Dear Editor,
Besides diagnostic tests for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) using reverse transcription-polymerase chain reaction (RT-PCR), some simple kits use immunochromatography to detect SARS-CoV-2-specific antigens. The immunochromatographic antigen test is less sensitive than RT-PCR but with equivalent specificity.1, 2, 3, 4 Therefore, in Japan, for a positive antigen test result within 9 days of onset, the coronavirus disease 2019 (COVID-19) can be diagnosed without performing RT-PCR. However, any test can produce false-positive. Herein, we report a case of false-positive SARS-CoV-2-specific antigen test.
A 40-year-old man was hospitalized with a diagnosis of COVID-19 because of a positive SARS-CoV-2-specific antigen test (ESPLINE SARS-CoV-2®; Fujirebio Inc. Tokyo, Japan). The patient had some symptoms consistent with COVID-19, but he had no history of close contact with known COVID-19 patients. On day 4 of admission, as part of the study to determine whether saliva samples could be used for SARS-CoV-2 RT-PCR tests, we performed SARS-CoV-2 RT-PCR tests (using a Cobas® z480 analyzer; Roche Diagnostics, Indianapolis, IN, USA) on saliva samples (saved after RNA extraction) collected from the patient on days 1 and 2 since admission, but the results for the patient's samples were negative. Consequently, we suspected that the original antigen-detecting rapid test result may have been a false-positive.
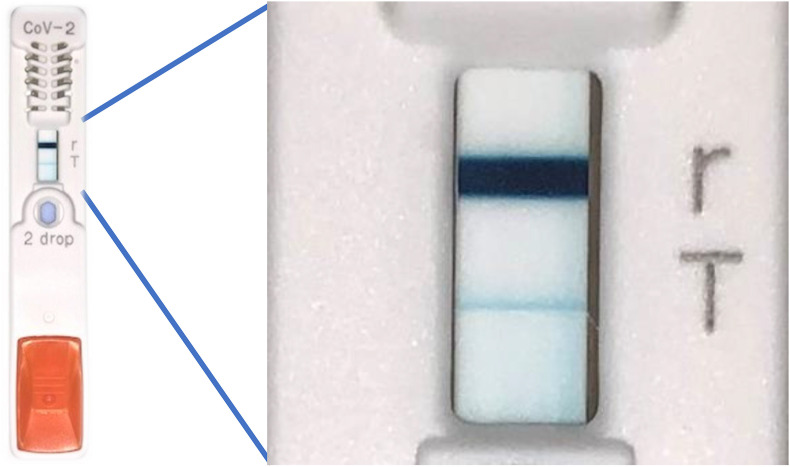
Two weeks after onset, an antibody test (Elecsys® Anti-SARS-CoV-2) was performed on a serum sample from the patient at LSI Medience (Tokyo, Japan), but the result was negative. Upon receiving these results, a second SARS-CoV-2-specific antigen test (ESPLINE SARS-CoV-2®) was conducted on a new nasopharyngeal swab sample collected from the patient 1.5 months after onset, which was weakly positive (Fig. 1 ) despite the patient experiencing no symptoms. Furthermore, the SARS-CoV-2 RT-PCR (using a Cobas® z480 analyzer) result for a nasopharyngeal swab sample collected at the same time was negative. Thus, we concluded that antigen test results were false positive.
Figure 1.
SARS-CoV-2-specific antigen test (ESPLINE SARS-CoV-2®, Fujirebio Inc.) results for this case at 1.5 months after onset. The figure on the right is an enlargement of a part of the kit. The r line is the reference line, and the T line is the judgment line. Because the judgment line was thinner and lighter than the reference line, we judged it weakly positive.
After that, 7 patients were admitted to our hospital because the same antigen test (ESPLINE SARS-CoV-2®) was positive. But 5 of them seemed to be false positives because the 5 patients had negative for RT-PCR performed thereafter. Other hospitals in Japan have been also encountering many false-positive cases in antigen tests. Thus, false-positive SARS-CoV-2-specific antigen test results may be more common than that currently thought.
Serious problems arise if a patient is diagnosed with COVID-19 based on a false-positive because not only is the patient forced into unnecessary hospitalization, but they also face the risk of becoming infected with SARS-CoV-2 from other COVID-19 patients. Therefore, if SARS-CoV-2-specific antigen test results are weakly positive, the infection route is unknown, or the symptoms are atypical, confirming the antigen test result using other test methods is necessary.
In some countries and regions where RT-PCR cannot be easily performed or when influenza and COVID-19 co-exist, immunochromatographic SARS-CoV-2-specific antigen tests may be widely used for COVID-19 diagnosis. However, as seen in this case, unless the sensitivity and specificity of the antigen-detecting rapid test are further improved, it is not recommended for clinical use as a single modality for COVID-19 diagnosis.
Declaration of Competing Interest
None.
Acknowledgments
We would like to express our gratitude to all the nurses, doctors, clinical laboratory technicians, and other medical and clerical staff at the Hiroshima Prefectural Hospital, as well as to other individuals involved in the medical care of patients with COVID-19, such as public health center employees.
References
- 1.Dinnes J., Deeks J.J., Adriano A., Berhane S., Davenport C., Dittrich S. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst Rev. 2020 doi: 10.1002/14651858.CD013705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Porte L., Legarraga P., Vollrath V., Agulera X., Munita J.M., Araos R. Evaluation of a novel antigen-based rapid detection test for the diagnosis of SARS-CoV-2 in respiratory samples. Int J Infect Dis. 2020;99:328–333. doi: 10.1016/j.ijid.2020.05.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention (CDC) Interim guidance for rapid antigen testing for SARS-CoV-2. 2020. https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antigen-tests-guidelines.html
- 4.Ministry of Health, Labor and Welfare Guidelines regarding use of SARS-CoV-2 antigen detection kits. https://www.mhlw.go.jp/content/000640554.pdf