Abstract
Objective
To screen pregnant women at risk of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection during delivery using reverse-transcription polymerase chain reaction (RT-PCR) test and serum immunoglobulin (Ig) testing.
Method
Between March 31 st and August 31 st of 2020, consecutive pregnant women admitted for labor and delivery in a single hospital were screened for SARS-CoV-2 with nasopharyngeal RT-PCR swab tests and detection of serum IgG and IgM.
Results
We studied 266 pregnant women admitted for labor and delivery. The prevalence of acute or past SARS-CoV-2 infection was 9.0 %, including (i) two cases with respiratory symptoms of SARS-Co-V-2 infection and positive RT-PCR; (ii) four asymptomatic women with positive RT-PCR without clinical symptoms and negative serological tests between two and 15 weeks later; and (iii) two women with false positive RT-PCR due to technical problems. All newborns of the 6 pregnant women with RT-PCR positive had negative RT-PCR and did not require Neonatal Intensive Care Unit admission. There were eighteen asymptomatic women with positive serological IgG tests and negative RT-PCR.
Conclusion
In our cohort of gravids, we found 2.2 % of women with positive RT-PRC tests and 6.7 % with positive serological tests during the first wave of the SARS-CoV-2 pandemic.
Keywords: SARS-CoV-2, COVID-19, Delivery, Reverse-transcription polymerase chain reaction (RT-PCR), serum immunoglobulins, Screening
1 Introduction
There are several strategies to diagnose the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection related to coronavirus disease (COVID-19) and to identify the current or past infection and immune status. The preferred primary method for screening is the reverse-transcription polymerase chain reaction (RT-PCR), using upper respiratory samples via nasopharyngeal or oropharyngeal swabs [1,2]. The procedure has been demonstrated to be highly specific (95 %) [3,4] and sensitive (70 %) in samples from non-pregnant women [4]. The RT-PCR may detect the current or past presence of viral material, whereas the serological tests assess the formation of antibodies to SARS-CoV-2 and may help to demonstrate a current infection [5]. The antibody tests for serum immunoglobulin (Ig) M (IgM), IgG, and IgA are based on the demonstration of those antibodies in human serum as a diagnostic tool of SARS-Co-V-2. These antibodies can be demonstrated in blood samples of patients RT-PCR positive 2–12 days after symptoms started and depending on sociodemographic factors [6].
In asymptomatic pregnant women admitted for delivery, the reported positive SARS−COV-2 screening with the RT-PCR tests is 86–88 %, which is similar to those in the general population [7,8]. However, the prevalence of those positive tests is variable depending on the study location and delivery facilities [[8], [9], [10], [11], [12]]. There are different techniques for antibody titration against SARS-CoV-2, including rapid IgM-IgG antibody tests, chemiluminescence immunoassay, and enzyme-linked immunosorbent assay (ELISA). The ELISA technique has a sensitivity of 89 % and a specificity of 91 % [13], although it varies according to the day of analysis since symptoms onset [14].
The objective of the present study is to evaluate the clinical manifestations and the performance of two different tests, RT-PCR and serological testing, for the screening of pregnant women admitted to the maternity ward for delivery.
2 Methods
This observational retrospective cohort study was conducted between the 31st of March and 31st of August 2020, at the Hospital Universitario General de Villalba, located in the North of Madrid, which attends 700–800 deliveries per year. The study was approved by the Fundación Jiménez Díaz Clinical Research Ethics Committee, Madrid, Spain (protocol EO107−20). A total of 266 pregnant women admitted to labor and delivery and scheduled procedures such as labor induction or caesarean delivery, were screened by RT-PCR in nasopharyngeal swabs and by a rapid blood antibodies rapid test. In cases with positive RT-PCR or positive antibodies rapid test for IgM and/or IgG, serological testing by ELISA was also carried out to confirm the results.
The RT-PCR measurements were carried out using the MagMAX Viral/Pathogen II Nucleic Acid Isolation reagents in a KinGFisher Flex Purification System. PCR reagents were the Viasure SARS-CoV-2 real-time RT-PCR detection is measured in a Bio-Rad CFX96 platform (TaqPath™ COVID-19 Combo Kit Multiplex Real Time RT-PCR). The rapid antibody test is a lateral flow immunochromatographic assay carried out using the test Biozek COVID-19 IgG/IgM Rapid Test Cassette. The ELISA serological presence of immunoglobulins was determined for IgG with Abbott reactive and for IgM with Vircell reactive.
We collected demographic, clinical (fever, cough, rhinorrhea, dyspnea, chest pain, diarrhea, myalgia, new anosmia or ageusia), obstetric and perinatal data for each woman admitted, as well as RT-PCR and serological results. Every woman was classified in one of the following three SARS-CoV-2 categories: (i) acute infection (positive RT-PCR); (ii) healed women (negative RT-PCR with positive IgG); (iii) and never infected women (both negative RT-PCR and IgG).
3 Results
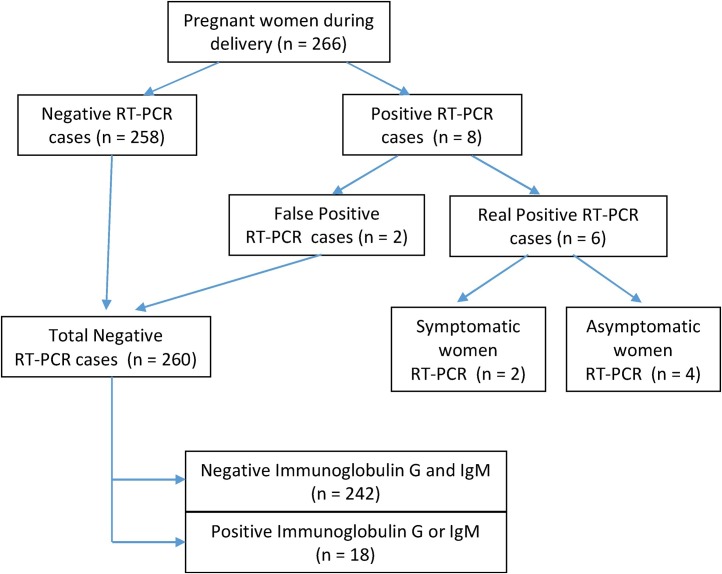
During the period of the study, 266 pregnant women admitted for labor and delivery were submitted to the SARS-Co-V-2 screening with RT-PCRs. The prevalence of acute or healed COVID-19 infection was 9.0 %, corresponding to 18 past SARS-CoV-2 exposures and six current infections (Fig. 1 ).
Fig. 1.
Flowchart of the SARS-CoV-2 screening and results in 266 pregnant women during delivery.
There were eight positive RT-PCR for SARS-CoV-2, although two of them were categorized as laboratory misinterpretation of results after women were discharged from the hospital. As expected, these two cases had no clinical symptoms and were negative for ELISA antibody tests. Therefore, we finally counted six positive RT-PCR women, of whom two had COVID-19 symptoms during labor or delivery (one patient was only IgM positive and the other had no serological test), and four were asymptomatic (Table 1 ). One of the two symptomatic cases with positive RT-PCR was diagnosed with intrauterine growth restriction. The four asymptomatic and positive RT-PCR pregnant women were negative in the ELISA study for both IgM and IgG during hospitalization. These four cases were submitted to a second ELISA immune tests five to 15 weeks after delivery being negative once again. All six cases were vaginal deliveries without neonatal acidosis, no newborn required for admission to the Neonatal Intensive Care Unit, and they all were RT-PCR negative. Symptomatic women were discharged on the third day and evolved favorably, as did their newborns.
Table 1.
Reverse transcription polymerase chain reaction (RT-PCR) positive cases in pregnant women (n = 8/266) admitted for delivery, maternal and newborn outcomes, and analytical results.
| Case | Maternal age (years); parity; delivery (weeks) | Maternal symptoms | Delivery | Newborn sex | Birth weight (grams) | Arterial umbilical cord blood pH | Apgar score at 5 min | Maternal RT-PCR | Maternal IgGb and IgMa (ELISAc) | Maternal IgGb and IgMa control (ELISAc) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 26; 2; 37 | Yes (fever and cough) | Vaginal | Female | 2525 | 7.28 | 10 | + | Not done | Not done |
| 2 | 35; 1; 40 | Yes (fever and cough) | Vaginal | Male | 3480 | 7.30 | 10 | + | + / + | Not done |
| 3 | 26; 3; 39 | No | Vaginal | Female | 3425 | 7.27 | 10 | + | Not done | - (15 weeks) |
| 4 | 32; 0; 40 | No | Vaginal | Male | 2805 | 7.20 | 10 | + | - / - | - (2 weeks) |
| 5 | 21; 0; 39 | No | Vaginal | Male | 3350 | 7.33 | 10 | + | + / - | - (12 weeks) |
| 6 | 27; 0; 39 | No | Vaginal | Female | 3054 | 7.33 | 10 | + | - / - | - (15 weeks) |
| 7 | 31; 0; 40 | No | Cesarean section (induction failure) | Male | 3950 | 7.31 | 10 | + (false positive) | - / - | Not done |
| 8 | 25; 0; 41 | No | Vaginal | Female | 3915 | 7.19 | 9 | + (false positive) | - / - | Not done |
IgM: immunoglobulin M.
IgG: immunoglobulin G.
ELISA: enzyme-linked immunosorbent assay.
All negative RT-PCR cases (n = 260) were asymptomatic throughout the whole hospitalization and 18 of them were positive for IgG, being considered as past SARS-CoV-2 exposure.
4 Discussion
In a group of 266 pregnant women, SARS-CoV-2 exposure was screened with RT-PCR tests during delivery. There were eight RT-PCR positive patients including two women with clinical evidence of SARS-CoV-2 infection, four past viral exposure, and two false positive due to technical problems. All these 8 neonates were healthy without clinical signs of virus infection and negative RT-PCR tests. Serological IgG specific antibodies addressed against the SARS-CoV-2 were present in 18 women with negative RT-PCR tests. Therefore, the prevalence of acute or past SARS-CoV-2 infection was 9.0 % in our cohort, which is similar to the prevalence in non-pregnant subjects studied by seroprevalence in the Madrid area [15]. The maternal ELISA tests, in the four RT-PCR positive and asymptomatic, repeated 2–15 weeks after delivery were negative.
Dust et al. [16] reported the performance of different commercial SARS−COV-2 RT-PCR assays testing clinical samples and reference material, ranging the sensitivity from 24 copies/mL to 574/mL specimen. However, the RT-PCR sensitivity, specificity, and positive or negative predictive values are still very difficult to determine without clear gold standard tests for SARS−COV-2 [17]. Previous studies have described positive RT-PCR in asymptomatic pregnant women rates ranging between 50 % and 89 % [8,9,11,12], our 66.7 % in our small sample seems to fit well within reported ranges.
Different studies have addressed the false-negative rate of the RT-PCR tests, ranging from 17.0–63.0 % [18]. We did not have patients with negative RT-PCR and symptoms suggestive of COVID-19. Less information is available about the false positive rate. Cohen et al. [19] reported a 2.3 % false-positive rate that was most likely related to contamination from other positive samples analyzed at the same time, target genes amplified from prior positive samples or positive controls, or misinterpretation of results.
SARS-CoV-2 serological testing can usually demonstrate IgM from 5th until the 21 st day of the infection and IgG within 10–20 days after the symptom onset, although it is still unknown for how long antibodies will be produced [20]. The serological test may reach a specificity of 98.7 % depending on the timing of sampling [5].
SARS-CoV-2 serology is complementary to RT-PCR for the COVID-19 diagnosis during at least 14 days after clinical infection initiation [21]. In a meta-analysis, the pooled ELISA methods have a sensitivity of 84 % for measuring IgG or IgM as compared to lateral flow immunoassays of 66.0 % and chemiluminescent immunoassays of 97.8 % in the general population [22]. Total antibody determination has low sensitivity during the first weeks with clinical symptoms (30.1 %), increasing during the second week to reach the highest levels during the third week. There is limited information beyond 35 days post-initiation of clinical symptoms [5].
There is scarce information concerning the antibody formation dynamic in pregnant women with SARS-Co-V-2 infection around the period of delivery. In an unselected cohort of German pregnant women, Zollkau et al. [23] reported a total of 225 PCRs and 180 IgG tests, finding only one case with a positive IgG test. We detected positive IgG serological tests in 18 asymptomatic women. None of our asymptomatic patients with positive RT-PCR developed antibodies during the study period. Pregnant women are a relatively low-risk group for COVID-19 since they are generally young [24,25]. However, there are also results suggesting that SARS-Co-V-2 is more likely associated with some adverse clinical conditions due to anatomic and physiological changes during pregnancy [26]. Besides, preeclampsia, excessive body weight, and socioeconomic disparities may be potential cofactors to worsen the obstetric and perinatal results [27]. On the other hand, pregnant women during their third trimester of gestation and labor may display atypical features, including the absence of fever as well as leukocytosis. From our own experience, in asymptomatic patients with positive RT-PCR, we had to review RT-PCR in search of false positives and take into account perform antibody tests.
5 Limitations
We found two false-positive RT-PCR for misinterpreting the test during the period of maximum incidence of the pandemic and probably related to the initial learning curve of the technique. The false-positive RT-PCR results may have a negative impact on clinical practice and emotions for pregnant women and their families, increasing specific assistance for suspicious women and biasing epidemiological statistics. Previous studies have reported both false positive and false negative rates for RT-PCR. Cohen and Kessel [19] meta-analyzed studies including at least 100 negative RT-PCR tests, and reported a global 3.2 % rate of false-positive results that could partially explain the large numbers of asymptomatic carriers of SARS-CoV-2.
Our two positive RT-PCR women were asymptomatic during the follow-up and were negative in the serological tests. We do not know if we have had any false negative RT-PCR in asymptomatic patients, although we did not have positive IgM serologies in these cases either. It is interesting to note that asymptomatic cases with positive RT-PCR have shown negative IgM and IgG SARS−COV-2 antibodies by ELISA testing during hospitalization and four weeks later. There are several possible explanations, including (i) false positive RT-PCR cases for sample contamination for the false negative of antibody testing cases; (ii) true positive RT-PCR patients that have not developed antibodies because of the theoretical B-cell response against SARS−COV-2 [28] or with lower viral load, which has been associated to lower rates of seropositivity [29].
New methods are currently under development to detect SARS-CoV-2, combining simplified extraction of RNA with reverse transcription followed by isothermal amplification and clustered regularly interspaced short palindromic repeats mediated detection. This new approach has a sensitivity of 93.1 % and a specificity of 98.5 % [30].
5.1 Stregths of the study
Our study points out the relevance that RT-PCR and antibody serologies are techniques that can be complementary in some circumstances. In particular, antibodies would be appropriate in symptomatic patients or with positive chest images with negative RT-PCR and in asymptomatic patients with positive RT-PCR to clarify false positives and negatives. The performance of antibodies has also allowed us to know which patients have overcome the disease.
5.2 Conclusion
The pandemic nature of the COVID-19 has allowed designing different strategies to manage pregnant women according to available resources in different health care systems. We found that the systematic RT-PCR assessment and serological studies of SARS-CoV-2 seem appropriated to identify women at risk during labor and delivery. There were 2.2 % of women with positive RT-PRC tests and 6.7 % with positive serological tests during the first wave of the SARS-CoV-2 pandemic in Madrid. There is a need to contrast different international experiences to effectively define the better models of clinical assistance during pregnancy and delivery since the pandemic nature of the virus.
Author contributions
RSC, JZ, MAG and FRPL contributed to the conception of the study. RSC, AV, LME and FRPL contributed to the design of the work. JZ and AV carried out data acquisition. All authors were involved in the interpretation of the study results, and the drafting and revision of the manuscript, and all approved the final version to be published.
Disclosure statement
The authors report no conflicts of interest and are alone responsible for the content and the writing of the article.
Funding / Source of support
This research did not receive any specific grant or was funded by any commercial or non-profit organization or public agency.
Details of ethics approval
The study was approved by the Fundación Jiménez Díaz Clinical Research Ethics Committee, Madrid, Spain (protocol EO107−20).
Data statement
The present study was based on clinical results obtained during the COVID-19 pandemic.
Declaration of Competing Interest
The authors report no conflicts of interest and are alone responsible for the content and the writing of the article.
Acknowledgments
None.
References
- 1.Savirón-Cornudella R., Altamirano-Barcia I.E., Chedraui P., Andeyro-García M., Tajada M., Pérez-López F.R. The coronavirus disease (COVID) 2019 and human pregnancy: A scoping review. Gynecol Reprod Endocrinol Metab. 2020;1(2):70–75. https://gremjournal.com/journal/02-2020/the-coronavirus-disease-covid-2019-and-human-pregnancy-a-scoping-review/ [Google Scholar]
- 2.Pascarella G., Strumia A., Piliego C. COVID-19 diagnosis and management: a comprehensive review. J Intern Med. 2020;288(2):192–206. doi: 10.1111/joim.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ai T., Yang Z., Hou H. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020;296(August (2)):E32–E40. doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang W., Xu Y., Gao R. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323(May (18)):1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deeks J.J., Dinnes J., Takwoingi Y. Antibody tests for identification of current and past infection with SARS-CoV-2. Cochrane Database Syst Rev. 2020;6(June(6)) doi: 10.1002/14651858.CD013652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flannery D.D., Gouma S., Dhudasia M.B. SARS-CoV-2 seroprevalence among parturient women in Philadelphia. Sci Immunol. 2020;5(July(49)) doi: 10.1126/sciimmunol.abd5709. eabd5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li R., Pei S., Chen B. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV2) Science. 2020;382(May (22)):2163–2164. doi: 10.1126/science.abb3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sutton D., Fuchs K., D’Alton M., Goffman D. Universal screening for SARS-CoV-2 in women admitted for delivery. N Engl J Med. 2020;382(May (22)):2163–2164. doi: 10.1056/NEJMc2009316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ceulemans D., Thijs I., Schreurs A. Screening for COVID-19 at childbirth: is it effective? Ultrasound Obstet Gynecol. 2020;56(July(1)):113–114. doi: 10.1002/uog.22099. [DOI] [PubMed] [Google Scholar]
- 10.Fassett M.J., Lurvey L.D., Yasumura L. Universal SARS-Cov-2 screening in women admitted for delivery in a large managed care organization. Am J Perinatol. 2020;37(September(11)):1110–1114. doi: 10.1055/s-0040-1714060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herraiz I., Folgueira D., Villalaín C., Forcén L., Delgado R., Galindo A. Universal screening for SARS-CoV-2 before labor admission during Covid-19 pandemic in Madrid. J Perinat Med. 2020;48(November (9)):981–984. doi: 10.1515/jpm-2020-0236. /j/jpme.ahead-of-print/jpm-2020-0236/jpm-2020-0236.xml. [DOI] [PubMed] [Google Scholar]
- 12.Prabhu M., Cagino K., Matthews K.C. Pregnancy and postpartum outcomes in a universally tested population for SARS-CoV-2 in New York City: a prospective cohort study. BJOG. 2020;127(November (12)):154–155. doi: 10.1111/1471-0528.16403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zullo F., Di Mascio D., Saccone G. COVID-19 antibody testing in pregnancy. Am J Obstet Gynecol MFM. 2020;2(August(3)) doi: 10.1016/j.ajogmf.2020.100142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao J., Yuan Q., Wang H. Antibody responses to SARS-CoV-2 in patients with novel coronavirus disease 2019. Clin Infect Dis. 2020;71(March (16)):2027–2034. doi: 10.1093/cid/ciaa344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pollán M., Pérez-Gómez B., Pastor-Barriuso R. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet. 2020;396(August (10250)):535–544. doi: 10.1016/S0140-6736(20)31483-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dust K., Hedley A., Nichol K. Comparison of commercial assays and laboratory developed tests for detection of SARS-CoV-2. J Virol Methods. 2020;285 doi: 10.1016/j.jviromet.2020.113970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahendiratta S., Batra G., Sarma P. Molecular diagnosis of COVID-19 in different biologic matrix, their diagnostic validity and clinical relevance: a systematic review. Life Sci. 2020;258(October (1)) doi: 10.1016/j.lfs.2020.118207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelly J.C., Dombrowksi M., O’Neil-Callahan M., Kernberg A.S., Frolova A.I., Stout M.J. False-negative testing for severe acute respiratory syndrome coronavirus 2: consideration in obstetrical care. Am J Obstet Gynecol MFM. 2020;2(3) doi: 10.1016/j.ajogmf.2020.100130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen A.N., Kessel B. medRxiv; 2020. False positives in reverse-transcription PCR testing for SARS-CoV-2. [DOI] [Google Scholar]
- 20.Long Q., Liu B., Deng H. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020;26(June (6)):845–848. doi: 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- 21.Marlet J., Petillon C., Ragot E. Clinical performance of four immunoassays for antibodies to SARS-CoV-2, including a prospective analysis for the diagnosis of COVID-19 in a real-life routine care setting. J Clin Virol. 2020;132 doi: 10.1016/j.jcv.2020.104633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lisboa Bastos M., Tavaziva G., Abidi S.K. Diagnostic accuracy of serological tests for covid-19: systematic review and meta-analysis. BMJ. 2020;370:m2516. doi: 10.1136/bmj.m2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zöllkau J., Baier M., Scherag A., Schleußner E., Groten T. Periodenprävalenz von SARS-CoV-2 in einer unselektierten Stichprobe schwangerer Frauen in Jena, Thüringen [Period Prevalence of SARS-CoV-2 in an Unselected Sample of Pregnant Women in Jena, Thuringia] Z Geburtshilfe Neonatol. 2020;224(4):194–198. doi: 10.1055/a-1206-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huntley B.J.F., Huntley E.S., Di Mascio D., Chen T., Berghella V., Chauhan S.P. Rates of maternal and perinatal mortality and vertical transmission in pregnancies complicated by severe acute respiratory syndrome coronavirus 2 (SARS-Co-V-2) infection: a systematic review. Obstet Gynecol. 2020;136(2):303–312. doi: 10.1097/AOG.0000000000004010. [DOI] [PubMed] [Google Scholar]
- 25.Khalil A., Kalafat E., Benlioglu C. SARS-CoV-2 infection in pregnancy: a systematic review and meta-analysis of clinical features and pregnancy outcomes. EClinicalMedicine. 2020;25 doi: 10.1016/j.eclinm.2020.100446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diriba K., Awulachew E., Getu E. The effect of coronavirus infection (SARS-CoV-2, MERS-CoV, and SARS-CoV) during pregnancy and the possibility of vertical maternal-fetal transmission: a systematic review and meta-analysis. Eur J Med Res. 2020;25(1):39. doi: 10.1186/s40001-020-00439-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barbero P., Mugüerza L., Herraiz I. SARS-CoV-2 in pregnancy: characteristics and outcomes of hospitalized and non-hospitalized women due to COVID-19. J Matern Fetal Neonatal Med. 2020:1–7. doi: 10.1080/14767058.2020.1793320. [DOI] [PubMed] [Google Scholar]
- 28.Soresina A., Moratto D., Chiarini M. Two X-linked agammaglobulinemia patients develop pneumonia as COVID-19 manifestation but recover. Pediatr Allergy Immunol. 2020;(April (22)) doi: 10.1111/pai.13263. 10.1111/pai.13263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wellinghausen N., Plonné D., Voss M., Ivanova R., Frodl R., Deininger S. SARS-CoV-2-IgG response is different in COVID-19 outpatients and asymptomatic contact persons. J Clin Virol. 2020;130(September) doi: 10.1016/j.jcv.2020.104542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joung J., Ladha A., Saito M. Detection of SARS-CoV-2 with SHERLOCK one-pot testing. N Engl J Med. 2020;383(September (15)):1492–1494. doi: 10.1056/NEJMc2026172. [DOI] [PMC free article] [PubMed] [Google Scholar]