Abstract
Purpose
To evaluate the feasibility of a multiscale deep learning algorithm for quantitative visualization and measurement of traumatic hemoperitoneum and to compare diagnostic performance for relevant outcomes with categorical estimation.
Materials and Methods
This retrospective, single-institution study included 130 patients (mean age, 38 years; interquartile range, 25–50 years; 79 men) with traumatic hemoperitoneum who underwent CT of the abdomen and pelvis at trauma admission between January 2016 and April 2019. Labeled cases were separated into five combinations of training (80%) and test (20%) sets, and fivefold cross-validation was performed. Dice similarity coefficients (DSCs) were compared with those from a three-dimensional (3D) U-Net and a coarse-to-fine deep learning method. Areas under the receiver operating characteristic curve (AUCs) for a composite outcome, including hemostatic intervention, transfusion, and in-hospital mortality, were compared with consensus categorical assessment by two radiologists. An optimal cutoff was derived by using a radial basis function–based support vector machine.
Results
Mean DSC for the multiscale algorithm was 0.61 ± 0.15 (standard deviation) compared with 0.32 ± 0.16 for the 3D U-Net method and 0.52 ± 0.17 for the coarse-to-fine method (P < .0001). Correlation and agreement between automated and manual volumes were excellent (Pearson r = 0.97, intraclass correlation coefficient = 0.93). The algorithm produced intuitive and explainable visual results. AUCs for automated volume measurement and categorical estimation were 0.86 and 0.77, respectively (P = .004). An optimal cutoff of 278.9 mL yielded accuracy of 84%, sensitivity of 82%, specificity of 93%, positive predictive value of 86%, and negative predictive value of 83%.
Conclusion
A multiscale deep learning method for traumatic hemoperitoneum quantitative visualization had improved diagnostic performance for predicting hemorrhage-control interventions and mortality compared with subjective volume estimation.
Supplemental material is available for this article.
© RSNA, 2020
Summary
A multiscale deep learning method for quantitative visualization of traumatic hemoperitoneum showed significantly improved accuracy for prediction of a composite outcome of surgical or angiographic hemostatic intervention, massive transfusion, and mortality compared with volume estimation using subjective categorical grading.
Key Points
■ Automated volumes were highly correlated with manual volumes (Pearson r = 0.97) and had excellent intermethod agreement (interclass correlation coefficient, 0.93); the mean Dice similarity coefficient of the multiscale method was 0.61, compared with 0.32 for a three-dimensional U-Net and 0.52 for a coarse-to-fine network (P < .0001).
■ Automated volumes had improved performance over subjective categorical estimation of hemoperitoneum volume (area under the receiver operating characteristic curve, 0.86 vs 0.77; P = .004).
■ A radial basis function–based support vector machine with automated volumes as inputs was used to determine an optimal cutoff of 278.9 mL for the composite outcome, with accuracy of 84%, sensitivity of 82%, specificity of 93%, positive predictive value of 86%, and negative predictive value of 83%.
Introduction
The amount of hemoperitoneum identified on contrast material–enhanced admission trauma CT scans was reported to be an important determinant of outcome after major abdominopelvic trauma by Federle and Jeffrey in 1983 (1). In their seminal work, they described a coarse categorical method for grading hemoperitoneum volumes using three ranges: minor (approximately 100–250 mL), moderate (approximately 250–500 mL), or major (>500 mL). The subjective method involves visual estimation together with a count of the number of compartments along pathways where free intraperitoneal blood tends to spread or pool dependently. These include the perihepatic region, perisplenic region, hepatorenal fossa (Morison pouch), right and left paracolic gutters, and intraperitoneal pelvis (1,2).
The estimated volume range of traumatic hemoperitoneum is an important factor in the decision to treat with surgical hemostasis or angioembolization in patients with both blunt and penetrating solid organ injuries who are sufficiently stable to undergo admission CT (1,3–7). A large estimated volume of hemoperitoneum has also been shown to be predictive of the need for massive transfusion (8), which is linked to mortality risk (9).
Automated methods for quantitative visualization of pathologic features in cross-sectional imaging can serve as a form of precision diagnostics to guide personalized point-of-care decision support and prognostication (10). Short-hand semiquantitative methods, such as measurement of the largest diameter, involve considerable information loss and may be particularly limited when injury is multicompartmental (11–13). In the past several years, convolutional neural networks of increasing depth and sophistication have become the primary computer-vision modality for segmentation tasks (14). Despite the information loss inherent to coarse subjective grading, the basic scheme described by Federle and Jeffrey has remained the primary method for assessing posttraumatic CT hemoperitoneum volume for more than 35 years (1,15). Manual or semiautomated volumetric techniques are prohibitively time-consuming for this task, precluding routine point-of-care use. Voxelwise segmentation and measurement could offer a rapid, objective, and explainable means of stratifying risk using hemoperitoneum as a truly quantitative imaging biomarker.
In this work, we implement and test a multiscale dilated convolutional neural network with an attentional module to address the problems of variability in lesion scale and multifocality. Method feasibility was assessed in terms of the Dice similarity coefficient (DSC), inference speed, and correlation between automated and manual measurements. Association with four key binary outcomes (intervention with surgical hemostasis, intervention with angioembolization, need for massive transfusion, and in-hospital mortality) was also assessed. Last, diagnostic performance of automated volumes for a composite outcome combining these four dependent variables was compared with consensus Federle scoring by two radiologists. An optimal cutoff volume was determined by using an established radial basis function–based support vector machine classifier.
Materials and Methods
Patient Dataset
This retrospective work is part of a Health Insurance Portability and Accountability Act–compliant study protocol determined to be exempt by the institution’s internal review board. The de-identified study dataset consisted of portal venous phase abdomen-pelvis series from admission contrast-enhanced trauma CT examinations in 130 adult (age ≥ 18 years) patients admitted to a level I trauma center for blunt or penetrating traumatic injury between January 1, 2016, and April 23, 2019.
We initially screened patients during the study period by using a keyword search for “hemoperitoneum” within their radiologic reports. The initial search resulted in 264 patients. Patients were excluded if CT was performed following angiography (n = 2) or postoperatively (n = 8), if the radiology report indicated (by using various phrases and diction) that the patient had no hemoperitoneum (n = 76), if the report contained ambiguity regarding the presence or absence of hemoperitoneum (eg, questionable trace hemoperitoneum) and there was no measurable hemoperitoneum at imaging review (n = 23), if patients were found to have extraperitoneal pelvic or retroperitoneal hematoma but no hemoperitoneum (n = 16), if the CT study was from an outside hospital with no repeat admission CT at our trauma center (n = 1), if the study was severely limited because of motion (n = 2), and if the portal venous phase images were only obtained for the upper abdomen (n = 6).
CT Imaging
Abdominopelvic studies were performed with trauma bay–adjacent 64-section (Brilliance; Philips Healthcare, Andover, Mass) or dual-source 128-section (Siemens Force; Siemens, Erlangen, Germany) scanners by using 100 mL of intravenous contrast material (Omnipaque [iohexol; 350 mg of iodine per milliliter]; GE Healthcare, Chicago Ill). In penetrating trauma, rectal contrast material (50 mL of iohexol; 300 mg of iodine per milliliter diluted in 1000 mL of water) is typically administered (16) and was present in 16 of 17 patients (94%) with penetrating wounds. Axial images were archived at 1.5- to 3-mm thickness, with a total of 58 135 images before preprocessing.
Ground Truth Image Labeling and Preprocessing
Digital Imaging and Communications in Medicine, or DICOM, images were converted into Neuroimaging Informatics Technology Initiative format before labeling. Manual labeling was performed by a board-certified radiologist (D.D.) with 8 years of dedicated experience in trauma imaging. Labeling was performed using the three-dimensional (3D) Slicer editor module (version 4.10.0; www.slicer.org). Attenuation values of hemoperitoneum can be widely variable, typically ranging from 0 to 80 HU, with values as low as 0–20 HU in one-quarter of measured regions of interest (17). Label masks of hemoperitoneum were created using a 3D threshold paint tool initially in the axial plane with supervision and editing in coronal and sagittal planes. Thresholding was set to a range of −20 to 100 HU to minimize noise while avoiding neighboring fat. Labeling around organs and at the edges of the body wall or muscle was performed with a small (3- to 5-mm diameter) spherical region of interest to carefully delineate the interface of blood with these structures, and a spherical region-of-interest diameter of up to 20 mm was used elsewhere within regions of confluent blood.
Neuroimaging Informatics Technology Initiative source images and labels were then upsampled to a 0.521- to 1.875-mm section thickness to achieve isotropic resolution following reconstruction of coronal and sagittal images, which yielded a total dataset of 199 680 images (512 × 512 matrix). Raw intensity values were truncated to within the range of −80 to 320 HU and were then normalized to 0 to 255 HU (18). Random image rotation was performed at 0° to 15° intervals for data augmentation.
Deep Learning Method: Multiscale Attentional Network
Background information on the multiscale attentional network (MSAN) can be found in Appendix E1 (supplement). The schematic of the network is found in Figure E1 (supplement). We believed that the same MSAN method (described in Appendix E1 [supplement]) would address the multifocality, spatial variability, and fine margins or weak boundaries inherent to hemoperitoneum.
Training and Implementation
The end-to-end MSAN algorithm was implemented on the TensorFlow (version 1.4.0) deep learning platform. Experiments were performed using an NVIDIA DGX (Santa Clara, Calif) machine with four Tesla V100 graphics processing units, each with 16 GB of memory running on Ubuntu. The MSAN was previously initialized by using ImageNet pretrained models (19). In our study, the network architecture, models, and hyperparameters were inherited from prior work by our group by using the MSAN for segmentation of intravenous contrast material extravasation. A learning policy with polynomial decay was used, with an initial learning rate of 0.05 and power of 0.9 for learning-rate decay. The algorithm was trained for 30 000 iterations with a minibatch size of 32. The mean training time was approximately 120 hours. Fivefold cross-validation was used by using an 80% training to 20% validation split in five unseen nonoverlapping combinations. Mean DSC and inference times (± standard deviations) were determined. Comparison experiments were performed by using a baseline reference deep learning toolkit 3D U-Net (20) and a coarse-to-fine algorithm called the Recurrent Saliency Transformation Network (21).
Statistical Analysis
The DSC summary statistic was used as a measure of spatial overlap and segmentation accuracy, and pairwise comparisons were performed by using Student t test. Pearson coefficient (r) and the single measure intraclass correlation coefficient were used for volumetric comparisons. Measurement bias and 95% limits of agreement were determined by using a Bland-Altman plot. Six months after ground truth manual labeling, 20 patients were randomly selected for assessment of test-retest reliability, and studies were relabeled by the same board-certified trauma radiologist (D.D.). The intraclass correlation coefficient, Pearson r, and DSC between the two sets of manual volumes were determined. Segmentation times (in minutes) were also recorded. Hemoperitoneum volumes were compared by using the Mann-Whitney U test for the following binary outcomes: surgical hemostatic intervention; angiographic hemostatic intervention; massive transfusion, defined as 10 U or more of packed red blood cells within 24 hours or 4 U or more of packed red blood cells within 4 hours; and in-hospital mortality. A composite dependent variable was generated that treated any patients with complete transfusion data as positive for hemoperitoneum if at least one of the four aforementioned outcomes were positive. Accurate transfusion data were available in 98 of 130 patients who were admitted directly to our trauma center following injury. The remaining 32 patients were transferred from another institution, and reliable transfusion data were not available. These patients were not considered in analyses involving transfusion or the composite outcome. Federle grading was performed in consensus by two board-certified radiologists (E.S., >20 years of experience, and D.D.) in the 98 patients with complete data. The areas under the receiver operating characteristic curve (AUCs) of automated and manual measurements for the composite outcome were compared with categorical grading by using the DeLong method (22). Stata (version 15.1; Stata, College Station, Tex) was used for all comparisons. SPSS Statistics (version 26.0; IBM, Armonk, NY) was used to generate receiver operating characteristic curves. For all analyses, P values less than .05 were considered to indicate statistical significance. An optimal cutoff value for predicting the composite outcome was reached by using the radial basis function–based support vector machine classifier described by Wang et al (23). The classifier was trained and tested by using n-1 cross-validation, with hemoperitoneum volumes and binary outcome data as inputs by using Matlab software (version 2019a; MathWorks, Natick, Mass).
Results
Patient Characteristics
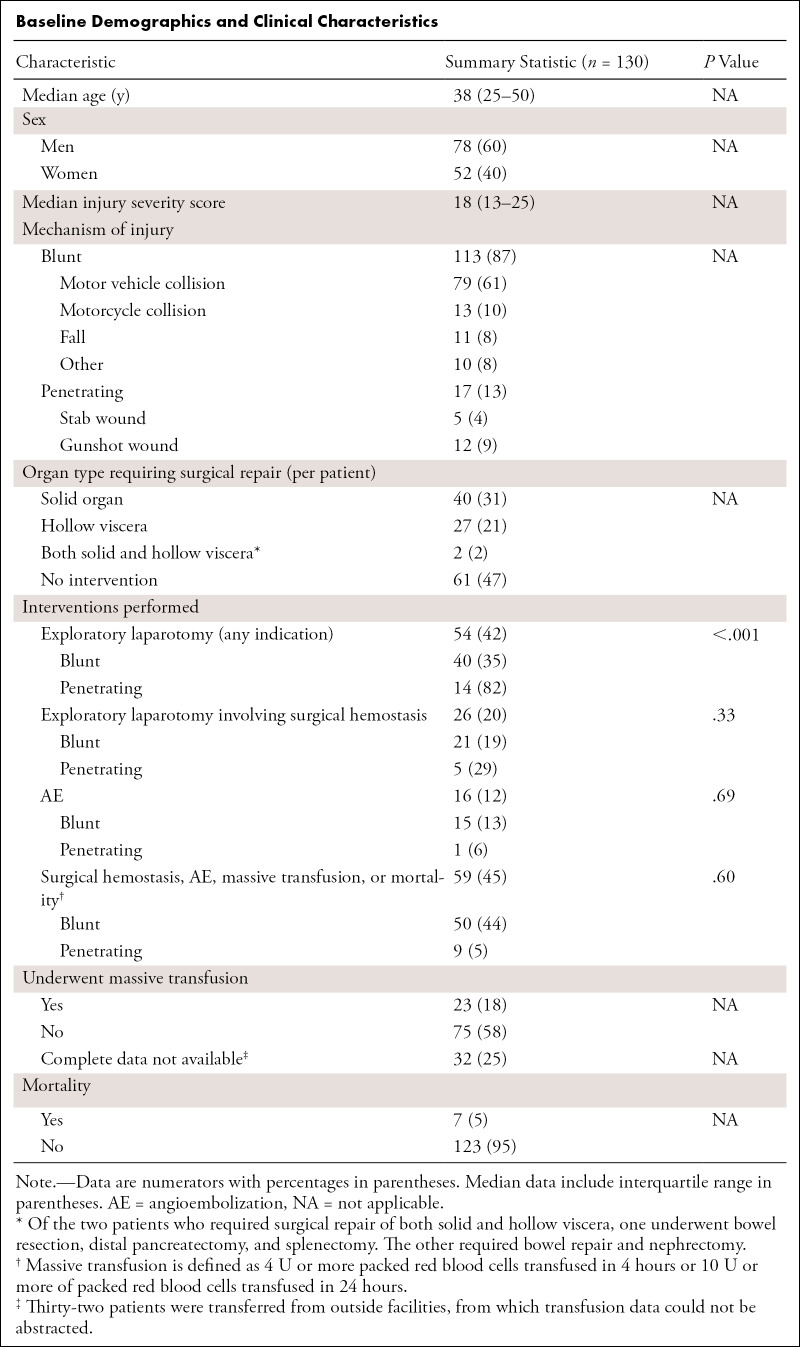
There were 130 patients included in this study (mean age, 38 years; interquartile range [IQR], 25–50 years; 79 men). Summary statistics for baseline demographic and clinical characteristics are shown in the Table. The range of hemoperitoneum volumes was broad (11–2036 mL), with highly skewed distributions. The mean and median manual hemoperitoneum volumes were 338.0 mL ± 370.9 and 180.9 (IQR, 68.5–473.7), respectively. Of the 130 patients, 113 patients sustained blunt trauma and 17 sustained penetrating wounds (five stab wounds and 12 gunshot wounds). More patients underwent exploratory laparotomy in the penetrating trauma cohort (P < .001); however, the proportions of patients requiring surgical hemostasis (P = .33), requiring angioembolization (P = .69), or who were positive for the composite outcome (P = .60) were not different between the two groups. Overall, 20 patients required massive transfusion. There were 51 patients who underwent exploratory laparotomy, 26 of whom required surgical hemostasis. Sixteen patients were treated with angioembolization. Twenty-three patients required massive transfusion, and seven patients died of their injuries. In the cohort of 98 patients with complete transfusion data, 40 patients were positive for the composite outcome, including all four end points.
Baseline Demographics and Clinical Characteristics
Hemoperitoneum Segmentation and Measurement
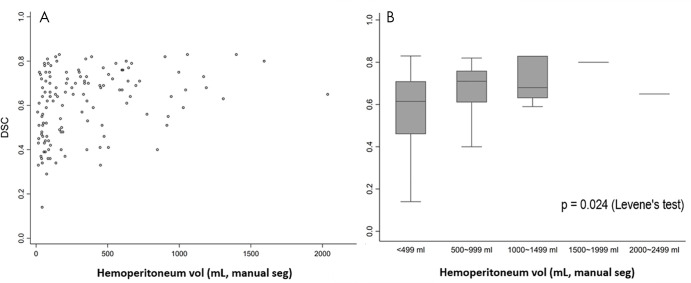
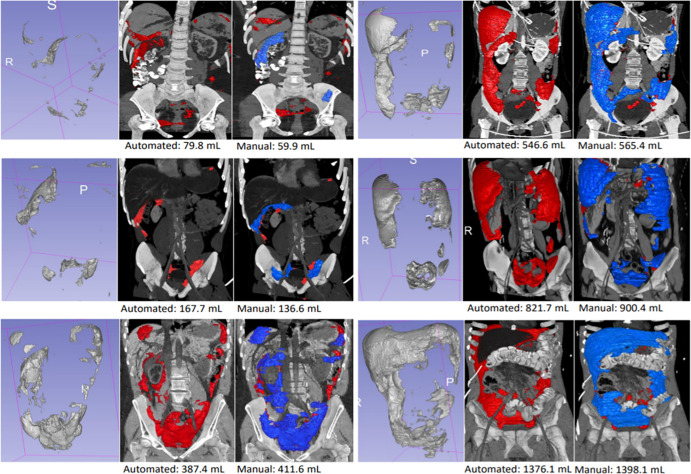
The mean DSC of the MSAN for hemoperitoneum segmentation was 0.61 ± 0.15, and the mean inference time was 151.3 seconds ± 2.8 (2.5 minutes ± 0.05). The DSC of the MSAN was higher than that of the 3D U-Net (0.32 ± 0.16; P < .0001) and that of the Recurrent Saliency Transformation Network (DSC of 0.52 ± 0.17; P < .0001). The DSC of MSAN corresponded with a high Pearson correlation coefficient between manual and automated measurements (r = 0.97; slope, y = 0.87x; 95% CI: 0.83, 0.91) (Fig 1). The single measure intraclass correlation coefficient was in the excellent range (intraclass correlation coefficient, 0.93; 95% CI: 0.90, 0.95) (24). Bland-Altman plots showed a low underestimation bias for automated measurements of 19.6 mL (Fig 2). We observed a pattern of decreasing DSCs with higher variance at lower-volume increments ([Fig 3]; P = .024, Levene test). Three-dimensional visualizations of six better-performing cases are illustrated from the smallest to the largest volume in Figure 4. These show the versatility of the algorithm for segmentation of numerous foci of hemoperitoneum of varying scales within and among patients. Figure E2 (supplement) shows images through the abdomen and pelvis from both high- and low-performing cases (DSCs above and below 1 standard deviation from the mean). Potential sources of error, ambiguity, and automated–manual label mismatch are summarized. The Movie (supplement) further enhances comparison of manual and automated hemoperitoneum labels in three dimensions.
Figure 1:
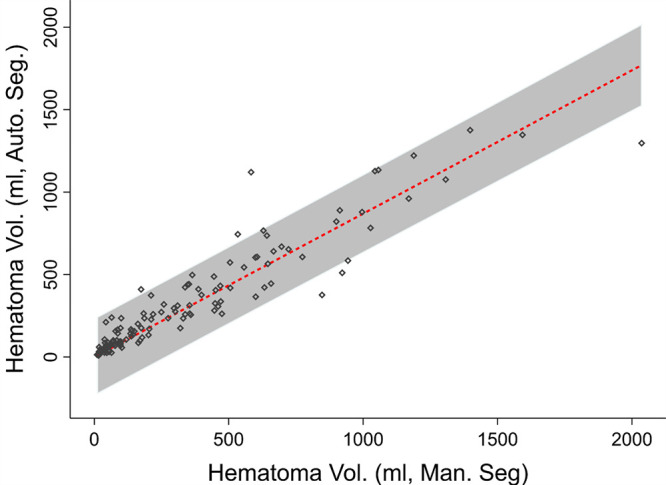
Dot matrix plot with best-fit line and 95% CI shows correlation between automated volume (vol.) and manual hemoperitoneum volume (Pearson r = 0.97; slope, 0.87; 95% CI: 0.83, 0.91). Auto. Seg. = automated segmentation, Man. Seg. = manual segmentation.
Figure 2:

Bland-Altman plot shows 95% limits of agreement and measurement bias. On average, there is a 19.6-mL underestimation by the deep learning algorithm. Diff. = difference in, SD = standard deviation, vol = volume.
Figure 3:
Distribution of Dice similarity coefficients (DSCs), A, on a per-patient basis and, B, grouped by hematoma volume increments of 500 mL are shown. The box plot in B shows decreasing interquartile ranges and 95% CIs with increasing hemoperitoneum volumes. The Levene test confirms unequal variance in DSCs across groups (P = .024). man seg = manual segmentation.
Figure 4:
Six high-performing cases are shown with ground truth (manual) volumes ranging from 59.9 to 1398.1 mL. For each patient, the image on the left provides an unobstructed view of the automated segmentation alone, the middle image shows the segmentation (red) superimposed on three-dimensional CT images, and the image on the right shows the overlay between manual (blue) and automated (red) segmentations. Images show blood distributed along common pathways, including perihepatic and perisplenic regions, paracolic gutters, hepatorenal fossa (Morison pouch), and the intraperitoneal pelvis.
Movie:
Video illustrates the similarity between manual (blue) and automated (red) hemoperitoneum volumes at the level of a midabdominal coronal cut-plane as the manual label is peeled back over the dome of the liver.
Assessment of test-retest reliability between two sets of manual measurements in the 20-patient subgroup revealed an intraclass correlation coefficient of 0.98 (95% CI: 0.94, 0.99), a Pearson correlation of 0.98 (P < .0001; slope of y = 1.07x; 95% CI: 1.00, 1.13), and a DSC of 0.70 ± 0.09. The median manual segmentation time was 16 minutes (mean, 21 minutes), with a range of 3–49 minutes for volumes ranging between 20.6 and 1309.2 mL.
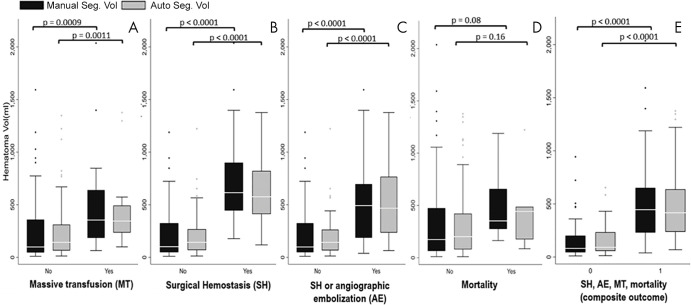
Median automated measurements (Fig 5) mirrored manual measurements in having higher hemoperitoneum volumes for those with massive transfusion (automated, 345.1 [IQR, 235.5–492.8], P = .0011; manual, 354.8 [IQR, 185.1–639.3], P = .009), surgical hemostatic intervention (automated, 624.0 [IQR, 414.5–872.7], P < .0001; manual, 635.5 [IQR, 410.4–910.1], P < .0001), and surgical hemostasis or angioembolization (automated: 434.4 [IQR, 191.1–779.0], P < .0001; manual, 456.1 [IQR, 180.2–754.7], P < .0001). Differences in volumes approached significance for mortality (automated, 338.8 [IQR, 205.5–445.8], P = .16; manual, 352.1 [IQR, 297.3–551.4], P = .08). This may have been due in part to the low number of deaths (n = 7). There was a strong positive association between the composite outcome and the hemoperitoneum volume (automated, 416.8 [IQR, 238.7–622.5], P < .0001; manual, 446.0 [IQR, 252.5–648.6], P < .0001).
Figure 5:
Clustered box and whisker plots show medians, interquartile ranges, and 95% CIs for, A, the need for massive transfusion, B, the need for surgical hemostasis, C, the need for surgical hemostasis or angioembolization, D, in-hospital mortality, and, E, a derived composite outcome including all end points (right side).
The AUCs of automated measurements, manual measurements, and Federle grading were 0.86 (95% CI: 0.79, 0.93), 0.86 (95% CI: 0.78, 0.93), and 0.77 (95% CI: 0.69, 0.86), respectively (Fig 6). The AUCs of automated and manual measurements were both higher than those of categorical grading (P = .004 and 0.015). There was no difference in AUCs between automated and manual measurements (P = .89). The optimal cutoff value determined by using the radial basis function–based support vector machine classifier was 278.9 mL, with accuracy of 84%, sensitivity of 82%, specificity of 93%, positive predictive value of 86%, and negative predictive value of 83%.
Figure 6:
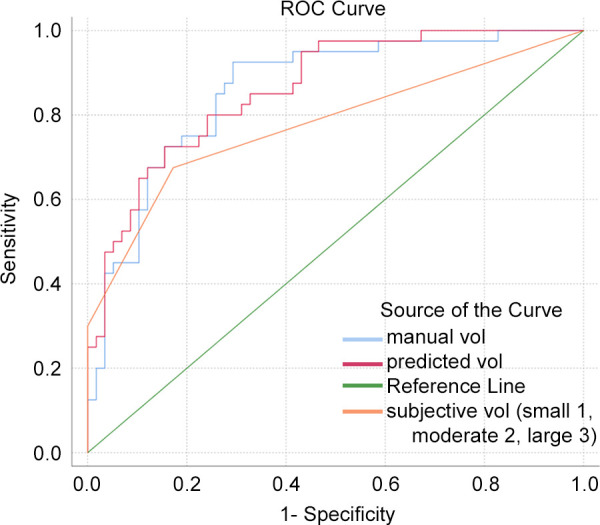
Receiver operating characteristic (ROC) curves for manual label volume (vol; blue), automated label volume (red), and subjective categorical Federle volume estimation (orange) are shown.
Discussion
In this setting of blunt or penetrating trauma in patients who are stable or who are transient responders (7,25), CT is a critical component of patient triage to surgical exploration or conventional angiography for control of potentially lethal hemorrhage and helps guide the transfusion strategy (10,26). By using a multiscale deep learning method that we originally described for segmentation of multifocal contrast material extravasation following pelvic fractures (27), we showed improved accuracy of automated volumes over subjective categorical grading (AUC, 0.86 vs 0.77; P = .004) for predicting a composite outcome. AUCs for manual and automated measurements were similar (both 0.86; P = .89). By using a support vector-machine classifier (23,28), an optimal cutoff of 278.9 mL was derived, with accuracy of 84%, sensitivity of 82%, specificity of 93%, positive predictive value of 86%, and negative predictive value of 83%.
An inference time of approximately 2.5 minutes could provide a point-of-care result if abdominal CT scans are routinely preprocessed on the basis of trauma resuscitation unit–ordering location. Speed will further improve with advances in graphics processing units and other artificial intelligence accelerators.
Hemoperitoneum segmentation cannot be suitably addressed with traditional fully convolutional networks such as the 3D U-Net (20), with a DSC of only 0.32 ± 0.16 in our dataset. The Recurrent Saliency Transformation Network (21), a cascaded coarse-to-fine neural network with an intermediate cropping step, is adequate for extraperitoneal pelvic hematoma segmentation in cases in which blood pools in closely connected extraperitoneal compartments within a comparably small anatomic space (29); however, when applied to hemoperitoneum, we found the DSC to be only 0.52 ± 0.17. Our multiscale algorithm achieved a higher DSC of 0.61 ± 0.15 (P < .0001).
Our confidence that the algorithm is precise and reliable for clinical purposes was supported by a high correlation and agreement between manual and automated volume measurements (r = 0.97; P < .0001; intraclass correlation coefficient, 0.93; 95% CI: 0.90, 0.95) and low bias (19.6 mL), given the large volume range. The trend of higher DSCs and decreased variance with higher volumes of hemoperitoneum suggests a greater degree of precision at higher volume ranges that have greater clinical import.
Hemoperitoneum volume estimation by using Federle grading has predictive value in trauma but is coarse and subjective (1,3,4,30,31). In the peritoneal cavity, varying quantities of blood pool discontinuously in dependent areas in widely disparate locations. This requires an approach that can handle multifocality and a wide variety of object scales.
Hemoperitoneum should be more formally defined to improve agreement in ground truth labeling. Sources of error include regions where the conditions of decompressed bowel and hemoperitoneum are indistinguishable (see Figure E2 [supplement]; this is also a known pitfall of subjective assessment [1,17,32]); imprecise definitions of hemoperitoneum regarding discrete mesenteric hematomas; missed areas of blood in the periphery of the peritoneal cavity; and potential uncertainty in cases with contiguous extraperitoneal pelvic hematoma, for which an automated segmentation method was already described (29).These issues could be formally addressed by updating the concept of hemoperitoneum through a formal lexicon such as RadLex, by using the Foundational Model of Anatomy reference ontology (33). Nevertheless, overall, the deep learning segmentation labels were explainable and intuitive (7,16). The algorithm produced fine-detail multifocal segmentations that largely avoided the viscera, vessels, and body wall.
A search of the literature did not reveal prior work that described automated methods for voxelwise hemoperitoneum segmentation, or related tasks (eg, ascites). Prior works only described shorthand semiquantitative or qualitative methods for estimating hemoperitoneum volume on focused abdominal sonographs in trauma or CT (1,34,35). Semiautomated region-growing has been used for measuring traumatic abdominopelvic free fluid to predict development of refractory abdominal compartment syndrome (36). However, Federle grading has remained the standard of care (1).
The decision to treat patients with surgical or angiographic hemostatic interventions rather than with an expectant approach can be challenging (7,25). Objective reliable measurements of hemoperitoneum volume could reduce morbidity, mortality, and costs associated with nontherapeutic laparotomies, unnecessary conventional angiographic examinations, treatment delays, and insufficiently aggressive transfusion strategies.
Hemoperitoneum is one of several volumetric features predictive of the outcome following abdominal injury, which also include organ lacerations, pseudoaneurysm, and active bleeding. Ultimately, volumetric measurements of each feature could be combined into multivariable decision support tools (37), but this was beyond the scope of our work.
The algorithm primarily serves a hemorrhage control–related purpose in patients who are sufficiently stable to undergo CT. It would not have as great an impact in patients with absolute surgical indications such as perforated viscus and is not intended for patients with refractory hypotension who require immediate surgery. Other limitations of our study pertained to inherent difficulty obtaining ground truth segmentations, labeling by a single radiologist (D.D.), the small size of our dataset, and the relative lack of diversity of acquisition systems at our institution. We started with what we perceived to be the most difficult task related to hemoperitoneum (voxelwise segmentation). As such, negative controls were not included. Finally, bleeding in multiple body compartments has always been a confounding factor in assessing the effects on the transfusion requirement. Combining the volumetric results from multiple algorithms (eg, three separate algorithms for pelvic hematoma, hemoperitoneum, and hemothorax) could give a more explanatory assessment of the patient’s overall CT hemorrhage burden index, which may more reliably guide the transfusion strategy.
In conclusion, we implemented a multiscale deep learning method for traumatic hemoperitoneum segmentation and assessed its feasibility. We found that the diagnostic performance of automated volumetric measurements for predicting hemorrhage-control interventions and mortality were significantly improved over standard-of-care subjective categorical hemoperitoneum volume estimation. The method offers a promising and clinically relevant approach for rapidly and objectively quantifying hemoperitoneum.
APPENDIX
SUPPLEMENTAL FIGURES
D.D. supported by the National Institutes of Health (NIH K08 EB027141-01A1) and RSNA Research Scholar Grant (RSCH1605). D.D., Y.Z., and A.Y. supported by an Accelerated Translational Incubator Pilot award, University of Maryland (D.D. principal investigator).
Disclosures of Conflicts of Interest: D.D. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: co-founder, TraumaVisual. Other relationships: disclosed no relevant relationships. Y.Z. disclosed no relevant relationships. S.F. disclosed no relevant relationships. Y.W. disclosed no relevant relationships. G.L. disclosed no relevant relationships. K.C. disclosed no relevant relationships. E.S. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: co-founder, TraumaVisual. Other relationships: disclosed no relevant relationships. Z.W. disclosed no relevant relationships. T.C. disclosed no relevant relationships. A.L.Y. disclosed no relevant relationships.
Abbreviations:
- AUC
- area under the receiver operating characteristic curve
- DSC
- Dice similarity coefficient
- IQR
- interquartile range
- MSAN
- multiscale attentional network
- 3D
- three-dimensional
References
- 1.Federle MP, Jeffrey RB Jr. Hemoperitoneum studied by computed tomography. Radiology 1983;148(1):187–192. [DOI] [PubMed] [Google Scholar]
- 2.Shuman WP. CT of blunt abdominal trauma in adults. Radiology 1997;205(2):297–306. [DOI] [PubMed] [Google Scholar]
- 3.Fang JF, Wong YC, Lin BC, Hsu YP, Chen MF. The CT risk factors for the need of operative treatment in initially hemodynamically stable patients after blunt hepatic trauma. J Trauma 2006;61(3):547–553; discussion 553–554. [DOI] [PubMed] [Google Scholar]
- 4.Goan YG, Huang MS, Lin JM. Nonoperative management for extensive hepatic and splenic injuries with significant hemoperitoneum in adults. J Trauma 1998;45(2):360–364; discussion 365. [DOI] [PubMed] [Google Scholar]
- 5.Berg RJ, Inaba K, Okoye O, et al. The contemporary management of penetrating splenic injury. Injury 2014;45(9):1394–1400. [DOI] [PubMed] [Google Scholar]
- 6.Teuben M, Spijkerman R, Pfeifer R, et al. Selective non-operative management for penetrating splenic trauma: a systematic review. Eur J Trauma Emerg Surg 2019;45(6):979–985 [Published correction appears in Eur J Trauma Emerg Surg 2019;45(6):987.]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dreizin D, Munera F. Multidetector CT for penetrating torso trauma: state of the art. Radiology 2015;277(2):338–355. [DOI] [PubMed] [Google Scholar]
- 8.Dreizin D. Commentary on “Multidetector CT in vascular injuries resulting from pelvic fractures”. RadioGraphics 2019;39(7):2130–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cancio LC, Wade CE, West SA, Holcomb JB. Prediction of mortality and of the need for massive transfusion in casualties arriving at combat support hospitals in Iraq. J Trauma 2008;64(2 Suppl):S51–S55; discussion S55–S56. [DOI] [PubMed] [Google Scholar]
- 10.Dreizin D, Zhou Y, Chen T, et al. Deep learning-based quantitative visualization and measurement of extraperitoneal hematoma volumes in patients with pelvic fractures: potential role in personalized forecasting and decision support. J Trauma Acute Care Surg 2020;88(3):425–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenkrantz AB, Mendiratta-Lala M, Bartholmai BJ, et al. Clinical utility of quantitative imaging. Acad Radiol 2015;22(1):33–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abramson RG, Burton KR, Yu JP, et al. Methods and challenges in quantitative imaging biomarker development. Acad Radiol 2015;22(1):25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dreizin D, Bodanapally UK, Neerchal N, Tirada N, Patlas M, Herskovits E. Volumetric analysis of pelvic hematomas after blunt trauma using semi-automated seeded region growing segmentation: a method validation study. Abdom Radiol (NY) 2016;41(11):2203–2208. [DOI] [PubMed] [Google Scholar]
- 14.Litjens G, Kooi T, Bejnordi BE, et al. A survey on deep learning in medical image analysis. Med Image Anal 2017;42:60–88. [DOI] [PubMed] [Google Scholar]
- 15.Lubner M, Menias C, Rucker C, et al. Blood in the belly: CT findings of hemoperitoneum. RadioGraphics 2007;27(1):109–125. [DOI] [PubMed] [Google Scholar]
- 16.Dreizin D, Boscak AR, Anstadt MJ, et al. Penetrating colorectal injuries: diagnostic performance of multidetector CT with trajectography. Radiology 2016;281(3):749–762. [DOI] [PubMed] [Google Scholar]
- 17.Levine CD, Patel UJ, Silverman PM, Wachsberg RH. Low attenuation of acute traumatic hemoperitoneum on CT scans. AJR Am J Roentgenol 1996;166(5):1089–1093. [DOI] [PubMed] [Google Scholar]
- 18.Erickson BJ. Magician’s Corner: 3. Image Wrangling. Radiol Artif Intell 2019;1(6):e190126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou Y, Dreizin D, Li Y, Zhang Z, Wang Y, Yuille A. Multi-scale attentional network for multi-focal segmentation of active bleed after pelvic fractures. In: Suk HI, Liu M, Yan P, Lian C, eds. Machine learning in medical imaging. MLMI 2019. Vol 11861, Lecture Notes in Computer Science. Cham, Switzerland: Springer, 2019; 461–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pawlowski N, Ktena SI, Lee MC, et al. Dltk: State of the art reference implementations for deep learning on medical images. ArXiv 1711.06853 [preprint] https://arxiv.org/abs/1711.06853. Posted November 18, 2017. Accessed December 2019. [Google Scholar]
- 21.Xie L, Yu Q, Zhou Y, Wang Y, Fishman EK, Yuille AL. Recurrent saliency transformation network for tiny target segmentation in abdominal CT scans. IEEE Trans Med Imaging 2020;39(2):514–525. [DOI] [PubMed] [Google Scholar]
- 22.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44(3):837–845. [PubMed] [Google Scholar]
- 23.Wang Z, Childress AR, Wang J, Detre JA. Support vector machine learning-based fMRI data group analysis. Neuroimage 2007;36(4):1139–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cicchetti DV. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol Assess 1994;6(4):284–290. [Google Scholar]
- 25.Dreizin D, Munera F. Blunt polytrauma: evaluation with 64-section whole-body CT angiography. RadioGraphics 2012;32(3):609–631. [DOI] [PubMed] [Google Scholar]
- 26.Costantini TW, Coimbra R, Holcomb JB, et al. Current management of hemorrhage from severe pelvic fractures: results of an American Association for the Surgery of Trauma multi-institutional trial. J Trauma Acute Care Surg 2016;80(5):717–723; discussion 723–725. [DOI] [PubMed] [Google Scholar]
- 27.Gan K, Xu D, Lin Y, et al. Artificial intelligence detection of distal radius fractures: a comparison between the convolutional neural network and professional assessments. Acta Orthop 2019;90(4):394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Z, Suh J, Duan D, et al. A hypo-status in drug-dependent brain revealed by multi-modal MRI. Addict Biol 2017;22(6):1622–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dreizin D, Zhou Y, Zhang Y, Tirada N, Yuille AL. Performance of a deep learning algorithm for automated segmentation and quantification of traumatic pelvic hematomas on CT. J Digit Imaging 2020;33(1):243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moon KL Jr, Federle MP. Computed tomography in hepatic trauma. AJR Am J Roentgenol 1983;141(2):309–314. [DOI] [PubMed] [Google Scholar]
- 31.van der Vlies CH, van Delden OM, Punt BJ, Ponsen KJ, Reekers JA, Goslings JC. Literature review of the role of ultrasound, computed tomography, and transcatheter arterial embolization for the treatment of traumatic splenic injuries. Cardiovasc Intervent Radiol 2010;33(6):1079–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Becker CD, Mentha G, Terrier F. Blunt abdominal trauma in adults: role of CT in the diagnosis and management of visceral injuries. Part 1: liver and spleen. Eur Radiol 1998;8(4):553–562. [DOI] [PubMed] [Google Scholar]
- 33.Mejino JL Jr, Rubin DL, Brinkley JF. FMA-RadLex: an application ontology of radiological anatomy derived from the foundational model of anatomy reference ontology. In: American Medical Informatics Association annual symposium proceedings program (abstr). Bethesda, Md: American Medical Informatics Association, 2008; 465. [PMC free article] [PubMed] [Google Scholar]
- 34.Charbit J, Mahul M, Roustan JP, et al. Hemoperitoneum semiquantitative analysis on admission of blunt trauma patients improves the prediction of massive transfusion. Am J Emerg Med 2013;31(1):130–136. [DOI] [PubMed] [Google Scholar]
- 35.McKenney KL, McKenney MG, Cohn SM, et al. Hemoperitoneum score helps determine need for therapeutic laparotomy. J Trauma 2001;50(4):650–654; discussion 654–656. [DOI] [PubMed] [Google Scholar]
- 36.Battey TWK, Dreizin D, Bodanapally UK, et al. A comparison of segmented abdominopelvic fluid volumes with conventional CT signs of abdominal compartment syndrome in a trauma population. Abdom Radiol (NY) 2019;44(7):2648–2655. [DOI] [PubMed] [Google Scholar]
- 37.Dreizin D, Bodanapally U, Boscak A, et al. CT prediction model for major arterial injury after blunt pelvic ring disruption. Radiology 2018;287(3):1061–1069. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.