Abstract
Current management of osteoarthritis (OA) is primarily focused on symptom control. Intra-articular corticosteroid (IACS) injections are often used for pain management of hip and knee OA in patients who have not responded to oral or topical analgesics. Recent case series suggested that negative structural outcomes including accelerated OA progression, subchondral insufficiency fracture, complications of pre-existing osteonecrosis, and rapid joint destruction (including bone loss) may be observed in patients who received IACS injections. This expert panel report reviews the current understanding of pain in OA, summarizes current international guidelines regarding indications for IACS injection, and considers preinterventional safety measures, including imaging. Potential profiles of those who would likely benefit from IACS injection and a suggestion for an updated patient consent form are presented. As of today, there is no established recommendation or consensus regarding imaging, clinical, or laboratory markers before an IACS injection to screen for OA-related imaging abnormalities. Repeating radiographs before each subsequent IACS injection remains controversial. The true cause and natural history of these complications are unclear and require further study. To determine the cause and natural history, large prospective studies evaluating the risk of accelerated OA or joint destruction after IACS injections are needed. However, given the relatively rare incidence of these adverse outcomes, any clinical trial would be challenging in design and a large number of patients would need to be included.
© RSNA, 2020
Learning Objectives:
After reading the article and taking the test, the reader will be able to:
■ List potential adverse outcomes of intra-articular corticosteroid (IACS) injections that can be recognized by imaging and potential pre-interventional safety measures
■ Identify the current recommendations by professional societies regarding the role of IACS injections for treatment of knee and hip osteoarthritis (OA) pain
■ Identify profiles of those patients who will likely benefit from IACS injections
Accreditation and Designation Statement
The RSNA is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to provide continuing medical education for physicians. The RSNA designates this journal-based SA-CME activity for a maximum of 1.0 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Disclosure Statement
The ACCME requires that the RSNA, as an accredited provider of CME, obtain signed disclosure statements from the authors, editors, and reviewers for this activity. For this journal-based CME activity, author disclosures are listed at the end of this article.
Summary
Intra-articular corticosteroid (IACS) injections have been associated with specific structural joint damage that may or may not be related to steroid treatment. There is a need for research to determine whether there is a risk for damage to joints as the result of IACS injections.
Essentials
■ Intra-articular corticosteroid (IACS) injections are recommended for pain management of hip and knee osteoarthritis (OA) in patients who have not responded to oral or topical analgesics.
■ Recent case series suggested that negative structural outcomes including accelerated OA progression, subchondral insufficiency fracture, complications of pre-existing osteonecrosis, and rapid joint destruction including bone loss may be observed after IACS injections.
■ To our knowledge, there are no studies with mid- to long-term follow-up available that provide data from before and after IACS injection compared with appropriate control participants.
■ Estimates of the prevalence of IACS injection-induced structural problems are highly variable.
■ As of today, there is no established recommendation or consensus regarding imaging, clinical, or laboratory markers before an IACS injection to screen for OA-related imaging abnormalities.
Introduction
Osteoarthritis (OA) is a highly prevalent, disabling, and costly condition that has been recognized by the United States Food and Drug Administration as a serious disease with unmet medical need (1–3). OA is understood as a disease of the whole joint, involving structural damage of the articular cartilage, subchondral bone, ligaments, capsule, synovial membrane, and periarticular muscles (4,5). Pain is the predominant symptom and is a major driving force in clinical decision making and health service attention (6).
Current management of OA is primarily focused on symptom control. Intra-articular corticosteroid (IACS) injections are often used for pain management of hip and knee OA in patients who have not responded to oral or topical analgesics and are frequently intended to delay total joint replacement surgery if patients are young or hesitant to undergo surgery right away (7). A recent case series presented data on possible negative outcomes of IACS injection as perceived from a radiologic perspective (8). These findings included accelerated OA progression, subchondral insufficiency fracture, complications of pre-existing osteonecrosis, and rapid joint destruction including bone loss. Another recent uncontrolled retrospective observational study that focused on IACS injection of the hip and subsequent joint events reported that almost half of the patients treated with IACS injections exhibited signs of radiographic progression and a minority also developed joint surface collapse (9). To our knowledge, there are no analyses of large observational cohorts or prospective randomized controlled studies with mid- to long-term follow-up available that provide data from before and after IACS injection and compare patients undergoing injection to patients not undergoing injection or undergoing injection with a different agent with similar levels of disease activity and severity. Such a controlled study is required to adequately address confounding by indications. On the basis of the observations available to date, no conclusions can be drawn regarding causality because it is uncertain whether the described findings or events were already present at the time of injection.
The aim of this expert panel report is to review the current understanding of pain in OA, summarize current international guidelines regarding indications for IACS injection, and consider potential preinterventional safety measures, including imaging. The interdisciplinary expert panel was composed of international experts in the field of rheumatology, orthopedics, epidemiology, and radiology who have been actively involved in clinical application and research regarding IACS injection (Appendix E1 [online]). Potential profiles of those who likely will benefit from the intervention and a suggestion for an updated patient consent form are presented. Points to consider regarding future radiologic and clinical research agendas are discussed.
OA Pain
The primary impact of OA is through its contribution to pain, which is often the primary driver for patients seeking medical care. The pain experienced in OA evolves over time with early stages accompanied by intermittent activity-related or weight-bearing pain, whereas later stages are accompanied by more constant pain that may be punctuated by intermittent intense pain (10). A so-called structure-symptom discordance in OA is recognized, wherein some patients with severe knee pain have minimal radiographic abnormalities and vice versa. For example, in the OA Initiative study only 9% of hips in patients with frequent pain showed radiographic hip OA, and 24% of hips with radiographic hip OA were frequently painful (11). Such observations highlight the complex, subjective, and multifactorial nature of pain in OA. If the between-person differences in knee pain are adequately controlled, a strong structure-symptom relationship can be observed (12). MRI data obtained in OA cohorts suggest that bone marrow edema, synovitis, and effusions appear to have the strongest relation to pain in OA (13). These structural pathologic lesions therefore provide rational therapeutic targets for addressing both structural pathology as well as the symptoms of disease. However, the success of treatments targeting these lesions have had mixed results to date and/or only short-term benefit in clinical trials (14–17).
Given the lack of disease-modifying agents to date, treatment guidelines have largely focused on an algorithmic approach to OA pain management. Guidelines typically recommend weight loss; exercise; self-management approaches; and pharmacologic options that include topical nonsteroidal anti-inflammatory drugs, oral therapies (eg, nonsteroidal anti-inflammatory drugs), and intra-articular therapies as appropriate for a patient’s clinical circumstances, comorbidities, and/or contraindications (18–20).
Recent guidelines have recognized the potential utility of providing local therapy, such as topical nonsteroidal anti-inflammatory drugs, preferentially as initial therapy to spare systemic exposure to oral therapies that may have an unfavorable adverse-effect profile or in patients with comorbid conditions where oral nonsteroidal anti-inflammatory drugs may be contraindicated (19,20). Intra-articular therapies in OA have traditionally been reserved for later stages in the treatment algorithm after topical and oral therapies have proven insufficient (18).
Treatment Recommendations
Several professional societies whose members treat patients with OA have formally evaluated IACS injections for inclusion in their OA treatment guidelines. The methods for guideline development differed somewhat by society but started in each case with a systematic review of literature on IACS injections in OA, which provided the foundation for each society’s final recommendation. IACS treatment guidelines of four professional societies—the American Association of Orthopedic Surgeons, American College of Rheumatology, European League Against Rheumatism, and Osteoarthritis Research Society International—are considered here (Fig 1). The American Association of Orthopedic Surgeons and American College of Rheumatology are the preeminent societies for orthopedic and rheumatology professionals, respectively, in the United States and are also recognized globally. The European League Against Rheumatism is the European equivalent of American College of Rheumatology, representing rheumatology professionals throughout Europe, and the Osteoarthritis Research Society International is an internationally recognized OA research organization.
Figure 1:
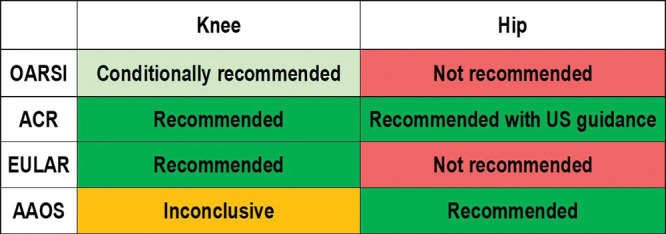
Osteoarthritis Research Society International (OARSI), American College of Rheumatology (ACR), European League Against Rheumatism (EULAR), and the American Association of Orthopedic Surgeons (AAOS) recommendations for intra-articular corticosteroid injections.
Recommendations for Knee OA
All four societies considered IACS injection in the knee in their OA treatment guidelines (18–21). Both the American College of Rheumatology and European League Against Rheumatism recommend IACS injection as treatment of patients with knee OA, citing evidence of short-term pain relief (19–22). The American College of Rheumatology guidelines stipulate that IACS injection should be reserved for those who do not respond to nonsteroidal anti-inflammatory drugs and acetaminophen (19,20). The recommendation from the European League Against Rheumatism is specific to patients with acute pain exacerbations, especially in the context of effusion (21,22). The Osteoarthritis Research Society International guidelines also supported IACS injection for treatment of knee OA through a conditional recommendation for patients with or without comorbid conditions (18). The conditionality of the Osteoarthritis Research Society International recommendation reflects the quality and strength of scientific support in the clinical literature and the potential toxicities of IACS injection. Strength of support for IACS injection was weakest in the recommendations of the American Association of Orthopedic Surgeons, which were inconclusive (23). This position reflects the panel’s finding a lack of compelling evidence for or against IACS injection as treatment for knee OA. The American Association of Orthopedic Surgeons Expert Panel suggests that, in the face of their inconclusive recommendation, clinicians should base treatment decisions on clinical judgement and patient preference (23).
Recommendations for Hip OA
These societies’ stances on IACS injections are less cohesive regarding hip OA. The American College of Rheumatology and American Association of Orthopedic Surgeons recommended IACS injections for treatment of hip OA with the American College of Rheumatology stipulating that US guidance is preferred, whereas European League Against Rheumatism and Osteoarthritis Research Society International did not recommend IACS hip injections (18–20,24,25). The American College of Rheumatology’s strong recommendation in favor of IACS injections is specific to patients who did not respond to nonsteroidal anti-inflammatory drugs (19,20). The American Association of Orthopedic Surgeons recommendation is not contingent on US guidance; however, American Association of Orthopedic Surgeons guidelines specify that IACS injections should only be used for short-term treatment of hip OA (25). In explaining the decision not to recommend IACS hip injections, Osteoarthritis Research Society International guidelines cited insufficient scientific support of their efficacy (18).
Prediction of Treatment Response
IACS injections are used widely for knee OA but in 20%–30% of patients they are ineffective in reducing knee pain, even transiently (26). For hip OA, IACS injections lead to a reduction in pain at 3–4 weeks after injection compared with control patients on average, but the quality of evidence is limited (27,28). Identifying patients in whom treatment is likely to be ineffective would lessen the risk of adverse outcomes and would help avoid treatments that do not work. We will focus on knee OA because there are only sparse data available regarding the hip joint.
Several studies have attempted to identify factors that predict both short- and long-term response to IACS treatment in knee OA. Maricar et al (29) performed a systematic review of these studies that focused on the knee and found that it was challenging to summarize their findings. Studies varied in the following: how they defined response, the time during which they assessed response (short- vs long-term), the treatment used, and the factors evaluated that might predict response. Findings were inconsistent across studies, and many studies were small, which limited their ability to robustly identify predictors. The review suggested that response to IACS injections diminished with increasing severity of radiographic disease and increased with more severe knee pain. Knees without clinical effusions were less likely to respond. Another systematic review of IACS injection for knee or hip OA over the same period was not able to identify strong evidence that any potential predictors were associated with clinical response, including age, body mass index, depression, different corticosteroid preparations, radiographic grade, and clinical or sonographic evidence of inflammation or synovial hypertrophy (30). A meta-analysis (31) of individual patient data on the efficacy of IACS injection for knee or hip OA from seven randomized clinical trials reported that there was a significant positive interaction between severe pain at baseline (ie, ≥70 on a 0–100 scale) and the treatment effect of IACS injection compared with placebo, with a larger reduction in short-term pain (ie, up to 4 weeks). Two additional large studies, each with over 100 patients administered IACS, examined factors affecting response. In one of these studies (32), the rate of response at 3 months varied depending on whether it was assessed as a greater than 50% reduction in the Western Ontario and McMaster Universities OA Index pain scale (only 16% of patients) or as a greater than 50% reduction in visual analog scale pain (39% of patients). Note that the percentage of persons responding to IACS injection was high in the weeks after the injection and falls by 3 months (33). Factors that lowered the likelihood of response at 3 months were worse radiographic severity, limited range of motion, and more tenderness at the knee. In the largest study of factors affecting response to steroids, Maricar et al (26) studied 199 patients who were administered injections and examined their response at 2 weeks and 6 months. Twenty-seven percent of patients had no Outcome Measures in Rheumatology–Osteoarthritis Research Society International response at 2 weeks. Those with a lower likelihood of even short-term response included persons with no joint line tenderness and those with a history of ligament or meniscus injury. Twenty percent were characterized as longer-term responders. Factors associated with lack of long-term response were chronic widespread pain and depressive symptoms, highlighting the need for understanding mechanisms underlying an individual’s pain experience to tailor the appropriate therapy toward those mechanisms. In a study that examined the relation of synovial fluid white blood cell count to pain reduction after IACS injection, compared with those with a white blood cell count of less than or equal to 100 cells/mm3 (referent), a greater within-person reduction in Knee injury and OA Outcome Score was observed in those with a white blood cell count between 101 and 250 cells/mm3 (β coefficient, 0.279, unstandardized coefficient of 11.1; 95% confidence interval: 0.03, 22.17; P = .049) and 251–1000 cells/mm3 (β coefficient, 0.320, unstandardized coefficient of 15.07; 95% confidence interval: 2.06, 28.09; P = .024). That is, lower synovial fluid white blood count (albeit still within the normal range) was associated with a lower likelihood of response to IACS injection (34).
Regarding structural features, higher MRI meniscal damage (odds ratio, 0.74; 95% confidence interval: 0.55, 0.98), increasing Kellgren and Lawrence maximal grade (odds ratio, 0.43; 95% confidence interval: 0.23, 0.82), and joint space narrowing maximal score (odds ratio, 0.60; 95% confidence interval: 0.36, 0.99) were each associated with a lower odds of longer term (∼6 months) responder status (35). Because the effects of IACS injections generally last less than 3 months, analyses of 6 months outcome are likely examining the factors predicting OA pain rather than response to IACS injections. One study suggested that obese patients (body mass index ≥ 30 kg/m2) may be less likely to experience symptom relief with IACS injection (36). In patients with a clinically apparent knee effusion and moderate knee pain, there was a significant association between larger synovitis volume on contrast material–enhanced MRI and greater pain relief, reflected in Knee Injury and OA Outcome Score pain subscale after IACS injection, although synovial tissue volume accounted for only a small proportion of the variance in change in pain (37).
One reason for inefficacy of steroid injections may be that some injections are inaccurately placed and outside the joint. Two reviews reported that US-guided IACS injections had superior efficacy to anatomic landmark-guided injections for accuracy of the injection placement and symptom relief (38,39). The accuracy of injection ranged from 63% to 100% with US, whereas the accuracy ranged from 39% to 100% with injections that were not guided with imaging (38,39). Accuracy was 95.8% with US guidance versus 77.8% without US guidance (39).
In summary, patients with greater pain at baseline, less severe radiographic disease, and clinically apparent knee effusion may be more likely to have symptom relief. In addition, the use of image-guided injection may increase the likelihood of a treatment response to IACS injection.
Minimizing Potential Adverse Events on the Basis of Imaging
Four radiologic entities have been reported after IACS injections of the knees and hips with a combined frequency of 8% in a recent noncontrolled case series of 459 IACS injections of the knee or hip (8,9), as follows: (a) rapid progressive OA (also termed accelerated OA) type 1 is defined on the basis of rapid loss of joint space on radiographs beyond the expected rate (ie, joint space loss of > 2 mm within a 12-month period [40]); (b) rapid progressive OA type 2 is defined as rapid articular destruction with accelerated bone loss not typically observed in patients with OA in a 12-month period (41); (c) complication of osteonecrosis, which is associated with subchondral femoral bone plate collapse and development of secondary OA; and (d) subchondral insufficiency fracture of the knee and hip, occult on radiographs at early stages and only recognized on MRI scans, which shows a subchondral hypointense fracture line of varying thickness and extent. A threshold of 4-mm thickness and/or 14 mm or more maximum length of the subchondral hypointense fracture line are considered relevant to define a negative outcome (ie, joint collapse) (42). There is an associated marked surrounding bone marrow edema that is more intense than would be expected for typical OA (8). Figures 2–5 show image examples of these radiologic entities from patients with OA who were referred to radiology for IACS injections. To our knowledge, it is not known whether these findings predated the IACS or were somehow induced by the IACS injection.
Figure 2a:
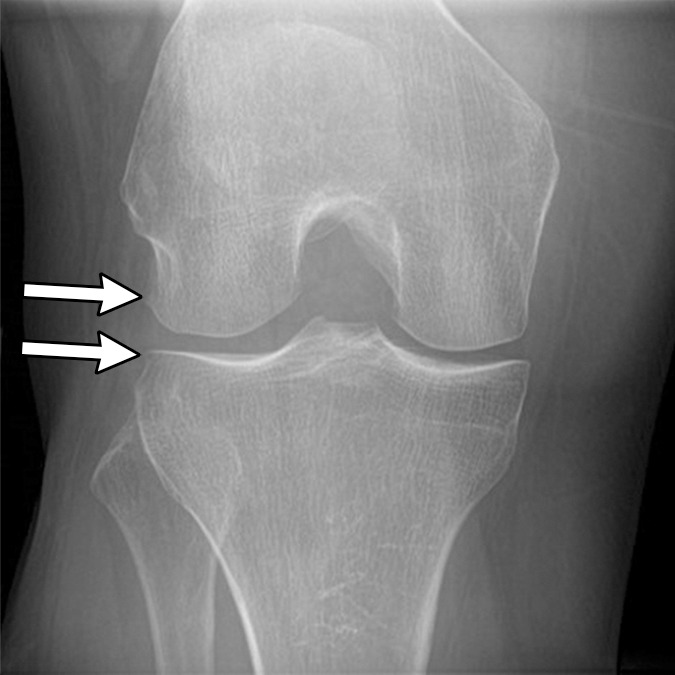
Subchondral insufficiency fracture in a 61-year-old woman with severe knee pain unrelated to trauma referred to radiology for intra-articular corticosteroid (IACS) injection. (a) Anteroposterior radiograph of the right knee obtained the day of the IACS injection shows mild osteoarthritis (OA) with small osteophytes of the lateral tibia and femur (arrows) and no joint space narrowing. (b) Coronal fat-suppressed proton density-weighted MRI performed 1 month after the IACS injection shows subchondral insufficiency fracture (arrow) with extensive bone marrow edema of the lateral femoral condyle (*) and adjacent soft tissue edema. There is also a severe lateral meniscus extrusion (arrowhead). (c) Repeat radiograph of the right knee 3 months later shows the subchondral insufficiency fracture with collapse of the articular contour of the lateral femoral condyle (arrow) surrounded by bone sclerosis (*) and lateral tibiofemoral joint space narrowing (arrowhead) likely secondary to the severe lateral meniscal subluxation. A normal or mild OA baseline radiograph in a patient with severe joint pain as in this case should trigger a preprocedural MRI to depict occult findings of clinical relevance such as subchondral insufficiency fracture.
Figure 5a:
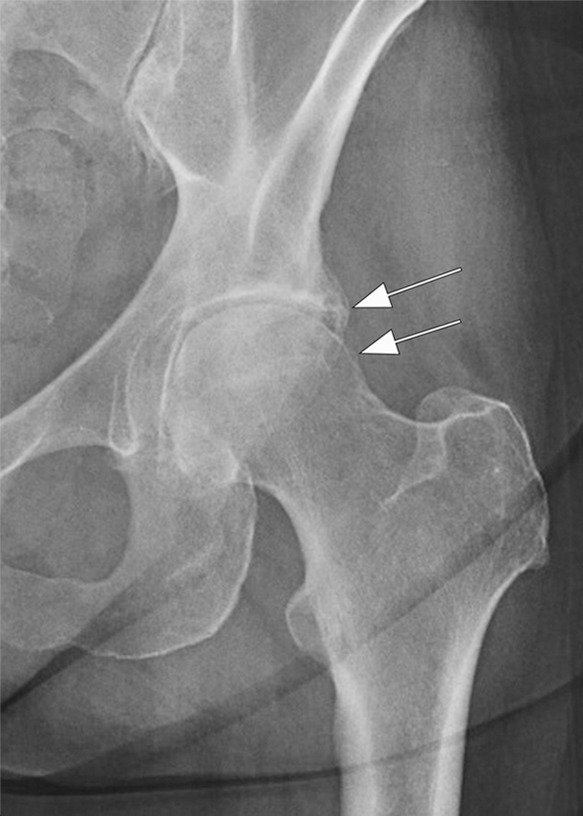
Rapid progressive osteoarthritis type 2 in a 38-year-old woman referred to radiology for intra-articular corticosteroid (IACS) injection. (a) Baseline anteroposterior radiograph of the left hip shows mild osteoarthritis with small definite osteophytes at the lateral acetabulum and femoral head (arrows) and no definite joint space narrowing. (b) Six months after the IACS injection a repeat anteroposterior radiograph of the left hip shows a complete collapse of the head of the femur with marked bone loss of the femoral head (arrow) and surface remodeling and flattening of the acetabulum (arrowheads). (c) Coronal fat-suppressed proton density-weighted MRI scan obtained on the same day demonstrates diffuse bone marrow edema of the femur and acetabulum (black and white *) and a large hip joint effusion (#) reflecting an ongoing process of marked synovial activation.
Figure 2b:
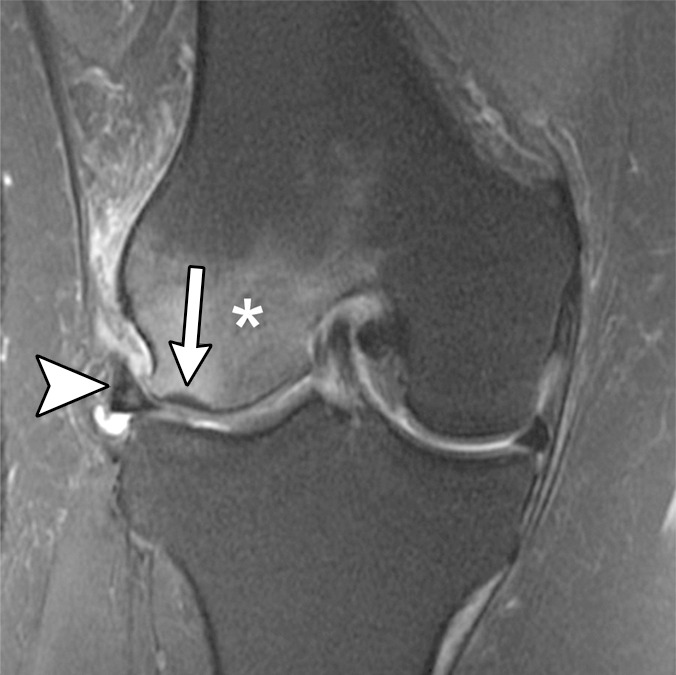
Subchondral insufficiency fracture in a 61-year-old woman with severe knee pain unrelated to trauma referred to radiology for intra-articular corticosteroid (IACS) injection. (a) Anteroposterior radiograph of the right knee obtained the day of the IACS injection shows mild osteoarthritis (OA) with small osteophytes of the lateral tibia and femur (arrows) and no joint space narrowing. (b) Coronal fat-suppressed proton density-weighted MRI performed 1 month after the IACS injection shows subchondral insufficiency fracture (arrow) with extensive bone marrow edema of the lateral femoral condyle (*) and adjacent soft tissue edema. There is also a severe lateral meniscus extrusion (arrowhead). (c) Repeat radiograph of the right knee 3 months later shows the subchondral insufficiency fracture with collapse of the articular contour of the lateral femoral condyle (arrow) surrounded by bone sclerosis (*) and lateral tibiofemoral joint space narrowing (arrowhead) likely secondary to the severe lateral meniscal subluxation. A normal or mild OA baseline radiograph in a patient with severe joint pain as in this case should trigger a preprocedural MRI to depict occult findings of clinical relevance such as subchondral insufficiency fracture.
Figure 2c:
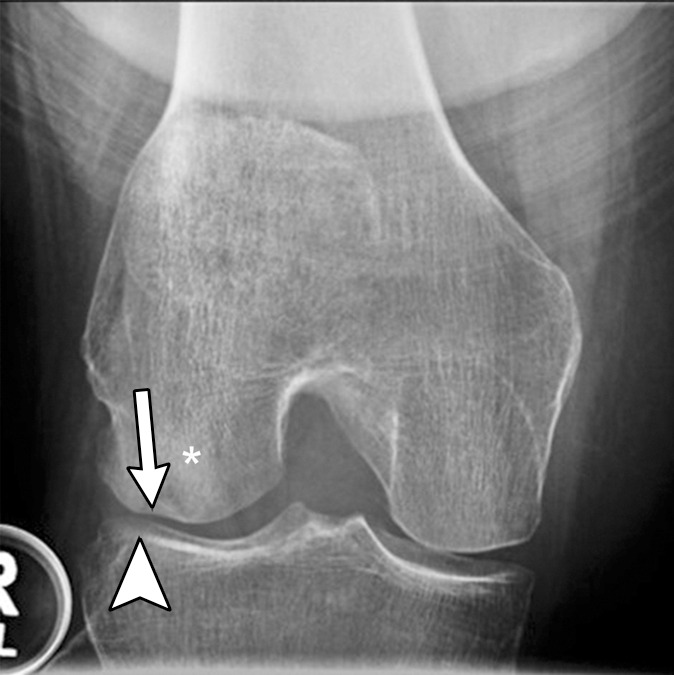
Subchondral insufficiency fracture in a 61-year-old woman with severe knee pain unrelated to trauma referred to radiology for intra-articular corticosteroid (IACS) injection. (a) Anteroposterior radiograph of the right knee obtained the day of the IACS injection shows mild osteoarthritis (OA) with small osteophytes of the lateral tibia and femur (arrows) and no joint space narrowing. (b) Coronal fat-suppressed proton density-weighted MRI performed 1 month after the IACS injection shows subchondral insufficiency fracture (arrow) with extensive bone marrow edema of the lateral femoral condyle (*) and adjacent soft tissue edema. There is also a severe lateral meniscus extrusion (arrowhead). (c) Repeat radiograph of the right knee 3 months later shows the subchondral insufficiency fracture with collapse of the articular contour of the lateral femoral condyle (arrow) surrounded by bone sclerosis (*) and lateral tibiofemoral joint space narrowing (arrowhead) likely secondary to the severe lateral meniscal subluxation. A normal or mild OA baseline radiograph in a patient with severe joint pain as in this case should trigger a preprocedural MRI to depict occult findings of clinical relevance such as subchondral insufficiency fracture.
Figure 3a:
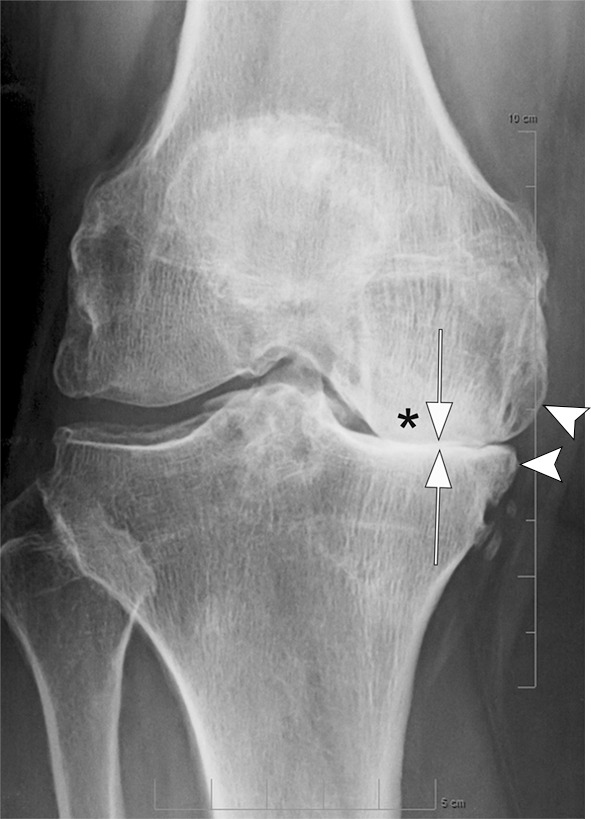
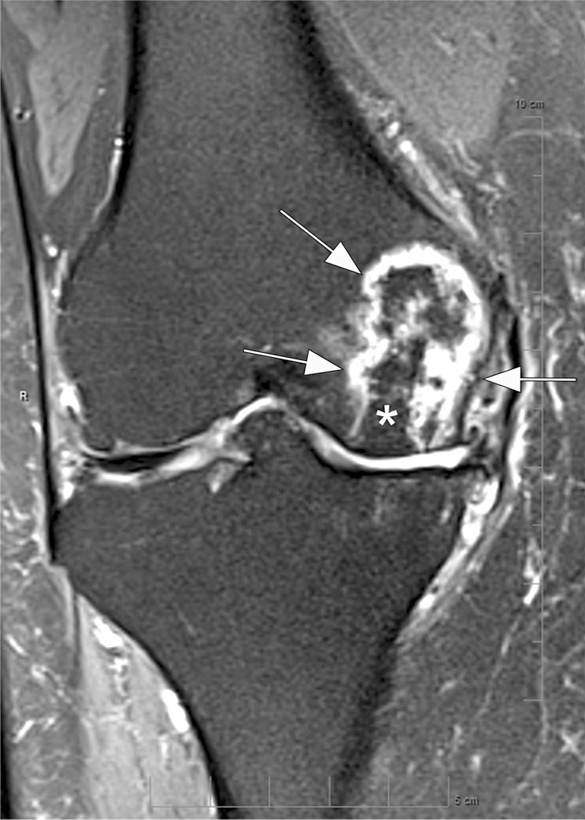
Osteonecrosis in a 59-year-old man referred to radiology for intra-articular corticosteroid (IACS) injection. (a) Anteroposterior radiograph of the right knee shows severe osteoarthritis with bone on bone appearance (arrows) and large definite osteophytes (arrowheads). There is a subchondral sclerosis of the medial femoral condyle (*), which is expected in advanced osteoarthritis. No sign of subchondral insufficiency fracture or osteonecrosis. Patient was experiencing an acute episode of pain exacerbation at time of presentation. (b) Coronal fat-suppressed intermediate-weighted MRI was performed before the IACS injection and discloses a large area of osteonecrosis of the medial femoral condyle (arrows) with pathognomonic serpiginous demarcation and fat-equivalent center of lesion (*). No collapse of the articular contour is seen. There is attrition (ie, surface remodeling) as part of the advanced osteoarthritis process. IACS injection was not performed.
Figure 3b:
Osteonecrosis in a 59-year-old man referred to radiology for intra-articular corticosteroid (IACS) injection. (a) Anteroposterior radiograph of the right knee shows severe osteoarthritis with bone on bone appearance (arrows) and large definite osteophytes (arrowheads). There is a subchondral sclerosis of the medial femoral condyle (*), which is expected in advanced osteoarthritis. No sign of subchondral insufficiency fracture or osteonecrosis. Patient was experiencing an acute episode of pain exacerbation at time of presentation. (b) Coronal fat-suppressed intermediate-weighted MRI was performed before the IACS injection and discloses a large area of osteonecrosis of the medial femoral condyle (arrows) with pathognomonic serpiginous demarcation and fat-equivalent center of lesion (*). No collapse of the articular contour is seen. There is attrition (ie, surface remodeling) as part of the advanced osteoarthritis process. IACS injection was not performed.
Figure 4a:
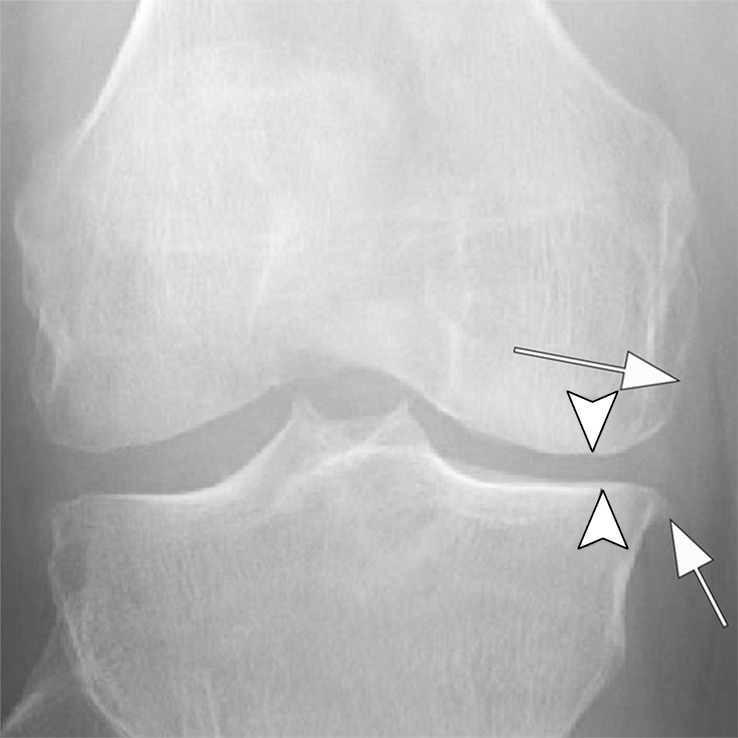
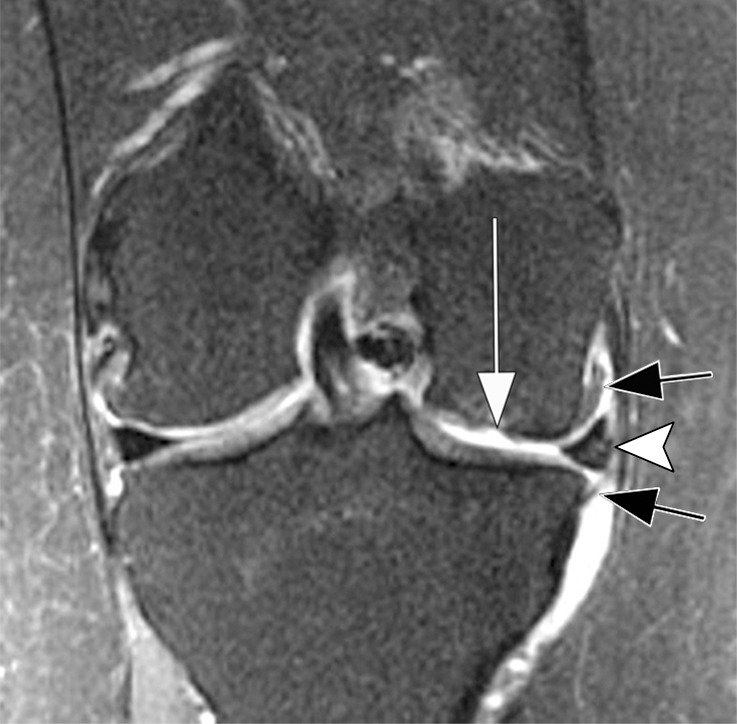
Rapid progressive osteoarthritis (RPOA) type 1 in a 52-year-old man referred to radiology for intra-articular corticosteroid (IACS) injection. (a) Anteroposterior radiograph of the right knee shows mild osteoarthritis with definite osteophytes (arrows) and minimal joint space narrowing of the medial tibiofemoral joint (arrowheads). (b) Baseline coronal fat-suppressed intermediate-weighted MRI scan confirms the osteophytes (black arrows) and shows diffuse cartilage loss at the medial femoral condyle (white arrow) with moderate subluxation of the medial meniscus (arrowhead). (c) Six months after the IACS injection, a repeat anteroposterior radiograph of the right knee shows severe medial tibiofemoral joint space narrowing (arrows) with loss of more than 2 mm of joint space width consistent with rapid progressive osteoarthritis type 1. (d) Coronal fat-suppressed intermediate-weighted MRI scan confirms extensive loss of cartilage at the medial femur and tibia (white arrows) and worsening of the medial meniscal subluxation (arrowhead). There is also subchondral bone marrow edema at the medial tibia and femur (black arrows).
Figure 4b:
Rapid progressive osteoarthritis (RPOA) type 1 in a 52-year-old man referred to radiology for intra-articular corticosteroid (IACS) injection. (a) Anteroposterior radiograph of the right knee shows mild osteoarthritis with definite osteophytes (arrows) and minimal joint space narrowing of the medial tibiofemoral joint (arrowheads). (b) Baseline coronal fat-suppressed intermediate-weighted MRI scan confirms the osteophytes (black arrows) and shows diffuse cartilage loss at the medial femoral condyle (white arrow) with moderate subluxation of the medial meniscus (arrowhead). (c) Six months after the IACS injection, a repeat anteroposterior radiograph of the right knee shows severe medial tibiofemoral joint space narrowing (arrows) with loss of more than 2 mm of joint space width consistent with rapid progressive osteoarthritis type 1. (d) Coronal fat-suppressed intermediate-weighted MRI scan confirms extensive loss of cartilage at the medial femur and tibia (white arrows) and worsening of the medial meniscal subluxation (arrowhead). There is also subchondral bone marrow edema at the medial tibia and femur (black arrows).
Figure 4c:
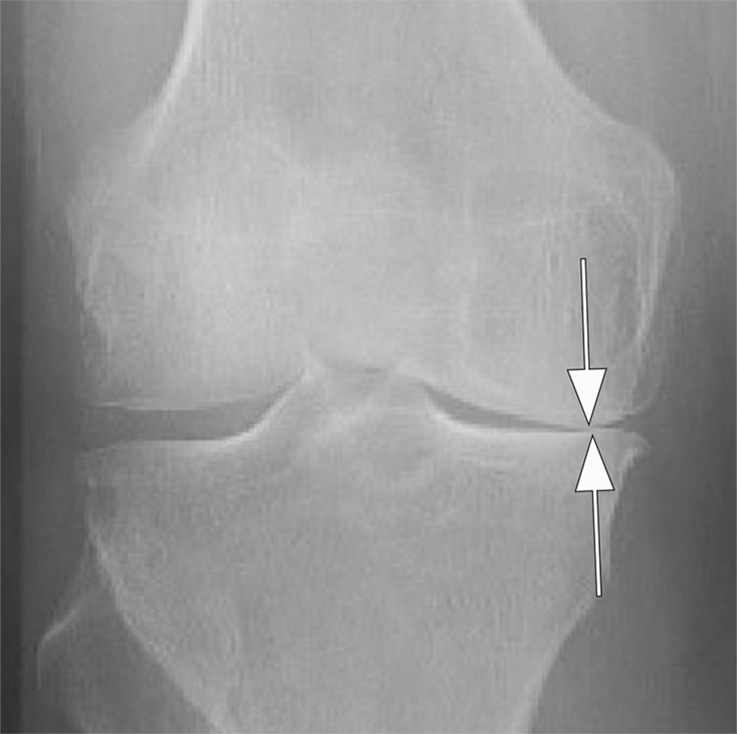
Rapid progressive osteoarthritis (RPOA) type 1 in a 52-year-old man referred to radiology for intra-articular corticosteroid (IACS) injection. (a) Anteroposterior radiograph of the right knee shows mild osteoarthritis with definite osteophytes (arrows) and minimal joint space narrowing of the medial tibiofemoral joint (arrowheads). (b) Baseline coronal fat-suppressed intermediate-weighted MRI scan confirms the osteophytes (black arrows) and shows diffuse cartilage loss at the medial femoral condyle (white arrow) with moderate subluxation of the medial meniscus (arrowhead). (c) Six months after the IACS injection, a repeat anteroposterior radiograph of the right knee shows severe medial tibiofemoral joint space narrowing (arrows) with loss of more than 2 mm of joint space width consistent with rapid progressive osteoarthritis type 1. (d) Coronal fat-suppressed intermediate-weighted MRI scan confirms extensive loss of cartilage at the medial femur and tibia (white arrows) and worsening of the medial meniscal subluxation (arrowhead). There is also subchondral bone marrow edema at the medial tibia and femur (black arrows).
Figure 4d:
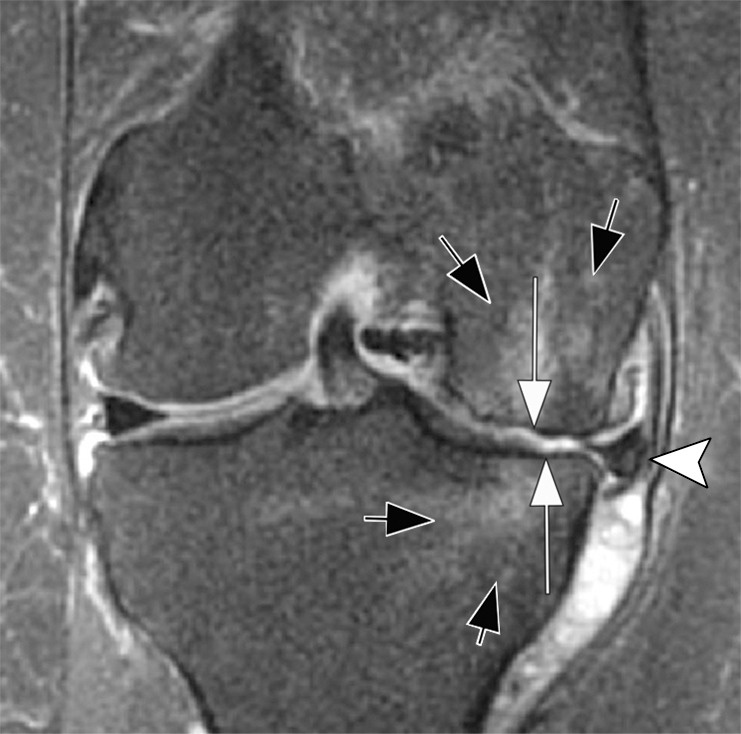
Rapid progressive osteoarthritis (RPOA) type 1 in a 52-year-old man referred to radiology for intra-articular corticosteroid (IACS) injection. (a) Anteroposterior radiograph of the right knee shows mild osteoarthritis with definite osteophytes (arrows) and minimal joint space narrowing of the medial tibiofemoral joint (arrowheads). (b) Baseline coronal fat-suppressed intermediate-weighted MRI scan confirms the osteophytes (black arrows) and shows diffuse cartilage loss at the medial femoral condyle (white arrow) with moderate subluxation of the medial meniscus (arrowhead). (c) Six months after the IACS injection, a repeat anteroposterior radiograph of the right knee shows severe medial tibiofemoral joint space narrowing (arrows) with loss of more than 2 mm of joint space width consistent with rapid progressive osteoarthritis type 1. (d) Coronal fat-suppressed intermediate-weighted MRI scan confirms extensive loss of cartilage at the medial femur and tibia (white arrows) and worsening of the medial meniscal subluxation (arrowhead). There is also subchondral bone marrow edema at the medial tibia and femur (black arrows).
Figure 5b:
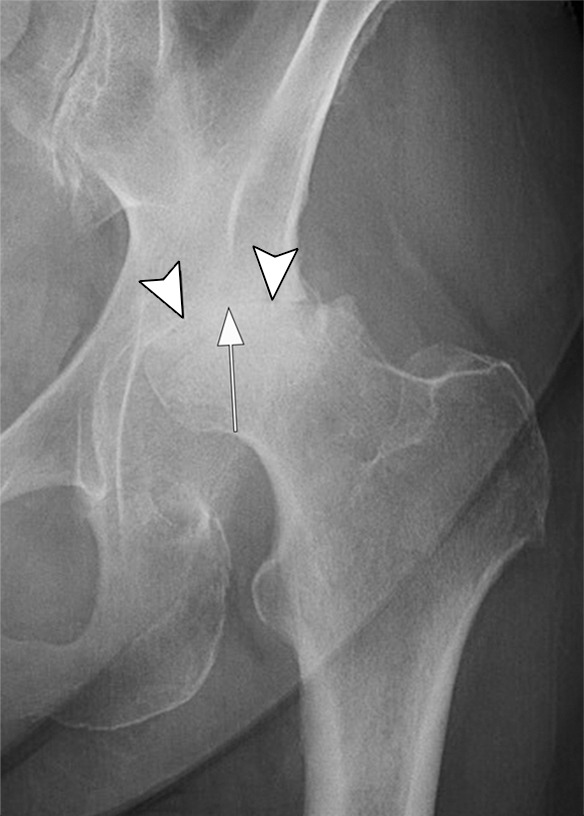
Rapid progressive osteoarthritis type 2 in a 38-year-old woman referred to radiology for intra-articular corticosteroid (IACS) injection. (a) Baseline anteroposterior radiograph of the left hip shows mild osteoarthritis with small definite osteophytes at the lateral acetabulum and femoral head (arrows) and no definite joint space narrowing. (b) Six months after the IACS injection a repeat anteroposterior radiograph of the left hip shows a complete collapse of the head of the femur with marked bone loss of the femoral head (arrow) and surface remodeling and flattening of the acetabulum (arrowheads). (c) Coronal fat-suppressed proton density-weighted MRI scan obtained on the same day demonstrates diffuse bone marrow edema of the femur and acetabulum (black and white *) and a large hip joint effusion (#) reflecting an ongoing process of marked synovial activation.
Figure 5c:
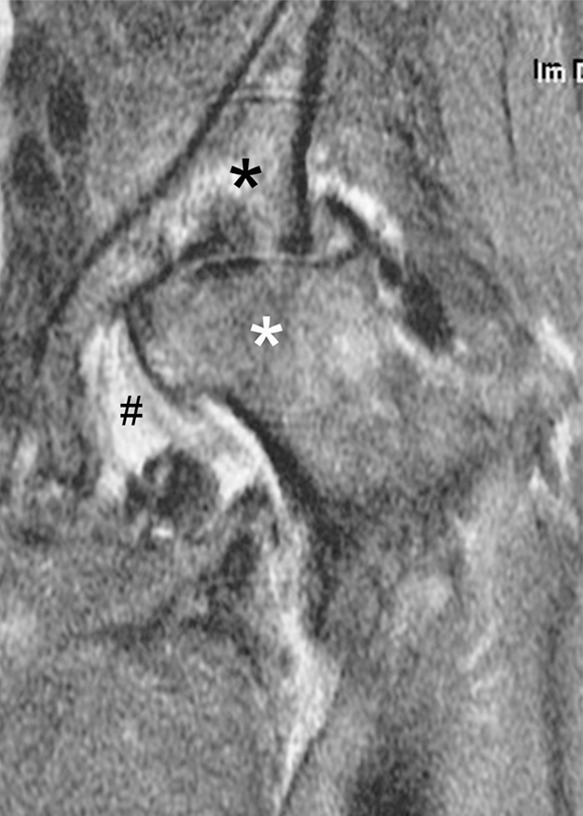
Rapid progressive osteoarthritis type 2 in a 38-year-old woman referred to radiology for intra-articular corticosteroid (IACS) injection. (a) Baseline anteroposterior radiograph of the left hip shows mild osteoarthritis with small definite osteophytes at the lateral acetabulum and femoral head (arrows) and no definite joint space narrowing. (b) Six months after the IACS injection a repeat anteroposterior radiograph of the left hip shows a complete collapse of the head of the femur with marked bone loss of the femoral head (arrow) and surface remodeling and flattening of the acetabulum (arrowheads). (c) Coronal fat-suppressed proton density-weighted MRI scan obtained on the same day demonstrates diffuse bone marrow edema of the femur and acetabulum (black and white *) and a large hip joint effusion (#) reflecting an ongoing process of marked synovial activation.
Given these four putative negative structural outcomes that have been reported in the context of IACS injections, the question arises regarding whether preinterventional imaging is able to reduce the occurrence of such events. By identifying these adverse joint findings, one could avoid administering IACS injection to a joint that may already be at risk for further deterioration. Currently, to our knowledge, there is no recommendation for imaging before administering IACS injection to detect such entities that may be regarded as so-called at-risk findings before the intervention. Subchondral insufficiency fracture and osteonecrosis are only rarely detectable on a radiograph, and the imaging findings are usually subtle or even occult, especially early in the process of these diseases. Administering an IACS injection in the presence of a subchondral insufficiency fracture may result in marked decrease in pain, and potentially result in increased weight bearing. As a consequence, the subchondral insufficiency fracture may progress to an osteochondral defect or frank articular joint collapse. If the underlying cause of pain is not known and patients who are referred for IACS injection exhibit no or only mild OA on a radiograph, the indication for IACS injection should be closely scrutinized as the cause of pain may be a process other than OA. Patients with a diagnosis of already collapsed osteonecrosis could likely be considered candidates for IACS injection, given that joint replacement would be their only other treatment option to relieve pain and no further damage to the joint is expected. A patient with femoral head osteonecrosis without collapse, however, may be at potential risk for collapse, which should be explained and carefully considered before intervention (Fig 6). We acknowledge that the evidence supporting such aggravation is anecdotal. Further, these types of injuries of the hip have been reported at least in case series in the literature, whereas virtually no such reports exist regarding the knee. Thus, observations from the hip should not be extrapolated to the knee.
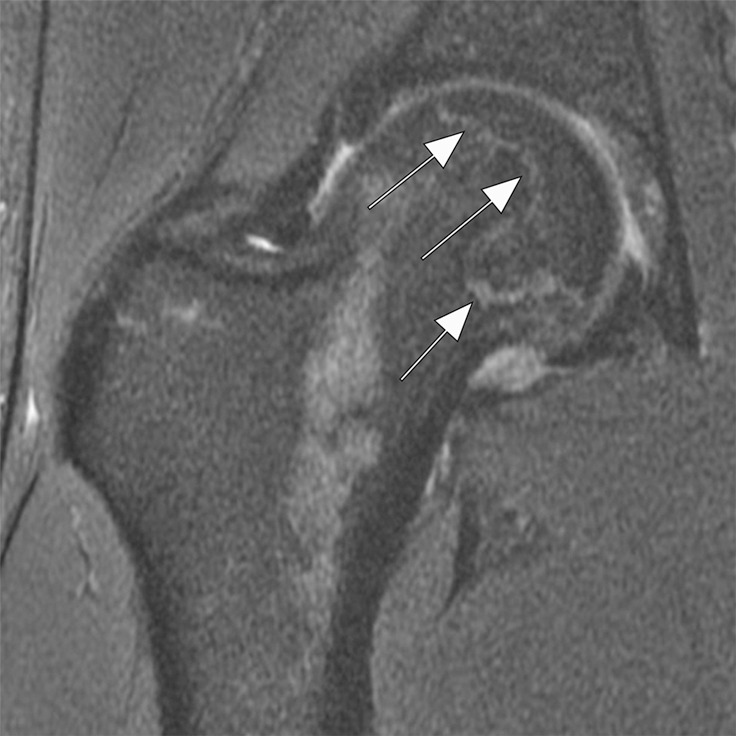
Figure 6a:
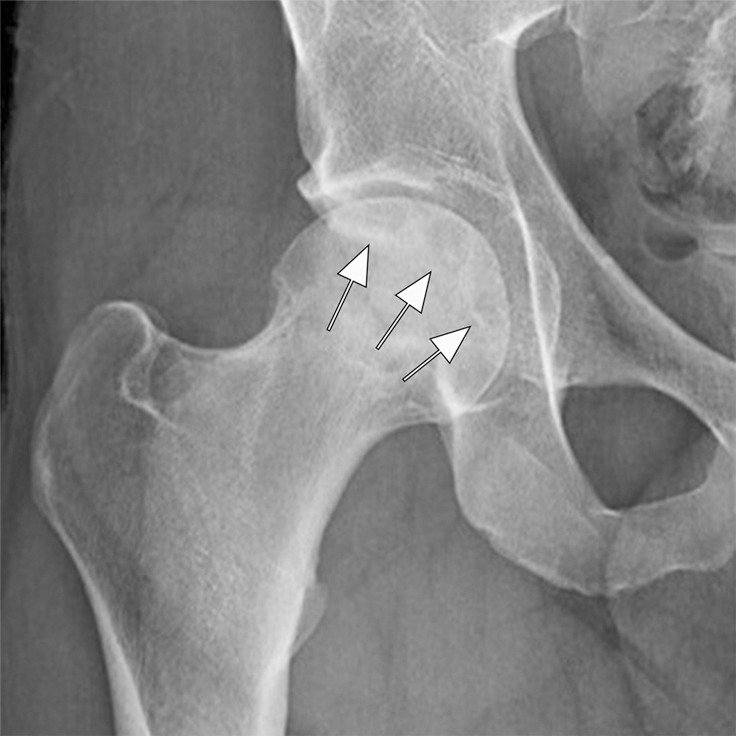
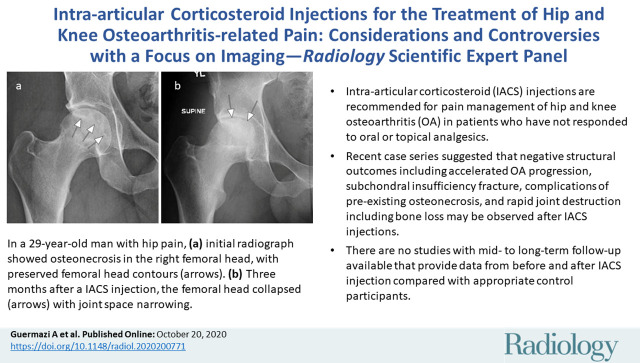
Osteonecrosis in a 29-year-old man who presented with right hip pain. (a) Anteroposterior radiograph and (b) coronal fat-suppressed proton density-weighted MRI of the right hip obtained on the same day show osteonecrosis in the right femoral head, with preserved femoral head contours (arrows). He subsequently went to the sports medicine clinic and was administered a right hip joint intra-articular corticosteroid (IACS) injection for pain. Three months later, he was referred to our institution for repeat IACS injection due to worsening pain. (c) Repeat anteroposterior right hip radiograph shows collapse of the superior femoral head articular surface (arrows). (d) Coronal reformatted CT image of the right hip confirms the collapse of the superior femoral head articular surface (arrows) and shows new hip joint space narrowing. Patient subsequently underwent right hip joint replacement.
Figure 6b:
Osteonecrosis in a 29-year-old man who presented with right hip pain. (a) Anteroposterior radiograph and (b) coronal fat-suppressed proton density-weighted MRI of the right hip obtained on the same day show osteonecrosis in the right femoral head, with preserved femoral head contours (arrows). He subsequently went to the sports medicine clinic and was administered a right hip joint intra-articular corticosteroid (IACS) injection for pain. Three months later, he was referred to our institution for repeat IACS injection due to worsening pain. (c) Repeat anteroposterior right hip radiograph shows collapse of the superior femoral head articular surface (arrows). (d) Coronal reformatted CT image of the right hip confirms the collapse of the superior femoral head articular surface (arrows) and shows new hip joint space narrowing. Patient subsequently underwent right hip joint replacement.
Figure 6c:
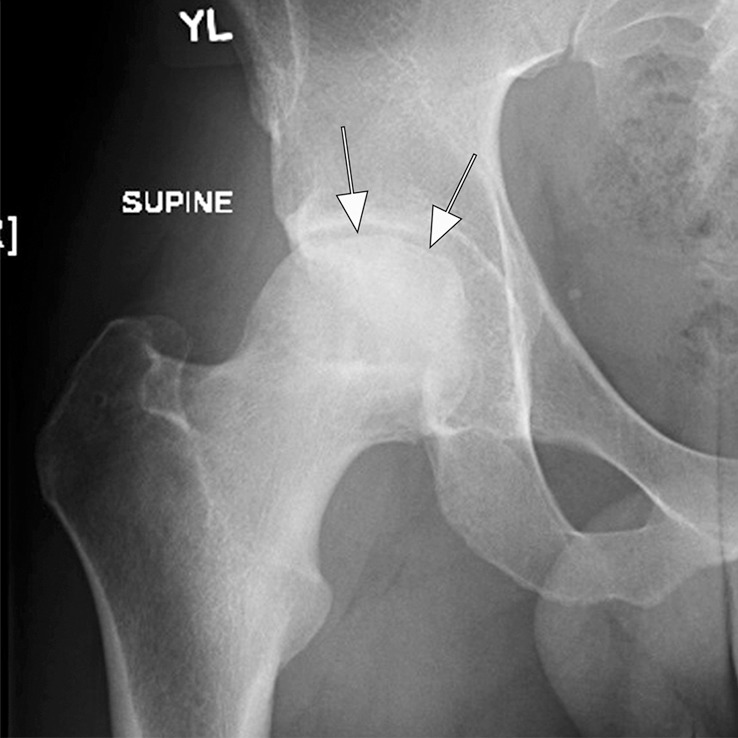
Osteonecrosis in a 29-year-old man who presented with right hip pain. (a) Anteroposterior radiograph and (b) coronal fat-suppressed proton density-weighted MRI of the right hip obtained on the same day show osteonecrosis in the right femoral head, with preserved femoral head contours (arrows). He subsequently went to the sports medicine clinic and was administered a right hip joint intra-articular corticosteroid (IACS) injection for pain. Three months later, he was referred to our institution for repeat IACS injection due to worsening pain. (c) Repeat anteroposterior right hip radiograph shows collapse of the superior femoral head articular surface (arrows). (d) Coronal reformatted CT image of the right hip confirms the collapse of the superior femoral head articular surface (arrows) and shows new hip joint space narrowing. Patient subsequently underwent right hip joint replacement.
Figure 6d:
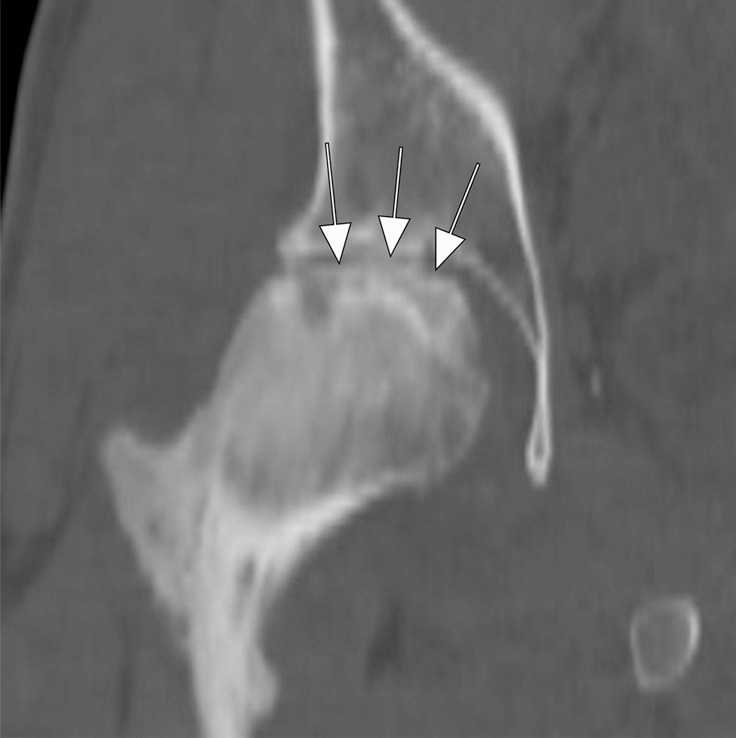
Osteonecrosis in a 29-year-old man who presented with right hip pain. (a) Anteroposterior radiograph and (b) coronal fat-suppressed proton density-weighted MRI of the right hip obtained on the same day show osteonecrosis in the right femoral head, with preserved femoral head contours (arrows). He subsequently went to the sports medicine clinic and was administered a right hip joint intra-articular corticosteroid (IACS) injection for pain. Three months later, he was referred to our institution for repeat IACS injection due to worsening pain. (c) Repeat anteroposterior right hip radiograph shows collapse of the superior femoral head articular surface (arrows). (d) Coronal reformatted CT image of the right hip confirms the collapse of the superior femoral head articular surface (arrows) and shows new hip joint space narrowing. Patient subsequently underwent right hip joint replacement.
In summary, certain patient characteristics, including but not limited to, acute change in pain not explained by a radiograph and no or only mild OA on a weight-bearing radiograph, should lead to careful reconsideration of planned IACS injection. In these circumstances, an MRI may be helpful to further evaluate the actual cause of pain (eg, subchondral insufficiency fracture, osteonecrosis, transient migratory osteoporosis, or occult stress fracture or reaction). However, if these are rare occurrences, such a strategy would be inefficient and cost prohibitive because screening vast numbers before IACS injection at MRI would be infeasible. Further, whether other strategies, such as counseling regarding weight bearing and/or physical therapy to minimize abnormal load through the joint postprocedure may help mitigate any risk is, to our knowledge, not known.
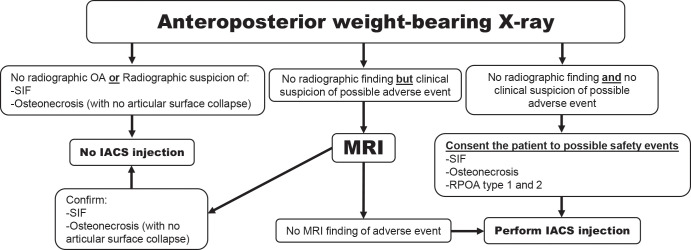
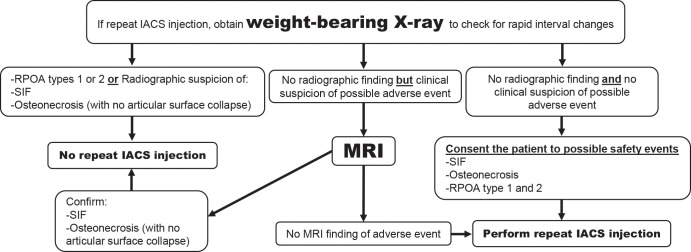
In Figure 7 the radiologist authors present a pragmatic preprocedural imaging algorithm from a radiologic perspective that may help recognize and potentially reduce adverse outcomes after IACS injection. We acknowledge that obtaining repeated radiographs before repeated IACS injections was not supported by the participating orthopedist and rheumatologists of this panel. Whether these concerns are limited to observations related to IACS injections only, or more broadly to other agents, such as intra-articular injection of hyaluronic acid, is not clear.
Figure 7a:
Suggestion of the use of imaging in the context of intra-articular corticosteroid (IACS) injection (to be tested for efficacy and cost-effectiveness). (a) First IACS injection and (b) repeat IACS injection. Obtaining weight-bearing imaging prior to repeat IACS injection is not supported by rheumatologists and orthopedic surgeons on the panel. OA = osteoarthritis, SIF = subchondral insufficiency fracture, RPOA = rapid progressive OA.
Figure 7b:
Suggestion of the use of imaging in the context of intra-articular corticosteroid (IACS) injection (to be tested for efficacy and cost-effectiveness). (a) First IACS injection and (b) repeat IACS injection. Obtaining weight-bearing imaging prior to repeat IACS injection is not supported by rheumatologists and orthopedic surgeons on the panel. OA = osteoarthritis, SIF = subchondral insufficiency fracture, RPOA = rapid progressive OA.
What Do We Need to Include in the Patient Consent on the Basis of What We Know?
As of now, clinicians typically tell the patients, “even if IACS injection does not help you with symptomatic relief, at least it will not harm you.” On the basis of clinical observations, this may not be true for some patients. In addition to standard risks for short-term (or early) complications (eg, bleeding, infection, damage to intra-articular and periarticular structures along the needle path, joint pain, swelling, and stiffness) we also need to explain to the patients that possible longer-term (or delayed) events may occur that may or may not be related to the actual IACS injection. Such complications may include, but are not limited to, accelerated OA progression (ie, rapid progressive OA type 1), subchondral insufficiency fracture leading potentially to articular collapse, osteonecrosis (also leading to articular collapse), and rapid joint destruction with bone loss (ie, rapid progressive OA type 2). According to a single institution report, these joint events can occur in up to 10% of patients after IACS injection in the hip and to a lesser extent in the knee joint (8). Whether these findings were apparent or already ongoing preinjection is not known. Thus, in addition to explaining the above potential risks at the time of informed consent, it will also be important to advise the patient that should they experience worsening joint pain after IACS injection, they should immediately seek clinical attention and potentially also imaging follow-up.
Future Research Agenda
Given the paucity of effective and tolerable analgesic therapies for OA, we need to optimize the usefulness of IACS injections while minimizing potential toxicity. This prompts a number of questions about this commonly used therapy: How effective are the IACS injections? What frequency should they be administered and for how long? What patient factors predict efficacy? What type of risks are associated with IACS injections and how likely are they? Who is at greatest risk for possible safety events? Can risk be attenuated with pre-IACS injection imaging or radiographs? Are there any other means of risk mitigation, such as through optimizing joint biomechanics or minimizing weight bearing after treatment? Are there potential risks with other intra-articular injections? Are there potential risks with joint pain relief more generally? Any potential studies addressing these questions will need large numbers and long duration.
Vast clinical experience of people who are administered repeat injections into a given joint do not suggest that immediate structural deleterious effects are common and worsening of symptoms in a short time period is also uncommon. The trial by McAlindon et al (17) and the multiple trials of extended release triamcinolone for knee OA support these statements. In a matched-cohort study embedded in the larger OA Initiative study, following knee OA patients over 4 years, rapid progressive OA and osteonecrosis were not reported (43). It is possible that some people presenting with apparent OA knee pain have a subchondral insufficiency fracture as the cause of their pain. These lesions may represent part of the structural spectrum of OA pathology that includes bone marrow edema (and may be more common than currently understood to be). Currently we believe many subchondral insufficiency fractures heal over time with non-or protected weight-bearing for at least 6 weeks (8). It is unclear if IACS injection interferes with healing, or if it helps the pain of subchondral insufficiency fracture without negative effects on fracture healing.
In terms of efficacy, we need to understand the benefits of repeat injections. There is a question as to whether IACS injections lose their efficacy over time (17,33). The trial by McAlindon and colleagues assessed pain at 3-month intervals (before next injection). Because the analgesic effect of injections tends to last less than 3 months, this trial cannot determine if patients experienced similar pain relief after multiple injections (17,33). An observational, two-injection study that used extended-release triamcinolone acetate showed that the second injection lasted as long as the first (44). We will need carefully designed randomized clinical trials to better understand the benefits of multiple injections.
Currently, the estimates on prevalence of IACS injections–induced structural problems are highly variable. The trial by McAlindon et al (17) demonstrated a small effect on cartilage and it is unclear how this translates to clinical end points such as pain severity, functional limitations, or joint replacement. Further, it is unknown how many patients have avoided total knee replacement by judicious use of IACS injections over many years, so long-term studies with sensitive MRI end points are required to determine if effects on pertinent joint tissues, such as cartilage and bone, are related to drug and importantly whether this association is causal or whether there is another underlying causal factor relating to both pain and structural progression.
Should There Be Imaging before Steroid Injections?
IACS injections have been associated with specific structural pathology that may or may not be related to steroid treatment versus pre-existing pathology that caused symptoms leading to IACS injection. To develop a rational clinical policy regarding the advisability of preprocedure radiographs we need to understand the prevalence of these radiographic findings before and after IACS injections compared with persons with similar disease severity who did not undergo an injection or who were administered another injection (eg, hyaluronic acid), whether there are patient factors associated with these findings, and the formal cost effectiveness of screening. These inputs would provide the rationale for a trial of radiographic screening before IACS injections. We recognize that this research agenda will require substantial funding and many years. If these complications are rare, were already present as causes of pain before the injection, or would have occurred irrespective of the intervention, then preinjection imaging would not be needed (45).
Conclusion
In summary, intra-articular corticosteroid (IACS) injection is a commonly performed procedure for pain relief in patients with knee or hip osteoarthritis (OA) in an outpatient setting. To our knowledge, there is no established recommendation or consensus regarding imaging before an IACS injection to screen for OA-related imaging abnormalities. It may be important to identify a subchondral insufficiency fracture before IACS injection because glucocorticoids may inhibit the healing process of such a fracture (46). However, the prevalence of subchondral insufficiency fracture is unknown and, if it is rare, screening for subchondral insufficiency fracture before an IACS injection would likely not be cost effective.
When patients with no radiographic OA or only mild radiographic OA are referred for IACS injection to achieve pain relief, the indication for IACS injection should potentially be questioned. Limited literature evidence has indicated that some patients who show a rapid decrease in joint space width or articular surface destruction with or without bone loss tend to have no radiographic OA or only mild OA at initial presentation, likely because there was an already-ongoing, underlying, non-OA related process present at that time (47).
To conclude, the exact causality and natural history of these articular complications are unclear and warrant further study. To do so, large prospective studies (optimally double-blind clinical trials) evaluating the natural history of accelerated OA or joint destruction in OA and after IACS injections would be ideal. Performing preinjection joint radiographs to identify patients with no or mild radiographic OA may be needed but that depends on the prevalence of these abnormalities and the likelihood of developing adverse outcomes. Additional imaging such as MRI may be helpful to further evaluate the actual cause of pain (especially acute pain) before a planned injection, with the recognition that there is often a structure-symptom discordance that makes it challenging to ascribe pain to particular image-based pathology. Further, as with preprocedural radiographic imaging, if these findings are rare, additional MRI will be prohibitively costly. Providers and patients need to continue engaging in shared decision making regarding IACS injections. To better inform such provider-patient communications and aid treatment decisions, the true prevalence of pathology, their relation to IACS injections or other OA treatments, and best risk mitigation strategies require urgent study.
APPENDIX
Acknowledgments
Acknowledgment
The authors thank Emma E. Williams, BA, Brigham and Women’s Hospital, for editorial assistance.
D.T.F. supported by a grant from the National Institutes of Health (NIH P30 AR072571).
Disclosures of Conflicts of Interest: A.G. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed consultancies from TissueGene, Pfizer, Galapagos, Roche, Merck Serono, AstraZeneca; disclosed stock/stock options in Boston Imaging Core Lab. Other relationships: disclosed no relevant relationships. T.N. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed consultancies from Pfizer/Lilly, Regeneron, EMD Merck Serono, Novartis. Other relationships: disclosed no relevant relationships. J.N.K. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed grants from Samumed, Flexion Therapeutics. Other relationships: disclosed no relevant relationships. C.K.K. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed consultancies from Taiwan Liposome Company, EMD Serono, Thusane, Amzell, Regenron, Express Scripts; grants from EMD Serono, Abbvie; payment for lectures from Prime Education, Focus Medical Communications; payment for development of educational presentations from Focus Medical Communications; and other from Astellas, Kolon Tissue Gene, and Regulus. Other relationships: disclosed no relevant relationships. P.G.C. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed consultancies from AbbVie, BMS, EMD Serono, Flexion Therapeutics, Novartis, Pfizer; disclosed payment for lectures from AbbVie, BMS, Flexion Therapeutics, Novartis, Pfizer, and Samumed. Other relationships: disclosed no relevant relationships. D.T.F. disclosed no relevant relationships. F.W.R. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed stock/stock options in Boston Imaging Core Lab. Other relationships: disclosed no relevant relationships.
Abbreviations:
- IACS
- intra-articular corticosteroid
- OA
- osteoarthritis
References
- 1.Turkiewicz A, Petersson IF, Björk J, et al. Current and future impact of osteoarthritis on health care: a population-based study with projections to year 2032. Osteoarthritis Cartilage 2014;22(11):1826–1832. [DOI] [PubMed] [Google Scholar]
- 2.GBD 2015 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388(10053):1545–1602 [Published correction appears in Lancet 2017;389(10064):e1.]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.U.S. Department of Health and Human Services, Food and Drug Administration . Osteoarthritis: Structural Endpoints for the Development of Drugs. Devices, and Biological Products for Treatment Guidance for Industry. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/osteoarthritis-structural-endpoints-development-drugs. Published 2018. Accessed September 24, 2020. [Google Scholar]
- 4.Guermazi A, Roemer FW, Haugen IK, Crema MD, Hayashi D. MRI-based semiquantitative scoring of joint pathology in osteoarthritis. Nat Rev Rheumatol 2013;9(4):236–251. [DOI] [PubMed] [Google Scholar]
- 5.Guermazi A, Roemer FW, Crema MD, Englund M, Hayashi D. Imaging of non-osteochondral tissues in osteoarthritis. Osteoarthritis Cartilage 2014;22(10):1590–1605. [DOI] [PubMed] [Google Scholar]
- 6.Neogi T. The epidemiology and impact of pain in osteoarthritis. Osteoarthritis Cartilage 2013;21(9):1145–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nelson AE, Allen KD, Golightly YM, Goode AP, Jordan JM. A systematic review of recommendations and guidelines for the management of osteoarthritis: The chronic osteoarthritis management initiative of the U.S. bone and joint initiative. Semin Arthritis Rheum 2014;43(6):701–712. [DOI] [PubMed] [Google Scholar]
- 8.Kompel AJ, Roemer FW, Murakami AM, Diaz LE, Crema MD, Guermazi A. Intra-articular corticosteroid injections in the hip and knee: perhaps not as safe as we thought? Radiology 2019;293(3):656–663. [DOI] [PubMed] [Google Scholar]
- 9.Simeone FJ, Vicentini JRT, Bredella MA, Chang CY. Are patients more likely to have hip osteoarthritis progression and femoral head collapse after hip steroid/anesthetic injections? A retrospective observational study. Skeletal Radiol 2019;48(9):1417–1426. [DOI] [PubMed] [Google Scholar]
- 10.Hawker GA, Davis AM, French MR, et al. Development and preliminary psychometric testing of a new OA pain measure--an OARSI/OMERACT initiative. Osteoarthritis Cartilage 2008;16(4):409–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim C, Nevitt MC, Niu J, et al. Association of hip pain with radiographic evidence of hip osteoarthritis: diagnostic test study. BMJ 2015;351:h5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neogi T, Felson D, Niu J, et al. Association between radiographic features of knee osteoarthritis and pain: results from two cohort studies. BMJ 2009;339:b2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hunter DJ, Guermazi A, Roemer F, Zhang Y, Neogi T. Structural correlates of pain in joints with osteoarthritis. Osteoarthritis Cartilage 2013;21(9):1170–1178. [DOI] [PubMed] [Google Scholar]
- 14.Laslett LL, Doré DA, Quinn SJ, et al. Zoledronic acid reduces knee pain and bone marrow lesions over 1 year: a randomised controlled trial. Ann Rheum Dis 2012;71(8):1322–1328. [DOI] [PubMed] [Google Scholar]
- 15.Callaghan MJ, Parkes MJ, Hutchinson CE, et al. A randomised trial of a brace for patellofemoral osteoarthritis targeting knee pain and bone marrow lesions. Ann Rheum Dis 2015;74(6):1164–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conaghan PG, Hunter DJ, Cohen SB, et al. Effects of a Single Intra-Articular Injection of a Microsphere Formulation of Triamcinolone Acetonide on Knee Osteoarthritis Pain: A Double-Blinded, Randomized, Placebo-Controlled, Multinational Study. J Bone Joint Surg Am 2018;100(8):666–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McAlindon TE, LaValley MP, Harvey WF, et al. Effect of Intra-articular Triamcinolone vs Saline on Knee Cartilage Volume and Pain in Patients With Knee Osteoarthritis: A Randomized Clinical Trial. JAMA 2017;317(19):1967–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bannuru RR, Osani MC, Vaysbrot EE, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage 2019;27(11):1578–1589. [DOI] [PubMed] [Google Scholar]
- 19.Kolasinski SL, Neogi T, Hochberg MC, et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Care Res (Hoboken) 2020;72(2):149–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kolasinski SL, Neogi T, Hochberg MC, et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Rheumatol 2020;72(2):220–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pendleton A, Arden N, Dougados M, et al. EULAR recommendations for the management of knee osteoarthritis: report of a task force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis 2000;59(12):936–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jordan KM, Arden NK, Doherty M, et al. EULAR Recommendations 2003: an evidence based approach to the management of knee osteoarthritis: Report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis 2003;62(12):1145–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jevsevar DS. Treatment of osteoarthritis of the knee: evidence-based guideline, 2nd edition. J Am Acad Orthop Surg 2013;21(9):571–576. [DOI] [PubMed] [Google Scholar]
- 24.Zhang W, Doherty M, Arden N, et al. EULAR evidence based recommendations for the management of hip osteoarthritis: report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis 2005;64(5):669–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.American Academy of Orthopaedic Surgeons . Management of Osteoarthritis of the Hip: Evidence-based Clinical Practice Guideline. https://aaos.org/quality/quality-programs/lower-extremity-programs/osteoarthritis-of-the-hip/. Published 2019. Accessed September 24, 2020. [DOI] [PubMed]
- 26.Maricar N, Parkes MJ, Callaghan MJ, Felson DT, O’Neill TW. Do Clinical Correlates of Knee Osteoarthritis Predict Outcome of Intraarticular Steroid Injections? J Rheumatol 2020;47(3):431–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCabe PS, Maricar N, Parkes MJ, Felson DT, O’Neill TW. The efficacy of intra-articular steroids in hip osteoarthritis: a systematic review. Osteoarthritis Cartilage 2016;24(9):1509–1517. [DOI] [PubMed] [Google Scholar]
- 28.Zhong HM, Zhao GF, Lin T, et al. Intra-Articular Steroid Injection for Patients with Hip Osteoarthritis: A Systematic Review and Meta-Analysis. BioMed Res Int 2020;2020:6320154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maricar N, Callaghan MJ, Felson DT, O’Neill TW. Predictors of response to intra-articular steroid injections in knee osteoarthritis--a systematic review. Rheumatology (Oxford) 2013;52(6):1022–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirsch G, Kitas G, Klocke R. Intra-articular corticosteroid injection in osteoarthritis of the knee and hip: factors predicting pain relief--a systematic review. Semin Arthritis Rheum 2013;42(5):451–473 10.1016/j.semarthrit.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 31.van Middelkoop M, Arden NK, Atchia I, et al. The OA Trial Bank: meta-analysis of individual patient data from knee and hip osteoarthritis trials show that patients with severe pain exhibit greater benefit from intra-articular glucocorticoids. Osteoarthritis Cartilage 2016;24(7):1143–1152. [DOI] [PubMed] [Google Scholar]
- 32.Fatimah N, Salim B, Raja EU, Nasim A. Predictors of response to intra-articular steroid injections in patients with osteoarthritis of the knee joint. Clin Rheumatol 2016;35(10):2541–2547. [DOI] [PubMed] [Google Scholar]
- 33.Jüni P, Hari R, Rutjes AW, et al. Intra-articular corticosteroid for knee osteoarthritis. Cochrane Database Syst Rev 2015;(10):CD005328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCabe PS, Parkes MJ, Maricar N, et al. Brief Report: Synovial Fluid White Blood Cell Count in Knee Osteoarthritis: Association With Structural Findings and Treatment Response. Arthritis Rheumatol 2017;69(1):103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maricar N, Parkes MJ, Callaghan MJ, et al. Structural predictors of response to intra-articular steroid injection in symptomatic knee osteoarthritis. Arthritis Res Ther 2017;19(1):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matzkin EG, Curry EJ, Kong Q, Rogers MJ, Henry M, Smith EL. Efficacy and Treatment Response of Intra-articular Corticosteroid Injections in Patients With Symptomatic Knee Osteoarthritis. J Am Acad Orthop Surg 2017;25(10):703–714. [DOI] [PubMed] [Google Scholar]
- 37.O’Neill TW, Parkes MJ, Maricar N, et al. Synovial tissue volume: a treatment target in knee osteoarthritis (OA). Ann Rheum Dis 2016;75(1):84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Daniels EW, Cole D, Jacobs B, Phillips SF. Existing Evidence on Ultrasound-Guided Injections in Sports Medicine. Orthop J Sports Med 2018;6(2):2325967118756576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berkoff DJ, Miller LE, Block JE. Clinical utility of ultrasound guidance for intra-articular knee injections: a review. Clin Interv Aging 2012;7:89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roemer FW, Hayes CW, Miller CG, Hoover K, Guermazi A. Imaging atlas for eligibility and on-study safety of potential knee adverse events in anti-NGF studies (Part 1). Osteoarthritis Cartilage 2015;23(Suppl 1):S22–S42. [DOI] [PubMed] [Google Scholar]
- 41.Roemer FW, Hayes CW, Miller CG, Hoover K, Guermazi A. Imaging atlas for eligibility and on-study safety of potential hip adverse events in anti-NGF studies (Part 2). Osteoarthritis Cartilage 2015;23(Suppl 1):S43–S58. [DOI] [PubMed] [Google Scholar]
- 42.Lecouvet FE, van de Berg BC, Maldague BE, et al. Early irreversible osteonecrosis versus transient lesions of the femoral condyles: prognostic value of subchondral bone and marrow changes on MR imaging. AJR Am J Roentgenol 1998;170(1):71–77. [DOI] [PubMed] [Google Scholar]
- 43.Zeng C, Lane NE, Hunter DJ, et al. Intra-articular corticosteroids and the risk of knee osteoarthritis progression: results from the Osteoarthritis Initiative. Osteoarthritis Cartilage 2019;27(6):855–862. [DOI] [PubMed] [Google Scholar]
- 44.Spitzer AI, Richmond JC, Kraus VB, et al. Safety and Efficacy of Repeat Administration of Triamcinolone Acetonide Extended-release in Osteoarthritis of the Knee: A Phase 3b, Open-label Study. Rheumatol Ther 2019;6(1):109–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Orchard JW. Is there a place for intra-articular corticosteroid injections in the treatment of knee osteoarthritis? BMJ 2020;368:l6923. [DOI] [PubMed] [Google Scholar]
- 46.Xie XH, Wang XL, Zhang G, et al. Impaired bone healing in rabbits with steroid-induced osteonecrosis. J Bone Joint Surg Br 2011;93(4):558–565. [DOI] [PubMed] [Google Scholar]
- 47.Batra S, Batra M, McMurtrie A, Sinha AK. Rapidly destructive osteoarthritis of the hip joint: a case series. J Orthop Surg Res 2008;3(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.