Abstract
The pathogenesis and etiology of schizophrenia (SCZ) remains unclear. Accumulating studies showed that complex interrelationships between brain-derived neurotrophic factor (BDNF) and an imbalanced redox system has a crucial role in the psychopathology of SCZ. However, the influence of the interrelationships of BDNF and superoxide dismutase (SOD) on cognitive impairment and clinical symptomatology in drug-naive first-episode (DNFE) SCZ patients has not been studied thoroughly. Serum BDNF levels, plasma total SOD, manganese-SOD (Mn-SOD), copper/zinc-containing SOD (CuZn-SOD) activities, and malondialdehyde (MDA) levels were measured in 327 DNFE patients with SCZ and 391 healthy controls. Cognitive functions were measured using the Repeatable Battery for the Assessment of Neuropsychological status (RBANS) and clinical symptoms were evaluated by the Positive and Negative Syndrome Scale (PANSS). Compared with the controls, the DNFE patients had increased activities of total SOD and CuZn-SOD, and reduced levels of BDNF and MDA. BDNF levels were positively correlated with CuZn-SOD activity in patients. In addition, we found that elevated Mn-SOD and CuZn-SOD activities were related to PANSS depression factor. Moreover, an interactive effect of BDNF levels and Mn-SOD activity was associated with attentional index score in the patients. Therefore, our findings suggested that interrelationships between BDNF and antioxidant mechanisms might underlie the pathological mechanisms of cognitive impairments and symptomatology in the DNFE patients with SCZ.
Keywords: schizophrenia, BDNF, superoxide dismutase, interactive effect, clinical symptoms, cognitive function
Introduction
There is increasing evidence that changes in redox regulation play a potential role in abnormal neurodevelopment in schizophrenia (SCZ).1 In a normal physiological state, the redox balance is controlled by an antioxidant defense system.2 When imbalance is present, oxidative stress occurs, which may be involved in the disease severity and cognitive decline in SCZ patients.2–4 Superoxide dismutase (SOD) is the first line in the defense responses to oxidative stress.5 Manganese SOD (Mn-SOD) in the mitochronia and copper/zinc-containing SOD (CuZn-SOD) in cytoplasm have fundamental roles in superoxide disproportionation and the rapid conversion of O2− to H2O2.6–7 Particularly, Mn-SOD scavenges 95% of the reactive oxygen species (ROS) and superoxide anions produced by mitochondrial oxygen consumption.8 CuZn-SOD is one of the most abundant SOD enzymes in the cytoplasm of cells (>90%) and contributes more to the total SOD activity than other enzymes.9 Mn-SOD has been shown to be related to SCZ, yet, CuZn-SOD enzyme was focused by limited researchers.10–11 Although mounting evidence has revealed diminished activity of total SOD and Mn-SOD in peripheral blood cells and brain of patients with SCZ,12–13 only 2 studies found a reduction of CuZn-SOD activity in cerebrospinal fluid of recent onset SCZ patients.14,15 Interestingly, a few of studies in first-episode drug-naive (DNFE) patients with SCZ found that total SOD activities were negatively related to the positive symptom score.16 More importantly, studies in aging animals showed overexpression of extracellular SOD significantly improved hippocampal synaptic plasticity and memory-related behavioral performance in aged mice.17 However, conflicting results about total SOD activity or concentrations in DNFE patients with SCZ have been reported.18–22
The levels of brain-derived neurotrophic factor (BDNF), the most abundant member of the neurotrophins, were found to be decreased and closely related to the pathologic mechanism and clinical manifestation of SCZ.23–27 Preclinical studies demonstrated that BDNF affects the production and release of dopamine in the brain circuit and induction of DA-related behaviors,28 indicating that BDNF may be closely linked to DA systems,28 which has been hypothesized to be associated with psychopathological symptoms of SCZ patients.29,30 Interestingly, studies also found that decreased serum BDNF levels in DNFE patients with SCZ were linked to clinical phenotypes, particularly cognitive impairments.31–34,35 In total, evidence provides support for the involvement of BDNF in the psychopathology of SCZ.
Complex interactive effects of between BDNF and oxidative stress have been linked to cognitive function in both preclinical and clinical studies. For example, a recent study in mice showed that antioxidant therapy significantly increased learning and memory by reducing antioxidant enzyme activities and increasing BDNF levels in the prefrontal cortex and hippocampus.36 Also, Canever et al revealed significant changes in the oxidative stress parameters including SOD activity and the neurotrophic factors levels including BDNF within the frontal cortex in the model of SCZ induced by ketamine. Moreover, supplementation of folic acid (FA), which had antioxidant properties, prevented the cognitive damage and improved these biochemical parameters in SCZ model, suggesting that FA may produce neuroprotective effects by regulating markers of oxidative stress and neurotrophin.37 Our recent study showed a significant association between the interactive effects of BDNF levels, total SOD activity and executive dysfunctions in chronic patients with SCZ.3 However, there has been no study on the interactive effect between BDNF and Mn-SOD or CuZn-SOD enzymes in DNFE patients with SCZ.
To our best knowledge, no study has been conducted to examine the interrelationships between BDNF, antioxidant enzymes, and clinical symptoms in the same group of DNFE patients with SCZ. This study therefore aimed to explore (1) whether BDNF levels and antioxidant enzyme activities (including total SOD, Mn-SOD, CuZn-SOD) were altered in DNFE patients with SCZ; (2) whether there was an association between BDNF and SOD enzyme activities; and (3) whether their interactive effect played a role in the psychopathological symptoms and cognitive dysfunction of SCZ.
Method
Subjects
A total of 350 patients with DNFE SCZ were recruited from Beijing Huilongguan Hospital and Henan Zhumadian Psychiatric Hospital. Six trained psychiatrists made the first diagnosis of SCZ for all patients at baseline and then made the second diagnosis after 3–6 months of follow-up based on the Structured Clinical Interview for DSM-IV (SCID).38 The remaining 23 patients were excluded from this study. Among them, 15 were excluded because of inconsistent diagnosis at these 2 time points, 4 due to inability to understand the consent procedure, and 4 due to being unable to perform the clinical assessments. The excluded patients were not different from those included in the study in any demographic parameters.
Beijing Huilongguan hospital is one of the largest psychiatric hospitals in China, which serves a catchment area population of about 20 million in Beijing, with inpatient bed capacity of about 1400. Also, the hospital is affiliated to Peking University. It is estimated that approximately 100 DNFE SCZ patients are admitted to the hospital each year. Generally, these DNFE patients will be treated in the hospital about 1–3 months and then followed for 3–6 months. Henan Zhumadian Psychiatric Hospital is a local psychiatric hospital in Henan province in the central China, providing psychiatric service for about 7 million people with 800 inpatients. About 300 DNFE patients with SCZ are hospitalized each year. Generally speaking, local mental patients are sent directly to the hospital after first episode. These DNFE patients are usually treated in the hospital about 1–3 months. The patients met the following inclusion criteria: providing informed consent; aged between 16 and 45 years; Han Chinese population; clinical course of less than 24 months; no previous history of psychotropic drug treatment; providing medical, psychological, and sociodemographic information; no major medical morbidity (eg, cancer, ongoing infection or infectious illness in the previous 2 weeks, diabetes, cerebrovascular disease, or hypertension); not taking any antihypertensive, anti-inflammatory, anti-lipidemic, or hypoglycemic agents; without abuse or substance dependence except tobacco; not breast-feeding or pregnant females.
Three hundred ninety-one unrelated, smoking status-, sex-matched controls of Han Chinese patients were recruited from local communities close to the hospital. Controls were excluded if they had a current or past psychiatric disorder determined by the SCID. Controls were also excluded if they were currently receiving antipsychotic medication (eg, mood stabilizing, anxiolytic, antidepressant, or antipsychotic drugs).
All subjects underwent physical examinations and laboratory tests, and they were all in good physical health. Their educational level was obtained through self-reported years of education. The socioeconomic status and educational level of the healthy controls and patients were comparable. In addition, we excluded any of subjects with medical illnesses or drug and alcohol abuse or dependence except tobacco.
This study was approved by the Medical Ethical Committee of Beijing Huilongguan Hospital. All subjects signed the informed consent before entry into the study.
Psychotic Symptoms and Cognition Assessment
Six psychiatrists participated in a training course in the use of the Positive and Negative Syndrome Scales (PANSS).39 Repeated assessment found that the interobserver correlation coefficient was maintained at >0.8 for the PANSS total score. Several previous studies have shown that the 5-factor model captures the effectiveness of the clinical symptom dimension.40 In this study, the original PANSS consisted of five factors, namely, positive factor (composed of P1, P3, P5, G9), negative factor (composed of N1, N2, N3, N4, N6, G7), disorganization factor (composed of P2, N5, G11), excitement factor (composed of P4, P7, G8, G14), and depressive factor (composed of G2, G3, G6).40
Four research psychologists administered the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS, Form A) to each subject on the same day or the next day after he/she signed the informed consent form. The RBANS includes a composite score and 5 age-adjusted index scores.41 The battery tests consist of immediate memory (comprised of List Learning and Story Memory tasks), visuospatial/construction (comprised of Figure Copy and Line Orientation tasks), language (comprised of Picture Naming and Semantic Fluency tasks), attention (comprised of Digit Span and Coding tasks), and delayed memory (comprised of List Recall, Story Recall, Figure Recall, and List Recognition tasks). It has been translated into Chinese and established the clinical validity and test–retest reliability in controls.42
Measurement of BDNF Levels and Oxidative Stress Markers in the Serum or Plasma
Usually, on the second day of admission, blood was drawn from the patient by a simple venipuncture between 7.00 and 9.00 am. The blood sampling time of the healthy controls was the same as that of the patients. BDNF levels in the serum were measured by the technicians who were blinded to the number of the sample using a commercially available kit (R&D, USA) as reported earlier.3 Malondialdehyde (MDA) and antioxidant enzymes (total SOD, Mn-SOD, and CuZn-SOD) in plasma were detected by spectrophotometer using commercially available kits (Jiancheng, Nanjing, China) as described in a prior literature.43 Serum BDNF levels and plasma MDA levels or antioxidant enzyme activities were measured in the biochemical laboratory of our research center through established procedures, which were reported in our previous studies.3 Moreover, most of the previous studies reported the level of BDNF in serum and the levels of MDA or the activity of antioxidant enzyme in plasma of patients with SCZ. Therefore, 2 different blood components were used in this study to detect different biomarkers in this study for comparison with the results of previous studies.
All samples were evaluated in duplicate and the inter- and intra-variation coefficients between the assays were below 10%.
Statistical Analysis
We carried out the first analysis to examine whether there was a difference in sociodemographic characteristics and cognitive functions between controls and patients. Further, the Kolmogorov–Smirnov one-sample test was performed to judge if BDNF levels, antioxidant enzyme activity and MDA levels were normally distributed in controls and patients. ANCOVA analysis were performed to analyze the differences of BDNF and oxidative stress markers among the patients and controls, with age, gender, smoke status, and body mass index (BMI) as covariates.
Next, we conducted the Pearson product moment correlation to explore the relationships between BDNF and SOD enzyme activities and MDA levels in patients and controls individually. Finally, the multiple regression were performed to explore the relationships between oxidative marker levels, BDNF levels, the interactive effects (BDNF × oxidative stress markers), and PANSS scores or cognitive function in the patient group. The calculation method of interactive effects was described in our previous study.44 In brief, the new interactive value was obtained via multiplying BDNF by each of SOD enzyme activity or MDA level, which was then entered into the multiple regression models. In the multiple regression model, cognitive function scores or PANSS scores were used as dependent variables, while oxidative stress markers and BDNF levels, as well as BDNF × oxidative stress markers were utilized as independent variables. Covariates in the regression models include age, education, smoking, sex, BMI, illness duration. We set the significance levels at P < .05. In addition, G*power 3.1.9.2 program was used to carry out the power calculation.
When conducting multiple analyses on the same dependent variable, the chance of committing a type I error (increased likelihood from a significant result by pure change) increases. To correct for this, a Bonferroni correction was conducted in this study. To get the Bonferroni corrected/adjusted P value, we divided the original α-value by the number of analyses on the dependent variable. In this study, a new α = 0.05/30 = 0.0023.
Results
Demographic Data and Cognitive Functions in Patients and Controls
Table 1 shows the general and clinical data of 327 DNFE patients with SCZ and 391 controls. The patients were significantly different from controls in BMI (P < .01). RBANS data was available from 256 patients and 180 healthy controls. The patients performed worse in total score and all subscales except visuospatial/constructional index controlling for sex, age, BMI and education (all P < .001; Bonferroni correction, P < .05). This is illustrated in table 2.
Table 1.
Demographic Characteristics, Clinical Data, and Cytokines in Drug-Naive First-Episode (DNFE) Patients With Schizophrenia and Healthy Controls
| Variable | DNFE Patients (n = 327) | Healthy Controls (n = 391) | F or χ2 (P Value) |
|---|---|---|---|
| Gender (male/female) | 160/118 | 208/181 | 0.3 (.31) |
| Age (years) | 26.9 ± 9.4 | 27.7 ± 7.4 | 3.0 (.12) |
| Education (years) | 9.7 ± 5.3 | 10.2 ± 3.1 | 1.2 (.25) |
| Smokers% | 28.3 | 33.0 | 1.3 (.26) |
| Body mass index (kg/m2) | 21.6 ± 3.6 | 24.5 ± 5.0 | 48.9 (.00) |
| BDNF (ng/ml) | 9.1 ± 3.6 | 11.8 ± 2.5 | 93.6 (.00) |
| MDA (nmol/ml) | 2.1 ± 1.3 | 2.9 ± 1.7 | 32.2 (.00) |
| CuZn-SOD (U/ml) | 53.5 ± 15.1 | 48.6 ±15.3 | 13.5 (.00) |
| LgMn-SOD (U/ml) | 1.25 ± 0.37 | 1.20 ± 0.4 | 2.5 (.11) |
| Total SOD (U/ml) | 76.2 ± 10.5 | 66.6 ± 11.4 | 103.0 (.00) |
| Age of onset (years) | 26.0 ± 9.4 | ||
| Duration of illness (months) | 23.7 ± 19.3 | ||
| PANSS score, mean ± SD | |||
| Positive symptoms | 21.5 ± 6.4 | ||
| Negative symptoms | 18.9 ± 7.0 | ||
| General psychopathology | 35.3 ± 9.4 | ||
| Total score | 75.8 ± 16.9 |
Table 2.
Comparison of RBANS Scores Between First-Episode Schizophrenia Patients and Healthy Control Subjects
| Cognitive Index | DNFE Patients n = 256 | Controls n = 180 | F(P)a | Effect Size | MD (95% CI) |
|---|---|---|---|---|---|
| Immediate memory | 65.3 ± 16.9 | 75.6 ± 17.6 | 57.4 (<.001) | 0.60 | −10.3 (−7.6 to −13.0) |
| Visuospatial/constructional | 77.4 ± 16.9 | 79.8 ± 15.4 | 3.4 (.066) | 0.15 | −2.3 (0.16 to −4.78) |
| Language | 75.2 ± 18.3 | 94.1 ± 13.2 | 249.4 (<.001) | 1.15 | −18.9 (−16.6 to −21.3) |
| Attention | 74.7 ± 19.9 | 87.5 ± 19.9 | 66.5 (<.001) | 0.64 | −12.7 (−9.7 to −15.8) |
| Delayed memory | 69.8 ± 20.1 | 86.5 ± 15.0 | 0.92 | −16.6 (−14.0 to −19.2) | |
| Total | 66.6 ± 15.9 | 80.2 ± 15.0 | 154.0 (<.001) | 0.88 | −13.5 (−11.2 to −15.9) |
Note: Adjusted F value controlling for gender, age, education, smoking status, and BMI between patients and control subjects; MD, mean difference between patients and health controls; CI, confidence interval.
The measure of effect size refers to Cohen’s d value here. Since Cohen suggested that d = 0.2 be considered a “small” effect size, 0.5 a “medium” effect size and 0.8 a “large” effect size, most of RBANS domain and total scores between patients and controls display large difference except for immediate memory (medium) and visuospatial/construction (small).
Interrelationships of BDNF, Antioxidant Enzyme and MDA in Patients and Controls
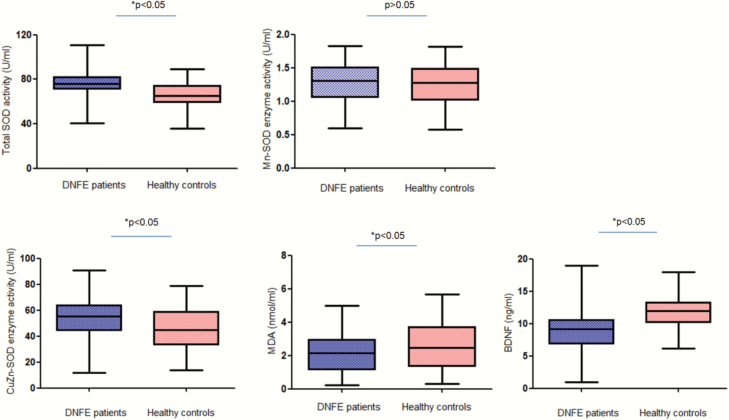
MDA and BDNF levels were reduced (MDA = −0.71 ± 0.18, 95% CI = −0.35 to −1.08; BDNF: −3.3 ± 0.35, 95% CI = −2.6 to −4.0), while total SOD and CuZn-SOD activities were increased in patients (total SOD = 10.53 ± 1.33, 95% CI = 7.92 to 13.14; CuZn-SOD: 4.49 ± 1.71, 95% CI = 1.12 to 7.86), when controlling for age, sex, BMI, smoking, and education (all P < .01; Bonferroni correction, P < .05, figure 1). The patients did not differ from controls in Mn-SOD (P > .05). The powers for measuring these biomarkers ranged from 0.96 to 1.0, except for Mn-SOD with a power of 0.36.
Fig. 1.
This figure presents the boxplots of BDNF and MDA levels and SOD, Mn-SOD, CuZn-SOD enzyme activities from patients with schizophrenia and from healthy controls. The sample means are indicated by the black bars.
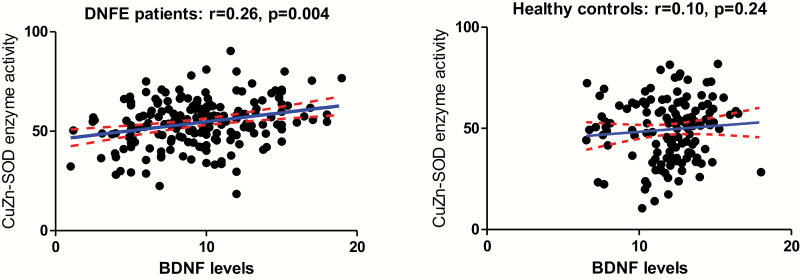
In DNFE patients, BDNF levels were positively correlated with total SOD (r = .20, P = .024) and CuZn-SOD activities (r = .26, P = .004) (table 3 and figure 2). However, only the CuZn-SOD activity survived Bonferroni correction, P < .05). The relationship between BDNF and CuZn-SOD activity was further verified by multiple regression analysis (β = 0.11, t = 2.65, P = .01). We also found an association between BDNF and MDA levels in control subjects (r = −.19, P = 0.047), which disappeared after controlling for age, gender, smoking, and BMI (P = .08).
Table 3.
Correlations Between BDNF Levels and Antioxidant Enzyme Activities in Both DNFE Patients and Healthy Controlsa
| BDNF | ||||||||
|---|---|---|---|---|---|---|---|---|
| Total SOD | CuZn-SOD | Mn-SOD | MDA | |||||
| r | P | r | P | r | P | r | P | |
| DNFE patientsb | .20 | .024 | .25 | .004 | −.06 | .48 | .02 | .81 |
| DNFE patientsc | .10 | .37 | .26 | .015 | −.14 | .20 | .03 | .82 |
| Healthy controlsb | −.04 | .66 | .10 | .34 | −.10 | .27 | .19 | .047 |
| Healthy controlsc | .17 | .14 | .16 | .15 | −.01 | .94 | .13 | .27 |
aPearson product moment.
bBefore adjusting for the confounding factors.
cAfter adjusting for the confounding factors.
Fig. 2.
There was a significant negative relationship between BDNF levels and CuZn-SOD activities (r = .26, P = .004) in patients, but not in healthy controls.
Relationships Between BDNF, MDA, Total SOD Enzymes and Psychotic Symptoms
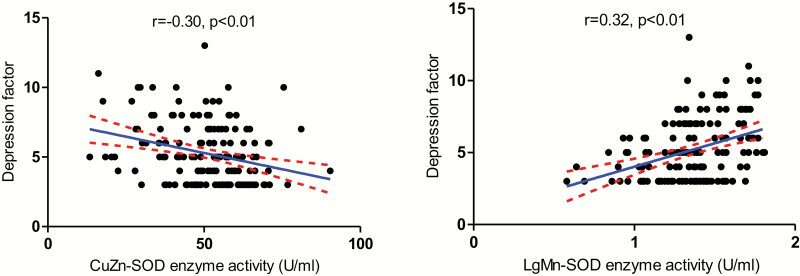
In the present study, a negative association between BDNF levels and PANSS positive factor (r = −.20, P = .04) was demonstrated. Moreover, the PANSS depressive factor was negatively correlated with CuZn-SOD activity (r = −.30, P < .001), positively correlated with Mn-SOD activity (r = .32, P < .001) and total SOD activity (r = .20, P = .011). After Bonferroni correction, only the relationship between depressive factor and CuZn-SOD activity or Mn-SOD activity remains significant (P < .01) (figure 3 and table 4).
Fig. 3.
There were significant relationships between antioxidant enzyme activities and psychotic symptoms (CuZn-SOD: r = −.30, P < .01; Mn-SOD: r = .32, P < .01) in patients.
Table 4.
Relationships Between BDNF, Oxidative Stress Markers, and Psychotic Symptom in DNFE Patientsa
| Positive Factor | Negative Factor | Cognitive Factor | Excited Factor | Depression Factor | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r | P | P b | r | P | P b | r | P | P b | r | P | P b | r | P | P b | |
| BDNF | −.20 | .04 | n.s. | .14 | .16 | n.s. | .02 | .83 | n.s. | .06 | .54 | n.s. | −.07 | .43 | n.s. |
| Total SOD | .04 | .61 | n.s. | .01 | .90 | n.s. | −.08 | .31 | n.s. | −.07 | .93 | n.s. | .20 | .01 | n.s. |
| CuZn-SOD | −.05 | .51 | n.s. | .01 | .93 | n.s. | .02 | .82 | n.s. | −.05 | .53 | n.s. | −.30 | <.001 | <.01 |
| Mn-SOD | .09 | .27 | n.s. | −.07 | .48 | n.s. | −.04 | .58 | n.s. | .10 | .22 | n.s. | .32 | <.001 | <.01 |
| MDA | .03 | .69 | n.s. | .07 | .42 | n.s. | −.01 | .89 | n.s. | −.14 | .09 | n.s. | −.13 | .12 | n.s. |
aPearson product moment.
bBonferroni correction was applied in the associations between biomarkers and psychotics symptoms.
Correlations Between Cognitive Functions and BDNF, MDA, and SOD Enzymes
For the combined subjects, the regression analysis showed the Mn-SOD activity (β = −0.26, t = −2.1, P = .04), age (β = 0.14, t = 2.4, P = .016), and education (β = 0.55, t = 9.9, P < .001) was the determine factor for the RBANS total score.
For the control subjects, significant positive associations were found between CuZn-SOD enzyme activities and visuospatial/constructional, attention index, and total scores. There was also a positive correlation between Mn-SOD activities and attention, visuospatial/constructional index, and total scores (all P < .05). After controlling for age, sex, education, smoking, and BMI, the multiple regression analysis revealed that no significant association was found between cognitive functions and BDNF, MDA, SOD enzyme activities (all P > .05). In addition, no any interactive effect among BDNF, oxidative stress markers on cognition was found in control subjects (all P > .05, table 5 and figure 4).
Table 5.
Relationships Between BDNF, Oxidative Stress Markers, and Cognitive Function in DNFE Patientsa
| Immediate Memory | Attention | Language | Visuospatial/Constructional | Delayed Memory | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r | P | P b | r | P | P b | r | P | P b | r | P | P b | r | P | P b | |
| DNFE patients | |||||||||||||||
| BDNF | −.09 | .20 | n.s. | −.08 | .25 | n.s. | −.04 | .60 | n.s. | .01 | .94 | n.s. | −.07 | .34 | n.s. |
| Total SOD | −.05 | .42 | n.s. | −.12 | .07 | n.s. | −.05 | .49 | n.s. | −.05 | .42 | n.s. | −.07 | .34 | n.s. |
| CuZn-SOD | .11 | .09 | n.s. | .16 | .02 | n.s. | .12 | .06 | n.s. | .15 | .03 | n.s. | .01 | .92 | n.s |
| Mn-SOD | −.17 | .01 | n.s. | −.25 | <.01 | s. | −.06 | .19 | n.s. | −.17 | .02 | n.s. | .05 | .50 | n.s. |
| MDA | −.06 | .51 | n.s. | −.18 | .01 | n.s. | .05 | .59 | n.s. | .05 | .59 | n.s. | .03 | .73 | n.s. |
| Healthy controls | |||||||||||||||
| BDNF | −.01 | .91 | n.s. | −.03 | .53 | n.s. | −.03 | .95 | n.s. | −.09 | .98 | n.s. | −.09 | .90 | n.s. |
| Total SOD | .03 | .73 | n.s. | .10 | .09 | n.s. | −.01 | .80 | n.s. | .07 | .14 | n.s. | .10 | .10 | n.s. |
| CuZn-SOD | −.07 | .30 | n.s. | −.18 | <.01 | n.s. | −.14 | <.01 | n.s. | −.15 | <.01 | n.s. | −.04 | .58 | n.s. |
| Mn-SOD | .08 | .23 | n.s. | −.19 | <.01 | n.s | .06 | .19 | n.s. | .19 | <.01 | n.s. | .15 | <.01 | n.s. |
| MDA | −.03 | .96 | n.s. | −.02 | .79 | n.s. | −.29 | .59 | n.s. | .11 | .04 | n.s. | .11 | .04 | n.s. |
Note: n.s., nonsignificant; s., significant.
aPearson product moment.
bAfter controlling for age, sex, smoking and BMI, no significant association was found between cognitive function and BDNF, MDA, SOD enzyme activities in the healthy controls (all P > .05).
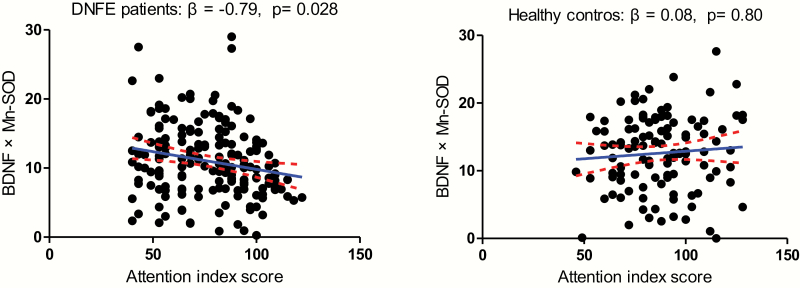
Fig. 4.
There was a significant relationship between the interactive effect of BDNF levels and Mn-SOD activity alteration with attention index score (β = −0.79, P = .028) in the first-episode and drug-naive patients with schizophrenia group, but not in healthy controls.
For the DNFE patients with SCZ, correlation analysis revealed a significant positive association between CuZn-SOD enzyme activity and visuospatial/constructional (r = .15, P = .02), attention index (r = .16, P = .02), and total scores (r = .14, P = .029). Also, there was negative association between Mn-SOD activity and immediate memory (r = −.17, P = .01), attention index (r = −.25, P = .000), or total score (r = −.23, P = .001) (table 5). Further multiple regression analyses with age, gender, education, smoking, BMI, onset age, PANSS total score or subscore and an interactive effect of BDNF and oxidative stress marker as covariates identified age (β = 2.9, t = 2.9, P = .005), onset age (β = −3.3, t = −3.2, P = .002), and BDNF × Mn-SOD (β = −0.79, t = −2.2, P = .028) as influencing factors for the attentional index of cognitive function measured by RBANS (figure 4). But there was no association between other interactive effect of BDNF and oxidative stress marker with cognitive function. Moreover, there was no significant association between BDNF and RBANS total score or index scores in patients.
Discussion
Alteration of SOD Enzymes, MDA, and BDNF in DNFE Patients With SCZ
This study was the first and the largest study to date to simultaneously report elevated activities of total SOD and CuZn-SOD enzyme, as well as reduced levels of MDA in DNFE patients with SCZ compared with controls. In agreement with these findings, our previous studies and other studies in DNFE patients with SCZ reported higher activity of SOD enzyme in peripheral blood.16,22,45 There have also been several studies in chronic patients which demonstrated similar findings.46,47 However, our results contradicted a few of recent studies in DNFE patients with SCZ,48,49 as some studies showed no difference between patients and controls.18,50,51 In addition, inconsistent results were found in Mn-SOD and CuZn-SOD enzymes activities in unmedicated patients with SCZ.10 We also found that MDA was reduced in the patient population, while other studies have reported inconsistent results in both neurotrophic-naive and chronic patients.51–53 Many factors, such as sources of sample, activation of endocrine stress axis, disease duration, smoking, and obesity, can contribute to this discrepancy.11,54,55 Although large discrepancies exist among various studies, the abnormalities found in antioxidant enzymes and MDA levels demonstrate severe dysregulations of redox system and subsequent oxidative stress persistent in the early stage of this disorder.
In addition, we also found that BDNF levels were diminished in the DNFE patients with SCZ. Abnormalities in BDNF levels in DNFE patients with SCZ have been reported in many prior studies22,32,34 and 2 recent meta-analysis.23,56 In line with our findings, most of these studies showed a reduction of BDNF levels in patients. Since the patients were drug-naive in the present study, the potential effects of antipsychotics on BDNF can be ruled out. This raises suspicion that the decreased BDNF may be related to the pathophysiology of SCZ. BDNF is well known to play a role in the synaptic plasticity, neuron growth, as well as neuronal function, so the insufficient BDNF in the patients may be suggestive of neuropathological changes in the patients.57,58 The results from our study in antipsychotic-naive, first-episode patients support the neurodevelopment hypothesis as the pathophysiologic mechanism of SCZ.
Association of BDNF With SOD Enzymes as well as MDA in DNFE Patients With SCZ and Healthy Controls
A positive relationship between CuZn-SOD and BDNF was shown in DNFE patients with SCZ, although there were decreased BDNF levels and elevated CuZn-SOD activity overall in patients compared with healthy controls. However, we did not find a relationship between BDNF and Mn-SOD or total SOD in patients.
Our finding of a positive association of BDNF levels and CuZn-SOD activity was in line with the results from another animal model study on compression-induced spinal cord injury.59 This study showed that continuous infusion of BDNF in the animal of spinal cord injury inhibited acute down-regulation of CuZn-SOD activity in glial cells and neurons. CuZn-SOD and BDNF are positively associated in the patients, even though there was an overall decrease in BDNF levels and increase in CuZn-SOD activities compared with control subjects. Consistent with our results, preclinical studies showed BDNF treatment alone stimulated and increased SOD activity by 108% and reduced oxidative stress.60–62 In the antioxidant defense system, CuZn-SOD is involved in the detoxification of superoxide radicals and redox balance, which transforms the superoxide radicals to hydrogen peroxide which can then be decomposed to water.6 The possible mechanism by which CuZn-SOD is elevated and correlated with BDNF is likely related to dopamine metabolism in patients with SCZ. It has been proposed that a significant elevation of presynaptic dopaminergic function is involved in the pathogenesis of SCZ, which causes excessive formation of free radicals through the metabolism of monoamine oxidase enzyme.63,64 We speculated the higher activities of CuZn-SOD enzyme in patients versus controls may be triggered by the oxidative stress produced by dopamine hyperfunction. Therefore, the CuZn-SOD activity is increased to scavenge ROS to protect the neuron or synapse from oxidative stress damage. Our findings of lower MDA levels in the patients provide further evidence for our postulations. The excess oxidative stress potentially upregulated the expression and activity of CuZn-SOD enzyme and relatively increased BDNF levels as a compensatory mechanism at the onset of psychosis. However, BDNF was not produced sufficiently to normal levels in the patients compared with controls, causing the overall levels in patients to be lower than that in healthy controls. Together, all findings indicate a interrelationship between BDNF and CuZn-SOD enzyme in the pathological mechanism of SCZ in the early stage of illness.
Interactive Effects of BDNF and Oxidative Stress Parameters With Clinical Symptoms in Patients
In the present study, we found Mn-SOD and CuZn-SOD enzyme activities were related to the depressive factor of 5-factor models of PANSS. In line with our findings, a previous study reported elevated SOD activity was associated with Beck Depression Inventory scores in peritoneal dialysis patients.65 In addition, higher Mn-SOD and CuZn-SOD enzyme activities were shown in previous studies in patients with depression. For example, a recent meta-analysis revealed that SOD enzyme activity was elevated in patients with depression disorders.66 Stanisavljevic et al67 also revealed that increased CuZn-SOD levels and SOD activity in hepatocyte solutes of rats existed when depression- and anxiety-like behaviors were induced by chronic social isolation exposure for 6 weeks. The mechanism by which antioxidant enzymes were correlated with depression may be related to the hyperactivity of the HPA axis and elevated cortisol levels, which have been widely noticed in depression.68 Hyperactivity of HPA axis correlated closely with chronic stress and is extensively present in the patients with SCZ. Hyperactivity of the HPA is related to an imbalance of redox system and produces deleterious ROS in the CNS.69 Additionally, the expression of CuZn-SOD protein is regulated by glucocorticoid and glucocorticoid receptors throughout the body.70 The burden of aggravating free radicals may induce an increase in Mn-SOD and CuZn-SOD enzyme activity. However, although both Mn-SOD and CuZn-SOD were associated with PANSS depression factors, a negative relationship between the two isoforms of SOD enzyme and depression scores existed in patients with SCZ. Given our study was cross-sectional design, we could not provide a rational explanation for it. Similar to this study, Gulesserian et al71 found different alterations of CuZn-SOD and Mn-SOD in the brains of patients with Down syndrome. Together, the underlying relationship of CuZn-SOD to depression in SCZ may be different from Mn-SOD and warrants further investigation.
Interactive Effects of BDNF and SOD Enzyme Activity With Cognitive Function in DNFE Patients
We found significant correlations between Mn-SOD and CuZn-SOD activities and RBANS attentional index and immediate memory index scores in DNFE patients with SCZ, although the correlation with the immediate memory did not survive Bonferroni correction. This suggests that abnormal peripheral levels of oxidative stress parameters are involved in the cognitive function impairments in SCZ patients. Further multiple regression analysis showed that an interactive effects of BDNF and Mn-SOD was positively correlated with attentional index scores in patients. Our results were consistent to a recent study by Li et al,72 which showed increased Mn-SOD activity and reduced BDNF levels were involved in the pathological mechanism of spatial memory impairments induced by bile duct ligation (BDL) in rats. In particular, minocycline treatment restored Mn-SOD activity and BDNF mRNA level to normal levels, thereby improving the spatial memory deficits in BDL rat model.
A combination of pathologic mediators of genetic and environmental factors was related to neurodevelopmental abnormalities in SCZ, which all converged on redox system imbalance and oxidative and nitrosative stress.4,12,73,74 Some studies have found that elevated production of ROS and decreased BDNF expression by downregulating the DNA-binding activities of activator protein-1, demonstrating that BDNF is significantly related to oxidative stress. However, given the cross-section design, the relationship between BDNF and Mn-SOD with attentional index scores does not provide an etiological explanation for cognitive decline in SCZ patients. Yet, it does provide more evidence that increased Mn-SOD enzyme activity impairs cognitive functioning.11,75,76 Although BDNF was decreased in patients in our current study, it was not correlated with cognitive impairments in patients, consistent to previous studies.31 However, the multiple regression analysis identified the interactive effect of BDNF and Mn-SOD as the influencing factors of attention index score, indicating the complex interplay of BDNF and oxidative stress is involved in the pathophysiology of cognitive impairments in DNFE SCZ. We currently cannot provide a good mechanism to explain why there was no relationship between BDNF and cognitive impairments in the patients. The brain is particularly susceptible to oxidative stress due to its high oxygen consumption, low antioxidant capacity, and relatively high levels of polyunsaturated fatty acid, especially in the basal forebrain and amygdale.77 Since these areas are important for specific functions of the brain, such as cognition and memory, damage to these areas can have significant neurological functions. For example, several studies have reported elevated lipid oxidation in the brain of patients with AD. The elevation of total SOD and CuZn-SOD enzyme activity suggests overexpression in the SCZ patients as a response to the oxidative stress and may be deleterious to neurodevelopment and cognitive functioning.71
It is worthy of mentioning that we are addressing an important topic in psychiatric disease research using a large patient cohort of the rare first-episode patients with SCZ. However, as numerous parameters were involved including cognitive function, clinical symptoms, as well as biomarkers (BDNF and several oxidative stress parameters), some major discrepancies occurred in our studies. For example, in the background, previous studied showed that increased SOD led to improved memory behavior, suggesting high SOD may be associated with better cognitive function.19 Moreover, exercise reduced oxidative stress markers and improved cognitive function.36,37 Especially, antioxidant therapy lead to improved memory behavior and increased BDNF.3 Taken together, it appears that lower oxidative stress (more SOD and lower MDA) is good for cognitive function. However, in this study, we found that SCZ patients had extensive cognitive deficits, higher total SOD and CuZn-SOD activities, but lower BDNF and MDA levels. We have put forward some speculations to explain these seemingly contradictory results. Due to still unknown causes, excess free radical productions and oxidative stress occur in DNFE patients with SCZ in the early stage, leading to cell damage or even death, which in turn results in decreases of BDNF levels and cognitive impairments. In this situation, the antioxidant defense system is activated, resulting in a compensation effect, which may induce an increase in the antioxidant enzyme activities, including total SOD and CuZn-SOD activities. Subsequently, these increased antioxidant enzyme activities may reduce the peroxidation, showing a decrease in MDA levels. In additional, interestingly, we found a significant positive association between BDNF and CuZn-SOD activity in patients only, suggesting an interrelationship between neurotrophin and oxidative stress as a pathological mechanisms for SCZ at the early stage. It is worth mentioning that although patients showed lower BDNF levels but high SOD activities in patients, the 2 processes appeared not to run in parallel possible due to underlying psychopathological mechanisms of SCZ. Maybe the positive correlation between BDNF and CuZn-SOD activity suggested that antioxidant SOD was elevated due to increased free radicals in SCZ and then subsequently stimulated an increase in BDNF to some degree to compensate the oxidative damage. However, these are our speculations, which clearly warrant further exploration.
Several limitations from our study need to be noted. First, whether peripheral levels or activities of BDNF and oxidative stress markers have a similar direction of changes in the central nervous system is still uncertain. Also, we still do not know whether SOD or BDNF in plasma originate from the brain, which deserves further investigation. Second, in this study, the biomarkers were only measured in the early stage of this disorder, rather than longitudinal comparisons before and after taking antipsychotics. This measurement could investigate whether alterations of biomarkers were related to the improvement of cognition. Third, only antioxidant enzyme activities were measured in the current study, we did not take other markers of antioxidant defense system and oxidative stress into account. Considering antioxidant protection involves complex cooperative and sequential actions including both enzymatic and nonenzymatic molecules, the activities of 3 antioxidant enzymes provide only partial insights into free radical-mediated neuronal dysfunction. Fourth, we did not analyze the influence of antipsychotics treatment on BDNF levels and SOD activity in this study. Fifth, in the present study, for those patients who were clinically unstable, the RBANS was assessed after starting treatment and not while their naive condition. Sixth, in this study, we utilized RBANS for cognition, rather than the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS), which represents the gold standard for cognition of SCZ. This is because when we carried out this study, we did not have MATRICS at that time. Seventh, in this study, we adopted a cross-sectional design that could not explain the causal relationship between biomarkers and cognitive deficits or clinical symptoms in SCZ patients. Eighth, our sample was the first-episode hospitalized SCZ patients, whose clinical symptoms were more serious, especially positive symptoms. Therefore, the findings in our current study may not be generalized to other patients, such as outpatients or community patients. Ninth, these first-episode SCZ patients in this study were stressed because the experience of acute psychosis itself was very stressful for them. Moreover, the effects of stress on biomarkers, such as BDNF and oxidative stress were reported before.78 Unfortunately, we did not evaluate the role of stress in this study, which should be remedied in future studies, for example, by measuring stress-related hormones such as cortisol.
In summary, abnormal SOD enzyme activities, MDA levels, and decreased BDNF levels were involved in the pathogenesis of DNFE patients with SCZ in this study. Particularly, BDNF levels were positively correlated with CuZn-SOD enzyme activity in patients, suggesting a relationship between the antioxidant defense system and insufficient compensation for BDNF levels which could be involved in the pathologic mechanism of SCZ. Furthermore, redox system interacts with BDNF to impact attention functioning and depression symptoms in the patients. In this study, we had several interesting results, showing altered biomarkers such as BDNF and oxidative free-related parameters in DNFE patients with SCZ, and also association between these biomarkers and cognitive or clinical symptom measures of patients. These results may have important clinical implication in early diagnostic identification from a translational approach. For example, identifying biomarkers in SCZ during the first episode is very important, since we may use these biomarkers to establish early diagnosis and then provide effective treatments, which can alleviate or prevent further episode and disease progression.
Funding
Funding for this study was provided by grants from the National Natural Science Foundation of China (81371477 and 81000509).
Acknowledgments
M.H.X. and X.Y.Z. were responsible for study design, statistical analysis, and article preparation. D.C.C., S.C., M.H.X., and Y.S.T. were responsible for recruiting the patients, performing the clinical rating, and collecting the clinical data. M.E.C., S.P.T., and H.E.W. were evolving the ideas and editing the article. Z.L., X.Y.Z., and M.H.X. were involved in writing the protocol, and cowrote the article. All authors have contributed to and have approved the final article. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Ali FT, Abd El-Azeem EM, Hamed MA, Ali MAM, Abd Al-Kader NM, Hassan EA. Redox dysregulation, immuno-inflammatory alterations and genetic variants of BDNF and MMP-9 in schizophrenia: pathophysiological and phenotypic implications. Schizophr Res. 2017;188:98–109. [DOI] [PubMed] [Google Scholar]
- 2. Yao JK, Keshavan MS. Antioxidants, redox signaling, and pathophysiology in schizophrenia: an integrative view. Antioxid Redox Signal. 2011;15(7):2011–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wei C, Sun Y, Chen N, Chen S, Xiu M, Zhang X. Interaction of oxidative stress and BDNF on executive dysfunction in patients with chronic schizophrenia. Psychoneuroendocrinology. 2020;111:104473. [DOI] [PubMed] [Google Scholar]
- 4. Fraguas D, Díaz-Caneja CM, Ayora M, et al. Oxidative stress and inflammation in first-episode psychosis: a systematic review and meta-analysis. Schizophr Bull. 2019;45(4):742–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Crapo JD, Oury T, Rabouille C, Slot JW, Chang LY. Copper, zinc superoxide dismutase is primarily a cytosolic protein in human cells. Proc Natl Acad Sci U S A. 1992;89(21):10405–10409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Romuk E, Jacheć W, Kozielska-Nowalany E, Birkner E, Zemła-Woszek A, Wojciechowska C. Superoxide dismutase activity as a predictor of adverse outcomes in patients with nonischemic dilated cardiomyopathy. Cell Stress Chaperones. 2019;24(3):661–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shimoda-Matsubayashi S, Hattori T, Matsumine H, et al. Mn SOD activity and protein in a patient with chromosome 6-linked autosomal recessive parkinsonism in comparison with Parkinson’s disease and control. Neurology. 1997;49(5):1257–1262. [DOI] [PubMed] [Google Scholar]
- 8. Robinson BH. The role of manganese superoxide dismutase in health and disease. J Inherit Metab Dis. 1998;21(5):598–603. [DOI] [PubMed] [Google Scholar]
- 9. Weydert CJ, Cullen JJ. Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat Protoc. 2010;5(1):51–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang DF, Cao B, Xu MY, et al. Meta-analyses of manganese superoxide dismutase activity, gene Ala-9Val polymorphism, and the risk of schizophrenia. Medicine (Baltim). 2015;94(36):e1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang XY, Chen DC, Xiu MH, et al. Cognitive function, plasma MnSOD activity, and MnSOD Ala-9Val polymorphism in patients with schizophrenia and normal controls. Schizophr Bull. 2014;40(3):592–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Upthegrove R, Khandaker GM. Cytokines, oxidative stress and cellular markers of inflammation in schizophrenia. Curr Top Behav Neurosci. 2020;44:49–66. [DOI] [PubMed] [Google Scholar]
- 13. Flatow J, Buckley P, Miller BJ. Meta-analysis of oxidative stress in schizophrenia. Biol Psychiatry. 2013;74(6):400–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Coughlin JM, Hayes LN, Tanaka T, et al. Reduced superoxide dismutase-1 (SOD1) in cerebrospinal fluid of patients with early psychosis in association with clinical features. Schizophr Res. 2017;183:64–69. [DOI] [PubMed] [Google Scholar]
- 15. Coughlin JM, Ishizuka K, Kano SI, et al. Marked reduction of soluble superoxide dismutase-1 (SOD1) in cerebrospinal fluid of patients with recent-onset schizophrenia. Mol Psychiatry. 2013;18(1):10–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wu Z, Zhang XY, Wang H, et al. Elevated plasma superoxide dismutase in first-episode and drug naive patients with schizophrenia: inverse association with positive symptoms. Prog Neuropsychopharmacol Biol Psychiatry. 2012;36(1):34–38. [DOI] [PubMed] [Google Scholar]
- 17. Hu D, Serrano F, Oury TD, Klann E. Aging-dependent alterations in synaptic plasticity and memory in mice that overexpress extracellular superoxide dismutase. J Neurosci. 2006;26(15):3933–3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Raffa M, Atig F, Mhalla A, Kerkeni A, Mechri A. Decreased glutathione levels and impaired antioxidant enzyme activities in drug-naive first-episode schizophrenic patients. BMC Psychiatry. 2011;11:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Khan NS, Das I. Oxidative stress and superoxide dismutase in schizophrenia. Biochem Soc Trans. 1997;25(3):418S. [DOI] [PubMed] [Google Scholar]
- 20. Mukherjee S, Decina P, Bocola V, Saraceni F, Scapicchio PL. Diabetes mellitus in schizophrenic patients. Compr Psychiatry. 1996;37(1):68–73. [DOI] [PubMed] [Google Scholar]
- 21. Pandya CD, Howell KR, Pillai A. Antioxidants as potential therapeutics for neuropsychiatric disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2013;46:214–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jordan W, Dobrowolny H, Bahn S, et al. Oxidative stress in drug-naïve first episode patients with schizophrenia and major depression: effects of disease acuity and potential confounders. Eur Arch Psychiatry Clin Neurosci. 2018;268(2):129–143. [DOI] [PubMed] [Google Scholar]
- 23. Fernandes BS, Steiner J, Berk M, et al. Peripheral brain-derived neurotrophic factor in schizophrenia and the role of antipsychotics: meta-analysis and implications. Mol Psychiatry. 2015;20(9):1108–1119. [DOI] [PubMed] [Google Scholar]
- 24. Buckley PF, Pillai A, Howell KR. Brain-derived neurotrophic factor: findings in schizophrenia. Curr Opin Psychiatry. 2011;24(2):122–127. [DOI] [PubMed] [Google Scholar]
- 25. Nurjono M, Lee J, Chong SA. A review of brain-derived neurotrophic factor as a candidate biomarker in schizophrenia. Clin Psychopharmacol Neurosci. 2012;10(2):61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nieto R, Kukuljan M, Silva H. BDNF and schizophrenia: from neurodevelopment to neuronal plasticity, learning, and memory. Front Psychiatry. 2013;4:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bora E. Neurodevelopmental origin of cognitive impairment in schizophrenia. Psychol Med. 2015;45(1):1–9. [DOI] [PubMed] [Google Scholar]
- 28. Narita M, Aoki K, Takagi M, Yajima Y, Suzuki T. Implication of brain-derived neurotrophic factor in the release of dopamine and dopamine-related behaviors induced by methamphetamine. Neuroscience. 2003;119(3):767–775. [DOI] [PubMed] [Google Scholar]
- 29. Nikulina EM, Johnston CE, Wang J, Hammer RP Jr. Neurotrophins in the ventral tegmental area: role in social stress, mood disorders and drug abuse. Neuroscience. 2014;282:122–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Koo JW, Chaudhury D, Han MH, Nestler EJ. Role of mesolimbic brain-derived neurotrophic factor in depression. Biol Psychiatry. 2019;86(10):738–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Man L, Lv X, Du XD, et al. Cognitive impairments and low BDNF serum levels in first-episode drug-naive patients with schizophrenia. Psychiatry Res. 2018;263:1–6. [DOI] [PubMed] [Google Scholar]
- 32. Yang Y, Liu Y, Wang G, et al. Brain-derived neurotrophic factor is associated with cognitive impairments in first-episode and chronic schizophrenia. Psychiatry Res. 2019;273:528–536. [DOI] [PubMed] [Google Scholar]
- 33. Heitz U, Papmeyer M, Studerus E, et al. Plasma and serum brain-derived neurotrophic factor (BDNF) levels and their association with neurocognition in at-risk mental state, first episode psychosis and chronic schizophrenia patients. World J Biol Psychiatry. 2019;20(7):545–554. [DOI] [PubMed] [Google Scholar]
- 34. Xiao W, Ye F, Liu C, et al. Cognitive impairment in first-episode drug-naïve patients with schizophrenia: relationships with serum concentrations of brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor. Prog Neuropsychopharmacol Biol Psychiatry. 2017;76:163–168. [DOI] [PubMed] [Google Scholar]
- 35. Ahmed AO, Mantini AM, Fridberg DJ, Buckley PF. Brain-derived neurotrophic factor (BDNF) and neurocognitive deficits in people with schizophrenia: a meta-analysis. Psychiatry Res. 2015;226(1):1–13. [DOI] [PubMed] [Google Scholar]
- 36. Ishola IO, Osele MO, Chijioke MC, Adeyemi OO. Isorhamnetin enhanced cortico-hippocampal learning and memory capability in mice with scopolamine-induced amnesia: role of antioxidant defense, cholinergic and BDNF signaling. Brain Res. 2019;1712:188–196. [DOI] [PubMed] [Google Scholar]
- 37. Canever L, Freire TG, Mastella GA, et al. Changes in behavioural parameters, oxidative stress and neurotrophins in the brain of adult offspring induced to an animal model of schizophrenia: the effects of FA deficient or FA supplemented diet during the neurodevelopmental phase. Prog Neuropsychopharmacol Biol Psychiatry. 2018;86:52–64. [DOI] [PubMed] [Google Scholar]
- 38. Phillips MR, Zhang J, Shi Q, et al. Prevalence, treatment, and associated disability of mental disorders in four provinces in China during 2001–05: an epidemiological survey. Lancet. 2009;373(9680):2041–2053. [DOI] [PubMed] [Google Scholar]
- 39. Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. [DOI] [PubMed] [Google Scholar]
- 40. Wallwork RS, Fortgang R, Hashimoto R, Weinberger DR, Dickinson D. Searching for a consensus five-factor model of the Positive and Negative Syndrome Scale for schizophrenia. Schizophr Res. 2012;137(1–3):246–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Randolph C, Tierney MC, Mohr E, Chase TN. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol. 1998;20(3):310–319. [DOI] [PubMed] [Google Scholar]
- 42. Zhang BH, Zhang WF, Wang ZR. Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) as a screening test in Chinese: reliability and validity. Chin Ment Health J. 2009;28:865–869. [Google Scholar]
- 43. Zhang XY, Chen DC, Tan YL, et al. The interplay between BDNF and oxidative stress in chronic schizophrenia. Psychoneuroendocrinology. 2015;51:201–208. [DOI] [PubMed] [Google Scholar]
- 44. Zhang X, Yang M, Du X, et al. Glucose disturbances, cognitive deficits and white matter abnormalities in first-episode drug-naive schizophrenia [published online ahead of print August 13, 2019]. Mol Psychiatry. 2019. doi: 10.1038/s41380-019-0478-1. [DOI] [PubMed] [Google Scholar]
- 45. Zhang XY, Zhou DF, Cao LY, Zhang PY, Wu GY. Elevated blood superoxide dismutase in neuroleptic-free schizophrenia: association with positive symptoms. Psychiatry Res. 2003;117(1):85–88. [DOI] [PubMed] [Google Scholar]
- 46. Gama CS, Salvador M, Andreazza AC, Kapczinski F, Silva Belmonte-de-Abreu P. Elevated serum superoxide dismutase and thiobarbituric acid reactive substances in schizophrenia: a study of patients treated with haloperidol or clozapine. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(3):512–515. [DOI] [PubMed] [Google Scholar]
- 47. Vidović B, Milovanović S, Dorđević B, et al. Effect of alpha-lipoic acid supplementation on oxidative stress markers and antioxidative defense in patients with schizophrenia. Psychiatr Danub. 2014;26(3):205–213. [PubMed] [Google Scholar]
- 48. Yuan X, Zhang P, Wang Y, et al. Changes in metabolism and microbiota after 24-week risperidone treatment in drug naïve, normal weight patients with first episode schizophrenia. Schizophr Res. 2018;201:299–306. [DOI] [PubMed] [Google Scholar]
- 49. Miljević ČD, Nikolić-Kokić A, Blagojević D, et al. Association between neurological soft signs and antioxidant enzyme activity in schizophrenic patients. Psychiatry Res. 2018;269:746–752. [DOI] [PubMed] [Google Scholar]
- 50. Şimşek Ş, Gençoğlan S, Yüksel T, Kaplan İ, Alaca R, Aktaş H. Oxidative stress and DNA damage in untreated first-episode psychosis in adolescents. Neuropsychobiology. 2016;73(2):92–97. [DOI] [PubMed] [Google Scholar]
- 51. Bai ZL, Li XS, Chen GY, et al. Serum oxidative stress marker levels in unmedicated and medicated patients with schizophrenia. J Mol Neurosci. 2018;66(3):428–436. [DOI] [PubMed] [Google Scholar]
- 52. Ranjekar PK, Hinge A, Hegde MV, et al. Decreased antioxidant enzymes and membrane essential polyunsaturated fatty acids in schizophrenic and bipolar mood disorder patients. Psychiatry Res. 2003;121(2):109–122. [DOI] [PubMed] [Google Scholar]
- 53. Dadheech G, Mishra S, Gautam S, Sharma P. Oxidative stress, α-tocopherol, ascorbic acid and reduced glutathione status in schizophrenics. Indian J Clin Biochem. 2006;21(2):34–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Đorđević VV, Lazarević D, Ćosić V, Knežević M, Đorđević VB. Age-related changes of superoxide dismutase activity in patients with schizophrenia. Vojnosanit Pregl. 2017;74(1):31–37. [DOI] [PubMed] [Google Scholar]
- 55. Zhang XY, Tan YL, Zhou DF, et al. Nicotine dependence, symptoms and oxidative stress in male patients with schizophrenia. Neuropsychopharmacology. 2007;32(9):2020–2024. [DOI] [PubMed] [Google Scholar]
- 56. Green MJ, Matheson SL, Shepherd A, Weickert CS, Carr VJ. Brain-derived neurotrophic factor levels in schizophrenia: a systematic review with meta-analysis. Mol Psychiatry. 2011;16(9):960–972. [DOI] [PubMed] [Google Scholar]
- 57. Durany N, Michel T, Zöchling R, et al. Brain-derived neurotrophic factor and neurotrophin 3 in schizophrenic psychoses. Schizophr Res. 2001;52(1–2):79–86. [DOI] [PubMed] [Google Scholar]
- 58. Weickert CS, Hyde TM, Lipska BK, Herman MM, Weinberger DR, Kleinman JE. Reduced brain-derived neurotrophic factor in prefrontal cortex of patients with schizophrenia. Mol Psychiatry. 2003;8(6):592–610. [DOI] [PubMed] [Google Scholar]
- 59. Ikeda O, Murakami M, Ino H, et al. Effects of brain-derived neurotrophic factor (BDNF) on compression-induced spinal cord injury: BDNF attenuates down-regulation of superoxide dismutase expression and promotes up-regulation of myelin basic protein expression. J Neuropathol Exp Neurol. 2002;61(2):142–153. [DOI] [PubMed] [Google Scholar]
- 60. Yang JL, Lin YT, Chuang PC, Bohr VA, Mattson MP. BDNF and exercise enhance neuronal DNA repair by stimulating CREB-mediated production of apurinic/apyrimidinic endonuclease 1. Neuromolecular Med. 2014;16(1):161–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zeng N, Xu J, Yao W, Li S, Ruan W, Xiao F. Brain-derived neurotrophic factor attenuates septic myocardial dysfunction via eNOS/NO pathway in rats. Oxid Med Cell Longev. 2017;2017:1721434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bodur A, İnce İ, Kahraman C, Abidin İ, Aydin-Abidin S, Alver A. Effect of a high sucrose and high fat diet in BDNF (+/−) mice on oxidative stress markers in adipose tissues. Arch Biochem Biophys. 2019;665:46–56. [DOI] [PubMed] [Google Scholar]
- 63. Howes OD, McCutcheon R, Owen MJ, Murray RM. The role of genes, stress, and dopamine in the development of schizophrenia. Biol Psychiatry. 2017;81(1):9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Stansley BJ, Yamamoto BK. L-dopa-induced dopamine synthesis and oxidative stress in serotonergic cells. Neuropharmacology. 2013;67:243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Eraldemir FC, Ozsoy D, Bek S, Kir H, Dervisoglu E. The relationship between brain-derived neurotrophic factor levels, oxidative and nitrosative stress and depressive symptoms: a study on peritoneal dialysis. Ren Fail. 2015;37(4):722–726. [DOI] [PubMed] [Google Scholar]
- 66. Jiménez-Fernández S, Gurpegui M, Díaz-Atienza F, Pérez-Costillas L, Gerstenberg M, Correll CU. Oxidative stress and antioxidant parameters in patients with major depressive disorder compared to healthy controls before and after antidepressant treatment: results from a meta-analysis. J Clin Psychiatry. 2015;76(12):1658–1667. [DOI] [PubMed] [Google Scholar]
- 67. Stanisavljevic A, Peric I, Pantelic M, Filipovic DM. Olanzapine alleviates oxidative stress in the liver of socially isolated rats. Can J Physiol Pharmacol. 2017;95(6):634–640. [DOI] [PubMed] [Google Scholar]
- 68. Yang SJ, Yu HY, Kang DY, et al. Antidepressant-like effects of salidroside on olfactory bulbectomy-induced pro-inflammatory cytokine production and hyperactivity of HPA axis in rats. Pharmacol Biochem Behav. 2014;124:451–457. [DOI] [PubMed] [Google Scholar]
- 69. van Bodegom M, Homberg JR, Henckens MJAG. Modulation of the hypothalamic-pituitary-adrenal axis by early life stress exposure. Front Cell Neurosci. 2017;11:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Cvijić G, Radojicić R, Matijasević Z, Davidović V. The effect of glucocorticoids on the activity of monoamine oxidase and superoxide dismutase in the rat interscapular brown adipose tissue. Zoolog Sci. 1994;11(5):707–711. [PubMed] [Google Scholar]
- 71. Gulesserian T, Seidl R, Hardmeier R, Cairns N, Lubec G. Superoxide dismutase SOD1, encoded on chromosome 21, but not SOD2 is overexpressed in brains of patients with Down syndrome. J Investig Med. 2001;49(1):41–46. [DOI] [PubMed] [Google Scholar]
- 72. Li SW, Chen YC, Sheen JM, et al. Minocycline restores cognitive-relative altered proteins in young bile duct-ligated rat prefrontal cortex. Life Sci. 2017;180:75–82. [DOI] [PubMed] [Google Scholar]
- 73. Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44(7):660–669. [DOI] [PubMed] [Google Scholar]
- 74. Dwir D, Giangreco B, Xin L, et al. MMP9/RAGE pathway overactivation mediates redox dysregulation and neuroinflammation, leading to inhibitory/excitatory imbalance: a reverse translation study in schizophrenia patients [published online ahead of print March 25, 2019]. Mol Psychiatry. 2019. doi: 10.1038/s41380-019-0393-5. [DOI] [Google Scholar]
- 75. Zhang XY, Chen DC, Xiu MH, et al. Clinical symptoms and cognitive impairment associated with male schizophrenia relate to plasma manganese superoxide dismutase activity: a case–control study. J Psychiatr Res. 2013;47(8):1049–1053. [DOI] [PubMed] [Google Scholar]
- 76. Sánchez-Rodríguez MA, Santiago E, Arronte-Rosales A, Vargas-Guadarrama LA, Mendoza-Núñez VM. Relationship between oxidative stress and cognitive impairment in the elderly of rural vs. urban communities. Life Sci. 2006;78(15):1682–1687. [DOI] [PubMed] [Google Scholar]
- 77. Martínez-Cengotitabengoa M, Mac-Dowell KS, Leza JC, et al. Cognitive impairment is related to oxidative stress and chemokine levels in first psychotic episodes. Schizophr Res. 2012;137(1–3):66–72. [DOI] [PubMed] [Google Scholar]
- 78. Chen DC, Wang J, Wang B, et al. Decreased levels of serum brain-derived neurotrophic factor in drug-naïve first-episode schizophrenia: relationship to clinical phenotypes. Psychopharmacology (Berl). 2009;207(3):375–380. [DOI] [PubMed] [Google Scholar]