Abstract
This report is based on proceedings from the Exposure Assessment Tools for Hypersensitivity Pneumonitis (HP) Workshop, sponsored by the American Thoracic Society, that took place on May 18, 2019, in Dallas, Texas. The workshop was initiated by members from the Environmental, Occupational, and Population Health and Clinical Problems Assemblies of the American Thoracic Society. Participants included international experts from pulmonary medicine, occupational medicine, radiology, pathology, and exposure science. The meeting objectives were to 1) define currently available tools for exposure assessment in evaluation of HP, 2) describe the evidence base supporting the role for these exposure assessment tools in HP evaluation, 3) identify limitations and barriers to each tool’s implementation in clinical practice, 4) determine which exposure assessment tools demonstrate the best performance characteristics and applicability, and 5) identify research needs for improving exposure assessment tools for HP. Specific discussion topics included history-taking and exposure questionnaires, antigen avoidance, environmental assessment, specific inhalational challenge, serum-specific IgG testing, skin testing, lymphocyte proliferation testing, and a multidisciplinary team approach. Priorities for research in this area were identified.
Keywords: hypersensitivity pneumonitis, extrinsic allergic alveolitis, interstitial lung disease, pulmonary fibrosis, exposure assessment
Contents
Executive Summary
Introduction
Methodology
Findings/Results
History-Taking and Exposure Questionnaires
Antigen Avoidance
Environmental Assessment
SIC
Serum-Specific Immunoglobulin Measurement
LPT
Multidisciplinary Team Approach
Summary and Future Directions
Executive Summary
Hypersensitivity pneumonitis (HP) is an immune-mediated interstitial lung disease (ILD) occurring in susceptible individuals after exposure to one or more antigens. Accurate antigen identification can be challenging yet is fundamental to diagnosis and management. Several tools are available for antigen identification in patients with suspected or confirmed HP, but there is limited evidence guiding their application in clinical practice. A comprehensive patient history to identify potential exposures associated with HP is an essential part of the clinical assessment. Validated and regionally relevant questionnaires would help supplement clinical history-taking and should be developed. Clinical improvement with antigen avoidance may inform the causative exposure in nonfibrotic cases but is less helpful in fibrotic HP. Environmental assessment by exposure scientists and/or industrial hygienists may identify otherwise unrecognized exposures and antigens. Residential, occupational, and avocational environmental sampling may be informative, but these tools need to be standardized, and guidelines should be developed for exposure abatement. Positive results from serum-specific immunoglobulin G (SS-IgG) testing indicate exposure sufficient for immunologic sensitization but do not prove causality. Negative test results do not exclude specific antigens as potential causes, and positive test results can occur in those with exposure but without disease. Specific inhalational challenge (SIC) and lymphocyte proliferation testing (LPT) provide information on immunologic sensitization but again do not establish causality, and their role in HP diagnosis and exposure assessment is unclear. Skin testing against specific antigens has no clinical role. Multidisciplinary evaluation of patients with suspected HP, guided by the integration of clinical history and questionnaire, environmental assessment and sampling, and in select cases, immunologic testing, likely would provide the most comprehensive approach to exposure assessment. No single approach will identify relevant antigen exposures in all situations. Future research should characterize the additive discriminative value of specific and well-standardized tests to identify causal antigens.
Introduction
HP is an inflammatory and/or fibrotic ILD, arising in susceptible individuals after inhalational exposure to one or more inciting antigens. HP can be acute and reversible or chronic and fibrotic (chronic HP [CHP]), the latter being associated with substantial morbidity and mortality (1–4). Diagnosing HP is challenging given the heterogeneity in clinical presentation, overlapping features with other pulmonary diseases, and historical lack of consensus on diagnostic criteria. A proportion of patients in whom idiopathic pulmonary fibrosis (IPF) is diagnosed are found to have CHP on the basis of explant studies or case reevaluation, suggesting that misdiagnosis is not infrequent (5, 6). This may result from not eliciting the relevant exposure history or identifying a putative causal antigen. Accurate and timely identification of inciting antigens improves diagnostic accuracy and may improve patient outcomes through exposure remediation. Recent efforts have established consensus diagnostic guidelines for HP, with exposure identification the first step toward achieving a confident diagnosis (see the American Thoracic Society [ATS] (7) and American College of Chest Physicians guidelines when published). Antigen identification can be challenging, given the variable reliability of exposure assessment tools and heterogeneity in their clinical use (8–10). Challenges arise in practice, as clinicians have little guidance on what exposure assessment techniques are informative, redundant, or even potentially misleading.
The objectives of this workshop were to 1) define currently available tools for exposure assessment in the evaluation of HP, 2) describe the evidence base supporting the role for these exposure assessment tools in HP evaluation, 3) identify limitations and barriers to each tool’s implementation in clinical practice, 4) determine which exposure assessment tools demonstrate the best performance characteristics and applicability, and 5) identify research needs for improving exposure assessment tools for HP. Specific topic areas for discussion included:
-
1.
History-taking and exposure questionnaires.
-
2.
Antigen avoidance.
-
3.
Environmental assessment.
-
4.
SIC.
-
5.
SS-IgG testing.
-
6.
Skin testing (see online supplement).
-
7.
LPT.
-
8.
Multidisciplinary team approach.
The ATS Workshop on Exposure Assessment Tools for HP was held in Dallas, Texas, on May 18, 2019. International experts convened to discuss the roles and limitations of each approach based on published literature. The purpose of the 1-day meeting was to lay the groundwork to critically appraise the evidence base for the exposure-assessment modalities listed above and identify research priorities for exposure assessment for antigens associated with HP. This report summarizes the workshop findings and recommendations.
Methodology
The Co–Chairs (K.A.J. and C.S.R.) invited an international team of HP experts to participate in the workshop. Potential conflicts of interest were disclosed and managed in accordance with policies and procedures of the ATS. All members participated in the workshop and subsequent document-development process. We assembled a multidisciplinary team of 22 members, representing nine countries and diverse specialties, including pulmonary medicine, occupational medicine, industrial hygiene, exposure science, chest radiology, and lung pathology. Before the workshop, subcommittees conducted literature reviews on each topic, with findings discussed and summarized at the workshop.
Findings/Results
History-Taking and Exposure Questionnaires
Clinical exposure assessment through history-taking involves the clinician leading the patient through a series of questions about their occupational, residential, and avocational environments to elicit possible exposures and temporal patterns associated with HP. Questions should also consider indirect exposures through contact with individuals who may carry antigens on their clothing or other materials.
Adequate history-taking in HP requires a high index of suspicion, time, and content expertise. Differences in approach and content among clinicians lead to varying reliability (11). Ideally, the history is structured, standardized, and formatted. Information-gathering may be iterative, and if the history suggests a potential exposure, further questioning should be conducted to obtain details of duration, extent, and frequency of exposure and relationship to symptoms. Questions should be relevant to the region of residence, considering seasonality and other temporal aspects of exposures. The history should be comprehensive even if the patient answers “yes” to one question, as subsequent questions may reveal additional potential antigen sources (e.g., an indoor hot tub, feather duvet, or moldy basement). The relevant history should be revisited at clinical follow-up. If a well-characterized antigen is identified by history in the context of other clinical findings of HP, further testing to verify the causal antigen may not be needed. If the exposure history does not clearly identify a source for antigens known to cause HP, the likelihood of HP is less certain, and further testing may be required.
Questionnaires help ensure consistency and comprehensiveness in eliciting potential exposures. There are published HP-specific questionnaires (9, 11) and online lists of ILD exposures available to guide clinical assessment (12). Currently, no HP questionnaire has been validated, nor is it likely that one tool would apply to all environmental and occupational settings. We encourage clinicians to use an HP questionnaire for exposures relevant to their regional patient population. In doing so, it is critical to recognize that not all exposures carry the same degree or likelihood of risk, and positive questionnaire responses must be further characterized before assuming likely causality. Not all affirmative responses are equally important. For example, breeding pigeons may need to be more heavily weighted than a report of visible mold between bathroom tiles, on the basis of reported exposures known to be associated with HP.
Antigen Avoidance
Antigen avoidance is considered the mainstay of HP management and is associated with improved clinical outcomes in some patients (13–15). Moreover, clinical improvement after antigen removal supports a causal association. Antigen avoidance can lead to disease resolution and normalization of lung function in some acute cases but is less likely in patients with more advanced fibrotic HP (16–19). Even in these patients, antigen avoidance may slow progression on the continuum from inflammation to fibrosis.
Approaches to addressing a suspected exposure through avoidance range from thorough home cleaning to job change to relocation. A general change of environment has been recommended in some studies, even when the specific HP-associated exposure is not clear (3, 20–23). However, no prospective studies have evaluated the efficacy of environmental change as a tool for antigen identification.
It is unknown how long patients must avoid a putative exposure before assessing disease causation. This is particularly problematic in fibrotic HP because causal antigens are frequently unknown or remote and, if identified, may be present at low concentrations. Importantly, lack of clinical improvement with antigen avoidance does not exclude the diagnosis of HP or rule out an antigen as causal. Furthermore, such measures may be expensive or impractical.
Environmental Assessment
A broad range of residential, avocational, industrial, agricultural, and office-based occupational settings have been associated with HP. The many discrete antigenic agents linked to disease can be categorized into animal products, plant products, microbial bioaerosols, and metal or chemical compounds. It is essential that clinicians develop an approach to environmental exposure assessment, including when and how to involve the expertise of industrial hygienists and other exposure scientists. Environmental professionals with knowledge of building systems and how they influence exposure pathways can make informed recommendations to reduce antigen exposures. Environmental assessment may be particularly important when concerns arise about indoor exposure to microbial antigens.
Several potential pathways link sources of dampness in indoor environments to risk for adverse health outcomes from exposure to microbial bioaerosols. The World Health Organization (24) describes both ambient (rain, groundwater, melting snow, and flooding) and anthropogenic (plumbing/sewage, water supply, and heating system) sources of indoor dampness that may lead to proliferation of molds and other microbial contaminants associated with HP. Furthermore, there is evidence-based guidance on questions clinicians should ask related to indoor dampness and mold (25). Responses to these questions help determine whether an on-site assessment is warranted. If so, it is vital that the environmental professional work closely with the clinician to formulate and test hypotheses for possible exposure hazards and clarify the scope and purpose of the dwelling assessment. Information-gathering begins with understanding the patient’s activities and where and how much time they typically spend in one location or possibly multiple locations of concern. In addition to questions about dampness and mold, details should be elicited about hobbies (e.g., woodworking, gardening, and activities involving organic dust and chemicals), animals (e.g., birds and grains used for feed), barns and workshops, crawlspaces and basements, recreational vehicles, subtle sources (e.g., feathers in decorations or duvets) (19), and activities such as hot-tub use at home and in recreational settings.
After initial information-gathering, an environmental hygienist may be enlisted to perform a qualitative or visual assessment of the indoor space. Published questionnaires (26) are helpful to assess specific components of building mechanical systems, including their operation, condition, and maintenance (27). In general, the on-site visual inspection is most useful for identifying obvious exposure sources and providing expert advice on abatement (24). The site visit is also important in decision-making about the need for quantitative air and/or bulk environmental antigen sampling. Photographs can document physical conditions, evidence of water intrusion or dampness, locations of suspect microbial colonization, and other exposure concerns and may support recommendations for mitigation and remediation.
There are no standardized or validated approaches to quantitative environmental antigen sampling. Surface microbial sampling using tape or swabs and bulk (water or vacuumed dust) samples are useful for identification (present/absent) but not quantification. Air sampling for quantifying fungal and other microbial bioaerosols represents exposure conditions only on the day of sampling and may not represent usual or “worst-case” conditions. Methods based on microbial culture are limited by uncertainties in choice of culture media, inability to detect nonviable species, and unclear thresholds for causal linkage with HP. Quantitative polymerase chain reaction may be advantageous for detecting nonviable microorganisms or those not easily detected in culture (28). Whatever sampling approach is chosen, it is essential to use an accredited environmental analytical laboratory for sample analysis, as most clinical laboratories lack the requisite expertise.
In some cases, the hygienist can obtain bulk samples from the environment for personalized antibody testing of the HP patient (29). In most clinical settings, however, environmental sampling to identify a causal antigen is limited by cost, expertise in sample collection and analysis, and challenges in linking sample results with health outcomes. On-site environmental assessment and targeted sampling may guide recommendations for exposure remediation and avoidance, yet antigen elimination often remains difficult, with little evidence to guide effective abatement. In circumstances in which preabatement sampling shows high concentrations of microbial contaminants or discordant species amplification indoors compared with outdoors, repeat sampling after remediation may be helpful to verify the adequacy of abatement.
SIC
SICs entail exposure to suspected causative antigens in controlled laboratory settings (30, 31). Procedures for SICs in diagnosis of occupational asthma have been standardized (31), and SICs have also been used in patients with suspected HP (32). A SIC may be performed by either inhalation of a nebulized solution of the suspected antigen (e.g., avian or mold) or by exposure in a challenge chamber (e.g., to isocyanates or wood dust); however, the approach to SIC has not been standardized for use in suspected HP. Several studies suggest their use can differentiate both acute and CHP from other ILDs (33–36). In certain clinical contexts, sensitivity and specificity of 73% and 84%, respectively, have been reported, and even higher sensitivity and specificity of 85% and 86% have been reported with avian or fungal antigens (36). A positive SIC result in a patient with ILD may increase the probability of HP diagnosis and preclude the need for invasive tests such as surgical lung biopsies (35).
There are several limitations to widespread implementation of SICs in clinical evaluation of patients with suspected HP. First, in contrast to occupational asthma, procedures for SIC in ILD and HP remain nonstandardized. There is no consensus on where SIC stands in the diagnostic algorithm for HP, which antigen(s) should be tested when none are elicited on a clinical questionnaire, how antigen extracts should be prepared, how provocations should be performed, what constitutes adequate patient monitoring, and what defines a positive response. Most expert centers consider a SIC result positive if patients experience a decline in lung function after antigen challenge or demonstrate a combination of lung-function decline and an increase in white blood cell count, a decrease in oxygen saturation, a change in chest imaging findings, an increased body temperature, or clinical symptoms (35). Moreover, SICs require laboratory resources and trained personnel not readily available outside of a limited number of expert centers. Adverse events can occur after SICs, most commonly transient influenza-like symptoms (36), and patients with severe lung-function impairment should not be subjected to SIC. Finally, it is unknown what SIC adds beyond a positive exposure history or other markers of sensitization. In summary, SICs can identify physiologic reactions to a causal antigen in a patient with suspected HP. However false-positive and false-negative test results can occur, and the accuracy of using SIC to identify a causative antigen is unclear.
Serum-Specific Immunoglobulin Measurement
Measurement of SS-IgG is a method used to identify an inciting antigen and is proposed as a diagnostic test for HP (8, 9, 36). For a summary of 22 relevant publications evaluating diagnostic test characteristics of SS-IgG measurement, see Table E1 in the online supplement (38–45).
Antibodies (immunoglobulins) against specific peptide components of organic antigens (e.g., molds, bacteria, and animal proteins) can be induced after exposure and measured in serum. The test antigen used to identify specific immunoglobulin is extracted from a bulk product (e.g., bird droppings, mixed feathers, or hay), from a cultured organism (e.g., a specific mold), or via immunoproteomic approaches that use recombination methods to produce the protein of interest (40, 46, 47). Some specialized centers extract antigens for testing from the patient’s own environment (29, 48), and some academic and commercial laboratories have developed so-called “HP panels” of antigens for testing (49).
A key question is the performance characteristics of SS-IgG measurement as a diagnostic test in patients with unspecified ILD. Four studies yielded a pooled sensitivity of 83% and specificity of 68% for identifying those with probable HP (6, 7, 41, 43, 44). Most studies evaluated test characteristics of SS-IgG measurement in patients with HP who have known exposure to a particular antigen, relative to exposed individuals without HP (Table E1). Eight of these studies showed a pooled sensitivity of 90% and a specificity of 91% (45–47, 50–54). However, methodologic issues, including variability in measurement methods, antigens tested, and thresholds used for positive findings limit the clinical utility of SS-IgG testing. Many practice settings employ commercially available antigen panels to test patients with undifferentiated ILD, aiming to accurately identify patients with HP. Published data suggest that use of SS-IgG testing for this purpose could produce a large number of false-positive results, limiting SS-IgG measurement as a “rule-in” confirmatory test for HP.
Routine SS-IgG testing of all patients with possible HP using a broad panel of antigens might help alert physicians to causative antigens not previously considered. However, studies evaluating this approach are limited (29, 55), and whether serologic testing is more useful for antigen identification than questionnaires or professional inspection of the patient’s environment is unknown (48). Immunoproteomic approaches to identification of proteins specific to HP from avian and farming exposures may hold promise for improved test performance (40, 46, 47). Recombinant laboratory methods to produce antigens commonly associated with disease could avoid issues with variability across batches and laboratories, a common problem when using extraction techniques that lead to higher test-to-test variability in SS-IgG measurement. Finally, the presence of SS-IgG is merely indicative of previous exposure and immunologic sensitization to the specific antigen and does not prove causality. However, the presence of the SS-IgG against a specific antigen may prompt the patient and physician to reassess potential exposure to occult sources of the antigen in the patient’s environment. A positive SS-IgG test result indicates enough antigen exposure to generate an immunologic reaction/sensitization that is B cell–mediated, causing specific antibody production. Antigen exposure can generate a positive SS-IgG reaction in people who do not have HP. It is a marker of exposure sufficient to cause an immunologic response. This finding by itself, without other clinical criteria for HP, is insufficient for diagnosis but, if present together with others, is a helpful diagnostic criterion.
LPT
Pulmonary lymphocyte accumulation is a hallmark of HP, reflecting in part an impaired T cell–suppressor response in the presence of inciting antigen (56–59). LPT has been proposed as a useful tool in HP diagnosis and antigen identification. Peripheral blood mononuclear cells or bronchoalveolar lavage (BAL) fluid lymphocytes are incubated with the suspected antigen and pulsed with [3H]thymidine. Cells are harvested, and [3H]thymidine incorporation is quantified using a scintillation counter. Results are expressed as a stimulation index.
Few studies have investigated the utility of LPT in HP antigen identification, and results are mixed. Most work has examined individuals with suspected bird-related HP (60–64). These studies suggest a role for LPT in antigen identification in patients who do not exhibit SS-IgG antibodies to the causative agent (65). In a study of 32 patients with bird breeder’s HP, testing results for SS-IgG antibodies against bird-dropping extracts were positive in only 35% of the insidious/chronic cases, whereas blood LPT results were positive in over 90% (61). In another small study, results for blood LPT using Trichosporon-related antigen were positive in all five subjects with chronic summer-type HP (16), but this was not tested in the total cohort of 14 patients or in exposed subjects without disease. For a summary of additional published data on BAL-fluid LPT in HP, see the online supplement.
There are several obstacles to using LPT as a tool for identifying offending antigens in patients with HP. Patients may be exposed to potential antigens from multiple sources in their home/workplace environments (48). However, at present, multiplex immunologic assessments of antigen candidates are not commercially available for use in LPT. There is no standardized methodology or guidance for what constitutes a positive test result, and testing is not widely available.
Multidisciplinary Team Approach
Accurate exposure assessment for HP relies on the integration of data collected through multiple domains. Pulmonary physicians often have limited training in occupational lung disease, environmental epidemiology, and exposure science, whereas many exposure scientists have limited clinical experience. However, each group brings valuable contributions to clinical exposure assessment for HP. Appropriate interpretation of results, whether by history, laboratory testing, or inhalational challenge, requires an understanding of the performance characteristics of each test, the relevance of positive or negative findings, and their contextualization within the clinical scenario. Similar to the multidisciplinary team discussion recommended as the gold standard for diagnosis of IPF/idiopathic interstitial pneumonias (IIPs) (66, 67), more information is needed on team-based approaches to exposure assessment in HP to enhance antigen recognition and abatement and to inform clinical diagnosis. Given resource limits and cost, it may be difficult outside of academic centers to build such collaborations among pulmonary/occupational medicine specialists and exposure experts. However, further research into the potential for multidisciplinary team evaluation to accurately identify exposures associated with HP is warranted.
Summary and Future Directions
There is a paucity of robust data guiding exposure assessment to identify causative antigens in patients with HP. Clinical exposure assessment is essential to establish a confident diagnosis of HP, guide management, and inform prognosis. Yet, the lack of a gold standard to inform the relationship between exposure and disease pathogenesis makes such assessment difficult. There is little doubt that a thorough exposure history, supplemented with a questionnaire, is a vital starting point to identify potential inciting antigens in patients with HP and, indeed, in all patients with ILD. Further research is needed to develop standardized, validated questionnaires that are appropriate for a wide range of cultural and geographic settings and that better elucidate the relevance of particular exposures. In addition, more work is needed to characterize the role of multiple antigenic exposures in causing HP. An overall assessment of each tool is summarized in Table 1.
Table 1.
Aggregate judgment of 20 independent expert raters on clinical applicability of exposure assessment tools
| Test Performance | Feasibility | Clinical Utility | Comments | |
|---|---|---|---|---|
| History | ++ | +++ | ++ | Fundamental to clinical assessment |
| Questionnaire | ++ | ++ | ++ | Should be locally adapted and validated |
| Improvement with antigen avoidance | ++ | ++ | ++ | May be informative in cases with a component of nonfibrotic HP |
| Environmental assessment | ++ | + | ++ | Limitations in availability of experts and sampling interpretation |
| Specific inhalational challenge | ++ | − | + | Limited role, requires experienced research laboratories |
| Serum specific IgG | + | ++ | + | Marker of antigen exposure and sensitization |
| Lymphocyte proliferation test | + | − | + | Limited role, needs more validation |
| Multidisciplinary assessment | ++ | ++ | ++ | Warrants further evaluation |
Definition of abbreviations: − = poor; + = fair; ++ = good; +++ = excellent; HP = hypersensitivity pneumonitis; IgG = immunoglobulin G.
The weighting of scores in this table represents a semiquantitative assessment of expert panel members’ overall interpretation of the workshop data and discussion. For each exposure assessment tool, workshop participants graded them as “poor = 0, fair = 1, good = 2, excellent = 3.” The scores were averaged and rounded to the nearest whole number. The system presented in the table corresponds to poor = 0 = −, fair = 1 = +, good = 2 = ++, excellent = 3 = +++.
Although clinical improvement after removal from suspected antigen exposure often occurs in acute HP, this occurs less frequently in chronic nonfibrotic HP and may not happen in fibrotic HP. Thus, although reasonable attempts at antigen remediation should be pursued, life-altering recommendations such as changing homes or occupations must be informed by the patient’s best interests and wishes. It is imperative that both clinicians and patients have a full understanding that such interventions may not point to a causative antigen or improve clinical outcomes and that they make informed decisions accordingly. To better elucidate the role of on-site environmental investigations, future studies should include standardized protocols for such investigations, clear criteria for when and how to perform air and surface sampling, practical and effective remediation actions for patients, and protocols for follow-up by hygienists to verify the adequacy and efficacy of recommended abatement efforts.
Several laboratory approaches to antigen testing are available, all with limitations for identifying relevant exposures. If a compelling exposure is identified by history, it is unclear whether further testing provides useful supplemental information. In cases with a less compelling exposure or in which multiple potential exposures are identified by history, SS-IgG testing likely increases the pretest probability of HP by demonstrating immunologic sensitization. However, a negative test result does not exclude a potential antigen as being inciting, given the limitations of most available assays. Furthermore, a positive test result does not prove causality, as these tests are primarily evidence of exposure and sensitization but are not by themselves diagnostic of HP. SS-IgG testing for antigen identification may be most useful with samples taken from the patient’s environment. Similarly, although SIC may provoke clinical features typical for HP, its role in improving diagnostic sensitivity beyond a positive exposure history is unknown. LPT may be a promising tool for antigen identification in some patients with HP, particularly in those with chronic disease; however, the lack of standardization and ambiguous performance characteristics limit its clinical applicability at present. Future research priorities for areas addressed at the workshop are summarized in Table 2.
Table 2.
Key areas for future research in exposure assessment tools for HP
| 1. Develop and validate regionally relevant HP exposure questionnaires |
| 2. Clarify the roles of SS-IgG, SIC, LPT, and preexposure/postexposure biomarkers in identifying immunologic sensitization and causal associations in HP |
| 3. Develop and validate biomarkers together with other clinical outcomes as endpoints for assessing effectiveness of antigen exposure avoidance and abatement |
| 4. Test the performance characteristics of exposure identification tools in diagnostic models for HP to determine their additional discriminative abilities |
| 5. Develop standardized protocols for on-site environmental investigation for relevant exposures including criteria for sampling before and after abatement |
| 6. Develop guidelines for practical and effective remediation actions for patients |
| 7. Characterize the role of multiple antigen exposures as causal for HP |
Definition of abbreviations: HP = hypersensitivity pneumonitis; LPT = lymphocyte proliferation testing; SIC = specific inhalational challenge; SS-IgG = serum specific immunoglobulin G.
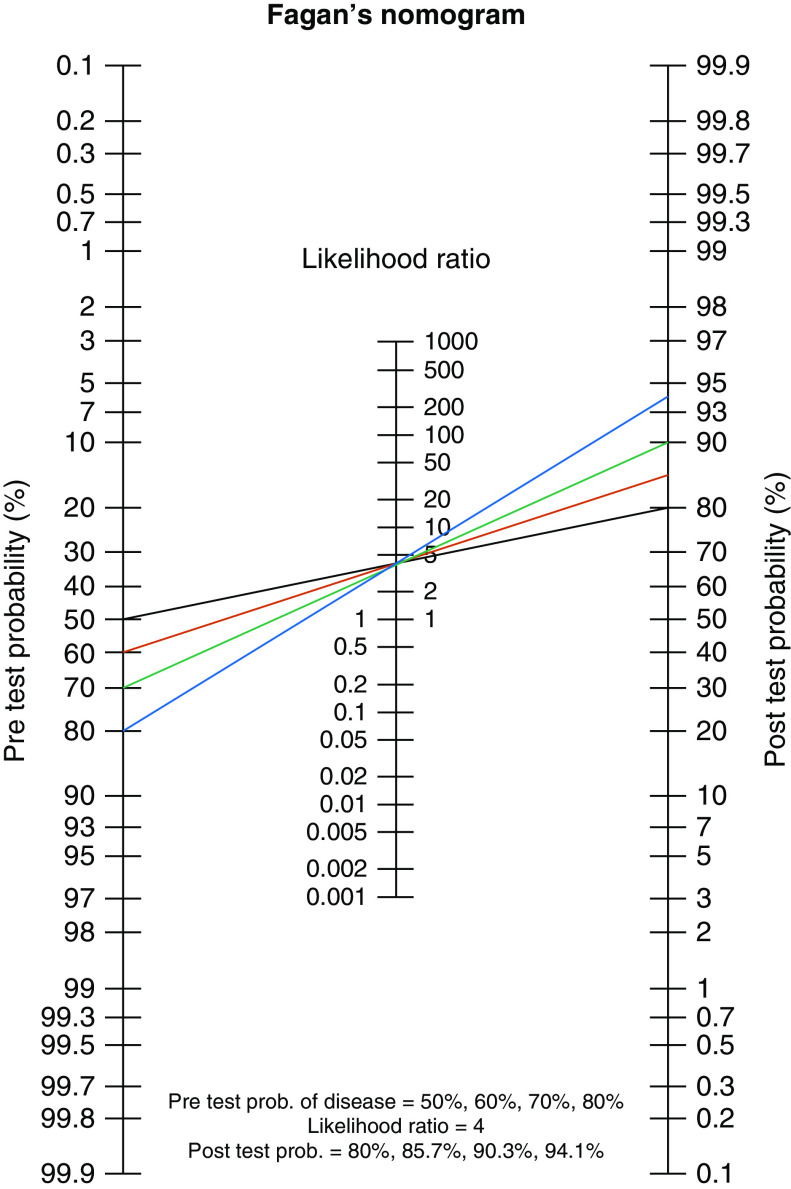
Finally, we propose a framework whereby interpretation of results of antigen identification tools are considered along a spectrum of probability, rather than dichotomously as positive or negative. Establishing a pretest probability of disease requires considering the weight of what a likely antigen exposure adds to HP diagnosis (Figure 1). Not all positive results from exposure histories or tests of sensitization are equally robust, and their accompanying pretest probability will vary on the basis of several factors. For example, in the appropriate clinical context, a positive exposure history for residential pigeon breeding likely confers a high pretest probability of HP. Results of clinical diagnostic evaluation (e.g., high-resolution computed tomography imaging, BAL, biopsy) will provide a likelihood ratio that impacts the post hoc test probability, or confidence, of HP diagnosis. A less compelling exposure, such as mold in the shower or use of an outdoor hot tub or residential feather duvet, would suggest a lower pretest probability of HP. These less compelling exposures may be supported by results from tests of sensitization. For example, in a patient reporting residential mold, a positive SS-IgG or SIC result may support the probability of sensitization and an HP-inducing immune response, increasing the pretest probability of HP. Less compelling exposure histories in the absence of objective sensitization would confer a low pretest probability of HP. We recognize that current evidence does not allow assignment of specific probabilities to each antigen exposure. That probability is also influenced by individual host factors such as genetic predisposition, history of infection, and smoking status. However, this general concept provides a foundation for assessing the likelihood of a relationship between an inciting antigen and subsequent development of HP.
Figure 1.
Conceptual framework describing how exposure identification and sensitization tests impact pretest probability of hypersensitivity pneumonitis (HP). Identification of plausible antigen(s) for HP will determine the pretest probability of disease. The likelihood ratio is determined by other aspects of the clinical assessment for suspected HP. Numbers are for example only. The black line indicates no compelling exposure by history; this is the lowest pretest and post hoc test probability of HP. The red line indicates a less-compelling exposure history (e.g., mold in bathroom) and shows a negative serum-specific immunoglobulin G (SS-IgG) test result. The green line indicates a less compelling exposure history (e.g., mold) and shows a positive SS-IgG test result. The blue line indicates identification of a compelling exposure (e.g., pigeons/farming) with no further testing needed. prob. = probability.
Supplementary Material
Acknowledgments
This official workshop report was prepared by an ad hoc subcommittee of the ATS Assembly on Environmental, Occupational, and Population Health and the Assembly on Clinical Problems.
Members of the subcommittee are as follows:
Kerri A. Johannson, M.D., M.P.H.1,2 (Co-Chair)
Cecile S. Rose, M.D., M.P.H.3,4 (Co-Chair)
Hayley Barnes, M.B.B.S., M.P.H.5,6
Anne-Pauline Bellanger, Ph.D.7
Jean-Charles Dalphin, M.D, Ph.D.8
Evans R. Fernández Pérez, M.D., M.S.3,4
Kevin R. Flaherty, M.D.9
Yuh-Chin T. Huang, M.D.10
Kirk D. Jones, M.D.11
Leticia Kawano-Dourado, M.D.12
Kevin Kennedy, Ph.D.13
Melissa Millerick-May, Ph.D.14
Yasunari Miyazaki, M.D.15
Julie Morisset, M.D.16
Ferran Morell, M.D.17
Ganesh R. Raghu, M.D.18
Coreen Robbins, Ph.D.19
Coralynn S. Sack, M.D.20
Margaret L. Salisbury, M.D., M.S.21
Moises Selman, M.D.22
Martina Vasakova, M.D., Ph.D.23
Simon L. F. Walsh, M.D.24
1Department of Medicine and 2Department of Community Health Sciences, University of Calgary, Calgary, Alberta, Canada; 3Department of Medicine, National Jewish Health, Denver, Colorado; 4Department of Medicine, University of Colorado, Denver, Colorado; 5Department of Medicine, University of California, San Francisco, California; 6Central Clinical School, Monash University, Melbourne, Australia; 7Department of Parasitology-Mycology, University Hospital of Besancon, Besancon, France; 8Department of Pneumology, University Hospital of Besancon, Besancon, France; 9Department of Medicine, University of Michigan, Ann Arbor, Michigan; 10Department of Medicine, Duke University Medical Center, Durham, North Carolina; 11Department of Pathology, University of California, San Francisco, California; 12Research Institute, Hospital do Coracao & Pulmonary Division, University of Sao Paulo, Sao Paulo, Brazil; 13Section of Toxicology and Environmental Health, Children’s Mercy Kansas City, Kansas City, Missouri; 14Division of Occupational and Environmental Medicine, Michigan State University, East Lansing, Michigan; 15Tokyo Medical and Dental University, Department of Respiratory Medicine, Tokyo, Japan; 16Département de Médicine, Centre Hospitalier de l’Université de Montréal, Montréal, Quebec, Canada; 17Vall d'Hebron Institut de Recerca (VHIR), CIBER de enfermedades respiratorias (CIBERES), Servei de Pneumologia, Hospital Vall d'Hebron, UAB, Barcelona, Catalonia, Spain; 18Department of Medicine, University of Washington, Seattle, Washington; 19J.S. Held, LLC, Redmond, Washington; 20Department of Medicine and Department of Environmental and Occupational Health Sciences, University of Washington, Seattle, Washington; 21Department of Medicine, Vanderbilt University Medical Center, Nashville, Tennessee; 22Instituto Nacional de Enfermedades Respiratorias “Ismael Cosio Villegas”, Mexico City, Mexico; 23Department of Respiratory Medicine, First Faculty of Medicine, Charles University and Thomayer Hospital, Prague, Czech Republic; and 24National Heart and Lung Institute, Imperial College, London, United Kingdom
Footnotes
Supported by the American Thoracic Society.
This official workshop report of the American Thoracic Society was approved September 2020
This work is dedicated to the memory of Dr. Jean-Charles Dalphin (1956–2019).
This document has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org.
Author Disclosures: K.A.J. served on an advisory committee for Blade Therapeutics, Boehringer Ingelheim, Roche; as a consultant for Blade Therapeutics, Boehringer Ingelheim, Theravance, Three Lakes Foundation; as a speaker for Boehringer Ingelheim and Roche; received research support from Chest Foundation, Pulmonary Fibrosis Society of Calgary, University of Calgary Cumming School of Medicine, UCB. J.-C.D. served on an advisory committee for Chiesi, Novartis, Teva; as a speaker for BIF, Boehringer Ingelheim, Menarini; received research support from Asten France, Novartis, SOS Oxygene; other transfers of value from GlaxoSmithKline, Roche. E.R.F.P. served as a speaker and received research support from Boehringer Ingelheim and Genentech; received research support from AI Grant CO and National Heart, Lung, and Blood Institute. K.R.F. served as a consultant for Bellerophon, Blade Therapeutics, Boehringer Ingelheim, Respivant., Roche/Genentech, Shionogi, Veracyte; received research support from Boehringer Ingelheim. J.M. served on an advisory committee for Roche and Boehringer Ingelheim; as a consultant for Boehringer Ingelheim. G.R.R. served as a consultant for Belleorphan, Biogen, BMS, Boehringer Ingelheim, Fibrogen, Genentech, Gilead, Nitto, Promedior, Respivant, Roche, Veracyte, Zambon; served on a data and safety monitoring board for Avalyn. M.L.S. served on an advisory committee, as a speaker and received research support from Boehringer Ingelheim; served as a consultant for Boehringer Ingelheim and Orinove; received research support from the National Institutes of Health. M.S. served as a consultant for Boehringer Ingelheim and Celgene. M.V. served on an advisory committee for Boehringer Ingelheim, Roche; as a consultant for Avalyn, Boehringer Ingelheim. S.L.F.W. served on an advisory committee for Boehringer Ingelheim, Fluidda, OncoArendi Therapeutics, Roche; as a consultant for Galapagos, OSIC, Sanofi; as a speaker for Bracco, Galapagos, Roche; received research support from Boehringer Ingelheim and National Institute for Health Research. C.S.R. served on an advisory committee for National Academies, National Institute for Occupational Safety and Health, Physicians for a National Health Program; received research support from the Alpha Foundation, Department of Defense and the Health Resources and Services Administration. H.B., A.-P.B., Y.-C.T.H., K.D.J., L.K.-D., K.K., M.M.-M., Y.M., F.M., C.R., C.S.S. reported no commercial or relevant non-commercial interests.
Contributor Information
Collaborators: on behalf of the American Thoracic Society Assembly on Environmental, Occupational, and Population Health and the Assembly on Clinical Problems
References
- 1.Fernández Pérez ER, Kong AM, Raimundo K, Koelsch TL, Kulkarni R, Cole AL. Epidemiology of hypersensitivity pneumonitis among an insured population in the United States: a claims-based cohort analysis. Ann Am Thorac Soc. 2018;15:460–469. doi: 10.1513/AnnalsATS.201704-288OC. [DOI] [PubMed] [Google Scholar]
- 2.Fernández Pérez ER, Swigris JJ, Forssén AV, Tourin O, Solomon JJ, Huie TJ, et al. Identifying an inciting antigen is associated with improved survival in patients with chronic hypersensitivity pneumonitis. Chest. 2013;144:1644–1651. doi: 10.1378/chest.12-2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Sadeleer LJ, Hermans F, De Dycker E, Yserbyt J, Verschakelen JA, Verbeken EK, et al. Effects of corticosteroid treatment and antigen avoidance in a large hypersensitivity pneumonitis cohort: a single-centre cohort study. J Clin Med. 2018;8:14. doi: 10.3390/jcm8010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mooney JJ, Elicker BM, Urbania TH, Agarwal MR, Ryerson CJ, Nguyen MLT, et al. Radiographic fibrosis score predicts survival in hypersensitivity pneumonitis. Chest. 2013;144:586–592. doi: 10.1378/chest.12-2623. [DOI] [PubMed] [Google Scholar]
- 5.Kern RM, Singer JP, Koth L, Mooney J, Golden J, Hays S, et al. Lung transplantation for hypersensitivity pneumonitis. Chest. 2015;147:1558–1565. doi: 10.1378/chest.14-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morell F, Villar A, Montero MA, Muñoz X, Colby TV, Pipvath S, et al. Chronic hypersensitivity pneumonitis in patients diagnosed with idiopathic pulmonary fibrosis: a prospective case-cohort study. Lancet Respir Med. 2013;1:685–694. doi: 10.1016/S2213-2600(13)70191-7. [DOI] [PubMed] [Google Scholar]
- 7.Raghu G, Remy-Jardin M, Ryerson CJ, Myers JL, Kreuter M, Vasakova M, et al. Diagnosis of hypersensitivity pneumonitis in adults. An Official ATS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med. 2020;202:e36–e69. doi: 10.1164/rccm.202005-2032ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salisbury ML, Myers JL, Belloli EA, Kazerooni EA, Martinez FJ, Flaherty KR. Diagnosis and treatment of fibrotic hypersensitivity pneumonia: where we stand and where we need to go. Am J Respir Crit Care Med. 2017;196:690–699. doi: 10.1164/rccm.201608-1675PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vasakova M, Morell F, Walsh S, Leslie K, Raghu G. Hypersensitivity pneumonitis: perspectives in diagnosis and management. Am J Respir Crit Care Med. 2017;196:680–689. doi: 10.1164/rccm.201611-2201PP. [DOI] [PubMed] [Google Scholar]
- 10.Fernández Pérez ER, Brown KK. Fibrotic hypersensitivity pneumonitis. Curr Respir Care Rep. 2014;3:170–178. [Google Scholar]
- 11.Barnes H, Morisset J, Molyneaux P, Westall G, Glaspole I, Collard HR CHP Exposure Assessment Collaborators. A systematically derived exposure assessment instrument for chronic hypersensitivity pneumonitis. Chest. 2020;157:1506–1512. doi: 10.1016/j.chest.2019.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu J, Barnes H, Johannson KA. hpLung.com. 2020 [accessed 2020 Jan 15]. Available from: http://www.hplung.com/
- 13.Gimenez A, Storrer K, Kuranishi L, Soares MR, Ferreira RG, Pereira CAC. Change in FVC and survival in chronic fibrotic hypersensitivity pneumonitis. Thorax. 2018;73:391–392. doi: 10.1136/thoraxjnl-2017-210035. [DOI] [PubMed] [Google Scholar]
- 14.de Gracia J, Morell F, Bofill JM, Curull V, Orriols R. Time of exposure as a prognostic factor in avian hypersensitivity pneumonitis. Respir Med. 1989;83:139–143. doi: 10.1016/s0954-6111(89)80230-6. [DOI] [PubMed] [Google Scholar]
- 15.Allen DH, Williams GV, Woolcock AJ. Bird breeder’s hypersensitivity pneumonitis: progress studies of lung function after cessation of exposure to the provoking antigen. Am Rev Respir Dis. 1976;114:555–566. doi: 10.1164/arrd.1976.114.3.555. [DOI] [PubMed] [Google Scholar]
- 16.Inase N, Ohtani Y, Usui Y, Miyazaki Y, Takemura T, Yoshizawa Y. Chronic summer-type hypersensitivity pneumonitis: clinical similarities to idiopathic pulmonary fibrosis. Sarcoidosis Vasc Diffuse Lung Dis. 2007;24:141–147. [PubMed] [Google Scholar]
- 17.Hanak V, Kalra S, Aksamit TR, Hartman TE, Tazelaar HD, Ryu JH. Hot tub lung: presenting features and clinical course of 21 patients. Respir Med. 2006;100:610–615. doi: 10.1016/j.rmed.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 18.Selman M, Pardo A, King TE., Jr Hypersensitivity pneumonitis: insights in diagnosis and pathobiology. Am J Respir Crit Care Med. 2012;186:314–324. doi: 10.1164/rccm.201203-0513CI. [DOI] [PubMed] [Google Scholar]
- 19.Morell F, Villar A, Ojanguren I, Muñoz X, Cruz MJ, Sansano I, et al. Hypersensitivity pneumonitis and (idiopathic) pulmonary fibrosis due to feather duvets and pillows. Arch Bronconeumol. doi: 10.1016/j.arbres.2019.12.003. [online ahead of print] 11 Feb 2020; DOI: 10.1016/j.arbres.2019.12.003. [DOI] [PubMed] [Google Scholar]
- 20.Kreiss K, Cox-Ganser J. Metalworking fluid-associated hypersensitivity pneumonitis: a workshop summary. Am J Ind Med. 1997;32:423–432. doi: 10.1002/(sici)1097-0274(199710)32:4<423::aid-ajim16>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 21.Ganier M, Lieberman P, Fink J, Lockwood DG. Humidifier lung: an outbreak in office workers. Chest. 1980;77:183–187. doi: 10.1378/chest.77.2.183. [DOI] [PubMed] [Google Scholar]
- 22.Bernstein DI, Lummus ZL, Santilli G, Siskosky J, Bernstein IL. Machine operator’s lung: a hypersensitivity pneumonitis disorder associated with exposure to metalworking fluid aerosols. Chest. 1995;108:636–641. doi: 10.1378/chest.108.3.636. [DOI] [PubMed] [Google Scholar]
- 23.Ojanguren I, Morell F, Ramón MA, Villar A, Romero C, Cruz MJ, et al. Long-term outcomes in chronic hypersensitivity pneumonitis. Allergy. 2019;74:944–952. doi: 10.1111/all.13692. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization Regional Office for Europe. Geneva, Switzerland: World Health Organization; 2009. WHO guidelines for indoor air quality: dampness and mould. [PubMed] [Google Scholar]
- 25.Chew GL, Horner WE, Kennedy K, Grimes C, Barnes CS, Phipatanakul W, et al. Environmental Allergens Workgroup. Procedures to assist health care providers to determine when home assessments for potential mold exposure are warranted. J Allergy Clin Immunol Pract. 2016;4:417–422, e2. doi: 10.1016/j.jaip.2016.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barnes CS, Horner WE, Kennedy K, Grimes C, Miller JD Environmental Allergens Workgroup. Home assessment and remediation. J Allergy Clin Immunol Pract. 2016;4:423–431, e15. doi: 10.1016/j.jaip.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 27.Office of Radiation and Indoor Air Indoor Environments Division, U.S. Environmental Protection Agency. Washington, DC: U.S. Environmental Protection Agency; 2020. Care for your air: a guide to indoor air quality. [accessed 2020 Jan 28]. Available from: www.epa.gov/indoor-air-quality-iaq/care-your-air-guide-indoor-air-quality. [Google Scholar]
- 28.Bellanger AP, Reboux G, Roussel S, Grenouillet F, Didier-Scherer E, Dalphin JC, et al. Indoor fungal contamination of moisture-damaged and allergic patient housing analysed using real-time PCR. Lett Appl Microbiol. 2009;49:260–266. doi: 10.1111/j.1472-765X.2009.02653.x. [DOI] [PubMed] [Google Scholar]
- 29.Bellanger AP, Reboux G, Rouzet A, Barrera C, Rocchi S, Scherer E, et al. Hypersensitivity pneumonitis: a new strategy for serodiagnosis and environmental surveys. Respir Med. 2019;150:101–106. doi: 10.1016/j.rmed.2019.02.019. [DOI] [PubMed] [Google Scholar]
- 30.Pepys J. New tests to assess lung function. inhalation challenge tests in asthma. N Engl J Med. 1975;293:758–759. doi: 10.1056/NEJM197510092931507. [DOI] [PubMed] [Google Scholar]
- 31.Vandenplas O, Suojalehto H, Aasen TB, Baur X, Burge PS, de Blay F, et al. ERS Task Force on Specific Inhalation Challenges with Occupational Agents. Specific inhalation challenge in the diagnosis of occupational asthma: consensus statement. Eur Respir J. 2014;43:1573–1587. doi: 10.1183/09031936.00180313. [DOI] [PubMed] [Google Scholar]
- 32.Hendrick DJ, Marshall R, Faux JA, Krall JM. Positive “alveolar” responses to antigen inhalation provocation tests: their validity and recognition. Thorax. 1980;35:415–427. doi: 10.1136/thx.35.6.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramírez-Venegas A, Sansores RH, Pérez-Padilla R, Carrillo G, Selman M. Utility of a provocation test for diagnosis of chronic pigeon Breeder’s disease. Am J Respir Crit Care Med. 1998;158:862–869. doi: 10.1164/ajrccm.158.3.9710036. [DOI] [PubMed] [Google Scholar]
- 34.Ohtani Y, Kojima K, Sumi Y, Sawada M, Inase N, Miyake S, et al. Inhalation provocation tests in chronic bird fancier’s lung. Chest. 2000;118:1382–1389. doi: 10.1378/chest.118.5.1382. [DOI] [PubMed] [Google Scholar]
- 35.Morell F, Roger A, Reyes L, Cruz MJ, Murio C, Muñoz X. Bird fancier’s lung: a series of 86 patients. Medicine (Baltimore) 2008;87:110–130. doi: 10.1097/MD.0b013e31816d1dda. [DOI] [PubMed] [Google Scholar]
- 36.Muñoz X, Sánchez-Ortiz M, Torres F, Villar A, Morell F, Cruz MJ. Diagnostic yield of specific inhalation challenge in hypersensitivity pneumonitis. Eur Respir J. 2014;44:1658–1665. doi: 10.1183/09031936.00060714. [DOI] [PubMed] [Google Scholar]
- 37.Richerson HB, Bernstein IL, Fink JN, Hunninghake GW, Novey HS, Reed CE, et al. Guidelines for the clinical evaluation of hypersensitivity pneumonitis: report of the Subcommittee on Hypersensitivity Pneumonitis. J Allergy Clin Immunol. 1989;84:839–844. doi: 10.1016/0091-6749(89)90349-7. [DOI] [PubMed] [Google Scholar]
- 38.Cormier Y, Bélanger J, LeBlanc P, Laviolette M. Bronchoalveolar lavage in farmers’ lung disease: diagnostic and physiological significance. Br J Ind Med. 1986;43:401–405. doi: 10.1136/oem.43.6.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soumagne T, Reboux G, Metzger F, Roussel S, Lefebvre A, Penven E, et al. Fungal contamination of wind instruments: immunological and clinical consequences for musicians. Sci Total Environ. 2019;646:727–734. doi: 10.1016/j.scitotenv.2018.07.284. [DOI] [PubMed] [Google Scholar]
- 40.Rouzet A, Reboux G, Dalphin JC, Gondouin A, De Vuyst P, Balliau T, et al. An immunoproteomic approach revealed antigenic proteins enhancing serodiagnosis performance of bird fancier’s lung. J Immunol Methods. 2017;450:58–65. doi: 10.1016/j.jim.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 41.Sandoval J, Bañales JL, Cortés JJ, Mendoza F, Selman M, Reyes PA. Detection of antibodies against avian antigens in bronchoalveolar lavage from patients with pigeon breeder’s disease: usefulness of enzyme-linked immunosorbent assay and enzyme immunotransfer blotting. J Clin Lab Anal. 1990;4:81–85. doi: 10.1002/jcla.1860040202. [DOI] [PubMed] [Google Scholar]
- 42.Suhara K, Miyazaki Y, Okamoto T, Yasui M, Tsuchiya K, Inase N. Utility of immunological tests for bird-related hypersensitivity pneumonitis. Respir Investig. 2015;53:13–21. doi: 10.1016/j.resinv.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 43.Fenoglio CM, Reboux G, Sudre B, Mercier M, Roussel S, Cordier JF, et al. Diagnostic value of serum precipitins to mould antigens in active hypersensitivity pneumonitis. Eur Respir J. 2007;29:706–712. doi: 10.1183/09031936.00001006. [DOI] [PubMed] [Google Scholar]
- 44.Lacasse Y, Selman M, Costabel U, Dalphin JC, Ando M, Morell F, et al. HP Study Group. Clinical diagnosis of hypersensitivity pneumonitis. Am J Respir Crit Care Med. 2003;168:952–958. doi: 10.1164/rccm.200301-137OC. [DOI] [PubMed] [Google Scholar]
- 45.Reboux G, Piarroux R, Roussel S, Millon L, Bardonnet K, Dalphin JC. Assessment of four serological techniques in the immunological diagnosis of farmers’ lung disease. J Med Microbiol. 2007;56:1317–1321. doi: 10.1099/jmm.0.46953-0. [DOI] [PubMed] [Google Scholar]
- 46.Roussel S, Reboux G, Rognon B, Monod M, Grenouillet F, Quadroni M, et al. Comparison of three antigenic extracts of Eurotium amstelodami in serological diagnosis of farmer’s lung disease. Clin Vaccine Immunol. 2010;17:160–167. doi: 10.1128/CVI.00129-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barrera C, Millon L, Rognon B, Quadroni M, Roussel S, Dalphin JC, et al. Immunoreactive proteins of Saccharopolyspora rectivirgula for farmer’s lung serodiagnosis. Proteomics Clin Appl. 2014;8:971–981. doi: 10.1002/prca.201400024. [DOI] [PubMed] [Google Scholar]
- 48.Millerick-May ML, Mulks MH, Gerlach J, Flaherty KR, Schmidt SL, Martinez FJ, et al. Hypersensitivity pneumonitis and antigen identification: an alternate approach. Respir Med. 2016;112:97–105. doi: 10.1016/j.rmed.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 49.Mayo Clinic Laboratories. Rochester, MN: Mayo Foundation for Medical Education and Research; 2019. Hypersensitivity pneumonitis FEIA panel II. [accessed 2019 Dec 15]. Available from: www.mayocliniclabs.com/test-catalog/Clinical+and+Interpretive/57595. [Google Scholar]
- 50.Boiron P, Drouhet E, Dupont B. Enzyme-linked immunosorbent-assay (ELISA) for IgG in bagasse workers’ sera: comparison with counter-immunoelectrophoresis. Clin Allergy. 1987;17:355–363. doi: 10.1111/j.1365-2222.1987.tb02025.x. [DOI] [PubMed] [Google Scholar]
- 51.Hébert J, Beaudoin J, Laviolette M, Beaudoin R, Bélanger J, Cormier Y. Absence of correlation between the degree of alveolitis and antibody levels to Micropolysporum faeni. Clin Exp Immunol. 1985;60:572–578. [PMC free article] [PubMed] [Google Scholar]
- 52.Huizinga M, Berrens L. Detection of class-specific antibodies against Micropolyspora faeni antigens in farmers’ lung. Clin Allergy. 1985;15:139–145. doi: 10.1111/j.1365-2222.1985.tb02265.x. [DOI] [PubMed] [Google Scholar]
- 53.Rodrigo MJ, Benavent MI, Cruz MJ, Rosell M, Murio C, Pascual C, et al. Detection of specific antibodies to pigeon serum and bloom antigens by enzyme linked immunosorbent assay in pigeon breeder’s disease. Occup Environ Med. 2000;57:159–164. doi: 10.1136/oem.57.3.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tillie-Leblond I, Grenouillet F, Reboux G, Roussel S, Chouraki B, Lorthois C, et al. Hypersensitivity pneumonitis and metalworking fluids contaminated by mycobacteria. Eur Respir J. 2011;37:640–647. doi: 10.1183/09031936.00195009. [DOI] [PubMed] [Google Scholar]
- 55.Sterclova M, Vasakova M, Metlicka M. Significance of specific IgG against sensitizing antigens in extrinsic allergic alveolitis: serological methods in EAA. Rev Port Pneumol. 2011;17:253–259. doi: 10.1016/j.rppneu.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 56.McSharry C, Banham SW, Lynch PP, Boyd G. Antibody measurement in extrinsic allergic alveolitis. Eur J Respir Dis. 1984;65:259–265. [PubMed] [Google Scholar]
- 57.Keller RH, Fink JN, Lyman S, Pedersen G. Immunoregulation in hypersensitivity pneumonitis: I. differences in T-cell and macrophage suppressor activity in symptomatic and asymptomatic pigeon breeders. J Clin Immunol. 1982;2:46–54. doi: 10.1007/BF00915978. [DOI] [PubMed] [Google Scholar]
- 58.Moore VL, Pedersen GM, Hauser WC, Fink JN. A study of lung lavage materials in patients with hypersensitivity pneumonitis: in vitro response to mitogen and antigen in pigeon breeders’ disease. J Allergy Clin Immunol. 1980;65:365–370. doi: 10.1016/0091-6749(80)90214-6. [DOI] [PubMed] [Google Scholar]
- 59.Agache IO, Rogozea L. Management of hypersensitivity pneumonitis. Clin Transl Allergy. 2013;3:5. doi: 10.1186/2045-7022-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yoshizawa Y, Ohtani Y, Hayakawa H, Sato A, Suga M, Ando M. Chronic hypersensitivity pneumonitis in Japan: a nationwide epidemiologic survey. J Allergy Clin Immunol. 1999;103:315–320. doi: 10.1016/s0091-6749(99)70507-5. [DOI] [PubMed] [Google Scholar]
- 61.Ohtani Y, Saiki S, Sumi Y, Inase N, Miyake S, Costabel U, et al. Clinical features of recurrent and insidious chronic bird fancier’s lung. Ann Allergy Asthma Immunol. 2003;90:604–610. doi: 10.1016/S1081-1206(10)61863-7. [DOI] [PubMed] [Google Scholar]
- 62.Ohtani Y, Saiki S, Kitaichi M, Usui Y, Inase N, Costabel U, et al. Chronic bird fancier’s lung: histopathological and clinical correlation: an application of the 2002 ATS/ERS consensus classification of the idiopathic interstitial pneumonias. Thorax. 2005;60:665–671. doi: 10.1136/thx.2004.027326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Inase N, Ohtani Y, Endo J, Miyake S, Yoshizawa Y. Feather duvet lung. Med Sci Monit. 2003;9:CS37–CS40. [PubMed] [Google Scholar]
- 64.Inase N, Ohtani Y, Sumi Y, Umino T, Usui Y, Miyake S, et al. A clinical study of hypersensitivity pneumonitis presumably caused by feather duvets. Ann Allergy Asthma Immunol. 2006;96:98–104. doi: 10.1016/S1081-1206(10)61047-2. [DOI] [PubMed] [Google Scholar]
- 65.Yoshizawa Y, Miyake S, Sumi Y, Hisauchi K, Sato T, Kurup VP. A follow-up study of pulmonary function tests, bronchoalveolar lavage cells, and humoral and cellular immunity in bird fancier’s lung. J Allergy Clin Immunol. 1995;96:122–129. doi: 10.1016/s0091-6749(95)70041-2. [DOI] [PubMed] [Google Scholar]
- 66.Travis WD, Costabel U, Hansell DM, King TE, Jr, Lynch DA, Nicholson AG, et al. ATS/ERS Committee on Idiopathic Interstitial Pneumonias. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188:733–748. doi: 10.1164/rccm.201308-1483ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Raghu G, Remy-Jardin M, Myers JL, Richeldi L, Ryerson CJ, Lederer DJ, et al. American Thoracic Society, European Respiratory Society, Japanese Respiratory Society, and Latin American Thoracic Society. Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2018;198:e44–e68. doi: 10.1164/rccm.201807-1255ST. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.