Abstract
Background
Levels of urinary microvesicles, which are increased during various kidney injuries, have diagnostic potential for renal diseases. However, the significance of urinary microvesicles as a renal disease indicator is dampened by the difficulty to ascertain their cell source.
Objectives
The aim of this study was to demonstrate that podocytes can release migrasomes, a unique class of microvesicle with size ranging between 400 and 2,000 nm, and the urine level of migrasomes may serve as novel non-invasive biomarker for early podocyte injury.
Method
In this study, immunofluorescence labeling, electronic microscopy, nanosite, and sequential centrifugation were used to purify and analyze migrasomes.
Results
Migrasomes released by podocytes differ from exosomes as they have different content and mechanism of release. Compared to podocytes, renal tubular cells secrete markedly less migrasomes. Moreover, secretion of migrasomes by human or murine podocytes was strongly augmented during podocyte injuries induced by LPS, puromycin amino nucleoside (PAN), or a high concentration of glucose (HG). LPS, PAN, or HG-induced podocyte migrasome release, however, was blocked by Rac-1 inhibitor. Strikingly, a higher level of podocyte migrasomes in urine was detected in mice with PAN-nephropathy than in control mice. In fact, increased urinary migrasome number was detected earlier than elevated proteinuria during PAN-nephropathy, suggesting that urinary migrasomes are a more sensitive podocyte injury indicator than proteinuria. Increased urinary migrasome number was also detected in diabetic nephropathy patients with proteinuria level <5.5 g/day.
Conclusions
Our findings reveal that podocytes release the “injury-related” migrasomes during migration and provide urinary podocyte migrasome as a potential diagnostic marker for early podocyte injury.
Keywords: Podocyte, Migrasome, Proteinuria, Rac-1, Puromycin amino nucleoside
Introduction
Podocytes control glomerular permeability with their exceptionally complex morphology. The development of proteinuria is often associated with podocyte injury, such as podocyte foot process effacement [1, 2] and cell lose [3, 4]. As podocytes are terminal differentiated cells and lack capacity of renewal, detecting early stage podocyte injury is thus critical for the success of glomerular disease treatment.
Previous studies by us [5, 6] and others [7, 8] have demonstrated that extracellular vesicles (EVs), particularly exosomes, are a class of signal molecules mediating intercellular communication. Although the molecular basis how exosome release is linked to cell injury remains poorly understood, the release of EVs by various cells is often increased at early stages of kidney diseases. This has generated a considerable interest in the potential of exosomes released by renal cells as sensitive biomarkers for nascent kidney diseases. Several studies have linked circulating EVs released from renal endothelial cells to the progression of different kidney diseases such as acute kidney injury, CKD, diabetic nephropathy (DN), lupus nephritis, and nephrotic syndrome [9, 10, 11]. As urine is also a rich source of EVs [12], increased levels of urinary EVs provide a possible noninvasive indicator of kidney injuries [12, 13]. Indeed, Burger et al. [14] reported that podocytes release EVs in vitro when treated with high glucose for 24 h. Interestingly, the same group detected significant increases in podocyte-EVs in type 1 diabetes patients in the absence of albuminuria, nephrinuria, or glomerular filtration rate (GFR) decline [15], implying that urinary podocyte-EVs may be a more sensitive marker for podocyte injury than proteinuria. In type 2 diabetes, urinary EVs were reported to be associated with DN progression [16, 17]. De et al. [17] observed a progressive increase in total urinary EVs and podocalyxin+ EVs in diabetic patients, along with increased albuminuria. In addition, an inverse correlation between urinary EVs and GFR was observed by Kamińska et al. [16] in diabetic patients. Higher levels of podocyte-derived EVs were associated with podocyte injury in patients with renovascular hypertension [18] or lupus nephritis [19]. Urinary EVs can be also derived from other cell types. Despite failing to detect increased podocyte-EVs in the urine of patients with renovascular hypertension and essential hypertension, Santelli et al. [20] reported an increase in tubule-derived p16+ EVs, which were directly associated with circulating proinflammatory biomarkers and negatively associated with GFR. In kidney transplant recipients, as well as a mouse model of adenine-CKD, urinary CD133+ EVs from progenitor cells were demonstrated to be released by the donor's glomeruli and proximal tubule cells [21, 22, 23]. Their study also showed that progenitor cell CD133+ EVs may have a protective role during the reestablishment of kidney function. Further complicating this issue, urinary EVs are also derived from extra-renal cell sources. Burbano et al. [24] observed higher levels of urinary EVs expressing specific markers of monocyte activation in lupus nephritis, implicating the presence of inflammatory infiltrates in renal parenchyma. Urinary EVs containing other biomarkers, such as monocyte chemoattractant protein-1 and neutrophil gelatinase-associated lipocalin, were also investigated in other kidney disease [25]. Given that urinary EVs particularly can be derived from different renal and extra-renal cells, and it is difficult to discern the cell source of urinary exosomes, the significance of urinary exosomes as noninvasive markers for podocyte injury is dampened.
Recent works by Yu's group [26, 27, 28, 29] discovered a unique class of microvesicles named as migrasomes can be secreted by cells during the process of cell migration. The studies showed that migrasomes have the following characteristics: (a) vesicles of 300–3,000 nm diameters with 1 monolar membrane, and (b) containing numerous smaller vesicles inside, and (c) expressing unique proteins such as NDST1 (bifunctionalheparan sulfate N-deacetylase/N-sulfotransferase 1), PIGK (phosphatidylinositol glycan anchor biosynthesis, class K), CPQ (carboxypeptidase Q), and EOGT (EGF domainspecific O-linked N acetylglucosaminetransferase). Migrasomes are different from exosomes not only in size but also in the content and the mechanism by which vesicles are released. More interestingly, although the biological purpose and function of cellular release of migrasomes remains incompletely understood, the production and release of migrasomes is highly dependent upon the motility of the cells. In other words, cells with high motility likely release more migrasomes than low motility cells.
Given podocytes possess a higher capacity of motility than other renal cells, we postulate that human and mouse podocyte can release a significant amount of migrasomes during migration. Since motility of podocytes, including the translocation to cover the space emptied by lost podocytes or the change of the podocyte foot processes from normal to an effaced morphology, are all tightly correlated with podocyte injury [30, 31, 32, 33], the migrasomes secreted by podocytes are likely increased when podocytes are injured. To test these hypotheses, we employed morphological, biochemical and molecular techniques to characterize the migrasomes released by podocytes and the association between the number of migrasomes released by podocytes and podocyte injury. Employing urine samples obtained from a puromycin amino nucleoside (PAN)-induced kidney injury mouse model and patients with active DN diseases, we further detected migrasomes in the urine and explored the possibility of urinary migrasomes serving as a non-invasive diagnostic marker for early podocyte injury.
Materials and Methods
Cell Culture and Treatment
Human podocyte cell line (HPC) was a kind gift from Professor M. Saleem (Children's Renal Unit, Bristol Royal Hospital for Children, University of Bristol, UK) and was cultured as previously described. HPCs were seeded onto culture plates and cultured in RPMI 1640 medium enriched with 10% FBS, 100 U/mL penicillin and 100 μg/mL streptomycin (Gibco, Gaithersburg, MD, USA), and ITS (Sigma-Aldrich, St. Louis, MO, USA). HPCs were cultured at 33°C and 5% CO2 for proliferation and were then shifted to 37°C and 5% CO2 for differentiation for 10–12 days. HPCs were incubated with or without different dose LPS (Sigma-Aldrich), PAN (Sigma-Aldrich), and HG (Sigma-Aldrich) for different time. HPCs were cultured with or without 50 μg/mL LPS, 75 μg/mL PAN, 60 mM HG, and different dose Rac-1 inhibitor (EHT 1864) (Tocris, 3872) for different time. In addition, HPCs were cultured with dynamin Inhibitor Dynasore (Merck, 324410) or blebbistatin (Merck, 203392) for 12 h.
Patient Sample Collection
Healthy volunteers (n = 5) and DN patients (n = 10) were enrolled in this study. The urine samples were collected from DN patients with mild podocyte injure and low proteinuria level (1.57–5.55 g/day). Patients were diagnosed based on renal biopsies at the National Clinical Research Center of Kidney Diseases, Jinling Hospital, Nanjing University School of Medicine. The urine was collected from DN patients within 24 h.
Cell Transfection
HPCs were seeded in a 6-well plate. Until they reached a confluence of 70–80%, HPCs were transfected, respectively, with 2-μg plasmid tetraspanin-4 (TSPAN4)-mcherry per well using LipofectamineTM 3000 (ThermoFisher Scientific). Then replace with new medium at 4 h post-transfection.
Immunofluorescence
HPCs transfected with plasmid TSPAN4-mcherry were seeded into 35-mm glass-bottom dishes coated with fibronectin (1 μg/mL) and cultured at 37°C for 15 h. Then HPCs transfected with plasmid TSPAN4-mcherry were fixed with 4% paraformaldehyde for 10 min at room temperature. Confocal images were taken using a two-photon laser confocal microscope (Lei TCS SP8-MaiTai MP). HPCs grown in 35 mm glass-bottom dishes were fixed with 4% paraformaldehyde for 10 min at room temperature. HPCs were washed with PBS 3 times, blocked in PBS containing 10% FBS, and then incubated with primary antibody anti-SIRPα (Abcam, ab8120) overnight at 4°C. After 3 PBS rinses, fluorophore-conjugated secondary antibody Alexa FluorTM488 donkey anti-rabbit (Invitrogen, A21206) was applied for 60 min at room temperature. After 3 PBS rinses, the stained samples were mounted in ProlongTM Diamond Antifade Mountant (Life Technologies, P36961) with DAPI (Santa Cruz Biotechnology, sc-3598).
Living-Cell Imaging
The night before imaging, HPCs transfected with TSPAN4-mcherry-expressing plasmid were cultured in a 96-well plate coated with fibronectin (1 μg/mL). Images were acquired using a high content screening (PerkElmer operetta), time interval is around 6 min. The images were analyzed by Columbus analysis software and Windows Movie Maker.
Isolation of Migrasomes and Exosomes
Migrasomes and exosomes produced by cultured podocytes were isolated by sequential centrifugation process. Briefly, for isolation of large amount of podocyte migrasomes and exosomes, podocytes were grown in 8–12 flasks (300 cm2 each flask) coated with 0.1 g/mL fibronectin. Migrasomes were isolated from 2 sources: migrasomes adhered to plates or connected to cell surface, and migrasomes in the supernatant of cultured podocytes. To isolate migrasomes in the supernatant, cell culture medium was collected in 50 mL tubes. To isolate the adherent migrasomes, flasks were treated with trypsin for 3–4 min after removal of supernatant, and the cell and migrasomes were also collected in 50 mL tubes. Cells and large debris were removed by centrifugation at 1,000 g for 10 min followed by 4,000 g for 20 min. Migrasomes were then collected as the pellet by centrifugation at 20,000 g for 30 min. The supernatant was then passed through a 0.22 m filter and centrifuged at 120,000 g for 90 min to obtain exosomes. Isolated migrasomes and endosomes were subjected to biological and morphological analysis. To isolate migrasomes from human urine samples, urine samples of the control group (n = 5), and the DN patients (n = 10) were collected in 50 mL tubes. After removal of cells and cell debris by centrifugation at 1,000 g for 10 min followed by 4,000 g for 20 min, urine samples were then centrifuged at 20,000 g for 30 min to collect crude urinary migrasomes.
Nanoparticle Tracking Analysis
The number and size of migrasomes were directly tracked using the NanoSight NS 300 system (NanoSight Technology, Malvern, UK), configured with a 488 nm laser and a high-sensitivity sCMOS camera. Migrasomes were resuspended in PBS to achieve between 20 and 100 particles per frame. Samples were manually injected into the sample chamber at ambient temperature. Each sample was measured in triplicate at camera setting 12 with acquisition time of 30 s and detection threshold of 7. At least 200 completed tracks were analyzed per video. The NTA analytical software version 2.3 was used for analyzing the data.
Migrasome Adhesion
Migrasomes isolated from the urine of healthy donors (control group) and DN patients were seeded into 35 mm glass-bottom dishes coated with fibronectin (1 μg/mL) and incubated at 37°C for 30 min. Migrasomes attached to dishes were stained with PKH67 Green Fluorescent Cell Linker (Sigma-Aldrich, MIDI67) in dark for 15 min. Afterward, migrasomes attached to dishes were washed with PBS and fixed with 4% paraformaldehyde for 10 min. After 3 washes with PBS, the stained samples were directly observed using a two-photon laser confocal microscope (Lei TCS SP8-MaiTai MP).
PAN-Induced Murine Kidney Injury Model
Animal protocols and procedures were approved by the Institutional Animal Care and Use Committee at Nanjing University (Nanjing, China). For PAN-induced podocyte injury, mice received 2 intravenous injections of PAN (180 mg/kg, on day 1 and day 3) [34]. Urine was collected for determination of albumin/Cr and NanoSight analysis on day 0, 2, 4, and 6. Kidneys were harvested and processed for transmission electron microscopy (TEM) on day 0, 2, 4, and 6. Urinary albumin and Cr were measured using mouse albumin ELISA (Bethyl Laboratories) and Cr assay (Sigma-Aldrich) kits according to the manufacturers' protocols. Urine albumin excretion was indicated by the ratio of albumin to Cr (mg/mg).
Electron Microscopy
Scanning electron microscopy was performed as previously described [26]. In brief, the culture cells were grown on coverslips, fixed with 2.5% glutaraldehyde at room temperature, and then post fixed with 1% osmium containing 1.5% potassium ferrocyanide for 60 min at room temperature. All samples were then dehydrated with a graded series of ethanol (50, 70, 90, 95, and 100%) for 8 min each. After changing ethanol with tertiary butyl alcohol, samples were frozen at −20°C and then dried with a freeze drier. The dried samples were coated with an approximately 10-nm-thick gold film by sputter coating before examination with a field emission scanning electron microscope. TEM was performed as previously described [35]. In brief, the samples were collected and fixed in 2.5% glutaraldehyde, followed by post-fixation in 2% osmium tetroxide, dehydrated in graded acetones ethanol, and embedded in epoxy resin (SPI Inc., Westchester, PA, USA), and 80- to 90-nm-ultrathin sections were stained for 15 min in 5% uranyl acetate followed by 0.1% lead citrate for 5 min. Electron micrographs were obtained and analyzed using the Hitachi 7,500 transmission electron microscope.
Western Blot
Western blot analyses were performed as previously described [35]. Briefly, proteins were extracted from cells, migrasomes, or exosomes was resolved by SDS-PAGE before being transferred onto the appropriate membrane and incubated with antibodies. Primary antibodies of ALIX (Abcam, ab186429), PIGK (Abcam, ab201693), podocin (Sigma-Aldrich, P0372), SGLT2 (Abcam, ab37296), and secondary antibody of goat anti-rabbit IgG-HRP (Santa Cruz, sc-2004) were used for detection.
microRNA Microarray
Total RNA was quantified by the NanoDrop ND-2000 (Thermo Scientific) and the RNA integrity was assessed using Agilent Bioanalyzer 2100 (Agilent Technologies). The sample labeling, microarray hybridization, and washing were performed based on the manufacturer's standard protocols. Briefly, total RNA was dephosphorylated, denaturated, and then labeled with Cyanine 3-CTP. After purification, the labeled RNAs were hybridized onto the microarray. After washing, the arrays were scanned with the Agilent Scanner G2505C (Agilent Technologies).
Statistical Analysis
Data were presented as mean ± SEM. Statistical analysis was performed in GraphPad Prism 7.0. All data were obtained from at least 3 independent experiments. Statistical comparisons between groups were analyzed for significance by two-tailed t test. Results were considered significant at p values of <0.05.
Results
Podocytes Secret Migrasomes, a Unique Class of Microvesicles Different from Exosomes
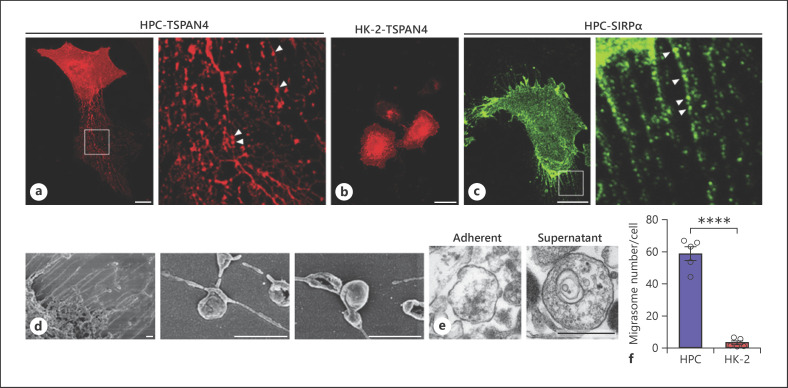
Recent work by Ma et al. [26] discovered that migrasomes, a unique class of microvesicles whose size vary between 300 and 3,000 nm, can be secreted by “migrating cells” during the process of cell migration. As podocytes possess a high motility capacity, we postulate that podocytes can also release migrasomes. To test this hypothesis, we transfected immobilized HPCs with TSPAN4-mCherry-expressing plasmid [26] and closely monitored the cell movement during in vitro culture. As shown in Figure 1a, corroborating the finding that migrasomes are released by rat podocytes in the opposing direction of cell migration [26], we observed that TSPAN4-positive migrasomes (arrowheads) were released along the tubular structures. In contrast, cultured renal tubular HK-2 cells, also transfected with TSPAN4-mCherry-expressing plasmid, showed neither significant TSPAN4-positive tubular extension nor migrasomes (Fig. 1b). Given that SIRPα is heavily expressed in podocytes [35], we also used SIRPα as a podocyte marker to validate migrasome secretion by HPCs. SIRPα-positive tubular structures (arrows) were found extended from podocytes, and along the tubular structures, SIRPα-positive migrasome particles (arrowheads) were observed (Fig. 1c). The dynamic process of migrasome release from a cultured podocyte was recorded (see online suppl. video; for all online suppl. material, see www.karger.com/doi/10.1159/000511504). Moreover, the scanning electron microscopy images showed numerous tubular structures protruding from the podocyte surface, and along these extended tubes, migrasomes with size of ∼500 nm were distally located from the cell boundary (Fig. 1d). Differing from exosomes, which are normally less than 100 nm in diameter, the migrasomes released from HPCs were arranged from 400 to 2,000 nm.
Fig. 1.
Cultured human podocytes release migrasomes. a, b, Migrasomes released by human podocyte (HPCs) (a) but not renal tubular HK-2 cells (b) observed by confocal fluorescence microscopy. HPCs and HK-2 cells were transfected with TSPAN4-mCherry-expressing plasmid and grown on coverslips. c Immunofluorescence staining of SIRPα in HPC observed by confocal microscopy. In panel a, c, enlarged sections were shown in the right panels, respectively. Scale bar, 25 μm. d Migrasomes released by HPC observed by SEM. Scale bar, 1 μm. e TEM images of HPC-released migrasomes adherent to the culture dish or purified from the supernatant. Scale bar, 500 nm. f Level of migrasomes secreted by HPCs and HK-2 cells in 3-h culture. HPCs, human podocyte cell lines; TEM, transmission electron microscopy; SEM, scanning electron microscopy; TSPAN4, tetraspanin-4.
As integrin α5 is enriched in migrasomes and plays a critical role in migrasome release [27], migrasomes secreted by podocytes should be much more adherent than exosomes released from the same cells. Indeed, we found that migrasomes rapidly adhered to fibronectin-coated culture plates following the release. Figure 1e shows the TEM images of adherent migrasomes (obtained after washing the culture dish) or free migrasomes (obtained from podocyte culture supernatant). Given that generation and release of migrasomes by a cell is dependent upon cell motility [26], we compared the generation and release of migrasomes in both human podocytes and renal tubular HK-2 cells. As shown in Figure 1f, compared to podocytes, HK-2 cells released significantly less migrasomes over the same 3-h duration. This observation supports the notion that podocytes possess significantly higher motility than tubular HK-2 cells.
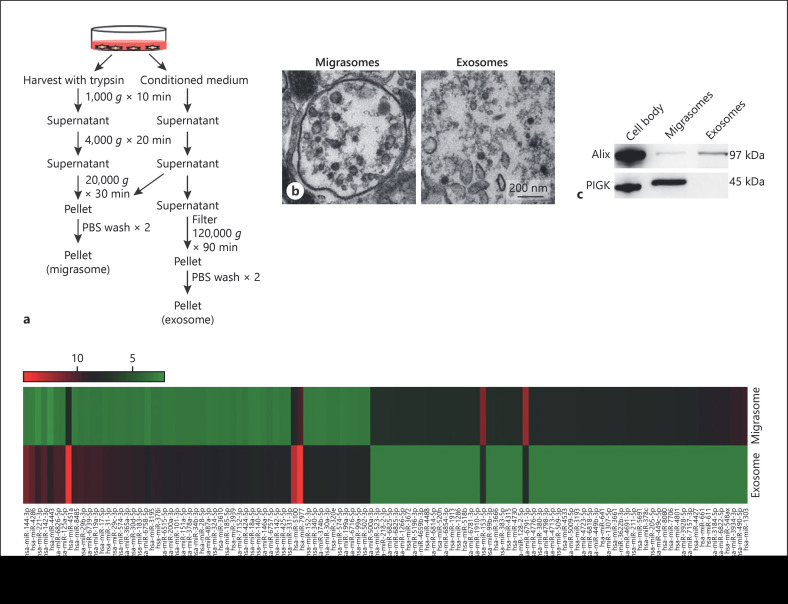
Based on the size difference between migrasomes and exosomes, we designed a simple sequential centrifugation strategy to directly isolate migrasomes and exosomes from the supernatant of cultured podocytes (Fig. 2a). As shown, following 1,000 g and 4,000 g centrifugation to remove cell debris, migrasomes were directly obtained by centrifugation at 20,000 g for 30 min. In contrast, exosomes were only harvested through ultracentrifugation (120,000 g for 90 min). The purity of isolated migrasomes and exosomes released by HPCs were validated by TEM (Fig. 2b). In agreement with a previous study showing that migrasomes contain a higher level of a class K gene (PIGK) whereas exosomes contain a higher level of Alix [29], Western blot analysis of isolated migrasomes and exosomes released by the same cultured podocytes detected a high level of PIGK in migrasomes but not in the exosomal fraction, whereas a high level of Alix was found in the exosomal fractions but not in migrasomes (Fig. 2c). Microarray data further showed a different expression profile of miRNAs in isolated podocyte migrasomes and exosomes (Fig. 2d). The detailed information of miRNA microarray can be obtained from GEO database (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE150459). For instance, significantly higher levels of miR-1303, miR-490-5p, miR-548a, miR-611, and miR-661 were detected in isolated migrasomes than in isolated exosomes, while isolated exosomes expressed more miR-144-3p, miR-221-3p, and miR-4286 than migrasomes. These results clearly suggest that migrasomes and exosomes released by podocytes are 2 distinct microvesicles.
Fig. 2.
Podocyte migrasomes and exosomes possess different protein and miRNA profiles. a Schematic illustration of migrasome and exosome purification procedure from cultured HPCs. b TEM images of migrasomes and exosomes. Scale bar, 200 nm. c Western blot analysis of HPCs (cell body), migrasomes, and exosomes using antibodies against Alix and PIGK. d Different miRNA expression profile in migrasomes and exosomes identified by miRNA microarray chip. HPCs, human podocyte cell lines; TEM, transmission electron microscopy.
Release of Migrasomes by Podocytes Is Augmented during Cell Injuries Induced by LPS, PAN, or HG
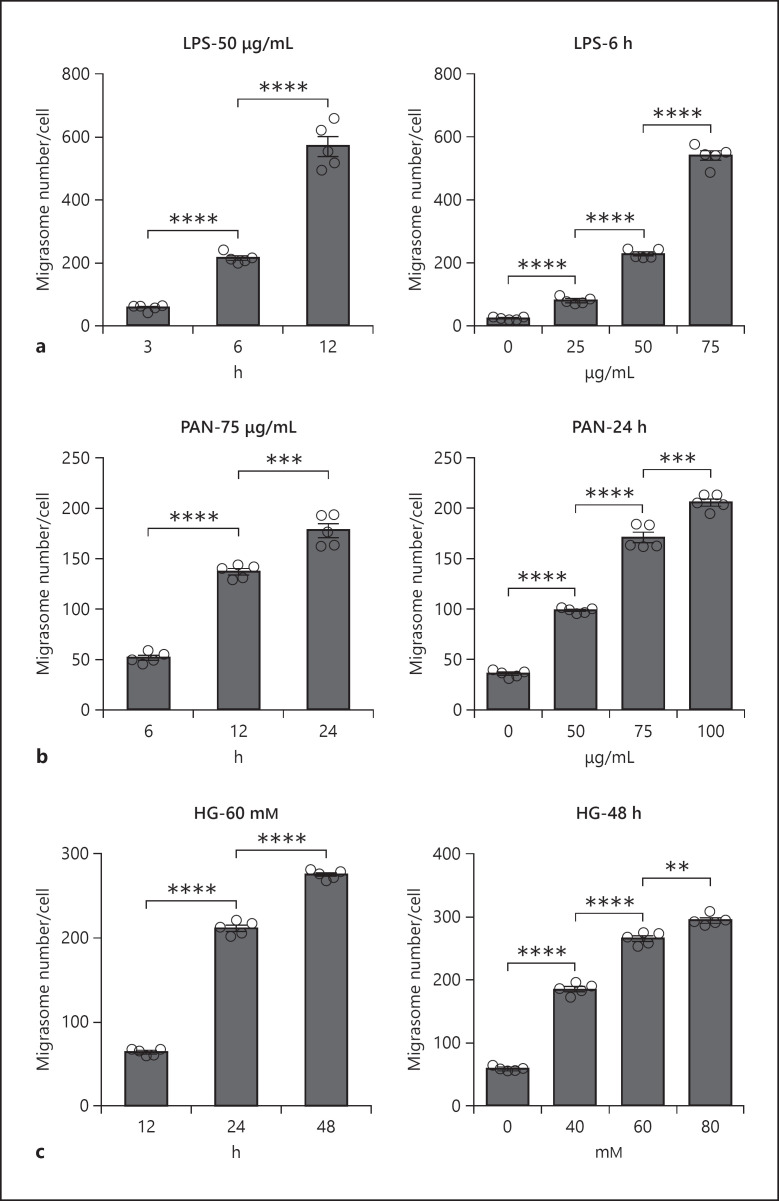
As motility of podocytes is linked to cell injury [31, 33], we next determined whether release of migrasomes by podocytes, which is positively correlated to podocyte migration, reflected podocyte injury. In this experiment, podocytes were injured by treatment with LPS, PAN, or HG at various dosages or for different durations, and the number of migrasomes released by podocytes was measured by NanoSight analysis. As shown in Figure 3, treatment with LPS (Fig. 3a), PAN (Fig. 3b), or HG (Fig. 3c) all significantly enhanced the release of migrasomes in a time-dependent and dose-dependent manner. Collectively, these results suggest that release of migrasomes from podocytes is augmented during podocyte injury.
Fig. 3.
Increased migrasome release during podocyte injury. HPCs were treated with LPS, PAN, or HG at different concentration or for different times. The number of migrasomes was detected and analyzed by NanoSight. a HPCs were treated with LPS at 50 μg/mL for different times (left panel) or various LPS concentration for 6 h (right panel). b HPCs were treated with PAN at 75 μg/mL for different times (left panel) or various concentration of PAN for 24 h (right panel). c HPCs were treated with HG at 60 mM for different times (left panel) or different concentration of glucose for 48 h (right panel). Data represent mean ± SEM, and p value was analyzed by 2-tailed Student's t test. **p < 0.01, ***p < 0.001, ****p < 0.0001. HPCs, human podocyte cell lines; PAN, puromycin amino nucleoside.
Release of Migrasomes Is Blocked by Inhibiting Rac-1 Activity in Podocytes
As podocyte migration requires a dynamic change or re-arrangement of the intracellular filament network, we next determined whether the release of migrasomes during podocyte injury is also dependent upon the intact intracellular filament network. For this experiment, migrasome release by injured podocytes was assessed in the presence of or absence of various inhibitors against filamentous actin or microtubulin. As shown in online suppl. Figure 1, migrasome release from HPCs was significantly reduced by Dynasore, an inhibitor of motor protein dynamin [36], and blebbistatin, a selective non-muscle myosin II inhibitor [37].
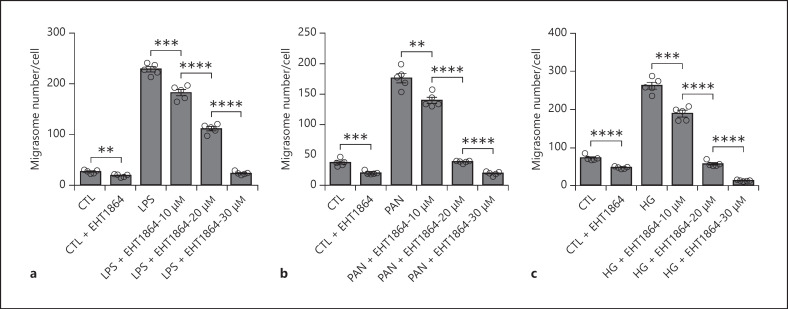
It has also been reported that Rac-1, a small Rho-family GTPase involved in modulating cellular signaling that control migration and inflammation, is over-activated during podocyte injury, and that inhibition of Rac-1 activity would protect podocyte structure and function [38, 39, 40]. To test whether Rac-1 activity is involved in migrasome release from injured podocytes, we treated podocytes with specific Rac-1 inhibitor (EHT 1864) in the presence of LPS, PAN, or HG. As shown in Figure 4, Rac-1 inhibitor dose-dependently suppressed the release of migrasomes from podocytes induced by LPS (Fig. 4a), PAN (Fig. 4b), or HG (Fig. 4c). The results strongly argue that the protective effect of Rac-1 inhibitor on podocyte injury may be due to inhibition of migrasome release by podocytes.
Fig. 4.
Rac-1 inhibitor blocks the release of migrasomes from podocytes induced by LPS, PAN, or HG. The number of migrasomes was detected and analyzed by NanoSight. a Dose-dependent inhibition of Rac-1 inhibitor (EHT 1864) on migrasome release from HPCs treated with or without LPS (50 μg/mL) for 6 h. b Dose-dependent inhibition of EHT 1864 on migrasome release from HPCs treated with or without PAN (75 μg/mL) for 24 h. c Dose-dependent inhibition of EHT 1864 on migrasome release from HPCs cultured in the normal concentration of glucose or HG (60 mM) for 48 h. Data represent mean ± SEM. p value was analyzed by 2-tailed Student's t test. **p < 0.01, ***p < 0.001, ****p < 0.0001. HPCs, human podocyte cell lines; PAN, puromycin amino nucleoside.
Urinary Migrasome Number Is a Sensitive Indicator for Early Podocyte Injury
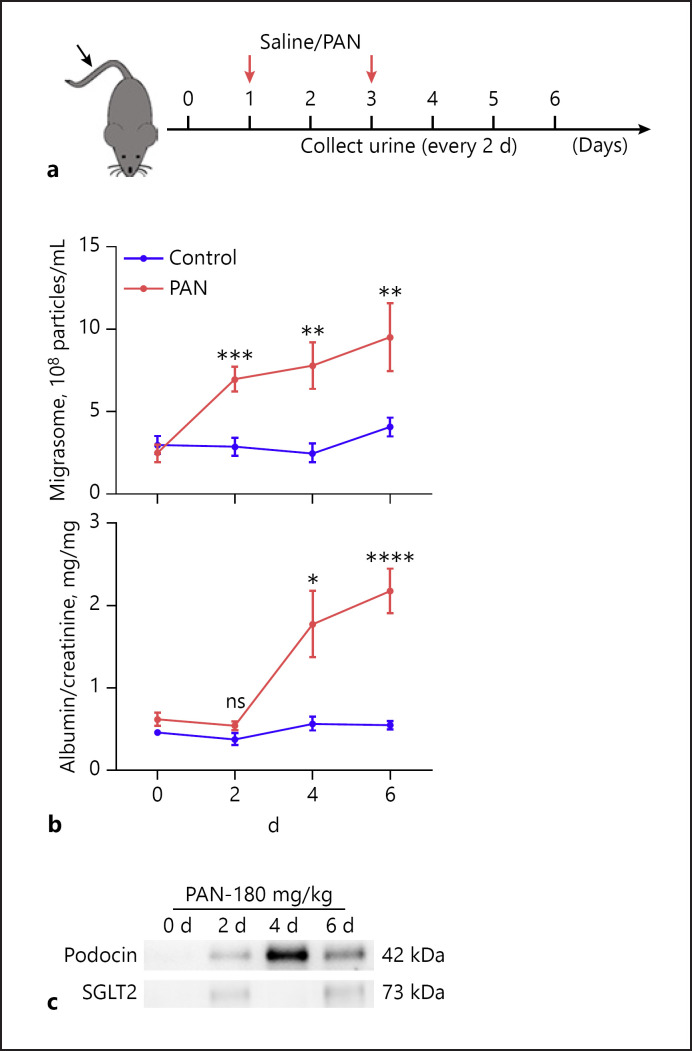
Given that migrasome release by injured podocytes is significantly increased compared to normal podocytes, we next explored whether podocyte migrasomes could be detected in mouse urine under normal or podocyte injury condition. To establish a mouse nephropathy model, mice were intravenously injected twice with PAN (180 mg/kg), with injections of saline serving as a control (Fig. 5a, red arrows). Mouse urine samples were collected every other day for migrasome, albumin, and Cr measurement. On the indicated day post-PAN treatment, mice were killed and kidneys were collected to examine renal tissue damage by transmission electronic microscopy. As shown in online suppl. Figure 2, ultrastructural analysis by TEM showed the appearance of disruption and effacement of podocyte foot processes even on day 2 after injection of PAN. PAN treatment significantly increased the level of migrasomes in mouse urine. Notably, increased level of migrasomes was detected in the urine at an earlier time point (day 2) than increased proteinuria (day 4) (Fig. 5b), suggesting that elevated levels of urinary migrasomes may serve as an indicator for early podocyte injury. Interestingly, Western blot analysis further showed that urinary migrasomes obtained from PAN-treated mice heavily expressed podocyte marker podocin but not tubular cell marker sodium-dependent glucose transporters 2 (SGLT2), implying that urinary migrasomes detected at this stage are mainly derived from mouse podocytes (Fig. 5c).
Fig. 5.
Detection of podocyte migrasomes in urine in PAN-treated mice. a Schematic of experimental design. Briefly, mice were injected with saline (control) or PAN (180 mg/kg) on day 1 and day 3 (indicated by red arrows). Urine was collected and tested on day 0, 2, 4, and 6. b Migrasome level and albumin/Cr ratio detected in control group (n = 8) and PAN group (n = 8). Data represent mean ± SEM, and p value was analyzed by 2-tailed Student's t test. ns, no significance. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. c Urinary migrasomes collected on day 2 and day 4 following PAN treatment expressed podocyte marker podocin but not tubular cell marker SGLT-2. PAN, puromycin amino nucleoside.
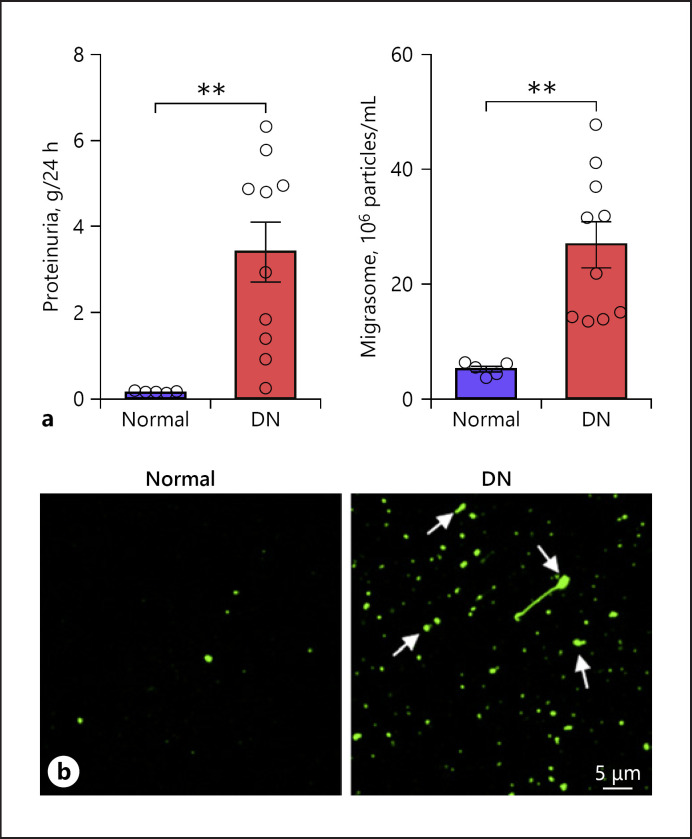
Next, elevated urinary migrasome concentration during kidney injury was validated in human urine samples. For this experiment, we collected urine samples from healthy volunteers (control group, n = 5) and patients with active DN (n = 10). The control group and DN patients had proteinuria level in a range of 0.10–0.15 and 1.57–5.55 g/day, respectively (Fig. 6a). As expected, migrasome levels in urine samples from DN patients were significantly higher than those in the control group (Fig. 6b). Given that integrin α5-containing migrasomes have high adhesive capacity [41], we also directly assessed the migrasome level in human urine samples via adhesion assay. Migrasomes isolated from the urine samples of the control group and DN patients were cultured in a fibronectin-coated culture dish for 30 min to allow adhesion. After gentle washing, adherent migrasomes were stained with PKH67 and observed under a confocal microscope. As shown in Figure 6c, significantly more PKH67-stained urinary migrasomes (arrows) were found in DN patients than in the control group.
Fig. 6.
Detection of migrasomes in urine samples from healthy volunteers (control) and patients with active DN. Urinary migrasome level was assayed and analyzed by NanoSight. a Proteinuria level of control (n = 5) and DN (n = 10) groups. b Migrasome levels in control (n = 5) and DN (n = 10) groups. c Immunofluorescence staining of migrasomes with PKH67. Migrasomes from urine samples of control group and DN group were incubated in culture dishes for 30 min for adhesion. Following gentle wash, migrasomes were then stained with PKH67. Scale bar, 5 μm. Data in a, b represent mean ± SEM, and p value was analyzed by 2-tailed Student's t test. **p < 0.01, ***p < 0.001, ****p < 0.0001. DN, diabetic nephropathy.
Discussion
In the present study, we report that podocytes can release significantly more migrasomes than renal tubular cells, and the release of migrasomes by podocytes is augmented during podocyte injury. As a markedly higher level of podocyte migrasomes in urine was detected in mice or human patients with kidney injury, our results collectively suggest that podocyte-released migrasomes in urine may serve as a non-invasive indicator for podocyte injury.
Migrasomes were first discovered by Yu's group recently [26, 27, 28]. As a new class of EVs, migrasomes are different from classic EVs released by cells, including exosomes. Supporting this, our study showed the size of migrasomes released by podocytes ranging 400–2,000 nm, which is significantly larger than ∼100-nm podocyte exosomes. In addition to the size difference, podocyte migrasomes contained different miRNAs compared to exosomes secreted by the same podocytes (Fig. 2). Morphological study also suggests that release of migrasomes and exosomes follow different pathway. Unlike exosomes which are generally stored in multivesicular bodies and released through fusion of multivesicular bodies with plasma membranes, migrasomes were released along the tubular structures that were directly extended from podocyte surface (Fig. 1). Although migrasome has just been named recently, such microvesicles might be observed previously. Analyzing EVs in urine samples from healthy donors and nephritic and nephrotic patients, Hera et al. [42] reported a subpopulation of EVs that had a mean size of over 200 nm and an absence of “exosomal” markers, such as CD24 and CD63, suggesting that these large EVs are not exosomes but migrasomes. Interestingly, 0.1- to 1.0-μm podocyte-derived EVs (termed as microparticles) were previously identified in urine samples and the urinary level of these microparticles was associated with diabetic glomerular injury [14, 15]. Given that cell shedding vesicles have a size of 100–500 nm [43], it would be difficult to separate the microparticles from migrasomes based on vesicle size. However, different from migrasomes, microvesicles, which are directly shed from cell plasma membranes, generally do not contain small vesicles inside. Employing TEM, we carefully analyzed the pellet isolated by centrifugation at 20,000 g for 30 min. The TEM data showed that >70% EVs isolated from the supernatant of cultured HPC contained small vesicles inside, suggesting that the majority of isolated EVs are migrasomes which directly link to podocyte motility and injury.
Although classic EVs particularly exosomes released by renal cells may reflect the physiologic or pathophysiologic status, the molecular basis underlying such exosomes as a potential indicator for renal cells is still missing. With different stimulation, non-injured cells can actively release exosomes. In other words, release of exosomes by renal cells such as podocytes or renal tubular cells is not an event specific for cell injury. In contrast, as generation and release of migrasomes is dependent upon cell motility, and in podocytes, cell motility is tightly correlated with cell injury, migrasomes are likely a novel class of true cell injury-related microvesicles. Therefore, the amount of migrasomes released by podocytes can directly reflect the degree of podocyte injury. Supporting the notion that generation and release of migrasomes is positively correlating with the capacity of cell motility, we compared the migrasomes released by different renal cells and found that cultured podocytes secreted significantly more migrasomes than cultured HK-2 cells. Although podocytes can possess a high capacity of motility like myeloid-derived leukocytes [44], accumulating evidence suggest that most migratory activities of podocytes, including lateral translocation, apical translocation, or even the stationary motility that is involved in podocyte cell retraction and foot process effacing [45], are associated with podocyte injury. Recent studies using in vivo multiphoton microscopy confirmed that healthy podocytes remain static and migration of podocytes only occur when podocytes are injured [46, 47]. Moreover, increased podocyte motility induced by various reagents that cause podocyte injury has been widely shown previously [34, 48, 49]. Indeed, our results showed that LPS, PAN, or HG treatment promoted migrasome release from podocytes in a time- and dose-dependent manner (Fig. 3). The amount of migrasomes released by cultured podocytes was positively correlated with the degree of cell injury. In line with previous studies showing that the dynamic cytoskeleton network plays an essential role in migrasome movement and release [26, 28], we found that release of podocyte migrasomes was significantly reduced by Dynasore and blebbistatin (online suppl. Fig. 1). Interestingly, we also observed a strong inhibitory effect of Rac-1 inhibitor on the release of podocyte migrasomes induced by LPS, PAN, or HG (Fig. 4). As Rac-1 activity plays a critical role in podocyte injury and inhibition of Rac-1 activity can protect podocytes [38, 39, 40], our results suggest that Rac-1 activity is involved in the release of podocyte migrasomes and that Rac-1-mediated release of podocyte migrasomes is a critical component of podocyte injury process. In other words, Rac-1 inhibitors may protect podocytes against various injuries via blocking the generation and secretion of podocyte migrasomes.
Another disadvantage of urinary exosomes as a non-invasive renal disease marker is the complexity of cell sources from which urinary exosomes are derived. Exosomes in urine can be derived from different renal cells as well as non-renal cells, and the originating cell source of urinary exosomes is difficult to ascertain. Therefore, alteration of whole urinary exosome level may fail to pinpoint which glomeruli cells are damaged. Unlike exosomes in urine, urinary migrasomes are mainly derived from podocytes at least at the early stage of nephropathy. As shown in Figure 5c, urinary migrasomes isolated from PAN-treated mice on day 2 and 4 posttreatment heavily expressed podocyte marker podocin but not renal tubular cell marker SGLT2. This notion of podocytes being the major cell source of urinary migrasomes is also supported by the observation that podocytes release significantly more migrasomes than other renal cells such as renal tubular HK-2 cells in vitro (Fig. 1f). Given the relevance of generation of urinary migrasomes to podocyte injury, levels of migrasomes in urine may serve as an ideal diagnostic indicator for early development of podocyte-based nephropathies.
As a novel class of microvesicles, the biological function of migrasomes, however, remains poorly understood up to date. Jiang et al. [50] recently reported that migrasomes may affect zebra fish organ morphogenesis through serving as chemoattractants to ensure the correct positioning of dorsal forerunner cells vegetally located to the embryonic shield. As podocyte migrasomes also carry different signal molecules and miRNAs and can be transferred between the cells, it would be very interesting to know whether they play a role in mediating cell-cell communication under various physiologic conditions. In conclusion, our study reveals that podocytes can release migrasomes during the injury-related migration process, and that detection of urinary migrasomes may serve as a diagnostic biomarker for early podocyte injury.
Statement of Ethics
All protocols concerning the use of patient samples in this study were approved by the Human Subjects Committee of Jinling Hospital, Nanjing University School of Medicine (2019NZKYKS-008-01). A signed consent form was obtained from each participant. The animal experiments conform to internationally accepted standards and have been approved by the Institutional Animal Care and Use Committee at Nanjing University.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This work was supported by grants from the Ministry of Science and Technology of China (2018YFA0507100), National Key Research and Development Program of China (2016YFC0901202), National Natural Science Foundation of China (31670917, 31770981, 81570644), Natural Science Foundation of Jiangsu Province (BK20170076), Project of Invigorating Health Care through Science, Technology and Education of Jiangsu Province Medical key Talent (ZDRCA2016098), and Project of Invigorating Health Care through Science, Technology, and Education of Jiangsu Province Medical key Talent (ZDRCA2016098).
Author Contributions
K.Z. and Z.H.L. designed the experiments. Y.L., S.L., W.W.R., X.D.Z., and Q.L.C. carried out the experiments. Y.L., C.H.Z., and L.M.L. analyzed data. Y.L. and S.L. made the Figure K.Z. and Y.L. wrote the manuscript.
Supplementary Material
Supplementary data
Supplementary data
Acknowledgements
The authors thank Dr. Koby Shane Kidder (Atlanta, GA) for critical reading and constructive discussion of the manuscript. All authors approved the final version of the manuscript.
References
- 1.Foster RR, Saleem MA, Mathieson PW, Bates DO, Harper SJ. Vascular endothelial growth factor and nephrin interact and reduce apoptosis in human podocytes. Am J Physiol Renal Physiol. 2005 Jan;288((1)):F48–57. doi: 10.1152/ajprenal.00146.2004. [DOI] [PubMed] [Google Scholar]
- 2.Petermann A, Floege J. Podocyte damage resulting in podocyturia: a potential diagnostic marker to assess glomerular disease activity. Nephron Clin Pract. 2007;106((2)):c61–6. doi: 10.1159/000101799. [DOI] [PubMed] [Google Scholar]
- 3.Pagtalunan ME, Miller PL, Jumping-Eagle S, Nelson RG, Myers BD, Rennke HG, et al. Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest. 1997 Jan 15;99((2)):342–8. doi: 10.1172/JCI119163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steffes MW, Schmidt D, McCrery R, Basgen JM. Glomerular cell number in normal subjects and in type 1 diabetic patients. Kidney Int. 2001 Jun;59((6)):2104–13. doi: 10.1046/j.1523-1755.2001.00725.x. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, Liu D, Chen X, Li J, Li L, Bian Z, et al. Secreted monocytic miR-150 enhances targeted endothelial cell migration. Mol Cell. 2010 Jul 9;39((1)):133–44. doi: 10.1016/j.molcel.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 6.Li J, Zhang Y, Liu Y, Dai X, Li W, Cai X, et al. Microvesicle-mediated transfer of microRNA-150 from monocytes to endothelial cells promotes angiogenesis. J Biol Chem. 2013 Aug 9;288((32)):23586–96. doi: 10.1074/jbc.M113.489302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mittelbrunn M, Gutiérrez-Vázquez C, Villarroya-Beltri C, González S, Sánchez-Cabo F, González MÁ, et al. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat Commun. 2011;2:282. doi: 10.1038/ncomms1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li D, Liu J, Guo B, Liang C, Dang L, Lu C, et al. Osteoclast-derived exosomal miR-214-3p inhibits osteoblastic bone formation. Nat Commun. 2016 Mar 7;7:10872. doi: 10.1038/ncomms10872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erdbrugger U, Le TH. Extracellular vesicles in renal diseases: more than novel biomarkers? J Am Soc Nephrol. 2016 Jan;27((1)):12–26. doi: 10.1681/ASN.2015010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Helmke A, von Vietinghoff S. Extracellular vesicles as mediators of vascular inflammation in kidney disease. World J Nephrol. 2016 Mar 6;5((2)):125–38. doi: 10.5527/wjn.v5.i2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nielsen CT, Rasmussen NS, Heegaard NH, Jacobsen S. “Kill” the messenger: targeting of cell-derived microparticles in lupus nephritis. Autoimmun Rev. 2016 Jul;15((7)):719–25. doi: 10.1016/j.autrev.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 12.Morrison EE, Bailey MA, Dear JW. Renal extracellular vesicles: from physiology to clinical application. J Physiol. 2016 Oct 15;594((20)):5735–48. doi: 10.1113/JP272182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abbasian N, Herbert KE, Pawluczyk I, Burton JO, Bevington A. Vesicles bearing gifts: the functional importance of micro-RNA transfer in extracellular vesicles in chronic kidney disease. Am J Physiol Renal Physiol. 2018 Nov 1;315((5)):F1430–F43. doi: 10.1152/ajprenal.00318.2018. [DOI] [PubMed] [Google Scholar]
- 14.Burger D, Thibodeau JF, Holterman CE, Burns KD, Touyz RM, Kennedy CR. Urinary podocyte microparticles identify prealbuminuric diabetic glomerular injury. J Am Soc Nephrol. 2014 Jul;25((7)):1401–7. doi: 10.1681/ASN.2013070763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lytvyn Y, Xiao F, Kennedy CR, Perkins BA, Reich HN, Scholey JW, et al. Assessment of urinary microparticles in normotensive patients with type 1 diabetes. Diabetologia. 2017 Mar;60((3)):581–4. doi: 10.1007/s00125-016-4190-2. [DOI] [PubMed] [Google Scholar]
- 16.Kamińska A, Platt M, Kasprzyk J, Kusnierz-Cabala B, Gala-Bladzinska A, Woznicka O, et al. Urinary extracellular vesicles: potential biomarkers of renal function in diabetic patients. J Diabetes Res. 2016;2016:5741518. doi: 10.1155/2016/5741518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De S, Kuwahara S, Hosojima M, Ishikawa T, Kaseda R, Sarkar P, et al. Exocytosis-mediated urinary full-length megalin excretion is linked with the pathogenesis of diabetic nephropathy. Diabetes. 2017 May;66((5)):1391–404. doi: 10.2337/db16-1031. [DOI] [PubMed] [Google Scholar]
- 18.Kwon SH, Woollard JR, Saad A, Garovic VD, Zand L, Jordan KL, et al. Elevated urinary podocyte-derived extracellular microvesicles in renovascular hypertensive patients. Nephrol Dial Transplant. 2017 May 1;32((5)):800–7. doi: 10.1093/ndt/gfw077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu J, Hu ZB, Chen PP, Lu CC, Zhang JX, Li XQ, et al. Urinary podocyte microparticles are associated with disease activity and renal injury in systemic lupus erythematosus. BMC Nephrol. 2019 Aug 5;20((1)):303. doi: 10.1186/s12882-019-1482-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santelli A, Sun IO, Eirin A, Abumoawad AM, Woollard JR, Lerman A, et al. Senescent kidney cells in hypertensive patients release urinary extracellular vesicles. J Am Heart Assoc. 2019 Jun 4;8((11)):e012584. doi: 10.1161/JAHA.119.012584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dimuccio V, Ranghino A, Praticò Barbato L, Fop F, Biancone L, Camussi G, et al. Urinary CD133+ extracellular vesicles are decreased in kidney transplanted patients with slow graft function and vascular damage. PLoS One. 2014;9((8)):e104490. doi: 10.1371/journal.pone.0104490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ranghino A, Bruno S, Bussolati B, Moggio A, Dimuccio V, Tapparo M, et al. The effects of glomerular and tubular renal progenitors and derived extracellular vesicles on recovery from acute kidney injury. Stem Cell Res Ther. 2017 Feb 7;8((1)):24. doi: 10.1186/s13287-017-0478-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gagnon L, Leduc M, Thibodeau JF, Zhang MZ, Grouix B, Sarra-Bournet F, et al. A newly discovered antifibrotic pathway regulated by two fatty acid receptors: GPR40 and GPR84. Am J Pathol. 2018 May;188((5)):1132–48. doi: 10.1016/j.ajpath.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 24.Burbano C, Gomez-Puerta JA, Munoz-Vahos C, Vanegas-Garcia A, Rojas M, Vasquez G, et al. HMGB1+ microparticles present in urine are hallmarks of nephritis in patients with systemic lupus erythematosus. Eur J Immunol. 2019 Feb;49((2)):323–35. doi: 10.1002/eji.201847747. [DOI] [PubMed] [Google Scholar]
- 25.Chirackal RS, Jayachandran M, Wang X, Edeh S, Haskic Z, Perinpam M, et al. Urinary extracellular vesicle-associated MCP-1 and NGAL derived from specific nephron segments differ between calcium oxalate stone formers and controls. Am J Physiol Renal Physiol. 2019 Dec 1;317((6)):F1475–F82. doi: 10.1152/ajprenal.00515.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma L, Li Y, Peng J, Wu D, Zhao X, Cui Y, et al. Discovery of the migrasome, an organelle mediating release of cytoplasmic contents during cell migration. Cell Res. 2015 Jan;25((1)):24–38. doi: 10.1038/cr.2014.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Y, Li Y, Ma L, Yu L. Detection of migrasomes. Methods Mol Biol. 2018;1749:43–9. doi: 10.1007/978-1-4939-7701-7_5. [DOI] [PubMed] [Google Scholar]
- 28.Huang Y, Zucker B, Zhang S, Elias S, Zhu Y, Chen H, et al. Migrasome formation is mediated by assembly of micron-scale tetraspanin macrodomains. Nat Cell Biol. 2019 Aug;21((8)):991–1002. doi: 10.1038/s41556-019-0367-5. [DOI] [PubMed] [Google Scholar]
- 29.Zhao X, Lei Y, Zheng J, Peng J, Li Y, Yu L, et al. Identification of markers for migrasome detection. Cell Discov. 2019;5:27. doi: 10.1038/s41421-019-0093-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee HW, Khan SQ, Faridi MH, Wei C, Tardi NJ, Altintas MM, et al. A Podocyte-based automated screening assay identifies protective small molecules. J Am Soc Nephrol. 2015 Nov;26((11)):2741–52. doi: 10.1681/ASN.2014090859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ivanova EA, Arcolino FO, Elmonem MA, Rastaldi MP, Giardino L, Cornelissen EM, et al. Cystinosin deficiency causes podocyte damage and loss associated with increased cell motility. Kidney Int. 2016 May;89((5)):1037–48. doi: 10.1016/j.kint.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 32.Widmeier E, Tan W, Airik M, Hildebrandt F. A small molecule screening to detect potential therapeutic targets in human podocytes. Am J Physiol Renal Physiol. 2017 Jan 1;312((1)):F157–F71. doi: 10.1152/ajprenal.00386.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cechova S, Dong F, Chan F, Kelley MJ, Ruiz P, Le TH. MYH9 E1841K mutation augments proteinuria and podocyte injury and migration. J Am Soc Nephrol. 2018 Jan;29((1)):155–67. doi: 10.1681/ASN.2015060707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shimo T, Adachi Y, Yamanouchi S, Tsuji S, Kimata T, Umezawa K, et al. A novel nuclear factor κB inhibitor, dehydroxymethylepoxyquinomicin, ameliorates puromycin aminonucleoside-induced nephrosis in mice. Am J Nephrol. 2013;37((4)):302–9. doi: 10.1159/000348803. [DOI] [PubMed] [Google Scholar]
- 35.Li L, Liu Y, Li S, Yang R, Zeng C, Rong W, et al. Signal regulatory protein α protects podocytes through promoting autophagic activity. JCI Insight. 2019 Mar 19;5:5. doi: 10.1172/jci.insight.124747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eleniste PP, Huang S, Wayakanon K, Largura HW, Bruzzaniti A. Osteoblast differentiation and migration are regulated by dynamin GTPase activity. Int J Biochem Cell Biol. 2014 Jan;46:9–18. doi: 10.1016/j.biocel.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duxbury MS, Ashley SW, Whang EE. Inhibition of pancreatic adenocarcinoma cellular invasiveness by blebbistatin: a novel myosin II inhibitor. Biochem Biophys Res Commun. 2004 Jan 23;313((4)):992–7. doi: 10.1016/j.bbrc.2003.12.031. [DOI] [PubMed] [Google Scholar]
- 38.Babelova A, Jansen F, Sander K, Löhn M, Schäfer L, Fork C, et al. Activation of Rac-1 and RhoA contributes to podocyte injury in chronic kidney disease. PLoS One. 2013;8((11)):e80328. doi: 10.1371/journal.pone.0080328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gee HY, Saisawat P, Ashraf S, Hurd TW, Vega-Warner V, Fang H, et al. ARHGDIA mutations cause nephrotic syndrome via defective RHO GTPase signaling. J Clin Invest. 2013 Aug;123((8)):3243–53. doi: 10.1172/JCI69134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robins R, Baldwin C, Aoudjit L, Côté JF, Gupta IR, Takano T. Rac1 activation in podocytes induces the spectrum of nephrotic syndrome. Kidney Int. 2017 Aug;92((2)):349–64. doi: 10.1016/j.kint.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 41.Wu D, Xu Y, Ding T, Zu Y, Yang C, Yu L. Pairing of integrins with ECM proteins determines migrasome formation. Cell Res. 2017 Nov;27((11)):1397–400. doi: 10.1038/cr.2017.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hara M, Yanagihara T, Hirayama Y, Ogasawara S, Kurosawa H, Sekine S, et al. Podocyte membrane vesicles in urine originate from tip vesiculation of podocyte microvilli. Hum Pathol. 2010 Sep;41((9)):1265–75. doi: 10.1016/j.humpath.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 43.van Niel G, D'Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018 Apr;19((4)):213–28. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 44.May CJ, Saleem M, Welsh GI. Podocyte dedifferentiation: a specialized process for a specialized cell. Front Endocrinol. 2014;5:148. doi: 10.3389/fendo.2014.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Endlich N, Siegerist F, Endlich K. Are podocytes motile? Pflugers Arch. 2017 Aug;469((7–8)):951–7. doi: 10.1007/s00424-017-2016-9. [DOI] [PubMed] [Google Scholar]
- 46.Peti-Peterdi J, Sipos A. A high-powered view of the filtration barrier. J Am Soc Nephrol. 2010;21((11)):1835–41. doi: 10.1681/ASN.2010040378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hackl MJ, Burford JL, Villanueva K, Lam L, Suszták K, Schermer B, et al. Tracking the fate of glomerular epithelial cells in vivo using serial multiphoton imaging in new mouse models with fluorescent lineage tags. Nat Med. 2013;19((12)):1661–6. doi: 10.1038/nm.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Srivastava T, Sharma M, Yew KH, Sharma R, Duncan RS, Saleem MA, et al. LPS and PAN-induced podocyte injury in an in vitro model of minimal change disease: changes in TLR profile. J Cell Commun Signal. 2013 Mar;7((1)):49–60. doi: 10.1007/s12079-012-0184-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lv Z, Hu M, Ren X, Fan M, Zhen J, Chen L, et al. Fyn mediates high glucose-induced actin cytoskeleton reorganization of podocytes via promoting ROCK activation in vitro. J Diabetes Res. 2016;2016:5671803. doi: 10.1155/2016/5671803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jiang D, Jiang Z, Lu D, Wang X, Liang H, Zhang J, et al. Migrasomes provide regional cues for organ morphogenesis during zebrafish gastrulation. Nat Cell Biol. 2019 Aug;21((8)):966–77. doi: 10.1038/s41556-019-0358-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data