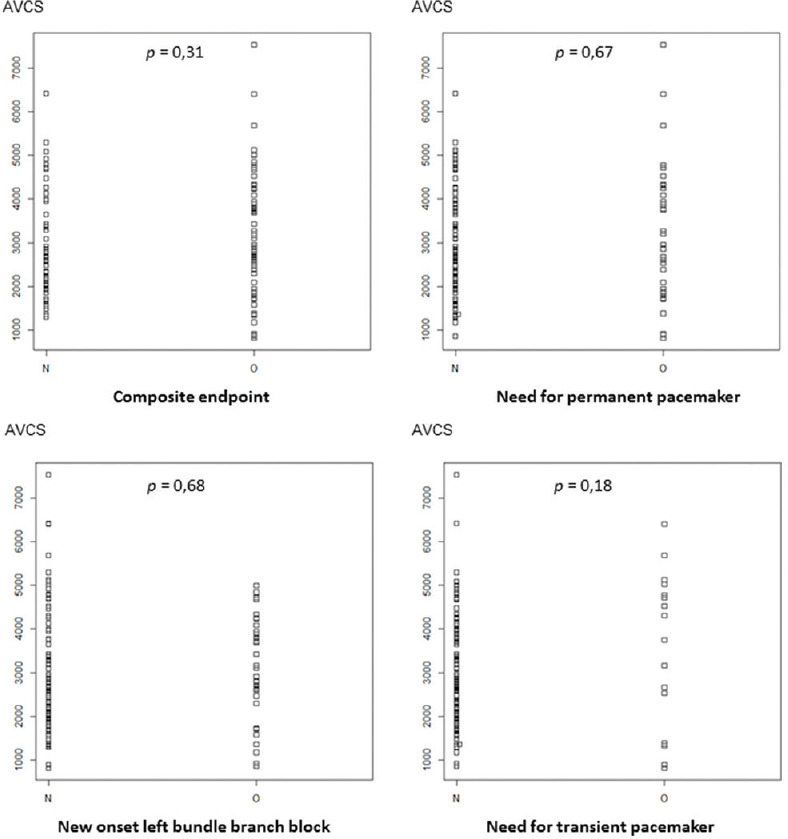
Impact of aortic valve calcium score levels on conductive disturbances after TAVR
Table 3.
Impact of valve’s model on the occurrence of conductive disturbances
| Sapien 3 (n=55) | Corevalve Evolut R (n=51) | P | |
|---|---|---|---|
| Composite endpoint (%) | 26 (47) | 33 (65) | 0.07 |
| Need for permanent pacemaker (%) | 16 (29) | 16 (31) | 0.79 |
| Need for transient pacemaker (%) | 8 (15) | 8 (16) | 0.87 |
| New onset LBBB (%) | 13 (24) | 19 (37) | 0.13 |
LBBB=Left bundle branch block
Finally, the incidence of significant aortic regurgitations within 1 month period after implantation was limited to 2 patients (1.4%). In our sample, calcium score was not statistically predictive of significant regurgitations.
DISCUSSION
The measurement of the aortic valve calcium score is a simple, fast, low-irradiation, contrast-free, reproducible technique. It intervenes in the diagnostic algorithm of patients with suspicion of paradoxical low flow aortic stenosis since a calcium score >1600 HU in women or 3000 HU in men was demonstrated to be strongly in favor of severe stenosis.[13] Aortic valve calcium score (AVCS) could also be helpful in primary prevention because a strong association between valvular calcifications and the occurrence of cardiovascular outcomes has been demonstrated.[14] Regarding its prognostic power in TAVR, the results of our study in 144 patients with new-generation prosthetic valves implantation showed no significant association between the value of the aortic valve calcium score and the occurrence of deaths or major cardiovascular outcomes. This result is in agreement with Akoda et al. who was interested in comparing the prognostic impact of the aortic valve calcium score based on the generation of the implanted valve.[9] In this study, the calcium score appeared to be statistically associated with the occurrence of cardiovascular events only with older generations of valves, as had already been shown in other studies.[8] However, with new-generation models, the results were not significant. The implementation of the latest generation of prosthetic valves was also accompanied in this study by a significant reduction in cardiovascular events (23.7 vs. 9.2%, P = 0.001), reflecting technical progresses in valve design and development, and the growing experience of the operators in implantation procedures. Over the years, the extension of the technique to younger patients with lower surgical risks is another explanation.
Guidelines for the indication of pacemaker implantation after TAVR were not precise at the time of our study,[15] but expert's consensus about this topic has been issued since.[16] It could be simple in patients with high-degree atrioventricular block after a 7-day observation period which can be shortened in case of prior conductive disturbances making a recovery unlikely. On the other hand, the management of patients with new-onset left bundle branch block was controversial. This may explain variations in the rate of pacemaker implantation in the literature. However, this complication is found in most studies and was identified as a serious risk factor of syncope, complete atrioventricular block, or sudden death. Several strategies have been evaluated: conservative treatment, systematic pacemaker implantation, electrophysiological exploration, and Holter implantation. In our center in 2017 and early 2018, the option of conducting an electrophysiological study to validate the indication of a pacemaker was chosen. As a result, the rate of the pacemaker in our study was close to the upper limit (29%) found in the literature. Rates of transient or definitive stimulation are not significantly different depending on the type of valve used despite a more pronounced trend with the CoreValve model, which seems to be in agreement with the various studies.[17] Indeed, although there is a clear predominance for pacemaker implantation for CoreValve with older generations of valves, this difference seems to decrease since the arrival of the last generations. The morphological evolution of the Sapien 3 valve, requiring a higher landing zone for implantation compared to these predecessors, resulted in an increase in the rate of pacemaker implantation.[18] Studies looking at the predictive value of the aortic valve calcium score on the occurrence of conduction disturbances following the implantation of a TAVR were mainly carried out with older generation valves, with conflicting results. Latsios et al. had shown the predictive value of calcifications of the anchor zone regarding the risk of pacemaker implantation after TAVR procedure with CoreValve older model.[19] Conversely, Koos et al. in 2011 and Al-Azzam et al. in 2017[20,21] did not show the influence of aortic valve calcium score on pacemaker implantation rates, the latest mixing valve's generations. Some authors have focused on assessing the impact of aortic root calcifications according to their location. They also did not show any strong association between the total calcium mass and risk of pacemaker implantation. However, they showed that calcifications of the right and left coronary cusps may influence the occurrence of conductive disorders.[22] In future, it might be interesting to analyze the role of valvular calcifications on the long-term dependence of stimulation in the case of pacemaker implantation after TAVR.
Correlation between aortic valve calcium score and the incidence of post-TAVR regurgitations had been demonstrated in several trials studying older generations of prostheses.[8] The emergence of new generations of valve prostheses has resulted in a significant decrease in the incidence of aortic regurgitations.[9] The Sapien 3 valve has a periprosthetic anti-regurgitation device, and the Evolut R valve can be recaptured to optimize its position. Akoda et al. had shown after Evolut R model implantation an association between aortic valve calcium score and the occurrence of significant aortic regurgitations. In our sample, the low incidence of moderate-to-severe aortic regurgitations did not allow a powerful analysis, but we noticed these patients were implanted with an Evolut R valve.
Finally, preoperative assessment of AVCS remains relevant to evaluate chances of a successful procedure until a negative association between valve calcifications and device success has been showed, particularly in case of self-expanding prostheses or asymmetric calcium distribution.[23]
The monocentric and retrospective nature of our study is a limitation. In addition, a large number of patients (37%) were excluded due to the lack of a calcium score measurement on the preoperative cardiac CT, significantly reducing the size of our sample. Finally, the low rate of events is also a statistical limit.
CONCLUSION
The results of our study did not show a prognostic impact of preoperative assessment of aortic valve calcium score on the occurrence of death, severe cardiovascular outcomes, or conductive disturbances after TAVR procedure using the new-generation Sapien 3 and CoreValve aortic valve models.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 2.Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. 2019;380:1695–705. doi: 10.1056/NEJMoa1814052. [DOI] [PubMed] [Google Scholar]
- 3.Siontis GC, Praz F, Pilgrim T, Mavridis D, Verma S, Salanti G, et al. Transcatheter aortic valve implantation vs.surgical aortic valve replacement for treatment of severe aortic stenosis: A meta-analysis of randomized trials. Eur Heart J. 2016;37:3503–12. doi: 10.1093/eurheartj/ehw225. [DOI] [PubMed] [Google Scholar]
- 4.Gilard M, Schlüter M, Snow TM, Dall'Ara G, Eltchaninoff H, Moat N, et al. The 2011-2012 pilot European Society of Cardiology sentinel registry of transcatheter aortic valve implantation: 12-month clinical outcomes. EuroIntervention. 2016;12:79–87. doi: 10.4244/EIJV12I1A15. [DOI] [PubMed] [Google Scholar]
- 5.Gilard M, Eltchaninoff H, Donzeau-Gouge P, Chevreul K, Fajadet J, Leprince P, et al. Late outcomes of transcatheter aortic valve replacement in high-risk patients: The FRANCE-2 registry. J Am Coll Cardiol. 2016;68:1637–47. doi: 10.1016/j.jacc.2016.07.747. [DOI] [PubMed] [Google Scholar]
- 6.Auffret V, Puri R, Urena M, Chamandi C, Rodriguez-Gabella T, Philippon F, et al. Conduction disturbances after transcatheter aortic valve replacement: Current status and future perspectives.Circulation. 2017;136:1049–69. doi: 10.1161/CIRCULATIONAHA.117.028352. [DOI] [PubMed] [Google Scholar]
- 7.Durand E, Doutriaux M, Bettinger N, Tron C, Fauvel C, Bauer F, et al. Incidence, prognostic impact, and predictive factors of readmission for heart failure after transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2017;10:2426–36. doi: 10.1016/j.jcin.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 8.Leber AW, Kasel M, Ischinger T, Ebersberger UH, Antoni D, Schmidt M, et al. Aortic valve calcium score as a predictor for outcome after TAVI using the Core valve revalving system. Int J Cardiol. 2013;166:652–7. doi: 10.1016/j.ijcard.2011.11.091. [DOI] [PubMed] [Google Scholar]
- 9.Akodad M, Lattuca B, Agullo A, Macia JC, Gandet T, Marin G, et al. Prognostic impact of calcium score after transcatheter aortic valve implantation performed with new generation prosthesis. Am J Cardiol. 2018;121:1225–30. doi: 10.1016/j.amjcard.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–32. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 11.Budoff MJ, Mao S, Takasu J, Shavelle DM, Zhao XQ, O'Brien KD. Reproducibility of electron-beam CT measures of aortic valve calcification. Acad Radiol. 2002;9:1122–7. doi: 10.1016/s1076-6332(03)80513-5. [DOI] [PubMed] [Google Scholar]
- 12.Bettinger N, Khalique OK, Krepp JM, Hamid NB, Bae DJ, Pulerwitz TC, et al. Practical determination of aortic valve calcium volume score on contrast-enhanced computed tomography prior to transcatheter aortic valve replacement and impact on paravalvular regurgitation: Elucidating optimal threshold cutoffs. J Cardiovasc Comput Tomogr. 2017;11:302–8. doi: 10.1016/j.jcct.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 13.Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2017;38:2739–91. doi: 10.1093/eurheartj/ehx391. [DOI] [PubMed] [Google Scholar]
- 14.Mazzone C, Cioffi G, Di Nora C, Barbati G, Guidetti F, Faggiano P, et al. Prognostic role of cardiac calcifications in primary prevention: A powerful marker of adverse outcome highly dependent on underlying cardiac rhythm. Int J Cardiol. 2018;258:262–8. doi: 10.1016/j.ijcard.2018.01.101. [DOI] [PubMed] [Google Scholar]
- 15.Brignole M, Auricchio A, Baron-Esquivias G, Bordachar P, Boriani G, Breithardt OA, et al. 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: The task force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC).Developed in collaboration with the European Heart Rhythm Association (EHRA) Eur Heart J. 2013;34:2281–329. doi: 10.1093/eurheartj/eht150. [DOI] [PubMed] [Google Scholar]
- 16.Rodés-Cabau J, Ellenbogen KA, Krahn AD, Latib A, Mack M, Mittal S, et al. Management of conduction disturbances associated with transcatheter aortic valve replacement: JACC scientific expert panel. J Am Coll Cardiol. 2019;74:1086–106. doi: 10.1016/j.jacc.2019.07.014. [DOI] [PubMed] [Google Scholar]
- 17.Giannini C, De Carlo M, Tamburino C, Ettori F, Latib AM, Bedogni F, et al. Transcatheter aortic valve implantation with the new repositionable self-expandable Evolut R versus Corevalve system: A case-matched comparison. Int J Cardiol. 2017;243:126–31. doi: 10.1016/j.ijcard.2017.05.095. [DOI] [PubMed] [Google Scholar]
- 18.De Torres-Alba F, Kaleschke G, Diller GP, Vormbrock J, Orwat S, Radke R, et al. Changes in the pacemaker rate after transition from Edwards Sapien XT to Sapien 3 transcatheter aortic valve implantation: The critical role of valve implantation height. JACC Cardiovasc Interv. 2016;3:805–13. doi: 10.1016/j.jcin.2015.12.023. [DOI] [PubMed] [Google Scholar]
- 19.Latsios G, Gerckens U, Buellesfeld L, Mueller R, John D, Yuecel S, et al. Device landing zone” calcification, assessed by MSCT, as a predictive factor for pacemaker implantation after TAVI. Catheter Cardiovasc Interv. 2010;76:431–9. doi: 10.1002/ccd.22563. [DOI] [PubMed] [Google Scholar]
- 20.Koos R, Mahnken AH, Aktug O, Dohmen G, Autschbach R, Marx N, et al. Electrocardiographic and imaging predictors for permanent pacemaker requirement after transcatheter aortic valve implantation. J Heart Valve Dis. 2011;20:83–90. [PubMed] [Google Scholar]
- 21.Al-Azzam F, Greason KL, Krittanawong C, Williamson EE, McLeod CJ, King KS, et al. The influence of native aortic valve calcium and transcatheter valve oversize on the need for pacemaker implantation after transcatheter aortic valve insertion. J Thorac Cardiovasc Surg. 2017;153:1056–62e1. doi: 10.1016/j.jtcvs.2016.11.038. [DOI] [PubMed] [Google Scholar]
- 22.Mauri V, Reimann A, Stern D, Scherner M, Kuhn E, Rudolph V, et al. Predictors of permanent pacemaker implantation after transcatheter aortic valve replacement with the Sapien 3. JACC Cardiovasc Interv. 2016;9:2200–9. doi: 10.1016/j.jcin.2016.08.034. [DOI] [PubMed] [Google Scholar]
- 23.Larroche J, Panh L, Lhermusier T, Bataille V, Marachet MA, Chollet T, et al. Impact of aortic valve calcification severity on device success after transcatheter aortic valve replacement. Int J Cardiovasc Imaging. 2020;36:731–40. doi: 10.1007/s10554-019-01759-7. [DOI] [PubMed] [Google Scholar]


