Abstract
The dog hookworm Ancylostoma caninum (Nematoda, Ancylostomatidae) is a blood-feeding intestinal parasitic nematode and can cause ancylostomiasis in humans. In this study, the complete mitochondrial genome of this anthropozoonotic hookworm was sequenced through Illumina deep sequencing technology. The whole genome was 13,721 bp in length and encoded 36 genes including 12 protein-coding genes, 22 transfer RNAs, and 2 ribosomal RNAs. Phylogeny revealed that A. caninum grouped with species from Ancylostomatinae and separated from species of Bunostominae in the family Ancylostomatidae. Amongst the subfamily Ancylostomatinae, three dog-originated A. caninum, regardless of isolate origins, clustered together and were more closely related to the cat hookworm A. tubaeforme and the human hookworm A. duodenale than to the dog/cat hookworm A. ceylanicum and the sea lion hookworm Uncinaria sanguinis. Taken together, the cumulative mitochondrial DNA data provides insights into phylogenetic studies among Ancylostomatidae nematodes.
Keywords: Hookworms, Ancylostoma caninum, mitochondrial genome, phylogeny
The dog hookworm Ancylostoma caninum (Nematoda, Ancylostomatinae) is a blood-feeding intestinal parasitic nematode and can cause zoonotic ancylostomiasis in almost all mammalian hosts including humans (Bowman et al. 2010). Adult hookworms parasitize in the intestines of dogs and shed millions of eggs to the environment through faeces. The eggs embryonate, develop and hatch as first stage larvae (L1) outside, and then the L1 molt twice through to the second stage (L2) and infective third stage larvae (iL3) that are capable of infecting dogs and humans. In dogs, A. caninum is regarded as a leading cause of acute, potentially fatal haemorrhagic enteritis in young puppies (Mulinge et al. 2019). Human infections typically relate to the larval migration of A. caninum under the skin and can cause cutaneous larvae migrans known as creeping eruptions (Prociv and Croese 1996). In addition, A. caninum was also sporadically reported to cause eosinophilic enteritis because its larvae can develop into pre-adult, non-patent worms in human intestines (Landmann and Prociv 2003). Recent increased molecular-based epidemiological evidence highlights that A. caninum and A. ceylanicum are emerging as important helminthic zoonosis in the Asia Pacific countries including Cambodia (Inpankaew et al. 2014), Laos (Sato et al. 2010), Malaysia (Ngui et al. 2012), Solomon Islands (Bradbury et al. 2017), Thailand (Jiraanankul et al. 2011), Australia (Smout et al. 2017) and China (Dai et al. 2009; Liu et al. 2013). However, current diagnosis of this zoonotic infection is still largely based on faecal microscopy and often misdiagnosed even by experienced microscopists due to the inability to morphologically distinguish A. caninum eggs from those of other hookworms (Monis et al. 2002). Therefore, it has become urgent to obtain a more efficient and reliable approach to identify A. caninum infection for clinical diagnosis and epidemiological investigation, and achieving this goal is foreseeable only through utilization of molecular methodologies (Rehman et al. 2017). Mitochondrial DNA (mtDNA) is regarded as an efficient molecular marker and has been widely used for species-specific identification and differentiation of many zoonotic nematodes (Hu et al. 2004; Hu and Gasser 2006). Herein, we reported the complete mitochondrial genome sequence of a representative A. caninum from China and added novel mtDNA data to this zoonotic nematode.
The parasite samples were obtained from an infected stray dog housed in an animal shelter at Wenjiang (30°44′N, 103°55′E), Sichuan Province of Southwest China, after treatment with pyrantel pamoate. After morphological identification, all worms (n = 5) were identified as A. caninum females according to the taxonomic key of Burrows (1962). One worm specimen was used for DNA extraction, and the others were fixed in 5% formalin solution and archived in the Parasitological Museum of Sichuan Agricultural University (Sichuan, China) under collection numbers XY2018_7-10. Total genomic DNA was isolated and sequenced using the Illumina HiSeq platform (Novogene, Tianjin, China). The mitochondrial genome assembly and gene annotation were performed as previously described (Xie et al. 2019).
The complete mitochondrial genome of A. caninum was 13,721 bp in length (GenBank accession no. MN215971) with 77.2% AT and encoded 12 protein-coding genes, 22 tRNA genes, and 2 rRNA genes. All genes were unidirectionally transcribed on the same strand, typical for other nematodes reported so far. Among the 12 protein-coding genes, except cox3 and nad5 deduced to use an incomplete stop codon ‘T’, the rest were predicted to use the typical TAA or TAG as the stop codons. Twenty-two tRNA genes ranged from 52 bp (tRNA-Pro) to 59 bp (tRNA-Ile) in length. Both 12S and 16S rRNAs were 695 bp and 960 bp in length, respectively, and located in the positions between tRNA-Glu and tRNA(UCN)-Ser and between tRNA-His and nad3, respectively. Three non-coding regions, namely NC1 (also known as AT-rich region; 267 bp), NC2 (104 bp) and NC3 (86 bp), were placed between tRNA-Ala and tRNA-Pro, between nad4 and cox1 and between nad3 and nad5, respectively, similar to other hookworm species, suggesting their conservation and function in regulation of transcription and control of DNA replication (Clayton 1991).
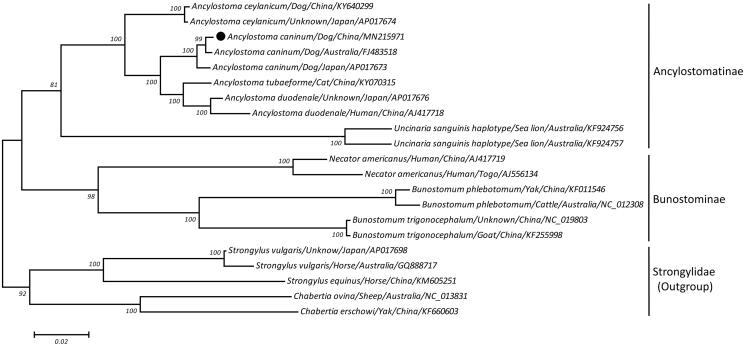
A maximum-likelihood (ML) phylogeny was reconstructed on a concatenated amino acid dataset of 12 protein-coding genes from 21 hookworms, using species of Strongylidae as outgroup. As shown in Figure 1, the phylogenic tree placed A. caninum together with species from Ancylostomatinae and separated from species of Bunostominae with high bootstrap confidence, supporting that the Ancylostomatinae and Bunostominae are monophyletic groups in the family Ancylostomatidae. Amongst the subfamily Ancylostomatinae, A. caninum from China and Australia were more closely related to each other than to that from Japan; nevertheless, these three dog-originated A. caninum clustered together and showed a closer genetic relationship to A. tubaeforme (cat hookworm) and A. duodenale (human hookworm) than to A. ceylanicum (dog/cat hookworm) and Uncinaria sanguinis (sea lion hookworm), consistent with recent molecular studies (Shi et al. 2017, 2018). In summary, the sequenced A. caninum mtDNA provides insights into phylogenetic studies among Ancylostomatidae nematodes.
Figure 1.
Maximum likelihood tree inferred from concatenated amino-acid sequences of 12 mt protein-coding genes of A. caninum and other related nematodes, utilizing MtArt model and after 100,000 bootstrap replications (<50% support not shown). The black circle sign represents the species in this study.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Bowman DD, Montgomery SP, Zajac AM, Eberhard ML, Kazacos KR. 2010. Hookworms of dogs and cats as agents of cutaneous larva migrans. Trends Parasitol. 26:162–167. [DOI] [PubMed] [Google Scholar]
- Bradbury RS, Hii SF, Harrington H, Speare R, Traub R. 2017. Ancylostoma ceylanicum hookworm in the Solomon Islands. Emerging Infect Dis. 23:252–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrows RB. 1962. Comparative morphology of Ancylostoma tubaeforme (Zeder, 1800) and Ancylostoma caninum (Ercolani, 1859). J Parasitol. 48:715–718. [PubMed] [Google Scholar]
- Clayton DA. 1991. Replication and transcription of vertebrate mitochondrial DNA. Annu Rev Cell Biol. 7:453–478. [DOI] [PubMed] [Google Scholar]
- Dai RS, Li ZY, Li F, Liu DX, Liu W, Liu GH, He SW, Tan MY, Lin RQ, Liu Y. 2009. Severe infection of adult dogs with helminths in Hunan Province, China poses significant public health concern. Vet Parasitol. 160:348–350. [DOI] [PubMed] [Google Scholar]
- Hu M, Chilton NB, Gasser RB. 2004. The mitochondrial genomics of parasitic nematodes of socio-economic importance: recent progress, and implications for population genetics and systematics. Adv Parasitol. 56:134–213. [DOI] [PubMed] [Google Scholar]
- Hu M, Gasser RB. 2006. Mitochondrial genomes of parasitic nematodes-progress and perspectives. Trends Parasitol. 22:78–84. [DOI] [PubMed] [Google Scholar]
- Inpankaew T, Schär F, Dalsgaard A, Khieu V, Chimnoi W, Chhoun C, Sok D, Marti H, Muth S, Odermatt P. 2014. High prevalence of Ancylostoma ceylanicum hookworm infections in humans, Cambodia, 2012. Emerging Infect Dis. 20:976–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiraanankul V, Aphijirawat W, Mungthin M, Khositnithikul R, Rangsin R, Traub RJ, Piyaraj P, Naanjlor T, Taamasri P, Leelayoova S. 2011. Incidence and risk factors of hookworm infection in a rural community of central Thailand. Am J Trop Med Hyg. 84:594–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landmann JK, Prociv P. 2003. Experimental human infection with the dog hookworm, Ancylostoma caninum. Med J Australia. 178:69–71. [DOI] [PubMed] [Google Scholar]
- Liu Y, Zheng G, Alsarakibi M, Zhang X, Hu W, Lu P, Lin L, Tan L, Luo Q, Li G. 2013. Molecular identification of Ancylostoma caninum isolated from cats in southern China based on complete ITS sequence. BioMed Res Int. 2013:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monis PT, Andrews RH, Saint CP. 2002. Molecular biology techniques in parasite ecology. Int J Parasitol. 32:551–562. [DOI] [PubMed] [Google Scholar]
- Mulinge E, Njenga SM, Odongo D. 2019. Molecular identification of zoonotic hookworms in dogs from four counties of Kenya. J Helminthol. 28:1–8. [DOI] [PubMed] [Google Scholar]
- Ngui R, Lim YAL, Traub R, Mahmud R, Mistam MS, Geiger SM. 2012. Epidemiological and genetic data supporting the transmission of Ancylostoma ceylanicum among human and domestic animals. PLOS Negl Trop Dis. 6:e1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prociv P, Croese J. 1996. Human enteric infection with Ancylostoma caninum: hookworms reappraised in the light of a “new” zoonosis. Acta Trop. 62:23–44. [DOI] [PubMed] [Google Scholar]
- Rehman A, Akhtar R, Akbar H, Riaz F, Rashid I, Shehzad W, Islam S, Bajwa AA, Waqas M. 2017. First report of the molecular detection of Ancylostoma caninum in Lahore, Pakistan: the threat from pets. Veterinarni Medicina. 62:559–564. [Google Scholar]
- Sato M, Sanguankiat S, Yoonuan T, Pongvongsa T, Keomoungkhoun M, Phimmayoi I, Boupa B, Moji K, Waikagul J. 2010. Copro-molecular identification of infections with hookworm eggs in rural Lao PDR. Trans Roy Soc Trop Med Hyg. 104:617–622. [DOI] [PubMed] [Google Scholar]
- Shi X, Wang M, Abdullahi AY, Fu Y, Yang F, Yu X, Pan W, Yan X, Hang J, Zhang P, Li G. 2018. Comparative analysis of Ancylostoma ceylanicum mitochondrial genome with other Ancylostoma species. Infect Genet Evol. 62:40–45. [DOI] [PubMed] [Google Scholar]
- Shi XL, Fu YQ, Abdullahi AY. 2017. The mitochondrial genome of Ancylostoma tubaeforme from cats in China. J Helminthol. 10:1–12. [DOI] [PubMed] [Google Scholar]
- Smout FA, Skerratt LF, Butler JRA, Johnson CN, Congdon BC, Thompson RCA. 2017. The hookworm Ancylostoma ceylanicum: an emerging public health risk in Australian tropical rainforests and Indigenous communities. One Health. 3:66–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Liu Y, Gu X, Meng X, Wang L, Li Y, Zhou X, Zheng Y, Zuo Z, Yang G. 2019. Complete mitogenome of the dog cucumber tapeworm Dipylidium caninum (Cestoda, Dilepididae) from Southwest China. Mitochondrial DNA B Resour. 4:2670–2672. [DOI] [PMC free article] [PubMed] [Google Scholar]