Abstract
The relationship between malignancy and coagulopathy is one that is well documented yet incompletely understood. Clinicians have attempted to quantify the hypercoagulable state produced in various malignancies using common coagulation tests such as prothrombin time, activated partial thromboplastin time, and platelet count; however, due to these tests’ focus on individual aspects of coagulation during one specific time point, they have failed to provide clinicians the complete picture of malignancy-associated coagulopathy (MAC). Viscoelastic tests (VETs), such as thromboelastography (TEG) and rotational thromboelastometry (ROTEM), are whole blood analyses that have the advantage of providing information related to the cumulative effects of plasma clotting factors, platelets, leukocytes, and red cells during all stages of the coagulation and fibrinolytic processes. VETs have gained popularity in the care of trauma patients to objectively measure trauma-induced coagulopathy (TIC), but the utility of VETs remains yet unrealized in many other medical specialties. The authors discuss the similarities and differences between TIC and MAC, and propose a mechanism for the hypercoagulable state of MAC that revolves around the thrombomodulin–thrombin complex as it switches between activating the protein C anticoagulation pathway or the thrombin activatable fibrinolysis inhibitor coagulation pathway. Additionally, they review the current literature on the use of TEG and ROTEM in patients with various malignancies. Although limited research is currently available, early results demonstrate the utility of both TEG and ROTEM in the prediction of hypercoagulable states and thromboembolic complications in oncologic patients.
Keywords: coagulopathy, thromboembolism, ROTEM, TEG, malignancy, tumors, hypercoagulable
“Omnis cellula e cellula” [All cells from pre-existing cells] R. Virchow, 1855.1,2
Despite increased awareness of venous thromboembolic prophylaxis, thromboemboli occur with increasing frequency in the United States, particularly in patients with malignancy.3 The association between thrombosis and malignancy was first described by Trousseau over a century ago.4,5 At minimum, an estimated 10 to 20% of patients with carcinoma of the pancreas, lung, or stomach also present with concomitant thromboembolic disease, which is associated with higher staging and increased thrombotic risk.5,6 Moreover, development of venous thrombosis within 1 year of diagnosis of a primary carcinoma increases the probability of detecting advanced disease.5,7,8 Among patients with solid malignant tumors and hematopoietic malignancies, activation of coagulation occurs despite the absence of clinically apparent thromboembolic disease.6,7,9–11 The process of ongoing activation of the coagulation system in malignancy is not well understood, but may involve tumor-induced activation of platelets, increased expression of tumor-related intravascular tissue factor (TF) in concert with circulating mononuclear phagocytes, and upregulation of endogenous inhibitors of fibrinolysis. These dysregulated host responses occur within the milieu of endothelial cell activation that exists as a principal component of a chronic, low-grade systemic inflammatory response.10,11 We refer to this dysregulated coagulation as malignancy-associated coagulopathy (MAC).
Common coagulation tests (CCTs), including PT/INR (prothrombin time/international normalized ratio) and aPTT (activated partial thromboplastin time), have traditionally been used for identifying hypocoagulable states. Although recently a shortened aPTT has been associated with thrombosis risk, there is still concern that such results are variably attributable to collection artifacts, reagent disparities, or other preanalytic issues.12 Further, CCTs are plasma-based assays that analyze a static snapshot of factor deficiencies in coagulation.3 Accordingly, they are poor predictors of the evolving clotting dynamics during a hypercoagulable state.3 Thus, it is critical to identify patients at risk for developing tumor-associated thrombosis using readily available biomarkers such as platelet count and plasma D-dimer levels, as well as research tools that identify circulating TF and P-selectin.13–21 These thrombotic biomarkers are only part of the story of MAC. Thus, recent American Society of Clinical Oncology guidelines counsel against assessing risk based on a single test, but rather emphasize scoring systems that consider multiple factors such as Khorana’s risk assessment model for assessing risk of venous thromboembolism in cancer patients.15–18,20–22
Over a century after Virchow foreshadowed the idea in the 1850s, literature regarding trauma resuscitation, liver transplantation, and cardiac surgery has brought to light the possibility of using whole blood viscoelastic tests (VETs) such as thromboelastography (TEG; Haemonetics) and rotational thromboelastometry (ROTEM; TEM International) to risk stratify cancer patients for venous thromboembolism.3,23,24 Recent literature has indicated that in noncancerous states, such as critically ill, septic, and postoperative states, VETs are useful in identifying a hypercoagulable state and guiding resuscitation.23–29 The use of VETs to guide hemostatic resuscitation of patients with trauma-induced coagulopathy (TIC) has also led to these point-of-care tests being used as predictors of thromboembolism and multiorgan failure (MOF) in trauma patients during the postresuscitation period.30–34 The utility of these assays has now been extended to surgical oncology patients with colon, rectal, and breast cancer who present with seemingly normal fibrinogen and platelets.35 High rates of thrombotic complications are observed with advanced pancreatic cancer and VETs have been able to accurately predict which patients will develop postoperative pulmonary emboli after pancreatic resection.35,36
Mechanisms of Hypercoagulable State in Cancer
Of interest for this review are the similarities of these derangements and hypercoagulability during the posttrauma resuscitation periods and the more prolonged cancer period. In cancer patients, the gradual and subclinical presentation of hemostatic activation and dysregulated coagulation results from structural, hemostatic, and biochemical derangements of coagulation factors, platelets, and endothelium. Thus, this complicated interplay of MAC is illustrated in ►Fig. 1. In TIC, there are several theories that explain the coagulopathy derangements as illustrated in ►Fig. 2. Then, as illustrated in ►Fig. 3, MAC is compared to TIC. The comparison with trauma is a useful starting point for discussing the utility of VETs in diagnosing and monitoring cancer patients at risk for thromboembolism.
Fig. 1.
Proposed molecules and cells involved in the hypercoagulable/hypofibrinolytic phenotype present in malignancy-associated coagulopathy (MAC). ADP, adenosine diphosphate; NETs, neutrophil extracellular traps; PAI-1, plasminogen activator inhibitor-1; TNF, tissue necrosis factor.
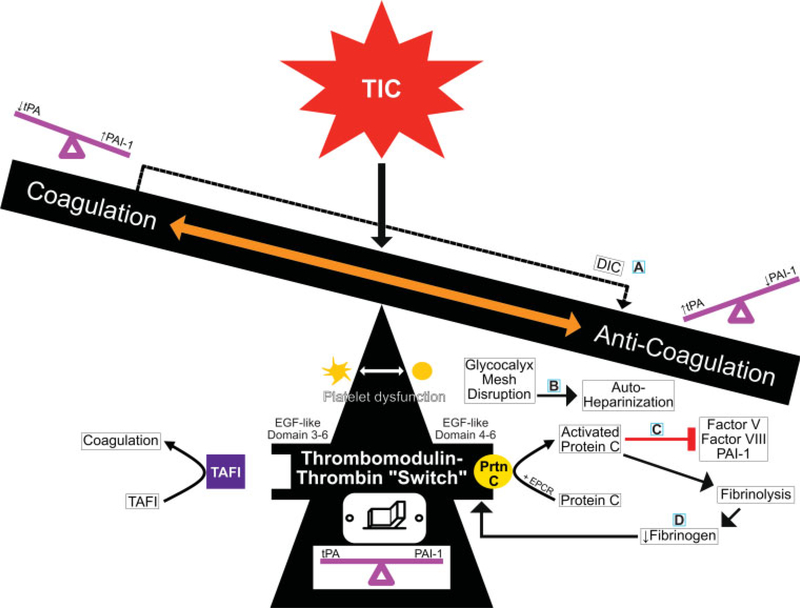
Fig. 2.
Proposed mechanisms for trauma-induced coagulopathy (TIC). This teeter-totter spectrum shows the balance and molecular interplay between hypocoagulable/hyperfibrinolytic and hypercoagulable/hypofibrinolytic phenotypes in TIC. There are four current hypotheses to explain TIC which are each centered around the balance of key molecules as follows: (A)DIC–fibrinolysis, (B) glycocalyx, (C) activated protein C, and (D) fibrinogen. These hypotheses are not mutually exclusive, but instead explain the mechanisms at play in TIC within minutes to hours following trauma. At the center, the TM–thrombin complex is the fulcrum of coagulation regulation. Structural and/or posttranslational covalent modifications of the TM–thrombin complex, along with certain cofactors and receptors, allow the TM–thrombin complex to switch between activating the protein C anticoagulation pathway or the TAFI coagulation pathway. (A) The DIC–fibrinolysis hypothesis centers around the consumption of clotting factors and platelets following hypoperfusion and endothelial injury; this consumption leads to a hypocoagulable/hyperfibrinolytic phenotype where fibrinolytic activity exceeds clot formation resulting in hemorrhage. (B) The glycocalyx hypothesis centers around endothelium injury resulting in glycocalyx shedding and systemic autoheparinization. (C) According to the protein C activation hypothesis, endothelium injury causes the activation of protein C by the TM–thrombin complex at the EPCR; protein C activation favors anticoagulation by inactivating PAI-1 and increasing levels of tPA. (D) The fibrinogen-centric hypothesis refers to the inverse relationship between fibrinogen levels and the activation of protein C. Accordingly, when fibrinolysis predominates, fibrinogen levels are low and protein C is activated on the TM–thrombin complex. DIC, disseminated intravascular coagulation; EGF, epidermal growth factor; EPCR, endothelial protein C receptor; PAI-1, plasminogen activator inhibitor 1; Prtn C, protein C; TAFI, thrombin activatable fibrinolysis inhibitor; TM, thrombomodulin; tPA, tissue plasminogen activator.
Fig. 3.
A double teeter-totter (or scale) spectrum showing the balance and molecular interplay between hypocoagulable/hyperfibrinolytic and hypercoagulable/hypofibrinolytic phenotypes in malignancy-associated coagulopathy (MAC) as compared with trauma-induced coagulopathy (TIC). This figure depicts the proposed mechanisms, endothelial interactions, and TEG/ROTEM tracings of both MAC and TIC; both MAC and TIC are centered around the thrombomodulin–thrombin complex as it switches between activating the protein C anticoagulation pathway and the TAFI coagulation pathway. While the majority of MAC patients favor coagulation and many TIC patients favor anticoagulation, they both exist within a fluid spectrum where the “switch” can be flipped back and forth from a procoagulant to an anticoagulant state when the conditions are favorable (i.e., protein C vs. TAFI activation and PAI-1 vs. tPA activity). The relative importance of PAI-1 and tPA ratios creates a milieu which favors thrombosis in patients with longstanding cancer and in trauma patients with persistent fibrinolytic shutdown following resuscitation. During the acute stage of severe trauma, the hyperfibrinolytic/anticoagulant phenotype gives way in minutes to hours to a fibrinolytic shutdown/procoagulant phenotype with successful resuscitation; this is similar to the hyperfibrinolytic phenotype of patients with early untreated acute promyelocytic leukemia. The four TIC hypotheses (right image) were included for the purposes of comparison with MAC. As explained in ►Fig. 2 and in-text, these hypotheses center around (A) DIC–fibrinolysis, (B) glycocalyx, (C) activated protein C, and (D) fibrinogen.49 ADP, adenosine diphosphate; DIC, disseminated intravascular coagulation; EGF, epidermal growth factor; EPCR, endothelial protein C receptor; FDPs, fibrin degradation products; G-CSF, granulocyte colony-stimulating factor; ICAM, intercellular adhesion molecule 1; IL-1, interleukin-1; NETs, neutrophil extracellular traps; PAI-1, plasminogen activator inhibitor-1; Prtn C, protein C; ROTEM, rotational thromboelastometry; TAFI, thrombin activatable fibrinolysis inhibitor; TEG, thromboelastography; TM, thrombomodulin; TNF, tumor necrosis factor; tPA, tissue plasminogen activator; VCAM, vascular cell adhesion molecule 1; VEGF, vascular endothelial growth factor; VTE, venous thromboembolism.
Proposed Molecules and Cells Involved in MAC
Malignancy-associated coagulopathy encompasses a spectrum of hemostatic derangements, platelet dysfunction, and vascular disturbances in patients with cancer. There are many proposed molecules and cells involved in the hypercoagulable/hypofibrinolytic phenotype of malignancy. Falanga et al categorize these molecules into different groups labeled as procoagulant factors, soluble mediators, and adhesion particles.10 ►Fig. 1 illustrates how the endothelial cells, tumor cells, free-floating cells, and particles interact with these procoagulant factors, soluble mediators, and adhesion particles.
Procoagulant factors include TF, TF-bearing microparticles, heparainase, and cancer procoagulant. TF is a transmembrane protein expressed by various cell types including some cancer cells. As shown in ►Fig. 1, TF stimulates factor X and factor IX by forming a complex with factor VII; this activates the coagulation cascade. Endothelial cells release tissue necrosis factor (TNF), interleukin-1 (IL-1), and P-selectin which induce expression of TF.10 TNF has multiple effects on coagulability including thrombomodulin (TM) downregulation, fibrinolytic activity suppression, and further expression of IL-1.10
Similar to TF, but without the transmembrane domain, TF-bearing microparticles are cancer-associated procoagulant proteins that free float in the plasma and also interact with factor X to activate the coagulation cascade.7,10,37 And, as shown in ►Fig. 1, monocytes can be stimulated to make TF and direct factor X activators.10 And, neutrophil cell-death is thought to play a role in hypercoagulability because their death releases a variety of proteins that create a mesh of chromatin called neutrophil extracellular traps (NETs).10,38 NETs react with surrounding structures and further the process of thrombin generation.10
Malignant cells can produce a variety of soluble mediators (i.e., adenosine diphosphate [ADP], IL-1, TNF, vascular endothelial growth factor, granulocyte-colony stimulating factor, thromboxane A2, and podoplanin) and adhesion molecules (i.e., vascular cell adhesion molecule, intercellular adhesion molecule) which have the ability to induce procoagulant states in other neighboring cell types.10,38,39 Cancer procoagulant is a molecule found only in malignant and fetal tissue and, as indicated in ►Fig. 1, directly activates factor X; the relative contribution of cancer procoagulant to malignancy-associated hypercoagulability is unknown at this time.10 MET oncogene is found to be expressed in multiple types of malignancies and, as demonstrated in ►Fig. 1, has been shown to increase plasminogen activator inhibitor-1 (PAI-1) and cyclooxygenase-2 production.7,40,41 Polyphosphate activates the intrinsic pathway. As indicated in ►Fig. 1, vesicles with high amounts of polyphosphate have been found in prostate cancer and shown to activate factor XII.42 Heparanase is expressed by a variety of cancer cells and its nonenzymatic domain enhances TF activity through phosphorylation and by acting as a cofactor for TF.10
Further, the release of activators from malignant cells stimulates platelets. Platelets can be activated via increased ADP expression by malignant cells, increased von Willebrand factor, tumor-induced thrombin creation, and/or increased expression of TF via release of P-selectin.10,38,43 For example, mucins released by mucinous adenocarcinomas activate platelets in the presence of P- and L-selectin.44 In myeloproliferative disorders, increased platelet clonality could also contribute to hypercoagulability.45,46 Furthermore, in malignancy-causing liver metastases or membranous nephropathy, for example, decreased circulating antithrombin could result in increased thrombin creation contributing to hypercoagulability.47,48
Proposed Mechanisms for TIC
For decades, the literature has described trauma patients—including those experiencing shock, hemorrhage, burns, cardiac arrest, and major surgery—with hemostatic disorders that we now call TIC.49 The endothelial TM–thrombin complex is the fulcrum in the in vivo regulation of the coagulation spectrum. The TM–thrombin complex can activate the protein C anticoagulation pathway as depicted on the right-hand side of ►Fig. 2. Or, the TM–thrombin complex can activate the thrombin activatable fibrinolysis inhibitor (TAFI) coagulation pathway as shown on the left-hand side of ►Fig. 2. The switch between the two pathways occurs within seconds to minutes after trauma and involves either structural or posttranslational covalent modifications of different sites on the TM–thrombin complex. Then, in the presence of their respective cofactors and/or receptors, the modified TM–thrombin complex binds and activates protein C or TAFI. The activation of protein C by the TM–thrombin complex is accelerated by endothelial protein C receptor (EPCR) binding.
There are four main theories of TIC in relation to the TM–thrombin activated protein C pathway. As shown in ►Figs. 2 and 3, current hypotheses to explain TIC include (A) the disseminated intravascular coagulation (DIC)–fibrinolysis hypothesis, (B) the glycocalyx hypothesis, (C) the protein C activation hypothesis, and (D) the fibrinogen hypothesis (correspondingly labeled in ►Fig. 3). (A) In DIC–fibrinolysis, the theory proposes that the anticoagulant state results from hypoperfusion and endothelial injury that leads to a consumption of clotting factors and platelets. This consumption results in hyperfibrinolysis as evidenced by increased fibrinogen degradation products (FDPs) and high FDP/D-dimer ratios. Following the DIC-fibrinolytic phase, there can be a DIC-thrombotic state that results from high levels of PAI-1 and inhibition of fibrinolysis.49 Thus, DIC is not synonymous with TIC because DIC’s anticoagulant state is the result of diffuse fibrin deposition leading to a state in which fibrinolytic activity exceeds clot formation and hemorrhage results.49 (B) The glycocalyx injury hypothesis also proposes that bleeding occurs secondary to endothelium injury from hypoperfusion resulting in a shedding of the glycocalyx layer on the luminal side of the endothelium. This leads to what has been called by Johansson et al as SHock INduced Endotheliopathy (SHINE).27,50 This glycocalyx breakdown signals further endothelial activation and results in systemic autoheparinization as evidenced by increased expression of syndecan-1.49 (C) Similar to the glycocalyx hypothesis’s focus on endothelium injury from hypoperfusion, the protein C activation hypothesis also proposes that endothelium injury and/or hypoperfusion results in an anticoagulant state. However, according to the activated protein C hypothesis, the anticoagulant state is the result of protein C activation by the TM–thrombin complex at the EPCR. Increased levels of activated protein C inhibit thrombin generation and favor hyperfibrinolysis by depleting PAI-1 levels which leads to increased tissue plasminogen activator (tPA) activity.49,51 (D) The fibrinogen hypothesis centers on the concentration of fibrinogen. The hypothesis is that the anticoagulant state is driven by the decrease in fibrinogen which reduces viscoelastic clot amplitudes. The hypofibrinogenemia occurs when fibrinolysis occurs at a greater rate than fibrinogen synthesis. Without sufficient fibrinogen, platelet function is altered and protein C activation on the TM–thrombin complex is increased leading to an anticoagulant state.49 The relationship between fibrinogen levels and activated protein C has been proposed as an inverse relationship. Accordingly, when fibrinolysis predominates, fibrinogen levels are low and protein C is activated on the TM–thrombin complex resulting in an anticoagulant state. Conversely, at high levels of fibrinogen, the TM–thrombin complex is inhibited from activating protein C.49,52
Comparison between MAC and TIC: Pathophysiologic Mechanisms Correlated to TEG/ROTEM Patterns
In both TIC and MAC, TF and its “activation” of the TM–thrombin complex are critical for maintaining normal hemostasis.10,49 The TM–thrombin activated protein C pathway is present on approximately 7,000 m2 of the endothelial surface in both trauma and cancer patients and can become dysregulated. Compared to trauma patients in whom dysregulation occurs over hours, the dysregulation in cancer patients occurs over a much longer period.6,10,31,49 However, whether over hours or weeks, dysregulated coagulopathy is driven by an imbalance of a number of tumor cells and factors. The balance between the anticoagulant/hypocoagulable and procoagulant/hypercoagulable states in cancer is a function of many interrelated factors as shown in ►Fig. 1 and many of these factors contribute to the increased incidence of thrombosis in cancer. As depicted in ►Fig. 2, these factors are similar to the factors in TIC. For example, the relative importance of PAI-1 and tPA ratios creates a milieu favoring thrombosis in patients with cancer and patients with persistent fibrinolytic “shutdown” following trauma resuscitation. As defined by Moore et al, a fibrinolytic “shutdown” occurs when a physiologic dysregulation of the plasminogen–plasmin system causes a relative resistance to tPA.31 During the acute stage of severe trauma, the hyperfibrinolytic phenotype may give way in minutes to hours to a fibrinolytic shutdown phenotype which is similar in many ways to the hyperfibrinolytic phenotype of patients with early untreated acute promyelocytic leukemia (APL).31,53–59 And, just as increased polyphosphate in prostate cancer has been shown to increase hypercoagulability, in the event of trauma, polyphosphate is released from platelets and strengthens fibrin aggregates while reducing sites for tPA and plasminogen.31,42,60,61
A classic comparative example of the spectrum of coagulopathies between TIC and MAC can be seen in ►Fig. 3. The hyperfibrinolytic phenotype in the severely injured patient with TIC is the hyperfibrinolysis-induced anticoagulant phenotype, while for hematologic malignancy the similar hyperfibrinolytic phenotype is the initial pretreatment anticoagulant state characteristic of APL. This APL-associated hyperfibrinolytic phenotype is dependent on increased expression of tPA, annexin A2, and urokinase-type plasminogen activator by the leukemic promyelocytes.53 Similar to the hyperfibrinolysis of TIC, the APL-associated hyperfibrinolytic phenotype may be quickly reversed and rapidly become a hypercoagulable state when treated with all-trans retinoic acid (ATRA) and other therapeutic agents.49,53–59 Inhibitors of the TF–FVIIa–FXa complex, such as TF pathway inhibitor are present in APL patients. Also, PAI-1 is complexed with tPA reducing both their activities in APL patients.53,54 Severe hyperfibrinolytic TIC bears similarities to the MAC present in APL because an early anticoagulant state is a function of hyperfibrinolysis but, following treatment in APL or resuscitation in TIC, patients become hypercoagulopathic. Therefore, whether caused by malignancy or trauma, TEG and the ROTEM allow for evaluation of the patient’s phenotype along the spectrum of hypocoagulable/hyperfibrinolytic to hypercoagulable/hypofibrinolytic.
TEG and ROTEM
Thromboelastography
The first documented use of TEG was in 1948 by Hellmut Hartert where it was used to demonstrate hemostatic function for whole blood samples.62,63 Since then, the use of TEG within the clinical setting has continued to grow.62,64–68
Thromboelastography is a method to graph and evaluate the entire process of blood coagulation from the beginning of clot formation to fibrinolysis.69,70 By measuring the viscoelastic properties of the blood clot, TEG has the advantage of providing information related to the cumulative effects of plasma clotting factors, platelets, leukocytes, and red cells during all stages of the coagulation and fibrinolytic processes.69
The standard kaolin TEG is a point-of-care whole blood test whereby citrated blood is placed in a warmed cup into which a pin descends. As the cup rotates and the clot forms, the connection between the rotating cup and the pin induces a torque on the pin, which is registered by a transducer that provides a characteristic curve that looks like a shovel. The TEG setup is illustrated on the right-hand side of ►Fig. 4A. A normal or physiologic TEG tracing (►Fig. 4A) resembles a wide flat (non-functional) “shovel” with a short handle. As enumerated in ►Table 1, the TEG parameters are abbreviated as follows: reaction time (R) for the transducer to be displaced 2 mm, clot formation/kinetics (K) or time to accomplish a specific level of clot strength, α-angle or rate of clot formation, maximum amplitude (MA) or overall clot strength, and lysis at 30 minutes (LY30) or percent decrease in amplitude 30 minutes after achieving MA.71
Fig. 4.
(A) Depiction of a TEG.28 TEG and ROTEM use independently labeled parameters that are equivalent. Reaction time (R) is the clotting time to cause a 2-mmdisplacement during initial clot formation. Kinetics (K) is the clot formation time. α-Angle is the rate of clot formation. Maximum amplitude (MA) is the maximum clot strength. Lysis at 30 minutes (LY30) is the percent decrease in amplitude 30minutes after achieving MA. As illustrated on the right-hand side, citrated blood is placed in a warmed cup into which a pin descends. As the cup rotates and the clot forms, the connection between the rotating cup and the pin induces torque on the pin, which is registered by a transducer as a characteristic shovel-shaped curve. Abbreviations: TEG, thromboelastography; ROTEM, rotational thromboelastometry; R, reaction time; K, kinetics; α-angle, rate of clot formation; MA, maximal amplitude; LY30, lysis at 30 minutes. (B)Depiction of a ROTEM tracing.28 TEG and ROTEM use independently labeled parameters that are equivalent. Clotting time (CT) is the reaction time to cause a 2-mm displacement during initial clot formation. Clot formation time (CFT) measures clot kinetics. α-Angle is the rate of clot formation. Maximum clot firmness (MCF) measures maximum clot strength. Lysis at 30 minutes (LI30) is the percent decrease in amplitude 30 minutes after MCF. As illustrated on the right-hand side, citrated blood is placed in a warmed cup with a pin. Unlike the TEG, the ROTEM pin revolves within a fixed cup. The resultant force of the clot on the pin is transduced and then traced as a characteristic shovel-shaped curve. TEG, thromboelastography; ROTEM, rotational thromboelastometry.
Table 1.
TEG/ROTEM normal parameters
| Parameter | TEG | ROTEM |
|---|---|---|
| Clotting time (2 mm amplitude) | R (reaction time)Normal (citrate/kaolin = 3–8 min | CT (clotting time)Normal (EXTEMa) = 42–74 s Normal (INTEMb) = 137–246 s |
| Clot formation/kinetics (20 mm amplitude) | K (kinetics)Normal(citrate/kaolin) = 1–3 min | CFT (clot formation time) Normal (EXTEM) = 46–148 s Normal (INTEM) 40–100 s |
| Clot strengthening (angle of clot formation) | α-Angle (slope between R and k points) Normal(citrate/kaolin) = 55–78° | α-Angle (slope of tangent at 2 mm amplitude) Normal (EXTEM) = 63–81° Normal (INTEM) = 71–82° |
| Amplitude/maximal firmness | MA (maximal amplitude)Normal (citrate/kaolin) = 51–69 mm | MCF (maximum clot firmness)Normal (EXTEM) = 49–71 mm Normal (INTEM) = 52–72 mm Normal (FIBTEMc) = 9–25 mm A5, A10, etc. – amplitudes at dedicated time points predicting the final clot firmness |
| Lysis | LY30, CL30, CL60, CL | LI30, LI60, ML |
Abbreviations: CFT, clot formation time; CT, clotting time; EXTEM, extrinsic activator thromboelastometry; FIBTEM, fibrin-based thromboelastometry; INTEM, intrinsic activator thromboelastometry; K, kinetics; LI, lysis; LY, lysis; MA, maximal amplitude; MCF, maximum clot firmness; R, reaction time; ROTEM, rotational thromboelastometry; TEG, thromboelastography; TF, tissue factor.
The EXTEM uses TF as the activator and is a test of the extrinsic pathway.
The INTEM uses ellagic acid and is a test of the intrinsic pathway.
FIBTEM uses both TF and platelet inhibition so it is a test of fibrin polymerization after inactivation of the platelets.
Rotational Thromboelastometry
Thromboelastography and ROTEM utilize identical principles with the exception that in the ROTEM the pin revolves within a fixed cup as illustrated on the right-hand side of ►Fig. 4B.72 The resulting ROTEM curve is identical to the TEG, but each assay uses different nomenclature (►Fig. 4B). As shown in ►Table 1, clot time (CT) corresponds to TEG’s R, clot formation time (CFT) corresponds to TEG’s K, maximum clot firmness (MCF) corresponds to TEG’s MA, and lysis after 30 minutes (LI30) corresponds to TEG’s LY30.64–68,72,73
Tissue factor is used to initiate the rapid TEG for the TEG and the extrinsic activator thromboelastometry (EXTEM) for ROTEM in assessing the hypocoagulopathic patient while kaolin TEG is more commonly employed to assess hypercoagulability for the TEG.3,29,72,74,75
Analysis of TEG or ROTEM
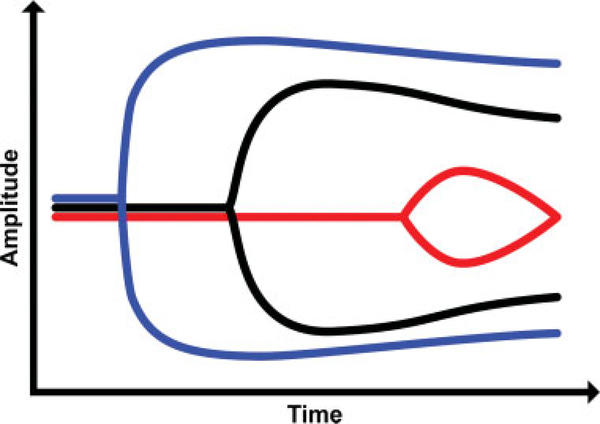
The authors suggest that the shapes of the TEG/ROTEM tracings can be analogized to the shape of a shovel. With hemostatic competence or normal hemostasis, when whole blood is neither hypocoagulable nor hypercoagulable, the shovel-shaped tracing has an ideal handle length, blade slope, blade width, and blade tip. This ideal shovel shape is shown in ►Fig. 5 as a black shovel. The shovel attributes correspond to TEG parameters as follows: the R is the handle, the α-angle is the slope of the blade, MA is the width of the blade, and LY30 is the point of the blade. The ends of the coagulation spectrum are represented by different shovels where the ease of tilling and moving the earth corresponds to the ease of moving blood. Continuing the analogy, the shovel tracing in hyperfibrinolysis has a long handle with a narrow and pointed blade (red shovel in ►Fig. 5) which makes it easy for the earth to be tilled and transported; similarly, in hyperfibrinolysis, the blood is easily transported because it is hypocoagulable. On the other end of the spectrum, the shovel tracing in a hypercoagulable state has a short handle and a wide blade with an absent tip (blue shovel in ►Fig. 5) which makes it very difficult for the earth to be broken up; by analogy, the blood in the hypercoagulable state has difficulty being broken up and is not easily transported. In summary, in ►Fig. 5, the black shovel represents hemostatic equilibrium, the red shovel represents hypocoagulable disequilibrium, and the blue shovel represents hypercoagulopathic disequilibrium.
Fig. 5.
Schematic of simplified TEG tracing.28 The TEG shovel-shaped tracing in black, with a normal handle length and moderately wide shovel blade with slight narrowing of the tip of the blade, represents physiologic hemostasis with normal R, α-angle, MA, and LY30. The superimposed TEG shovel tracing in red depicts a tracing with a prolonged R, narrow α-angle, a narrow MA, and pointed and increased LY30; these parameters are indicative of a hypocoagulopathic/hyperfibrinolytic phenotype. The superimposed TEG shovel tracing in blue with a very short handle and wide blade with very little tapering of the tip of the blade depicts a tracing with a decreased R and a wide α-angle, MA, and very low LY30. The blue shovel tracing is indicative of a hypercoagulable/fibrinolytic shutdown phenotype. α-Angle, rate of clot formation; K, kinetics; LY30, lysis at 30 minutes; MA, maximal amplitude; R, reaction time; TEG, thromboelastography.
The hyperfibrinolytic TEG tracings at the bottom left and right corners of ►Fig. 3 demonstrate a prolonged reaction time (R or CT), decreased kinetics (K or CFT) or time to accomplish a specific level of clot formation, flat α-angle or rate of clot formation, small MA (MCF) or overall clot strength/firmness, and increased LY30 (LI30) or percent decrease in amplitude 30 minutes after achieving MA. These values indicate a systemic coagulopathy with hyperfibrinolysis.64–68,73 In response to TEG/ROTEM values, appropriate therapy can be planned. Fresh frozen plasma or prothrombin complex concentrate is given for a prolonged R (CT), cryoprecipitate or fibrinogen concentrate is given for a flat α-angle, platelets are given for a small MA (MCF), and tranexamic acid (TXA) can be given for an increased LY30 (LI30).28,29,64–70,73,76
Coagulation index is another way to interpret the data from TEG. It is the sum of the R, K, MA, and α-angle values. The resulting value represents an individual’s hemostatic balance and has been suggested as a way to assess hemorrhagic or thrombotic risk.77
Efficacy of VETs to Define the Hypercoagulable State in Patients
Recently, it has been suggested that VETs such as TEG should replace CCTs for the guidance of blood component therapy (BCT) in the trauma population.78 Whereas VETs evaluate whole blood samples, CCTs are plasma-based assays that assess only a part of the story.3 CCTs not only fail to identify deficiencies in coagulation factors, fibrinogen, and fibrin, but they also do not evaluate blood cellular contribution to clotting function and fibrinolysis.78–82 VETs provide a rapid and accurate assessment of clot formation, stability, and firmness which allows for individualized treatment of patients with TIC.3,29,78,83
Sepsis-induced coagulopathy and MAC have been determined by ROTEM.84 Reductions in the MA, CFT, α-angles have been associated with increased mortality at 28 days in the critical care unit in septic patients with and withoutDIC.85 The ROTEM parameters for hypocoagulability have demonstrated reduced fibrinolysis or fibrinolytic shutdown in patients with overt DIC defined by the International Society on Thrombosis and Haemostasis or Japanese Association for Acute Medicine criteria alone.86 At the other end of the coagulopathy spectrum, VETs were a better indicator of the postinjury hypercoagulable state than CCTs.87 Postoperative patients with and without cancer in the intensive care unit have been found to be at risk for venous thromboembolism and this risk can be predicted by ROTEM and TEG.23,34 Specifically, the MA value of TEG has been found to be predictive of increased risk of deep vein thrombosis and pulmonary embolism in patients with major orthopedic trauma.34
In patients with malignancy, both TEG and ROTEM parameters can predict hypercoagulable states. Specifically in patients with malignancy, TEG parameters have shown decreased R and K, and increased MA.37,88,89 ROTEM parameters demonstrated hypercoagulability in patients with solid tumors by identifying the contribution of fibrinogen and platelets to clot strength.24 MCF of the ROTEM tracings on all assays—the intrinsic, extrinsic, fibrin, and aprotinin activation thromboelastometry tests (INTEM [intrinsic activator thromboelastometry], EXTEM, FIBTEM [fibrin-based thromboelastometry], APTEM [aprotinin thromboelastometry])—has revealed a statistically significant shortening of CFT and increasing of MCF in cancer patients compared to routine laboratory tests.23,24,90,91 Also, venous thromboembolism and arterial thromboembolismmay also precede the diagnosis of cancer by months or years and, therefore, ROTEM and TEG data may function as a marker for occult malignancy.10,37,92,93
VETs and the Hypercoagulable State in the Noncancer Setting
Multiorgan failure and thrombosis result from severe inflammation and hemostatic derangement in the emergent and intensive care unit populations.26,75,94,95 Recently, in an attempt to further categorize the hemostatic competence of critically ill patients, VETs have been proposed as an adjunctive point-of-care methodology to further delineate the hypercoagulable states of these patients.25,34,87,96–102
The first studies of VETs in critically ill patients were performed by Starzl’s liver transplantation group in Colorado in the 1960s. They first identified hyperfibrinolysis during the anhepatic phase of liver transplantation and began using routine epsilon aminocaproic acid (EACA) to treat hyperfibrinolysis.103,104 However, in their subsequent experience, this led to fatal pulmonaryemboli. This led to the recommendation of EACA for select patients with posttransplant hyperfibrinolysis as documented by TEG.64,105 Another antifibrinolytic agent, aprotinin, was known to be associated with both venous thromboemboli and MOF following cardiac surgery.106
Also, CCTs do not adequately predict thrombosis because of “rebalanced hemostasis” associated with chronic liver failure.105 “Rebalanced hemostasis” in patients with chronic liver disease refers to deficits in the production of proteins C and S resulting in abnormalities in prohemostatic drivers that are then compensated for by commensurate changes in antihemostatic drivers. For example, the clot dissolution phase of hemostasis is defective in patients with cirrhosis due to diminished levels of profibrinolytic proteins and depressed levels of protein C from decreased hepatic biosynthesis. These patients maintain a fragile balance of hemostasis due to such deficiencies and compensation measures. Standard CCTs, such as INR, do not adequately assess the fragility of this state of balance, but VETs are much more informative. On the other side of the coagulation spectrum, VETs reveal the presence of hypercoagulability and a prothrombotic state and also provide information about the presence of endogenous heparinoids with vascular endothelial injury associated with liver failure, sepsis, or acute and chronic inflammation.105,107–109
Since the early Starzl studies, extensive research has been conducted on the use of VETs in the acute setting. Moore et al have proposed that hypofibrinolysis in the postoperative period was the “missing link” that caused Starzl to use the TEG in order to identify those patients at greatest risk for post-liver transplant venous thromboembolism.75 Recent studies have confirmed Starzl’s prescient utilization of the TEG by identifying a group of patients with severe trauma and shock who developed MOF and venous thromboembolism following resuscitation and TXA administration. TXA has been recently demonstrated in a large, retrospective study of more than 21,000 severely injured patients from the University of Pittsburgh to be associated with a significantly increased risk of thromboemboli with no survival benefit in patients with multiple trauma.110 In this study of 21,931 patients, 189 pairs werewellmatched across propensity score variables and had a median injury severity score of 19. TXA was not significantly associated with survival (adjusted odds ratio [aOR]: 0.86; 95% confidence interval [CI]: 0.23–3.25; p = 0.83) and was associated with more than threefold increase in the odds of venous thromboembolism (aOR: 3.3; 95% CI: 1.3–9.1; p = 0.02). Risk of venous thromboembolism remained elevated in the TXA cohort despite accounting for mortality. In other studies, patients have demonstrated a similar hypofibrinolysis in the postresuscitation and post-TXA period which has been called a fibrinolytic shutdown.30,31,110–112 As defined by Moore et al, a fibrinolytic shutdown occurs when a physiologic dysregulation of the plasminogen–plasmin system causes a relative resistance to tPA and thus, a hypercoagulable state.31 MAC exhibits a similar state with a decrease in tPA activity.113
The pathophysiologic causes that drive the hypercoagulability and fibrinolytic shutdown after trauma are a matter of speculation. In trauma, the early fibrinolytic response is believed to be the release of tPA from the vasculature as a response to hypotension and shock; this shock is absent in cancer.75,114 Rather, in the absence of shock and with tissue injury such as malignancy, a hypercoagulable state exists. For example, tissue injury in the absence of shock in animal models promotes fibrinolysis resistance and a hypercoagulable state.115,116 However, this translation to the human scenarios remains a challenge because most patients have variable combinations of tissue injury and shock after experiencing trauma. With a cancer patient, there is less incidence of acute shock and therefore a greater incidence of a hypercoagulopathic state. It is appropriate then to apply the knowledge learned from more than a half century use of VETs, such as in liver transplantation and trauma, to predict coagulopathy at both ends of the coagulation spectrum in the cancer patient.
Thromboelastography-identified postoperative hypercoagulability has been demonstrated following hepatic and intra-abdominal surgery, and neurosurgery.117–120 In addition, TEG-identified hypercoagulability has been assessed in coronary heart disease, dialysis patients, cut-down intravenous catheters, oral contraceptives, and burn patients.102,121–124 And, TEG-identified hypercoagulability has been used in major orthopedic trauma injuries to predict venous thromboemboli during hospitalization.34
VETs and Specific Malignancies
Solid Tumors
Liver and Pancreaticobiliary Malignancies
Blasi et al and Moore et al have described a positive correlation Between ROTEM and TEG parameters and venous thromboembolism (►Table 2).35,125 Specifically, cholangiocarcinoma has demonstrated correlation between increased CFT and MCF in patients with venous thromboembolism.125 Based on their study, they recommended addition of ROTEM abnormalities to an expanded Khorana risk model for venous thromboembolism.125 Moore et al noted increased α-angle and low LY30 in 11% of patients with pancreatic neoplasms who developed venous thromboembolism.35 De Pietri et al noticed prolonged PT/INR despite normal TEGs and a reduction in antithrombin and platelet count which reflected the rebalanced hemostasis in patients with liver cancer.126
Table 2.
Solid tumors
| Study | Cancer/number | Test | Findings |
|---|---|---|---|
| Blasi et al, 2018125 | Cholangiocarcinoma | ROTEM | Recommended addition of ROTEM to expanded Khorana risk model for VTE after identifying ↑CFT, ↑MCF, and a 22% incidence of VTE. |
| Patients: 27 | |||
| Scărlătescu et al, 201884 | Solid tumors/sepsis | ROTEM | Organ dysfunction associated with reduced fibrinolysis. Standard coagulation tests and plasmatic indicators of fibrinolysis are inaccurate predictors of bleeding. |
| Patients: 35 | |||
| Moore et al, 201835 | Pancreatic neoplasms | Native TEG | Pulmonary embolism with ↓ R, ↑α-angle, and ↓ LY30. 11% incidence of VTE. |
| Patients: 100 | |||
| Jansohn et al, 2017133 | Glioma | ROTEM | ↓ CT with concomitant local fibrinolysis in tissue-spiked tumor samples. |
| Patients: 21 | |||
| Wang and Ye, 201689 | Nonsmall cell lung cancer | TEG | ↓ R, ↓ K, ↑MA, ↑α-angle, ↑fibrinogen, and ↑d-dimer in 15 of 48 lung cancer acute cerebral infarction patients. |
| Patients: 48 | |||
| Wikner et al, 2015129 | Free flap in head/neck | ROTEM | Heparin guided by ROTEM was noninferior to aPTT. ROTEM may augment traditional coagulation tests in microvascular tissue surgery. |
| Patients: 25 | |||
| Toukh et al, 201437 | Prostate cancer | TEG | 68% patients were hypercoagulable based on ↓ R, ↑α-angle, ↑MA, and ↑CI, including ↑microparticles. |
| Patients: 32 | |||
| Thorson et a, 201491 | Intra-abdominal malignancies | ROTEM | In resectable malignancies, ↓ CFT, ↑α-angle, and ↑MCF in pancreatic compared to esophageal/liver cancers, and in all cancers compared to benign disease. Pancreatic neoplasms have greatest risk for hypercoagulability. |
| Patients: 72 | |||
| Hincker et al, 201423 | Solid tumor surgery | ROTEM | Preoperative EXTEM and INTEM indicated patients (147/313 or 47% had cancer) had significantly ↓ CFT, ↑α-angle, and ↑MCF associated with increased thromboembolic complications. |
| Patients: 147 | |||
| Akay et al, 2014132 | Gynecologic malignancies | ROTEM | Hetastarch causes hypocoagulopathic state in patients with gynecologic cancers as shown by ↑CT, ↑CFT, and ↓ MCF. HES may reduce VTE incidence in gynecologic cancer surgery. |
| Patients: 22 | |||
| Thorson et al, 201390 | Intra-abdominal malignancies | ROTEM | Postsurgical hypercoagulability with ↓ CFT, ↑α-angle, and ↑MCF within 1 wk of surgery. Patients were hypercoagulable at 3–4 wk. Supports postdischarge thromboprophylaxis in high-risk cancer patients. |
| Patients: 35 | |||
| Attaran et al, 2010131 | Lung tumor | TEG | Advocated TEG use in patients with hypercoagulable states to monitor LMWH. |
| Patients: 60 | |||
| De Pietri et al, 2010126 | Liver and pancreatic cancer | TEG | In postoperative period of liver patients, TEG parameters remained normal while PT/INR remained prolonged despite ↓ antithrombin III, ↓ platelets, normal aPTT, and normal fibrinogen. Pancreatic patients revealed transient ↑R and ↑K. |
| Patients: 56 | |||
| Liang et al, 201088 | Colon cancer Patients: 35 |
TEG | TEG parameters in patients with colon cancer indicated ↓ R, ↓ K, and ↑MA. TEG parameters indicated that presurgical loading with HES inhibited platelet activation. |
| Akay et al, 200924 | Solid tumors | ROTEM | Hypercoagulability reflected by ROTEM ↓ CFT and ↑MCF. Contribution of fibrinogen and platelets to clot strength was determined by ROTEM. |
| Patients: 78 | |||
| Papa et al, 2007127 | Digestive tract cancers | ROTEM | ↑Maximum velocity and ↓ time to maximum velocity reflect hypercoagulability. |
| Patients: 50 | |||
| Wen et al, 1997130 | Solid tumors | TEG | Hypercoagulable states in cancer patients do not have direct contribution as determined by TEG. |
| Patients: 76 | |||
| Goh et al, 1997118 | Brain tumors | TEG | In postoperative patients without hematoma, TEG demonstrated ↓ K, ↑α-angle, and ↑MA. In patients with hematoma, there was increased fibrinolysis and ↑R. |
| Patients: 50 | |||
| Francis et al, 1994128 | Solid tumors | TEG | TEG demonstrated ↓ R in patients with colon cancer, breast cancer, and miscellaneous cancers. Patients with breast cancer and colon cancer also had ↑MA and ↑α-angle. Sonoclot Analyzer demonstrated similar coagulopathy. |
| Patients: 17 |
Abbreviations: aPTT, activated partial thromboplastin time; CFT, clot formation time; CI, coagulation index; CT, clotting time; EXTEM, extrinsic activator thromboelastometry; HES, hetastarch; INR, international normalized ratio; INTEM, intrinsic activator thromboelastometry; K, kinetics; LI, lysis; LMWH, low molecular weight heparin; LY, lysis; MA, maximal amplitude; MCF, maximum clot firmness; PT, prothrombin time; R, reaction time; ROTEM, rotational thromboelastometry; TEG, thromboelastography; VTE, venous thromboembolism.
Intra-abdominal Malignancies
In 2013, Thorson et al described postoperative abnormalities of the ROTEM which predicted a hypercoagulable state at 1 and 4 weeks postoperatively in patients with intra-abdominal malignancies.90 At 1 week, 86% had more rapid CFT, high α-angles, and elevated MCF; all of these patients remained hypercoagulable at 4 weeks. In total, 81% of patients with abnormal ROTEM values demonstrating hypercoagulability had normal coagulation profiles preoperatively. Thorson et al also published a follow-up study which confirmed their previous study showing the following hypercoagulable parameters associated with intra-abdominal malignancies: low CFT, high α-angle, and elevated MCF.91 They noted that pancreatic neoplasms have the greatest risk for hypercoagulability, and that pancreas, esophageal, and liver cancers had more hypercoagulability measured by ROTEM than benign diseases. Papa et al similarly described a shortened time to maximum velocity (i.e., low CFT) in patients with “digestive tract” cancers.127 Specifically for colon cancer patients, Francis et al and Liang et al noted that TEG parameters demonstrated a hypercoagulable state with decreased R and an increased MA.88,128 To address the hypercoagulable state and increased platelet activation, Liang et al found that presurgical loading with HES 200/0.5 inhibited platelet activation as evidenced by TEG parameter changes.88
Head and Neck Malignancies
Wikner et al observed that ROTEM was noninferior to aPTT in guiding heparin use in patients with free flaps following head and neck cancer surgery.129 Once it becomes standardized, ROTEM may augment traditional coagulation tests in microvascular tissue surgery.
Solid Tumors (Not Otherwise Specified)
Scărlătescu et al used ROTEM to associate fibrinolytic shutdown with MOF.84 In those cases, standard coagulation tests and plasma indicators of fibrinolysis were inaccurate indicators of bleeding and thrombosis. Hincker et al described patients undergoing noncardiac surgery including 147 patients (147/313 or 47% of patients) with cancer. In all patients with thromboembolic complications, preoperative EXTEM and INTEM revealed reduced CFT, elevated α-angle, and elevated MCF in patients with thromboembolic complications.23 There was no difference using FIBTEM, which excludes platelets. In 2009, Akay et al noted significant correlation between ROTEM parameters and hypercoagulability including decreased CFT and increased MCF.24 The strongest correlations were in the INTEM, EXTEM, FIBTEM, and APTEM for CFT, MCF, and α-angle. Similarly in 1994, Francis et al noted hypercoagulable TEGs in patients with solid tumors as evidenced by a short R and an elevated MA.128 In comparison, in 1997Wen et al found no correlation between TEG parameters and thrombosis of implantable venous catheters in patients with solid tumors.130
Lung Cancer
Wang et al in 2016 noted that patients with nonsmall cell carcinoma of the lung have hypercoagulability as demonstrated by increased MA, α-angle, and fibrinogen.89 Attaran et al advocated the use of TEG in patients with hypercoagulable states to monitor low molecular weight heparin (LMWH) thromboprophylaxis.131
Gynecologic Malignancies
In 2014, Akay et al performed a similar study of gynecologic malignancies and demonstrated that hetastarch caused a hypocoagulable state in patients with gynecologic cancers.132 Using ROTEM, they identified an increased CT and CFT, and a reduced MCF compared to controls. This study was done to demonstrate that hetastarch could potentially reduce the incidence of venous thromboembolism in gynecologic surgery.
Prostate Malignancies
Toukh et al in 2014 noted that 68% of patients with prostate malignancies showed hypercoagulable TEG tracings as demonstrated by short R, elevated α-angle, increased MA, and increased coagulation index.37 They also noticed increased microparticles in the patients who were hypercoagulable.
Central Nervous System Malignancies
In one study, Jansohn et al noted decreased clotting times with maintained local fibrinolysis in tissue-spiked tumor samples of patientswithglioblastomas.133 In another study by Gohet al of patients with primary brain tumors, TEG tracings demonstrated both hyper- and hypocoagulable patients in the pre- and postoperative periods based on changes in R, α-angle, MA, and LY30.118 Specifically, postoperative patients without hematoma had decreased K, increased α-angle, and increased MA. Comparatively, patients with hematoma demonstrated a fibrinolysis phenotype with an increased R.
Hematopoietic Malignancies
As noted above, a spectrum of coagulopathy from the hypocoagulopathic and hyperfibrinolytic to the hypercoagulopathic and hypofibrinolytic states exists in MAC, much as it does in TIC (►Table 3). We have seen that the paradigm for hypocoagulability and hyperfibrinolytic hematologic malignancy is observed in APL. Patients with this form of leukemia are often hypocoagulable at presentation and the hypocoagulability progressed with chemotherapy in the pre-ATRA era; these patients’ coagulopathy is mostly abated by 7 to 10 days with ATRA treatment.53,54,56–58,134 To date there are no studies of VETs in this group of patients. However, there have been reported cases of the utilization of TEG/ROTEM to predict thromboembolic events and hypercoagulability in patients with hematologic malignancies.
Table 3.
Hematopoietic malignancies
| Study | Cancer/number | Test | Findings |
|---|---|---|---|
| Crowley et al, 2017137 | Multiple myeloma | TEG | ↓ K and ↑α-angle after induction. Hypercoagulability markers directly proportional to myeloma burden. |
| Patients: 16 | |||
| Giaccherini et al, 201645 | Myeloproliferative neoplasms Patients: 39 |
ROTEM | ↓ CFT and ↑MCF. MCF values indicated lower platelet reactivity in MPN patients. |
| Gracheva et al, 2015135 | Multiple myeloma | TEG | Thrombin generation test and TEG parameters (R, K, and MA) reveal hypercoagulability in patients with multiple myeloma. |
| Patients: 20 | |||
| Crowley et al, 2015138 | Myeloma/MGUS Patients: 24 |
TEG | No difference in TEG parameters between multiple myeloma and MGUS. |
| Ko et al, 2015136 | Acute lymphoblastic leukemia | TEG | ↓ R and ↑MA during induction. |
| Patients: 80 | |||
| Akay et al, 2015140 | Hematologic malignancies | ROTEM | No difference in TEG parameters in low-dose or high-dose random or single-donor platelet transfusions. |
| Patients: 100 | |||
| Flisberg et al, 2009139 | Thrombocytopenia due to hematologic malignancy | ROTEM | Platelet transfusion in patients with hematologic malignancies is associated with ↓ CFT and ↑MCF. |
| Patients: 20 |
Abbreviations: CFT, clot formation time; K, kinetics; MA, maximal amplitude; MCF, maximum clot firmness; MGUS, monoclonal gammopathy of undetermined significance; MPN, myeloproliferative neoplasm; R, reaction time; ROTEM, rotational thromboelastometry; TEG, thromboelastography.
Giaccherini et al found a hypercoagulable profile in patients with myeloproliferative neoplasms and ROTEM analysis demonstrated increased MCF and shorter CFT in this population.45 When MCF values were corrected for platelet counts, the parameters supported the hypothesis that platelet exhaustion occurred from clotting activation. Gracheva et al in 2015 described 20 patients utilizing TEG (specifically the R, K, and MA parameters), thrombin generation test, and thrombodynamics which revealed hypercoagulability in patients with multiple myeloma.135 Ko et al in 2017 demonstrated a short R and an increased MA in 80 patients with acute lymphoblastic leukemia during induction.136 Crowley et al in 2017 noted borderline shortened K and increased α-angle of the TEG in patients with multiple myeloma that reflected the burden of disease in the postinduction period.137 However, previously in 2015 Crowley et al found no difference in TEG parameters between multiple myeloma, monoclonal gammopathy of undetermined significance, and control.138
Flisberg et al in 2009 demonstrated that patients with hematologic malignancies who were given platelets prior to central venous port placement responded with shortened CFT and increased MCF.139 The implication was that TEG measurements are a useful parameter for the necessity of platelet transfusion in patients with hematologic malignancies needing this procedure. Akay et al noted in 2015 that ROTEM measurements had no statistical difference between low-dose and high-dose, random or single-donor platelet transfusion in patients with hematologic malignancies.140 Therefore, they proposed that based on ROTEM, low-dose platelet transfusion should be considered because of its reduced likelihood of transfusion reactions and economic burden.
The Spectrum of MAC
The recent application of the description of the spectrum of fibrinolytic phenotypes in trauma may allow adaptation of this phenotype classification to patients with MAC. The trauma patient can evolve in seconds to minutes from one phenotype to another in what has been called “phenotype switching.”114,141 Patients who remain in a prolonged state of hypofibrinolysis or fibrinolytic shutdown are at increased risk for venous thromboembolism and MOF.30 In posttrauma resuscitation hypercoagulability, this shutdown occurs in the days following trauma and is comparable to the hypercoagulability of cancer that evolves over months to years.
The importance of the fibrinolytic shutdown in the pathophysiology of trauma and cancer-related hypercoagulability has long been a matter of intense investigation. The parameters for the definition of fibrinolytic shutdown as determined by VETs and used to guide BCT in bleeding patients recently have been refined and are matters of continued investigation.25,142–147
As has been studied in trauma and liver transplant patients, it would be reasonable to infer that a similar fibrinolytic spectrum exists in cancer patients; there are theoretical and clinical studies that confirm this inference. In fact, there has been a significant body of work regarding the fibrinolytic system and cancer. This work has demonstrated a similar event to fibrinolytic shutdown occurring in the 24-hour postoperative period following cancer surgeries. For example, Galster et al and others have noted that differences in the activation of coagulation depend on the cancer type, which suggests that this process is mainly related to procoagulants released from the neoplastic cells.148,149 This clinical observation has fueled research into the fibrinolytic spectrum including fibrinolytic shutdown, whether in trauma or cancer, as a reflection of the nascent state of hypercoagulability.35,75,150–152
We have seen that there are similarities between the mechanisms of coagulopathies of TIC and MAC. While TIC evolves over a much more rapid period and has fewer variables causing the hemostatic derangements than MAC, the basic influences of endothelial dysfunction, the TF effects on the TM–thrombin activated protein C pathway, and the balances between PAI-1, antifibrinolytics, and anticoagulants are similar between TIC and MAC. In both TIC and MAC, these changes result in an eventual hypercoagulable state for trauma patients who survive the initial insult and for cancer patients who do not succumb to initial hypocoagulable complications. The switch from bleeding due to thrombocytopenia, DIC, or immediate fibrinolysis to a hypercoagulable state has been documented in successfully treated APL patients.53,54,56–59,134
In trauma and in malignancy, PAI-1 is one of the main factors responsible for hypercoagulability, increased rates of venous thromboemboli, and MOF.112 It has been shown that elevated levels of PAI-1 are associated with increased risk of venous thromboembolism in orthopedic surgery, and in particular, in patients with acute hip fractures who have been given TXA.153,154 Specific ratios of PAIs to activators may shut down fibrinolysis after certain surgeries. It has been noted that following postoperative hip replacement, endothelial cells cultured in the plasma of these patients have high PAI-1 levels.154,155 Antifibrinolytics are not recommended in the treatment of acute hip fractures since an increased rate of venous thromboembolism has been noted in those patients treated with TXA confirming the above-mentioned recent large study demonstrating increased risk of thromboemboli without mortality benefits in patients given TXA.110,153,155 In MAC, elevation of PAI-1 and reduced levels of tPA and antithrombin have been noted and associated with a hypofibrinolysis phenotype.38,47,113 The use of more modern assays revealed similar elevation of PAI-1 and other natural antifibrinolytics, as well as reductions in fibrinolytic proteins.38,156
Potential use of Precision-Based Medicine in MAC and TIC
Large randomized controlled trials regarding the empiric management of bleeding trauma and obstetrical patients have been met with differing levels of acceptances as the histories of the CRASH-2 and WOMAN trials have shown.27,157–163 Because of the unintended consequence of “one-size-fits-all” randomized controlled trial philosophy touted for the advancement of evidence-based clinical science, it is more difficult to engage in smaller proof-of-concept studies that evaluate the influence of specific pathophysiological derangements and phenotypes on clinical outcomes.164,165
The concept of precision-based medicine has been developed to allow for the integration of epidemiologic findings from randomized controlled trials with select patient-group-specific clinical findings.165,166 For example, the field of proteomics can help identify patients with specific fibrinolytic phenotypes and these subsets of patients can be studied in a focused manner with attention to precision-based medicine.158,167
Conclusion
Virchow’s prescient anticipation of the cell-based theory of hemostasis in 1855 with the words: “Omnis cellulae cellula” [All cells from pre-existing cells], sets the stage for the next phase in delineating hemostatic competence of patients along the spectra of coagulopathies.1,2 Currently, the VETs, TEG, and ROTEM are the most commonly used whole-blood assays that avail themselves of the cell-based theory of hemostasis in order to predict bleeding and coagulation, and to guide BCT in cancer patients. However, these point-of-care tests have barriers because there is no universal system that is agreed upon resulting in interobserver variability and institution-specific procedures. The TEG and ROTEM parameters are not directly interchangeable and separate expertise is required in order to utilize each test.168 These tests are not yet at the level of reproducibility that the plasma-based tests are and therefore, these tests are in their relative infancy in the areas of trauma resuscitation and assessment of MAC.169 Yet, as seen in ►Tables 2 and 3, there is substantial literature that describes the utilization of the TEG and ROTEM to guide decisions regarding coagulopathic patients with MAC. The concern regarding interobserver reproducibility and institutional variability has now been addressed with cartridge system VETs such as the TEG6s and the ROTEM Sigma. These cartridge VETs facilitate a more standardized performance of assays that detect the lifespan of the clot from initiation through amplification, propagation, and termination via fibrinolysis. These cartridge VETs also provide a reproducible quantification of the efficacy of anti-Xa and direct thrombin inhibitors. These new cartridge VETs such as the TEG6s and the ROTEM Sigma may hold the future to a more vigorous and refined utilization of VETs to define and guide therapy and prevention of thrombosis in cancer patients.170,171 Moreover, recent development of multichannel microfluidic assays that include endothelium may allow us soon to advance confidence in quantifying hemostasis based on plasma, platelets, and endothelium. Thus, emerging endothelium-based assays may be used to further refine the assessment of hemostatic integrity, not just in trauma patients, but also in patients with hypercoagulopathic states in cancer.172–174 The future of assaying coagulopathy in cancer, but also in all areas of surgery, transplantation, and trauma, remains an exciting, open field that promises to bear fruit with the expansion of these whole-blood-based technologies. The history of the utilization of VETs to guide BCT and to predict venous and arterial thrombosis in transplant patients and in trauma patients has formed a pathophysiologic foundation that will usher in an era where VETs may be used in order to risk stratify patients with MAC. This new era will allow for more refined prevention and treatment of thrombosis in cancer patients. Guidelines concerning the dosage and timing of administration of venous thromboembolism prophylaxis in cancer patients are not clear. Recently, direct oral anticoagulants (DOACs) have been shown to be noninferior to LMWH in the treatment of venous thromboembolism from MAC.175 Because TEG has been validated in monitoring the dosage of LMWH and may be validated to monitor DOACs, the role of TEG as a resource for guiding prevention and treatment of MAC in cancer patients is likely to continue to evolve.131,176,177
Acknowledgements
Financial support or grants: Haemonetics Inc., Braintree, Massachusetts.
Conflict of Interest
Dr. Walsh reports grants from Haemonetics, outside the submitted work. Dr. H. Moore received research support from Instrument Laboratories and Haemonetics. He also shares intellectual property with Haemonetics and Thrombo Therapeutics Incorporated. He is also on the board of Thrombo Therapeutics Incorporated. Dr. E. Moore reports research grant from Haemonetics, outside the submitted work. In addition, he has a patent tPA TEG issued. Dr. Achneck reports having served as an employee of Haemonetics in the position of Director of Medical Affairs and Clinical Development during writing of the manuscript. The other authors have no conflict of interest to disclose.
References
- 1.Virchow RLK. Cellular Pathology: As Based upon Physiological and Pathological Histology. Twenty Lectures Delivered in the Pathological Institute of Berlin during the Months of February, March and April, 1858. New York, NY: RM De Witt; 1860 [DOI] [PubMed] [Google Scholar]
- 2.Bagot CN, Arya R. Virchow and his triad: a question of attribution. Br J Haematol 2008;143(02):180–190 [DOI] [PubMed] [Google Scholar]
- 3.Kashuk JL, Moore EE, Sabel A, et al. Rapid thrombelastography (r-TEG) identifies hypercoagulability and predicts thromboembolic events in surgical patients. Surgery 2009;146(04):764–772, discussion 772–774 [DOI] [PubMed] [Google Scholar]
- 4.Trousseau A Clinique médicale de l’Hôtel-Dieu de Paris. Vol 2 Paris: Baillière; 1861 [Google Scholar]
- 5.Sørensen HT, Mellemkjaer L, Olsen JH, Baron JA. Prognosis of cancers associated with venous thromboembolism. N Engl J Med 2000;343(25):1846–1850 [DOI] [PubMed] [Google Scholar]
- 6.Rickles FR, Edwards RL. Activation of blood coagulation in cancer: Trousseau’s syndrome revisited. Blood 1983;62(01):14–31 [PubMed] [Google Scholar]
- 7.Falanga A, Marchetti M, Vignoli A. Coagulation and cancer: biological and clinical aspects. J ThrombHaemost 2013;11(02):223–233 [DOI] [PubMed] [Google Scholar]
- 8.Blom JW, Osanto S, Rosendaal FR. High risk of venous thrombosis in patients with pancreatic cancer: a cohort study of 202 patients. Eur J Cancer 2006;42(03):410–414 [DOI] [PubMed] [Google Scholar]
- 9.Falanga A, Barbui T, Rickles FR, Levine MN; For the Scientific and Standardization Committee of the Subcommittee on Haemostasis and Malignancy International Society of Thrombosis and Haemostasis. Guidelines for clotting studies in cancer patients. Thromb Haemost 1993;70(03):540–542 [PubMed] [Google Scholar]
- 10.Falanga A, Schieppati F, Russo D. Cancer tissue procoagulant mechanisms and the hypercoagulable state of patients with cancer. Semin Thromb Hemost 2015;41(07):756–764 [DOI] [PubMed] [Google Scholar]
- 11.Falanga A, Ofosu FA, Delaini F, et al. The hypercoagulable state in cancer patients: evidence for impaired thrombin inhibitions. Blood Coagul Fibrinolysis 1994;5(01, Suppl Suppl 1):S19–S23, discussion 59–64 [DOI] [PubMed] [Google Scholar]
- 12.Lippi G, Salvagno GL, Ippolito L, Franchini M, Favaloro EJ. Shortened activated partial thromboplastin time: causes and management. Blood Coagul Fibrinolysis 2010;21(05):459–463 [DOI] [PubMed] [Google Scholar]
- 13.Ay C, Dunkler D, Marosi C, et al. Prediction of venous thromboembolismin cancer patients. Blood 2010;116(24):5377–5382 [DOI] [PubMed] [Google Scholar]
- 14.Ay C, Vormittag R, Dunkler D, et al. D-dimer and prothrombin fragment 1þ2 predict venous thromboembolism in patients with cancer: results from the Vienna Cancer and Thrombosis Study. J Clin Oncol 2009;27(25):4124–4129 [DOI] [PubMed] [Google Scholar]
- 15.Khorana AA, Francis CW, Culakova E, Lyman GH. Risk factors for chemotherapy-associated venous thromboembolism in a prospective observational study. Cancer 2005;104(12):2822–2829 [DOI] [PubMed] [Google Scholar]
- 16.Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood 2008;111(10):4902–4907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mandala M, Clerici M, Corradino I, et al. Incidence, risk factors and clinical implications of venous thromboembolism in cancer patients treated within the context of phase I studies: the ‘SENDO experience’. Ann Oncol 2012;23(06):1416–1421 [DOI] [PubMed] [Google Scholar]
- 18.Moore RA, Adel N, Riedel E, et al. High incidence of thromboembolic events in patients treated with cisplatin-based chemotherapy: a large retrospective analysis. J Clin Oncol 2011;29(25):3466–3473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palumbo A, Rajkumar SV, Dimopoulos MA, et al. ; International Myeloma Working Group. Prevention of thalidomide- and lenalidomide-associated thrombosis in myeloma. Leukemia 2008;22 (02):414–423 [DOI] [PubMed] [Google Scholar]
- 20.Verso M, Agnelli G, Barni S, Gasparini G, LaBianca R. A modified Khorana risk assessment score for venous thromboembolism in cancer patients receiving chemotherapy: the Protecht score. Intern Emerg Med 2012;7(03):291–292 [DOI] [PubMed] [Google Scholar]
- 21.Ay C, Simanek R, Vormittag R, et al. High plasma levels of soluble P-selectin are predictive of venous thromboembolism in cancer patients: results from the Vienna Cancer and Thrombosis Study (CATS). Blood 2008;112(07):2703–2708 [DOI] [PubMed] [Google Scholar]
- 22.Lyman GH, Khorana AA, Falanga A, et al. ; American Society of Clinical Oncology. American Society of Clinical Oncology guideline: recommendations for venous thromboembolism prophylaxis and treatment in patients with cancer. J Clin Oncol 2007;25 (34):5490–5505 [DOI] [PubMed] [Google Scholar]
- 23.Hincker A, Feit J, Sladen RN, Wagener G. Rotational thromboelastometry predicts thromboembolic complications after major non-cardiac surgery. Crit Care 2014;18(05):549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akay OM, Ustuner Z, Canturk Z, Mutlu FS, Gulbas Z. Laboratory investigation of hypercoagulability in cancer patients using rotation thrombelastography. Med Oncol 2009;26(03):358–364 [DOI] [PubMed] [Google Scholar]
- 25.Levi M, Hunt BJ. A critical appraisal of point-of-care coagulation testing in critically ill patients. J Thromb Haemost 2015;13(11): 1960–1967 [DOI] [PubMed] [Google Scholar]
- 26.Ostrowski SR, Berg RM, Windeløv NA, et al. Discrepant fibrinolytic response in plasma and whole blood during experimental endotoxemia in healthy volunteers. PLoS One 2013;8(03):e59368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Curry NS, Davenport R. Transfusion strategies for major haemorrhage in trauma. Br J Haematol 2019; 184(04):508–523 [DOI] [PubMed] [Google Scholar]
- 28.Walsh M, Jbara M, Miller A, Lawson J. Thromboelastographic goal-directed blood component therapy for severe hemorrhage. Blood Bulletin 2014. Available at: http://www.cbccts.org/media/38370/BloodBulletin_March2014.pdf. Accessed April 10, 2019
- 29.Schöchl H, Maegele M, Solomon C, Görlinger K, Voelckel W. Early and individualized goal-directed therapy for trauma-induced coagulopathy. Scand J Trauma Resusc Emerg Med 2012;20:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meizoso JP, Karcutskie CA, Ray JJ, Namias N, Schulman CI, Proctor KG. Persistent fibrinolysis shutdown is associated with increased mortality in severely injured trauma patients. J Am Coll Surg 2017;224(04):575–582 [DOI] [PubMed] [Google Scholar]
- 31.Moore EE, Moore HB, Gonzalez E, et al. Postinjury fibrinolysis shutdown: rationale for selective tranexamic acid. J Trauma Acute Care Surg 2015;78(06, Suppl Suppl 1):S65–S69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valle EJ, Allen CJ, Van Haren RM, et al. Do all trauma patients benefit from tranexamic acid? J Trauma Acute Care Surg 2014;76 (06):1373–1378 [DOI] [PubMed] [Google Scholar]
- 33.Roberts DJ, Kalkwarf KJ, Moore HB, et al. Time course and outcomes associated with transient versus persistent fibrinolytic phenotypes after injury: a nested, prospective, multicenter cohort study. J Trauma Acute Care Surg 2019;86(02):206–213 [DOI] [PubMed] [Google Scholar]
- 34.Gary JL, Schneider PS, Galpin M, et al. Can thrombelastography predict venous thromboembolic events in patients with severe extremity trauma? J Orthop Trauma 2016;30(06):294–298 [DOI] [PubMed] [Google Scholar]
- 35.Moore HB, Paniccia A, Lawson PJ, et al. Utility of viscoelastic assays beyond coagulation: can preoperative thrombelastography indices predict tumor histology, nodal disease, and resect-ability in patients undergoing pancreatectomy? J Am Coll Surg 2018;227(01):55–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tun NM, Guevara E, Oo TH. Benefit and risk of primary thromboprophylaxis in ambulatory patients with advanced pancreatic cancer receiving chemotherapy: a systematic review and meta-analysis of randomized controlled trials. Blood Coagul Fibrinolysis 2016;27(03):270–274 [DOI] [PubMed] [Google Scholar]
- 37.Toukh M, Siemens DR, Black A, et al. Thromboelastography identifies hypercoagulablilty and predicts thromboembolic complications in patients with prostate cancer. Thromb Res 2014;133(01):88–95 [DOI] [PubMed] [Google Scholar]
- 38.Hisada Y, Mackman N. Cancer-associated pathways and biomarkers of venous thrombosis. Blood 2017;130(13):1499–1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pinto S, Gori L, Gallo O, Boccuzzi S, Paniccia R, Abbate R. Increased thromboxane A2 production at primary tumor site in metastasizing squamous cell carcinoma of the larynx. Prostaglandins Leukot Essent Fatty Acids 1993;49(01):527–530 [DOI] [PubMed] [Google Scholar]
- 40.Boccaccio C, Sabatino G, Medico E, et al. The MET oncogene drives a genetic programme linking cancer to haemostasis. Nature 2005;434(7031):396–400 [DOI] [PubMed] [Google Scholar]
- 41.Varki A Trousseau’s syndrome: multiple definitions and multiple mechanisms. Blood 2007;110(06):1723–1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nickel KF, Ronquist G, Langer F, et al. The polyphosphate-factor XII pathway drives coagulation in prostate cancer-associated thrombosis. Blood 2015;126(11):1379–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zucchella M, Dezza L, Pacchiarini L, et al. Human tumor cells cultured “in vitro” activate platelet function by producing ADP or thrombin. Haematologica 1989;74(06):541–545 [PubMed] [Google Scholar]
- 44.Shao B, Wahrenbrock MG, Yao L, et al. Carcinoma mucins trigger reciprocal activation of platelets and neutrophils in a murine model of Trousseau syndrome. Blood 2011;118(15):4015–4023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Giaccherini C, Verzeroli C, Marchetti M, et al. PO-26 - Whole blood rotational thromboelastometry (ROTEM) to detect hypercoagulability in patients with myeloproliferative neoplasms (MPN). Thromb Res 2016;140(Suppl 1):S185–S186 [DOI] [PubMed] [Google Scholar]
- 46.Kessler CM. Propensity for hemorrhage and thrombosis in chronic myeloproliferative disorders. Semin Hematol 2004;41 (02, Suppl 3):10–14 [DOI] [PubMed] [Google Scholar]
- 47.Honegger H, Anderson N, Hewitt LA, Tullis JL. Antithrombin III profiles in malignancy, relationship primary tumors and metastatic sites. Thromb Haemost 1981;46(02):500–503 [PubMed] [Google Scholar]
- 48.Ponticelli C Membranous nephropathy. J Nephrol 2007;20(03): 268–287 [PubMed] [Google Scholar]
- 49.Dobson GP, Letson HL, Sharma R, Sheppard FR, Cap AP. Mechanisms of early trauma-induced coagulopathy: the clot thickens or not? J Trauma Acute Care Surg 2015;79(02):301–309 [DOI] [PubMed] [Google Scholar]
- 50.Johansson PI, Stensballe J, Ostrowski SR. Shock induced endotheliopathy (SHINE) in acute critical illness - a unifying pathophysiologic mechanism. Crit Care 2017;21(01):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brohi K, Cohen MJ, Ganter MT, Matthay MA, Mackersie RC, Pittet JF. Acute traumatic coagulopathy: initiated by hypoperfusion: modulated through the protein C pathway? Ann Surg 2007;245 (05):812–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Díez N, Montes R, Alonso A, et al. Association of increased fibrinogen concentration with impaired activation of anticoagulant protein C. J Thromb Haemost 2006;4(02):398–402 [DOI] [PubMed] [Google Scholar]
- 53.Kwaan HC, Cull EH. The coagulopathy in acute promyelocytic leukaemia–what have we learned in the past twenty years. Best Pract Res Clin Haematol 2014;27(01):11–18 [DOI] [PubMed] [Google Scholar]
- 54.Kwaan HC, Rego EM. Role of microparticles in the hemostatic dysfunction in acute promyelocytic leukemia. Semin Thromb Hemost 2010;36(08):917–924 [DOI] [PubMed] [Google Scholar]
- 55.Liu Y, Wang Z, Jiang M, et al. The expression of annexin II and its role in the fibrinolytic activity in acute promyelocytic leukemia. Leuk Res 2011;35(07):879–884 [DOI] [PubMed] [Google Scholar]
- 56.Stein E, McMahon B, Kwaan H, Altman JK, Frankfurt O, Tallman MS. The coagulopathy of acute promyelocytic leukaemia revisited. Best Pract Res Clin Haematol 2009;22(01):153–163 [DOI] [PubMed] [Google Scholar]
- 57.Tallman MS, Kwaan HC. Reassessing the hemostatic disorder associated with acute promyelocytic leukemia. Blood 1992;79 (03):543–553 [PubMed] [Google Scholar]
- 58.Zakarija A, Kwaan HC. Adverse effects on hemostatic function of drugs used in hematologic malignancies. Semin Thromb Hemost 2007;33(04):355–364 [DOI] [PubMed] [Google Scholar]
- 59.Kwaan HC, Rego EM, McMahon B, Weiss I, Marvin J. Microparticles in acute promyelocytic leukemia. Blood 2011;118(21): 3346 [Google Scholar]
- 60.Mutch NJ, Engel R, Uitte de Willige S, Philippou H, Ariëns RAJB. Polyphosphate modifies the fibrin network and down-regulates fibrinolysis by attenuating binding of tPA and plasminogen to fibrin. Blood 2010;115(19):3980–3988 [DOI] [PubMed] [Google Scholar]
- 61.Esmon CT. Molecular circuits in thrombosis and inflammation. Thromb Haemost 2013;109(03):416–420 [DOI] [PubMed] [Google Scholar]
- 62.Hardaway R, Bredenberg C. Monitoring hematology laboratory values In: Care of Wounded in Vietnam. Manhattan, KS: Sunflower University Press; 1988:139–220 [Google Scholar]
- 63.Hartert H, Schaeder J. The physical and biological constants of thrombelastography. Biorheology 1962;1(01):31–39 [Google Scholar]
- 64.Kang YG, Martin DJ, Marquez J, et al. Intraoperative changes in blood coagulation and thrombelastographic monitoring in liver transplantation. Anesth Analg 1985;64(09):888–896 [PMC free article] [PubMed] [Google Scholar]
- 65.Enriquez LJ, Shore-Lesserson L. Point-of-care coagulation testing and transfusion algorithms. Br J Anaesth 2009;103(01, Suppl Suppl 1):i14–i22 [DOI] [PubMed] [Google Scholar]
- 66.Shore-Lesserson L, Manspeizer HE, DePerio M, Francis S, Vela-Cantos F, Ergin MA. Thromboelastography-guided transfusion algorithm reduces transfusions in complex cardiac surgery. Anesth Analg 1999;88(02):312–319 [DOI] [PubMed] [Google Scholar]
- 67.Johansson PI, Stissing T, Bochsen L, Ostrowski SR. Thrombelastography and tromboelastometry in assessing coagulopathy in trauma. Scand J Trauma Resusc Emerg Med 2009;17(01):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kaufmann CR, Dwyer KM, Crews JD, Dols SJ, Trask AL. Usefulness of thrombelastography in assessment of trauma patient coagulation. J Trauma 1997;42(04):716–720, discussion 720–722 [DOI] [PubMed] [Google Scholar]
- 69.Spiel AO, Mayr FB, Firbas C, Quehenberger P, Jilma B. Validation of rotation thrombelastography in a model of systemic activation of fibrinolysis and coagulation in humans. J Thromb Haemost 2006; 4(02):411–416 [DOI] [PubMed] [Google Scholar]
- 70.Traverso CI, Caprini JA, Arcelus JI. The normal thromboelastogram and its interpretation. Semin Thromb Hemost 1995;21 (Suppl (Suppl 4):7–13 [DOI] [PubMed] [Google Scholar]
- 71.Walsh M, Fritz S, Hake D, et al. Targeted thromboelastographic (TEG) blood component and pharmacologic hemostatic therapy in traumatic and acquired coagulopathy. Curr Drug Targets 2016;17(08):954–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gorlinger K, Bhardwaj V, Kapoor PM. Simulation in coagulation testing using rotational thromboelastometry: a fast emerging, reliable point of care technique. Ann Card Anaesth 2016;19(03): 516–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Johansson PI. Coagulation monitoring of the bleeding traumatized patient. Curr Opin Anaesthesiol 2012;25(02):235–241 [DOI] [PubMed] [Google Scholar]
- 74.Gonzalez E, Pieracci FM, Moore EE, Kashuk JL. Coagulation abnormalities in the trauma patient: the role of point-of-care thromboelastography. Semin Thromb Hemost 2010;36(07):723–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moore HB, Moore EE, Gonzalez E, et al. Hyperfibrinolysis, physiologic fibrinolysis, and fibrinolysis shutdown: the spectrum of postinjury fibrinolysis and relevance to antifibrinolytic therapy. J Trauma Acute Care Surg 2014;77(06):811–817, discussion 817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Görlinger K, Fries D, Dirkmann D, Weber CF, Hanke AA, Schöchl H. Reduction of fresh frozen plasma requirements by perioperative point-of-care coagulation management with early calculated goal-directed therapy. Transfus Med Hemother 2012;39(02): 104–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Goldenberg NA, Hathaway WE, Jacobson L, Manco-Johnson MJ. A new global assay of coagulation and fibrinolysis. Thromb Res 2005;116(04):345–356 [DOI] [PubMed] [Google Scholar]
- 78.Holcomb JB, Minei KM, Scerbo ML, et al. Admission rapid thrombelastography can replace conventional coagulation tests in the emergency department: experience with 1974 consecutive trauma patients. Ann Surg 2012;256(03):476–486 [DOI] [PubMed] [Google Scholar]
- 79.Davenport R, Manson J, De’Ath H, et al. Functional definition and characterization of acute traumatic coagulopathy. Crit Care Med 2011;39(12):2652–2658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kitchens CS. To bleed or not to bleed? Is that the question for the PTT?. J Thromb Haemost 2005;3(12):2607–2611 [DOI] [PubMed] [Google Scholar]
- 81.Segal JB, Dzik WH; Transfusion Medicine/Hemostasis Clinical Trials Network. Paucity of studies to support that abnormal coagulation test results predict bleeding in the setting of invasive procedures: an evidence-based review. Transfusion 2005;45 (09):1413–1425 [DOI] [PubMed] [Google Scholar]
- 82.Toulon P, Ozier Y, Ankri A, Fléron M-H, Leroux G, Samama CM. Point-of-care versus central laboratory coagulation testing during haemorrhagic surgery. A multicenter study. Thromb Haemost 2009;101(02):394–401 [PubMed] [Google Scholar]
- 83.Schöchl H, Solomon C, Voelckel W. Thromboelastometry in the perioperative setting. Neth J Crit Care 2010;14(01):23–31 [Google Scholar]
- 84.Scărlătescu E, Lancé MD, White NJ, Aramă SS, Tomescu DR. Effects of malignancy on blood coagulation in septic intensive care patients. Blood Coagul Fibrinolysis 2018;29(01):92–96 [DOI] [PubMed] [Google Scholar]
- 85.Ostrowski SR, Windeløv NA, Ibsen M, Haase N, Perner A, Johansson PI. Consecutive thrombelastography clot strength profiles in patients with severe sepsis and their association with 28-day mortality: a prospective study. J Crit Care 2013;28(03):317. e1–317.e11 [DOI] [PubMed] [Google Scholar]
- 86.Kander T, Larsson A, Taune V, Schött U, Tynngård N. Assessment of haemostasis in disseminated intravascular coagulation by use of point-of-care assays and routine coagulation tests, in critically ill patients; a prospective observational study. PLoS One 2016;11 (03):e0151202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Park MS, Martini WZ, Dubick MA, et al. Thromboelastography as a better indicator of hypercoagulable state after injury than prothrombin time or activated partial thromboplastin time. J Trauma 2009;67(02):266–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liang H, Yang CX, Li H, Wen XJ, Zhou QL, Gu MN. The effects of preloading infusion with hydroxyethyl starch 200/0.5 or 130/0.4 solution on hypercoagulability and excessive platelet activation of patients with colon cancer. Blood Coagul Fibrinolysis 2010;21 (05):406–413 [DOI] [PubMed] [Google Scholar]
- 89.Wang Z-W, Ye P-J. Clinical analysis of acute cerebral infarction accompanied with lung cancer. J Acute Dis 2016;5(04):307–310 [Google Scholar]
- 90.Thorson CM, Van Haren RM, Ryan ML, et al. Persistence of hypercoagulable state after resection of intra-abdominal malignancies. J Am Coll Surg 2013;216(04):580–589, discussion 589–590 [DOI] [PubMed] [Google Scholar]
- 91.Thorson CM, Van Haren RM, Ryan ML, et al. Pre-existing hypercoagulability in patients undergoing potentially curative cancer resection. Surgery 2014;155(01):134–144 [DOI] [PubMed] [Google Scholar]
- 92.Bagatin D, Šakić K, Bagatin T, Šturm D, Milošević M. Does rotation thrombelastometry (ROTEM) improve early prediction of coagulopathy in breast tumor? Period Biol 2015;117(02):291–296 [Google Scholar]
- 93.Cohen E, Caprini J, Zuckerman L, Vagher P, Robinson B. Evaluation of three methods used to identify accelerated coagulability. Thromb Res 1977;10(04):587–604 [DOI] [PubMed] [Google Scholar]
- 94.Levi M. Diagnosis and treatment of disseminated intravascular coagulation. Int J Lab Hematol 2014;36(03):228–236 [DOI] [PubMed] [Google Scholar]
- 95.Asakura H, Takahashi H, Uchiyama T, et al. ; DIC subcommittee of the Japanese Society on Thrombosis and Hemostasis. Proposal for new diagnostic criteria for DIC from the Japanese Society on Thrombosis and Hemostasis. Thromb J 2016;14(01):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Brenner T, Schmidt K, Delang M, et al. Viscoelastic and aggregometric point-of-care testing in patients with septic shock – cross-links between inflammation and haemostasis. Acta Anaesthesiol Scand 2012;56(10):1277–1290 [DOI] [PubMed] [Google Scholar]
- 97.Collins PW, Macchiavello LI, Lewis SJ, et al. Global tests of haemostasis in critically ill patientswith severe sepsis syndrome compared to controls. Br J Haematol 2006;135(02):220–227 [DOI] [PubMed] [Google Scholar]
- 98.Daudel F, Kessler U, Folly H, Lienert JS, Takala J, Jakob SM. Thromboelastometry for the assessment of coagulation abnormalities in early and established adult sepsis: a prospective cohort study. Crit Care 2009;13(02):R42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dempfle C-E, Borggrefe M. Point of care coagulation tests in critically ill patients. Semin Thromb Hemost 2008;34(05): 445–450 [DOI] [PubMed] [Google Scholar]
- 100.Johansson PI, Stensballe J, Vindeløv N, Perner A, Espersen K. Hypocoagulability, as evaluated by thrombelastography, at admission to the ICU is associated with increased 30-day mortality. Blood Coagul Fibrinolysis 2010;21(02):168–174 [DOI] [PubMed] [Google Scholar]
- 101.Müller MC, Meijers JC, Vroom MB, Juffermans NP. Utility of thromboelastography and/or thromboelastometry in adults with sepsis: a systematic review. Crit Care 2014;18(01):R30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Meizoso JP, Ray JJ, Allen CJ, et al. Hypercoagulability and venous thromboembolism in burn patients. Semin Thromb Hemost 2015;41(01):43–48 [DOI] [PubMed] [Google Scholar]
- 103.Starzl TE. The saga of liver replacement, with particular reference to the reciprocal influence of liver and kidney transplantation (1955–1967). J Am Coll Surg 2002;195(05):587–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Groth CG, Pechet L, Starzl TE. Coagulation during and after orthotopic transplantation of the human liver. Arch Surg 1969; 98(01):31–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Stravitz RT. Potential applications of thromboelastography in patients with acute and chronic liver disease. Gastroenterol Hepatol (N Y) 2012;8(08):513–520 [PMC free article] [PubMed] [Google Scholar]
- 106.Fergusson DA, Hébert PC, Mazer CD, et al. ; BART Investigators. A comparison of aprotinin and lysine analogues in high-risk cardiac surgery. N Engl J Med 2008;358(22):2319–2331 [DOI] [PubMed] [Google Scholar]
- 107.Agarwal B, Wright G, Gatt A, et al. Evaluation of coagulation abnormalities in acute liver failure. J Hepatol 2012;57(04): 780–786 [DOI] [PubMed] [Google Scholar]
- 108.Mallett SV. Clinical utility of viscoelastic tests of coagulation (TEG/ROTEM) in patients with liver disease and during liver transplantation. Semin Thromb Hemost 2015;41(05):527–537 [DOI] [PubMed] [Google Scholar]
- 109.Stravitz RT, Lisman T, Luketic VA, et al. Minimal effects of acute liver injury/acute liver failure on hemostasis as assessed by thromboelastography. J Hepatol 2012;56(01):129–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Myers SP, Kutcher ME, Rosengart MR, et al. Tranexamic acid administration is associated with an increased risk of posttraumatic venous thromboembolism. J Trauma Acute Care Surg 2019;86(01):20–27 [DOI] [PubMed] [Google Scholar]
- 111.Harvin JA, Peirce CA, Mims MM, et al. The impact of tranexamic acid on mortality in injured patients with hyperfibrinolysis. J Trauma Acute Care Surg 2015;78(05):905–909, discussion 909–911 [DOI] [PubMed] [Google Scholar]
- 112.Moore HB, Moore EE, Liras IN, et al. Acute fibrinolysis shutdown after injury occurs frequently and increases mortality: a multicenter evaluation of 2,540 severely injured patients. J Am Coll Surg 2016;222(04):347–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sawaya R, Ramo OJ, Glas-Greenwalt P, Wu SZ. Plasma fibrinolytic profile in patients with brain tumors. Thromb Haemost 1991;65 (01):15–19 [PubMed] [Google Scholar]
- 114.Chapman MP, Moore EE, Moore HB, et al. Overwhelming tPA release, not PAI-1 degradation, is responsible for hyperfibrinolysis in severely injured trauma patients. J Trauma Acute Care Surg 2016;80(01):16–23, discussion 23–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Moore HB, Moore EE, Lawson PJ, et al. Fibrinolysis shutdown phenotype masks changes in rodent coagulation in tissue injury versus hemorrhagic shock. Surgery 2015;158(02):386–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Prat NJ, Montgomery R, Cap AP, et al. Comprehensive evaluation of coagulation in swine subjected to isolated primary blast injury. Shock 2015;43(06):598–603 [DOI] [PubMed] [Google Scholar]
- 117.Butler MJ. Thrombelastography during and after elective abdominal surgery. Thromb Haemost 1978;39(02):488–495 [PubMed] [Google Scholar]
- 118.Goh KY-C, Tsoi W-C, Feng C-S, Wickham N, Poon WS. Haemostatic changes during surgery for primary brain tumours. J Neurol Neurosurg Psychiatry 1997;63(03):334–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Howland WS, Castro EB, Fortner JB, Gould P. Proceedings: hypercoagulability. Thrombelastographic monitoring during extensive hepatic surgery. Arch Surg 1974;108(04):605–608 [DOI] [PubMed] [Google Scholar]
- 120.Mahla E, Lang T, Vicenzi MN, et al. Thromboelastography for monitoring prolonged hypercoagulability after major abdominal surgery. Anesth Analg 2001;92(03):572–577 [DOI] [PubMed] [Google Scholar]
- 121.Artang R, Jensen E, Pedersen F, Frandsen NJ. Thrombelastography in healthy volunteers, patients with stable angina and acute chest pain. Thromb Res 2000;97(06):499–503 [DOI] [PubMed] [Google Scholar]
- 122.Holloway DS, Vagher JP, Caprini JA, Simon NM, Mockros LF. Thrombelastography of blood from subjects with chronic renal failure. Thromb Res 1987;45(06):817–825 [DOI] [PubMed] [Google Scholar]
- 123.Fisch IR, Freedman SH. Oral contraceptives, ABO blood groups, and in vitro fibrin formation. Obstet Gynecol 1975;46(04):473–479 [PubMed] [Google Scholar]
- 124.Spillert CR, McGovern PJ, Vidaver RM, Lazaro EJ. Thromboelastographic evaluation of cut down-induced accelerated coagulation. Thromb Haemost 1981;46(02):567–567 [PubMed] [Google Scholar]
- 125.Blasi A, Molina V, Sanchez-Cabús S, Balust J, Garcia-Valdecasas JC, Taura P. Prediction of thromboembolic complications after liver resection for cholangiocarcinoma: is there a place for thromboelastometry? Blood Coagul Fibrinolysis 2018;29(01):61–66 [DOI] [PubMed] [Google Scholar]
- 126.De Pietri L, Montalti R, Begliomini B, et al. Thromboelastographic changes in liver and pancreatic cancer surgery: hypercoagulability, hypocoagulability or normocoagulability? Eur J Anaesthesiol 2010;27(07):608–616 [DOI] [PubMed] [Google Scholar]
- 127.Papa ML, Capasso F, Pudore L, et al. Thromboelastographic profiles as a tool for thrombotic risk in digestive tract cancer. Exp Oncol 2007;29(02):111–115 [PubMed] [Google Scholar]
- 128.Francis JL, Francis DA, Gunathilagan GJ. Assessment of hypercoagulability in patients with cancer using the Sonoclot Analyzer and thromboelastography. Thromb Res 1994;74(04):335–346 [DOI] [PubMed] [Google Scholar]
- 129.Wikner J, Beck-Broichsitter BE, Schlesinger S, et al. Thromboelastometry: a contribution to perioperative free-flap management. J Craniomaxillofac Surg 2015;43(07):1065–1071 [DOI] [PubMed] [Google Scholar]
- 130.Wen YR, Ho WY, Sun WZ, et al. Thromboelastographic study of thrombosis in the implantable central venous access device. Acta Anaesthesiol Sin 1997;35(04):223–228 [PubMed] [Google Scholar]
- 131.Attaran S, Somov P, Awad WI. Randomised high- and low-dose heparin prophylaxis in patients undergoing thoracotomy for benign and malignant disease: effect on thrombo-elastography. Eur J Cardiothorac Surg 2010;37(06):1384–1390 [DOI] [PubMed] [Google Scholar]
- 132.Akay MO, Bilir A, Öge T, Kuş G, Mutlu FŞ. The evaluation of hydroxyethyl starch (6% HES 130/0.4) solution’s potential preventive effects on coagulation status inwomen with gynecologic malignancies using rotation thromboelastography. Turk J Haematol 2014;31(03):261–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jansohn E, Bengzon J, Kander T, Schött U. A pilot study on the applicability of thromboelastometry in detecting brain tumourinduced hypercoagulation. Scand J Clin Lab Invest 2017;77(04): 289–294 [DOI] [PubMed] [Google Scholar]
- 134.Kwaan HC, Wang J, Boggio LN. Abnormalities in hemostasis in acute promyelocytic leukemia.HematolOncol 2002;20(01):33–41 [DOI] [PubMed] [Google Scholar]
- 135.Gracheva MA, Urnova ES, Sinauridze EI, et al. Thromboelastography, thrombin generation test and thrombodynamics reveal hypercoagulability in patients with multiple myeloma. Leuk Lymphoma 2015;56(12):3418–3425 [DOI] [PubMed] [Google Scholar]
- 136.Ko RH, Sposto R, Goodarzian F, Carmona R, Vasquez S, Young G. Thromboelastography to detect hypercoagulability in patients with newly diagnosed acute lymphoblastic leukemia. Blood 2015;126(23):111826170031 [Google Scholar]
- 137.Crowley MP, Quinn S, Coleman ET, O’Shea SI, Gilligan OM. The evolving hemostatic profile of patients with myeloma receiving treatment. Comp Clin Pathol 2017;26(03):713–717 [Google Scholar]
- 138.Crowley MP, Quinn S, Coleman E, Eustace JA, Gilligan OM, O’Shea SI. Differing coagulation profiles of patients with monoclonal gammopathy of undetermined significance and multiple myeloma. J Thromb Thrombolysis 2015;39(02):245–249 [DOI] [PubMed] [Google Scholar]
- 139.Flisberg P, Rundgren M, Engström M. The effects of platelet transfusions evaluated using rotational thromboelastometry. Anesth Analg 2009;108(05):1430–1432 [DOI] [PubMed] [Google Scholar]
- 140.Akay OM, Goren Sahin D, Andic N, et al. The utility of thromboelastometry in prophylactic platelet transfusion for hematological malignancies. Transfus Apheresis Sci 2015;53(01):64–68 [DOI] [PubMed] [Google Scholar]
- 141.Sur S, Sugimoto JT, Agrawal DK. Coronary artery bypass graft: why is the saphenous vein prone to intimal hyperplasia? Can J Physiol Pharmacol 2014;92(07):531–545 [DOI] [PubMed] [Google Scholar]
- 142.Cardenas JC, Wade CE, Cotton BA, et al. TEG lysis shutdown represents coagulopathy in bleeding trauma patients: analysis of the proppr cohort. Shock 2019;51(03):273–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gall LS, Davenport RA. Fibrinolysis and antifibrinolytic treatment in the trauma patient. Curr Opin Anaesthesiol 2018;31 (02):227–233 [DOI] [PubMed] [Google Scholar]
- 144.Gall LS, Vulliamy P, Gillespie S, et al. The S100A10 pathway mediates an occult hyperfibrinolytic subtype in trauma patients. Ann Surg 2018. (e-pub ahead of print). doi: 10.1097/SLA.0000000000002733 [DOI] [PubMed] [Google Scholar]
- 145.Hunt H, Stanworth S, Curry N, et al. Thromboelastography (TEG) and rotational thromboelastometry (ROTEM) for trauma induced coagulopathy in adult trauma patients with bleeding. Cochrane Database Syst Rev 2015;(02):CD010438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wikkelsø A, Wetterslev J, Møller AM, Afshari A. Thromboelastography (TEG) or rotational thromboelastometry (ROTEM) to monitor haemostatic treatment in bleeding patients: a systematic review with meta-analysis and trial sequential analysis. Anaesthesia 2017;72(04):519–531 [DOI] [PubMed] [Google Scholar]
- 147.Wikkelsø A, Wetterslev J, Møller AM, Afshari A. Thromboelastography (TEG) or thromboelastometry (ROTEM) to monitor haemostatic treatment versus usual care in adults or children with bleeding. Cochrane Database Syst Rev 2016;(08):CD007871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Galster H, Kolb G, Kohsytorz A, Seidlmayer C, Paal V. The pre-, peri-, and postsurgical activation of coagulation and the thromboembolic risk for different risk groups. Thromb Res 2000;100 (05):381–388 [DOI] [PubMed] [Google Scholar]
- 149.Garcia-Avello A, Galindo-Alvarez J, Martinez-Molina E, Cesar-Perez J, Navarro JL. Coagulative system activation and fibrinolytic system inhibition activities arise from tumoral draining vein in colon carcinoma. Thromb Res 2001;104(06):421–425 [DOI] [PubMed] [Google Scholar]
- 150.Gall L, Davenport R, Brohi K. Effect of early tranexamic acid on the coagulation system in patients with suspected traumatic haemorrhage: a prospective cohort study. Lancet 2016; 387:S46 [Google Scholar]
- 151.Raza I, Davenport R, Rourke C, et al. The incidence and magnitude of fibrinolytic activation in trauma patients. J Thromb Haemost 2013;11(02):307–314 [DOI] [PubMed] [Google Scholar]
- 152.Gomez-Builes JC, Acuna SA, Nascimento B, Madotto F, Rizoli SBJA. Harmful or physiologic: diagnosing fibrinolysis shutdown in a trauma cohort with rotational thromboelastometry. Anesth Analg 2018;127(04):840–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zufferey PJ, Miquet M, Quenet S, et al. ; Tranexamic acid in hipfracture surgery (THIF) study. Tranexamic acid in hip fracture surgery: a randomized controlled trial. Br J Anaesth 2010;104 (01):23–30 [DOI] [PubMed] [Google Scholar]
- 154.Kassis J, Hirsh J, Podor TJ. Evidence that postoperative fibrinolytic shutdown is mediated by plasma factors that stimulate endothelial cell type I plasminogen activator inhibitor biosynthesis. Blood 1992;80(07):1758–1764 [PubMed] [Google Scholar]
- 155.Yukizawa Y, Inaba Y, Watanabe S, et al. Association between venous thromboembolism and plasma levels of both soluble fibrin and plasminogen-activator inhibitor 1 in 170 patients undergoing total hip arthroplasty. Acta Orthop 2012;83(01):14–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kleiss SF, Adelmeijer J, Meijers JCM, Porte RJ, Lisman T. A sustained decrease in plasma fibrinolytic potential following partial liver resection or pancreas resection. Thromb Res 2016; 140:36–40 [DOI] [PubMed] [Google Scholar]
- 157.Roberts I, Shakur H, Coats T, et al. The CRASH-2 trial: a randomised controlled trial and economic evaluation of the effects of tranexamic acid on death, vascular occlusive events and transfusion requirement in bleeding trauma patients. Health Technol Assess 2013;17(10):1–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Letson HL, Dobson GP. Tranexamic acid for post-partum haemorrhage in the WOMAN trial. Lancet 2017;390(10102):1581–1582 [DOI] [PubMed] [Google Scholar]
- 159.The CRASH trial management group; The CRASH trial collaborators. The CRASH trial protocol (Corticosteroid randomisation after significant head injury) [ISRCTN74459797]. BMC Emerg Med 2001;1(01):1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Shakur H, Elbourne D, Gülmezoglu M, et al. The WOMAN Trial (World Maternal Antifibrinolytic Trial): tranexamic acid for the treatment of postpartum haemorrhage: an international randomised, doubleblind placebo controlled trial. Trials 2010;11(01):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Walsh M, Thomas S, Moore E, et al. Tranexamic acid for trauma resuscitation in the United States of America. Semin Thromb Hemost 2017;43(02):213–223 [DOI] [PubMed] [Google Scholar]
- 162.Henriquez DDCA, Bloemenkamp KWM, van der Bom JG. Management of postpartum hemorrhage: how to improve maternal outcomes? J Thromb Haemost 2018;8:1523–1534 [DOI] [PubMed] [Google Scholar]
- 163.Sentilhes L, Winer N, Azria E, et al. ; Groupe de Recherche en Obstétrique et Gynécologie. Tranexamic acid for the prevention of blood loss after vaginal delivery. N Engl J Med 2018;379(08): 731–742 [DOI] [PubMed] [Google Scholar]
- 164.Tignanelli CJ, Napolitano LM. The fragility index in randomized clinical trials as a means of optimizing patient care. JAMA Surg 2018;154:74–79 [DOI] [PubMed] [Google Scholar]
- 165.Beckmann JS, Lew D. Reconciling evidence-based medicine and precision medicine in the era of big data: challenges and opportunities. Genome Med 2016;8(01):134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med 2015;372(09):793–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Moore HB, Moore EE, Morton AP, et al. Shock-induced systemic hyperfibrinolysis is attenuated by plasma-first resuscitation. J Trauma Acute Care Surg 2015;79(06):897–903, discussion 903–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hildyard C, Curry N. Point-of-care testing: a standard of care? Anaesthesia 2015;70(10):1113–1118 [DOI] [PubMed] [Google Scholar]
- 169.Korpallová B, Samoš M, Bolek T, et al. Role of thromboelastography and rotational thromboelastometry in the management of cardiovascular diseases. Clin Appl Thromb Hemost 2018;24 (08):1199–1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Duffield C, Davies R, Barclay P. Advances in thromboelastograph technology. Anaesthesia 2018;73(03):398–399 [DOI] [PubMed] [Google Scholar]
- 171.Hans GA, Besser MW. The place of viscoelastic testing in clinical practice. Br J Haematol 2016;173(01):37–48 [DOI] [PubMed] [Google Scholar]
- 172.Walsh M, Shreve J, Thomas S, et al. Fibrinolysis in trauma: “myth,” “reality,” or “something in between”. Semin Thromb Hemost 2017;43(02):200–212 [DOI] [PubMed] [Google Scholar]
- 173.Li R, Elmongy H, Sims C, Diamond SL. Ex vivo recapitulation of trauma-induced coagulopathy and preliminary assessment of trauma patient platelet function under flow using microfluidic technology. J Trauma Acute Care Surg 2016;80(03):440–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kim D, Wu X, Young AT, Haynes CL. Microfluidics-based in vivo mimetic systems for the study of cellular biology. Acc Chem Res 2014;47(04):1165–1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Raskob GE, van Es N, Verhamme P, et al. ; Hokusai VTE Cancer Investigators. Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med 2018;378(07):615–624 [DOI] [PubMed] [Google Scholar]
- 176.Gottlieb A, Mraovic B, Sprung J, Potter P, Kottke-Marchant K. The usefulness of thromboelastography in determining the coagulation effects of low-molecular-weight heparin. Anesth Analg 1999;88(2S):274S [Google Scholar]
- 177.Zmuda K, Neofotistos D, Ts’ao CH. Effects of unfractionated heparin, low-molecular-weight heparin, and heparinoid on thromboelastographic assay of blood coagulation. Am J Clin Pathol 2000;113(05):725–731 [DOI] [PubMed] [Google Scholar]