Abstract
Deficits in learning and memory are often associated with disruption of hippocampal neurogenesis, which is regulated by numerous processes, including precursor cell proliferation, survival, migration, and differentiation to mature neurons. Recent studies demonstrate that adult born neurons in the dentate gyrus (DG) in the hippocampus can functionally integrate into the existing neuronal circuitry and contribute to hippocampal-dependent learning and memory. Here, we demonstrate that relatively short-term deltamethrin exposure (3 mg/kg every 3 days for 1 month) inhibits adult hippocampal neurogenesis and causes deficits in learning and memory in mice. Hippocampal-dependent cognitive functions were evaluated using 2 independent hippocampal-dependent behavioral tests, the novel object recognition task and Morris water maze. We found that deltamethrin-treated mice exhibited profound deficits in novel object recognition and learning and memory in water maze. Deltamethrin exposure significantly decreased bromodeoxyuridine (BrdU)-positive cells (39%) and Ki67+ cells (47%) in the DG of the hippocampus, indicating decreased cellular proliferation. In addition, deltamethrin-treated mice exhibited a 44% decrease in nestin-expressing neural progenitor cells and a 38% reduction in the expression of doublecortin (DCX), an early neuronal differentiation marker. Furthermore, deltamethrin-exposed mice exhibited a 25% reduction in total number of granule cells in the DG. These findings indicate that relatively short-term exposure to deltamethrin causes significant deficits in hippocampal neurogenesis that is associated with impaired learning and memory.
Keywords: pyrethroid, adult neurogenesis, mice, cognition
Cognitive impairment reflects the failure of brain functions including attention, thought, remembering, learning, or decision-making that affects a person’s daily life, causing substantial financial burden and stress to their families and caregivers when they lose their ability to live independently (CDC, 2009; DeFries et al., 2009; Glisky, 2007; Zhao et al., 2019). In 2009, the Center for Disease Control and Prevention (CDC) reported that over 16 million people aged 18 and older in the United States are living with cognitive impairment (CDC, 2009). Approximately 6% of people ages 18–49 and 11% of those over 50 years of age exhibit cognitive impairment in 5 states (CA, FL, IA, LA, and MI) (CDC, 2009).
Although age is the primary risk factor, several other factors, including brain injury and environmental exposures may contribute to cognitive deficits (CDC, 2009; Rohlman et al., 2007). Deltamethrin is a type II pyrethroid found to be extensively used in households, including home, lawn, garden, and pet care, cloth treatment, and mosquito control, which leads to public health concerns as cognitive impairment has been reported in pesticide applicators and their families (Calvert et al., 2003; Kamel et al., 2005; Kimata et al., 2009; Muller-Mohnssen, 1999). Recently, we published data demonstrating that repeated low level exposure to the pyrethroid pesticide deltamethrin is associated with endoplasmic reticulum (ER) stress-mediated apoptosis and learning deficits in mice (Hossain et al., 2015).
Adult neurogenesis is thought to be an important mechanism for maintaining plasticity in the adult brains and is associated with cognitive function in adulthood (Baptista and Andrade, 2018; Kempermann et al., 2015; Kozareva et al., 2019; Toda et al., 2019). Adult neurogenesis comprises several processes, including precursor cell proliferation, survival, migration, and differentiation into mature neurons. This occurs throughout life in the subventricular zone of the lateral ventricle and the subgranular zone (SGZ) of the dentate gyrus (DG) in the hippocampus of most mammals, including humans under normal conditions (Eriksson et al., 1998; Ming and Song, 2005; Thuret et al., 2009). Although the functional role of adult neurogenesis in humans is still being established, it has been demonstrated that adult-born neurons are important for cognitive plasticity in rodents (Kee et al., 2007b; Latchney et al., 2013; Spalding et al., 2013; Zhao et al., 2008). Several recent studies defined the magnitude of adult neurogenesis in animals and humans by quantification of the number of neural precursor cells (NPCs) in the DG of the hippocampus (Kheirbek and Hen, 2013; Sanai et al., 2011; Spalding et al., 2013). A study has shown that approximately 1400 newborn neurons are added to adult human hippocampus every day and provide about 2% annual turnover during aging, suggesting that adult neurogenesis contributes significantly to human hippocampal function (Spalding et al., 2013).
Over the past 2 decades, more attention has been paid on the effects of environmental chemicals and contaminants, including carbofuran, mercury, and 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on cognitive dysfunction and impairment of hippocampal neurogenesis in developing rodents (Falluel-Morel et al., 2007; Gilbert et al., 2005; Mishra et al., 2012; Seth et al., 2017). However, few studies have focused on the potential effects of environmental exposures on adult neurogenesis. Exposure to the agrichemicals maneb and paraquat resulted in a significant reduction in neuronal precursors and proliferating cells in hippocampal DG by altering transcriptional regulation of neurogenesis-related genes in adult mice (Desplats et al., 2012). Recently, we have published data demonstrating that exposure of adult mice to deltamethrin at a dose close to the Lowest Observed Adverse Effect Level (LOAEL) caused ER stress in the hippocampus that is accompanied by cognitive dysfunction and impaired proliferation of progenitor cells in the DG, suggestive of a potential effect on adult hippocampal neurogenesis (Hossain et al., 2015).
Here, we investigated whether relatively short-term exposure to deltamethrin is associated with inhibition of adult hippocampal neurogenesis and cognitive deficits in mice.
MATERIALS AND METHODS
Animals
Thirty-six 10-week-old C57BL/6 male mice were purchased from Jackson Laboratory (Bar Harbor, Maine). Mice were housed 5 per cage in barrier facilities with 12-h light-dark cycle and provided with PicoLabVerified 75 IF Irradiated Diet (Cat No. 0039980, LabDiet, St Louis, Missouri) and Hydropac sterile water (HydropacAWS-2500, Seaford, Delaware) ad libitum throughout the study. Mice were acclimated to the animal colony environment for 2 weeks before experiments began. Animal handling and experiments were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals at Northeast Ohio Medical University.
Treatment
A total of 36 mice were randomly divided into 2 groups for control (n = 18) and deltamethrin treatment (n = 18). Deltamethrin (Cat No. N-11579-250MG, purity 99%, Chem Service, Inc, West Chester, Pennsylvania) was dissolved in corn oil in a dosing volume of 1 ml/kg and administered 3 mg/kg body weight of deltamethrin via gavage every 3 days for 30 days. The dose of deltamethrin was chosen based on our previous study that did not show any signs of toxicity such as tremor, salivation, ataxia, or decreased body weight gain (Hossain et al., 2015). Control animals received corn oil on the same schedule. Three-day dosing interval was chosen for the clearance of deltamethrin from the body, and was based on a recent physiologically based pharmacokinetic study demonstrating that deltamethrin is rapidly metabolized and almost completely eliminated within 48 h following oral administration of 3 mg/kg to rodents (Godin et al., 2010). Body weight was measured and recorded prior to dosing throughout and at the end of the study. At the end of dosing paradigm, 12 animals (n = 6 per group) were used for neurogenesis study and the remaining 24 animals (n = 12 per group) were used for behavioral studies.
Novel object recognition
Three days after the final deltamethrin exposure, the novel object recognition test was performed to evaluate recognition memory (Supplementary Figure 2). On day 1, mice (n = 12/group) were habituated to the open field arenas (27.3 cm × 27.3 cm × 20.3 cm, Cat No. ENV-510, Med Associates, Inc, Fairfax, Vermont) for 30 min. Twenty-four hours after habituation, mice were exposed to the familiar area containing 2 identical (size, shape, and color) plastic objects (red color blocks) and allowed to explore them for 10 min. At the end of the trials, mice were placed back into their home cages. An hour later, mice were allowed to explore the open field arena for 5 min in the presence of 1 of these 2 familiar (identical) objects (red block) and 1 novel object with similar size but different shape and color (blue cylinder shape plastic) to test their recognition memory. Both objects were placed at equal distance. The order of objects and object locations were changed randomly. The objects and the test arena were thoroughly cleaned with 70% ethanol between the sessions to make sure no residual or telltale odors remain on the objects to prevent the use of odor cues during test trials (Lueptow, 2017; Moore et al., 2013). The mice were considered to be exploring the object when the head of the animal is facing the object at a distance of no more than 2 cm and or touching or sniffing the object. The time spent exploring each object was recorded with the video tracking software (EthoVision, Noldus). The amount of time spent exploring the novel object per location and familiar object per location was used to determine their recognition memory.
Morris water maze
Following the novel object recognition test, mice were tested in the Morris water maze (MWM) task (Morris, 1984) as previously described by our group (Hossain et al., 2015). Data were acquired and analyzed with a live video tracking system (EthoVision, Noldus). The maze consists of a circular pool (100 cm diameter × 30 cm height) filled with water at 23 ± 1°C and made white opaque with powdered milk (Cat No. 902887, MP Biomedicals, Solon, Ohio). Briefly, to acquire learning acquisition, each animal received 4 trials daily at 15 min intertrial interval for 5 days. Each trial was 60 s to allow the animal to find the platform (5 cm diameter × 18 cm height). At the end of each trial, mice were towel dried and kept in a cage under a heating lamp until the next trial. Latency, distance traveled to reach the platform, swimming speed, and percentage time spent in each quadrant were recorded. The memory probe was conducted 2 and 24 h after the last training without the hidden platform. The percentage of time spent in each quadrant was considered to determine memory deficits. A visible platform (2.5 cm above the water surface) test was performed at the beginning and end of the experiment to evaluate their visual acuity and motor function.
BrdU administration and tissue preparation
Mice were administered 4 ip injections of 50 mg/kg BrdU at 2-h intervals 24 h after the last dose of deltamethrin (Figure 1B). Bromodeoxyuridine (BrdU) incorporates into the newly synthesized DNA in S-phase of the mitosis and labels proliferating cells (Falluel-Morel et al., 2007; Hossain et al., 2015; Mishra et al., 2012; Winocur et al., 2006). A cohort of animals (n = 6/group) were sacrificed 2 h after the last BrdU injection for neural cell proliferation and neural stem cells (NSCs) population studies. Animals were anesthetized with sodium pentobarbital (50 mg/kg), and transcardially perfused with PBS, followed by 4% paraformaldehyde. Brains were removed, fixed in 4% paraformaldehyde overnight, and thereafter transferred into 30% sucrose and 0.1% sodium azide in PBS and stored at 4°C until they were sectioned. Sections (30 μm) were cut coronally on a sliding microtome and were processed for immunofluorescence and immunohistochemistry.
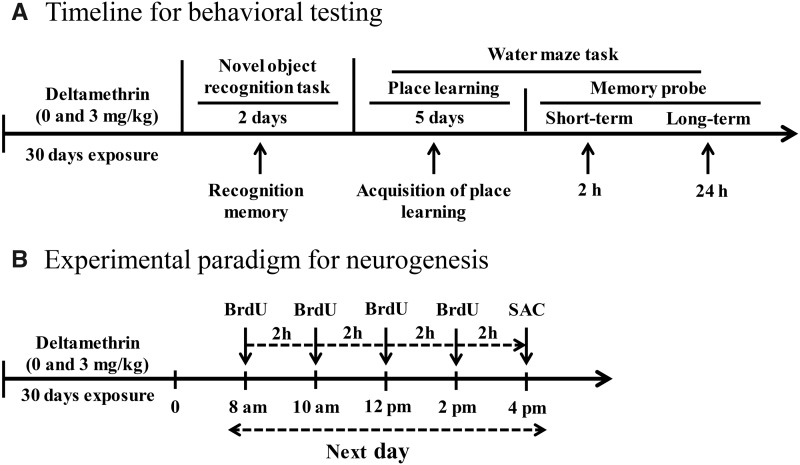
Figure 1.
Experimental timeline for behavioral testing (A). Mice were exposed to 0 and 3 mg/kg of deltamethrin via gavage every 3 days for 30 days. Three days after the final deltamethrin exposure, the novel object recognition test was performed to evaluate recognition memory. Following the novel object recognition test, mice were tested in the Morris water maze task. These animals were not used for neurogenesis. Experimental paradigm used to investigate neural precursor cell proliferation (B). Abbreviation: BrdU, bromodeoxyuridine
Immunohistochemistry
Immunohistochemistry was performed on free-floating sections as described previously (Hossain et al., 2015). Briefly, every 10th section through the entire extent of hippocampus per animal was taken for immunohistochemical analysis. Sections were washed in PBS, followed by permeabilization in 0.3% triton X-100 prepared in PBS. Heat-induced antigen retrieval was then performed by steaming sections in 0.1-M citrate buffer (pH 6.0) for 10 min at 97°C. To inactivate endogenous peroxidases, sections were incubated with 0.3% hydrogen peroxide in methanol for 10 min. Tissue sections were then blocked in 10% normal goat serum and 0.3% triton X-100 in BPS for 1 h and incubated with the antibodies against Nestin (1:200; Cat No. MAB 353, Millipore) overnight at 4°C. Following 3 rinses in PBS, sections were incubated with biotinylated horse anti-mouse or goat anti-rabbit IgG (Vector Laboratories, Burlingame, California) secondary antibody for 1 h at room temperature, followed by incubation in avidin-biotin peroxidase complex (ABC kit; Vector Laboratories) for 1 h at room temperature for amplification. Diaminobenzidine (DAB) fast-tab solution (Vector Laboratories) was used as the chromogen. Sections were rinsed in PBS, mounted onto slides (VWR, West Chester, Pennsylvania), and counterstained with 0.5% cresyl violet (Sigma, St Louis, Missouri). Positive cells from every 10th coronal section containing hippocampus were visualized using an FSX 100 microscope (Olympus, Tokyo, Japan). The positive cells from bilateral hippocampi were manually counted at higher magnification (×40) from a total of 10 sections per animal and 5 animals per group. The mean for each animal (from the 10 sections) then was averaged to obtain the group mean, and compared by an unpaired t test (Hossain et al., 2015).
For immunofluorescence staining, free-floating sections were processed for BrdU, Ki67, and DCX. Heat-induced antigen retrieval and blocking was performed according to the procedure described above. For BrdU staining, sections were pretreated in 2N HCl for 30 min at room temperature to denature DNA. Sections were incubated overnight at 4°C with anti-BrdU (1:200; Cat No. 5292, Cell Signalling), Ki67 (1:200; Cat No. 550609, BD Pharmingen) and anti-DCX (1:1000; Cat No. AB2253, Millipore), in 5% normal goat serum and 0.3% Triton X-100 containing PBS. Sections were washed and incubated with appropriate secondary antibody-conjugated to Alexa Fluor 488 or 594 for 1 h at room temperature in the dark. Sections then were rinsed, mounted onto slides, dried, and coverslipped with Prolong Gold containing 4′,6-diamidino-2-phenylindole. There was no signal when the primary antibody was omitted, thus serving as a negative control. Section was visualized on a Carl Zeiss Axiophot El-Einsatz microscope (Zeiss, Inc, Germany) equipped with a ProgRes C14plus camera - and ProgRes CapturePro 2.8 software (JENOPTIK Optical Systems, Germany). The positive cells from bilateral hippocampi were manually counted at higher magnification (×40) from a total of 10 sections per animal and 5 animals per group as described above.
Unbiased stereology for total cell counts
Following toluidine blue staining, unbiased stereology was performed using the Leica DM2500 microscope and Stereologer Software (3.0) coupled to an ASI MS-2000 3-axis stage system to quantify the number of cells in the granular zone in every 10th section through the entire extent of the hippocampus per animal (Genestine et al., 2015; Obiorah et al., 2015). Boundary of the DG in the hippocampus was traced at low magnification (×5 objective) and then the granule cells were counted under high magnification (×63 objective) in oil. The counting frame = 25 µm × 25 µm, the sampling grid area = 200 µm × 200 µm, a counting height = 10 µm, and the guard height = 3 μm was selected. Cells were only counted if the recognizable profile came into focus within the counting frame. Quantification of cells observed on unilateral hippocampi was performed for 5 animals per group.
Statistical analysis
All of the data were statistical analyzed using Prism 5.01 software (GraphPad, San Diego, California). All of the values were presented as mean ± SEM. Body weights were analyzed by repeated measure ANOVA. For the histological assays and objects recognition, an unpaired t test was used to determine significant difference between control and treatment. For analysis water maze data, 2-way ANOVA and Bonferroni’s post hoc multiple comparison test was used to determine the effects of treatment with day as the repeated measurement and latency as the dependent variable and the daily blocks from the average of 4 trials within each day were used for analysis. p Values ≤ .05 were considered to be statistically significant.
RESULTS
Body Weight and Physical Appearance of Mice Following Exposure to Deltamethrin
Throughout the entire study, no changes in physical appearance (i.e., rough coat) and body weight (f = 0.014; df = 34; p < .907) were observed in mice in the deltamethrin exposure group (final weight: 28.81 ± 0.56 g) when compared with mice in control group (final weight: 28.17 ± 0.32 g) (Supplementary Figure 1). Importantly, no animals exhibited any signs of toxicity such as tremors, salivation, diarrhea, or ataxia following deltamethrin administration throughout the experiment.
Impairment of Learning and Memory in Adult Mice Following Short-term Repeated Exposure to Deltamethrin
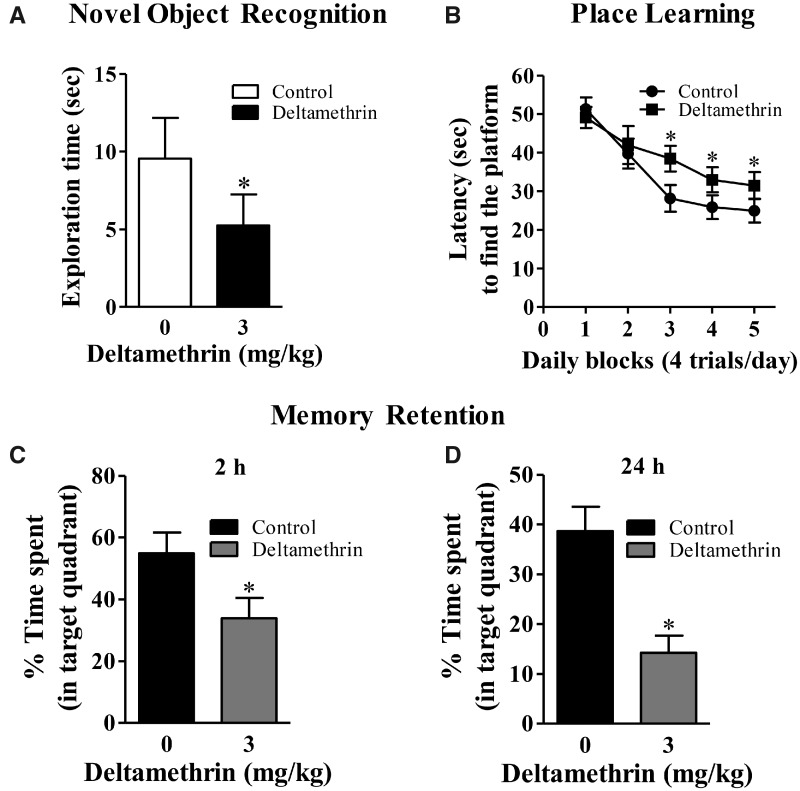
We utilized 2 independent tests of hippocampal-dependent cognitive function following exposure to deltamethrin (Figure 1A). First, we employed the novel object recognition task (Sık et al., 2003), which primarily relies on the animal’s innate exploratory behavior and was performed in the absence of external cues and no training. Second, we evaluated spatial-learning and memory with a MWM (Morris, 1984), which incorporates training and the use of external cues. Three days after the final deltamethrin exposure, the novel object recognition test was performed to evaluate recognition memory. Deltamethrin-treated animals exhibited profound deficits (t = 1.73; df = 22; p < .048) in novel object recognition (Figure 2A) as control animals explored the novel objects significantly more time (9.91 ± 2.40 s) than the deltamethrin-treated animals (5.16 ± 1.33 s). Following the novel object recognition test, mice underwent the MWM task. In this exposure paradigm, deltamethrin-exposed mice did reach criterion for learning, although it was significantly slower (f = 17.08; df = 22; p < .001) than the controls (Figure 2B). A memory probe was conducted 2 and 24 h after the last training without the hidden platform (Figs. 2C and 2D). The percentage of time spent in each quadrant was considered to determine memory deficits. Findings from the memory probe indicate that deltamethrin impaired both short-term (t = 2.72; df = 22; p < .012) and long-term (t = 4.48; df = 22; p < .0002) memory in mice (Figs. 2C and 2D). A visible platform test was performed at the beginning of training and at the end of last memory test to identify whether there were any visual or motor deficits with the mice that impaired their performance. The difference between groups was not the result of motor or visual impairment, because neither swim speed (control: 11.51 ± 1.16 cm/s; deltamethrin: 11.80 ± 1.216 s/s in pre-MWM task and control: 12.38 ± 0.69 cm/s; deltamethrin: 12.14 ± 0.79 cm/s in post-MWM task) nor visual acuity (control: 29.13 ± 3.74 s; deltamethrin: 27.58 ± 5.29 s to find the platform in pre-MWM task and control: 28.82 ± 6.64 s; deltamethrin: 31.43 ± 6.85 s to find the platform in post-MWM task) was affected by deltamethrin treatment (Supplementary Figure 3).
Figure 2.
Repeated deltamethrin (3 mg/kg, every 3 days for 30 days) exposure impairs hippocampal-dependent learning and memory in adult mice. (A) novel object recognition, (B) acquisition of place learning, (C) short-term memory retention, (D) long-term memory retention, Data represent mean ± SEM of 12 individual animals in each group. For objects recognition and memory probe, an unpaired t test was used to determine significant difference between control and treatment. For analysis place learning data, 2-way ANOVA was used to determine the effects of treatment. Asterisk indicates significant treatment effect (object recognition: t = 1.73; df = 22; p < .048; learning: f = 17.08; df = 22; p < .001 and memory: t = 2.72; df = 22; p < .012 for short-term and t = 4.48; df = 22; p < .0002 for long-term).
Deltamethrin Exposure Decreases Cell Proliferation in the DG of the Hippocampus
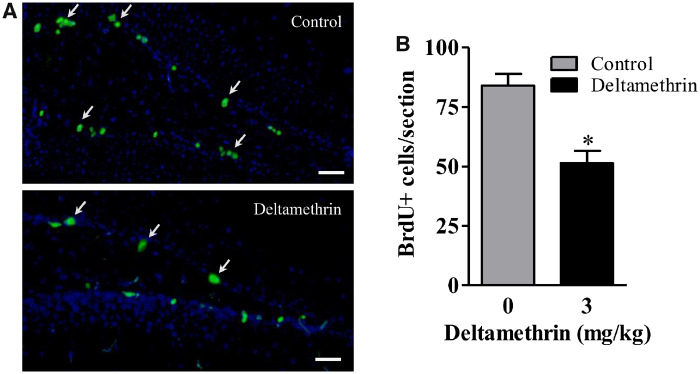
Reduction in neurogenesis in DG effects hippocampal-dependent learning and memory (Sokolowski et al., 2013). As decreased neurogenesis could be due to the effects on proliferation, differentiation, or survival that comprise neurogenesis we investigated these components through the use of BrdU labeling of dividing cells. To examine the effects of deltamethrin on NSC proliferation, mice were given 4 ip injections of BrdU (50 mg/kg) at 2-h intervals 24 h after the last dose of deltamethrin and sacrificed 2 h later (Figure 1B). Deltamethrin exposure decreased BrdU-positive cells by 39% (t = 4.55; df = 8; p < .02) in the DG (Figs. 3A and 3B).
Figure 3.
Short-term repeated exposure to deltamethrin reduced the number of BrdU+ cells or cell proliferation in the dentate gyrus (DG) in hippocampus of adult mice. BrdU+ (green) cells were visualized by immunofluorescence staining in the DG region of the hippocampus (A). The number of BrdU+ cells is presented in bar graphs (B). The values represent mean cell number ± SEM per section from 10 sections per animal and 5 animals per group. An unpaired t test was used to determine significant difference between control and treatment. Asterisk indicates significantly different from control (t = 4.55; df = 8; p < .02). Scale bar = 400 µm.
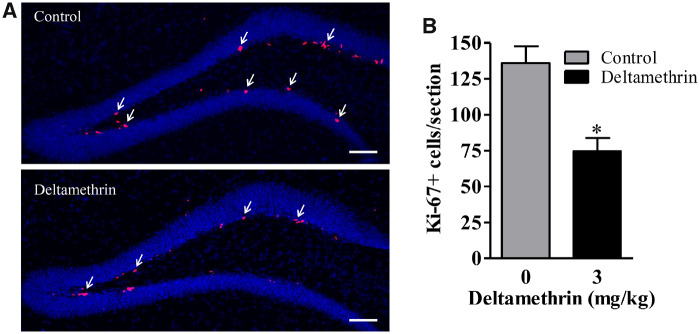
To validate the BrdU results, levels of Ki-67, an endogenous marker of proliferating cells was examined. The Ki-67 immunolabeling was similar to the BrdU results and spread throughout the SGZ in the DG. The Ki-67-labeled cells were decreased by 47% (t = 4.15; df = 8; p < .03) in deltamethrin-exposed mice compared with control (Figs. 4A and 4B), indicating decreased cellular proliferation in the DG.
Figure 4.
Deltamethrin exposure reduces the number of Ki-67+ cells in the dentate gyrus (DG) in hippocampus of adult mice. Immunostaining of Ki-67 (pink) in the DG region of the hippocampus (A). The number of Ki-67+ cells is presented in bar graphs (B). The values represent mean cell number ± SEM per section from 10 sections per animal and 5 animals per group. An unpaired t test was used to determine significant difference between control and treatment. Asterisk indicates significantly different from control (t = 4.15; df = 8; p < .03). Scale bar = 800 µm.
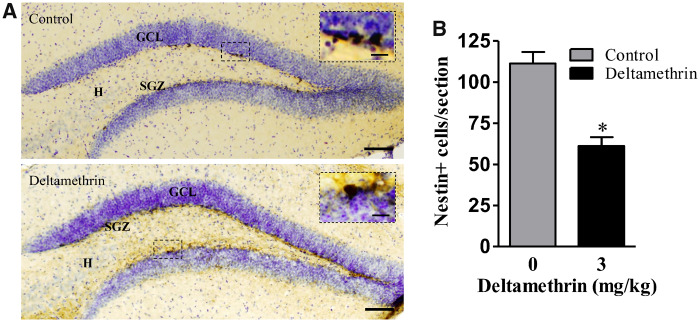
Deltamethrin Decreases Nestin-positive Neural Progenitor Cells in the DG of the Hippocampus
Next, we determined whether reduction in cell proliferation is the result of a change in the number of nestin-positive NSCs. Nestin is an intermediate filament protein expressed in neural stem and progenitor cells. We found that 30 days deltamethrin exposure significantly reduced nestin-positive NPCs/NSCs by 44% (t = 5.68; df = 8; p < .01) in the DG region of the hippocampus compared with control animals (Figs. 5A and 5B), suggesting that reduction of cellular proliferation could be secondary to loss of NSCs. These results indicate that deltamethrin diminishes the proportion of neuroblast cells that are in the mitotic phase in the adult hippocampus.
Figure 5.
Deltamethrin exposure reduces the number of nestin+ cells in the dentate gyrus (DG) in hippocampus of adult mice. . Immunostaining of nestin (dark brown) in the DG region of the hippocampus (A). The number of nestin+ cells is presented in bar graphs (B). An unpaired t test was used to determine significant difference between control and treatment. The values represent mean cell number ± SEM per section from 10 sections per animal and 5 animals per group. Asterisk indicates significantly different from control (t = 5.68; df = 8; p < .01). Abbreviations: GCL, granule cell layer; H, hilus; SGZ, subgranular zone. Scale bar = 1600 µm. Inset scale bar 200 µm.
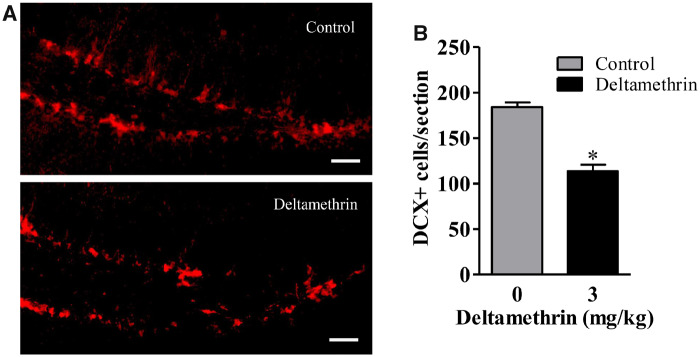
Deltamethrin Exposure Leads to Reduced Neurogenesis in the Hippocampus in Adult Mice
To determine whether short-term deltamethrin exposure affected neurogenesis in adult mice, DCX expression (a marker for immature newborn neurons) was examined. DCX is expressed transiently by early postmitotic neurons but the expression of this marker ceases as neurons mature. We found DCX-positive cells throughout the superior and inferior edges of the DG of the hippocampus in both the control and deltamethrin-exposed animals. Mice exposed to deltamethrin had 38% less (t = 3.36; df = 8; p < .04) immature neurons (DCX+ cells) compared with control mice (Figs. 6A and 6B). These data suggest that exposure to deltamethrin disrupted adult hippocampal neurogenesis in mice.
Figure 6.
Short-term repeated exposure to deltamethrin reduces the number of DCX+ cells in the dentate gyrus (DG) in hippocampus of adult mice. . Immunofluorescence staining of DCX+ (red) in the DG region of the hippocampus (A). The number of DCX+ cells is presented in bar graphs (B). An unpaired t test was used to determine significant difference between control and treatment. The values represent mean cell number ± SEM per section from 10 sections per animal and 5 animals per group. Asterisk indicates significantly different from control (t = 3.36; df = 8; p < .04). Scale bar = 400 µm.
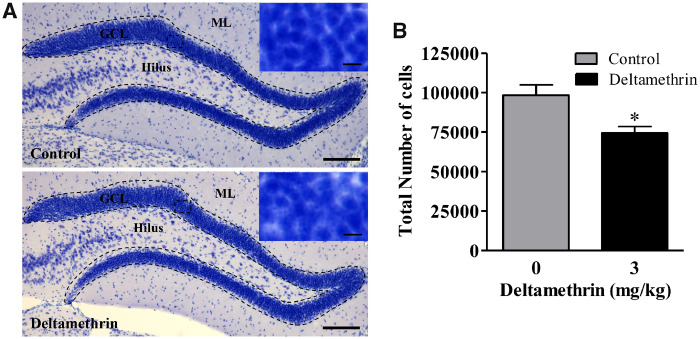
Repeated Deltamethrin Exposure Decreases Granule Cells in the DG of the Hippocampus in Adult Mice
Because exposure to deltamethrin decreased NSC populations and inhibited cell proliferation in the hippocampus, it is possible that deltamethrin may affect the mature neuronal cell in DG. To test this hypothesis, we counted the total number of granule cells in the DG using unbiased stereology (Figs. 7A and 7B). After analyzing the number of total cells, we found that deltamethrin-exposed animals exhibited a 25% decrease (t = 3.042; df = 8; p < .05) in total number of cells in the granule cell layer (GCL) when compared with control (Fig. 7B).
Figure 7.
Short-term repeated exposure to deltamethrin reduces the number of granule cells in the dentate gyrus in hippocampus of adult mice. Granule cells (blue) were visualized with 0.25% Toluidine Blue (A). Unbiased stereology count of total granule cells is presented in bar graphs (B). An unpaired t test was used to determine significant difference between control and treatment. The values represent mean cell number ± SEM from 5 animals per group. Asterisk indicates significantly different from control (t = 3.04; df = 8; p < .05). Abbreviations: GCL, granule cell layer; H, hilus; ML, molecular layer. Scale bar = 1600 µm. Inset scale bar = 200 µm.
DISCUSSION
Pesticide exposure may pose a substantial public health risks as increasing evidence has linked pesticide exposure to development of several neurological disorders and diseases, including pesticide classes such as organophosphates and carbamates (Chuang et al., 2017; Jokanovic, 2018; Kamel and Hoppin, 2004; Sanchez-Santed et al., 2016). However, the potential effects of pyrethroid pesticides are less understood. A nationally representative cohort study of 2116 adults aged 20 years and older demonstrates that environmental exposure to pyrethroid insecticides was significantly associated with an increased risk of all-cause and cardiovascular disease and cancer mortality in the U.S. general adult population (Bao et al., 2020). Recently, occupational exposure to pyrethroids during adulthood have been associated with increased risk of Parkinson disease (Furlong et al., 2015) and developmental exposures have been linked to increased risk of developing autism spectrum disorders (Shelton et al., 2014) and attention deficit hyperactivity disorder (Richardson et al., 2015; Wagner-Schuman et al., 2015). Furthermore, developmental exposure to deltamethrin has been shown to cause memory deficits in water maze, conditioned fear, and/or object recognition (Pitzer et al., 2019; Zhang et al., 2018). Deltamethrin is one of the most commonly used type II pyrethroid insecticides in home and agriculture and is also found as an ingredient in flea and tick repellents and are available at sporting goods stores for treating fabrics (Furlong et al., 2017; Md Meftaul et al., 2020). Previously, we reported that mice repeatedly exposed to deltamethrin (3 mg/kg for 60 days) exhibited profound deficits in learning in the MWM (Hossain et al., 2015) and was accompanied by decreased BrdU incorporation, suggesting a potential effect on neurogenesis. Given the magnitude of this effect, this study was performed to determine whether 3 mg/kg of deltamethrin causes similar effects after 1 month of exposure. Here, in addition to the MWM, we performed the novel object recognition task as a 2nd independent test of hippocampal-dependent cognitive function. We found that short-term exposure to deltamethrin causes profound deficits in novel object recognition and spatial-learning and memory in mice. These deficits were associated with significant reduction of proliferating cells (BrdU- and Ki67-positive cells) and decreased nestin-positive NSCs, immature neurons (DCX-positive cells), and total granule cell population in the DG of the hippocampus, suggesting potential impairment of hippocampal neurogenesis caused by deltamethrin. Together, these results are consistent with our previously published data suggesting that repeated exposure to deltamethrin causes deficits in hippocampal-dependent learning and memory in adult mice.
Environmental factors, including pesticide exposure, are thought to be significant contributors to neurodegeneration and cognitive dysfunction in many neurological diseases (Richardson et al., 2019) but little is known about the potential effect of pesticide exposure on adult neurogenesis. Previously, we demonstrated that repeated exposure to low levels (3 mg/kg) close to the LOAEL of pyrethroid pesticide deltamethrin for 60 days causes reduction of progenitor cell proliferation (BrdU+ cells) in the hippocampal DG in adult mice (Hossain et al., 2015), suggesting that exposure to deltamethrin may inhibit hippocampal neurogenesis in adult. In this study, we find that a shorter 30 days of adult exposure to the same dose of deltamethrin decreased neurogenesis as evidence by a substantial reduction of BrdU-, Ki67-, nestin-, and DCX-positive cells in the hippocampal DG and impaired learning and memory in mice. The observed impairment in neurogenesis resulting from marked reduction of cellular proliferation, decreased NSCs and immature neurons that ultimately become mature neurons in the DG of the adult hippocampus.
To probe the mechanism of deltamethrin-induced inhibition of adult neurogenesis, we conducted a systematic assessment of different components that contribute to neurogenesis. We examined markers of NSCs, cell proliferation and neuronal differentiation using nestin, BrdU, Ki67, and DCX, respectively. All nestin, BrdU, Ki67, and DCX immunoreactive cells were significantly decreased in deltamethrin-exposed mice compared with control. These results suggest that deltamethrin is detrimental to neuronal stem cell development, cell proliferation, and immature neurons in adult brain. The DCX is a widely accepted marker to assess adult neurogenesis (Dennis et al., 2016; Kuhn et al., 2016; Rao and Shetty, 2004). In this study, we observed that the decreased DCX+ cells corresponded to reduced BrdU- and Ki67-labeling cells, indicating decreased neurogenesis following exposure to deltamethrin. It also cannot be ruled out that deltamethrin may directly kill the NSCs or impact a process that reduces their generation. We will address these questions in our future study by colabeling NSCs with caspase-3 and by performing in vitro experiment with isolated NSCs.
There is rising evidence that adult neurogenesis in the hippocampus is important for learning and memory (Cameron and Glover, 2015; Deng et al., 2010; Lemaire et al., 2012; Yau et al., 2015). Hippocampal-dependent learning and memory in the adult rodent has been found to be associated with hippocampal neurogenesis (Baptista and Andrade, 2018; Mishra et al., 2012). Existing studies suggest that adult-born neurons in the DG are functionally integrated into the existing neuronal circuitry and contribute to hippocampal-dependent learning and memory (Adeosun et al., 2014; Thuret et al., 2009; Zhao et al., 2008) whereas inhibition of neurogenesis can interfere with hippocampal-dependent memory (Winocur et al., 2006). Indeed, neurogenesis, which declines with age (Knoth et al., 2010; Molofsky et al., 2006; Pekcec et al., 2008), is impaired by various stressors including stress, sleep deprivation, chronic drug abuse (Mirescu and Gould, 2006; Mirescu et al., 2006; Sudai et al., 2011; Walter et al., 2011) and pesticide exposure (Bosma et al., 2000; Dardiotis et al., 2019; Kim et al., 2019), all of which are associated with cognitive dysfunction.
Several studies have suggested that adult born neurons in the DG play an important role in hippocampal functions because the proliferating newly born cells differentiate into mature neurons, establish synapses with existing hippocampal circuitry, and contribute to hippocampus-dependent learning and memory processes (Adeosun et al., 2014; Baptista and Andrade, 2018; Kempermann et al., 2015; Kozareva et al., 2019; Thuret et al., 2009; Toda et al., 2019; Toni et al., 2008; Yau et al., 2015). Furthermore, disruption of adult neurogenesis causes deficits in hippocampal-dependent learning and memory in rodents (Jessberger et al., 2009). Thus, the disruption of hippocampal neurogenesis by deltamethrin may lead to observed behavioral deficits in mice. Accordingly, we report that the inhibition of adult neurogenesis by deltamethrin accompanied with marked deficits in the hippocampal-dependent learning and memory in adult mice. This finding is consistent with recent studies reporting that reduced neurogenesis is associated with learning and memory deficits in rats following exposure to MeHg, TCDD, and carbamate pesticide (Falluel-Morel et al., 2007; Latchney et al., 2013; Mishra et al., 2012). Thus, our data suggest that inhibition of adult hippocampal neurogenesis following exposure to pyrethroid insecticides may contribute to cognitive deficits. To uncover this fact, additional studies will be conducted in the future with positive controls to determine whether similar magnitude of impairments of neurogenesis produce similar behavioral deficits. In future studies, we will also examine whether fostering neurogenesis ameliorates deltamethrin-induced functional deficits in mice.
Granule cells in the DG are thought to function in the formation of spatial memories, as loss of dentate granule neurons in the hippocampal has been found to associated with spatial memory deficits (Colicos and Dash, 1996; Diamantaki et al., 2016; Kee et al., 2007a). Therefore, we counted granule cells in DG and found that deltamethrin exposure significantly (25%) reduced granular cells when compared with control. It is also possible that deltamethrin may lead cell death of mature granular cells and could occur through activation of ER stress. Previously, we reported that deltamethrin causes cell death in hippocampus through ER stress pathways (Hossain and Richardson, 2020; Hossain et al., 2019). Thus, the loss of progenitors and death of mature granular cells together may contribute to the reduction of total granule cells in the DG. Stereological quantification of granular cells in the DG of the hippocampus suggests that deltamethrin induces a decrease in cells in the GCL. The magnitude of neuronal loss appears to be greater than the normal turnover and replacement following exposure to deltamethrin. Adult neurogenesis in the DG generates nearly 6% of the total granule cell population every month in adult rats (Cameron and McKay, 2001). Furthermore, we cannot exclude the possibility that deltamethrin exposure altered the ability of new-born cells to migrate into the GCL, an additional mechanism to reduce mature neuron numbers. This question could be addressed in our future study by prelabeling precursors with BrdU, and then tracking their migration rates after deltamethrin exposure.
Emerging evidence on the contribution of adult neurogenesis to hippocampal function suggest that dysregulation of neurogenesis by pyrethroid exposure likely contributes to impairments in hippocampal-dependent learning and memory and may have implications for the development of age-related cognitive dysfunction. Taken together, these findings indicate that exposure to deltamethrin insecticide may contribute to disruption of adult hippocampal neurogenesis leading significant deficits in learning and memory in adults, which was further strengthened by the results of others (Souza et al., 2018, 2020) who observed similar cognitive and behavioral deficits in open field, object recognition, and fear conditioning in adult rats, however, their routes of administration were different (intranasal and intracerebroventricular). Therefore, long-term adult exposure to deltamethrin could increase the risk for the development of cognitive deficits in later life of those occupationally expose to these insecticides. Further research is warranted to identify the precise mechanism by which adult pyrethroid exposure perturbs hippocampal neurogenesis to determine the potential contribution of this environmental toxicant in developing age-related cognitive disorders.
SUPPLEMENTARY DATA
Supplementary data are available at Toxicological Sciences online.
FUNDING
National Institute of Environmental Health Sciences (R01ES027481 to M.M.H. and R01ES026057 to J.R.R.) and National Institute of Neurological Disorders and Stroke (U01NS108956 to J.R.R).
DECLARATION OF CONFLICTING INTERESTS
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplementary Material
REFERENCES
- Adeosun S. O., Hou X., Zheng B., Stockmeier C., Ou X., Paul I., Mosley T., Weisgraber K., Wang J. M. (2014). Cognitive deficits and disruption of neurogenesis in a mouse model of apolipoprotein E4 domain interaction. J. Biol. Chem. 289, 2946–2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao W., Liu B., Simonsen D. W., Lehmler H. J. (2020). Association between exposure to pyrethroid insecticides and risk of all-cause and cause-specific mortality in the general us adult population. JAMA Intern. Med. 180, 367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baptista P., Andrade J. P. (2018). Adult hippocampal neurogenesis: Regulation and possible functional and clinical correlates. Front. Neuroanat. 12, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosma H., van Boxtel M. P., Ponds R. W., Houx P. J., Jolles J. (2000). Pesticide exposure and risk of mild cognitive dysfunction. Lancet 356, 912–913. [DOI] [PubMed] [Google Scholar]
- Calvert G. M., Mehler L. N., Rosales R., Baum L., Thomsen C., Male D., Shafey O., Das R., Lackovic M., Arvizu E. (2003). Acute pesticide-related illnesses among working youths, 1988–1999. Am. J. Public Health 93, 605–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron H. A., Glover L. R. (2015). Adult neurogenesis: Beyond learning and memory. Annu. Rev. Psychol. 66, 53–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron H. A., McKay R. D. (2001). Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J. Comp. Neurol. 435, 406–417. [DOI] [PubMed] [Google Scholar]
- CDC. 2009. Centers for Disease Control and Prevention: Cognitive Impairment Available at: https://www.cdc.gov/aging.
- Chuang C. S., Su H. L., Lin C. L., Kao C. H. (2017). Risk of Parkinson disease after organophosphate or carbamate poisoning. Acta Neurol. Scand. 136, 129–137. [DOI] [PubMed] [Google Scholar]
- Colicos M. A., Dash P. K. (1996). Apoptotic morphology of dentate gyrus granule cells following experimental cortical impact injury in rats: Possible role in spatial memory deficits. Brain Res. 739, 120–131. [DOI] [PubMed] [Google Scholar]
- Dardiotis E., Siokas V., Moza S., Kosmidis M. H., Vogiatzi C., Aloizou A. M., Geronikola N., Ntanasi E., Zalonis I., Yannakoulia M., et al. (2019). Pesticide exposure and cognitive function: Results from the Hellenic Longitudinal Investigation of Aging and Diet (HELIAD). Environ. Res. 177, 108632. [DOI] [PubMed] [Google Scholar]
- DeFries E. L., McGuire L. C., Andresen E. M., Brumback B. A., Anderson L. A. (2009). Caregivers of older adults with cognitive impairment. Prev. Chronic Dis. 6, A46. [PMC free article] [PubMed] [Google Scholar]
- Deng W., Aimone J. B., Gage F. H. (2010). New neurons and new memories: How does adult hippocampal neurogenesis affect learning and memory? Nat. Rev. Neurosci. 11, 339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis C. V., Suh L. S., Rodriguez M. L., Kril J. J., Sutherland G. T. (2016). Human adult neurogenesis across the ages: An immunohistochemical study. Neuropathol. Appl. Neurobiol. 42, 621–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desplats P., Patel P., Kosberg K., Mante M., Patrick C., Rockenstein E., Fujita M., Hashimoto M., Masliah E. (2012). Combined exposure to Maneb and Paraquat alters transcriptional regulation of neurogenesis-related genes in mice models of Parkinson's disease. Mol. Neurodegen. 7, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamantaki M., Frey M., Berens P., Preston-Ferrer P., Burgalossi A. (2016). Sparse activity of identified dentate granule cells during spatial exploration. eLife 5, e20252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson P. S., Perfilieva E., Bjork-Eriksson T., Alborn A. M., Nordborg C., Peterson D. A., Gage F. H. (1998). Neurogenesis in the adult human hippocampus. Nat. Med. 4, 1313–1317. [DOI] [PubMed] [Google Scholar]
- Falluel-Morel A., Sokolowski K., Sisti H. M., Zhou X., Shors T. J., Dicicco-Bloom E. (2007). Developmental mercury exposure elicits acute hippocampal cell death, reductions in neurogenesis, and severe learning deficits during puberty. J. Neurochem. 103, 1968–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlong M. A., Barr D. B., Wolff M. S., Engel S. M. (2017). Prenatal exposure to pyrethroid pesticides and childhood behavior and executive functioning. Neurotoxicology 62, 231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlong M., Tanner C. M., Goldman S. M., Bhudhikanok G. S., Blair A., Chade A., Comyns K., Hoppin J. A., Kasten M., Korell M., et al. (2015). Protective glove use and hygiene habits modify the associations of specific pesticides with Parkinson's disease. Environ. Int. 75, 144–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genestine M., Lin L., Durens M., Yan Y., Jiang Y., Prem S., Bailoor K., Kelly B., Sonsalla P. K., Matteson P. G., et al. (2015). Engrailed-2 (En2) deletion produces multiple neurodevelopmental defects in monoamine systems, forebrain structures and neurogenesis and behavior. Hum. Mol. Genet. 24, 5805–5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert M. E., Kelly M. E., Samsam T. E., Goodman J. H. (2005). Chronic developmental lead exposure reduces neurogenesis in adult rat hippocampus but does not impair spatial learning. Toxicol. Sci. 86, 365–374. [DOI] [PubMed] [Google Scholar]
- Glisky E. L. 2007. Changes in cognitive function in human aging In Brain Aging: Models, Methods, and Mechanisms Riddle D. R., (Ed.). CRC Press/Taylor & Francis, Boca Raton, FL. [PubMed] [Google Scholar]
- Godin S. J., DeVito M. J., Hughes M. F., Ross D. G., Scollon E. J., Starr J. M., Setzer R. W., Conolly R. B., Tornero-Velez R. (2010). Physiologically based pharmacokinetic modeling of deltamethrin: Development of a rat and human diffusion-limited model. Toxicol. Sci. 115, 330–343. [DOI] [PubMed] [Google Scholar]
- Hossain M. M., DiCicco-Bloom E., Richardson J. R. (2015). Hippocampal ER stress and learning deficits following repeated pyrethroid exposure. Toxicol. Sci. 143, 220–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain M. M., Richardson J. R. (2020). Nerve growth factor protects against pyrethroid-induced endoplasmic reticulum (ER) stress in primary hippocampal neurons. Toxicol. Sci. 174, 147–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain M. M., Sivaram G., Richardson J. R. (2019). Regional susceptibility to ER stress and protection by salubrinal following a single exposure to deltamethrin. Toxicol. Sci. 167, 249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessberger S., Clark R. E., Broadbent N. J., Clemenson G. D. Jr, Consiglio A., Lie D. C., Squire L. R., Gage F. H. (2009). Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn. Mem. 16, 147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokanovic M. (2018). Neurotoxic effects of organophosphorus pesticides and possible association with neurodegenerative diseases in man: A review. Toxicology 410, 125–131. [DOI] [PubMed] [Google Scholar]
- Kamel F., Engel L. S., Gladen B. C., Hoppin J. A., Alavanja M. C., Sandler D. P. (2005). Neurologic symptoms in licensed private pesticide applicators in the agricultural health study. Environ. Health Perspect. 113, 877–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamel F., Hoppin J. A. (2004). Association of pesticide exposure with neurologic dysfunction and disease. Environ. Health Perspect. 112, 950–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kee N., Teixeira C. M., Wang A. H., Frankland P. W. (2007. a). Imaging activation of adult-generated granule cells in spatial memory. Nat. Protoc. 2, 3033–3044. [DOI] [PubMed] [Google Scholar]
- Kee N., Teixeira C. M., Wang A. H., Frankland P. W. (2007. b). Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat. Neurosci. 10, 355–362. [DOI] [PubMed] [Google Scholar]
- Kempermann G., Song H., Gage F. H. (2015). Neurogenesis in the adult hippocampus. Cold Spring Harb. Perspect. Biol. 7, a018812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheirbek M. A., Hen R. (2013). (Radio)active neurogenesis in the human hippocampus. Cell 153, 1183–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. Y., Park S. J., Kim S. K., Kim C. S., Kim T. H., Min S. H., Oh S. S., Koh S. B. (2019). Pesticide exposure and cognitive decline in a rural south Korean population. PLoS One 14, e0213738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimata A., Kondo T., Ueyama J., Yamamoto K., Yoshitake J., Takagi K., Suzuki K., Inoue T., Ito Y., Hamajima N., et al. (2009). Comparison of urinary concentrations of 3-phenoxybenzoic acid among general residents in rural and suburban areas and employees of pest control firms. Int. Arch. Occup. Environ. Health 82, 1173–1178. [DOI] [PubMed] [Google Scholar]
- Knoth R., Singec I., Ditter M., Pantazis G., Capetian P., Meyer R. P., Horvat V., Volk B., Kempermann G. (2010). Murine features of neurogenesis in the human hippocampus across the lifespan from 0 to 100 years. PLoS One 5, e8809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozareva D. A., Cryan J. F., Nolan Y. M. (2019). Born this way: Hippocampal neurogenesis across the lifespan. Aging Cell 18, e13007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn H. G., Eisch A. J., Spalding K., Peterson D. A. (2016). Detection and phenotypic characterization of adult neurogenesis. Cold Spring Harb. Perspect. Biol. 8, a025981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latchney S. E., Hein A. M., O'Banion M. K., DiCicco-Bloom E., Opanashuk L. A. (2013). Deletion or activation of the aryl hydrocarbon receptor alters adult hippocampal neurogenesis and contextual fear memory. J. Neurochem. 125, 430–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire V., Tronel S., Montaron M. F., Fabre A., Dugast E., Abrous D. N. (2012). Long-lasting plasticity of hippocampal adult-born neurons. J. Neurosci. 32, 3101–3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lueptow L. M. (2017). Novel object recognition test for the investigation of learning and memory in mice. J. Vis. Exp. 126, e55718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Md Meftaul I., Venkateswarlu K., Dharmarajan R., Annamalai P., Megharaj M. (2020). Pesticides in the urban environment: A potential threat that knocks at the door. Sci. Total Environ. 711, 134612. [DOI] [PubMed] [Google Scholar]
- Ming G. L., Song H. (2005). Adult neurogenesis in the mammalian central nervous system. Annu. Rev. Neurosci. 28, 223–250. [DOI] [PubMed] [Google Scholar]
- Mirescu C., Gould E. (2006). Stress and adult neurogenesis. Hippocampus 16, 233–238. [DOI] [PubMed] [Google Scholar]
- Mirescu C., Peters J. D., Noiman L., Gould E. (2006). Sleep deprivation inhibits adult neurogenesis in the hippocampus by elevating glucocorticoids. Proc. Natl. Acad. Sci. U.S.A. 103, 19170–19175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra D., Tiwari S. K., Agarwal S., Sharma V. P., Chaturvedi R. K. (2012). Prenatal carbofuran exposure inhibits hippocampal neurogenesis and causes learning and memory deficits in offspring. Toxicol. Sci. 127, 84–100. [DOI] [PubMed] [Google Scholar]
- Molofsky A. V., Slutsky S. G., Joseph N. M., He S., Pardal R., Krishnamurthy J., Sharpless N. E., Morrison S. J. (2006). Increasing p16iNK4a expression decreases forebrain progenitors and neurogenesis during ageing. Nature 443, 448–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore S. J., Deshpande K., Stinnett G. S., Seasholtz A. F., Murphy G. G. (2013). Conversion of short-term to long-term memory in the novel object recognition paradigm. Neurobiol. Learn. Mem. 105, 174–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R. (1984). Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods 11, 47–60. [DOI] [PubMed] [Google Scholar]
- Muller-Mohnssen H. (1999). Chronic sequelae and irreversible injuries following acute pyrethroid intoxication. Toxicol. Lett. 107, 161–176. [DOI] [PubMed] [Google Scholar]
- Obiorah M., McCandlish E., Buckley B., DiCicco-Bloom E. (2015). Hippocampal developmental vulnerability to methylmercury extends into prepubescence. Front. Neurosci. 9, 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekcec A., Baumgartner W., Bankstahl J. P., Stein V. M., Potschka H. (2008). Effect of aging on neurogenesis in the canine brain. Aging Cell 7, 368–374. [DOI] [PubMed] [Google Scholar]
- Pitzer E. M., Sugimoto C., Gudelsky G. A., Huff Adams C. L., Williams M. T., Vorhees C. V. (2019). Deltamethrin exposure daily from postnatal day 3–20 in Sprague-Dawley rats causes long-term cognitive and behavioral deficits. Toxicol. Sci. 169, 511–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao M. S., Shetty A. K. (2004). Efficacy of doublecortin as a marker to analyse the absolute number and dendritic growth of newly generated neurons in the adult dentate gyrus. Eur. J. Neurosci. 19, 234–246. [DOI] [PubMed] [Google Scholar]
- Richardson J. R., Fitsanakis V., Westerink R. H. S., Kanthasamy A. G. (2019). Neurotoxicity of pesticides. Acta Neuropathol. 138, 343–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson J. R., Taylor M. M., Shalat S. L., Guillot T. S., Caudle W. M., Hossain M. M., Mathews T. A., Jones S. R., Cory‐Slechta D. A., Miller G. W. (2015). Developmental pesticide exposure reproduces features of attention deficit hyperactivity disorder. FASEB J. 29, 1960–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohlman D. S., Lasarev M., Anger W. K., Scherer J., Stupfel J., McCauley L. (2007). Neurobehavioral performance of adult and adolescent agricultural workers. Neurotoxicology 28, 374–380. [DOI] [PubMed] [Google Scholar]
- Sanai N., Nguyen T., Ihrie R. A., Mirzadeh Z., Tsai H.-H., Wong M., Gupta N., Berger M. S., Huang E., Garcia-Verdugo J.-M., et al. (2011). Corridors of migrating neurons in the human brain and their decline during infancy. Nature 478, 382–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Santed F., Colomina M. T., Herrero H. E. (2016). Organophosphate pesticide exposure and neurodegeneration. Cortex 74, 417–426. [DOI] [PubMed] [Google Scholar]
- Seth B., Yadav A., Agarwal S., Tiwari S. K., Chaturvedi R. K. (2017). Inhibition of the transforming growth factor-beta/SMAD cascade mitigates the anti-neurogenic effects of the carbamate pesticide carbofuran. J. Biol. Chem. 292, 19423–19440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton J. F., Geraghty E. M., Tancredi D. J., Delwiche L. D., Schmidt R. J., Ritz B., Hansen R. L., Hertz-Picciotto I. (2014). Neurodevelopmental disorders and prenatal residential proximity to agricultural pesticides: The charge study. Environ. Health Perspect. 122, 1103–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Şık A., van Nieuwehuyzen P., Prickaerts J., Blokland A. (2003). Performance of different mouse strains in an object recognition task. Behav. Brain Res. 147, 49–54. [DOI] [PubMed] [Google Scholar]
- Sokolowski K., Obiorah M., Robinson K., McCandlish E., Buckley B., DiCicco-Bloom E. (2013). Neural stem cell apoptosis after low-methylmercury exposures in postnatal hippocampus produce persistent cell loss and adolescent memory deficits. Dev. Neurobiol. 73, 936–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza M. F., Freire M. A. M., Medeiros K. A. A. L., Lins L. C. R. F., Bispo J. M. M., Gois A. M., Leal P. C., Engelberth R. C. G. J., Ribeiro A. M., Silva R. H., et al. (2018). Deltamethrin intranasal administration induces memory, emotional and tyrosine hydroxylase immunoreactivity alterations in rats. Brain Res. Bull. 142, 297–303. [DOI] [PubMed] [Google Scholar]
- Souza M. F., Medeiros K., Lins L., Bispo J. M. M., Gois A. M., Freire M. A. M., Marchioro M., Santos J. R. (2020). Intracerebroventricular injection of deltamethrin increases locomotion activity and causes spatial working memory and dopaminergic pathway impairment in rats. Brain Res. Bull. 154, 1–8. [DOI] [PubMed] [Google Scholar]
- Spalding K. L., Bergmann O., Alkass K., Bernard S., Salehpour M., Huttner H. B., Bostrom E., Westerlund I., Vial C., Buchholz B. A., et al. (2013). Dynamics of hippocampal neurogenesis in adult humans. Cell 153, 1219–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudai E., Croitoru O., Shaldubina A., Abraham L., Gispan I., Flaumenhaft Y., Roth-Deri I., Kinor N., Aharoni S., Ben-Tzion M., et al. (2011). High cocaine dosage decreases neurogenesis in the hippocampus and impairs working memory. Addict. Biol. 16, 251–260. [DOI] [PubMed] [Google Scholar]
- Thuret S., Toni N., Aigner S., Yeo G. W., Gage F. H. (2009). Hippocampus-dependent learning is associated with adult neurogenesis in MRL/MpJ mice. Hippocampus 19, 658–669. [DOI] [PubMed] [Google Scholar]
- Toda T., Parylak S. L., Linker S. B., Gage F. H. (2019). The role of adult hippocampal neurogenesis in brain health and disease. Mol. Psychiatry 24, 67–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toni N., Laplagne D. A., Zhao C., Lombardi G., Ribak C. E., Gage F. H., Schinder A. F. (2008). Neurons born in the adult dentate gyrus form functional synapses with target cells. Nat. Neurosci. 11, 901–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner-Schuman M., Richardson J. R., Auinger P., Braun J. M., Lanphear B. P., Epstein J. N., Yolton K., Froehlich T. E. (2015). Association of pyrethroid pesticide exposure with attention-deficit/hyperactivity disorder in a nationally representative sample of U.S. children. Environ. Health 14, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter J., Keiner S., Witte O. W., Redecker C. (2011). Age-related effects on hippocampal precursor cell subpopulations and neurogenesis. Neurobiol. Aging 32, 1906–1914. [DOI] [PubMed] [Google Scholar]
- Winocur G., Wojtowicz J. M., Sekeres M., Snyder J. S., Wang S. (2006). Inhibition of neurogenesis interferes with hippocampus-dependent memory function. Hippocampus 16, 296–304. [DOI] [PubMed] [Google Scholar]
- Yau S. Y., Li A., So K. F. (2015). Involvement of adult hippocampal neurogenesis in learning and forgetting. Neural Plast. 2015, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Xu Q., Xiao X., Li W., Kang Q., Zhang X., Wang T., Li Y. (2018). Prenatal deltamethrin exposure-induced cognitive impairment in offspring is ameliorated by memantine through NMDAR/BDNF signaling in hippocampus. Front. Neurosci. 12, 615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B., Shang S., Li P., Chen C., Dang L., Jiang Y., Wang J., Huo K., Deng M., Wang J., et al. (2019). The gender- and age-dependent relationships between serum lipids and cognitive impairment: A cross-sectional study in a rural area of Xi'an, China. Lipids Health Dis. 18, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C., Deng W., Gage F. H. (2008). Mechanisms and functional implications of adult neurogenesis. Cell 132, 645–660. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.