The coronavirus disease (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has severely restricted pulmonary diagnostic testing because of the concern of droplet and aerosol generation by procedures conducted in small test rooms. SARS-CoV-2 infection is characterized by viral shedding from the upper and lower respiratory tracts; in addition, SARS-CoV-2 RNA has been detected in sampled air throughout a hospital, which leads to this concern (1–4). Pulmonary function laboratories are justifiably concerned because test maneuvers involve forceful breathing, which may generate infectious particles. Normal speaking has also been reported to generate small-droplet aerosols, increasing the potential exposure risk in close contact with infected individuals (5–7). Currently, there are no studies evaluating particle generation during pulmonary function tests (PFTs). To better understand the risk associated with PFTs, we sought to quantify and characterize the amount of detectable aerosol and droplet generation during routine pulmonary function studies at prespecified distances.
Methods
This was a single-center prospective study conducted at the Mayo Clinic in Florida. Five adult volunteer subjects without pulmonary disease consented to perform tidal breathing (Vt), normal speaking, forced vital capacity (FVC), and maximum voluntary ventilation (MVV) maneuvers. The Mayo Clinic Institutional Review Board approved this study (20–005544).
Particle measurement
A light-scattering particle counter (FLUKE 983) was used to simultaneously measure six channels of particle size distribution (0.3, 0.5, 1, 2, 5, and 10 μm), temperature, and humidity while each maneuver was being performed at each measured location. A minimum sampling volume of 1 L was obtained per measurement.
Test conditions
To control for humidity, temperature, and other indeterminate ambient variables, room occupancy was limited to three persons (the subject and two investigators) during the study. The pulmonary function test room is 936 cubic feet and has 12.8 room air exchanges per hour. The ambient baseline measurement was obtained at the center of the room before and after each maneuver, with the subject and two investigators in the room and the door closed. The testing room was used only for this study on the day of the testing. The particle counter was positioned as follows: For the speaking portion, the particle counter was positioned 12 inches directly in front of the speaker. For the pulmonary function test portion, the air was sampled at the following three locations: 1) at a 90° angle to and touching the exhalation port (0 ft) on the pulmonary function equipment (Masterscreen PFT; Vyaire Medical), 2) at 1.5 ft from the exhalation port, halfway between where the patient and respiratory therapist sat, and 3) at 3 ft from the exhalation port. The number of particles counted and the volume of air sampled were recorded. The particle counter was zeroed before each test condition. Spirometry was performed outside the plethysmography box. A Microgard II filter (Vyaire Medical) was interposed between the mouthpiece and intake port for all maneuvers, as per manufacturer. For the speaking portion, each subject read “The Rainbow Passage” by Grant Fairbanks for 30 seconds. The subject then performed Vt, FVC, and MVV through the pulmonary function test machine. If the subject coughed during the maneuver, the measurement was discarded, and the study was repeated once ambient particle count had returned to baseline. Each subject performed the test conditions twice.
Analysis
A nonparametric distribution of measurements was assumed. The Mann-Whitney test for two group comparisons and Wilcoxon/Kruskal-Wallis test for multiple group comparisons was used. If the Kruskal-Wallis test was significant, a Steel test would be performed to compare each group to a single control. This was designated to be the ambient measurement obtained before the respective test. A P value of less than 0.05 was considered significant.
Results
The five subjects included four men (80%). The mean age was 37.6 ± 8.4 years, the mean height was 177.6 ± 3.9 cm, and the mean weight was 80.4 ± 4.5 kg. Volunteers were white (60%), East Asian Indian (20%), and Asian (20%). Ambient room humidity was measured at 48%, and temperature was 23°C. Table 1 shows the increase in particle counts under different test conditions, with small respirable particles in the 0.3-μm range being generated the most. The FVC and MVV maneuvers generated increased small particles when compared with the ambient measurements with Vt, FVC, and MVV.
Table 1.
Quantity of particles per liter of sampled air separated by particle size for each maneuver and ambient room measurement
| Maneuver | Particle Quantity Per Liter of Sampled Air by Size |
|||||
|---|---|---|---|---|---|---|
| 0.3 μm [Mean (95% CI)] | 0.5 μm [Mean (95% CI)] | 1 μm [Mean (95% CI)] | 2 μm [Mean (95% CI)] | 5 μm [Mean (95% CI)] | 10 μm [Mean (95% CI)] | |
| Speaking | ||||||
| Ambient | 1,269.6 (1,230.6–1,308.6) | 79.5 (51.2–107.8) | 21.3 (10.3–32.3) | 37.6 (17.3–57.9) | 4.9 (3.2–6.6) | 3.3 (1.4–5.2) |
| Speaking for 30 s, 1 ft | 1,293.4 (1,242.9–1,343.9) | 131.7 (47.3–216.1) | 30.2 (23.6–36.8) | 47.6 (38.8–56.4) | 7.7 (4.0–11.4) | 2.7 (0.0–5.4) |
| Vt | ||||||
| Ambient | 1,299.2 (1,222.7–1,375.7) | 95.2 (80.1–110.3) | 33.3 (15.8–50.8) | 59.6 (44.0–75.1) | 8.9 (5.3–12.5) | 4.2 (1.8–6.6) |
| 0 ft | 6,466.6(5,991.3–6,941.9) | 770.3 (730.5–810.1) | 24.7 (16.7–32.7) | 21.7 (13.8–29.6) | 3.0 (1.4–4.6) | 1.4 (0.1–2.7) |
| 1.5 ft | 1,250.8 (1,201.8–1,299.8) | 88.8 (72.9–104.7) | 28.6 (22.5–34.7) | 41.6 (36.1–47.1) | 5.6 (3.2–8.0) | 2.5 (1.0–4.0) |
| 3 ft | 1,250.3 (1,190.0–1,310.6) | 77.2 (67.3–87.1) | 24.2 (17.6–30.8) | 32.8 (24.8–40.8) | 5.0 (3.4–6.6) | 2.2 (1.1–3.3) |
| FVC | ||||||
| Ambient | 1,334.8 (1,195.9–1,473.7) | 101.8 (71.7–131.9) | 31.6 (19.8–43.4) | 38.8 (25.4–52.2) | 4.7 (1.9–7.5) | 2.8 (2.2–3.4) |
| 0 ft | 10,739.3 (8,634.7–12,843.9) | 1,408.4 (1,076.4–1,740.4) | 26.0 (16.1–35.9) | 21.1 (12.6–29.6) | 2.1 (1.8–2.4) | 0.8 (0.2–1.4) |
| 1.5 ft | 1,258.2 (1,218.2–1,298.2) | 79.0 (73.0–85.0) | 23.4 (18.4–28.4) | 29.8 (25.0–34.6) | 5.0 (2.7–7.3) | 1.8 (0.7–2.9) |
| 3 ft | 1,266.9 (1,208.3–1,325.5) | 73.6 (61.9–85.3) | 19.6 (14.2–25.0) | 27.0 (22.0–32.0) | 2.4 (1.3–3.5) | 1.8 (0.4–3.2) |
| MVV | ||||||
| Ambient | 1,323.7 (1,226.1–1,421.3) | 88.6 (83.3–93.9) | 30.8 (14.3–47.3) | 40.8 (34.8–46.8) | 4.6 (3.9–5.3) | 2.4 (1.7–3.1) |
| 0 ft | 8,845.2 (6,439.1–11,251.3) | 1,251 (444.1–2,057.9) | 33.2 (17.5–48.9) | 20.8 (14.2–27.4) | 3.4 (2.0–4.8) | 0.6 (0.0–1.3) |
| 1.5 ft | 1,278.1 (1,214.3–1,341.9) | 81.0 (58.5–103.5) | 29.2 (26.2–32.2) | 38.0 (35.5–40.5) | 2.8 (1.0–4.6) | 2.8 (1.4–4.2) |
| 3 ft | 1,283.6 (1,231.2–1,336.0) | 82.6 (77.4–87.8) | 19.4 (16.2–22.6) | 34.6 (29.6–39.6) | 4.2 (1.8–6.6) | 1.4 (0.0–2.8) |
Definition of abbreviations: CI = confidence interval; FVC = forced vital capacity; MVV = maximum voluntary ventilation; Vt = tidal volume.
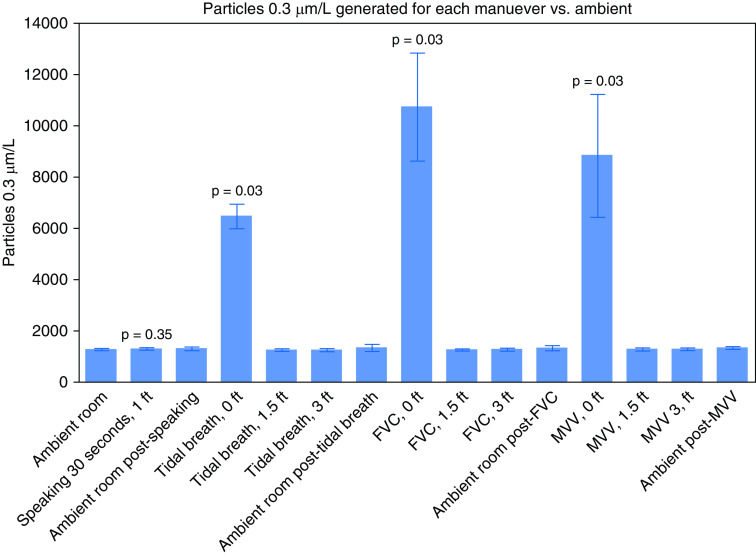
Proximity to the source was associated with significant increases in particle generation. With normal speaking, there was not an increase of generated 0.3-μm particles at 12 inches. When sampled close to the exhalation port position, all the maneuvers generated an increase in respirable 0.3-μm particles when compared with baseline (Figure 1). Other aerosol-sized particles measured at 0.5 μm were also increased at this close position. At 1.5 ft and 3 ft from the exhalation port, there was no increase in particle generation for any of the trialed maneuvers.
Figure 1.
Graph of the concentration of 0.3-μm particles per liter of sampled air generated for each maneuver. P values shown for significant results. FVC = forced vital capacity; MVV = maximum voluntary ventilation.
Conclusions
This report documents and characterizes aerosol particle generation from routine PFTs and supports precautions to mitigate the transmission of SARS-CoV-2. These precautions include the patient wearing a mask during the instructional phase, maintaining a distance of at least 1.5 ft between the subject and respiratory therapist, using disposable plastic covers over the PFT equipment, and using N95 and personal protective equipment for the respiratory therapist. This study provides valuable information guiding infectious control measures to ensure personnel and patient safety during pulmonary function testing. A limitation of this study was the small sample size of healthy volunteers, which increases the risk of multiple comparisons and decreases the overall stability of the results, but the results were consistent between volunteers, providing reassurance that larger sample sizes may provide similar results. In addition, the high room exchange and humidity may have contributed to particle dispersion and evaporation. Because the volunteers were healthy, the amount and size of particles generated could be considered the minimum, as a patient with nasal secretions or a productive cough may theoretically generate more particles. We did not assess the composition or infectivity of the measured particles, so the possibility of transmission is unknown but possible given the increase in particle generation.
In conclusion, PFTs and normal breathing all generate aerosols rather than just droplets. The room air exchange, room turnaround time between testing, and distance between the patient and technician in the testing room are important. Particle generation close to the exhalation port warrants using a single-use plastic covering over the device, with the mouthpiece port and the exhalation port exposed, to avoid equipment contamination. The generation of aerosol-sized particles warrants the use of N95 masks and personal protective equipment during routine PFTs in patients who potentially have an airborne transmissible infectious disease.
Supplementary Material
Footnotes
Author Contributions: S.A.H., K.G.L., A.S.L., A.S.N., and N.M.P. had substantial contributions to the conception and design of this work; acquisition, analysis, and interpretation of the data; drafting and revising this manuscript; and final approval of this version to be published; and all agree to be accountable for all aspects of the work.
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Sungnak W, Huang N, Bécavin C, Berg M, Queen R, Litvinukova M, et al. HCA Lung Biological Network. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020;26:681–687. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wölfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Müller MA, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 3.Zou L, Ruan F, Huang M, Liang L, Huang H, Hong Z, et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Y, Ning Z, Chen Y, Guo M, Liu Y, Gali NK, et al. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. 2020;582:557–560. doi: 10.1038/s41586-020-2271-3. [DOI] [PubMed] [Google Scholar]
- 5.Anfinrud P, Stadnytskyi V, Bax CE, Bax A. Visualizing speech-generated oral fluid droplets with laser light scattering. N Engl J Med. 2020;382:2061–2063. doi: 10.1056/NEJMc2007800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asadi S, Wexler AS, Cappa CD, Barreda S, Bouvier NM, Ristenpart WD. Effect of voicing and articulation manner on aerosol particle emission during human speech. PLoS One. 2020;15:e0227699. doi: 10.1371/journal.pone.0227699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duguid JP. The size and the duration of air-carriage of respiratory droplets and droplet-nuclei. J Hyg (Lond) 1946;44:471–479. doi: 10.1017/s0022172400019288. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.