Abstract
Introduction
Although collecting faeces and blood samples are considered non-invasive methods of monitoring Crohn's disease (CD), these methods are less preferred by some patients. This study utilized urine as an alternative to evaluate four disease biomarkers in young adults with active CD before and after exclusive enteral nutrition (EEN) therapy.
Methods
Urine samples collected at baseline (W0) and after 8 weeks (W8) of EEN therapy were assayed by ELISA for levels of intestinal fatty acid-binding protein (I-FABP), liver fatty acid-binding protein (L-FABP), claudin-3, and calprotectin. Levels of each biomarker were also compared with standard clinical parameters, including disease indexes, nutrient, and inflammatory markers.
Results
Of the paired urine samples from 14 patients, 10 were female and 12 were newly diagnosed with CD. Urinary I-FABP: Cr (standardized to urine Cr) levels were significantly reduced, while urinary L-FABP: Cr levels increased following EEN therapy. Urinary L-FABP: Cr correlated positively with serum insulin-like growth factor 1 (IGF-1) (r = 0.60, p = 0.02). Urinary CLND3: Cr and calprotectin: Cr levels were not significantly different after treatment.
Conclusion
I-FABP is a potential urinary biomarker of disease activity in adults with CD, while urinary L-FABP may be an indirect marker of nutritional status in adults with CD. CLND3 and calprotectin do not appear to have roles as urinary biomarkers in CD. These findings warrant further investigations using a larger sample size.
Keywords: Crohn's disease, Calprotectin, Claudin-3, Intestinal fatty acid-binding protein, Liver fatty acid-binding protein, Urine
Introduction
Crohn's disease (CD), one of the main types of inflammatory bowel disease (IBD), is a life-long relapsing-remitting condition characterized by inflammation of the gastrointestinal tract (GIT), most commonly involving the ileum and colon [1]. After diagnosis, the initial treatment goal is to induce remission, followed by maintenance of disease remission and the prevention of complications. Exclusive enteral nutrition (EEN) is commonly used to induce remission in children with CD [2]. This therapy, which typically involves solely drinking a nutritionally complete liquid formula for up to 8 weeks, also has the potential to treat CD in young adults [3].
The current treat-to-target goal for CD is to achieve endoscopic resolution of mucosal ulceration, termed mucosal healing [4], which is associated with sustained clinical remission and reduced hospitalization and surgery [5, 6, 7]. However, repeat endoscopy is limited by invasiveness, cost, and patient preference [8]. Less invasive tests such as serum C-reactive protein (CRP) and faecal calprotectin (FC) have been validated against endoscopic assessment with modest sensitivities and specificities [9, 10]. The collection of urine samples is reportedly more convenient and more acceptable to patients than faeces collection [11]. To date, however, little consideration has been given to the measurement of biomarkers in urine as an alternative way to monitor CD non-invasively. The current pilot study aimed to assess the utility of four candidate biomarkers (intestinal fatty acid-binding protein [I-FABP, also known as FABP2], liver fatty acid-binding protein [L-FABP, also known as FABP1], claudin-3 [CLDN3], and calprotectin) in urine samples previously collected from young adults with active CD before and after EEN therapy.
Materials and Methods
Participants
This was a sub-study of a previously reported prospective clinical trial involving young adults aged between 16 and 40 years with active CD from Christchurch Hospital, New Zealand, treated with EEN [3]. Diagnosis of CD was based on the Montreal classification [12], with eligible patients required to have at least ileal disease. Active disease was defined as disease visible endoscopically or noted radiologically. Study exclusion criteria were as reported previously [3]. Concomitant use of 5-aminosalicylic acid, biologic agents, or immunomodulators was allowed as long as the dosage was stable. Maintenance medical therapy, such as a thiopurine, could be introduced after four weeks of EEN treatment.
Standard baseline (W0) clinical disease assessments were taken prior to patients starting EEN and again after eight weeks (W8) of EEN treatment [3]. These indicators included weight, BMI, Harvey-Bradshaw Index (HBI), Crohn's Disease Activity Index (CDAI), serum insulin-like growth factor 1 (IGF-1), CRP, erythrocyte sedimentation rate (ESR), and FC. IGF-1 and CRP were measured by a reference laboratory. FC was measured using BÜHLMANN fCAL® ELISA (BÜHLMANN Laboratories AG, Schönenbuch, Switzerland) as per the manufacturer's instructions. BMI was calculated using weight in kilograms divided by height in metres squared.
All enrolled patients gave their written informed consent. This study was approved by the New Zealand Northern B Health and Disability Ethics Committee (reference: 13/NTB/11) and registered with the Australia New Zealand Clinical Trial Registry (trial number: 363665).
EEN Treatment
As previously described, the patients were required to drink only polymeric nutritional formula (Ensure® Plus; Abbott Laboratories, The Netherlands) for 8 weeks. A dietician regularly reviewed participants' nutritional requirements throughout the treatment and adjusted their intake accordingly. Additional fluids, such as water and/or unsweetened black tea, coffee or herbal tea, were allowed.
Urine Samples
Urine samples were collected at W0 and W8. Prior to storage at −80 Celsius, 100 μL of sodium azide 0.02% was added per 10 mL of urine. Thawed urines were centrifuged at 1,000 g for 20 min prior to testing and the supernatant used for the specific biomarker ELISAs. Cr levels in each supernatant were measured by a reference laboratory (using Abbott reagent on the chemistry analyzer; Abbott Architect c16000 Analyzer, Abbott Park, IL, USA), as a means to standardize urine concentrations [13].
Measurement of Urinary Biomarkers
Urinary levels of each biomarker were determined (in duplicate) using commercially available ELISA kits that detect human I-FABP (Hycult®Biotech, Uden, The Netherlands), L-FABP (Hycult®Biotech), CLDN3 (MyBioSource.com, San Diego, CA, USA), and calprotectin (Immundiagnostik, Bensheim, Germany). Assays were performed according to each manufacturer's instructions.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism 8 version 8.3.0 (GraphPad Software, San Diego, CA, USA). Descriptive results are presented with median (interquartile range, [IQR]), with continuous variables analyzed using Wilcoxon matched pairs signed rank test and Spearman's r for correlations. p < 0.05 was assumed to be statistically significant.
Results
Characteristics of Participants
Fourteen patients completed 8 weeks of EEN treatment with paired urine at W0 and W8 (Table 1). Ten of these patients were female and 12 were newly diagnosed with CD. Nine patients had ileocolonic involvement and five had isolated terminal ileal disease. Two also had upper gastrointestinal involvement. One patient had multiple GIT strictures. The remaining 13 patients had inflammatory disease only.
Table 1.
Background characteristics of patients with CD treated with EEN
| N = 14 | N = 7a | |
|---|---|---|
| Age, median (IQR), years | 25.04 (20.45–33.06) | 23.04 (15.58–26.89) |
| Female, N (%) | 10 (71) | 4 (57) |
| Ethnicity, N | ||
| New Zealand European | 14 | 7 |
| Duration of disease, median (range), years | 0 (0–14.43) | 0 (0–0) |
| New diagnosis, N (%) | 12 (86) | 7 (100) |
| Disease location, N | ||
| Terminal ileum (L1) | 5 | 3 |
| Colon (L2) | − | − |
| Ileocolonic (L3) | 9 | 4 |
| Upper GI (L4) | 2 | 1 |
| L1 + L4 | − | − |
| L3 + L4 | 2 | 1 |
| Disease behaviour, N | ||
| Non-stricturing/penetrating (B1) | 13 | 7 |
| Stricturing (B2) | 1 | − |
| Penetrating (B3) | − | − |
| Perianal | − | − |
| Concurrent medication, N | ||
| None | 5 | 2 |
| Antibioticb | 1 | − |
| 5-ASA | 6 | 4 |
| Immunomodulator | 2 | 1 |
| Biologic agent | 1 | − |
EEN, exclusive enteral nutrition; 5-ASA, 5-aminosalicylic acid; IQR, interquartile range; L-FABP, liver fatty acid-binding protein; N, sample size.
Patients tested for urinary L-FABP.
Doxycycline.
Median (IQR) of CDAI and HBI clinical disease activity indexes reduced significantly with EEN treatment (W0 vs. W8), 137.5 (100.8–176.0) versus 53.5 (35.3–76.5) and 4 (2.8–6.0) versus 1 (0.0–2.0), respectively (p < 0.01 for each comparison) (Table 2). In contrast, median serum IGF-1 levels increased significantly post-treatment (156.0 [138.8–174.0] versus 180.0 [154.5–225.3] μg/L, p = 0.01). Inflammatory markers including FC, CRP, and ESR were not statistically different, whereas median weight and BMI reduced over the 8 weeks (Table 2).
Table 2.
Clinical parameters of patients, (N = 14) at baseline (W0) and after EEN treatment (W8)
| W0, median (IQR) | W8, median (IQR) | p value | |
|---|---|---|---|
| Weight, kg | 64.9 (57.9–83.8) | 63.6 (58.6–78.4) | 0.01 |
| BMI, kg/m2 | 23.7 (21.5–26.3) | 23.3 (20.7–25.2) | 0.01 |
| CDAI | 137.5 (100.8–176.0) | 53.5 (35.3–76.5) | <0.01 |
| HBI | 4 (2.8–6.0) | 1 (0.0–2.0) | <0.01 |
| Cr, g/L | 1.0 (0.4–2.4) | 2.2 (1.1–3.1) | NS |
| IGF-1, µg/L | 156.0 (138.8–174.0) | 180.0 (154.5–225.3) | 0.01 |
| FC, µg/g | 895.0 (225.0–1,496) | 557.5 (161.5–1,353.0) | NS |
| CRPa, mg/L | 14.0 (7.5–23.0) | 11.0 (6.0–19.5) | NS |
| ESRa, mm/h | 7.0 (3.5–24.5) | 3.0 (3.0–10.0) | NS |
EEN, exclusive enteral nutrition; FC, faecal calprotectin; CDAI, Crohn's Disease Activity Index; HBI, Harvey-Bradshaw Index; IQR, interquartile range; IGF-1, insulin-like growth factor 1; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; N, sample size; NS, not statistically significant.
Sample size is 13 patients.
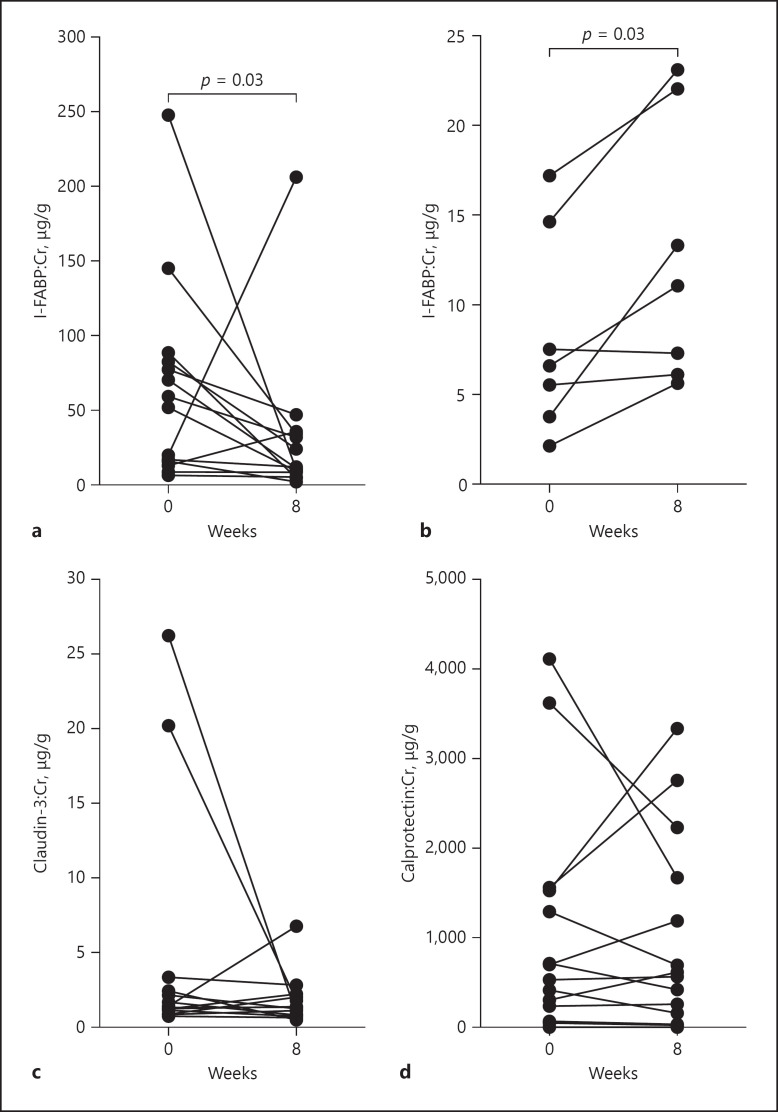
Urinary I-FABP
Urinary I-FABP: Cr (standardized to urine Cr) levels reduced significantly with EEN treatment; median (IQR) 55.7 (15.1–84.1) versus 11.7 (7.8–34.5) ng/g, p = 0.03 (Fig. 1a). No significant correlations between urine I-FABP: Cr and standard clinical parameters (HBI, CDAI, serum IGF-1, CRP, ESR, and FC) were found (data not shown).
Fig. 1.
Novel urinary biomarkers standardized to Cr measured at baseline and after 8 weeks of EEN treatment (assessed using Wilcoxon matched-pairs signed rank test). a Urinary (I-FABP) levels (N = 14) were significantly reduced (p = 0.03). b Urinary L-FABP levels measured in 7 patients were significantly elevated (p = 0.03). c, d Urinary CLDN3 and urinary calprotectin levels (N = 14) were unchanged. EEN, exclusive enteral nutrition; I-FABP, intestinal fatty acid-binding protein; L-FABP, liver fatty acid-binding protein; CLDN3, claudin-3.
Urinary L-FABP
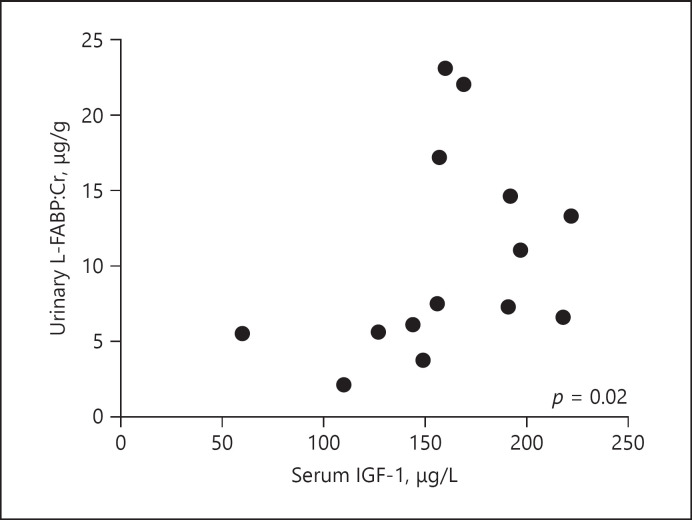
Lack of samples meant that urinary L-FABP was only able to be measured in samples from 7 of the 14 patients (Table 1). The results showed that urinary L-FABP: Cr levels increased significantly following EEN treatment (median 6.6 [3.8–14.6] versus 11.1 [6.1–22.0] μg/g, p = 0.03) (Fig. 1b). L-FABP: Cr levels in urine correlated positively with serum IGF-1 levels (W0 and W8), r = 0.60, p = 0.02 (Fig. 2). Apart from IGF-1, no other correlations between urine L-FABP: Cr and standard clinical parameters (HBI, CDAI, CRP, ESR, and FC) were found (data not shown).
Fig. 2.
Urinary levels of L-FABP, standardized to Cr, correlated positively with serum IGF-1 levels in the W0 and W8 samples from each of the 7 patients tested (Spearman's r = 0.60, p = 0.02). IGF-1, insulin growth-like factor 1; L-FABP, liver fatty acid-binding protein.
Urinary CLDN-3
Following Cr standardization, urine CLN3: Cr levels were not significantly different following treatment (median 1.4 [1.1–2.7] versus 1.3 [0.8–2.1] μg/g, p > 0.05) (Fig. 1c). In addition, there were no significant correlations with standard clinical parameters (HBI, CDAI, serum IGF-1, CRP, ESR, and FC) (data not shown). This did not change when sub-analysis of those patients with L3 disease phenotype (N = 9) was performed to take into account that CLD3 is predominantly expressed in the large intestine [14] (data not shown).
Urinary Calprotectin
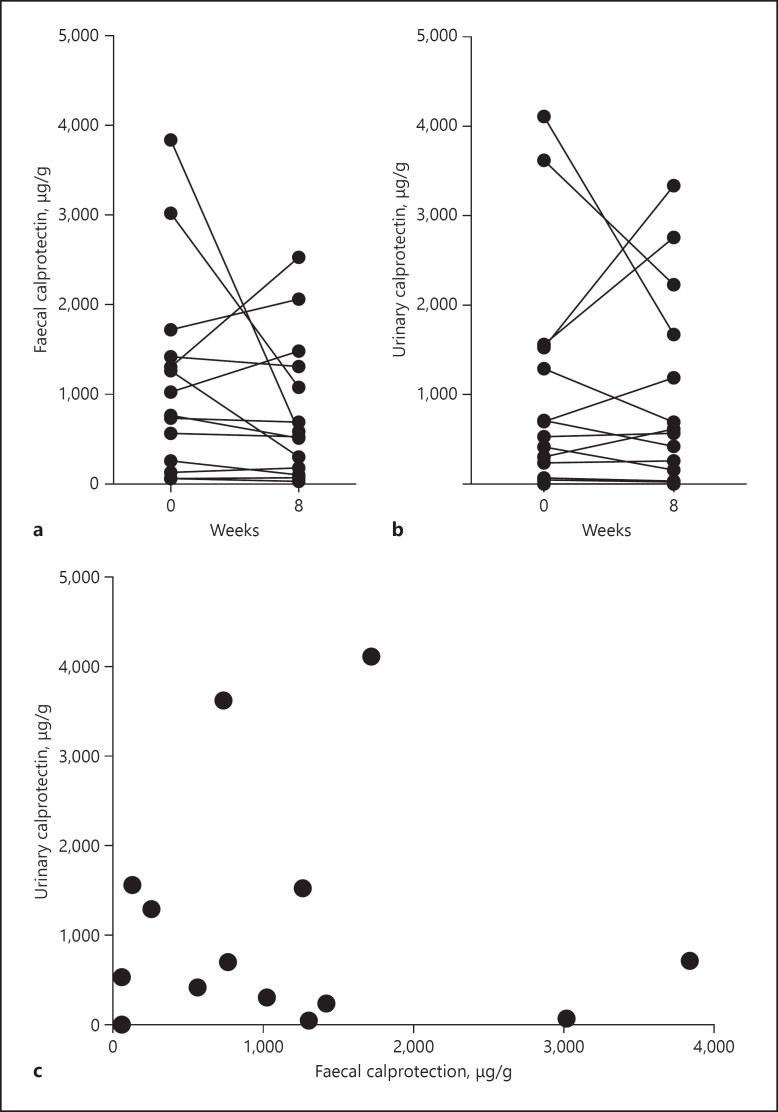
Urinary calprotectin standardized to Cr did not change significantly after EEN treatment (median 615.0 [194.9–1,535] versus 591.1 [125.9–1,811] μg/g, p > 0.05) (Fig. 1d). For comparison, urinary calprotectin: Cr levels were compared to FC levels in the same patients. Although urinary calprotectin: Cr values showed a similar trend to FC, these 2 biomarkers were not significantly correlated (Fig. 3). Further, there was no significant correlation between urinary calprotectin: Cr and other clinical parameters (HBI, CDAI, serum IGF-1, CRP, ESR, and FC) (data not shown).
Fig. 3.
Comparison between faecal and urinary calprotectin. a, b Faecal and urinary calprotectin levels (the latter standardized to Cr), measured in 14 patients, were unchanged after EEN treatment (assessed using Wilcoxon matched-pairs signed rank test). c No correlation between urine calprotectin: Cr and FC at W0, Spearman's r = 0.002, p = 0.99 was observed. EEN, exclusive enteral nutrition; FC, faecal calprotectin.
Discussion
The present study provides a proof of concept that urine, when standardized with Cr to take into account variations in protein concentration, can potentially be used as an alternative non-invasive biological fluid to measure disease biomarkers in patients with CD. Moreover, the observed reduction in I-FABP levels and concomitant elevation of L-FABP in urine following EEN therapy may each have a different role in monitoring the disease. In contrast, urinary CLDN3 and calprotectin were not useful in this cohort and do not appear to be useful as urinary biomarkers.
I-FABP and L-FABP (13–14 kDa) are 2 of the 9 known cytoplasmic proteins that regulate long-chain fatty acid transportation intracellularly, as well as having their own tissue-specific functions [15]. Both are expressed along the GIT, with mature enterocytes in the jejunal villi generating higher levels than colonocytes [16]. In contrast to I-FABP, which is only expressed in intestinal tissue, L-FABP is also found in hepatocytes, renal tubular cells, pancreas, and lung [17]. When enterocytes are damaged, L-FABP is released into the blood circulation and cleared rapidly by the kidneys (plasma half-life of 11 min) [18]. Given the close molecular resemblance of I-FABP to L-FABP [19], it is presumed both proteins have similar circulating half-lives.
The current study determined that urinary I-FABP levels reduced following 8 weeks of EEN therapy in young adults with active CD, suggesting this may be a sensitive indicator of disease activity. In contrast to these findings, another study evaluating urinary and plasma I-FABP levels in 5 children with CD before and after EEN treatment did not show any differences from levels measured in control children [20]. The reason for this difference is not clear.
Although urinary I-FABP did not correlate with any of the standard clinical CD markers in the current study, other studies have shown that serum I-FABP levels correlate with CDAI, CRP [21], and tumour necrosis factor-α levels [22], but not endoscopic disease activity [23]. Collectively, these findings suggest that I-FABP may reflect disease activity in patients with CD. Future studies should include direct comparison of urinary I-FABP to endoscopic disease activity.
Another key finding from the present study is the elevation of urinary L-FABP and positive correlation with IGF-1 levels following EEN treatment. There is evidence that IGF-1 levels are low in patients with IBD, reportedly attributed to malnutrition and/or GIT inflammation [24, 25, 26]. Furthermore, levels of IGF-1 increase within 1–2 weeks in children and adults with active CD following EEN therapy [3, 27, 28]. Moreover, this increase in IGF-1 levels is concomitant with reduced inflammatory markers (including CRP, ESR, and FC levels) and improved nutritional status. IGF-1 and L-FABP are both expressed by the liver; thus, the correlation seen in the present study between increased levels of urinary L-FABP and serum levels of IGF-1 suggests a potential role for urinary L-FABP levels as a non-invasive, indirect marker of nutritional status. However, this is tempered by the finding that L-FABP is also considered a potential diagnostic marker in other conditions that include non-alcoholic fatty liver disease [29] and various renal diseases [30]. Hence, future research is needed to more fully interpret urinary L-FABP levels in the context of a patient's response to EEN.
CLDN3 is a 22-kDa transmembrane protein that forms part of the complex intestinal tight junctions between epithelial cells. Expressed predominately in the colon [14], CLDN3 levels are reduced in colonocytes of patients with active CD and when colonocytes are exposed to tumour necrosis factor-α [31, 32]. Thuijls et al. [32] also correlated loss of CLDN3 in the GIT to increased CLDN3 levels in urine, suggesting that CLDN3 released into the circulation during active CD is cleared by the kidneys. The present study, however, found low urinary CLDN3 levels at baseline that did not change following EEN therapy. This finding was retained despite further analysis of urinary CLDN3: Cr levels in the patients with ileocolonic phenotype. These variations may reflect inter-study differences in the choice of assay used to measure CLDN3.
Calprotectin is a 36-kDa heterodimer of the S100A8 and S100A9 proteins, both members of the S100 calcium-binding family. It is predominantly expressed in neutrophils, to a lesser extent in monocytes and macrophages and found in various body fluids [33, 34]. Typically, calprotectin is released in response to infection or inflammation. Calprotectin in faeces has been studied extensively in the setting of IBD. A meta-analysis evaluated 13 studies, including 744 patients with UC and 727 with CD and found that FC had a higher area under the curve for UC than CD, 0.93 (95% confident interval [CI] 0.89–0.97) and 0.88 (95% CI 0.83–0.93), respectively [35]. However, while serum calprotectin levels in patients with IBD, in particular CD, correlate with CRP, this does not extend to a correlation with FC levels and/or active endoscopic disease [36, 37, 38]. This suggests that serum (and consequently urinary) levels of calprotectin may be more useful as biomarkers of systemic inflammation than specifically of GIT inflammation. Although there are few supportive data, 1 study does report no difference in urinary calprotectin levels in children suspected of having appendicitis (including those with gangrenous or perforated appendicitis) and a control group without appendicitis [39].
The current study has several limitations. First, the study sample size is small, and the cohort presented with relatively mild disease activity. The inclusion of more subjects with a wider range of disease activity would be helpful. Second, the same urinary biomarkers were not measured in urine samples from age-matched healthy controls, so no comparison can be made between levels of each protein in health and disease. Third, this study measured the novel biomarker levels in urine, without comparing to serum or plasma levels. In addition, W8 mucosal healing data were not available for direct comparison to the urinary biomarkers at that time. Finally, the study findings may not be able to be extrapolated to older adults or to individuals with renal conditions, prolonged fasting, or excessive meat consumption where Cr may be elevated or reduced. In contrast, the inclusion criteria of the study ensured selection of young adults with no significant past medical history, including liver disease, thereby reducing additional confounding factors.
In conclusion, this pilot study provides preliminary data on the utility of specific urinary biomarkers in the setting of CD. I-FABP is a potential marker of disease activity and L-FABP may be an indirect nutritional marker. These findings warrant further studies involving larger populations to further explore these biomarkers.
Statement of Ethics
This research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. All enrolled patients gave their written informed consent. This study was approved by the New Zealand Northern B Health and Disability Ethics Committee (reference: 13/NTB/11) and registered with the Australia New Zealand Clinical Trial Registry (trial number: 363665).
Conflict of Interest Statement
All authors have no conflict of interest to declare.
Funding Sources
This research was supported by a project grant from the New Zealand Society of Gastroenterology. Additional overall support was from Cure Kids to ASD. Both organizations have no role in the preparation of data or the manuscript.
Author Contributions
S.S.H. was involved in study design, completed laboratory experiments, analyzed and interpreted the data, and wrote the initial draft of the manuscript. C.W. was involved in study conception, patient recruitment, and sample collection. R.G. was involved in study conception and supervision. J.K. was involved in study conception, study design, guided study methods, and contributed to analysis and interpretation of the data. A.S.D. was involved in study conception and design, analysis and interpretation of data arising, and supervision. All authors were involved in the critical review of the manuscript, and all have approved and are accountable for the final version of the manuscript.
Acknowledgement
We would like to acknowledge Freemasons New Zealand for their support of SSH via the Freemasons Paediatric Postgraduate Scholarship.
References
- 1.Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361((21)):2066–78. doi: 10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ho SSC, Day AS. Exclusive enteral nutrition in children with inflammatory bowel disease: physician perspectives and practice. J Gastroenterol Hepatol Open. 2019;3((2)):148–53. doi: 10.1002/jgh3.12121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wall CL, Gearry RB, Day AS. Treatment of active crohn's disease with exclusive and partial enteral nutrition: a pilot study in adults. Inflamm Intest Dis. 2018;2((4)):219–27. doi: 10.1159/000489630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peyrin-Biroulet L, Sandborn W, Sands BE, Reinisch W, Bemelman W, Bryant RV, et al. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): determining therapeutic goals for treat-to-target. Am J Gastroenterol. 2015;110((9)):1324–38. doi: 10.1038/ajg.2015.233. [DOI] [PubMed] [Google Scholar]
- 5.Pineton de Chambrun G, Peyrin-Biroulet L, LémannLemann M, Colombel JF. Clinical implications of mucosal healing for the management of IBD. Nat Rev Gastroenterol Hepatol. 2010;7((1)):15–29. doi: 10.1038/nrgastro.2009.203. [DOI] [PubMed] [Google Scholar]
- 6.Shah SC, Colombel JF, Sands BE, Narula N. Mucosal healing is associated with improved long-term outcomes of patients with ulcerative colitis: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2016;14((9)):1245–e8. doi: 10.1016/j.cgh.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 7.Shah SC, Colombel JF, Sands BE, Narula N. Systematic review with meta-analysis: mucosal healing is associated with improved long-term outcomes in Crohn's disease. Aliment Pharmacol Ther. 2016;43((3)):317–33. doi: 10.1111/apt.13475. [DOI] [PubMed] [Google Scholar]
- 8.Papay P, Ignjatovic A, Karmiris K, Amarante H, Milheller P, Feagan B, et al. Optimising monitoring in the management of Crohn's disease: a physician's perspective. J Crohns Colitis. 2013;7((8)):653–69. doi: 10.1016/j.crohns.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 9.Mosli MH, Zou G, Garg SK, Feagan SG, MacDonald JK, Chande N, et al. C-reactive protein, fecal calprotectin, and stool lactoferrin for detection of endoscopic activity in symptomatic inflammatory bowel disease patients: a systematic review and meta-analysis. Am J Gastroenterol. 2015;110((6)):802–20. doi: 10.1038/ajg.2015.120. [DOI] [PubMed] [Google Scholar]
- 10.Rokkas T, Portincasa P, Koutroubakis IE. Fecal calprotectin in assessing inflammatory bowel disease endoscopic activity: a diagnostic accuracy meta-analysis. J Gastrointestin Liver Dis. 2018;27((3)):299–306. doi: 10.15403/jgld.2014.1121.273.pti. [DOI] [PubMed] [Google Scholar]
- 11.Ho SSC, Keenan JI, Day AS. Adult perceptions of Inflammatory bowel disease diagnostic and monitoring tests [abstract] New Zealand Society of Gastroenterology Annual Scientific Meeting. 2019.
- 12.Silverberg MS, Satsangi J, Ahmad T, Arnott ID, Bernstein CN, Brant SR, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19((Suppl A)):5A–36A. doi: 10.1155/2005/269076. [DOI] [PubMed] [Google Scholar]
- 13.Needleman SB, Porvaznik M, Ander D. Creatinine analysis in single collection urine specimens. J Forensic Sci. 1992;37((4)):1125–33. [PubMed] [Google Scholar]
- 14.Lameris AL, Huybers S, Kaukinen K, MäkeläMakela TH, Bindels RJ, Hoenderop JG, et al. Expression profiling of claudins in the human gastrointestinal tract in health and during inflammatory bowel disease. Scand J Gastroenterol. 2013;48((1)):58–69. doi: 10.3109/00365521.2012.741616. [DOI] [PubMed] [Google Scholar]
- 15.Ho SSC, Keenan JI, Day AS. The role of gastrointestinal-related fatty cid-binding proteins as biomarkers in gastrointestinal diseases. Dig Dis Sci. 2020;65((2)):376–90. doi: 10.1007/s10620-019-05841-x. [DOI] [PubMed] [Google Scholar]
- 16.Pelsers MM, Namiot Z, Kisielewski W, Namiot A, Januszkiewicz M, Hermens WT, et al. Intestinal-type and liver-type fatty acid-binding protein in the intestine. Tissue distribution and clinical utility. Clin Biochem. 2003;36((7)):529–35. doi: 10.1016/s0009-9120(03)00096-1. [DOI] [PubMed] [Google Scholar]
- 17.Furuhashi M, Hotamisligil GS. Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets. Nat Rev Drug Discov. 2008;7((6)):489–503. doi: 10.1038/nrd2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van de Poll MC, Derikx JP, Buurman WA, Peters WH, Roelofs HM, Wigmore SJ, et al. Liver manipulation causes hepatocyte injury and precedes systemic inflammation in patients undergoing liver resection. World J Surg. 2007;31((10)):2033–8. doi: 10.1007/s00268-007-9182-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Storch J, Thumser AE. Tissue-specific functions in the fatty acid-binding protein family. J Biol Chem. 2010;285((43)):32679–83. doi: 10.1074/jbc.R110.135210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clark CM, Kountouri A, MacKinder M, Hansen R, Russell RK, Gerasimidis K. Intestinal fatty acid binding protein as a biomarker of intestinal damage in children with coeliac and Crohn's disease. J Crohns Colitis. 2016;10((Suppl 1)):S129. [Google Scholar]
- 21.Sarikaya M, Ergül B, Doğan Z, Filik L, Can M, Arslan L. Intestinal fatty acid binding protein (I-FABP) as a promising test for Crohn's disease: a preliminary study. Clin Lab. 2015;61((1–2)):87–91. doi: 10.7754/clin.lab.2014.140518. [DOI] [PubMed] [Google Scholar]
- 22.Al-Saffar AK, Meijer CH, Gannavarapu VR, Hall G, Li Y, Diaz Tartera HO, et al. Parallel changes in harvey-bradshaw index, TNF. Gastroenterol Res Pract. 2017;2017:1745918. doi: 10.1155/2017/1745918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bodelier AGL, Pierik MJ, Lenaerts K, de Boer E, Olde Damink SW, Hameeteman WM, et al. Plasma intestinal fatty acid-binding protein fails to predict endoscopic disease activity in inflammatory bowel disease patients. Eur J Gastroenterol Hepatol. 2016;28((7)):807–13. doi: 10.1097/MEG.0000000000000616. [DOI] [PubMed] [Google Scholar]
- 24.Eivindson M, Nielsen JN, Gronbaek H, Flyvbjerg A, Hey H. The insulin-like growth factor system and markers of inflammation in adult patients with inflammatory bowel disease. Horm Res. 2005;64((1)):9–15. doi: 10.1159/000087190. [DOI] [PubMed] [Google Scholar]
- 25.Katsanos KH, Tsatsoulis A, Christodoulou D, Challa A, Katsaraki A, Tsianos EV. Reduced serum insulin-like growth factor-1 (IGF-1) and IGF-binding protein-3 levels in adults with inflammatory bowel disease. Growth Horm IGF Res. 2001;11((6)):364–7. doi: 10.1054/ghir.2001.0248. [DOI] [PubMed] [Google Scholar]
- 26.Krakowska-Stasiak M, Cibor D, Domagala-Rodacka R, Salapa K, Szczeklik K, Owczarek D. Insulin-like growth factor system in remission and flare of inflammatory bowel diseases. Pol Arch Intern Med. 2017;127((12)):832–9. doi: 10.20452/pamw.4136. [DOI] [PubMed] [Google Scholar]
- 27.Bannerjee K, Camacho-Hubner C, Babinska K, Dryhurst KM, Edwards R, Savage MO, et al. Anti-inflammatory and growth-stimulating effects precede nutritional restitution during enteral feeding in Crohn disease. J Pediatr Gastroenterol Nutr. 2004;38((3)):270–5. doi: 10.1097/00005176-200403000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Day AS, Aurangzeb B, Leachemberg ST, Lemberg DA, Walker J. The effects of exclusive enteral nutrition upon the insulin-like growth factor pathway in children with Crohn's disease. J Gastroenterol Hepatol. 2008;23((Suppl 4)):A303–A303. [Google Scholar]
- 29.Akbal E, Kocak E, Akyurek O, Koklu S, Batgi H, Senes M. Liver fatty acid-binding protein as a diagnostic marker for non-alcoholic fatty liver disease. Wien Klin Wochenschr. 2016;128((1–2)):48–52. doi: 10.1007/s00508-014-0680-8. [DOI] [PubMed] [Google Scholar]
- 30.Xu Y, Xie Y, Shao X, Ni Z, Mou S. L-FABP: a novel biomarker of kidney disease. Clin Chim Acta. 2015;445:85–90. doi: 10.1016/j.cca.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 31.Prasad S, Mingrino R, Kaukinen K, Hayes KL, Powell RM, MacDonald TT, et al. Inflammatory processes have differential effects on claudins 2, 3 and 4 in colonic epithelial cells. Lab Invest. 2005;85((9)):1139–62. doi: 10.1038/labinvest.3700316. [DOI] [PubMed] [Google Scholar]
- 32.Thuijls G, Derikx JP, de Haan JJ, Grootjans J, de Bruine A, Masclee AA, et al. Urine-based detection of intestinal tight junction loss. J Clin Gastroenterol. 2010;44((1)):e14–9. doi: 10.1097/MCG.0b013e31819f5652. [DOI] [PubMed] [Google Scholar]
- 33.Brandtzaeg P, Dale I, Fagerhol MK. Distribution of a formalin-resistant myelomonocytic antigen (L1) in human tissues. I. Comparison with other leukocyte markers by paired immunofluorescence and immunoenzyme staining. Am J Clin Pathol. 1987;87((6)):681–99. doi: 10.1093/ajcp/87.6.681. [DOI] [PubMed] [Google Scholar]
- 34.Johne B, Fagerhol MK, Lyberg T, Prydz H, Brandtzaeg P, Naess-Andresen CF, et al. Functional and clinical aspects of the myelomonocyte protein calprotectin. Mol Pathol. 1997;50((3)):113–23. doi: 10.1136/mp.50.3.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin JF, Chen JM, Zuo JH, Yu A, Xiao ZJ, Deng FH, et al. Meta-analysis: fecal calprotectin for assessment of inflammatory bowel disease activity. Inflamm Bowel Dis. 2014;20((8)):1407–15. doi: 10.1097/MIB.0000000000000057. [DOI] [PubMed] [Google Scholar]
- 36.Carlsen K, Malham M, Hansen LF, Petersen JJH, Paerregaard A, Houen G, et al. Serum calprotectin in adolescents with inflammatory bowel disease: a pilot investigation. J Pediatr Gastroenterol Nutr. 2019;68((5)):669–75. doi: 10.1097/MPG.0000000000002244. [DOI] [PubMed] [Google Scholar]
- 37.Meuwis MA, Vernier-Massouille G, Grimaud JC, Bouhnik Y, Laharie D, Piver E, et al. Serum calprotectin as a biomarker for Crohn's disease. J Crohns Colitis. 2013;7((12)):e678–83. doi: 10.1016/j.crohns.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 38.Suárez Ferrer C, Abadia Barno M, Martin Arranz E, Jochems A, Garcia Ramirez L. The use of serum calprotectin as a biomarker for inflammatory activity in inflammatory bowel disease. Rev Esp Enferm Dig. 2019;111((10)):744–9. doi: 10.17235/reed.2019.5797/2018. [DOI] [PubMed] [Google Scholar]
- 39.Salo M, Roth B, Stenstrom P, Arnbjornsson E, Ohlsson B. Urinary biomarkers in pediatric appendicitis. Pediatr Surg Int. 2016;32((8)):795–804. doi: 10.1007/s00383-016-3918-x. [DOI] [PubMed] [Google Scholar]