Abstract
Fusarium oxysporum f. sp. lactucae 016-086 is a plant pathogenic filamentous fungus isolated from wilted lettuce in Korea. We reported complete mitochondrial genome sequence of F. oxysporum f. sp. lactucae 016-086. Total length of this mitogenome is 41,826 bp and it encoded 42 genes (14 protein-coding genes, 2 rRNAs, and 26 tRNAs). Nucleotide sequence of coding region takes over 30.6%, and overall GC content is 32.5%. Phylogenetic tree of Fusarium mitochondrial genomes presented distinct clades along with nine formae speciales. This mitogenome will contribute distinguishing formae speciales of F. oxysoporum claearly with additional mitogenomes sequenced in the near future.
Keywords: Fusarium oxysporum f. sp. lactuace, mitochondrial genome, formae speciales, Ascomycota
Fusarium oxysporum is an ascomycete, soilborne, and pathogenic fungus (Alabouvette and Couteaudier 1992; Fravel et al. 2003). Owing to next generation sequencing technologies, more than 60 fungal mitogenomes have been assembled (Pantou et al. 2008; Fourie et al. 2013; Brankovics et al. 2017; Park et al., in preparation). These mitogenomes cover only nine out of around 120 formae speciales, suggesting mitogenomes of more formae speciales should be investigated to understand their genetic diversities as well as host-specificity. F. oxysporum f. sp. lactucae 16-086 isolated from wilted lettuces in Iksan area, South Korea (36.041085 N, 126.976071E) and identified based on translation elongation factor 1-a gene sequences (Samson et al. 2004) confirmed strong pathogenicity to lettuces as other F. oxysporum did. To understand mitogenomic background of F. oxysporum f. sp. lactuace, we sequenced its complete mitochondrial genome.
Its DNA was extracted from the hyphae of F. oxysporum f. sp. lactuace 16-086, taken from Horticultural and Herbal Crop Environment Division (16-086), by using HiGene™ Genomic DNA Prep Kit (BIOFACT, Korea). Raw data generated by HiSeq4000 were subject to de novo assembly done by Velvet 1.2.10 (Zerbino and Birney 2008), gap filling with SOAPGapCloser 1.12 (Zhao et al. 2011), and base confirmation with BWA 0.7.17 and SAMtools 1.9 (Li et al. 2009; Li 2013). Geneious R11 11.0.5 (Biomatters Ltd, Auckland, New Zealand) was used to annotate its mitochondrial genome by comparing with that of F. oxysporum f. sp. cucumerinum (LT906315; Brankovics et al. 2017).
The length of F. oxysporum f. sp. lactuace 16-086 mitochondrial genome (Genbank accession is MN259514) is 41,826 bp. Its mitochondrial genome encoded 42 genes consisting of 14 protein-coding genes (PCGs), 2 rRNAs, and 26 tRNAs, different from that of F. oxysporum f. sp. cucumerinum (LT906315). Nucleotide sequence of coding region takes over 30.6%, and overall GC content is 32.5%.
Based on alignment between 16-086 and F. oxysporum f. sp. cucumerinum (LT906315), 3,889-bp deletion on 16-086 mitogenome covering three genes and one pair of direct repeat sequences were found. Except it, only six single nucleotide polymorphisms (SNPs) and 18 insertions and deletions (INDELs) are identified. There are 62 SNPs and 180 INDELs except 3,861-bp deletion on 16-086 mitogenome based on alignment between 16-086 and Fusarium oxysporum f. sp. cubense race 1 (LT906350), presenting that F. oxysporum f. sp. lactuace is very close to F. oxysporum f. sp. cucumerinum.
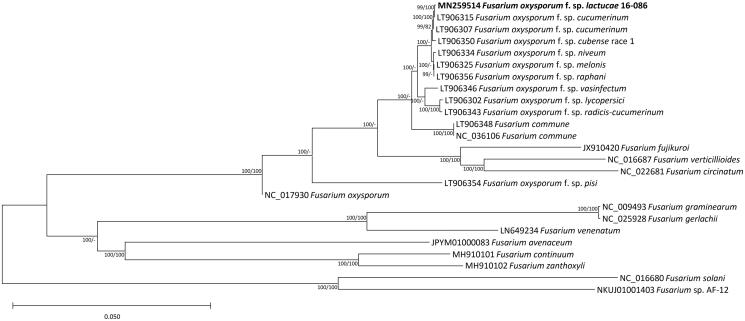
Sequence alignment of 14 conserved PCGs extracted from twenty-five Fusarium mitochondrial genomes was conducted by MAFFT 7.388 (Katoh and Standley 2013). The bootstrapped neighbor joining and maximum likelihood phylogenetic trees were constructed using MEGA X (Kumar et al. 2018) and IQ-TREE 1.6.6 (Nguyen et al. 2014), respectively. Based on phylogenetic tree, nine formae speciales of F. oxysporum show distinct phylogenetic position and it is clearly distinct with Fusarium commune (Figure 1). In addition, F. oxysporum f. sp. lactuace and F. oxysporum f. sp. cucumerinum (LT906315) are clustered tightly, agreeing with number of SNPs and INDELs between two mitogenomes. Our mitogenome can contribute for distinguishing formae speciales clearly with more F. oxysporum mitogenomes in the near future.
Figure 1.
Neighbor joining (bootstrap repeat is 10,000) and maximum likelihood (bootstrap repeat is 1,000) phylogenetic trees of Fusarium based on 25 Fusarium mitochondrial genomes: F. oxysporum f. sp. lactucae (MN259514 in this study), F. oxysporum f. sp. cucumerinum (LT906315, LT906307), F. oxysporum f. sp. cubense race 1 (LT906350), F. oxysporum f. sp. niveum (LT906334), F. oxysporum f. sp. melonis (LT906325), F. oxysporum f. sp. raphani (LT906356), F. oxysporum f. sp. vasinfectum (LT906346), F. oxysporum f. sp. lycopersici (LT906302), F. oxysporum f. sp. radicis-cucumerinum (LT906343), F. oxysporum f. sp. pisi (LT906354), F. oxysporum (NC_017930), F. commune (LT906348, NC_036106), F. fujikuroi (JX910420), F. verticillioides (NC_016687), F. circinatum (NC_022681), F. graminearum (NC_009493), F. gerlachii (NC_025928), F. venenatum (LN649234), F. avenaceum (JPYM01000083), F. continuum (MH910101), F. zanthoxyli (MH910102), F. solani (NC_016680), and Fusarium sp. AF-12 (NKUJ01001403). Phylogenetic tree was drawn based on neighbour joining tree from alignment of complete mitochondiral genomes. The numbers above branches indicate bootstrap support values of neighbor joining and maximum likelihood phylogenetic trees, respectively.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Disclosure statement
The authors declare that they have no competing interests.
References
- Alabouvette C, Couteaudier Y. 1992. Biological control of Fusarium wilts with nonpathogenic Fusaria. Biological control of plant diseases. Berlin: Springer; p. 415–426. [Google Scholar]
- Brankovics B, van Dam P, Rep M, de Hoog GS, van der Lee TA, Waalwijk C, van Diepeningen AD. 2017. Mitochondrial genomes reveal recombination in the presumed asexual Fusarium oxysporum species complex. BMC Genomics. 18:735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourie G, Van der Merwe NA, Wingfield BD, Bogale M, Tudzynski B, Wingfield MJ, Steenkamp ET. 2013. Evidence for inter-specific recombination among the mitochondrial genomes of Fusarium species in the Gibberella fujikuroi complex. BMC Genomics. 14:605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fravel D, Olivain C, Alabouvette C. 2003. Fusarium oxysporum and its biocontrol. New Phytol. 157:493–502. [DOI] [PubMed] [Google Scholar]
- Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 30:772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K. 2018. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 35:1547–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R. 2009. The sequence alignment/map format and SAMtools. Bioinformatics. 25:2078–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. 2013. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv preprint arXiv:13033997. [Google Scholar]
- Nguyen L-T, Schmidt HA, von Haeseler A, Minh BQ. 2014. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol. 32:268–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantou MP, Kouvelis VN, Typas MA. 2008. The complete mitochondrial genome of Fusarium oxysporum: insights into fungal mitochondrial evolution. Gene. 419:7–15. [DOI] [PubMed] [Google Scholar]
- Samson RA, Hoekstra ES, Frisvad JC. 2004. Introduction to food-and airborne fungi. Centraalbureau voor Schimmelcultures (CBS). Ed. 7). [Google Scholar]
- Zerbino DR, Birney E. 2008. Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 18:821–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao QY, Wang Y, Kong YM, Luo D, Li X, Hao P. 2011. Optimizing de novo transcriptome assembly from short-read RNA-Seq data: a comparative study. BMC Bioinformatics. BioMed Central. 15 :S2. [DOI] [PMC free article] [PubMed] [Google Scholar]