Abstract
Rationale: Patients with pulmonary arterial hypertension (PAH) and chronic thromboembolic pulmonary hypertension (CTEPH) typically undergo frequent clinical evaluation. The incidence and outcomes of coronavirus disease (COVID-19) and its impact on routine management for patients with pulmonary vascular disease is currently unknown.
Objectives: To assess the cumulative incidence and outcomes of recognized COVID-19 for patients with PAH/CTEPH followed at accredited pulmonary hypertension centers, and to evaluate the pandemic’s impact on clinic operations at these centers.
Methods: A survey was e-mailed to program directors of centers accredited by the Pulmonary Hypertension Association. Descriptive analyses and linear regression were used to analyze results.
Results: Seventy-seven center directors were successfully e-mailed a survey, and 58 responded (75%). The cumulative incidence of COVID-19 recognized in individuals with PAH/CTEPH was 2.9 cases per 1,000 patients, similar to the general U.S. population. In patients with PAH/CTEPH for whom COVID-19 was recognized, 30% were hospitalized and 12% died. These outcomes appear worse than the general population. A large impact on clinic operations was observed including fewer clinic visits and substantially increased use of telehealth. A majority of centers curtailed diagnostic testing and a minority limited new starts of medical therapy. Most centers did not use experimental therapies in patients with PAH/CTEPH diagnosed with COVID-19.
Conclusions: The cumulative incidence of COVID-19 recognized in patients with PAH/CTEPH appears similar to the broader population, although outcomes may be worse. Although the total number of patients with PAH/CTEPH recognized to have COVID-19 was small, the impact of COVID-19 on broader clinic operations, testing, and treatment was substantial.
Keywords: outcomes, clinic operations, telehealth, COVID-19
Guidelines for the care of patients with pulmonary arterial hypertension (PAH) and chronic thromboembolic pulmonary hypertension (CTEPH) recommend regular clinic visits and frequent diagnostic testing at referral centers (1, 2). During the coronavirus disease (COVID-19) pandemic, the Centers for Disease Control and Prevention (CDC) has recommended exploring alternatives to face-to-face visits and suggested rescheduling nonurgent visits and testing (3). Consistent with this recommendation, many institutions temporarily stopped performing nonurgent cardiac catheterizations (4). Unfortunately, the definition of urgency is uncertain in a chronic serious illness like PAH or CTEPH, and its application to routine care in these populations may be variable. Owing to frequent clinic visits and high use of outpatient testing and procedures, patients with PAH or CTEPH are likely to be particularly affected by the current pandemic.
Although several studies have explored the incidence of specific diseases in individuals with COVID-19 (5, 6), few have focused on the incidence of COVID-19 among individuals with PAH or CTEPH (7). A panel of experts recently highlighted challenges encountered while caring for patients with PAH or CTEPH during this pandemic; however, this paper did not attempt to provide quantitative data (8). In addition, outside of experience posted on a few websites, description of the impact of the pandemic on routine clinic operations is sparse (9, 10). Although these websites suggest a large decrease in outpatient clinic volumes, their data have not been published or peer reviewed.
We conducted a cross-sectional survey of program directors at accredited pulmonary hypertension referral centers to better understand the early impact of the pandemic on pulmonary vascular disease patients. We specifically report the cumulative incidence of COVID-19 recognized by center directors in their programs, the outcomes in those affected, and the impact on the process of care for pulmonary hypertension programs more broadly.
Methods
The Pulmonary Hypertension (PH) Care Center network began in 2014 and is composed of pulmonary vascular disease programs that have been accredited by the Pulmonary Hypertension Association (PHA) as centers with special expertise in the care of patients with PAH and/or CTEPH (11). A survey of program directors was undertaken to better understand the impacts of COVID-19 on patients with PAH or CTEPH (PAH/CTEPH) and on routine clinical operations of pulmonary vascular disease programs. An online survey was created using REDCap electronic data tools hosted at the University of Washington. The survey was designed so that most physicians could complete the task in 5–10 minutes. The project and questionnaire were reviewed by the University of Washington Human Subjects Division and ruled to be exempt from further human subjects review.
A list of directors at accredited centers was obtained from the PHA. In the case of centers with multiple named directors, only the first listed director was contacted. Directors were offered the opportunity to complete the survey themselves or forward the survey to a physician designee with a better knowledge of day-to-day clinical operations. The goal was to have each program complete only one survey. Invitations with an electronic link to an anonymous online survey were e-mailed to identified program directors on April 17, 2020. The survey link was disabled 1 week later on April 24, 2020. For comparison with our target population, CDC-reported state and national data on the COVID-19 pandemic were recorded at the midpoint of the survey (April 20, 2020) (12). A brief follow-up survey was conducted from April 27, 2020, until May 1, 2020, to collect the outcomes of patients with COVID-19 reported in the first survey.
Statistical Analysis
Results were largely descriptive and included percentages, medians (with interquartile range) for highly skewed data, and mean (with standard deviation) for data with a more normal distribution. Cumulative incidence was calculated as the number of patients with PAH or CTEPH who were recognized to have COVID-19 divided by the total number of patients with PAH or CTEPH followed by centers who responded to the survey. To better understand the impact of the pandemic on a pulmonary vascular disease program within the context of its surrounding community, programs were divided into those residing in states with a COVID-19 incidence above the median versus below the median as recorded on April 20, 2020, by the CDC. The choice of state as the unit of measure, rather than a smaller unit like a county or city, was done for convenience and because local practices at this point in the pandemic were largely dictated at the gubernatorial and state health department level. Average cumulative incidence was an average of individual state-specific cumulative incidences (and not an aggregate of cases divided by an aggregate of population) to better reflect the local experience. Linear regression was used to evaluate relationships between center characteristics and the impact on the weekly number of clinic visits. Center characteristics considered included cumulative incidence of COVID-19 in the state (high or low), presence of one or more patients with PAH or CTEPH with COVID-19 at the center, type of center accreditation (pediatric, adult, or regional), weekly visit volume before the outbreak, and the total number of patients followed by the center. Because the absolute change in the number of clinic visits would likely be influenced by the volume of visits before the outbreak, regressions were repeated looking at the relationship between centers characteristics and percent change in the volume of weekly clinic visits before and after the pandemic.
Missing Data
Among surveys that were returned, there were no missing data for any question used in quantitative estimates of cumulative incidence or in any of the demographic queries for a center. For the questions assessing the number of weekly clinic visits, participants were asked not to provide an answer if they did not feel confident estimating within 20% of the truth. Five respondents (8.6%) did not answer this question and were not included in analyses including weekly visit volume. Three survey respondents (5.2%) did not answer questions about the impact of the pandemic on testing and two survey respondents (3.4%) did not answer questions about the impact of the pandemic on treatment. As a result, the denominator of centers was adjusted to 55 for descriptions of testing impact and 56 for treatment impact.
Results
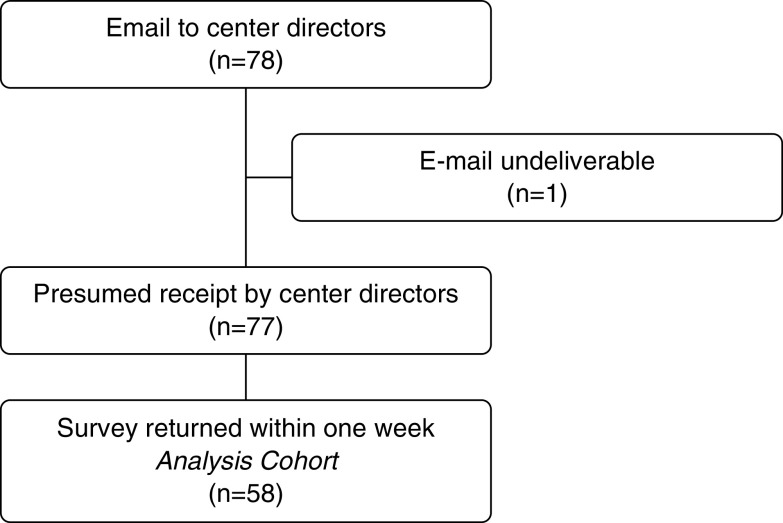
The survey was distributed to 78 center directors. One e-mail was automatically returned for an incorrect address, and of the 77 presumed successful deliveries, 58 center directors or their designees returned the survey from 30 unique states for a response rate of 75% (Figure 1). The majority of participating centers were Adult Centers for Comprehensive Care (CCC), most had at least one clinic on the same campus or within a hospital, and the majority were actively participating in the national PHA longitudinal registry of patients with PAH and CTEPH. The typical program was large, with a median of 300 patients with PAH or CTEPH, three physicians, an advanced practice provider, and a PH nurse (Table 1). Centers from states with high COVID-19 incidence were similar but tended to be slightly smaller.
Figure 1.
Study design.
Table 1.
Characteristics of participating pulmonary vascular disease centers
| Center Location |
|||
|---|---|---|---|
| All | Low-COVID-19 State* | High-COVID-19 State† | |
| Number of centers | 58 | 25 | 33 |
| Number of unique states | 30 | 13 | 17 |
| Clinic location, n (%) | |||
| Free-standing | 4 (7) | 3 (12) | 1 (3) |
| Within a hospital | 43 (74) | 14 (56) | 29 (88) |
| Both | 11 (19) | 8 (32) | 3 (9) |
| Type of accreditation, n (%) | |||
| Pediatric center (CCC) | 7 (12) | 3 (12) | 4 (12) |
| Adult center (CCC) | 42 (72) | 20 (80) | 22 (67) |
| Regional clinical program (RCP) | 9 (16) | 2 (8) | 7 (21) |
| Participating in the National PHA Registry, n (%) | 38 (66) | 19 (76) | 19 (58) |
| Number of patients with PAH/CTEPH followed, median (IQR) | 300 (120–375) | 350 (175–500) | 225 (100–350) |
| Staffing at the center, median (IQR) | |||
| PH physicians | 3 (2–4) | 3 (2–4) | 3 (1–4) |
| PH advanced practice providers | 1 (0–2) | 1 (0–2) | 1 (1–2) |
| PH nurses | 1 (1–2) | 1 (1–2) | 2 (1–2) |
Definition of abbreviations: CCC = Centers for Comprehensive Care; CDC = Centers for Disease Control and Prevention; COVID-19 = coronavirus disease; CTEPH = chronic thromboembolic pulmonary hypertension; IQR = interquartile range; PAH = pulmonary arterial hypertension; PH = pulmonary hypertension; PHA = Pulmonary Hypertension Association; RCP = regional clinical program.
States with a COVID-19 incidence below the median on April 20, 2020, as reported by the CDC with an average incidence of 0.7 infected individuals per 1,000 inhabitants (from the CDC on April 20, 2020; https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html#2.).
States with a COVID-19 incidence above the median on April 20, 2020, as reported by the CDC with an average incidence of 3.1 infected individuals per 1,000 inhabitants (from the CDC on April 20, 2020; https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html#2.).
Center directors reported a total of 50 patients with recognized COVID-19 out of a total of 16,979 patients with PAH or CTEPH followed at their respective centers. The cumulative incidence of COVID-19 in patients with PAH or CTEPH that was recognized by their center director during the fourth week of April 2020 was approximately 2.9 cases of COVID-19 per 1,000 patients (95% confidence interval [95% CI], 2.2–3.9 cases per 1,000 patients). Because errors in estimation are not necessarily predictable, another way to convey uncertainty in the estimate is to select an arbitrary “error” range for estimation. When accounting for up to a 30% error in estimates of program size by center directors (e.g., a collective range of patients with PAH and CTEPH at these centers that was actually between 11,885 and 22,073), this would reflect a range of 2.3–4.2 cases of COVID-19 recognized per 1,000 patients at risk. Both estimates were similar to concurrent CDC population estimate of COVID-19 cumulative incidence in the United States (approximately 2.4 cases per 1,000 people). The cumulative incidence of recognized COVID-19 in patients with PAH/CTEPH in states with a high population burden of COVID-19 appeared higher than states with a low reported incidence at the time (Table 2). Most PH care centers did not have any patients with PAH or CTEPH with COVID-19 infection; however, among those that had at least one, there were similar numbers of centers with one, two, or three total infected patients. Most centers (62%) did not use experimental therapies for their patients with PAH/CTEPH with COVID-19 infection; however, among centers that did try a COVID-19–targeted therapy, most used more than one agent, and hydroxychloroquine was the most commonly used. Of the 50 patients with COVID-19, 15 (30%) were hospitalized, 11 of whom (22%) were admitted to an intensive care unit, and 6 (12%) died. At the time of the survey, one patient with COVID-19 remained critically ill with a high risk of dying.
Table 2.
Patients with PAH or CTEPH who were recognized to have COVID-19
| Patients with PAH/CTEPH with recognized COVID-19 | 50 |
| Hospitalizations among patients with recognized COVID-19 | 15 |
| Intensive care unit stays among patients with recognized COVID-19 | 11 |
| Deaths among patients with recognized COVID-19 | 6 |
| Total number of reported patients with PAH/CTEPH followed by centers | 16,979 |
| Cumulative incidence of recognized COVID-19 among patients with PAH/CTEPH (per 1,000 patients) | |
| Overall cumulative incidence of recognized COVID-19 | 2.9 (95% CI, 2.2–3.9) |
| Cumulative incidence in patients from states with a low COVID-19 incidence | 1.4 (95% CI, 0.8–2.4) |
| Cumulative incidence in patients from states with a high COVID-19 incidence | 4.6 (95% CI, 3.4–6.4) |
| U.S. cumulative incidence of COVID-19 (per 1,000 inhabitants)* | 2.4 |
| Centers with patients with PAH/CTEPH recognized to have COVID-19, n (%) | |
| No patients | 37 (64) |
| One patient | 8 (14) |
| Two patients | 6 (10) |
| Three patients | 5 (9) |
| Four or more patients | 2 (3) |
| Among centers with at least one patient with COVID-19, centers that used a potential targeted therapy, n (%) | |
| Hydroxychloroquine | 6 (29) |
| Chloroquine | 2 (9) |
| Azithromycin | 3 (14) |
| Remdesivir | 2 (9) |
| Steroids | 3 (14) |
| Vitamin C | 3 (14) |
| Convalescent sera | 1 (5) |
| Tocilizumab (or other interleukin therapy) | 2 (9) |
| iNO | 1 (5) |
| Centers who tried none of these therapies | 13 (62) |
Definition of abbreviations: CDC = Centers for Disease Control and Prevention; CI = confidence interval; COVID-19 = coronavirus disease; CTEPH = chronic thromboembolic pulmonary hypertension; iNO = inhaled nitric oxide; PAH = pulmonary arterial hypertension.
From the CDC on April 20, 2020; https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html#2.
Although the number of patients with PAH/CTEPH with recognized COVID-19 infection was quite low, the impact on clinical operations at centers was substantial (Tables 3 and E1 in the online supplement). The median number of weekly clinic visits, whether in person or via telehealth, declined to less than half of preoutbreak volumes. Nearly half (45%) of centers reported a reduction in overall program staffing, and the majority of programs (85%) reported a reduction of in-person staffing during clinic. The majority of centers transitioned from not routinely using telephone or video-enabled visits to using both of these approaches in the less than 2 months since the outbreak began. The impact on routine diagnostic testing for pulmonary vascular disease was similarly large, with more than 80% of centers reporting that they were obtaining fewer echocardiograms, right heart catheterizations, and ventilation–perfusion scans. A minority of centers completely stopped obtaining these tests (Table 3). Although fewer centers reported impact on treatment, a minority reported decreased numbers of new prescriptions for oral therapy, parenteral therapy, and transplant referral. The most common reason for declining visits was a hospital or health system mandate (more than 80% of centers) followed by physician or patient fear that the patient would acquire COVID-19 at the health center (more than 60% of centers). Very few centers attributed the decline in visits or testing to a lack of staff (<15%). Centers prioritized seeing patients for visits or testing who reported worsening to clinic staff (60% of centers) and/or those who had severe disease (47% of centers). A smaller number of centers (<25%) prioritized seeing new patient evaluations, World Health Organization Group 1 or 4 patients, or those who lived locally. No center prioritized seeing patients with mild disease.
Table 3.
Impact of the coronavirus outbreak on routine clinic operations
| Relative to the Outbreak |
||
|---|---|---|
| Before | After | |
| Clinical visits | ||
| Typical number of weekly outpatient visits, median (IQR) | 30 (20–45) | 12 (5–25) |
| Centers with routine use of formal telephone visits, n (%) | 4 (7) | 48 (83) |
| Centers with routine use of video-enabled visits, n (%) | 5 (9) | 54 (93) |
| Testing (n = 55 centers responding), n (%) | ||
| Centers obtaining fewer echocardiograms | Ref | 50 (91) |
| Of these, centers with 60–99% reduction | 30 (60) | |
| Of these, centers with total cessation | 5 (10) | |
| Centers obtaining fewer RHCs | Ref | 49 (89) |
| Of these, centers with 60–99% reduction | 34 (69) | |
| Of these, centers with total cessation | 4 (8) | |
| Centers obtaining fewer CTPAs | Ref | 35 (64) |
| Of these, centers with 60–99% reduction | 18 (51) | |
| Of these, centers with total cessation | 5 (14) | |
| Centers obtaining fewer scans | Ref | 50 (91) |
| Of these, centers with 60–99% reduction | 23 (46) | |
| Of these, centers with total cessation | 12 (24) | |
| Centers that have switched to Q scans only | Ref | 21 (38) |
| Treatment (n = 56 centers responding), n (%) | ||
| Centers limiting new oral therapy prescriptions | Ref | 10 (18) |
| Of these, centers with 60–99% reduction | 2 (20) | |
| Of these, centers with total cessation | 0 (0) | |
| Centers limiting new i.v./s.c. prescriptions | Ref | 17 (30) |
| Of these, centers with 60–99% reduction | 5 (29) | |
| Of these, centers with total cessation | 3 (18) | |
| Centers with fewer transplant referrals | Ref | 15 (27) |
| Of these, centers with 60–99% reduction | 2 (13) | |
| Of these, centers with total cessation | 3 (20) | |
Definition of abbreviations: CTPA = computed tomographic pulmonary angiogram; IQR = interquartile range; i.v. = intravenous; Q = perfusion; Ref = referent; RHC = right heart catheterization; s.c. = subcutaneous; = ventilation–perfusion.
The impact on program operations was more pronounced in centers located in states with a high cumulative incidence of COVID-19 (Tables 4 and E2). This impact was most apparent in the reduction in clinic visits, adoption of telephone and telehealth visits, and limitation of new parenteral therapies or transplant referrals. A series of unadjusted linear regressions suggested the absolute decrease in clinic visits was greater in programs from states with a high burden of COVID-19 (8.0 fewer weekly visits in high-cumulative-incidence states; 95% CI, 3.8–15.6; P = 0.04), programs with at least one patient who developed COVID-19 (3.5 fewer weekly visits per additional patient diagnosed with COVID-19; 95% CI, 0.7–6.2; P = 0.01), Adult CCCs (Adult CCCs had 15.1 fewer visits than Regional Clinical Programs; 95% CI, 4.5–27.5; P = 0.006; and 10.3 fewer visits than pediatric CCCs; 95% CI, 0.3–20.3; P = 0.04), and programs with a large weekly visit volume before the outbreak (0.4 fewer visits after the outbreak per additional typical weekly visit before the outbreak; 95% CI, 0.1–0.6; P = 0.002). The overall number of patients followed by a program was not associated with the impact on the weekly number of clinic visits. Many of the features associated with a greater absolute decline in weekly visits were also associated with a larger typical volume of weekly visits before the pandemic. After accounting for differences in baseline weekly visit volume before the outbreak (by looking at the percent change in outpatient clinic volume), only high COVID-19 incidence in the state was independently associated with the magnitude in reduction of clinic visits after the outbreak (19.1% fewer visits in states with high cumulative incidence; 95% CI, 16.2–36.6%; P = 0.03).
Table 4.
Impact of the coronavirus outbreak on routine clinic operations of centers in states with a high reported incidence of COVID-19 relative to a low reported incidence
| Low COVID-19 Incidence |
High COVID-19 Incidence |
||||
|---|---|---|---|---|---|
| Before | After | Before | After | ||
| Clinic visits | |||||
| Typical number of weekly outpatient visits, median (IQR) | 35 (25–50) | 25 (6–40) | 30 (20–40) | 9 (5–20) | |
| Centers with routine formal telephone visits, n (%) | 1 (4) | 18 (72) | 3 (9) | 30 (91) | |
| Centers with routine video-enabled visits, n (%) | 2 (8) | 22 (88) | 3 (9) | 32 (97) | |
| Testing, n (%) |
n = 23 responding |
n = 32 responding |
|||
| Centers obtaining fewer echocardiograms | Ref | 20 (87) | Ref | 30 (94) | |
| Of these, centers with 60–99% reduction | 10 (50) | 20 (67) | |||
| Of these, centers with total cessation | 2 (10) | 3 (10) | |||
| Centers obtaining fewer RHCs | Ref | 21 (91) | Ref | 28 (88) | |
| Of these, centers with 60–99% reduction | 11 (52) | 23 (82) | |||
| Of these, centers with total cessation | 3 (14) | 1 (4) | |||
| Centers obtaining fewer CTPAs | Ref | 13 (57) | Ref | 22 (69) | |
| Of these, centers with 60–99% reduction | 4 (31) | 14 (64) | |||
| Of these, centers with total cessation | 2 (15) | 3 (14) | |||
| Centers obtaining fewer scans | Ref | 22 (96) | Ref | 28 (88) | |
| Of these, centers with 60–99% reduction | 8 (36) | 15 (54) | |||
| Of these, centers with total cessation | 6 (27) | 6 (21) | |||
| Centers who have switch to Q scans only | Ref | 12 (52) | Ref | 9 (28) | |
| Treatment, n (%) |
n = 23 responding |
n = 33 responding |
|||
| Centers limiting new oral therapy prescriptions | Ref | 5 (22) | Ref | 5 (15) | |
| Of these, centers with 60–99% reduction |
1 (20) | 1 (20) | |||
| Of these, centers with total cessation |
0 (0) | 0 (0) | |||
| Centers limiting new i.v./s.c. prescriptions | Ref | 6 (26) | Ref | 11 (33) | |
| Of these, centers with 60–99% reduction |
1 (17) | 4 (36) | |||
| Of these, centers with total cessation |
2 (33) | 1 (9) | |||
| Centers with fewer transplant referrals | Ref | 4 (17) | Ref | 11 (33) | |
| Of these, centers with 60–99% reduction |
1 (25) | 1 (9) | |||
| Of these, centers with total cessation | 1 (25) | 2 (18) | |||
Definition of abbreviations: COVID-19 = coronavirus disease; CTPA = computed tomographic pulmonary angiogram; IQR = interquartile range; i.v. = intravenous; Q = perfusion; Ref = referent; RHC = right heart catheterization; s.c. = subcutaneous; = ventilation–perfusion.
The outbreak also had a substantial impact on patient-oriented research at centers. Only three centers (5%) continued to enroll new participants into clinical studies. For subjects already participating in clinical research, about half of centers (48%) continued to follow them normally per protocol, whereas the other centers modified their follow-up relative to the original protocol.
Discussion
In this cross-sectional survey, the cumulative incidence of COVID-19 infection recognized in patients with PAH and CTEPH was similar to the cumulative incidence recognized in the general public at both the local and national level. Although we found a qualitatively similar incidence, which may be cautiously reassuring, care must be taken not to overinterpret this similarity (13). We focused on “recognized” COVID-19. This is likely to underestimate the overall burden of COVID-19 owing to a lack of widespread testing and incomplete awareness by center directors of COVID-19 among their patients. On the other hand, patients with PAH and CTEPH have a greater interface with the medical system and more limited physiologic reserve. As such, it is possible that patients with PAH or CTEPH were tested at a higher rate than the general population. If true, this could falsely elevate the incidence in patients with PAH/CTEPH relative to the general population (which suffers from the same or more pronounced lack of access to testing) (14).
Although the total number of patients recognized as having COVID-19 was limited, those that were recognized experienced high rates of hospitalization and death. These outcomes appear worse than those reported for the population at large. For instance, 73% of hospitalized patients in this survey required intensive care whereas only 14% of hospitalized patients in New York required intensive care (15–19). Morbidity and mortality were modestly worse than previously suggested by a research letter speculating that patients with PAH may be at lower risk than expected for severe COVID-19. In that survey-based analysis, they found that 53% of patients with recognized COVID-19 were hospitalized and 8% of hospitalized patients died. Although our results suggested fewer patients required hospitalization (30%), a greater fraction of the overall number of patients had died (we did not specifically query whether deaths occurred among patients who were hospitalized) (7). We did not find evidence that PAH or CTEPH protect from morbidity and mortality in COVID-19 relative to the general population. That said, it is again worth stressing that the lack of widespread testing makes these estimates preliminary. Case fatality and hospitalization rates could be substantially different if all cases of COVID-19 in individuals with PAH or CTEPH were identified or all individuals who died with COVID-19 were recognized (20).
In contrast to the relatively small percentage of patients recognized to have COVID-19, the total impact on clinical care for patients with PAH or CTEPH was substantial. We discovered a large decrease in clinic visits, which not surprisingly appeared to be most pronounced in states with a high cumulative incidence of COVID-19. There was a similarly large decrease in pulmonary vascular–focused testing, and some programs reported a total cessation of testing. Although the frequency of testing in pulmonary hypertension has been identified as potentially excessive before the pandemic, this assertion is debated and guidelines incorporate routine testing in the current standard of care (2, 21, 22). Finally, we found that a minority of centers limited new prescriptions and referred a smaller number of patients to transplant.
Advances in PAH management have markedly improved patient survival over the past 20 years (23). Limitations in access to evaluation, testing, and therapy may have detrimental effects on patient health, even in patients with PAH/CTEPH who do not contract COVID-19. We show that centers rapidly incorporated formal telephone and video-enabled visits over a small window of time to mitigate the impact of these disruptions. Although encouraging, barriers to effective implementation of telehealth are well recognized (24). It will be important to measure longitudinal outcomes in the PAH and CTEPH communities to assess the impact of these widespread disruptions and attempts at mitigation.
A number of experimental therapies for COVID-19 have been proposed (25). At the time of the survey, none of these therapies were strongly supported by randomized trial data (25, 26). The majority of centers in our survey did not use experimental therapies for targeted treatment of COVID-19. Centers that did try a potential targeted therapy most commonly used hydroxychloroquine. There has also been some speculation that endothelin receptor antagonists could modulate the body's response to COVID-19 (akin to targeted therapy); however, no centers either initiated or stopped endothelin receptor antagonists in response to this pandemic (27).
Our study has clear methodologic limitations. Central among these is reliance on program directors to estimate the number of patients they follow, the number of COVID-19 cases, the outcomes of these cases, and the impact on clinical operations. This may introduce error when compared with aggregated surveys of individuals or widespread screening. Surveys also have other well-documented biases that could influence our results (28, 29). Although the response rate was high, a quarter of centers did not respond, and nonrepresented centers may differ from those that responded. In addition, outcomes of patients with PAH/CTEPH with COVID-19 should be interpreted cautiously because of the small total number of patients in our survey with COVID-19 and potential inaccuracies from underdiagnosed infection due to the widespread lack of testing.
Despite its limitations, a survey of accredited center directors can be an effective early approach to efficiently estimate incidence, outcomes, and impact of COVID-19 within a rare disease population like PAH or CTEPH. There is no universal national monitoring system for COVID-19, and approaches relying on administrative coding have well-recognized limitations, particularly in pulmonary hypertension research (30). By leveraging a well-organized network of expert centers, this study increases the likelihood that results are specific to patients with PAH or CTEPH and allows early estimates from a nationally representative sample.
Conclusion
We present survey-based estimates of cumulative incidence and outcomes for patients with PAH or CTEPH who were cared for at PH care centers and who were recognized to have COVID-19. The cumulative incidence of COVID-19 in this population appears to be similar to the overall U.S. population, and outcomes appear worse, but not universally poor. The fraction of patients with PAH or CTEPH who contracted COVID-19 was small; however, the impact the pandemic had on clinic operations, routine diagnostic testing, and treatment for the broader PAH/CTEPH community was substantial. Whether these impacts will ultimately affect outcomes is unknown, but at this time, these indirect impacts of the pandemic affect a significantly higher proportion of patients with PAH or CTEPH than the infection itself.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank the Pulmonary Hypertension Association for supporting the effort to conduct this survey and the directors of accredited centers for providing their experience.
Footnotes
Author Contributions: J.D.L. and P.J.L. contributed to survey design, analyzed the data, and drafted the manuscript. G.B.D. contributed to survey design, collected the data, and revised the manuscript. C.D.B., D.G., D.D.R., S.G.R., J.J.R., Z.S., C.E.V., and R.T.Z. contributed to survey design and revised the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Chakinala MM, Duncan M, Wirth J. Managing the patient with pulmonary hypertension: specialty care centers, coordinated care, and patient support. Cardiol Clin. 2016;34:489–500. doi: 10.1016/j.ccl.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 2.Galiè N, Humbert M, Vachiery J-L, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Respir J. 2015;46:903–975. doi: 10.1183/13993003.01032-2015. [DOI] [PubMed] [Google Scholar]
- 3.CDC. Centers for Disease Control and Prevention; 2020. Coronavirus Disease 2019 (COVID-19) [accessed 2020 May 2]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-hcf.html. [Google Scholar]
- 4.Welt FGP, Shah PB, Aronow HD, Bortnick AE, Henry TD, Sherwood MW, et al. Catheterization laboratory considerations during the coronavirus (COVID-19) pandemic: from ACC’s Interventional Council and SCAI. J Am Coll Cardiol. 2020;75:2372–2375. doi: 10.1016/j.jacc.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Emami A, Javanmardi F, Pirbonyeh N, Akbari A. Prevalence of underlying diseases in hospitalized patients with COVID-19: a systematic review and meta-analysis. Arch Acad Emerg Med. 2020;8:e35. [PMC free article] [PubMed] [Google Scholar]
- 7.Horn EM, Chakinala M, Oudiz R, Joseloff E, Rosenzweig EB. Could pulmonary arterial hypertension patients be at a lower risk from severe COVID-19? Pulm Circ. 2020;10:2045894020922799. doi: 10.1177/2045894020922799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ryan JJ, Melendres-Groves L, Zamanian RT, Oudiz RJ, Chakinala M, Rosenzweig EB, et al. Care of patients with pulmonary arterial hypertension during the coronavirus (COVID-19) pandemic. Pulm Circ. 2020;10:2045894020920153. doi: 10.1177/2045894020920153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Primary Care & COVID-19. Primary Care Collaborative; 2020. Week 6 survey. [accessed 2020 May 2]. Available from: https://www.pcpcc.org/2020/04/23/primary-care-covid-19-week-6-survey. [Google Scholar]
- 10. Mehrotra A, Chernew M, Linetsky D, Hatch H, Cutler D. The impact of the COVID-19 pandemic on outpatient visits: a rebound emerges. To the point (blog), Commonwealth fund, 2020 [updated May 19, 2020; accessed 2020 Oct 28]. Available from: [DOI]
- 11.Polanco-Briceno S, Glass D, Caze A. Self-reported physician practices in pulmonary arterial hypertension: diagnosis, assessment, and referral. Contemp Clin Trials Commun. 2015;2:54–60. doi: 10.1016/j.conctc.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.CDC. Coronavirus disease 2019 (COVID-19): Cases in the U.S. 2020 [accessed 2020 Apr 20]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html.
- 13.Yue M, Clapham HE, Cook AR. Estimating the size of a COVID-19 epidemic from surveillance systems. Epidemiology. 2020;31:567–569. doi: 10.1097/EDE.0000000000001202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lipsitch M, Riley S, Cauchemez S, Ghani AC, Ferguson NM. Managing and reducing uncertainty in an emerging influenza pandemic. N Engl J Med. 2009;361:112–115. doi: 10.1056/NEJMp0904380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murthy S, Gomersall CD, Fowler RA. Care for critically ill patients with COVID-19. JAMA. 2020;323:1499. doi: 10.1001/jama.2020.3633. [DOI] [PubMed] [Google Scholar]
- 16.Baud D, Qi X, Nielsen-Saines K, Musso D, Pomar L, Favre G. Real estimates of mortality following COVID-19 infection. Lancet Infect Dis. 2020;20:773. doi: 10.1016/S1473-3099(20)30195-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rajgor DD, Lee MH, Archuleta S, Bagdasarian N, Quek SC. The many estimates of the COVID-19 case fatality rate. Lancet Infect Dis. 2020;20:776–777. doi: 10.1016/S1473-3099(20)30244-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garg S. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019 - COVID-NET, 14 states, March 1-30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:458–464. doi: 10.15585/mmwr.mm6915e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. and the Northwell COVID-19 Research Consortium. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. doi: 10.1001/jama.2020.4683. [online ahead of print] 23 Mar 2020; DOI: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 21.Kirson NY, Birnbaum HG, Ivanova JI, Waldman T, Joish V, Williamson T. Excess costs associated with patients with pulmonary arterial hypertension in a US privately insured population. Appl Health Econ Health Policy. 2011;9:293–303. doi: 10.2165/11592430-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 22.Kirson NY, Birnbaum HG, Ivanova JI, Waldman T, Joish V, Williamson T. Excess costs associated with patients with chronic thromboembolic pulmonary hypertension in a US privately insured population. Appl Health Econ Health Policy. 2011;9:377–387. doi: 10.2165/11592440-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 23.Badlam JB, Bull TM. Steps forward in the treatment of pulmonary arterial hypertension: latest developments and clinical opportunities. Ther Adv Chronic Dis. 2017;8:47–64. doi: 10.1177/2040622317693218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith WR, Atala AJ, Terlecki RP, Kelly EE, Matthews CA. Implementation guide for rapid integration of an outpatient telemedicine program during the COVID-19 pandemic. J Am Coll Surg. 2020;231:216–222, e2. doi: 10.1016/j.jamcollsurg.2020.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. doi: 10.1001/jama.2020.6019. [online ahead of print] 13 Apr 2020; DOI: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 26.CDC. Centers for Disease Control and Prevention; 2020. Information for clinicians on investigational therapeutics for patients with COVID-19. [accessed 2020 May 2]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/therapeutic-options.html. [Google Scholar]
- 27.Badagliacca R, Sciomer S, Petrosillo N. Endothelin receptor antagonists for pulmonary arterial hypertension and COVID-19: friend or foe? J Heart Lung Transplant. 2020;39:729–730. doi: 10.1016/j.healun.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khanji C, Schnitzer ME, Bareil C, Perreault S, Lalonde L. Concordance of care processes between medical records and patient self-administered questionnaires. BMC Fam Pract. 2019;20:92. doi: 10.1186/s12875-019-0979-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Safdar N, Abbo LM, Knobloch MJ, Seo SK. Research methods in healthcare epidemiology: survey and qualitative research. Infect Control Hosp Epidemiol. 2016;37:1272–1277. doi: 10.1017/ice.2016.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Link J, Glazer C, Torres F, Chin K. International classification of diseases coding changes lead to profound declines in reported idiopathic pulmonary arterial hypertension mortality and hospitalizations: implications for database studies. Chest. 2011;139:497–504. doi: 10.1378/chest.10-0837. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.