Graphical Abstract
Graphical Abstract.
Keywords: Percutaneous coronary intervention, Clinical Trials, Drug-coated balloon, Drug-eluting stent, In-stent restenosis, Meta-analysis, Mortality, Paclitaxel
Abstract
Aims
Consensus is lacking regarding the best treatment for coronary in-stent restenosis (ISR). The two most effective treatments are angioplasty with paclitaxel-coated balloon (PCB) and repeat stenting with drug-eluting stent (DES) but individual trials were not statistically powered for clinical endpoints, results were heterogeneous, and evidence about comparative efficacy and safety in relevant subsets was limited.
Methods and results
The Difference in Anti-restenotic Effectiveness of Drug-eluting stent and drug-coated balloon AngiopLasty for the occUrrence of coronary in-Stent restenosis (DAEDALUS) study was a comprehensive, investigator-initiated, collaborative, individual patient data meta-analysis comparing angioplasty with PCB alone vs. repeat stenting with DES alone for the treatment of coronary ISR. The protocol was registered with PROSPERO (CRD42017075007). All 10 available randomized clinical trials were included with 1976 patients enrolled, 1033 assigned to PCB and 943 to DES. At 3-year follow-up, PCB was associated with a significant increase in the risk of target lesion revascularization (TLR) compared with DES [hazard ratio (HR) 1.32, 95% CI 1.02–1.70, P = 0.035; number-needed-to-harm 28.5]. There was a significant interaction between treatment effect and type of restenosed stent (P = 0.029) with a more marked difference in patients with DES-ISR and comparable effects in patients with bare-metal stent-ISR. At 3-year follow-up, the primary safety endpoint of all-cause death, myocardial infarction, or target lesion thrombosis was comparable between treatments (HR 0.80, 95% CI 0.58–1.09, P = 0.152). A pre-specified subgroup analysis indicated a significant interaction between treatment effect and type of DES used to treat ISR (P = 0.033), with a lower incidence of events associated with PCB compared with first-generation DES and similar effect between PCB and second-generation DES (HR 1.06, 95% CI 0.71–1.60, P = 0.764). Long-term all-cause mortality was similar between PCB and DES (HR 0.81, 95% CI 0.53–1.22, P = 0.310); results were consistent comparing PCB and non-paclitaxel-based DES (HR 1.42, 95% CI 0.80–2.54, P = 0.235). Myocardial infarction and target lesion thrombosis were comparable between treatments.
Conclusions
In patients with coronary ISR, repeat stenting with DES is moderately more effective than angioplasty with PCB at reducing the need for TLR at 3 years. The incidence of a composite of all-cause death, myocardial infarction, or target lesion thrombosis was similar between groups. The rates of individual endpoints, including all-cause mortality, were not significantly different between groups.
See page 3729 for the editorial comment on this article (doi: 10.1093/eurheartj/ehz731)
Introduction
In-stent restenosis (ISR) represents the most common cause of treatment failure after percutaneous coronary intervention.1 ISR not infrequently presents as an acute coronary syndrome and is associated with worse long-term outcomes compared with treatment of de novo coronary artery disease.2,3
Although newer generation drug-eluting stent (DES) has significantly reduced the incidence of ISR compared with previous devices, all-comers randomized clinical trials comparing contemporary devices showed cumulative rates of target lesion revascularization (TLR) of ∼7–10% at 5-year follow-up.4,5 Trials with extended follow-up out to 10 years are rare and a recent report showed that approximately one-fifth of patients required TLR at this time points.6 In addition, bare-metal stents continue to be used occasionally and are associated with high rates of ISR.7,8
Several therapies for coronary ISR have been tested in clinical trials.9 However, paclitaxel-coated balloon (PCB) angioplasty and repeat stenting with DES implantation have emerged as the most effective therapeutic options.10,11 Indeed, several randomized clinical trials have compared outcomes of patients treated with the two types of device, though none were powered for clinical endpoints and considerable heterogeneity exists in terms of characteristics of included patients, type of restenotic stent, generation of DES used in the repeat stenting arm, and duration of follow-up.10,11 In addition, concerns have recently emerged regarding a possible higher risk of death in patients treated with paclitaxel-eluting devices for the treatment of peripheral arterial disease.12
Against this background, we conducted a comprehensive, collaborative meta-analysis of individual patient data from all available randomized clinical trials comparing the angioplasty with PCB and repeat stenting with DES in patients undergoing treatment for ISR.
Methods
Study design and search strategy
The Difference in Anti-restenotic Effectiveness of Drug-eluting stent and drug-coated balloon AngiopLasty for the occUrrence of coronary in-Stent restenosis (DAEDALUS) study was an investigator-initiated, collaborative individual patient data meta-analysis of randomized clinical trials. Trials could be pooled when all the following eligibility criteria were satisfied: (i) random allocation of treatments; (ii) angioplasty with PCB alone vs. repeat stenting with DES alone; (iii) treatment of coronary ISR; and (iv) clinical follow-up of at least 12 months.
Multiple electronic databases (PubMed, Scopus, ScienceDirect, Web of Science) and archives of major scientific societies and international conferences in the field were searched from 13 November 2006 (date of publication of the first randomized clinical trial on ISR testing PCB) to 15 April 2019. Reports retrieved by literature search were screened for eligibility. Further details on the search strategy and reports selection process are provided in the Supplementary material online. After protocol drafting, the primary investigator of each trial eligible for inclusion was invited to contribute to the DAEDALUS study. Data extraction was coordinated by the primary investigator of each trial. Variables of interest were selected at the study protocol stage according to the clinical relevance and consistency across trials by cross check on original publications. Additional unpublished data, including extension of duration of follow-up and variable standardization, were provided when available in the original databases. All the variables of interest were independently checked for each trial at the German Heart Center of Munich with satisfactory results before generating the dedicated electronic database of the DAEDALUS study. The final database was then created and stored at the coordinating centre.
The study was designed and conducted in keeping with the PRISMA-IPD guidelines (Supplementary material online, Table S1)13 and the protocol was registered with PROSPERO (CRD42017075007). The project was funded in part by the German Ministry of Education and Research (BMBF) through a research grant (#KS2017-236).
The local institutional review boards approved each of the included trials and all patients signed informed, written consent before randomization. Clinical events and angiographic measurements in each trial were adjudicated and assessed by independent clinical events committee and core laboratories, respectively.
Endpoints
The primary efficacy endpoint was TLR defined as any revascularization, either percutaneous or surgical, at the target segment (i.e. in-segment ISR). The primary safety endpoint was a composite of all-cause death, myocardial infarction, or target lesion thrombosis. Death was classified as cardiac or non-cardiac according to the cause; generally, when a clear non-cardiac cause could not be established, the event was considered as cardiac. Myocardial infarction was defined according to clinical symptoms, electrocardiogram, and cardiac biomarkers as defined elsewhere.14 Academic Research Consortium criteria for definite or probable stent thrombosis were used to define target lesion thrombosis.14 Ischaemia-driven TLR definition included any revascularization at the target lesion site driven by typical symptoms and objective signs of myocardial ischaemia at non-invasive or invasive testing rather than only binary restenosis at angiography follow-up. Target vessel revascularization was defined as any revascularization, either percutaneous or surgical, of any segment of the target vessel including the target lesion. The composite of all-cause death, myocardial infarction, target lesion thrombosis, or TLR as well as the composite of all-cause death, myocardial infarction, target lesion thrombosis, or target vessel revascularization were included among secondary endpoints to describe the net benefit associated with the two treatments.
Statistical analysis
Statistical analysis was conducted at the German Heart Center of Munich. Nominal variables were reported as counts and percentages and compared by the Pearson χ2 or Fisher’s exact test as appropriate. Continuous variables distribution was assessed by the Shapiro–Wilk test and reported accordingly as mean and standard deviation or median and interquartile range (IQR); continuous variables were compared by the Student’s t or Mann–Whitney–Wilcoxon U test as appropriate.
Outcomes were assessed as time-to-first event according to the intention-to-treat principle. Cumulative incidences were computed according to the Kaplan–Meier method, survival curves plotted along with 95% confidence intervals and numbers at risk, and comparisons performed by the log rank test.15–17 For each outcome, primary results were obtained by one-stage mixed-effects Cox proportional hazards regression model with treatment assignment as the fixed component and the original trial as the random component.13,18,19 Resulting risk estimates were reported as HR and 95% confidence interval and P-values provided by the Wald test.15 Proportional hazards assumption was assessed by testing the correlation between Schoenfeld scaled residuals and follow-up time and by inspecting the scaled residuals against transformed time;15,17 when required Aalen’s additive hazards model and time splitting (data-driven landmark analysis) were applied.15,17 When outcomes resulted significantly different after statistical testing, the number-needed-to-treat or number-needed-to-harm (NNH) was computed as described for survival analysis.20
Multivariable adjustment of risk estimates for age, gender, diabetes, hypertension, hypercholesterolaemia, smoking history, prior myocardial infarction, clinical presentation, lesion site, left ventricular ejection fraction, multivessel disease, DES generation, ISR type, ISR length, ISR class, reference vessel diameter, minimum lumen diameter, pre-dilation, and maximum pressure of application after multiple imputation by chained equations for missing data and pooling of datasets by Rubin rules (overall ∼3% of missing values) was conducted by mixed-effects model with an additional random effect accounting for multiple lesions per patient.21
A two-stage meta-analysis with individual trial risk estimates extraction by Cox proportional hazards regression and subsequent pooling by fixed- and random-effects models was conducted as sensitivity analysis for each outcome.13,18,19,22 Forest plots reporting pooled and trial-specific effects along with the corresponding relative weight according to the inverse of variance were drawn.22,23 Heterogeneity between trials was formally explored by the Q test and described by between-trial variance τ2 and I2 statistic, with values <25%, between 25% and 50%, and >50% describing low, intermediate, and severe heterogeneity, respectively.22,24
Planned subgroup analysis by Cox mixed-effects model for the primary safety and efficacy endpoints included the following subsets: age < or ≥65 years old, gender, region of trial conduct, diabetes, smoking history, acute coronary syndrome at admission, ISR angiographic pattern, ISR type, DES generation used in the trial, reference vessel diameter < or ≥2.75 mm, minimum lumen diameter < or ≥median value, and ISR length <20 or ≥20 mm.
Finally, potential sources of bias were assessed by using the Cochrane Collaboration tool,25 publication bias/small-study effect was explored by funnel plots and Egger test,22 and overall reliability of the conclusions was presented according to the GRADE system.26
Results
Ten prospective, randomized clinical trials27–36 identified by literature search were eligible for inclusion in the DAEDALUS study. Details about the results of search and screening processes are shown in the Supplementary material online, Figure S1 and Table S2. After formal invitation, the primary investigator of each trial agreed to the collaborative project.
A total of 1976 patients was included (2080 lesions), 1033 (1084 lesions) assigned to PCB and 943 (996 lesions) assigned to DES. Details about the included trials are shown in the Table 1 and Supplementary material online. The two groups of patients were balanced with respect to baseline clinical characteristics (Table 2), though there were some differences in baseline lesion and procedural characteristics (Table 3). Patients assigned to PCB received most frequently an iopromide-excipient-based device (84.8%). Patients assigned to repeat stenting with DES received paclitaxel-eluting stent in the three earlier trials (32.0%), everolimus-eluting stent in the six subsequent trials (60.3%), and biolimus-eluting stent (7.6%) in the most recent trial. Follow-up duration was comparable between PCB and DES groups (P = 0.357) with a median of 1015 (403–1095) days in the study population.
Table 1.
Main characteristics of the included randomized clinical trials
| Trial | Design | Centres Region | Investigation time | Patients (lesions) Total |
PCB type | DES type | Restenotic stent | |
|---|---|---|---|---|---|---|---|---|
| PCB | DES | |||||||
| PEPCAD II |
|
|
Jan 2006 – Dec 2006 | 131 (131) |
|
|
Bare-metal | |
| 66 (66) | 65 (65) | |||||||
| ISAR DESIRE 3 |
|
|
Aug 2009 – Oct 2011 | 268 (340) |
|
|
Drug-eluting | |
| 137 (172) | 131 (168) | |||||||
| PEPCAD China ISR |
|
|
Mar 2011 – Apr 2012 | 215 (221) |
|
|
Drug-eluting | |
| 109 (113) | 106 (108) | |||||||
| RIBS V |
|
|
Jan 2010 – Jan 2012 | 189 (189) |
|
|
Bare-metal | |
| 95 (95) | 94 (94) | |||||||
| SEDUCE |
|
|
Jun 2009 – Oct 2011 | 49 (49) |
|
|
Bare-metal | |
| 24 (24) | 25 (25) | |||||||
| RIBS IV |
|
|
Jan 2010 – Aug 2013 | 309 (309) |
|
|
Drug-eluting | |
| 154 (154) | 155 (155) | |||||||
| TIS |
|
|
Jan 2012 – Aug 2014 | 136 (148) |
|
|
Bare-metal | |
| 68 (74) | 68 (74) | |||||||
| DARE |
|
|
May 2010 – Jun 2015 | 278 (278) |
|
|
|
|
| 137 (137) | 141 (141) | |||||||
| RESTORE |
|
|
Apr 2013 – Oct 2016 | 172 (172) |
|
|
Drug-eluting | |
| 86 (86) | 86 (86) | |||||||
| BIOLUX-RCT |
|
|
Aug 2012 – Jan 2015 | 229 (243) |
|
|
|
|
| 157 (163) | 72 (80) | |||||||
BTHC, butyryl-tri-hexyl citrate; CEC, clinical events committee.
Table 2.
Baseline clinical characteristics
| PCB (n = 1033) | DES (n = 943) | P-value | |
|---|---|---|---|
| Age (years) | 66.7 [59.0–74.0] | 66.3 [59.0–73.3] | 0.282 |
| Female | 242 (23.4) | 207 (22.0) | 0.434 |
| Diabetes | 383 (37.1) | 325 (34.5) | 0.226 |
| Insulin-requiring | 123 (31.9) | 121 (37.3) | 0.131 |
| Hypertension | 780 (75.5) | 720 (76.4) | 0.661 |
| Hypercholesterolemia | 729 (70.6) | 657 (69.7) | 0.662 |
| Ever-smoked | 525 (50.8) | 450 (47.7) | 0.162 |
| Prior myocardial infarction | 518 (50.1) | 429 (45.5) | 0.041 |
| Clinical presentation | 0.965 | ||
| Silent ischaemia/stable angina | 623 (59.7) | 559 (59.3) | |
| Unstable angina | 348 (33.7) | 327 (34.7) | |
| NSTEMI | 48 (4.6) | 43 (4.6) | |
| STEMI | 5 (0.5) | 4 (0.4) | |
| Left ventricular ejection fraction (%) | 60 [50–65] | 60 [51–65] | 0.282 |
| Multivessel disease | 475 (46.0) | 408 (43.3) | 0.378 |
Data are n (%) or median [interquartile range].
NSTEMI, non-ST-segment elevation myocardial infarction; STEMI, ST-segment elevation myocardial infarction.
Table 3.
Angiographic and procedural characteristics
| PCB (n = 1084) | DES (n = 996) | P-value | |
|---|---|---|---|
| Target lesion site | 0.102 | ||
| Left main | 0 | 5 (0.5) | |
| Left anterior descending | 451 (41.6) | 432 (43.4) | |
| Left circumflex | 238 (22.0) | 228 (22.9) | |
| Right coronary artery | 377 (34.8) | 316 (31.8) | |
| Saphenous vein graft | 17 (1.6) | 13 (1.3) | |
| Restenotic device | 0.810 | ||
| Bare-metal stent | 379 (35.0) | 345 (34.6) | |
| Drug-eluting stent | 693 (63.9) | 645 (64.8) | |
| In-stent restenosis morphology | 0.048 | ||
| Focal | 606 (55.9) | 527 (52.9) | |
| Diffuse | 322 (29.7) | 301 (30.2) | |
| Proliferative | 75 (6.9) | 81 (8.1) | |
| Occlusive | 28 (2.6) | 46 (4.6) | |
| Focal in-stent restenosis morphology | 0.364 | ||
| Edge or gap | 146 (24.1) | 131 (24.9) | |
| Body | 369 (60.9) | 293 (55.6) | |
| Multifocal | 35 (5.8) | 38 (7.2) | |
| Restenosis length (mm) | 9.9 [6.7–15.7] | 10.9 [7.6–17.1] | 0.0002 |
| Diameter stenosis (%) | 68.2 [57.1–77.4] | 69.1 [59.6–79.4] | 0.004 |
| Minimum lumen diameter (mm) | 0.86 [0.60–1.14] | 0.79 [0.55–1.10] | 0.006 |
| Reference vessel diameter (mm) | 2.72 [2.40–3.04] | 2.71 [2.41–3.05] | 0.874 |
| Pre-dilation | 1011 (93.3) | 891 (89.5) | 0.002 |
| Maximum balloon pressure | 14 [12–18] | 16 [14–20] | <0.0001 |
Data are n (%) or median [interquartile range].
DES, drug-eluting stent; PCB, paclitaxel-coated balloon.
Primary efficacy endpoint
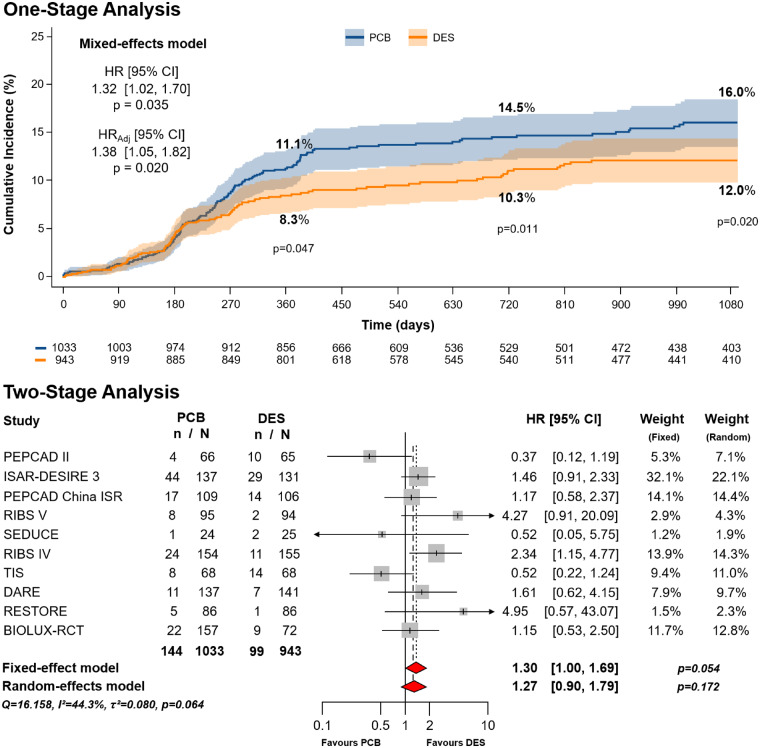
Clinical outcomes are shown in Table 4. With respect to the primary efficacy endpoint, at 3-year follow-up a total of 243 events occurred, 144 in the PCB group (7.14 per 100 person-years) and 99 in the DES group (5.14 per 100 person-years), corresponding to cumulative incidences of 16.0% (IQR 13.5–18.4%) and 12.0% (IQR 9.7–14.3%), respectively (P = 0.020) (Figure 1). Patients assigned to PCB showed a 32% relative risk increase in TLR compared with those assigned to DES (HR 1.32, 95% CI 1.02–1.70, P = 0.035; NNH 28.5). After multivariable adjustment, results remained consistent [adjusted hazard ratio (HRadj) 1.38, 95% CI 1.05–1.82, P = 0.020].
Table 4.
Three-year clinical outcomes
| PCB (n = 1033) | DES (n = 943) | P LR | HR (95% CI) | P W | HRadj (95% CI) | P adj | |
|---|---|---|---|---|---|---|---|
| Target lesion revascularization (primary efficacy endpoint) | 144 (16.0) | 99 (12.0) | 0.020 | 1.32 (1.02–1.70) | 0.035 | 1.38 (1.05–1.82) | 0.020 |
| All-cause death, myocardial infarction, or target lesion thrombosis (primary safety endpoint) | 75 (9.0) | 85 (10.9) | 0.182 | 0.80 (0.58–1.09) | 0.152 | 0.74 (0.52–1.04) | 0.085 |
| Death | 42 (5.5) | 48 (6.6) | 0.334 | 0.81 (0.53–1.22) | 0.310 | 0.68 (0.42–1.10) | 0.116 |
| Cardiac death | 16 (2.0) | 24 (3.3) | 0.134 | 0.61 (0.32–1.15) | 0.128 | 0.61 (0.32–1.19) | 0.148 |
| Non-cardiac death | 26 (3.6) | 24 (3.4) | 0.964 | 1.01 (0.58–1.76) | 0.973 | 0.80 (0.44–1.46) | 0.474 |
| Myocardial infarction | 41 (4.7) | 38 (4.4) | 0.941 | 0.95 (0.61–1.48) | 0.820a | 0.95 (0.59–1.53) | 0.829 |
| Target lesion thrombosis | 10 (1.2) | 8 (0.9) | 0.765 | 1.14 (0.45–2.90) | 0.777 | 1.09 (0.39–3.03) | 0.869 |
| Ischaemia-driven target lesion revascularization | 129 (14.3) | 84 (10.1) | 0.011 | 1.39 (1.06–1.84) | 0.018 | 1.43 (1.07–1.92) | 0.016 |
| Target vessel revascularization | 161 (17.9) | 126 (15.2) | 0.173 | 1.15 (0.91–1.46) | 0.235 | 1.19 (0.92–1.55) | 0.184 |
| All-cause death, myocardial infarction, target lesion thrombosis, or target lesion revascularization | 197 (22.1) | 167 (20.6) | 0.384 | 1.07 (0.87–1.32) | 0.518b | 1.07 (0.84–1.35) | 0.593 |
| All-cause death, myocardial infarction, target lesion thrombosis, or target vessel revascularization | 207 (23.0) | 191 (23.2) | 0.945 | 0.97 (0.80–1.19) | 0.796c | 0.98 (0.78–1.23) | 0.851 |
CI, confidence interval; DES, drug-eluting stent; HR, hazard ratio; Padj, P-value of the Wald test after multivariable adjustment; PLR, P-value of the log rank test; PW, P-value of the Wald test; PCB, paclitaxel-coated balloon.
Aalen additive hazards model with penalization: P = 0.392.
Aalen additive hazards model with penalization: P = 0.944.
Aalen additive hazards model with penalization: P = 0.910.
Figure 1.
Primary efficacy endpoint (target lesion revascularization). Cumulative incidence of primary efficacy endpoint in patients allocated to angioplasty with paclitaxel-coated balloon vs. repeat stenting with drug-eluting stent. The upper panel shows the results of the one-stage analysis. The lower panel shows the results of the two-stage analysis. CI, confidence interval; DES, drug-eluting stent; HR, hazard ratio; HRadj, adjusted hazard ratio; n, number of patients with event; N, number of patients assigned to the treatment; PCB, paclitaxel-coated balloon. The numbers of patients at risk in the treatment groups are shown below the graphs.
Two-stage sensitivity meta-analysis with fixed- and random-effects models showed, respectively, borderline and non-statistically significant differences in the risk of TLR between groups (Figure 1). The highest relative weights were associated with the ISAR-DESIRE 3, PEPCAD ISR China, and RIBS IV trials. Heterogeneity across the included trials was moderate (τ2 = 0.080; I2 = 44.3%).
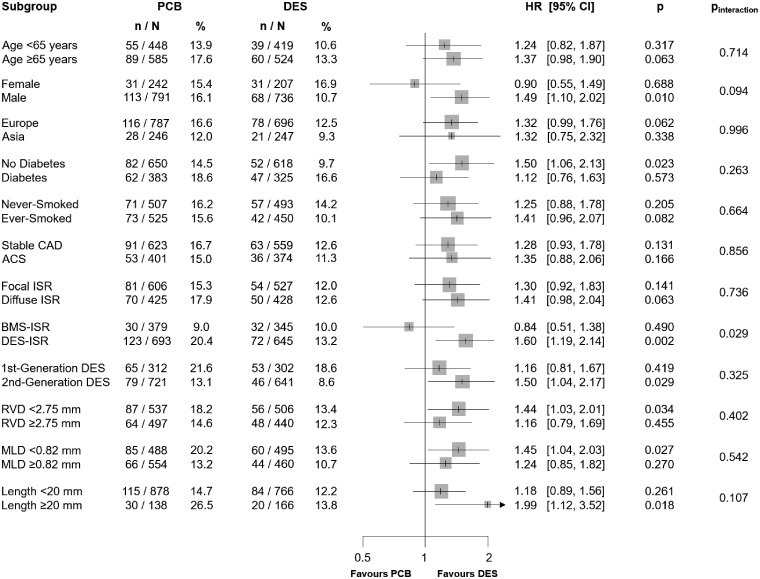
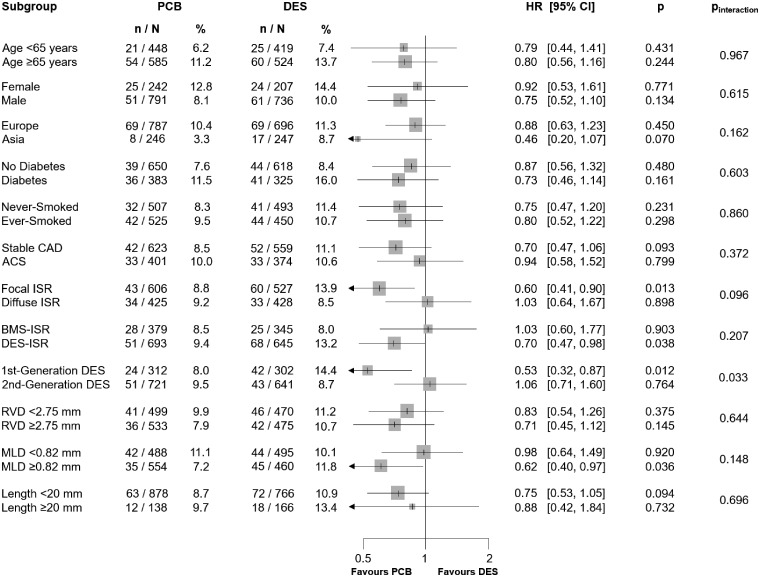
The analysis of major clinical and angiographic subgroups revealed a significant (P = 0.029) interaction between treatments effect and type of restenotic stent (Figure 2). Indeed, a similar risk of TLR between PCB and DES was observed in patients who had bare-metal stent-ISR (HR 0.84, 95% CI 0.51–1.38, P = 0.490) and an increased risk associated with PCB (HR 1.60, 95% CI 1.19–2.14, P = 0.002) was detected in patients who had DES-ISR.
Figure 2.
Subgroup analysis for the primary efficacy endpoint. ACS, acute coronary syndrome; BMS, bare-metal stent; CAD, coronary artery disease; CI, confidence interval; DES, drug-eluting stent; HR, hazard ratio; ISR, in-stent restenosis; MLD, minimum lumen diameter; n, number of patients with event; N, number of patients assigned to the treatment; RVD, reference vessel diameter.
Primary safety endpoint
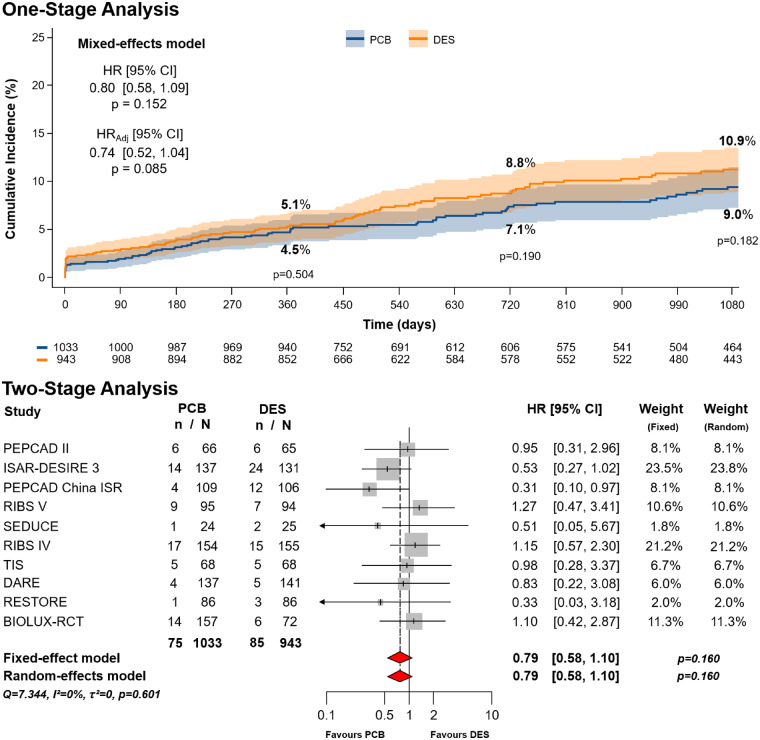
With respect to the primary safety endpoint, at 3-year follow-up a total of 160 events occurred, 75 in the PCB group (3.42 per 100 person-years) and 85 in the DES group (4.20 per 100 person-years), corresponding to 3-year cumulative incidences of 9.0% (IQR 7.0–11.0%) vs. 10.9% (IQR 8.6–13.1%), respectively (P = 0.182). At primary analysis, the risk of all-cause death, myocardial infarction, or target lesion thrombosis was similar between groups (HR 0.80, 95% CI 0.58–1.09, P = 0.152) (Figure 3). After multivariable adjustment, the numerical trend favouring PCB remained non-statistically significant (HRadj 0.74, 95% CI 0.52–1.04, P = 0.085).
Figure 3.
Primary safety endpoint (all-cause death, myocardial infarction, or target lesion thrombosis). Cumulative incidence of primary safety endpoint in patients allocated to angioplasty with paclitaxel-coated balloon vs. repeat stenting with drug-eluting stent. The upper panel shows the results of the one-stage analysis. The lower panel shows the results of the two-stage analysis. CI, confidence interval; DES, drug-eluting stent; HR, hazard ratio; HRadj, adjusted hazard ratio; n, number of patients with event; N, number of patients assigned to the treatment; PCB, paclitaxel-coated balloon. The numbers of patients at risk in the treatment groups are shown below the graphs.
The main results did not change after two-stage meta-analysis, regardless of the model applied (HR 0.79, 95% CI 0.58–1.10, P = 0.160) (Figure 3). The highest relative weights were associated with the ISAR-DESIRE 3, RIBS IV, BIOLUX-RCT, and RIBS V trials. Heterogeneity was not detected (τ2 = 0; I2 = 0%).
Subgroup analysis revealed a significant interaction between treatment effect and generation of DES used for the treatment of ISR (P = 0.033) (Figure 4): PCB led to lower incidence of adverse events compared with first-generation DES (HR 0.53, 95% CI 0.32–0.87, P = 0.012) and similar incidence when compared with second-generation DES (HR 1.06, 95% CI 0.71–1.60, P = 0.764).
Figure 4.
Subgroup analysis for the primary safety endpoint. ACS, acute coronary syndrome; BMS, bare-metal stent; CAD, coronary artery disease; CI, confidence interval; DES, drug-eluting stent; HR, hazard ratio; ISR, in-stent restenosis; MLD, minimum lumen diameter; n, number of patients with event; N, number of patients assigned to the treatment; RVD, reference vessel diameter.
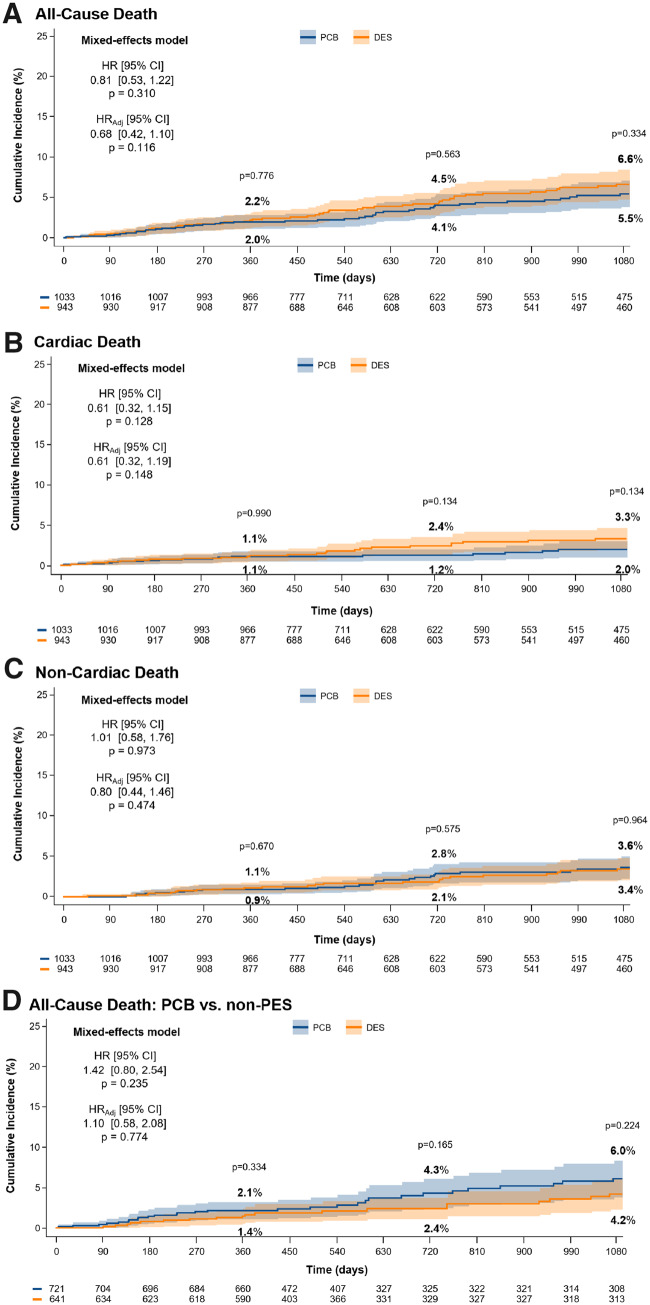
All-cause death, cardiac death, non-cardiac death, and mortality between paclitaxel-coated balloon and non-paclitaxel-based drug-eluting stent
The incidence of all-cause death was similar between PCB and DES (42 events, 1.87 per 100 person-years and 48 events, 2.30 per 100 person-years; cumulative incidence of 5.5% vs. 6.6%, P = 0.334; HR 0.81, 95% CI 0.53–1.22, P = 0.310) (Figure 5). After multivariable adjustment, results remained consistent (HRadj 0.68, 95% CI 0.42–1.10, P = 0.116). The risk of cardiac and non-cardiac death was similar between PCB and DES (HR 0.61, 95% CI 0.32–1.15, P = 0.128 and HR 1.01, 95% CI 0.58–1.76, P = 0.973, respectively) (Figure 5).
Figure 5.
(A) All-cause death, (B) cardiac death, (C) non-cardiac death, for paclitaxel-coated balloon vs. drug-eluting stent, and (D) mortality after paclitaxel-coated balloon vs. non-paclitaxel-eluting stent. Incidence and type of death in patients allocated to angioplasty with paclitaxel-coated balloon vs. repeat stenting with drug-eluting stent (A–C) and paclitaxel-coated balloon vs. non-paclitaxel-eluting stent (D). CI, confidence interval; DES, drug-eluting stent; HR, hazard ratio; HRadj, adjusted hazard ratio; PCB, paclitaxel-coated balloon. The numbers of patients at risk in the treatment groups are shown below the graphs.
Pooling only trials using PCB vs. non-paclitaxel-based DES, the risk of all-cause death was similar between groups (HR 1.42, 95% CI 0.80–2.54, P = 0.235), without significant changes after adjustment (HRadj 1.08, 95% CI 0.58–2.08, P = 0.774) (Figure 5).
Two-stage sensitivity analyses showed consistent results (Supplementary material online, Table S3).
Other secondary endpoints
The risk of myocardial infarction at 3-year follow-up was similar between PCB and DES (HR 0.95, 95% CI 0.61–1.48, P = 0.820) (Table 4). A different distribution in the occurrence of myocardial infarction over time was observed between the two treatment groups with an early post-procedural trend towards an increased incidence after DES implantation compared with PCB application followed by an opposite trend favouring DES compared with PCB between 7 and 400 days (Supplementary material online, Table S4); late occurrence of myocardial infarction was similar between treatments. The risk of target lesion thrombosis at 3-year follow-up was comparable between groups (HR 1.14, 95% CI 0.45–2.90, P = 0.777) (Table 4). The net composite secondary endpoint deriving from the combination of the primary efficacy and safety endpoints was similar between groups (HR 1.07, 95% CI 0.87–1.32, P = 0.514) (Supplementary material online, Figures S2 and S3). Similarly, the other net composite of all-cause death, myocardial infarction, target lesion thrombosis, or target vessel revascularization was comparable between groups (HR 0.97, 95% CI 0.80–1.19, P = 0.796) (Table 4; Supplementary material online, Table S4).
Two-stage sensitivity analyses showed consistent results for all the individual and composite secondary endpoints (Supplementary material online, Table S3).
Assessment of bias and reliability of results
Overall, the qualitative assessment of individual trials did not reveal significant sources of bias related to the design and the risk of publication bias/small-study effect was quantified as low (Supplementary material online, Figures S4 and S5). The reliability of the conclusions of the study was generally good (Supplementary material online, Table S5).
Discussion
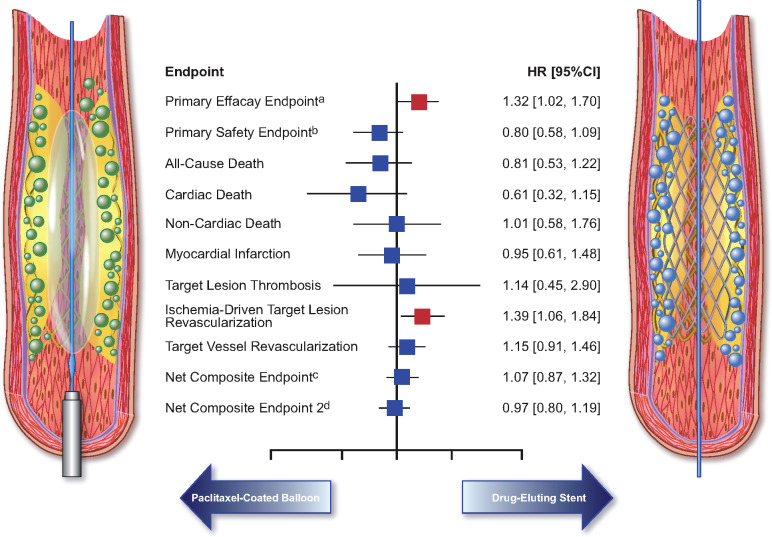
In a large-scale, collaborative, individual patient data meta-analysis of patients undergoing treatment for coronary ISR enrolled in the 10 randomized clinical trials comparing angioplasty with PCB and repeat stenting with DES conducted thus far to the best of our knowledge, the main results were as follows (see Take home figure):
Take home figure.
Summary of the treatment effects for angioplasty with paclitaxel-coated balloon vs. repeat stenting with drug-eluting stent in patients treated for coronary in-stent restenosis. aPrimary efficacy endpoint was target lesion revascularization; bprimary safety endpoint was the composite of death, myocardial infarction, or target lesion thrombosis; cnet composite endpoint 1 refers to the composite of death, myocardial infarction, target lesion thrombosis, or target lesion revascularization; dnet composite endpoint 2 refers to the composite of death, myocardial infarction, target lesion thrombosis, or target vessel revascularization.
Angioplasty with PCB is moderately less effective than repeat stenting with DES in terms of the primary efficacy endpoint of TLR;
The incidence of the primary safety endpoint of all-cause death, myocardial infarction, or target lesion thrombosis is similar between treatments, though a numerical increase associated with repeat DES implantation after multivariable adjustment is observed;
The rates of a composite endpoint including both efficacy and safety components are similar between groups.
The rates of all-cause death, cardiac death, and non-cardiac death are similar between treatments and PCB use in the setting of coronary artery disease does not increase long-term mortality compared with non-paclitaxel-based DES.
The findings from the main analysis of the DAEDALUS study should be interpreted in light of a number of considerations. Indeed, the clinical magnitude of the benefit in TLR is moderate and the statistical significance of the risk reduction associated with DES was not confirmed in the two-stage sensitivity analysis as a result of the relatively small difference between the two treatments against an intermediate degree of between-trial heterogeneity.37 In the primary analysis, we estimated that about 29 patients with ISR need to be treated with repeat stenting with DES compared with angioplasty with PCB in order to prevent one TLR.
We observed a significant interaction between treatment effect and type of restenosed stent, with a more pronounced difference in favour of repeat stenting in patients undergoing intervention for DES-ISR and similar effect in patients with bare-metal stent restenosis. This is an interesting finding that found possible correlation with the dissimilar characteristics in types of restenotic tissue after bare-metal and DES implantation.38 Mixed outcomes after repeat revascularization according to the anatomic pattern have been reported, with DES-ISR generally more challenging to treat and associated with a higher rate of subsequent adverse clinical events compared with bare-metal stent-ISR regardless of the interventional approach.39
Although the incidence of the primary safety endpoint of all-cause death, myocardial infarction, or target lesion thrombosis was similar in the two groups, after multivariable adjustment a trend towards a signal of harm after repeat DES implantation was observed. However, there was also evidence of interaction between treatment effect and type of DES used for repeat stenting, with adverse safety signal restricted to patients receiving first-generation DES compared with PCB and quite similar risk of all-cause death, myocardial infarction, and target lesion thrombosis between second-generation DES and PCB at long-term follow-up.
The observations in relation to all-cause death, cardiac death, and non-cardiac death are of some relevance in light of recent analyses suggesting higher all-cause mortality in patients treated with PCB in peripheral arterial disease.12 In contrast, we did not detect statistically significant differences between angioplasty with PCB and repeat stenting with DES for the treatment of coronary ISR. Importantly, by comparing patients enrolled in trials comparing PCB with non-paclitaxel-based DES (i.e. everolimus- and biolimus-eluting stents), no significant difference in long-term survival was observed.
The risk of myocardial infarction between groups was similar at long-term follow-up. Indeed, the somewhat inferior performance of PCB in terms of acute gain and minimum lumen diameter at surveillance angiography observed in some trials31,33,35,36 as well as the higher number of TLR during follow-up emerged from our study do not to translate into higher rates of myocardial infarction. Similarly, the incidence of definite or probable target lesion thrombosis was low and comparable between groups proving in a general subset that both possible minor dissections after angioplasty with PCB and double metallic layers after repeat stenting with DES implantation do not seem to significantly influence long-term safety.1,9
Current European guidelines on myocardial revascularization recommend the use of either PCB or DES for the treatment of coronary ISR (class of recommendation I, level of evidence A).40 The results of the DAEDALUS study support the use of both types of device in a mixed population of patients with coronary ISR. The moderate advantage in efficacy of repeat stenting with DES should be weighted against the potential advantages of avoiding additional layers of stent and the absence of significant differences in terms of safety.
Limitations
The present individual patient data meta-analysis shares some of the limitations of the original trials. For example, type of restenotic bare-metal or DES, time from implantation to index intervention for ISR, or endovascular imaging-guided procedures were not uniformly collected across trials. However, the improvement of consistency across trials for several variables, the use of additional unpublished data available in the original databases, and the extension of the follow-up when possible are notable strengths of the study. Specific additional considerations are the following. First, despite inclusion of studies with random treatment allocation, significant differences for some angiographic characteristics were observed at baseline. However, the main findings remained unchanged after multivariable statistical adjustment and some differences are related to the specific technical requirements of angioplasty with PCB (systematic pre-dilation, lower pressure of application, etc.) and DES implantation for ISR (post-dilation, higher pressure of application, etc.). Second, all trials incorporated planned angiographic follow-up as part of the study protocol. This has the advantage of adding information about the mechanisms of recurrent target lesion failure, describing the pattern of reappearance of the disease, and verifying explicitly by standardized measurements the success of the revascularization. However, it has also the potential disadvantage of influencing the natural clinical course of events, producing more revascularizations and related events (e.g. myocardial infarctions) than otherwise would be the case. Nevertheless, restricting analysis to ischaemia-driven TLR did not reveal any significant change from main results. Third, the definition of myocardial infarction was made uniform across trials when possible, but trivial differences could not be overcome in two trials that applied only the definition used in the series of studies of the same research group.30,32 Fourth, the interesting findings emerging from subgroup analyses need to be interpreted bearing in mind the reduced statistical power after grouping.41 Finally, although the DAEDALUS study reports the longest available large-scale follow-up of PCB vs. DES for ISR thus far, additional significant benefits or unexpected safety issues related to the two strategies might become apparent only after additional years of observation.
Conclusions
In patients with coronary ISR, angioplasty with PCB is moderately less effective than repeat stenting with DES in reducing TLR at 3-year follow-up. The composite of death, myocardial infarction, or target lesion thrombosis was similar between groups. Individual endpoints, including all-cause death, were not significantly different between groups.
Funding
The work was funded by a research grant from the German Ministry of Education and Research (BMBF; #KS2017-236). The sponsors of the original trials had no role in the study design, data analysis, interpretation and preparation of the manuscript, and submission of the results. The authors had final responsibility for the decision to submit the manuscript for publication.

Conflict of interest: D.G. reports research grant from the European Association of Percutaneous Coronary Intervention. R.A.B. reports lecture fees/honoraria from B Braun Melsungen AG, Biotronik, and Boston Scientific and Micell Technologies, and research funding to the institution from Boston Scientific and Celonova Biosciences. All other authors declared no conflict of interest.
Supplementary Material
References
- 1. Byrne RA, Joner M, Kastrati A. Stent thrombosis and restenosis: what have we learned and where are we going? The Andreas Gruntzig Lecture ESC 2014. Eur Heart J 2015;36:3320–3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cassese S, Byrne RA, Schulz S, Hoppman P, Kreutzer J, Feuchtenberger A, Ibrahim T, Ott I, Fusaro M, Schunkert H, Laugwitz KL, Kastrati A. Prognostic role of restenosis in 10004 patients undergoing routine control angiography after coronary stenting. Eur Heart J 2015;36:94–99. [DOI] [PubMed] [Google Scholar]
- 3. Farooq V, Gogas BD, Serruys PW. Restenosis: delineating the numerous causes of drug-eluting stent restenosis. Circ Cardiovasc Interv 2011;4:195–205. [DOI] [PubMed] [Google Scholar]
- 4. Iqbal J, Serruys PW, Silber S, Kelbaek H, Richardt G, Morel MA, Negoita M, Buszman PE, Windecker S. Comparison of zotarolimus- and everolimus-eluting coronary stents: final 5-year report of the RESOLUTE all-comers trial. Circ Cardiovasc Interv 2015;8:e002230.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vlachojannis GJ, Smits PC, Hofma SH, Togni M, Vazquez N, Valdes M, Voudris V, Slagboom T, Goy JJ, den Heijer P, van der Ent M. Biodegradable polymer biolimus-eluting stents versus durable polymer everolimus-eluting stents in patients with coronary artery disease: final 5-year report from the COMPARE II Trial. JACC Cardiovasc Interv 2017;10:1215–1221. [DOI] [PubMed] [Google Scholar]
- 6. Kufner S, Joner M, Thannheimer A, Hoppman P, Ibrahim T, Mayer K, Cassese S, Laugwitz KL, Schunkert H, Kastrati A, Byrne RA. Ten-year clinical outcomes from a trial of three limus-eluting stents with different polymer coatings in patients with coronary artery disease: results from the ISAR-TEST 4 randomized trial. Circulation 2019;139:325–333. [DOI] [PubMed] [Google Scholar]
- 7. Colombo A, Giannini F, Briguori C. Should we still have bare-metal stents available in our catheterization laboratory? J Am Coll Cardiol 2017;70:607–619. [DOI] [PubMed] [Google Scholar]
- 8. Cassese S, Byrne RA, Tada T, Pinieck S, Joner M, Ibrahim T, King LA, Fusaro M, Laugwitz K-L, Kastrati A. Incidence and predictors of restenosis after coronary stenting in 10004 patients with surveillance angiography. Heart 2014;100:153–159. [DOI] [PubMed] [Google Scholar]
- 9. Alfonso F, Byrne RA, Rivero F, Kastrati A. Current treatment of in-stent restenosis. J Am Coll Cardiol 2014;63:2659–2673. [DOI] [PubMed] [Google Scholar]
- 10. Giacoppo D, Gargiulo G, Aruta P, Capranzano P, Tamburino C, Capodanno D. Treatment strategies for coronary in-stent restenosis: systematic review and hierarchical Bayesian network meta-analysis of 24 randomised trials and 4880 patients. BMJ 2015;351:h5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Siontis GC, Stefanini GG, Mavridis D, Siontis KC, Alfonso F, Perez-Vizcayno MJ, Byrne RA, Kastrati A, Meier B, Salanti G, Jüni P, Windecker S. Percutaneous coronary interventional strategies for treatment of in-stent restenosis: a network meta-analysis. Lancet 2015;386:655–664. [DOI] [PubMed] [Google Scholar]
- 12. Katsanos K, Spiliopoulos S, Kitrou P, Krokidis M, Karnabatidis D. Risk of death following application of paclitaxel-coated balloons and stents in the femoropopliteal artery of the leg: a systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc 2018;7:e011245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stewart LA, Clarke M, Rovers M, Riley RD, Simmonds M, Stewart G, Tierney JF. Preferred Reporting items for systematic review and meta-analyses of individual participant data: the PRISMA-IPD statement. JAMA 2015;313:1657–1665. [DOI] [PubMed] [Google Scholar]
- 14. Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, Steg PG, Morel MA, Mauri L, Vranckx P, McFadden E, Lansky A, Hamon M, Krucoff MW, Serruys PW. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation 2007;115:2344–2351. [DOI] [PubMed] [Google Scholar]
- 15. Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. New York: Springer; 2000. [Google Scholar]
- 16. Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958;53:457–481. [Google Scholar]
- 17. Aalen OO, Borgan Ø, Gjessing H. Survival and Event History Analysis. New York: Springer; 2008. [Google Scholar]
- 18. Bowden J, Tierney JF, Simmonds M, Copas AJ, Higgins JP. Individual patient data meta-analysis of time-to-event outcomes: one-stage versus two-stage approaches for estimating the hazard ratio under a random effects model. Res Synth Meth 2011;2:150–162. [DOI] [PubMed] [Google Scholar]
- 19. Debray TP, Moons KG, van Valkenhoef G, Efthimiou O, Hummel N, Groenwold RH, Reitsma JB. Get real in individual participant data (IPD) meta-analysis: a review of the methodology. Res Synth Meth 2015;6:293–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Altman DG, Andersen PK. Calculating the number needed to treat for trials where the outcome is time to an event. BMJ 1999;319:1492–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van Buuren S, Groothuis-Oudshoorn K. mice: multivariate imputation by chained equations in R. J Stat Soft 2011;45:1–67. [Google Scholar]
- 22. Borenstein M. Introduction to Meta-Analysis. Chichester: John Wiley & Sons; 2009. [Google Scholar]
- 23. Schriger DL, Altman DG, Vetter JA, Heafner T, Moher D. Forest plots in reports of systematic reviews: a cross-sectional study reviewing current practice. Int J Epidemiol 2010;39:421–429. [DOI] [PubMed] [Google Scholar]
- 24. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version.5.1.0. 2011. https://handbook-5-1.cochrane.org/ (15 May 2019).
- 26. Schünemann H, Brozek J, Guyatt G, Oxman A. Handbook for Grading the Quality of Evidence and the Strength of Recommendations Using the GRADE Approach https://gdt.gradepro.org/app/handbook/handbook.html (15 May 2019).
- 27. Unverdorben M, Vallbracht C, Cremers B, Heuer H, Hengstenberg C, Maikowski C, Werner GS, Antoni D, Kleber FX, Bocksch W, Leschke M, Ackermann H, Boxberger M, Speck U, Degenhardt R, Scheller B. Paclitaxel-coated balloon catheter versus paclitaxel-coated stent for the treatment of coronary in-stent restenosis. Circulation 2009;119:2986–2994. [DOI] [PubMed] [Google Scholar]
- 28. Byrne RA, Neumann FJ, Mehilli J, Pinieck S, Wolff B, Tiroch K, Schulz S, Fusaro M, Ott I, Ibrahim T, Hausleiter J, Valina C, Pache J, Laugwitz KL, Massberg S, Kastrati A. Paclitaxel-eluting balloons, paclitaxel-eluting stents, and balloon angioplasty in patients with restenosis after implantation of a drug-eluting stent (ISAR-DESIRE 3): a randomised, open-label trial. Lancet 2013;381:461–467. [DOI] [PubMed] [Google Scholar]
- 29. Xu B, Gao R, Wang J, Yang Y, Chen S, Liu B, Chen F, Li Z, Han Y, Fu G, Zhao Y, Ge J. A prospective, multicenter, randomized trial of paclitaxel-coated balloon versus paclitaxel-eluting stent for the treatment of drug-eluting stent in-stent restenosis: results from the PEPCAD China ISR trial. JACC Cardiovas Interv 2014;7:204–211. [DOI] [PubMed] [Google Scholar]
- 30. Alfonso F, Pérez-Vizcayno MJ, Cárdenas A, García del Blanco B, Seidelberger B, Iñiguez A, Gómez-Recio M, Masotti M, Velázquez MT, Sanchís J, García-Touchard A, Zueco J, Bethencourt A, Melgares R, Cequier A, Dominguez A, Mainar V, López-Mínguez JR, Moreu J, Martí V, Moreno R, Jiménez-Quevedo P, Gonzalo N, Fernández C, Macaya C. A randomized comparison of drug-eluting balloon versus everolimus-eluting stent in patients with bare-metal stent-in-stent restenosis: the RIBS V clinical trial. J Am Coll Cardiol 2014;63:1378–1386. [DOI] [PubMed] [Google Scholar]
- 31. Adriaenssens T, Dens J, Ughi G, Bennett J, Dubois C, Sinnaeve P, Wiyono S, Coosemans M, Belmans A, D'hooge J, Vrolix M, Desmet W. Optical coherence tomography study of healing characteristics of paclitaxel-eluting balloons vs. everolimus-eluting stents for in-stent restenosis: the SEDUCE randomised clinical trial. EuroIntervention 2014;10:439–448. [DOI] [PubMed] [Google Scholar]
- 32. Alfonso F, Pérez-Vizcayno MJ, Cárdenas A, García del Blanco B, García-Touchard A, López-Minguéz JR, Benedicto A, Masotti M, Zueco J, Iñiguez A, Velázquez M, Moreno R, Mainar V, Domínguez A, Pomar F, Melgares R, Rivero F, Jiménez-Quevedo P, Gonzalo N, Fernández C, Macaya C. A prospective randomized trial of drug-eluting balloons versus everolimus-eluting stents in patients with in-stent restenosis of drug-eluting stents: the RIBS IV randomized clinical trial. J Am Coll Cardiol 2015;66:23–33. [DOI] [PubMed] [Google Scholar]
- 33. Pleva L, Kukla P, Kusnierova P, Zapletalova J, Hlinomaz O. Comparison of the efficacy of paclitaxel-eluting balloon catheters and everolimus-eluting stents in the treatment of coronary in-stent restenosis: the treatment of in-stent restenosis study. Circ Cardiovasc Interv 2016;9:e003316.. [DOI] [PubMed] [Google Scholar]
- 34. Baan J, Claessen BE, Dijk K. B-V, Vendrik J, van der Schaaf RJ, Meuwissen M, van Royen N, Gosselink ATM, van Wely MH, Dirkali A, Arkenbout EK, de Winter RJ, Koch KT, Sjauw KD, Beijk MA, Vis MM, Wykrzykowska JJ, Piek JJ, Tijssen JGP, Henriques JPS. A randomized comparison of paclitaxel-eluting balloon versus everolimus-eluting stent for the treatment of any in-stent restenosis: the DARE trial. JACC Cardiovasc Interv 2018;11:275–283. [DOI] [PubMed] [Google Scholar]
- 35. Wong YTA, Kang DY, Lee JB, Rha SW, Hong YJ, Shin ES, Her SH, Nam CW, Chung WY, Kim MH, Lee CH, Lee PH, Ahn JM, Kang SJ, Lee SW, Kim YH, Lee CW, Park SW, Park DW, Park SJ. Comparison of drug-eluting stents and drug-coated balloon for the treatment of drug-eluting coronary stent restenosis: a randomized RESTORE trial. Am Heart J 2018;197:35–42. [DOI] [PubMed] [Google Scholar]
- 36. Jensen CJ, Richardt G, Tölg R, Erglis A, Skurk C, Jung W, Neumann FJ, Stangl K, Brachmann J, Fischer D, Mehilli J, Rieber J, Wiemer M, Schofer J, Sack S, Naber CK. Angiographic and clinical performance of a paclitaxel-coated balloon compared to a second-generation sirolimus-eluting stent in patients with in-stent restenosis: the BIOLUX randomised controlled trial. EuroInterv 2018;14:1096–1103. [DOI] [PubMed] [Google Scholar]
- 37. Burke DL, Ensor J, Riley RD. Meta-analysis using individual participant data: one-stage and two-stage approaches, and why they may differ. Stat Med 2017;36:855–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Byrne RA, Joner M, Tada T, Kastrati A. Restenosis in bare metal and drug-eluting stents: distinct mechanistic insights from histopathology and optical intravascular imaging. Minerva Cardioangiol 2012;60:473–489. [PubMed] [Google Scholar]
- 39. Byrne RA, Cassese S, Windisch T, King LA, Joner M, Tada T, Mehilli J, Pache J, Kastrati A. Differential relative efficacy between drug-eluting stents in patients with bare metal and drug-eluting stent restenosis; evidence in support of drug resistance: insights from the ISAR-DESIRE and ISAR-DESIRE 2 trials. EuroInterv 2013;9:797–802. [DOI] [PubMed] [Google Scholar]
- 40. Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet JP, Falk V, Head SJ, Jüni P, Kastrati A, Koller A, Kristensen SD, Niebauer J, Richter DJ, Seferovic PM, Sibbing D, Stefanini GG, Windecker S, Yadav R, Zembala MO. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J 2019;40:87–165. [DOI] [PubMed] [Google Scholar]
- 41. Assmann SF, Pocock SJ, Enos LE, Kasten LE. Subgroup analysis and other (mis)uses of baseline data in clinical trials. Lancet 2000;355:1064–1069. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.