Abstract
The polygenic risk score (PRS) allows for quantification of the relative contributions of genes and environment in population-based studies of mental health. We analyzed the impact of transdiagnostic schizophrenia PRS and measures of familial and environmental risk on the level of and change in general mental health (Short-Form-36 mental health) in the Netherlands Mental Health Survey and Incidence Study-2 general population sample, interviewed 4 times over a period of 9 years, yielding 8901 observations in 2380 individuals. Schizophrenia PRS, family history, somatic pain, and a range of environmental risks and social circumstances were included in the regression model of level of and change in mental health. We calculated the relative contribution of each (group of) risk factor(s) to the variance in (change in) mental health. In the combined model, familial and environmental factors explained around 17% of the variance in mental health, of which around 5% was explained by age and sex, 30% by social circumstances, 16% by pain, 22% by environmental risk factors, 24% by family history, and 3% by PRS for schizophrenia (PRS-SZ). Results were similar, but attenuated, for the model of mental health change over time. Childhood trauma and gap between actual and desired social status explained most of the variance. PRS for bipolar disorder, cross-disorder, and depression explained less variance in mental health than PRS-SZ. Polygenic risk for mental suffering, derived from significance-testing in massive samples, lacks impact in analyses focusing on prediction in a general population epidemiological setting. Social-environmental circumstances, particularly childhood trauma and perceived status gap, drive most of the attributable variation in population mental health.
Keywords: schizophrenia, psychotic disorder, genetics, environment, polygenic risk, mental health
Introduction
Heritability estimates of mental disorders, derived from twin and extended family studies, are typically in the range of 40%–80%. With the advent of molecular genetic testing, however, it has become clear that twin-based heritability estimates do not translate into direct effects of specific molecular genetic variation.1 Molecular genetic analysis allows the estimation of a model that predicts trait values from genotype data, expressed as a polygenic risk score (PRS).2 The amount of phenotypic variance explained by PRS typically is much lower than the amount of additive genetic variance estimated across twin studies. For arguably the most investigated mental disorder, schizophrenia, with an estimated twin heritability of 60%–80%, tens of thousands of markers explain only 7% of the variance on the liability scale and around 20% of the variance on the observed 0–1 scale derived from the logistic regression model.3,4 Thus, a considerable “heritability gap” remains, the origin of which may represent environmental effects, indirect genetic effects within the family, rare genetic variants, gene–environment interplay, assortative mating, or other factors.5
PRS is increasingly used as a measure of risk, etiology, or clinical utility in epidemiological studies.6,7 In psychiatry, the PRS has been used in some epidemiological studies to examine the prediction of mental disorders and related traits8 and to test the aspects of gene–environment interplay.9 These studies, however, have mostly focused on diagnosis-specific models and not on the relative contribution of PRS in population-based models of mental health. In addition, transdiagnostic molecular genetic analyses indicate that the majority of common genetic variants are non‐specifically associated with a range of mental disorders.10,11 Around two-thirds of genetic associations are common to schizophrenia, bipolar disorder, and major depressive disorder, and overlaps also exist with genetic variants contributing to autism, attention‐deficit/hyperactivity disorder, and intellectual disabilities.10,11 These findings suggest that PRS for mental disorders to a large extent represents transdiagnostic risk for mental suffering. PRS for schizophrenia (PRS-SZ) in particular is associated with a variety of disorders,12–14 quality of life,15 and subclinical multidimensional phenotypes.16–23 Indeed, investigation of electronic health records from the United States reveal that PRS-SZ is associated with not only a diagnosis of schizophrenia but also diagnoses of other related psychiatric and medical conditions.13
Given that schizophrenia, in a transdiagnostic psychopathology perspective, can be considered as the selection at the extreme end of the mental disorder severity spectrum, PRS-SZ, in comparison with other possible nonspecific PRS constructs, arguably should have the greatest probability of showing impact on mental health at the population level. In addition, PRS-SZ is better powered than genome-wide association studies of any other mental disorder. The transdiagnostic perspective of PRS thus opens the way to test the basic question to what degree PRS may contribute, in a population-based setting, to variation in mental health, and how this compares to known risk factors of mental ill-health. To our knowledge, no previous study has addressed this basic question. If a transdiagnostic mental health PRS predicts mental suffering in a population-based sample, over and above traditional measures of environmental and familial risk, significant progress could be made in elucidating the role of genetics in the diagnosis and treatment of mental suffering. In addition, showing the impact of PRS on mental health in population-based, epidemiological settings would considerably increase the scope for preventative usage of PRS.
The expectation, guided the existing literature,8 is that measures of PRS will have little or no predictivity in an epidemiological setting, as their contribution typically is evaluated on the basis of statistical significance-testing in massive samples, in which minute effects can acquire statistical significance.24 For prediction in a general population, epidemiological setting, however, a minimum clinical effect size is required to generate a statistical signal.24
As this aspect of PRS has not been analyzed previously in an epidemiological setting, in comparison with established clinical predictors, this study set out to comparatively quantify the prediction of PRS in a general population setting. To this end, we examined the contribution of PRS-SZ and other known risk factors to the variance in level and change of mental health in a large population-based cohort that was examined 4 times over a period of 9 years. Guided by previous work in this sample, we used a mental health phenotype that was responsive to variation in PRS-SZ.25
Methods
Study Population
All 4 waves of the Netherlands Mental Health Survey and Incidence Study-2 (NEMESIS-2) were used. NEMESIS-2 was conducted to study the prevalence, incidence, course, and consequences of mental disorders in the Dutch general population. The baseline data of NEMESIS-2 were collected from 2007 to 2009, and the follow-up was until 2018. The study was approved by the Medical Ethics Review Committee for Institutions on Mental Health Care, and written informed consent was collected from participants at each wave. To ensure the representativeness of the sample in terms of age (between the ages of 18 and 65 at baseline), region, and population density, a multistage random sampling procedure was applied. Dutch illiteracy was an exclusion criterion. Non-clinician, trained interviewers applied the Composite International Diagnostic Interview (CIDI) version 3.026,27 and additional questionnaires during home visits. Details of NEMESIS-2 are provided elsewhere.28,29 The first wave (T0) enrolled 6646 participants (response rate 65.1%; average interview duration: 95 min), who were followed up in 3 visits within 9 years: successive response rates at year 3 (T1), year 6 (T2), and year 9 (T3) were 80.4% (n = 5,303; excluding those who deceased; interview duration: 84 min), 87.8% (n = 4618; interview duration: 83 min), and 86.8% (n = 4007; interview duration: 102 min), respectively. Thus, more than 60% of the sample had follow-up from baseline to T3. Rates at baseline reflect lifetime occurrence; rates at T1 to T3 reflect approximately 3-year interval (baseline-T1, T1-T2, and T2-T3) occurrence. Attrition between T0 and T3 was not significantly associated with any of the individual 12-month mental disorders at T0 after controlling for sociodemographic characteristics.30,31
Measurements
Mental Health
The Short-Form-36 (SF-36) Health Survey 32 consists of 8 subscales, each scale ranging from poor (0) to good (100) functioning. Mental health, role limitations due to emotional problems, social functioning, and vitality were averaged into a single mental health dimension, while general health perceptions, physical functioning, role limitation due to physical health problems, and bodily pain were averaged into the physical health dimension.33 The SF-36 was assessed at each time-point and refers to the past 4 weeks. As per previous work in this sample examining PRS,25 the SF-36 mental health dimension at each time point was used in the analyses as the dependent variable, scored reversely so that higher scores reflect less mental health. In addition, the SF-36 dimension of bodily pain was used as an independent variable, given the fact that: mental ill-health and pain are strongly associated with each other; pain affects between one-third and one-half of the population; and pain represents one of the most prominent causes of disability worldwide according to the Global Burden of Disease reviews.34–36
Adverse Social Circumstances
Age was expressed in years, and sex was coded as male (“0”) or female (“1”). Marital status at each interview was coded married/widowed vs divorced/never married. Unemployment at each interview was coded as having no employment vs employment/homemaker/student/retired. Educational level at baseline was a 4-level variable (primary, lower and higher secondary, and higher professional/university education); income at each interview was net annual household income, rated on a scale from 1 to 14 (not rated at one interview and predicted linearly from the values at the interviews before and after). Having ever been on disability benefit over the period of observation was analyzed as a binary variable (5% of the sample). The variable “debts” was rated present at each interview (not rated at one interview and predicted linearly from the values at the interviews before and after) if the participant had arrears in payment or acquired debts. The variable “living alone” at each interview indicated that the participant was the only person in the household. The perceived status gap was assessed at T1, T2, and T3 using 2 questions. First, the MacArthur Scale of Subjective Social Status37 was used to rate subjective social status. In an easy pictorial format, it presents a “social ladder” with 10 steps and asks individuals to place an “X” on the step on which they feel they stand. The second question was about a similar ladder, but this time with regard to the desired level of social status. The difference between the subjective desired and actual social status was used as an independent variable in the analyses. It was treated as a person-level variable in the analyses.
Family History and Parental History
Family history was assessed as a person-level characteristic across 2 variables, as described in a previous publication.38 First, for participants who screened positive for the following psychiatric diagnoses, presence of the disorder in direct relatives was assessed at each interview wave: alcohol/drugs abuse/dependence, depression, mania, and anxiety disorders (panic disorder, social phobia, agoraphobia, and generalized anxiety disorder). This variable will be referred to as “family history.” A total of 51% of the sample screened positive for this variable at any of the 4 interview waves. Second, at T1, self-reported parental history of “problems with alcohol,” “problems with drugs,” “any psychiatric treatment or admission,” “severe anxiety or phobias,” “severe depression,” “suicide,” and “delusions or hallucinations” were assessed in the entire sample. A total of 31% screened positive for positive parental family history. This variable will be referred to as “parental history.”
Childhood Adversity
Childhood adversity was assessed at T0 using a questionnaire based on the NEMESIS trauma questionnaire.28 Whenever a subject reported having experienced 1 of 5 types of childhood adversity before the age of 16 years (emotional neglect [not listened to, ignored, or unsupported], physical abuse [kicked, hit, bitten, or hurt with object or hot water], psychological abuse [yelled at, insulted, unjustly punished/treated, threatened, belittled, or blackmailed], peer victimization [bullying], and one time or more sexual abuse [any unwanted sexual experience]), they were asked to state how often it had occurred. The item “sexual abuse” was rated on a scale of 1 (once) to 5 (very often), while all other items (namely, emotional neglect, physical abuse, psychological abuse, and peer victimization or bullying) were rated and on a scale of 1 (sometimes) to 4 (very often). The total childhood adversity score was used in the analyses.
Cannabis Exposure
Lifetime cannabis use was assessed with the section substance use disorders of the CIDI 3.0 at baseline (T0). If subjects reported cannabis use, they were rated on the frequency of use in the period of most frequent use on a scale of 1 (never) to 7 (every day). Consistent with previous work,38,39 a binary variable (absent = “0” and present = “1”) was constructed by using the cutoff value of once per week or more in the period most frequent use.
Urbanicity
The extent of the exposure to the urban environment until age 16 years was constructed at 5 levels based on the Dutch classification of population density: (1) countryside (distances to amenities is larger), (2) village (<25 000 inhabitants), (3) small city (25 000–50 000 inhabitants), (4) medium city (50 000–100 000 inhabitants), and (5) large city (>100 000 inhabitants).
Adulthood Stressful Life Events
Based on the “Brugha Life events section,” 40 participants were asked at each interview whether they experienced 1 of 9 life events within the last 12 months (T0) or since the last interview (T1–T3). Examples of items are serious sickness, death of family member or close friend, and serious financial problems. The continuous life event score at the 4 interview occasions was used in the analyses.
Polygenic Risk Score for Schizophrenia
PRS-SZ was created from best-guess genotypes at 6 different P-thresholds (.5, .1, .05, 5 ×10–3, 5 × 10–5, 5 ×·10–8). For our primary analyses, we used the P-threshold of < .05, as this threshold explained most variation in the phenotype in the Psychiatric Genomics Consortium analysis41 and was previously shown to perform well for the current phenotype of SF-36 mental health.42 For details on the genotyping, see the supplementary material. Statistical analyses were adjusted for 3 principal components.
Use of Schizophrenia Polygenic Risk as Transdiagnostic Measure
We used PRS-SZ as a measure of transdiagnostic genetic liability. In explaining 7% of the variance on the liability scale, PRS-SZ clearly outperforms the rest of the PRSs for mental disorder phenotypes that have been estimated so far and appears to be the forerunner for developing PRS-based clinical applications.13
In a sensitivity analysis, we also examined results using the following other PRS: PRS bipolar disorder, PRS educational achievement, PRS cross-disorder, PRS IQ, and PRS MDD,10,43–45 and finally we examined a model with the joint multivariable contribution of all PRS that contributed in univariable models.
Statistical Analyses
Risk Set
Material for DNA analysis of sufficient quality was available for 3104 individuals (47%) at T0 (see supplementary material). Excluding individuals who at interview had been assessed as a member of an ethnic minority, given a lack of generalizability of PRS in this group, left 3052 participants. Of the 3052, 2380 had non-missing values for all variables used in the analyses, yielding 8901 observations over the 4 interviews. Values for all variables were very similar in a comparison between the 8901 included and the 10 127 non-included observations (table 1).
Table 1.
Sample Characteristics, Stratified by Risk Set Included for Analysis (n = 8901 Observations) or Excluded From Analysis (n = 10 127 Observations)
| Sample | PRS | Parental History | Family History | Childhood Trauma | Regular Cannabis Use | Urbanicity | Living Alone | Life Events | No Partner | Unemployment | Income | Education | Status Gap | Disability | Pain | Debts | Age | Female Sex |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | % | % | Mean | % | Mean | % | Mean | % | % | Mean | Mean | Mean | % | Mean | % | Mean | % | |
| Excluded | −130.95 | 0.31 | 0.52 | 0.2 | 0.02 | 2.99 | 0.73 | 0.22 | 0.39 | 0.12 | 6.86 | 2.99 | 0.95 | 0.05 | 0.1 | −84.39 | 48.59 | 0.54 |
| SD | 4.48 | 0.4 | 1.34 | 0.41 | 2.49 | 0.9 | 1.33 | 0.3 | 13.1 | |||||||||
| N | 1081 | 8979 | 10 127 | 10 127 | 9590 | 10 106 | 10 009 | 10 127 | 10 126 | 10 127 | 9364 | 10 127 | 8756 | 10 119 | 8959 | 10 120 | 10 127 | 10 127 |
| Included | −131.29 | 0.31 | 0.53 | 0.19 | 0.02 | 2.99 | 0.68 | 0.18 | 0.35 | 0.12 | 7.08 | 3.07 | 0.8 | 0.04 | 0.1 | −84.46 | 48.98 | 0.56 |
| SD | 4.33 | 0.39 | 1.34 | 0.39 | 2.4 | 0.88 | 1.15 | 0.29 | 12.71 | |||||||||
| N | 8901 | 8901 | 8901 | 8901 | 8901 | 8901 | 8901 | 8901 | 8901 | 8901 | 8901 | 8901 | 8901 | 8901 | 8901 | 8901 | 8901 | 8901 |
| Total | −131.25 | 0.31 | 0.52 | 0.2 | 0.02 | 2.99 | 0.71 | 0.2 | 0.37 | 0.12 | 6.97 | 3.03 | 0.87 | 0.05 | 0.1 | −84.43 | 48.77 | 0.55 |
| SD | 4.35 | 0.4 | 1.34 | 0.4 | 2.45 | 0.89 | 1.24 | 0.3 | 12.92 | |||||||||
| N | 9982 | 17 880 | 19 028 | 19 028 | 18 491 | 19 007 | 18 910 | 19 028 | 19 027 | 19 028 | 18 265 | 19 028 | 17 657 | 19 020 | 17 860 | 19 021 | 19 028 | 19 028 |
Analyses
All analyses were performed using Stata, version 16.46P < .05 (2-tailed) was considered nominally statistically significant. We fitted cross-sectional regression models, adjusted for time, to test the effects of the independent variables on mental health as dependent variables. As each person contributed 4 observations in the cross-sectional model, the data were hierarchically structured. The Stata cluster option was, therefore, used to take into account intra-group correlations occasioned by clustering of observations within individuals. Some variables were assessed at each time-point and, therefore, time-varying; other variables were demographics or antecedents and time-invariant. Models including PRS-SZ were adjusted for 3 principal components. Shapely decomposition (Stata shapley2 command) was used to calculate the relative contribution of each (group of) regressor(s) to the R2 statistic.
The contribution of each (group of) regressor(s) to the model was statistically evaluated using likelihood ratio tests with the Stata test postestimation command.
Regressor groups (jointly) evaluated were: (1) PRS; (2) family history and parental history (family history); (3) urbanicity, cannabis use, childhood trauma, and life events (environmental risks); (4) somatic pain; and (5) living alone, no partner, unemployment, household income, educational status, perceived status gap, received disability, and debts (social circumstances).
Analyses were conducted separately for (1) level of mental health: a cross-sectional analysis of the 4 measures of mental health and (2) change in mental health which was similar to (1) but with adjustment for the baseline value of mental health, thus effectively assessing the effect of predictors of change of mental health over time.
Models were developed by adding more groups of variables across 5 steps.
In addition, we calculated, in separate regression analyses, the standardized effect sizes (beta) and contributions to the explained variance of all the individual factors in the regressor groups.
Results
Sample characteristics and representativeness are shown in table 1. Of the participants included in the analysis, mean age was 50.0 years (SD = 12.7), and 56% was female. Distributions of variables did not differ between participants included and excluded from the analysis.
Results are summarized in tables 2 and 3 and figure 1.
Table 2.
Contributions of Proxy Genetic and Non-Genetic Risksa to Level of and Change in Mental Health
| Level of Mental Health | Model 1 (%) | Model 2 (%) | Model 3 (%) | Model 4 (%) | Model 5 (%) | % Model 5 (%) |
|---|---|---|---|---|---|---|
| −Time | 1.8 | 1.8 | 1.8 | 1.8 | 1.8 | |
| −PRS | 0.6 | 0.5 | 0.4 | 2.5 | ||
| −Age/sex | 1.7 | 1.2 | 1.2 | 1.1 | 0.9 | 5.3 |
| −Family history | 6.1 | 6.0 | 4.1 | 24.3 | ||
| −Environmental risks | 4.5 | 3.7 | 22.2 | |||
| −Pain | 2.9 | 2.6 | 15.6 | |||
| −Social circumstances | 5.5 | 5.1 | 30.2 | |||
| Totalb | 2.3 | 7.3 | 7.7 | 14.0 | 16.7 | |
| Change in mental health | ||||||
| −Time | 1.8 | 1.8 | 1.8 | 1.8 | 1.8 | |
| −PRS | 0.3* | 0.3* | 0.2* | 2.1 | ||
| −Age/sex | 1.0 | 0.8 | 0.8 | 0.7 | 0.6 | 5.5 |
| −Family history | 3.7 | 3.7 | 2.7 | 23.4 | ||
| −Environmental risks | 3.2 | 2.8 | 23.7 | |||
| −Pain | 2.1 | 2.0 | 16.8 | |||
| −Social circumstances | 3.5 | 3.3 | 28.6 | |||
| Totalb | 1.3 | 4.5 | 4.7 | 9.6 | 11.7 |
Note: Model 1: PRS-SZ only; model 2: family history only; model 3: PRS-SZ and family history; model 4: environmental risks (childhood trauma, regular cannabis use, and urban environment), pain, and social circumstances (living alone, jobless, income, educational level, recent life events, no partner, perceived status gap, disability payment, and debts); model 5: all factors of models 3 and 4 combined; %model 5: as a percentage of total variance explained. All associations with regressor groups displayed in the table are statistically significant except marked with *.
aContributions of genetic principal components not displayed.
bExcludes contribution of factor “time.”
Table 3.
Individual Factor Effect Size and Variance Explained
| Table 2; model 5* | Level of Mental Health | Change in Mental Health | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Beta | t | P | R 2 (%) | Beta | t | P | R 2 (%) | ||
| PRS | PRS | .054 | 3.702 | .000 | .4 | .015 | 1.554 | .120 | .3 |
| Family history | Parental history | .044 | 2.938 | .003 | .8 | .026 | 2.369 | .018 | .6 |
| Family history | .147 | 10.560 | .000 | 3.6 | .069 | 7.052 | .000 | 2.5 | |
| Environmental risks | Childhood trauma | .095 | 4.825 | .000 | 2.3 | .049 | 3.788 | .000 | 1.6 |
| Regular cannabis use | .017 | 1.309 | .191 | .1 | .007 | 0.849 | .396 | .1 | |
| Urbanicity | −.005 | −0.367 | .713 | .0 | −.008 | −0.728 | .467 | .0 | |
| Life events | .096 | 8.405 | .000 | 1.5 | .077 | 8.283 | .000 | 1.2 | |
| Social circumstances | Living alone | .077 | 4.912 | .000 | 1.0 | .040 | 3.196 | .001 | .7 |
| No partner | .043 | 2.666 | .008 | .8 | −.001 | -0.104 | .917 | .6 | |
| Unemployed | .009 | 0.648 | .517 | .6 | .002 | 0.198 | .843 | .5 | |
| Income | −.037 | −2.476 | .013 | .3 | −.015 | −1.369 | .171 | .2 | |
| Educational level | .047 | 2.941 | .003 | .1 | .021 | 1.856 | .063 | .0 | |
| Perceived status gap | .070 | 3.760 | .000 | 1.1 | .025 | 1.893 | .058 | .8 | |
| Disability | .075 | 3.672 | .000 | 1.2 | .039 | 2.541 | .011 | .9 | |
| Debts | .049 | 3.596 | .000 | .8 | .035 | 3.483 | .000 | .6 | |
| Somatic pain | Pain | .121 | 8.183 | .000 | 2.6 | .079 | 6.589 | .000 | 1.9 |
| Age/sex | Age | −.026 | −1.572 | .116 | .1 | −.028 | −2.315 | .021 | .1 |
| Female sex | .059 | 4.266 | .000 | .8 | .028 | 2.807 | .005 | .5 |
Note: Beta: standardized regression coefficient; t: test statistic t; P: P-value; R2: percentage variance explained.
*The sum of the R2 of individual factors may not correspond exactly to the combined R2 in table 2 because of small differences in Stata shapley2 model specification.
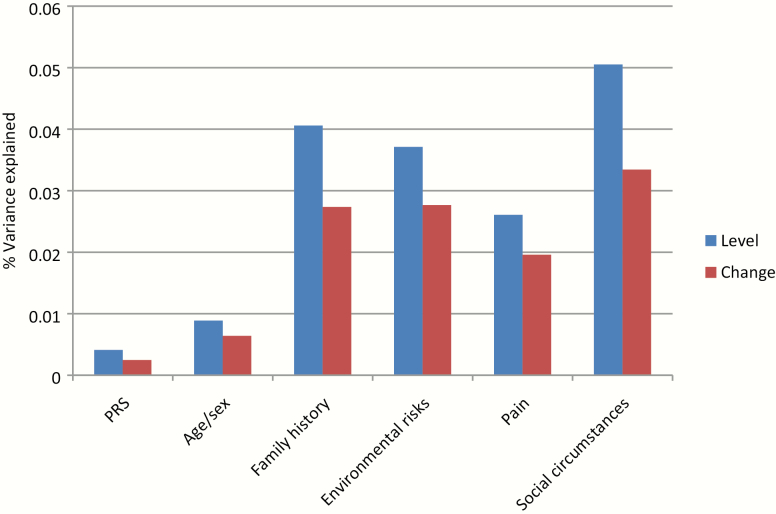
Fig. 1.
Contribution of factors used to explain mental health variance in models of level of mental health and change of mental health (all statistically significant except polygenic risk score in the model of change).
Level of Mental Health
PRS-SZ contributed significantly to mental health across all models; however, the relative contribution to R2 was very small. In the combined model (table 2; model 5), proxy genetic and environmental factors explained around 17% of the variance in mental health, of which around 5% was explained by age and sex, 30% by social circumstances, 16% by pain, 22% by environmental risk factors, 24% by family history, and 3% by PRS-SZ (figure 1).
Of the environmental risks, childhood trauma had the largest impact, followed by life events, whereas urbanicity and cannabis use did not contribute significantly. Of the social circumstances, perceived status gap had the largest impact, although other variables contributed comparatively, with the exception of unemployment that did not contribute. Of the variables age and sex, only sex contributed significantly (table 3).
The effect of family history was not reducible to PRS-SZ (only 2% reduction; model 3). In contrast, the contribution of the PRS-SZ was reduced by 20% when the family history variables were added to the model (table 2). The contributions of both “family history” and PRS-SZ were reduced by around 30% from the model with only time and age/sex, to the full model with all independent variables (table 2). Conversely, the contributions of environmental risks and social circumstances were not affected much by adding PRS-SZ and family history information to the model (table 2).
Change in Mental Health
PRS-SZ did not contribute significantly to mental health in any of the models of mental health change (table 2).
In the model of mental health change over time, proxy genetic and environmental factors explained around 12% of the variance, of which around 6% was explained by age and sex, 29% by social circumstances, 17% by pain, 24% by environmental risk factors, 24% by family history, and 2% by PRS-SZ, the latter not statistically significant (figure 1). Of the different environmental risks, childhood trauma had the largest impact, followed by life events, whereas urbanicity and cannabis use did not contribute significantly. Of the different social circumstances, having received disability benefit had the largest impact, although other variables contributed comparatively, with the exception of educational level, having no partner, household income, and unemployment. Both age and sex contributed significantly (table 3).
Sensitivity Analyses
PRS depression, PRS bipolar disorder, and PRS cross-disorder contributed less than PRS-SZ. PRS educational achievement and PRS-IQ did not contribute at all (table 4). The different PRS only marginally added to each other: the multivariable contribution of PRS depression, PRS bipolar disorder, PRS cross-disorder, and PRS-SZ rose from 0.4% to 0.6% in the full model of level of mental health and from 0.2% to 0.4% in the full model of change of mental health (tables 2 and 4).
Table 4.
Sensitivity Analysis With Cross-Disorder Polygenic Score: Contributions of Proxy Genetic and Non-Genetic Risks to Level of and Change in Mental Health
| Level of Mental Health | Model 1 (%) | Model 2 | Model 3 (%) | Model 4 | Model 5 (%) |
|---|---|---|---|---|---|
| PRS schizophrenia | 0.6 | — | 0.5 | — | 0.4 |
| PRS cross-disorder | 0.2 | — | 0.1 | — | 0.1 |
| PRS bipolar disorder | 0.2 | — | 0.1 | — | 0.1 |
| PRS depression | 0.4 | — | 0.3 | — | 0.2 |
| PRS IQ | 0.0 | — | 0.0 | — | 0.0 |
| PRS educational achievement | 0.0 | — | 0.0 | — | 0.0 |
| PRS depression/bipolar/schizophrenia/cross-disorder entered together | 1.0 | — | 0.7 | — | 0.6 |
| Change in mental health | |||||
| PRS schizophrenia | 0.3 | — | 0.3 | — | 0.2 |
| PRS cross-disorder | 0.1 | — | 0.1 | — | 0.1 |
| PRS bipolar disorder | 0.1 | — | 0.1 | — | 0.1 |
| PRS depression | 0.2 | — | 0.2 | — | 0.1 |
| PRS IQ | 0.0 | — | 0.0 | — | 0.0 |
| PRS educational achievement | 0.0 | — | 0.0 | — | 0.0 |
| PRS depression/bipolar/schizophrenia /cross-disorder entered together | 0.6 | — | 0.5 | — | 0.4 |
Note: Model 1: PRS only; model 2: family history only; model 3: PRS and family history; model 4: environmental risks (childhood trauma, regular cannabis use, and urban environment), pain, and social circumstances (living alone, jobless, income, educational level, recent life events, no partner, perceived status gap, disability payment, and debts); model 5: all factors of models 3 and 4 combined.
Discussion
Summary of Findings
The results of this study suggest that the transdiagnostic PRS-SZ is associated cross sectionally with a phenotype of mental health in the general population, in line with emerging work showing small statistical associations between PRS and various mental health phenotypes in the general population.8 PRS-SZ was not associated with change in mental health over time.
Contrary to case-control studies, however, in which the PRS-SZ explains a proportion of the variance of the latent liability (7%) or the observed scale (20%),3,4 the contribution of PRS-SZ to the variance of mental health was very small in the cross sectional and nonsignificant in the change model of mental health. The lack of contribution of PRS-SZ contrasted sharply with traditional measures of familial and environmental risk; socio-environmental circumstances were responsible for the bulk of the explained variance, particularly childhood trauma and perceived status gap.
Interpretation of Findings
These results cannot be interpreted as showing that genetic factors are not important. Indeed, all measures of environmental and social circumstances that were used may in fact reflect, to a degree, genetic effects.47 Conversely, measures of family history also mediate environmental effects such as higher rates of birth and pregnancy complications,48–50 growing up in an unfavorable home environment,51 out-of-home placement,52 elevated divorce rate, alterations in parental communication,53 altered school functioning,54 and the psychosocial impact of growing up with a parent with mental illness.55 What the results do indicate, however, is that current transdiagnostic measures of polygenic risk lack impact in epidemiological general population studies, beyond very small but statistically significant associations. Genetic factors may contribute to variance of mental health in population-based samples, but it appears that they are not captured by the current version of various transdiagnostic PRS.
The effect of family history was not reducible to PRS. This is compatible with previous work showing that in psychotic disorder, only a fraction of the effect of family history is mediated by PRS.56
Some environmental factors, such as cannabis, did not predict in the multivariable model, which may be considered unexpected. However, in a post hoc univariable model, cannabis did contribute strongly (P = .007); adding other environmental risks indicated that some of its effects were reducible to other variables, such as childhood trauma.
The lack of relevance of PRS-SZ is not related to the choice of phenotype, as associations between PRS-SZ and the range of mental health phenotypes used to date, similarly, are very small although sometimes showing statistical significance.16–23
The Contribution of Epidemiological Predictors
There is a large literature on the impact of environmental risks and social circumstances on mental health, and how this may inform policy.57 Our results do not suggest that traditional socio-environmental risks are reducible to the genetic factors that are captured by transdiagnostic polygenic risk, although genes and environment may show a degree of synergistic interaction.9 The results are compatible with the suggestion that mental health and mental health research may be productively approached from the perspective of public health.58 In addition, pain was confirmed as a major factor impacting health, as expected given its strong association with mental health, high prevalence, and prominent contribution to disability worldwide.34–36
Methodological Issues
The predictivity of even the full model of mental health was low at less than 20%. This, however, is conform expectation in the domain of behavioral and mental science, where predictivity of models typically is limited.59
It could be argued that modeling other phenotypes for PRS analysis would be more productive. This is unlikely, however, as previous work examining associations between PRS-SZ and a range of mental disorders and associated trait phenotypes has shown similar weak and ambiguous associations.16–23 Given the comorbid nature of psychopathology, it is highly unlikely that PRS-SZ would show robust associations with another, hitherto untested phenotype.
Similarly, we showed that other measures of PRS did not improve PRS performance and that different PRS only minimally added to each other.
The analyses included less than half of the original sample. However, it is unlikely that this would have resulted in bias as there was no evidence of differential attrition from the analysis.
Conclusion
These findings suggest that the examination of molecular genetic risk for mental suffering, derived from theoretical analyses focusing on significance-testing, lack impact in analyses focusing on prediction in epidemiological settings.24
Supplementary Material
Acknowledgments
NEMESIS-2 is conducted by the Netherlands Institute of Mental Health and Addiction (Trimbos Institute) in Utrecht. The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Funding
Financial support for this study has been received from the Ministry of Health, Welfare and Sport, The Netherlands, with supplementary support from the Netherlands Organisation for Health Research and Development (ZonMw). This work was supported by the European Community’s Seventh Framework Program under grant agreement No. HEALTH-F2-2009-241909 (Project EU-GEI). B.P.F.R. was funded by a VIDI award number 91718336 from the Netherlands Scientific Organisation.
References
- 1. Kendler KS. A joint history of the nature of genetic variation and the nature of schizophrenia. Mol Psychiatry. 2015;20(1):77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Purcell SM, Wray NR, Stone JL, et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature 2009;460:748–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pardiñas AF, Holmans P, Pocklington AJ, et al. ; GERAD1 Consortium; CRESTAR Consortium Common schizophrenia alleles are enriched in mutation-intolerant genes and in regions under strong background selection. Nat Genet. 2018;50(3):381–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ripke S, O’Dushlaine C, Chambert K, et al. ; Multicenter Genetic Studies of Schizophrenia Consortium; Psychosis Endophenotypes International Consortium; Wellcome Trust Case Control Consortium 2 Genome-wide association analysis identifies 13 new risk loci for schizophrenia. Nat Genet. 2013;45(10):1150–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Young AI. Solving the missing heritability problem. PLoS Genet. 2019;15(6):e1008222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Calafato MS, Thygesen JH, Ranlund S, et al. ; Genetic Risk and Outcome of Psychosis (GROUP) consortium Use of schizophrenia and bipolar disorder polygenic risk scores to identify psychotic disorders. Br J Psychiatry. 2018;213(3):535–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schijven D, Veldink JH, Luykx JJ. Genetic cross-disorder analysis in psychiatry: from methodology to clinical utility. Br J Psychiatry. 2020;216(5):246–249. [DOI] [PubMed] [Google Scholar]
- 8. Martin AR, Daly MJ, Robinson EB, Hyman SE, Neale BM. Predicting polygenic risk of psychiatric disorders. Biol Psychiatry. 2019;86(2):97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guloksuz S, Pries LK, Delespaul P, et al. ; Genetic Risk and Outcome of Psychosis (GROUP) investigators Examining the independent and joint effects of molecular genetic liability and environmental exposures in schizophrenia: results from the EUGEI study. World Psychiatry. 2019;18(2):173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cross-Disorder Group of the Psychiatric Genomics Consortium. Genomic relationships, novel loci, and pleiotropic mechanisms across eight psychiatric disorders. Cell 2019;179(7):1469–1482 e1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cross-Disorder Group of the Psychiatric Genomics Consortium, Lee SH, Ripke S, et al. Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat Genet. 2013;45(9):984–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Richardson TG, Harrison S, Hemani G, Davey Smith G. An atlas of polygenic risk score associations to highlight putative causal relationships across the human phenome. Elife. 2019;8:e43657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zheutlin AB, Dennis J, Karlsson Linnér R, et al. Penetrance and pleiotropy of polygenic risk scores for schizophrenia in 106,160 patients across four health care systems. Am J Psychiatry. 2019;176(10):846–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mistry S, Harrison JR, Smith DJ, Escott-Price V, Zammit S. The use of polygenic risk scores to identify phenotypes associated with genetic risk of schizophrenia: systematic review. Schizophr Res. 2018;197:2–8. [DOI] [PubMed] [Google Scholar]
- 15. Pazoki R, Lin BD, van Eijk KR, Schijven D, Guloksuz S, GROUP investigators, Luykx JJ. Polygenic risk scores are associated with quality of life in schizophrenia. bioRxiv 2019;744045.
- 16. Hatzimanolis A, Bhatnagar P, Moes A, et al. Common genetic variation and schizophrenia polygenic risk influence neurocognitive performance in young adulthood. Am J Med Genet B Neuropsychiatr Genet. 2015;168B(5):392–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jones HJ, Stergiakouli E, Tansey KE, et al. Phenotypic manifestation of genetic risk for schizophrenia during adolescence in the general population. JAMA Psychiatry. 2016;73(3):221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mistry S, Harrison JR, Smith DJ, Escott-Price V, Zammit S. The use of polygenic risk scores to identify phenotypes associated with genetic risk of schizophrenia: systematic review. Schizophr Res. 2018;197:2–8. [DOI] [PubMed] [Google Scholar]
- 19. Riglin L, Collishaw S, Richards A, et al. Schizophrenia risk alleles and neurodevelopmental outcomes in childhood: a population-based cohort study. Lancet Psychiatry. 2017;4(1):57–62. [DOI] [PubMed] [Google Scholar]
- 20. Nivard MG, Gage SH, Hottenga JJ, et al. Genetic overlap between schizophrenia and developmental psychopathology: longitudinal and multivariate polygenic risk prediction of common psychiatric traits during development. Schizophr Bull. 2017;43(6):1197–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Power RA, Steinberg S, Bjornsdottir G, et al. Polygenic risk scores for schizophrenia and bipolar disorder predict creativity. Nat Neurosci. 2015;18(7):953–955. [DOI] [PubMed] [Google Scholar]
- 22. Hatzimanolis A, Avramopoulos D, Arking DE, et al. Stress-dependent association between polygenic risk for schizophrenia and schizotypal traits in young army recruits. Schizophr Bull. 2018;44(2):338–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. van Os J, Pries LK, Delespaul P, et al. Replicated evidence that endophenotypic expression of schizophrenia polygenic risk is greater in healthy siblings of patients compared to controls, suggesting gene-environment interaction. The EUGEI study [published online ahead of print August 15, 2019]. Psychol Med. doi: 10.1017/S003329171900196X. [DOI] [PubMed] [Google Scholar]
- 24. Lo A, Chernoff H, Zheng T, Lo SH. Why significant variables aren’t automatically good predictors. Proc Natl Acad Sci U S A. 2015;112(45):13892–13897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pries L-K, van Os J, ten Have M, et al. Association of recent stressful life events with mental and physical health in the context of genomic and exposomic liability for schizophrenia [published online ahead of print August 5, 2020]. Jama Psychiat. doi: 10.1001/jamapsychiatry.2020.2304. [DOI] [PMC free article] [PubMed]
- 26. Alonso J, Angermeyer MC, Bernert S, et al. Sampling and methods of the European Study of the Epidemiology of Mental Disorders (ESEMeD) project. Acta Psychiatr Scand Suppl. 2004;420:8–20. [DOI] [PubMed] [Google Scholar]
- 27. de Graaf R, ten Have M, Burger H, Buist-Bouwman M. Mental disorders and service use in the Netherlands. Results from the European Study of the Epidemiology of Mental Disorders (ESEMeD) In: Ustun T, Kessler R, eds. The WHO World Mental Health Surveys: Global Perspectives on the Epidemiology of Mental Disorders. New York, NY: Cambridge University Press; 2008:388–405. [Google Scholar]
- 28. de Graaf R, Ten Have M, van Dorsselaer S. The Netherlands Mental Health Survey and Incidence Study-2 (NEMESIS-2): design and methods. Int J Methods Psychiatr Res. 2010;19(3):125–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. de Graaf R, ten Have M, van Gool C, van Dorsselaer S. Prevalence of mental disorders and trends from 1996 to 2009. Results from the Netherlands Mental Health Survey and Incidence Study-2. Soc Psychiatry Psychiatr Epidemiol. 2012;47(2):203–213. [DOI] [PubMed] [Google Scholar]
- 30. Nuyen J, Tuithof M, de Graaf R, van Dorsselaer S, Kleinjan M, ten Have M. The bidirectional relationship between loneliness and common mental disorders in adults: findings from a longitudinal population-based cohort study [published online ahead of print September 19, 2019]. Soc Psychiatry Psychiatr Epidemiol. doi: 10.1007/s00127-019-01778-8. [DOI] [PubMed] [Google Scholar]
- 31. de Graaf R, van Dorsselaer S, Tuithof M, ten Have M. Sociodemographic and Psychiatric Predictors of Attrition in the Third Follow-Up of the Netherlands Mental Health Survey and Incidence Study-2 (NEMESIS2). Utrecht: Trimbos Institute; 2018. [DOI] [PubMed] [Google Scholar]
- 32. Stewart AL, Ware JE. Measuring Functioning and Well-Being: The Medical Outcomes Study Approach. Durham: Duke University Press; 1992. [Google Scholar]
- 33. Loge JH, Kaasa S. Short Form 36 (SF-36) health survey: normative data from the general Norwegian population. Scand J Soc Med. 1998;26(4):250–258. [PubMed] [Google Scholar]
- 34. Fayaz A, Croft P, Langford RM, Donaldson LJ, Jones GT. Prevalence of chronic pain in the UK: a systematic review and meta-analysis of population studies. BMJ Open. 2016;6(6):e010364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2163–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tegethoff M, Belardi A, Stalujanis E, Meinlschmidt G. Comorbidity of mental disorders and chronic pain: chronology of onset in adolescents of a national representative cohort. J Pain. 2015;16(10):1054–1064. [DOI] [PubMed] [Google Scholar]
- 37. Adler NE, Epel ES, Castellazzo G, Ickovics JR. Relationship of subjective and objective social status with psychological and physiological functioning: preliminary data in healthy white women. Health Psychol. 2000;19(6):586–592. [DOI] [PubMed] [Google Scholar]
- 38. Radhakrishnan R, Guloksuz S, Ten Have M, et al. Interaction between environmental and familial affective risk impacts psychosis admixture in states of affective dysregulation. Psychol Med. 2019;49(11):1879–1889. [DOI] [PubMed] [Google Scholar]
- 39. Pries LK, Guloksuz S, Ten Have M, et al. Evidence that environmental and familial risks for psychosis additively impact a multidimensional subthreshold psychosis syndrome. Schizophr Bull. 2018;44(4):710–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Brugha T, Bebbington P, Tennant C, Hurry J. The list of threatening experiences: a subset of 12 life event categories with considerable long-term contextual threat. Psychol Med. 1985;15(1):189–194. [DOI] [PubMed] [Google Scholar]
- 41. Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature 2014;511(7510):421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pries LK, Klingenberg B, Menne-Lothmann C, et al. Polygenic liability for schizophrenia and childhood adversity influences daily-life emotion dysregulation and psychosis proneness. Acta Psychiatr Scand. 2020;141(5):465–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Stahl EA, Breen G, Forstner AJ, et al. ; eQTLGen Consortium; BIOS Consortium; Bipolar Disorder Working Group of the Psychiatric Genomics Consortium Genome-wide association study identifies 30 loci associated with bipolar disorder. Nat Genet. 2019;51(5):793–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lee JJ, Wedow R, Okbay A, et al. ; 23andMe Research Team; COGENT (Cognitive Genomics Consortium); Social Science Genetic Association Consortium Gene discovery and polygenic prediction from a genome-wide association study of educational attainment in 1.1 million individuals. Nat Genet. 2018;50(8):1112–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Savage JE, Jansen PR, Stringer S, et al. Genome-wide association meta-analysis in 269,867 individuals identifies new genetic and functional links to intelligence. Nat Genet. 2018;50(7):912–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. StataCorp. STATA Statistical Software: Release 16. College: Station, TX: StataCorp; 2019. [Google Scholar]
- 47. Van Os J, Sham P. Gene-environment interactions. In: Murray RM, Jones PB, Susser E, Van Os J, Cannon M, eds. The Epidemiology of Schizophrenia. Cambridge: Cambridge University Press; 2003:235–254. [Google Scholar]
- 48. Vigod SN, Fung K, Amartey A, et al. Maternal schizophrenia and adverse birth outcomes: what mediates the risk? Soc Psychiatry Psychiatr Epidemiol. 2020;55(5):561–570. [DOI] [PubMed] [Google Scholar]
- 49. Vigod SN, Kurdyak PA, Dennis CL, et al. Maternal and newborn outcomes among women with schizophrenia: a retrospective population-based cohort study. BJOG. 2014;121(5):566–574. [DOI] [PubMed] [Google Scholar]
- 50. Zhong QY, Gelaye B, Fricchione GL, Avillach P, Karlson EW, Williams MA. Adverse obstetric and neonatal outcomes complicated by psychosis among pregnant women in the United States. BMC Pregnancy Childbirth. 2018;18(1):120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gantriis DL, Thorup AAE, Harder S, et al. Home visits in the Danish High Risk and Resilience Study – VIA 7: assessment of the home environment of 508 7-year-old children born to parents diagnosed with schizophrenia or bipolar disorder. Acta Psychiatr Scand. 2019;140(2):126–134. [DOI] [PubMed] [Google Scholar]
- 52. Simoila L, Isometsa E, Gissler M, et al. Maternal schizophrenia and out-of-home placements of offspring: a national follow-up study among Finnish women born 1965–1980 and their children. Psychiatry Res. 2019;273:9–14. [DOI] [PubMed] [Google Scholar]
- 53. de Sousa P, Varese F, Sellwood W, Bentall RP. Parental communication and psychosis: a meta-analysis. Schizophr Bull. 2014;40(4):756–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ranning A, Laursen T, Agerbo E, et al. School performance from primary education in the adolescent offspring of parents with schizophrenia and bipolar disorder – a national, register-based study. Psychol Med. 2018;48(12):1993–2000. [DOI] [PubMed] [Google Scholar]
- 55. Kallquist A, Salzmann-Erikson M. Experiences of having a parent with serious mental illness: an interpretive meta-synthesis of qualitative literature. J Child Fam Stud. 2019;28:2056–2068. [Google Scholar]
- 56. Agerbo E, Sullivan PF, Vilhjalmsson BJ, et al. Polygenic risk score, parental socioeconomic status, family history of psychiatric disorders, and the risk for schizophrenia: a Danish population-based study and meta-analysis. JAMA Psychiatry. 2015;72(7):635–641. [DOI] [PubMed] [Google Scholar]
- 57. Lund C, Brooke-Sumner C, Baingana F, et al. Social determinants of mental disorders and the sustainable development goals: a systematic review of reviews. Lancet Psychiatry. 2018;5(4):357–369. [DOI] [PubMed] [Google Scholar]
- 58. Forsman AK, Wahlbeck K, Aarø LE, et al. ; ROAMER Consortium Research priorities for public mental health in Europe: recommendations of the ROAMER project. Eur J Public Health. 2015;25(2):249–254. [DOI] [PubMed] [Google Scholar]
- 59. Kerlinger FN, Pedhazur EJ. Multiple Regression in Behavioral Research. New York, NY: Holt, Rinehart and Winston; 1973. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.