Abstract
Background
Cardiovascular diseases (CVDs) are the leading cause of morbidity and mortality in advanced CKD. The major pathological changes of CKD-associated CVD are severe vascular media calcification, aberrant cardiac remodeling such as hypertrophy and fibrosis, as well as accelerated atherosclerosis. α-Klotho is proposed as an anti-aging gene, which is primarily expressed in the kidney. Recent studies reveal that α-Klotho deficiency is associated with profound cardiovascular dysfunction. Of note, CKD represents extremely declined α-Klotho levels, hinting that α-Klotho deficiency may be implicated in the pathogenesis of CKD-associated CVD.
Summary
Based on the pathogenic mechanism of α-Klotho deficiency and decreased Klotho levels in the circulation even early in stage 1 of CKD, α-Klotho serves as a sensitive biomarker for renal insufficiency and also a novel predictor of risk of overall mortality of CVD events in CKD. Meanwhile, loss of Klotho resulted from kidney dysfunction markedly contributes to the progressive development of CKD and CVD. By contrast, prevention of Klotho decline using exogenous supplementation or genetically activated ways by several mechanisms can dramatically mitigate cardiac dysfunction, prevent vascular calcification, and retard the progression of CKD-accelerated atherosclerosis.
Key Messages
Klotho deficiency is proposed as a novel predictive biomarker as well as a pathogenic contributor to CVD events in CKD. In the future, Klotho may be a crucial potential therapeutic strategy to decrease the burden of CVD comorbidity with CKD in clinics.
Keywords: Chronic kidney disease, Cardiovascular diseases, Klotho
Introduction
CKD is a worldwide disease with a high incidence and prevalence, which puts a huge burden on public health. CKD is a progressive systemic disease, in which kidney structure and function are irreversibly damaged. Except for renal injury, CKD is always accompanied by multiple pathological changes of other organs, especially cardiovascular system [1]. Cardiovascular diseases (CVDs) are the primary cause of death in advanced CKD. The major pathological changes of CKD-associated CVD are severe vascular calcification (VC), aberrant cardiac remodeling with hypertrophy and fibrosis, and accelerated atherosclerosis [2]. Over the past decades, although considerable efforts were devoted to investigate the etiology and pathogenesis involved in the progression of CVD associated with CKD, the high-risk factor and its underlying mechanisms are still far from being elucidated.
The earliest discovered Klotho was named as α-Klotho, which was found in the animal experiment of primary hypertension by Kuro-o et al. [3]. Given its robust anti-aging effect, α-Klotho was initially considered as an anti-aging gene. α-Klotho is mainly expressed in the kidney, parathyroid gland, and choroid plexus, where it exists as a membrane-bound protein. α-Klotho also can be found as a soluble protein in the blood, urine, and cerebrospinal fluid. Deficiency of α-Klotho results in VC, atherosclerosis, skin atrophy, and osteoporosis, a syndrome resembling human aging. Interestingly, these phenotypes of α-Klotho deficiency are similar to CKD subjects, suggesting that α-Klotho is tightly related to the pathogenesis mechanisms of CKD [4]. Meanwhile, many researchers and our group have found that CKD is a state of severe α-Klotho deficiency, which is always along with progressive renal insufficiency and an extremely high incidence of CVD such as VC, cardiac hypertrophy, and uremic vasculopathy [5, 6, 7, 8]. Conversely, exogenous supplement of α-Klotho protein or genetic overexpression of α-Klotho can remarkably attenuate renal dysfunction and decline morbidity and mortality of CVD complications [9], suggesting that α-Klotho might be a predictor and a potential therapeutic target for renal and extra-renal outcomes in CKD. Based on the important relationship between α-Klotho and CKD, we will review the physiopathological functions and potential applications of α-Klotho in CKD and its complications below. Hereinafter the term Klotho is referred to as α-Klotho.
An Introduction of Klotho
Structure of Klotho Protein
Klotho gene consists of 5 exons in both mice and humans. The Klotho protein is composed of a large extracellular domain and a short C-terminal intracellular region [10]. The extracellular domain consists of 2 repetitive sequences termed as Kl1 and Kl2. The encoded protein of full-length transcript can be cleaved by α-secretase metalloproteinase domain-containing proteins 10 and 17 (ADAM10 and ADAM17) as well as β-secretase β-site APP-cleaving enzyme 1 (BACE1). The cleaved Klotho is composed of Kl1 and Kl2, and both Kl1 and Kl2 fragments can release into circulation [11]. Secreted Klotho is synthesized by alternative transcription splicing. Soluble Klotho includes cleaved Klotho and secreted Klotho. Membrane-bound Klotho exerts its functions mainly as co-receptor of fibroblast growth factor (FGF) family by increasing affinity of FGF receptors (FGFRs) to FGF [12]. Soluble Klotho acts as a hormone in the circulation to exert its role on the whole body.
Recently, 2 other homologous types of Klotho, β- and γ-Klotho, were identified. β-Klotho contains Kl1 and Kl2 domains and is mainly expressed in liver, gastrointestinal tract, kidney, and adipose tissue. γ-Klotho contains only Kl1 domain and is expressed in adipose tissue, kidney, and skin [8]. We only focus on the role of α-Klotho.
Biological Functions of Klotho
Soluble Klotho exerts its broad biological functions on targets, even several distal organs, as a pleiotropic endocrine/paracrine factor or a hormone, while membrane-bound Klotho always exerts its functions as co-receptors [13]. Of note, accumulating evidence suggest that soluble Klotho also can act as co-receptors for some special soluble ligands [14]. Thus, it is necessary to classify the different mechanisms underlying the Klotho functions.
Membrane-Bound Klotho Acts as a Co-Receptor with FGF23
Membrane Klotho mainly forms co-receptors with FGFRs (FGFR1c, FGFR3c, and FGFR4) to promote the binding of FGF23 and FGFRs and regulate calcium and phosphorus metabolism. FGF23 is a bone-derived growth factor that belongs to FGF family [15]. Due to lack of a heparan sulfate-binding domain, FGF23 requires Klotho to convert the FGFR from canonical form into specific high-affinity form to function on target organs such as kidney and parathyroid glands. In this process, Klotho serves as a massive scaffold, tethering both FGFR and FGF23 to itself, enforcing proximity and augmenting binding affinity between these 2 molecules. The membrane Klotho-FGFRs-FGF23 complex could inhibit phosphate reabsorption by inhibiting NaPi-IIa in renal proximal tubule. At the same time, the complex can also downregulate the expression of renal 1α-hydroxylase. As a result, the synthesis of 1,25-dihydroxyvitamin D3 (calcitriol) decreases, and the intestinal absorption of phosphate is reduced [16].
Membrane-Bound Klotho Acts as a Competitor for Binding Sites
FGF2 was found to be the founding member of the FGF family, and it was primarily identified as mitogens promoting proliferation of fibroblasts and vascular endothelial cells [17]. Unlike FGF23, FGF2 has heparin-binding domains, through which FGF2 tethers themselves to heparan sulfate in the extracellular matrices and functions primarily in an autocrine or paracrine manner [18]. Increased FGF2 and reduced Klotho have both been reported to be closely associated with renal fibrosis. Our group has demonstrated there was a competitive relationship between Klotho and FGF2, increase in FGF2 might weaken the function of Klotho, whereas the increase in Klotho would suppress the activity of FGF2 signaling pathway [19]. In addition, Klotho restrained FGF2-induced tubulo-epithelial plasticity and fibroblast proliferation and activation. The inhibitory effect of Klotho on the activity of FGF2 was likely due to its potent ability to compete with FGF2 binding to FGFR1, suggesting that there is a negative feedback loop exist between Klotho depletion and FGF2 activation [19].
Soluble Klotho Acts as a Co-Receptor for Soluble Ligands
Kusaba et al. [20] suggested that vascular endothelium in Klotho-deficient mice showed hyperpermeability with increased apoptosis and decreased vascular endothelial-cadherin because of an increase in vascular endothelial growth factor (VEGF)-mediated internal calcium concentration Ca2+ influx and hyperactivation of Ca2+-dependent proteases. Nevertheless, Klotho supplement could directly strengthen the binding affinity between VEGF receptor 2 and endothelial transient receptor potential canonical Ca2+ channel 1 (TRPC1) to promote their co-internalization, the resulting regulating TRPC1-mediated Ca2+ entry to maintain endothelial integrity.
Soluble Klotho Acts as an Enzyme
Previous studies showed that soluble Klotho possessed sialidase activity and regulated several ion channels via this activity. Removal of terminal sialic acids from N-glycan chains of the epithelial Ca2+ channel TRPV5 and the renal K+ channel renal outer medullary potassium channel 1 (ROMK1) by soluble Klotho exposes the underlying ligand for galectin-1. Binding to galectin-1 at the extracellular surface prevents internalization and leads to accumulation of the channels on the plasma membrane [21]. Soluble Klotho also downregulates transient receptor potential Ca2+ canonical isoform 6 (TRPC6)-mediated calcium signaling by binding to α2,3-sialyllactose moiety of gangliosides in lipid rafts [22]. Recent study has shown that soluble Klotho downregulated PI3K/Akt signaling via binding to ganglioside-enriched lipid [23]. These results suggest that Klotho regulates ion transport by modifying sugar moieties of their transporters and affecting cell surface abundance via sialidase or β-glucuronidase activity [24].
Soluble Klotho Acts as a Decoy Receptor for Soluble Factors
Wnt proteins are a large family of highly conserved signal molecules that involved in process of nephron formation and renal development. Increasing evidence indicates that abnormal activation of Wnt/β-catenin signaling pathways plays a crucial role in renal fibrosis. There is an inverse correlation between Klotho and canonical Wnt signaling. Soluble Klotho directly inhibits Wnt signaling by binding to several Wnt factors, such as Wnt1, Wnt4, and Wnt7a [25].
Soluble Klotho Decreases Receptor Affinity for Ligands
Soluble Klotho protein can directly bind to the type-II TGF-β receptor (TGFβR2) and inhibit TGF-β1 binding to cell surface receptors, thereby inhibiting TGF-β1 signaling-induced epithelial-to-mesenchymal transition responses in cells. In addition to TGF-β1 signaling, soluble Klotho has been shown to act as a circulating hormone that binds to a cell surface receptor and represses intracellular signals of insulin and insulin-like growth factor 1 (IGF1), an evolutionarily conserved mechanism for extending life span [26, 27, 28].
Antioxidative Stress and Anti-inflammatory Effects of Klotho Protein
Oxidative stress results from the imbalance between excessive oxidant free radicals and insufficient degradation of these prooxidants by anti-oxidants. CKD is a state of severe oxidative stress due to decreased antioxidants and excessive reactive oxygen species (ROS) generation [29]. Excessive ROS production in CKD state is involved in many kinds of CKD-associated complication [30]. On the contrary, controlling ROS is regarded as an effective way to prevent CKD-induced disease. Klotho is considered as an important antioxidative factor, and previous studies have found that there is a close connection between Klotho and ROS production. Klotho could inhibit insulin/IGF-1/PI3K signaling, then activating the FoxO forkhead transcription factors and inducing the expression of manganese superoxide dismutase to resist oxidative stress [31]. Interestingly, this antioxidative effect induced by Klotho also inhibits the production of mitochondrial-derived ROS and protects mitochondrial dysfunction [32]. In addition, it has been reported that increased endogenous ROS production in Klotho-deficient mice and reduced ROS production in Klotho overexpression are closely related to the regulation of ASK1-signalosome-p38 MAPK signal pathway [33].
CKD is regarded as a typical example of inflammatory disease and premature ageing. Plenty of pro-inflammatory factors accumulated during CKD progression. These inflammatory factors play an important role in CKD-induced complication. There are multiple studies that have shown a bidirectional relationship between Klotho and inflammation. On the one hand, inflammation can decrease the expression of Klotho through transcriptional and epigenetic mechanisms [34]. On the other hand, Klotho is reported to have powerful anti-inflammatory effect. Our group found that Klotho could inhibit indoxyl sulfate (IS)-activated RIG-I/NF-κB and the production of inflammatory factors including IL-6 and TNF-α in cultured monocytes. Interestingly, higher serum levels of IL-6 and TNF-α were observed in Klotho heterozygous mice with CKD than that in single CKD group. Conversely, Klotho treatment can dramatically inhibit inflammation in monocytes. Previous report has shown that supplementation or overexpression of Klotho in vitro could suppress NF-κB [35] and RIG-I-mediated inflammation [36], which is consistent with our results. Moreover, it has been reported that post-treatment with recombinant Klotho could suppress the inflammatory responses in aging endotoxemic mice through modulating levels of heat shock protein 70 [37]. These findings suggest that Klotho has a powerful antioxidative stress and anti-inflammatory ability.
The Role of Klotho in CKD-Induced CVD
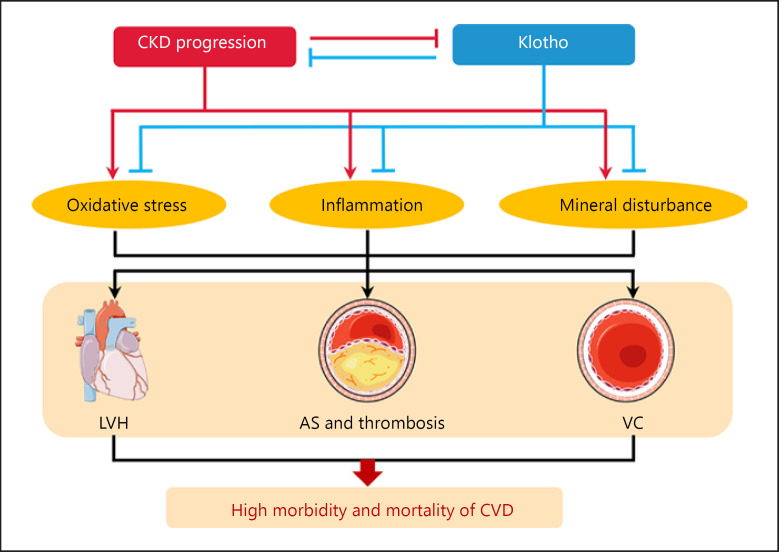
Physiologically, kidney is the major organ to maintain soluble Klotho homeostasis. Yet in CKD, a large number of renal tubular epithelial cells are injured and replaced by extracellular matrix, and the rest cannot produce and release enough soluble Klotho into the circulation. Therefore, CKD is proposed as a state of soluble Klotho deficiency [38]. Even in the younger CKD population such as children, serum level of Klotho was still lower than in healthy subjects. As CKD and Klotho-deficient mice have an extremely high incidence of CVD [4], the morbidity and mortality of CVD can be repressed by Klotho gene overexpression or protein supplementation in those animal models [39, 40]. We summarized the in vivo studies directly related to the protective effects of Klotho (shown in Table 1). These phenomena suggest that loss of Klotho is likely to be directly pathogenic and probably a prerequisite for CKD-associated CVD (shown in Fig. 1).
Table 1.
The verified and potential mechanisms for cardiovascular protection of Klotho in CKD
| Animal model | Outcome of CVD | Mechanism | Reference |
|---|---|---|---|
| EFmKL46 (Tg-K1) mice | VC | Inhibition of Pi influx into VSMC | Hu et al., 2011 [48] |
| Klotho−/– mice + rapamycin | VC | Inhibition of mammalian target of rapamycin (mTOR) | Zhao et al., 2015 [110] |
| NaPi2a−/–/Klotho−/– mice | VC | Regulating the phosphate associated co-transporter | Ohnishi et al., 2009a [51] |
| Cyp27b1−/–/Klotho−/– mice | VC | Reducing vitamin D levels through inactivation of Cyp27b1 activity | Ohnishi et al., 2009b [52] |
| Uremic mice + Klotho i.p. | LVH | Blocking Nox2/Nox4-derived ROS production and inhibiting p38 and ERK1/2 signaling pathways | Yang et al., 2015 [6] |
| Diabetic mice + Klotho i.p. | LVH | Suppressing cardiac inflammatory cytokines and oxidative stress | Guo et al., 2018 [68] |
| Uremic rats + rKlotho | LVH | Increasing myocardial fibroblast growth factor 21 expression | Suassuna et al., 2020 [73] |
| Mice received subcutaneous injections of Ang II + Klotho | LVH | Modifying the TGF-β1-miR-132 axis | Ding et al., 2019 [67] |
| Klotho−/– mice | AS | Regulating TRPC-1-mediated Ca2+ entry | Kusaba et al., 2016 [20] |
| OLETF rats + Ad-Klotho | AS | Increasing nitric oxide production | Saito et al., 2000 [111] |
| DKD mice + pcMV-Klotho | AS | Inhibiting macrophage M1 polarization and inducing macrophage M2 polarization | Jia et al., 2019 [95] |
| CKD mice + Klotho i.p. | AS | Inhibiting platelet hyperactivity | Yang et al., 2017 [7] |
AS, atherosclerosis; CVD, cardiovascular disease; LVH, left ventricular hypertrophy; VC, vascular calcification.
Fig. 1.
The role of Klotho in CKD-associated CVD. CKD is a public health epidemic. CVD, including uremic cardiomyopathy, vascular calcification, and atherosclerosis, was found in CKD subjects. Klotho deficiency, which results from renal insufficiency, is always associated with poor outcomes in CKD. By contrast, prevention of Klotho decline by inhibiting oxidative stress and inflammation as well as rectifying mineral disturbance can dramatically mitigate cardiac dysfunction, prevent vascular calcification, and retard the progression of CKD-accelerated atherosclerosis. AS, atherosclerosis; CVD, cardiovascular disease; LVH, left ventricular hypertrophy; VC, vascular calcification.
The Role of Klotho in VC
VC is one of the most potent risk factors of cardiovascular mortality and morbidity in CKD patients, and CKD is often related to vascular media calcification. Even in the early stage of CKD, the rate of VC increases significantly [41]. VC is mainly related to the transdifferentiation of vascular smooth muscle cells (VSMCs) into osteogenic-like cells. CKD-induced mineral bone disorder syndrome is a potent contributor to VC [42]. Plenty of evidence revealed that disturbance of serum phosphate and calcium can directly lead to VC [43, 44]. Previous study has found that hyperphosphatemia was regarded as a main inducer for the onset and progression of VC. Under the influence of CKD, both hyperphosphatemia and VC represent an extremely higher incidence than the health population. However, CKD patients with normal serum phosphate levels still showed severe VC, suggesting that several factors other than hyperphosphatemia can lead to VC [45]. Recently, the role of Klotho is considered increasingly important in CKD-induced VC. It has been reported that either in CKD patients or peritoneal dialysis patients, low serum levels of soluble Klotho are independently related to VC [46, 47]. In CKD mice, Klotho deficiency induces a higher serum phosphate level and more severe VC than that in CKD mice. On the contrary, overexpression or supplementation of Klotho can reduce serum phosphate level, improve renal function, and protect against VC [48]. In vitro, using Klotho siRNA pretreatment accelerates CKD-induced VSMCs transformation to a calcifying phenotype, which is characterized by the loss of inhibitory factors, such as MGP (matrix Gla protein) and OPN (osteopontin), and upregulation of Runx2 (runt-related transcription factor 2) [49]. These results suggest that the deficiency of Klotho is not only a marker CKD, but is also an important risk factor that contributes to the onset and progression of VC in CKD.
The mechanism of Klotho deficiency aggravating CKD-induced VC is closely related to the homeostasis of phosphorus metabolism. Klotho could regulate phosphate metabolism by enhancing phosphaturia and preserving glomerular filtration, as complete Klotho deficiency induces high serum phosphate levels [48]. Previous studies indicated that restricted dietary phosphate of Klotho-deficient mice might increase renal expression of Klotho and partially rescued the ectopic calcification [50, 51]. Besides, it is reported that NaPi2a−/− Klotho−/− mice showed lower phosphate levels and reduced VC, while the features of VC reestablished as long as the mice were given the same mice high-phosphate diets [51]. It is therefore conceivable that in the setting of Klotho deficiency, elevated phosphate is indispensable to VC [52]. In fact, despite lower phosphate diets, serum phosphate eventually increased to the point of inducing VC. This may be mediated by increased activation of NaPi2a induced by elevated FGF23 and lack of compensatory action resulting from Klotho deficiency. In contrast to low-phosphate diets, low-calcium diets could effectively restrain the progression of calcification [53]. These effects suggest that in the setting of Klotho deficiency, calcium may be another rate-limiting factor for VC pathophysiology.
Supplementation or overexpression of Klotho could play a protective role in CKD-induced VC. Hum et al. [54] reported that injection of recombinant Klotho downregulated the expression of renal sodium-phosphate co-transporter Npt2a, which reduced hyperphosphatemia and eventually rescued the CKD-associated VC. Moreover, CKD mice with overexpression of Klotho showed a less severe VC than wild-type CKD mice. In vitro, it has been found that overexpression of Klotho directly inhibited phosphate influx into VSMC, which in turn suppressed the differentiation of VSMC and rescued the VC [48]. These results demonstrate that Klotho is an endogenous inhibitor of VC [49]. The mechanism of protective role of Klotho in VC is related to the ability to inhibit the uptake of phosphate through phosphate sodium-dependent co-transporter in VSMCs [55]. In addition, we have investigated that local expression of Klotho in artery had ability to inhibit VC by regulating phosphate uptake. And the expression of Klotho is decreased with the peroxisome proliferator-activated receptor-gamma (PPAR-γ) reduction in VSMCs after high phosphate treatment [56]. Another mechanism is that Klotho has anti-oxidative and anti-apoptotic effects in VSMCs to decease VC. Oxidative stress and apoptosis are important factors to induce VC in CKD [45, 57]. Wang et al. [58] found that Klotho gene transfer significantly decreased superoxide production by decreasing the expression of Nox2 in VSMCs in protein level through inhibiting cAMP-PKA pathway. It has also been reported that exogenous Klotho ameliorated the development of VC and ROS generation induced by salusin-β [59]. Besides, it has also been found that Klotho can protect AngII-induced VSMCs apoptosis. Finally, Klotho can be inducible by other substances and then acts as an induced protective factor to inhibit VC. Upregulation of Klotho expression by inhibiting mTOR signaling via oral rapamycin ameliorates VC and protects against vascular disease in CKD [60]. Another study showed that intermedin 1–53 attenuates VC in rats with CKD by upregulating membrane-bound Klotho expression in the vessel wall [61]. All of these suggested that Klotho could be a hopeful therapeutic target for CKD-induced VC and whether maintaining or increasing the Klotho level could improve VC in CKD patients is needed to be proven in future research.
The Role of Klotho in Left Ventricular Hypertrophy
The epidemiological studies show that the progressively declined renal function accompanied by a high incidence of left ventricular hypertrophy (LVH), which accounts for nearly 40 and 74% in patients with early stage of CKD and ESRD, respectively. LVH is the pathological basis of heart failure or ventricular arrhythmias, which is regarded as an important independent risk factor in patients with CKD [62]. Previous studies indicated that pressure and volume overload were the major contributors to LVH in CKD. However, LVH still develops even after mitigating these 2 factors [63]. Recent data prefer to pay more attention on the role of Klotho in CKD-induced LVH. It has been reported that maintenance hemodialysis patients with lower circulating sKlotho levels were more often associated with larger interventricular septal thickness and greater ratios of interventricular septal thickness and posterior wall thickness, which are typical indicators of LVH in patients [64]. Consistently, in a survey of 86 patients with CKD, we found that increased left ventricular mass was more apparent in patients with low Klotho level [6]. In mice, it has been reported that Klotho-deficient CKD mice showed worsening LVH, while LVH in Klotho-overexpressing CKD mice was relieved [65]. These suggested that there was an independent association between Klotho and CKD-induced LVH, and Klotho could be a potential risk factor of uremic cardiomyopathy. Therefore, discussing the role of Klotho in this pathological process is helpful to understand the underlying mechanism of CKD-induced LVH and find effective treatment.
It has been indicated that administered injections or genetically active expression of Klotho could apparently attenuate cardiac remodeling, manifested as reduced ventricular wall thickness, left ventricular internal diameter at end-diastole (LVIDD), and posterior wall thickness in mice [6, 65]. The cardioprotective role of Klotho in CKD is mainly related to its robust antioxidative stress effect. Recent data showed that Klotho could protect against cardiomyocyte hypertrophy induced by isoproterenol [66], Ang II [67], or hyperglycemia [68] by suppressing ROS-stimulated signaling pathways both in vitro and in vivo experiments. We proved that Klotho protects IS-induced LVH by inhibiting oxidative stress signaling pathway in vivo and in vitro. In detail, we found that IS induced cardiomyocyte hypertrophy by activating oxidative stress and its downstream signaling pathways p38 and ERK1/2 and Klotho showed powerful antioxidative ability to ameliorate the pathological changes [6].
Second, Klotho can act as circulating co-receptor that can bind circulating FGF23 and FGFRs on the cell surface, which makes it possible for Klotho to regulate FGF23-mediated signaling [69]. As a novel cardiovascular uremic toxin, FGF23 directly participates in the development of LVH in CKD. Evidence showed that FGF23 directly targeted cardiomyocytes by activating FGFR4/PLCγ/calcineurin/NFAT signaling pathway and induced cardiac hypertrophy [70]. Interestingly, it is suggested that Klotho can regulate myocardial FGF23 effects by blocking the binding of circulating FGF23 to FGFR4 on cardiomyocyte membrane [71, 72]. Besides, Klotho could also play a role of co-receptor for FGFR1c to redirect the action of FGF23-induced cardioprotective pathway. One of the possible protective pathways is to induce the expression of FGF21 [73]. In addition, Klotho can ameliorate cardiac hypertrophy through inhibiting TRPC6 currents by blocking phosphoinositide-3-kinase-dependent exocytosis of TRPC6 channels in uremic hearts [74]. Further study made it clear that this effect of Klotho-inhibited TRPC6 was independent of FGF23 [22, 75].
Finally, Klotho can directly prevent cardiac remodeling and fibrosis to protect the heart. Cardiac remodeling is characterized by cardiomyocyte hypertrophy and fibrosis and has been regarded as an important risk factor for the development of heart failure in CKD [76, 77]. Recent study showed that Klotho protein can protect Ang II-induced cardiac remodeling through regulating TGF-β-miR-132 axis. Liu et al. [78] reported that Klotho is expressed in human atrial appendage tissues. Further, Klotho knockdown or overexpression in primary human cardiomyocytes aggravates or mitigates TGF-β1-induced cardiac fibrosis and activation of the Wnt signaling. These results suggest that endogenous Klotho in cardiomyocytes plays an important role in CKD-induced cardiac fibrosis and subsequent activation of Wnt signaling pathway. On the contrary, upregulation of endogenous Klotho can inhibit activation of Wnt signaling pathway induced by CKD, which may provide a new strategy for the treatment of myocardial fibrosis in CKD patients. All of these evidence show that Klotho participates in the pathogenesis of CKD-associated LVH and treatment with Klotho is a potential therapy for CKD-induced LVH.
The Role of Klotho in CKD-Accelerated Atherosclerosis
As a chronic inflammatory disease with complex etiology and the most potent pathological basis of multiple cardiovascular events, atherosclerosis is seriously harmful to human health. The major clinical outcomes of atherosclerosis, such as myocardial infarction or stoke, are not a result of gradual narrowing of lumen but rather the cause of thrombotic events [79]. Of note, atherosclerosis is the leading cause of cardiovascular morbidity and mortality in CKD patients and its progression is often accelerated [80]. Over the past decades, considerable efforts have been devoted to investigate the risk factors as well as their underlying mechanisms that accelerated development and progression of atherosclerosis in CKD.
As an early predictor of atherosclerosis, lower levels of Klotho are positively correlated with lower values of flow-mediated dilation, larger values of epicardial fat thickness and carotid artery intima-media thickness, all of which are the admitted predictors of atherosclerosis [81]. Moreover, lower Klotho levels coexist with higher inflammatory status in patients with atherosclerosis [82]. In the hemodialysis population, Klotho acts as an atherosclerosis predictor as well. Increasing numbers of studies showed that Klotho deficiency can aggravate the progress of atherosclerosis in CKD, while supplement of Klotho played a protective role in this process [83]. Therefore, it is significant to focus on the effects of Klotho in this pathological process, and here, we will discuss the role of Klotho in the pathogenesis of atherosclerosis in CKD to provide clues for future research.
Protection against Endothelial Dysfunction
Endothelial dysfunction plays a vital role in the onset and progression of CKD-induced atherosclerosis [84]. Previous study has found that endothelial dysfunction was a common feature of arteries in Klotho-deficient mice [20]. Conversely, many endothelial protective factors such as reduced systolic blood pressure, coronary perivascular fibrosis, and medial hypertrophy were observed in Klotho transgenic mice [85]. It has been reported that membrane-form Klotho enhanced manganese superoxide dismutase expression by approximately twofold, partially via activation of the cAMP/PKA-dependent pathway and increase of nitric oxide production in human umbilical vein endothelial cells [86]. This finding provides new insights into the mechanisms of Klotho action and support the therapeutic potential of membrane-form Klotho to regulate endothelial function. In addition, Cui et al. [87] suggested that Klotho protected H2O2-induced oxidative injury by activating the PI3K/AKT pathway in endothelial cells. Similarly, Klotho can attenuate ox-LDL-induced oxidative stress in human umbilical vein endothelial cells through upregulating oxidative scavengers (superoxide dismutase and NO) via activating the PI3K/Akt/eNOS pathway and suppressing LOX-1 expression [88]. Moreover, we demonstrated that Klotho could attenuate the effects of uremic toxin-induced activation of p38 MAPK, NF-κB, and ROS production and protect endothelial cells from the injury [5]. Also, Klotho had an ability to inhibit endothelial senescence via mitogen-activated protein kinase kinase and extracellular signal-regulated kinase pathways [89]. Besides, Liu et al. [90] demonstrated that Klotho participated in micro-RNA335-5p-induced endothelial senescence. These evidence suggested the protective effects of Klotho in protecting endothelial dysfunction.
Protection against VSMC Injuries
In the early stage of atherosclerosis, the proliferation and migration of VSMCs dramatically promote the progression of atherosclerosis [91]. Interestingly, Klotho can inhibit proliferation and migration of Ang II-induced VSMCs by regulating NF-κB/p65/Akt/ERK signaling pathways. Klotho also inhibited the expression of proliferation phenotype marker protein, PCNA, and increased the expression of contractile phenotype marker proteins, suggesting that Klotho plays a major role in Ang II-induced phenotype modulation of VSMCs [92]. Moreover, Klotho was proposed to protect VSMCs by inhibiting oxidative stress. It is suggested that VSMCs with overexpression of Klotho significantly reduced the expression of Nox2 and ROS production through regulating cAMP/PKA pathways [58]. In addition, it has been shown that Klotho supplement can enhance the expression of oxygenase-1 (HO-1) and peroxiredoxin-1 (Prdx-1), which are powerful antioxidant enzymes to protect against VSMC dysfunction. During this process, Nrf2 activation was found after the supplement of Klotho, and silencing of Nrf2 attenuated the induction of HO-1 and Prx-1 induced by Klotho [93]. These results suggested that Nrf2-mediated antioxidant roles of Klotho in VSMCs.
Inhibiting Macrophage/Monocyte-Associated Inflammation and Phenotypic Transition
Recently, we found that lack of Klotho aggravates systemic inflammation in CKD, while supplement of Klotho might mitigate this process by decreasing the expression of inflammatory factors or related signaling pathways [94], which hints that Klotho could prevent atherosclerosis progression through inhibiting monocyte-associated inflammation. In addition, Klotho participated human serum albumin and MiR-199a-5p induced macrophage M1 polarization, which is a pro-inflammatory phenotype. Further, Klotho induces macrophage M2 polarization through the Toll-like receptor 4 pathway both in vivo and in vitro experiments [95]. These findings hint that Klotho might be involved in the phenotype modulation of macrophage and the progression of atherosclerosis. Finally, researchers suggested that Klotho can inhibit atherosclerosis by lowering lipid. Pretreatment of Klotho protein before incubating with phorbol myristate acetate and ox-LDL can significantly decrease total cholesterol level and suppressing transition of THP-1 macrophages to foam cells. Furthermore, Klotho-induced upregulation of reverse cholesterol transport capacity promotes cholesterol efflux and reduces lipid accumulation by suppressing the Wnt/β-catenin pathway in foam cells [96].
Inhibiting Platelet Hyperactivity
Activated platelets are present in the circulating blood of atherosclerotic individuals throughout the atherosclerotic process, which play an important part in the atherosclerotic lesions. Activated platelets prefer to bind leukocytes and form platelet-leukocyte aggregates [97]. In both in vivo and in vitro studies, it had been proved that aggregation of the circulating activated platelets and platelet-leukocyte promoted the development of atherosclerosis [98]. In both CKD and IS-treated mice, we found that the expression of P-selectin and the circulating level of PMPs were remarkably increased, while activated GPIIb/IIIa induced by collagen and thrombin was more obvious and a high tendency of thrombus formation [7]. These suggested that IS plays an important role in hyperactivation of platelets and the formation of thrombosis in CKD. Furthermore, we found that the activation of platelets induced by IS in CKD state is caused by the activation of ROS/p38 MAPK signaling. As expected, Klotho treatment markedly ameliorated IS-induced platelet hyperactivity through inhibiting IS-induced activation of ROS/p38 MAPK signaling and reduced the formation of thrombosis both in vitro and in vivo [7]. Thus, we concluded that Klotho plays a key protective role in CKD-induced atherosclerosis and thrombosis through inhibiting IS-induced platelet hyperactivity.
The Prospect of Clinical Application of Klotho in CKD-Associated CVD
We have discussed the definite protective role and the underlying mechanisms of Klotho in CKD-associated CVD. Therefore, the prospect of clinical application of Klotho is of great concern. CKD-associated CVD is the main cause of death in patients with CKD, which occurs in the early stage of CKD and progresses rapidly. It is very difficult to make an early and accurate diagnosis. And there are no biomarkers that are able to be measured easily, specially, and sensitively, in correlation with CKD-associated CVD complications [99, 100]. Recently, several clinical studies found that there was an excellent correlation between Klotho and cardiovascular events or mortality in CKD patients. In dialysis patients, Marçais et al. [101] reported that conservation of serum Klotho above 280 ng/L is associated with a better 2-year cardiovascular protection. This is consistent with a recent prospective study − lower serum level of Klotho is associated with cardiovascular events in hemodialysis patients [102]. In CKD patients without dialysis, we demonstrated that lower serum Klotho levels are independently associated with overall mortality and CVD events [103]. Collectively, it is clear that Klotho is an independent predictor of CVD complication and cardiovascular events in CKD patients. However, large multicenter clinical cohort studies are required to determine the cutoff value of Klotho for predicting the onset of CVD complication in CKD patients. It is definitely helpful for clinicians to judge the patient's cardiovascular-related condition and prognosis.
In addition, the clinical transformation studies of Klotho should be carried out in a planned way, and it will make it clear that whether the increase of serum Klotho exerts protective effects on cardiovascular events in patients. Potential drugs to increase the serum Klotho level include PPAR-γ agonists, angiotensin II-type I receptor antagonists, vitamin D active derivatives, intermedin, and dihydromyricetin, all of which had been proved to upregulate the expression of Klotho in vivo and/or in vitro [38, 61, 104, 105, 106]. Furthermore, direct supplementation of soluble Klotho protein is also a hopeful treatment. However, potential side effects of increasing serum Klotho level should also be taken into account. Some researchers have reported the potential side effects of Klotho in inducing insulin resistance and participating in obesity and obesity-associated complications [107, 108]. Furthermore, mechanism of Klotho mediated-insulin resistance is through prevention of GLUT4 translocation and interfering with phosphorylation of Akt, GSK3β, and PFKf3β intracellular signaling mediators by insulin [108]. However, this point of view is controversial, as Lorenzi et al. [109] provided evidence against a direct role of Klotho in insulin resistance in HEK293, L6, and HepG2 cells. Therefore, more studies are needed to clearly determine the potential side effects of Klotho protein.
Conclusion
The identification of Klotho helps us to understand more about how Klotho functions as a circulating hormone or local autocrine/paracrine factor. Klotho exerts pleiotropic functions in body. In future study, the crystal structure, functional pattern, as well as the regulatory mechanisms of Klotho are needed to be discovered. What is more, it is also unclear whether there is a receptor on the cell membranes that directly binds to Klotho. A large number of basic and clinical studies have shown that Klotho is an excellent predictor of CKD-related cardiovascular complications. The in-depth researches of the relation between Klotho and CVD complications in CKD as well as its potential applications are the potential therapeutic strategies for CKD-associated CVD in the future.
Conflict of Interest Statement
All the authors declared no competing interests.
Funding Sources
This study was supported by research grants from the Natural Science Foundation of China (No. 81873605, 81700379), the Young Elite Scientist Sponsorship Program by CAST (No. 2018QNRC001), Personal Training Program for Clinical Medicine Research of Army Medical University (No. 2018XLC1007), and Frontier Specific Projects of Xinqiao Hospital (No. 2018YQYLY004).
Author Contributions
J.Z. decided on the topics. X.B. and K.Y. wrote the manuscript and prepared the figure and the table. B.Z. provided suggestions for the revision of the manuscript.
References
- 1.Zoccali C, Vanholder R, Massy ZA, Ortiz A, Sarafidis P, Dekker FW, et al. The systemic nature of CKD. Nat Rev Nephrol. 2017 Jun;13((6)):344–58. doi: 10.1038/nrneph.2017.52. [DOI] [PubMed] [Google Scholar]
- 2.Sadeghi-Alavijeh O, Tadayyon M, Caplin B. Chronic kidney disease-associated cardiovascular disease: scope and limitations of animal models. Cardiovasc Endocrinol. 2017;6((4)):120–7. doi: 10.1097/XCE.0000000000000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997 Nov 6;390((6655)):45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 4.Zou D, Wu W, He Y, Ma S, Gao J. The role of klotho in chronic kidney disease. BMC Nephrol. 2018 Oct 22;19:285. doi: 10.1186/s12882-018-1094-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang K, Nie L, Huang Y, Zhang J, Xiao T, Guan X, et al. Amelioration of uremic toxin indoxyl sulfate-induced endothelial cell dysfunction by Klotho protein. Toxicol Lett. 2012 Nov 30;215((2)):77–83. doi: 10.1016/j.toxlet.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Yang K, Wang C, Nie L, Zhao X, Gu J, Guan X, et al. Klotho protects against indoxyl sulphate-induced myocardial hypertrophy. J Am Soc Nephrol. 2015 Oct;26((10)):2434–46. doi: 10.1681/ASN.2014060543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang K, Du C, Wang X, Li F, Xu Y, Wang S, et al. Indoxyl sulfate induces platelet hyperactivity and contributes to chronic kidney disease-associated thrombosis in mice. Blood. 2017 May 11;129((19)):2667–79. doi: 10.1182/blood-2016-10-744060. [DOI] [PubMed] [Google Scholar]
- 8.Kuro-o M. The Klotho proteins in health and disease. Nat Rev Nephrol. 2019 Jan;15((1)):27–44. doi: 10.1038/s41581-018-0078-3. [DOI] [PubMed] [Google Scholar]
- 9.Neyra JA, Hu MC. Potential application of klotho in human chronic kidney disease. Bone. 2017 Jul;100:41–9. doi: 10.1016/j.bone.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olejnik A, Franczak A, Krzywonos-Zawadzka A, Kałużna-Oleksy M, Bil-Lula I. The biological role of Klotho protein in the development of cardiovascular diseases. Biomed Res Int. 2018 Dec 24;2018:5171945. doi: 10.1155/2018/5171945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lim K, Halim A, Lu T-S, Ashworth A, Chong I. Klotho: a major shareholder in vascular aging enterprises. Int J Mol Sci. 2019 Sep 19;20:4637. doi: 10.3390/ijms20184637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muñoz-Castañeda JR, Rodelo-Haad C, Pendon-Ruiz de Mier MV, Martin-Malo A, Santamaria R, Rodriguez M. Klotho/FGF23 and Wnt signaling as important players in the comorbidities associated with chronic kidney disease. Toxins. 2020 Mar 16;12:185. doi: 10.3390/toxins12030185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuro-O M. Molecular mechanisms underlying accelerated aging by defects in the FGF23-klotho system. Int J Nephrol. 2018;2018:9679841. doi: 10.1155/2018/9679841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dalton GD, Xie J, An SW, Huang CL. New insights into the mechanism of action of soluble Klotho. Front Endocrinol. 2017;8:323–3. doi: 10.3389/fendo.2017.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schumacher D, Schuh A. Cardiac FGF23: a new player in myocardial infarction. Discoveries. 2019 Sep 30;7:e97. doi: 10.15190/d.2019.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richter B, Faul C. FGF23 actions on target tissues-with and without klotho. Front Endocrinol. 2018;9:189. doi: 10.3389/fendo.2018.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuro-O M. Klotho and endocrine fibroblast growth factors: markers of chronic kidney disease progression and cardiovascular complications? Nephrol Dial Transpl. 2019 Jan 1;34:15–21. doi: 10.1093/ndt/gfy126. [DOI] [PubMed] [Google Scholar]
- 18.Schlessinger J, Plotnikov AN, Ibrahimi OA, Eliseenkova AV, Yeh BK, Yayon A, et al. Crystal structure of a ternary FGF-FGFR-heparin complex reveals a dual role for heparin in FGFR binding and dimerization. Mol Cell. 2000 Sep;6((3)):743–50. doi: 10.1016/s1097-2765(00)00073-3. [DOI] [PubMed] [Google Scholar]
- 19.Guan X, Nie L, He T, Yang K, Xiao T, Wang S, et al. Klotho suppresses renal tubulo-interstitial fibrosis by controlling basic fibroblast growth factor-2 signalling. J Pathol. 2014 Dec;234((4)):560–72. doi: 10.1002/path.4420. [DOI] [PubMed] [Google Scholar]
- 20.Kusaba T, Okigaki M, Matui A, Murakami M, Ishikawa K, Kimura T, et al. Klotho is associated with VEGF receptor-2 and the transient receptor potential canonical-1 Ca2+ channel to maintain endothelial integrity. Proc Natl Acad Sci U S A. 2010 Nov 9;107((45)):19308–13. doi: 10.1073/pnas.1008544107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cha SK, Hu MC, Kurosu H, Kuro-o M, Moe O, Huang CL. Regulation of renal outer medullary potassium channel and renal K(+) excretion by Klotho. Mol Pharmacol. 2009 Jul;76((1)):38–46. doi: 10.1124/mol.109.055780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wright JD, An S-W, Xie J, Lim C, Huang C-L. Soluble klotho regulates TRPC6 calcium signaling lipid rafts, independent of the FGFR-FGF23 pathway. FASEB J. 2019 Aug;33((8)):9182–93. doi: 10.1096/fj.201900321R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dalton G, An SW, Al-Juboori SI, Nischan N, Yoon J, Dobrinskikh E, et al. Soluble klotho binds monosialoganglioside to regulate membrane microdomains and growth factor signaling. Proc Natl Acad Sci U S A. 2017 Jan 24;114((4)):752–7. doi: 10.1073/pnas.1620301114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang C-L. Regulation of ion channels by secreted Klotho: mechanisms and implications. Kidney Int. 2012;77((10)):855–60. doi: 10.1038/ki.2010.73. [DOI] [PubMed] [Google Scholar]
- 25.Satoh M, Nagasu H, Morita Y, Yamaguchi TP, Kanwar YS, Kashihara N. Klotho protects against mouse renal fibrosis by inhibiting Wnt signaling. Am J Physiol Renal Physiol. 2012 Dec 15;303((12)):F1641–51. doi: 10.1152/ajprenal.00460.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, et al. Suppression of aging in mice by the hormone Klotho. Science. 2005 Sep 16;309((5742)):1829–33. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wolf I, Levanon-Cohen S, Bose S, Ligumsky H, Sredni B, Kanety H, et al. Klotho: a tumor suppressor and a modulator of the IGF-1 and FGF pathways in human breast cancer. Oncogene. 2008 Nov 27;27((56)):7094–105. doi: 10.1038/onc.2008.292. [DOI] [PubMed] [Google Scholar]
- 28.Doi S, Zou Y, Togao O, Pastor JV, John GB, Wang L, et al. Klotho inhibits transforming growth factor-beta1 (TGF-beta1) signaling and suppresses renal fibrosis and cancer metastasis in mice. J Biol Chem. 2011 Mar 11;286((10)):8655–65. doi: 10.1074/jbc.M110.174037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ravarotto V, Simioni F, Pagnin E, Davis PA, Calò LA. Oxidative stress: chronic kidney disease: cardiovascular disease: a vicious circle. Life Sci. 2018 Oct 1;210:125–31. doi: 10.1016/j.lfs.2018.08.067. [DOI] [PubMed] [Google Scholar]
- 30.Huang M, Zheng L, Xu H, Tang D, Lin L, Zhang J, et al. Oxidative stress contributes to vascular calcification in patients with chronic kidney disease. J Mol Cell Cardiol. 2020 Jan;138:256–68. doi: 10.1016/j.yjmcc.2019.12.006. [DOI] [PubMed] [Google Scholar]
- 31.Yamamoto M, Clark JD, Pastor JV, Gurnani P, Nandi A, Kurosu H, et al. Regulation of oxidative stress by the anti-aging hormone klotho. J Biol Chem. 2005 Nov 11;280((45)):38029–34. doi: 10.1074/jbc.M509039200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lim SW, Jin L, Luo K, Jin J, Shin YJ, Hong SY, et al. Klotho enhances FoxO3-mediated manganese superoxide dismutase expression by negatively regulating PI3K/AKT pathway during tacrolimus-induced oxidative stress. Cell Death Dis. 2017 Aug 3;8((8)):e2972. doi: 10.1038/cddis.2017.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsieh CC, Kuro-o M, Rosenblatt KP, Brobey R, Papaconstantinou J. The ASK1-Signalosome regulates p38 MAPK activity in response to levels of endogenous oxidative stress in the Klotho mouse models of aging. Aging (Albany NY) 2010 Sep;2((9)):597–611. doi: 10.18632/aging.100194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ruiz-Andres O, Sanchez-Niño MD, Moreno JA, Ruiz-Ortega M, Ramos AM, Sanz AB, et al. Downregulation of kidney protective factors by inflammation: role of transcription factors and epigenetic mechanisms. Am J Physiol Renal Physiol. 2016 Dec 1;311((6)):F1329–40. doi: 10.1152/ajprenal.00487.2016. [DOI] [PubMed] [Google Scholar]
- 35.Jin M, Lv P, Chen G, Wang P, Zuo Z, Ren L, et al. Klotho ameliorates cyclosporine A-induced nephropathy via PDLIM2/NF-kB p65 signaling pathway. Biochem Biophys Res Commun. 2017 Apr 29;486((2)):451–7. doi: 10.1016/j.bbrc.2017.03.061. [DOI] [PubMed] [Google Scholar]
- 36.Liu F, Wu S, Ren H, Gu J. Klotho suppresses RIG-I-mediated senescence-associated inflammation. Nat Cell Biol. 2011 Mar;13((3)):254–62. doi: 10.1038/ncb2167. [DOI] [PubMed] [Google Scholar]
- 37.Hui H, Zhai Y, Ao L, Cleveland JC, Liu H, Fullerton DA, et al. Klotho suppresses the inflammatory responses and ameliorates cardiac dysfunction in aging endotoxemic mice. Oncotarget. 2017 Feb 28;8((9)):15663–76. doi: 10.18632/oncotarget.14933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Y, Bi X, Xiong J, Han W, Xiao T, Xu X, et al. MicroRNA-34a promotes renal fibrosis by downregulation of klotho in tubular epithelial cells. Mol Ther. 2019 May 8;27((5)):1051–65. doi: 10.1016/j.ymthe.2019.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Donate-Correa J, Martín-Núñez E, Mora-Fernández C, Muros-de-Fuentes M, Pérez-Delgado N, Navarro-González JF. Klotho in cardiovascular disease: current and future perspectives. World J Biol Chem. 2015 Nov 26;6((4)):351–7. doi: 10.4331/wjbc.v6.i4.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takenaka T, Kobori H, Inoue T, Miyazaki T, Suzuki H, Nishiyama A, et al. Klotho supplementation ameliorates blood pressure and renal function in DBA/2-pcy mice, a model of polycystic kidney disease. Am J Physiol Renal Physiol. 2020 Mar 1;318((3)):F557–64. doi: 10.1152/ajprenal.00299.2019. [DOI] [PubMed] [Google Scholar]
- 41.Dai L, Qureshi AR, Witasp A, Lindholm B, Stenvinkel P. Early vascular ageing and cellular senescence in chronic kidney disease. Comput Struct Biotechnol J. 2019;17:721–9. doi: 10.1016/j.csbj.2019.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ray M, Jovanovich A. Mineral bone abnormalities and vascular calcifications. Adv Chronic Kidney Dis. 2019 Nov;26:409–16. doi: 10.1053/j.ackd.2019.09.004. [DOI] [PubMed] [Google Scholar]
- 43.Ritter CS, Slatopolsky E. Phosphate toxicity in CKD: the killer among us. Clin J Am Soc Nephrol. 2016 Jun 6;11((6)):1088–100. doi: 10.2215/CJN.11901115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamada S, Giachelli CM. Vascular calcification in CKD-MBD: roles for phosphate, FGF23, and Klotho. Bone. 2017 Jul;100:87–93. doi: 10.1016/j.bone.2016.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kukida M, Mogi M, Kan-No H, Tsukuda K, Bai H-Y, Shan B-S, et al. AT2 receptor stimulation inhibits phosphate-induced vascular calcification. Kidney Int. 2019 Jan;95:138–48. doi: 10.1016/j.kint.2018.07.028. [DOI] [PubMed] [Google Scholar]
- 46.Kitagawa M, Sugiyama H, Morinaga H, Inoue T, Takiue K, Ogawa A, et al. A decreased level of serum soluble Klotho is an independent biomarker associated with arterial stiffness in patients with chronic kidney disease. PLoS One. 2013;8((2)):e56695. doi: 10.1371/journal.pone.0056695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fan Q. Decreased level of serum soluble Klotho is a biomarker associated with vascular calcification in peritoneal dialysis patients. Hong Kong J Nephrol. 2015;17((2)):S125–6. [Google Scholar]
- 48.Hu MC, Shi M, Zhang J, Quiñones H, Griffith C, Kuro-o M, et al. Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol. 2011 Jan;22((1)):124–36. doi: 10.1681/ASN.2009121311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lim K, Lu TS, Molostvov G, Lee C, Lam FT, Zehnder D, et al. Vascular Klotho deficiency potentiates the development of human artery calcification and mediates resistance to fibroblast growth factor 23. Circulation. 2012 May 8;125((18)):2243–55. doi: 10.1161/CIRCULATIONAHA.111.053405. [DOI] [PubMed] [Google Scholar]
- 50.Morishita K, Shirai A, Kubota M, Katakura Y, Nabeshima Y, Takeshige K, et al. The progression of aging in klotho mutant mice can be modified by dietary phosphorus and zinc. J Nutr. 2001 Dec;131((12)):3182–8. doi: 10.1093/jn/131.12.3182. [DOI] [PubMed] [Google Scholar]
- 51.Ohnishi M, Razzaque MS. Dietary and genetic evidence for phosphate toxicity accelerating mammalian aging. FASEB J. 2010 Sep;24((9)):3562–71. doi: 10.1096/fj.09-152488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ohnishi M, Nakatani T, Lanske B, Razzaque MS. Reversal of mineral ion homeostasis and soft-tissue calcification of klotho knockout mice by deletion of vitamin D 1alpha-hydroxylase. Kidney Int. 2009 Jun;75((11)):1166–72. doi: 10.1038/ki.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nicoll R, Howard JM, Henein MY. A review of the effect of diet on cardiovascular calcification. Int J Mol Sci. 2015 Apr 21;16((4)):8861–83. doi: 10.3390/ijms16048861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hum JM, O'Bryan LM, Tatiparthi AK, Cass TA, Clinkenbeard EL, Cramer MS, et al. Chronic hyperphosphatemia and vascular calcification are reduced by stable delivery of soluble Klotho. J Am Soc Nephrol. 2017 Apr;28((4)):1162–74. doi: 10.1681/ASN.2015111266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hanazaki A, Ikuta K, Sasaki S, Sasaki S, Koike M, Tanifuji K, et al. Role of sodium-dependent Pi transporter/Npt2c on Pi homeostasis in klotho knockout mice different properties between juvenile and adult stages. Physiol Rep. 2020 Feb;8((3)):e14324. doi: 10.14814/phy2.14324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu L, Liu Y, Zhang Y, Bi X, Nie L, Liu C, et al. High phosphate-induced downregulation of PPARγ contributes to CKD-associated vascular calcification. J Mol Cell Cardiol. 2018 Jan;114:264–75. doi: 10.1016/j.yjmcc.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 57.Duni A, Liakopoulos V, Roumeliotis S, Peschos D, Dounousi E. Oxidative stress in the pathogenesis and evolution of chronic kidney disease: untangling Ariadne's thread. Int J Mol Sci. 2019 Jul 29;20((15)):3711. doi: 10.3390/ijms20153711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang Y, Kuro-o M, Sun Z. Klotho gene delivery suppresses Nox2 expression and attenuates oxidative stress in rat aortic smooth muscle cells via the cAMP-PKA pathway. Aging Cell. 2012 Jun;11((3)):410–7. doi: 10.1111/j.1474-9726.2012.00796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun H, Zhang F, Xu Y, Sun S, Wang H, Du Q, et al. Salusin-β promotes vascular calcification via nicotinamide adenine dinucleotide phosphate/reactive oxygen species-mediated klotho downregulation. Antioxid Redox Signal. 2019 Dec 20;31((18)):1352–70. doi: 10.1089/ars.2019.7723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mizusaki K, Hasuike Y, Kimura T, Nagasawa Y, Kuragano T, Yamada Y, et al. Inhibition of the mammalian target of rapamycin may augment the increase in soluble klotho levels in renal transplantation recipients. Blood Purif. 2019;47((Suppl 2)):12–8. doi: 10.1159/000496630. [DOI] [PubMed] [Google Scholar]
- 61.Chang JR, Guo J, Wang Y, Hou YL, Lu WW, Zhang JS, et al. Intermedin1-53 attenuates vascular calcification in rats with chronic kidney disease by upregulation of α-Klotho. Kidney Int. 2016 Mar;89((3)):586–600. doi: 10.1016/j.kint.2015.12.029. [DOI] [PubMed] [Google Scholar]
- 62.Di Lullo L, Gorini A, Russo D, Santoboni A, Ronco C. Left ventricular hypertrophy in chronic kidney disease patients: from pathophysiology to treatment. Cardiorenal Med. 2015;5((4)):254–66. doi: 10.1159/000435838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grande D, Gioia MI, Terlizzese P, Iacoviello M. Heart failure and kidney disease. Adv Exp Med Biol. 2018;1067:219–38. doi: 10.1007/5584_2017_126. [DOI] [PubMed] [Google Scholar]
- 64.Zhang A-H, Guo W-K, Yu L, Liu W-H. Relationship of serum soluble klotho levels and echocardiographic parameters in patients on maintenance hemodialysis. Kidney Blood Press Res. 2019;44((3)):396–404. doi: 10.1159/000499200. [DOI] [PubMed] [Google Scholar]
- 65.Xie J, Wu YL, Huang CL. Deficiency of soluble α-Klotho as an independent cause of uremic cardiomyopathy. Vitam Horm. 2016;101:311–30. doi: 10.1016/bs.vh.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 66.Tang G, Shen Y, Gao P, Song S-S, Si L-Y. Klotho attenuates isoproterenol-induced hypertrophic response in H9C2 cells by activating Na/K-ATPase and inhibiting the reverse mode of Na/Ca-exchanger. In Vitro Cell Dev Biol Anim. 2018 Mar;54((3)):250–6. doi: 10.1007/s11626-017-0215-5. [DOI] [PubMed] [Google Scholar]
- 67.Ding J, Tang Q, Luo B, Zhang L, Lin L, Han L, et al. Klotho inhibits angiotensin II-induced cardiac hypertrophy, fibrosis, and dysfunction in mice through suppression of transforming growth factor-β1 signaling pathway. Eur J Pharmacol. 2019 Sep 15;859:172549. doi: 10.1016/j.ejphar.2019.172549. [DOI] [PubMed] [Google Scholar]
- 68.Guo Y, Zhuang X, Huang Z, Zou J, Yang D, Hu X, et al. Klotho protects the heart from hyperglycemia-induced injury by inactivating ROS and NF-κB-mediated inflammation both in vitro and in vivo. Biochim Biophys Acta. 2018 Jan;1864((1)):238–51. doi: 10.1016/j.bbadis.2017.09.029. [DOI] [PubMed] [Google Scholar]
- 69.Razzaque MS. The FGF23-Klotho axis: endocrine regulation of phosphate homeostasis. Nat Rev Endocrinol. 2009;5((11)):611–9. doi: 10.1038/nrendo.2009.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, et al. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011 Nov;121((11)):4393–408. doi: 10.1172/JCI46122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Leifheit-Nestler M, Große Siemer R, Flasbart K, Richter B, Kirchhoff F, Ziegler WH, et al. Induction of cardiac FGF23/FGFR4 expression is associated with left ventricular hypertrophy in patients with chronic kidney disease. Nephrol Dial Transplant. 2016 Jul;31((7)):1088–99. doi: 10.1093/ndt/gfv421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Han X, Cai C, Xiao Z, Quarles LD. FGF23 induced left ventricular hypertrophy mediated by FGFR4 signaling in the myocardium is attenuated by soluble Klotho in mice. J Mol Cell Cardiol. 2020 Jan;138:66–74. doi: 10.1016/j.yjmcc.2019.11.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Suassuna PGdA, Cherem PM, de Castro BB, Maquigussa E, Cenedeze MA, Lovisi JCM, et al. αKlotho attenuates cardiac hypertrophy and increases myocardial fibroblast growth factor 21 expression in uremic rats. Exp Biol Med. 2020 Jan;245((1)):66–78. doi: 10.1177/1535370219894302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xie J, Cha SK, An SW, Kuro-O M, Birnbaumer L, Huang CL. Cardioprotection by Klotho through downregulation of TRPC6 channels in the mouse heart. Nat Commun. 2012;3:1238. doi: 10.1038/ncomms2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xie J, Yoon J, An SW, Kuro-o M, Huang CL. Soluble Klotho protects against uremic cardiomyopathy independently of fibroblast growth factor 23 and phosphate. J Am Soc Nephrol. 2015 May;26((5)):1150–60. doi: 10.1681/ASN.2014040325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Paterson MR, Geurts AM, Kriegel AJ. miR-146b-5p has a sex-specific role in renal and cardiac pathology in a rat model of chronic kidney disease. Kidney Int. 2019 Dec;96((6)):1332–45. doi: 10.1016/j.kint.2019.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang B, Zhang A, Wang H, Klein JD, Tan L, Wang Z-M, et al. miR-26a limits muscle wasting and cardiac fibrosis through exosome-mediated microRNA transfer in chronic kidney disease. Theranostics. 2019;9((7)):1864–77. doi: 10.7150/thno.29579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu Q, Zhu L-J, Waaga-Gasser AM, Ding Y, Cao M, Jadhav SJ, et al. The axis of local cardiac endogenous Klotho-TGF-β1-Wnt signaling mediates cardiac fibrosis in human. J Mol Cell Cardiol. 2019 Nov;136:113–24. doi: 10.1016/j.yjmcc.2019.09.004. [DOI] [PubMed] [Google Scholar]
- 79.Libby P, Buring JE, Badimon L, Hansson GK, Deanfield J, Bittencourt MS, et al. Atherosclerosis. Nat Rev Dis Primers. 2019;5((1)):56. doi: 10.1038/s41572-019-0106-z. [DOI] [PubMed] [Google Scholar]
- 80.Drüeke TB, Massy ZA. Atherosclerosis in CKD: differences from the general population. Nat Rev Nephrol. 2010;6((12)):723–35. doi: 10.1038/nrneph.2010.143. [DOI] [PubMed] [Google Scholar]
- 81.Keles N, Caliskan M, Dogan B, Keles NN, Kalcik M, Aksu F, et al. Low serum level of klotho is an early predictor of atherosclerosis. Tohoku J Exp Med. 2015 Sep;237((1)):17–23. doi: 10.1620/tjem.237.17. [DOI] [PubMed] [Google Scholar]
- 82.Martín-Núñez E, Donate-Correa J, Ferri C, López-Castillo Á, Delgado-Molinos A, Hernández-Carballo C, et al. Association between serum levels of Klotho and inflammatory cytokines in cardiovascular disease: a case-control study. Aging (Albany NY) 2020 Jan 27;12((2)):1952–64. doi: 10.18632/aging.102734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kuro-O M. The Klotho proteins in health and disease. Nat Rev Nephrol. 2019 Jan;15((1)):27–44. doi: 10.1038/s41581-018-0078-3. [DOI] [PubMed] [Google Scholar]
- 84.Kim HY, Yoo TH, Hwang Y, Lee GH, Kim B, Jang J, et al. Indoxyl sulfate (IS)-mediated immune dysfunction provokes endothelial damage in patients with end-stage renal disease (ESRD) Sci Rep. 2017 Jun 8;7((1)):3057. doi: 10.1038/s41598-017-03130-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ohta J, Rakugi H, Ishikawa K, Yang J, Ikushima M, Chihara Y, et al. Klotho gene delivery suppresses oxidative stress in vivo. Geriatr Gerontol Int. 2007;7((3)):293–9. [Google Scholar]
- 86.Rakugi H, Matsukawa N, Ishikawa K, Yang J, Imai M, Ikushima M, et al. Anti-oxidative effect of Klotho on endothelial cells through cAMP activation. Endocrine. 2007 Feb;31((1)):82–7. doi: 10.1007/s12020-007-0016-9. [DOI] [PubMed] [Google Scholar]
- 87.Cui W, Leng B, Wang G. Klotho protein inhibits H2O2-induced oxidative injury in endothelial cells via regulation of PI3K/AKT/Nrf2/HO-1 pathways. Can J Physiol Pharmacol. 2019;97((5)):370–6. doi: 10.1139/cjpp-2018-0277. [DOI] [PubMed] [Google Scholar]
- 88.Yao Y, Wang Y, Zhang Y, Liu C. Klotho ameliorates oxidized low density lipoprotein (ox-LDL)-induced oxidative stress via regulating LOX-1 and PI3K/Akt/eNOS pathways. Lipids Health Dis. 2017;16((1)):77. doi: 10.1186/s12944-017-0447-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ikushima M, Rakugi H, Ishikawa K, Maekawa Y, Yamamoto K, Ohta J, et al. Anti-apoptotic and anti-senescence effects of Klotho on vascular endothelial cells. Biochem Biophys Res Commun. 2006;339((3)):827–32. doi: 10.1016/j.bbrc.2005.11.094. [DOI] [PubMed] [Google Scholar]
- 90.Liu Y, Lai P, Deng J, Hao Q, Li X, Yang M, et al. sKlotho Micro-RNA335-5p targeted inhibition of and promoted oxidative stress-mediated aging of endothelial cells. Biomark Med. 2019 Apr;13((6)):457–66. doi: 10.2217/bmm-2018-0430. [DOI] [PubMed] [Google Scholar]
- 91.Uhrin P, Wang D, Mocan A, Waltenberger B, Breuss JM, Tewari D, et al. Vascular smooth muscle cell proliferation as a therapeutic target. Part 2: natural products inhibiting proliferation. Biotechnol Adv. 2018;36((6)):1608–21. doi: 10.1016/j.biotechadv.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 92.Yu S, Chen Y, Chen S, Ye N, Li Y, Sun Y. Klotho inhibits proliferation and migration of angiotensin II-induced vascular smooth muscle cells (VSMCs) by modulating NF-κB p65, Akt, and extracellular signal regulated kinase (ERK) signaling activities. Med Sci Monit. 2018 Jul 13;24:4851–60. doi: 10.12659/MSM.908038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Maltese G, Psefteli PM, Rizzo B, Srivastava S, Gnudi L, Mann GE, et al. The anti-ageing hormone klotho induces Nrf2-mediated antioxidant defences in human aortic smooth muscle cells. J Cell Mol Med. 2017 Mar;21((3)):621–7. doi: 10.1111/jcmm.12996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.He T, Xiong J, Huang Y, Zheng C, Liu Y, Bi X, et al. Klotho restrain RIG-1/NF-κB signaling activation and monocyte inflammatory factor release under uremic condition. Life Sci. 2019 Aug 15;231:116570. doi: 10.1016/j.lfs.2019.116570. [DOI] [PubMed] [Google Scholar]
- 95.Jia Y, Zheng Z, Xue M, Zhang S, Hu F, Li Y, et al. Extracellular vesicles from albumin-induced tubular epithelial cells promote the M1 macrophage phenotype by targeting klotho. Mol Ther. 2019 Aug 7;27((8)):1452–66. doi: 10.1016/j.ymthe.2019.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu W, Chen X, Wu M, Li L, Liu J, Shi J, et al. Recombinant Klotho protein enhances cholesterol efflux of THP-1 macrophage-derived foam cells via suppressing Wnt/β-catenin signaling pathway. BMC Cardiovasc Disord. 2020 Mar 5;20((1)):120. doi: 10.1186/s12872-020-01400-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.André P, Denis CV, Ware J, Saffaripour S, Hynes RO, Ruggeri ZM, et al. Platelets adhere to and translocate on von Willebrand factor presented by endothelium in stimulated veins. Blood. 2000 Nov 15;96((10)):3322–8. [PubMed] [Google Scholar]
- 98.Huo Y, Schober A, Forlow SB, Smith DF, Hyman MC, Jung S, et al. Circulating activated platelets exacerbate atherosclerosis in mice deficient in apolipoprotein E. Nat Med. 2003 Jan;9((1)):61–7. doi: 10.1038/nm810. [DOI] [PubMed] [Google Scholar]
- 99.Liu M, Li XC, Lu L, Cao Y, Sun RR, Chen S, et al. Cardiovascular disease and its relationship with chronic kidney disease. Eur Rev Med Pharmacol Sci. 2014 Oct;18((19)):2918–26. [PubMed] [Google Scholar]
- 100.Matsushita K, Ballew SH, Coresh J. Cardiovascular risk prediction in people with chronic kidney disease. Curr Opin Nephrol Hypertens. 2016;25((6)):518–23. doi: 10.1097/MNH.0000000000000265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Marçais C, Maucort-Boulch D, Drai J, Dantony E, Carlier MC, Blond E, et al. Circulating Klotho associates with cardiovascular morbidity and mortality during hemodialysis. J Clin Endocrinol Metab. 2017 Sep 1;102((9)):3154–61. doi: 10.1210/jc.2017-00104. [DOI] [PubMed] [Google Scholar]
- 102.Memmos E, Sarafidis P, Pateinakis P, Tsiantoulas A, Faitatzidou D, Giamalis P, et al. Soluble Klotho is associated with mortality and cardiovascular events in hemodialysis. BMC Nephrol. 2019 Jun 11;20((1)):217. doi: 10.1186/s12882-019-1391-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yang K, Yang J, Bi X, Yu Z, Xiao T, Huang Y, et al. Serum klotho, cardiovascular events, and mortality in nondiabetic chronic kidney disease. Cardiorenal Med. 2020;10((3)):175–87. doi: 10.1159/000506380. [DOI] [PubMed] [Google Scholar]
- 104.Zhang R, Zheng F. PPAR-gamma and aging: one link through klotho? Kidney Int. 2008 Sep;74((6)):702–4. doi: 10.1038/ki.2008.382. [DOI] [PubMed] [Google Scholar]
- 105.Forster RE, Jurutka PW, Hsieh JC, Haussler CA, Lowmiller CL, Kaneko I, et al. Vitamin D receptor controls expression of the anti-aging klotho gene in mouse and human renal cells. Biochem Biophys Res Commun. 2011 Oct 28;414((3)):557–62. doi: 10.1016/j.bbrc.2011.09.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yoon HE, Ghee JY, Piao S, Song JH, Han DH, Kim S, et al. Angiotensin II blockade upregulates the expression of Klotho, the anti-ageing gene, in an experimental model of chronic cyclosporine nephropathy. Nephrol Dial Transplant. 2011 Mar;26((3)):800–13. doi: 10.1093/ndt/gfq537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ohnishi M, Kato S, Akiyoshi J, Atfi A, Razzaque MS. Dietary and genetic evidence for enhancing glucose metabolism and reducing obesity by inhibiting klotho functions. FASEB J. 2011 Jun;25((6)):2031–9. doi: 10.1096/fj.10-167056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hasannejad M, Samsamshariat SZ, Esmaili A, Jahanian-Najafabadi A. Klotho induces insulin resistance possibly through interference with GLUT4 translocation and activation of Akt, GSK3β, and PFKfβ3 in 3T3-L1 adipocyte cells. Res Pharm Sci. 2019 Aug;14((4)):369–77. doi: 10.4103/1735-5362.263627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lorenzi O, Veyrat-Durebex C, Wollheim CB, Villemin P, Rohner-Jeanrenaud F, Zanchi A, et al. Evidence against a direct role of klotho in insulin resistance. Pflugers Arch. 2010 Feb;459((3)):465–73. doi: 10.1007/s00424-009-0735-2. [DOI] [PubMed] [Google Scholar]
- 110.Zhao Y, Zhao M, Cai Y, Zheng M, Sun W, Zhang S, et al. Mammalian target of rapamycin signaling inhibition ameliorates vascular calcification via Klotho upregulation. Kidney Int. 2015 June 10;88((4)):711–21. doi: 10.1038/ki.2015.160. [DOI] [PubMed] [Google Scholar]
- 111.Saito Y, Nakamura T, Ohyama Y, Suzuki T, Iida A, Shiraki-Iida T, et al. In vivo klotho gene delivery protects against endothelial dysfunction in multiple risk factor syndrome. Biochem Biophys Res Commun. 2000 Sep 24;276((2)):767–72. doi: 10.1006/bbrc.2000.3470. [DOI] [PubMed] [Google Scholar]