Abstract
The recent renaissance of psychedelic science has reignited interest in the similarity of drug-induced experiences to those more commonly observed in psychiatric contexts such as the schizophrenia-spectrum. This report from a multidisciplinary working group of the International Consortium on Hallucinations Research (ICHR) addresses this issue, putting special emphasis on hallucinatory experiences. We review evidence collected at different scales of understanding, from pharmacology to brain-imaging, phenomenology and anthropology, highlighting similarities and differences between hallucinations under psychedelics and in the schizophrenia-spectrum disorders. Finally, we attempt to integrate these findings using computational approaches and conclude with recommendations for future research.
Keywords: psychedelics, psychosis, hallucinations, serotonin, Bayesian, computational
Introduction
Hallucinations, that is, percepts without corresponding stimulus, are common in psychiatric disorders (eg, schizophrenia spectrum disorders, a heterogeneous category with variable course and expressions; henceforth SCZs), in neurological disorders (eg, Parkinson’s disease, Lewy body dementia), while they can be observed in the general population too. They are also engendered by psychotomimetic drugs, including serotonergic agonists (ie, psychedelics). Since the nineteenth century, scientists have posited that clinical and pharmacological experiences could be related and that psychedelics might constitute a model of psychosis.1 The discovery of lysergic acid diethylamide (LSD) in 1943 was a boon to this “model psychosis theory,” spurring researchers to understand psychosis by administering psychedelics to healthy volunteers and by self-experimentation.2,3
The recent revival of psychedelic science generated new data and ideas, sparking great interest in the relevance of those compounds to psychosis. Do psychosis-related and drug-induced hallucinations share a similar etiology? Do they involve similar or overlapping neural mechanisms? How similar or different are these experiences phenomenologically and how are they each affected by culture?
This review from the International Consortium on Hallucinations Research (ICHR) aims to compare and contrast hallucinations under psychedelics with those observed in SCZs. Our working-group adopted a multiscale approach spanning multiple levels of understanding. First, we reviewed the underlying neural mechanisms, with a special focus on microscopic (synaptic) and macroscopic (network) mechanisms. Then, we described the subjective features of the two experiences, emphasizing their commonalities and differences and the impact of cultural factors. Finally, we described how computational models might connect these levels of analysis, from synapses to society.
Pharmacology
At the synaptic level, SCZs has been linked to dopaminergic (DA) alterations, while classical psychedelic drugs, such as LSD, mescaline, and psilocybin, are serotonin (5-HT) receptor agonists. Psychedelics can be divided into three main structural classes: phenethylamines, tryptamines, and ergolines. The phenethylamines are relatively selective for 5-HT2 subtypes, whereas the tryptamines bind to a larger number of sites, including most 5-HT receptors and σ 1 sites. Ergolines, by contrast, are even less selective and interact with serotonergic, dopaminergic, adrenergic, and histaminergic receptors. There is now a consensus that the 5-HT2A receptor is the primary target for serotonergic hallucinogens in the brain. The first evidence linking the 5-HT2A receptor to hallucinogenesis was derived from animal behavioral models (see table 1). For example, Glennon and colleagues found that 5-HT2A antagonists, such as pirenperone and ketanserin, block the effects of psychedelics in drug discrimination (DD) studies conducted in rats.4 Those investigators also found that the potencies (ED50 values) of hallucinogens in the DD paradigm are robustly correlated with their 5-HT2A affinity.5 The head-twitch response (HTR) assay is another behavioral paradigm that has been used in mechanistic studies of serotonergic hallucinogens. The HTR is a rapid reciprocal head movement that occurs in rodents after administration of serotonergic hallucinogens.6,7 Similar to the DD paradigm, selective 5-HT2A receptor antagonists such as M100907 also block the HTR induced by hallucinogens.8,9 Likewise, LSD and other hallucinogens do not induce the HTR in 5-HT2A knockout mice.10,11 The HTR paradigm has become increasingly popular in recent years because it is one of the few behavioral effects produced by hallucinogens that are not observed when animals are treated with non-hallucinogenic 5-HT2A agonists such as lisuride, an LSD analog.10,12,13 There is also a robust correlation between the ED50 values of hallucinogens in the HTR paradigm and their potencies in humans and rat DD studies.7 Therefore, although the HTR assay does not directly model the psychedelic effects produced by hallucinogens, it serves as a behavioral readout of 5-HT2A receptor activation that has considerable cross-species translational relevance.
Table 1.
The pharmacology of psychedelics
| Drug Discrimination (DD) | Head-Twitch Response (HTR) | Prepulse Inhibition (PPI) | Exploratory and Investigatory Behavior | |
|---|---|---|---|---|
| Behavioral effect | Rats can be trained to discriminate hallucinogens from vehicle. | Rats and mice treated with hallucinogens express the HTR. | Hallucinogens reduce PPI in rats. | Hallucinogens reduce exploratory and investigatory behavior in rats. |
| Receptor mechanism for phenylalkylamine hallucinogens (eg, mescaline) | Selective 5-HT2A receptor antagonists (eg, M100907 and MDL 11,939) block the effect. | Selective 5-HT2A receptor antagonists (eg, M100907 and MDL 11,939) block the effect. | Selective 5-HT2A receptor antagonists (eg, M100907 and MDL 11,939) block the effect. | Selective 5-HT2A receptor antagonists (eg, M100907 and MDL 11,939) block the effect. |
| Receptor mechanism for indoleamine hallucinogens (eg, LSD and psilocybin) | The mechanism for the DD effects for tryptamine hallucinogens often mediated by both 5-HT1A and 5-HT2A receptors. The mechanism for the effect of LSD is time-dependent; at short intervals between injection and testing (eg, 15–30 min), the effect of LSD is blocked by selective 5-HT2A antagonists; however, if the interval is increased to 90 min then the effect of LSD is blocked by antagonists of D2-like receptors. | Selective 5-HT2A receptor antagonists (eg, M100907 and MDL 11,939) block the effect. | Selective 5-HT2A receptor antagonists (eg, M100907 and MDL 11,939) block the effect. | Indoleamine hallucinogens act through both 5-HT1A and 5-HT2A receptor mechanisms. |
| Sensitivity to lisuride | Lisuride produces hallucinogen-like effects in some DD studies but not in others. | Lisuride does not induce the HTR in rats or mice. | Lisuride reduces PPI in rats but the effect is mediated by D2/3 receptors rather than the 5-HT2A receptor. | Lisuride does not produce LSD-like effects on exploratory or investigatory behavior in rats. |
In addition to DD and HTR, several other behavioral paradigms are commonly used to study the effects and pharmacology of hallucinogens in rodents. Prepulse inhibition (PPI) of the startle reflex is one example. PPI refers to the phenomenon where a weak prestimulus will inhibit the response to a subsequent startle-inducing pulse. This effect is commonly used as an operational measure of sensorimotor gating. LSD and other hallucinogens inhibit PPI in rats, an effect that can be blocked by pretreatment with selective 5-HT2A receptor antagonists (eg, M100907 and MDL 11,939).11,14 Although lisuride also reduces PPI in rats, its effect is blocked by DA D2/3 receptor antagonists but not by MDL 11,939. Similar findings have also emerged from studies of exploratory behavior in rats. Although hallucinogens reduce exploratory locomotor activity in a novel environment via 5-HT2A receptor activation,15,16 lisuride produces a qualitatively different behavioral profile similar to the effect of DA receptor agonists.17 Hallucinogens also alter timing behavior in rats and mice via 5-HT2A receptor activation.18–20
Although the 5-HT2A receptor was first linked to the mechanism of action of hallucinogens in 1984, it took more than a decade to generate relevant evidence in humans. In 1998, a clinical study conducted by Franz Vollenweider and colleagues confirmed that ketanserin can block the subjective effects of psilocybin.21 The 5-HT2A/D2 receptor antagonist risperidone can also block the subjective response to psilocybin, whereas the D2 antagonist haloperidol was not effective.21 More recently, similar findings were reported for LSD. Although there has been speculation that D2 receptor activation may contribute to the psychopharmacology of LSD, ketanserin seems to have little effect on D2 sites but is capable of blocking the subjective and neural response to LSD.22–24 Notably, it was also reported recently that the intensity of the subjective response to psilocybin is correlated with the level of central 5-HT2A receptor occupancy.25
Brain-Imaging Markers
At the network level, SCZs and psychedelics exhibit interesting commonalities and differences. A first line of work comes from fMRI capture studies which compare ON and OFF periods for hallucinations and detect the phasic neural changes associated with hallucinatory ON states. In SCZs, these studies suggest a role for modality-dependent associative cortex overactivations during hallucinations.26–30 When recruited, the primary cortices were associated with more vivid experiences.28 Interestingly, the onset of hallucinations has been found associated with various aberrant activation/deactivation patterns. Hyperactivity was found in the hippocampal complex, as well as within associative cortices related to the hallucinatory content, while the default-mode network was found concomitantly deactivated.28,31
Brain imaging studies conducted to explore psychedelic states did not try to specifically capture hallucinatory events, but rather focused on neural changes in relation to sensory experiences during the psychedelic intoxication, making links with hallucinations more indirect. Regarding visual hallucinations (VH), a greater cerebral blood flow was measured in the visual cortex under LSD.32 Increased early visual activity but decreased processing in associative visual areas was also observed after psilocybin administration,33 suggesting that a combination of enhanced early sensory and reduced associative processing may contribute to the psychedelic experience.23,34
The second contribution comes from large-scale neural connectivity analyses, based on functional connectivity (FC; correlations between signals measured in different brain areas that define intrinsic brain networks), and effective connectivity, namely the effect one neuronal system exerts over another. We first look at FC studies and then briefly look at selective changes in directed effective connectivity.
A well-replicated finding in healthy individuals is an antagonistic activity between the default-mode resting-state network (DMN) and the task-related central-executive network (CEN).35–40 Some authors proposed that the orthogonality of these networks might break down in psychotic states.41 A functional disconnection between the nodes of the DMN and CEN might notably engender impaired self-monitoring as observed in SCZs42 and manifest as weak anti-correlation between these intrinsic brain networks.
According to the triple-network theory,43 the antagonistic activity of these resting-state networks (DMN and CEN) putatively reflects competing modes of information processing that may be regulated by the salience network (SN).44 Recent experimental data using intracranial EEG reported temporal profiles of task-evoked activity compatible with the hypothesis of SN acting as a switch between the CEN and DMN.45 Impairments of the triple-network was proposed broadly involved in psychopathology,46–48 and more specifically in intrusive experiences, such as flash-backs,49 obsessive ideas,50 or hallucinations in SCZs.31 In this vein, it has been proposed that SN impairments may reflect a disturbance in ascribing salience properly,51 while DMN instabilities seem to be a shared characteristic across multiple sensory domains in patients with hallucinations.41
Classical psychedelics also induce pervasive changes in network-dynamics that can generally be described as a transition from regularity to increased instability. The coherence of classical resting-state networks was found diminished (disintegrated), while FC of the primary visual cortex expanded—desegregated.32,52 In complement to its reduced activity-level, the DMN was found to potentially co-activate with the CEN, a phenomenon which may underlie the reported confusion between internally and externally generated mental contents.53 Analyzing global brain connectivity with fMRI after the administration of LSD and psilocybin also revealed an increased integration of sensory and somatomotor information together with a disintegration of information from associative networks.23,54 Additionally, a general decrease in directed FC, and concurrently an increase in undirected FC after the administration of LSD was observed using MEG imaging and may point to increased instability in psychedelic states.55
Another influential theory in SCZs is the thalamic filter hypothesis (wherein the thalamus gates sensory information to prevent the information overflow in the cortex56). Resting-state fMRI studies in patients at various stages of the illness showed that prefrontal–thalamic FC was decreased, while thalamic FC with somatosensory and motor areas was strengthened during disease progression, in a manner that correlates with positive symptoms.57,58 However, findings regarding the exact relationship between thalamocortical dysconnectivity and clinical symptoms are mixed.59
Thalamocortical connectivity was found altered in psychedelic states. Specifically, LSD was found to selectively increase effective connectivity from the thalamus to certain DMN areas, while other connections are attenuated.60 Furthermore, increased thalamic connectivity with the right fusiform gyrus and the anterior insula correlated with visual and auditory hallucinations (AH), respectively.61
In summary (see table 2), hallucinations relate more to associative network overactivations in SCZs, while they are linked with primary cortex overactivations under psychedelics. Second, in both cases, the experience is associated with reduced internal integration of functional networks, an enhanced correlation between internally and externally oriented networks as well as an impaired thalamocortical connectivity. This phenomenon may notably blur the differentiation between self-generated and perceived mental contents.
Table 2.
Comparison of the brain-imaging markers of psychotic and serotonergic hallucinations
| Schizophrenia Spectrum | 5-HT2A Agonists | Comparison | |
|---|---|---|---|
| Major networks a)During rest b)During task |
a) DMN hypoactivation Decreased connectivity within and (mostly) between RSN b) Lack of DMN suppression during tasks—decreased DMN and CEN anticorrelation |
a) DMN hypoactivation Decreased connectivity within—increased connectivity between RSN Decreased DMN and CEN anticorrelation b) Not enough evidence |
a) Partially similar Differences in changes in connectivity between RSN |
| Hallucinations a) Symptom capture b) Resting-state analysis |
a) Activation of hippocampus and modality-specific secondary cortex with deactivation of DMN and activation of SN and CEN b) Thalamic connectivity with prefrontal cortex lowered—thalamic connectivity with somatosensory cortex increased |
a) Not available b) Increased activity in primary visual areas—decreased activity in associative areas Preserved thalamic connectivity with DMN—increased thalamic connectivity with CEN |
a) Directly incomparable—mostly primary cortices in psychedelics—mostly associative cortices in SCZs |
| Link with experience | AH altered resting-state connectivity in left temporal areas VH increased RS connectivity between visual cortex and amygdala in SCZs (AH and VH) |
VH / imagery expanded connectivity and activity of V1—VH/imagery correlated with CEN activation |
RSNs, resting-state networks; DMN, default mode network; CEN, central-executive network; SN, salience network.
Phenomenology
In terms of the sensory modalities involved, AH are the most common modality of hallucinations in SCZs, with a prevalence of around 79%.62 AH are three times as frequent as VH, which have a mean prevalence of approximately 27%.62,63 The exact prevalence of hallucinations in other modalities is largely unknown, with significant variation between studies. Estimates vary for olfactory hallucinations (6–26%), gustatory hallucinations (1–31%), and somatic or tactile hallucinations (4–19%).62,64,65 AH occur alone approximately half of the time,66,67 while hallucinations in other modalities almost never occur alone.66,68 Some studies report that multimodal or “fused” hallucinations (MMH; eg, seeing a talking head)69 are highly prevalent in SCZs,65,70–72 whereas other reports suggest that these hallucinations are rare.73 By contrast, hallucinations induced by 5-HT2A agonists occur primarily in the visual domain74 (a shared feature with neurological disorders, such as Parkinson’s disease and Lewy body dementia). Nevertheless, distortions of body image, tactile hallucinations, and auditory alterations are not uncommon, especially when hallucinogens such as DMT or psilocybin are administered at high doses.75–77 Audio-visual experiences have frequently been reported, but whether they qualify as hallucinations (or synesthesias) is still debated.77 Olfactory and gustatory hallucinations are very rare in comparison, but have occasionally been reported.78 Synesthesia-like experiences are also very common with serotonergic hallucinogens79 but are uncommon in SCZs.63
With respect to the content of VH, serotonergic hallucinogens induce both elementary (brightly colored geometric form constants such as lattices, cobwebs, tunnels, and spirals)78 and complex hallucinations.74,76,80 Complex hallucinations are images of scenes or landscapes, often containing “ordinary” (humans, animals, artifacts, etc.) and “extraordinary” entities (chimeras, spirits, aliens, monsters, etc.). The prevalence of complex hallucinations increases with drug dose76,81 and as the psychedelic experience progresses over time.82 In SCZs, VH more often includes life-size images of faces, people, objects, or events, which may be bizarre or frightening. Typically, the hallucinations experienced in SCZs are detailed, concrete, and well-anchored in space.83
A series of experiential changes often precede the onset of psychosis, including AH (for a review, see Refs. 84,85). The occurrence of these prodromal hallucinations often provokes intense emotions; they may be attributed to a supernatural origin and viewed as a sign of a larger meaning or fate.86 Similarly, the VH induced by 5-HT2A agonists are often very meaningful and can be imbued with strong existential, metaphysical, and religious overtones.80,87–89
Psychosis is often accompanied by very rich and detailed hallucinations that are experienced as vivid, real, and beyond volitional control.83,90 There may be profound changes in attention, reality testing, and memory.91 Although the hallucinations induced by 5-HT2A agonists can be extremely vivid and may even feel more real than everyday sensory experiences, insight about their etiology is typically preserved; in other words, reality testing is not impaired and subjects using hallucinogens can typically distinguish between drug effects and normal waking consciousness.80,92,93 In contrast, in SCZs, hallucinations tend to be more difficult to discriminate from every-day perception. An important contributing factor is the contextual differences between the two states: while psychotic episodes in SCZs occur recurrently and unpredictably, the psychedelic state is transient (the nature and prevalence of chronic perceptual abnormalities, such as acid flashbacks and the hallucinogen persisting perceptual disorder are still debated77,94), purposeful and voluntarily initiated, thus marked by a special sense of agency (see Anthropology section).
As summarized in table 3, psychotic and serotonergic hallucinations differ in many respects: most notably in the modalities involved in the types of hallucinatory objects, and in the reality status ascribed to hallucinations. Yet, some commonalities can also be identified, especially as regards the meaningfulness, the emotional significance, and the metaphysical/spiritual quality of hallucinations (cf. Ref. 95).
Table 3.
Comparison of the phenomenology of psychotic and serotonergic hallucinations
| Schizophrenia Spectrum | 5-HT2A Agonists | Comparison | |
|---|---|---|---|
| Sensory modalities | Mainly AH (multimodal in some cases) | Mainly VH (multimodal in some cases) | Different |
| Content | No geometric hallucinations Complex hallucinations (mostly ordinary entities) |
Geometric hallucinations Complex hallucinations (ordinary and extraordinary entities) |
Different |
| Meaning | Strong existential/metaphysical meaning | Strong existential/metaphysical meaning | Similar |
| Reality monitoring/insight | Poor reality monitoring and insight | Reality monitoring and insight often preserved | Different |
| Duration | Recurrent psychotic episodes; they can last from several weeks to several months. Hallucinatory episodes during psychotic episodes can last several seconds or minutes; continuously present in some individuals. |
Transient states, lasting a few hours. Long-term perceptual effects are rare. |
Different |
Anthropology
Both in relation to psychedelic use and SCZs pathology, anthropological studies reveal enormous cultural variation that would benefit from a more systematic study. Comparative anthropological studies show that some features of the experiences induced by hallucinogenic plants and mushrooms are similar across cultures (eg, geometric VH), while others vary extensively cross-culturally (eg, subjective feeling tone, meaning, or content of the hallucinations).96–98 Hallucinogenic substances such as serotonergic plants and mushrooms have been traditionally employed in a variety of sociocultural purposes. For example, species of Anadenanthera and Virola, psilocybin mushrooms, and peyote have been used for divinatory and healing purposes.99–106 Some of these plants have also been employed in initiation rituals.107,108 It is worth highlighting that these hallucinogens have also traditionally been used for “non-ritualistic” purposes, for instance, in warfare109,110 and hunting.111 Finally, as illustrated by the case of “psilocybin mushrooms parties” held in Mexico, the pre-Columbian recreational use of these plants has been documented.112
Observing homogeneity in the features of the hallucinations produced by psychedelics within the same culture, many ethnographers have defended a culturalist approach to psychedelic hallucinations.97,107,113–117 For instance, terms such as “culturally influenced visions” 117 or “stereotypic visions” 97 have been used to argue that cultural variables are significant in shaping the hallucinogenic experience. Several candidates have been proposed to shed light on the vectors of this enculturation of the hallucinatory content: mythological and cosmological knowledge,118 kinship system and gender,118 iconographic representations,117 verbal exchanges and ritual interactions.119,120 However, these factors, the underpinnings of their effectiveness, and the sensitivity of different psychedelic substances to their effects require further study.
In the laboratory context, there have been few attempts to identify and experimentally manipulate nondrug variables in studies of serotonergic psychedelics (see Refs. 121,122 for an overview). In one exception, Studerus et al123 analyzed data from 23 controlled experimental studies, concluding that: the personality trait of absorption (“openness to cognitive, perceptual, imagistic, and other experiences”), the state of mind immediately prior to drug intake and having had few psychological problems in the prior weeks, were most strongly associated with positive experiences, while emotional excitability, young age, and an equipment-heavy experimental setting, were most strongly associated with negative experiences. In the resurgence of clinical therapeutics, extra-pharmacological variables considered especially important for therapeutic outcomes include a safe and supported treatment space, bespoke therapeutic support from trusted guides and appropriate music to accompany psychedelic sessions.124–126
There has also been little cross-cultural research on variability in hallucinations in SCZs. Nuevo et al127 conducted a cross-country study of prevalence of hallucinations finding high variability (eg, from 0.8% in Vietnam to 31.4% in Nepal), but did not analyze this further in order to uncover any potential cross-cultural patterns or correlations between specific cultural factors and the phenomenology of hallucinations. Luhrmann et al128 compared AH in SCZs patients in the United States, India, and Ghana, arguing that the negative content of AH varied according to culture. However, this was a qualitative, interview-based study, with small numbers, and groups were not compared or matched in terms of co-attendant clinical variables. A large number of questions remain unanswered in terms of what role culture may play in shaping hallucinations (for more detail, see Ref. 129). The relationship between hallucinations and culture in SCZs and in the use of psychedelics, and the possible overlap between these two research areas merits further study, not least because techniques traditionally mobilized to shape the phenomenology of psychedelic hallucinations in native societies in the Americas may enrich the therapeutic engagement with hallucinations in non-native contexts.130 This could be especially useful in cases where hallucinations respond minimally to antipsychotic medication.
Computational Modeling
In previous sections, we described psychedelic experiences and contrasted them with psychotic experiences in SCZs. We notably focused on the potential neural mechanisms that may support those experiences, both at the level of synapses (pharmacology) and networks (brain-imaging). Then, we explored the first-person experience (phenomenology) and described how it can be shaped by the social and cultural milieu (anthropology). Despite such a multi-scale approach, our endeavor would be incomplete without discussing the links between them. Besides, another relevant question remains open: Could hallucinations with different phenomenology and neurobiology be underlain by (partially) similar mechanisms? To address those questions, we turn to the burgeoning field of computational psychiatry131 and discuss how information processing might hold the key to both answers.
Computational models conceive the brain as an information processing system and provide normative accounts of those processes, which are then mapped onto existing neural structures.131 We will focus on one particular type of computational models: Bayesian models.132,133 The main idea behind this framework is that the brain learns generative models, that is, internal, hierarchical representations of the causal structure of the world.134,135 When new inputs enter the system through the sensors, they are combined with prior information (accumulated knowledge which might include expectations, memories, etc.) to generate predictions about the causes of the sensory input. In short, Bayesian models conceptualize the brain as an inference machine that tests multiple hypotheses about the state of the world, the body or the brain itself and picks the most probable one. We will summarize Bayesian theories that situate the synaptic disconnections implicit in the neuropharmacology of psychedelics (and hallucinations) in the larger context of abnormal functional and effective connectivity studies reviewed above. The basic premise rests on linking false (perceptual) inference to disconnections or disintegration of the psyche (in the sense of Bleuler), conceiving of hallucinations as aberrant perceptual inference due to abnormal belief updating, particularly in terms of how abnormal synaptic connectivity can lead to false inference via inappropriate weighting of sensory evidence and prior beliefs. This inappropriate weighting, via neuromodulation, could underwrite hallucinations in both SCZs and psychedelic states.
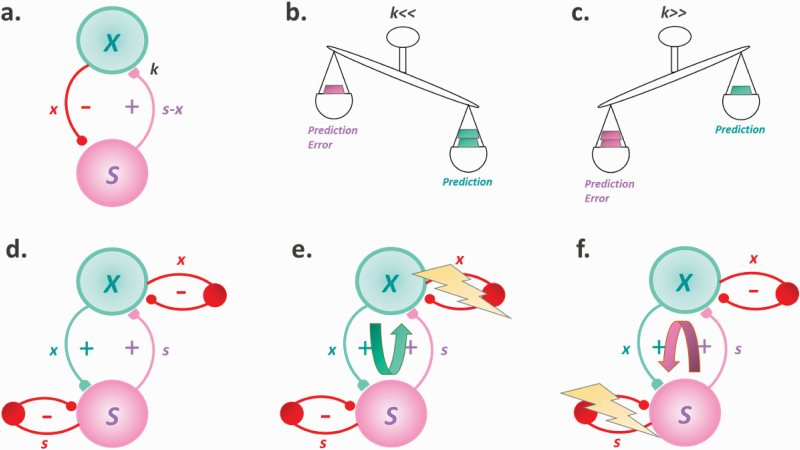
Inference can be implemented in various ways. According to predictive coding136 (ie, Kalman filtering137,138 or variational free-energy minimization139), new sensory inputs are constantly explained-away by inhibitory feedback signals (sent from higher level areas to lower level areas, that “modulate” sensory inputs according to the behavioral context; ie, predictions; figure 1a). When predictions cannot fully explain the input, a residual error-signal (ie, prediction error [PE]) is sent up in the hierarchy to update the dominant hypothesis (belief), thereby reducing surprise (or surprisal). Conversely, when predictions and inputs match, no PE is generated and thus, the current model is sustained. It is worth noting that, under certain formulations, surprise can also be minimized by appropriate action (active sampling of the environment, ie, active inference140), also explaining exploratory behavior and long-term minimization of PE.141 Crucially, both predictions and inputs are weighted according to their reliability (parameter k in figure 1; Kalman gain), resulting in precision-weighted PE. In one of the first articles to suggest a computational account of psychedelics, Corlett and colleagues suggested that psychedelics act by increasing the prior weight (thus decreasing k), which results in inferences being mainly driven by expectations (figure 1b).142 The group also suggested a tentative neural mechanism for this prior overweighting, namely “excessive AMPA-receptor signaling, in the absence of NMDA-receptor impairment.” Importantly, it has been argued that the same mechanism might underlie hallucinations in SCZs,143–145 with a recent study validating this theory and, additionally, providing evidence for over-weighted priors in a group of nonclinical voice hearers.146 Taken together, those theories and evidence suggest that hallucinations might reflect the same underlying computational mechanism, regardless of the exhibited phenomenology or clinical context.
Fig. 1.
Illustration of different Bayesian models of hallucinations. (a–c) The predictive coding framework. (d–f) The circular inference framework. X, hidden cause; S, sensory variable; x and s, predictions and sensory messages; s-x, prediction error; k, relative weight of inputs as compared to predictions (Kalman gain).
The idea that serotonergic agonists increase prior weight is not unanimously accepted. In a recent article, Carhart-Harris and Friston suggested that the opposite might also be true, namely a relaxation of the priors that increases k (figure 1c).147 Their REBUS theory explains, among other things, the potential therapeutic effects of psychedelics (eg, in depressive disorders), mediated by a relaxation of pathological priors associated with those illnesses. Intriguingly, although the REBUS and the strong-prior theory seem at first sight incompatible, this is not necessarily true. In particular, priors, can be both over- and under-weighted, but at different levels in the cortical hierarchy, for example, weak low-level priors (high k) might be compensated by stronger high-level priors (low k).148
Although predictive coding is a powerful inference scheme, it is not the only one. For example, one could replace inhibitory priors with excitatory priors, resulting in a closely related algorithm in which beliefs are not updated by error-signals, but by the sensory inputs per se (Belief Propagation [BP]; figure 1d). Despite its generality and simplicity, BP postulates recurrent, excitatory connections. Without well-tuned control mechanisms (eg, inhibitory control), it results in information loops, a form of “run-away excitation” where beliefs are erroneously amplified and the feed-forward (input) and feedback (prediction) messages become aberrantly correlated (Circular Inference149,150). There are two types of loops: descending (overcounted priors; figure 1e) and ascending (overcounted inputs; figure 1f). Importantly, different loops result in different types of aberrant percepts: while ascending loops induce unimodal hallucinations (eg, AH in SCZs), descending loops give rise to multisensory phenomena (eg, synesthesia-like experiences; MMH induced by DMT).151 Although the former link between ascending loops and SCZs has already been empirically established,152 the latter between descending loops and psychedelics remains purely theoretical and still needs experimental support.
Conclusion
In this article, we sought to compare and contrast hallucinations in SCZs and under psychedelics. We identified several interesting common features: both experiences are related to a reduced integration and stability of functional networks, as well as a distorted anti-correlation between resting-state and task-positive networks. Furthermore, both experiences are afforded a strong metaphysical meaning. We also highlighted various crucial differences: First, psychedelics over-engage primary sensory cortices, hallucinations in SCZs, on the other hand, are mostly related to overactivation of associative networks. Furthermore, while drug-induced psychosis mostly encompasses VH (often geometric) with preserved insight, SCZs is characterized by AH (mostly voices) and poor reality monitoring. Additionally, we pointed out a number of topics that need further investigation, more particularly the role of serotonin in SCZs, the prevalence of MMH in both experiences and the potential cultural impact on hallucinations in SCZs. Finally, we suggested that psychotic experiences, regardless of their diagnostic categorization, might be underlain by the same computational mechanisms that tie together subjectivity and neural implementation, namely altered predictive processing. Future studies will have to clarify whether the same (eg, strong priors) or different (eg, climbing vs descending loops) impairments underscore these different psychotic experiences.
Acknowledgments
We would like to dedicate this work to the memory of our beloved friend and colleague, Martin Fortier-Davy.
Funding
D.D. was supported by a Wellcome Trust Grant (WT108720). A.L.H. was supported by the National Institute on Drug Abuse, USA [grant number 5R01 DA041336]. Y.Z. and E.K. were supported by the Internal Grant Agency of the Ministry of Health of the Czech Republic [grant number AZV 17-32957A]. K.H.P was supported by the Heffter Research Institute, USA [grant number 2020-03-01]. T.N. was partially supported by Junior Research Fellowship COFUNDed between Durham University and the European Union [grant number 609412].
References
- 1. Osmond H. A review of the clinical effects of psychotomimetic agents. Ann N Y Acad Sci. 1957;66(3):418–434. [DOI] [PubMed] [Google Scholar]
- 2. Dyck E. Psychedelic Psychiatry : LSD from Clinic to Campus. Baltimore, Maryland: Johns Hopkins University Press; 2008. [Google Scholar]
- 3. Hermle L, Kraehenmann R. Experimental psychosis research and schizophrenia—similarities and dissimilarities in psychopathology. In: Halberstadt AL, Vollenweider F, Nichols D, eds. Behavioral Neurobiology of Psychedelic Drugs. Current Topics in Behavioral Neurosciences. Vol 36 Berlin: Springer; 2018:313–332. [DOI] [PubMed] [Google Scholar]
- 4. Glennon RA, Young R, Rosecrans JA. Antagonism of the effects of the hallucinogen DOM and the purported 5-HT agonist quipazine by 5-HT2 antagonists. Eur J Pharmacol. 1983;91(2–3):189–196. [DOI] [PubMed] [Google Scholar]
- 5. Glennon RA, Titeler M, McKenney JD. Evidence for 5-HT2 involvement in the mechanism of action of hallucinogenic agents. Life Sci. 1984;35(25):2505–2511. [DOI] [PubMed] [Google Scholar]
- 6. Canal CE, Morgan D. Head-twitch response in rodents induced by the hallucinogen 2,5-dimethoxy-4-iodoamphetamine: a comprehensive history, a re-evaluation of mechanisms, and its utility as a model. Drug Test Anal. 2012;4(7–8):556–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Halberstadt AL, Chatha M, Klein AK, Wallach J, Brandt SD. Correlation between the potency of hallucinogens in the mouse head-twitch response assay and their behavioral and subjective effects in other species. Neuropharmacology. 2020;167:107933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schreiber R, Brocco M, Audinot V, Gobert A, Veiga S, Millan MJ. (1-(2,5-dimethoxy-4 iodophenyl)-2-aminopropane)-induced head-twitches in the rat are mediated by 5-hydroxytryptamine (5-HT) 2A receptors: modulation by novel 5-HT2A/2C antagonists, D1 antagonists and 5-HT1A agonists. J Pharmacol Exp Ther. 1995;273(1):101–112. [PubMed] [Google Scholar]
- 9. Fantegrossi WE, Harrington AW, Eckler JR, et al. Hallucinogen-like actions of 2,5-dimethoxy-4-(n)-propylthiophenethylamine (2C-T-7) in mice and rats. Psychopharmacology (Berl). 2005;181(3):496–503. [DOI] [PubMed] [Google Scholar]
- 10. González-Maeso J, Weisstaub NV, Zhou M, et al. Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron. 2007;53(3):439–452. [DOI] [PubMed] [Google Scholar]
- 11. Halberstadt AL, Geyer MA. LSD but not lisuride disrupts prepulse inhibition in rats by activating the 5-HT(2A) receptor. Psychopharmacology (Berl). 2010;208(2):179–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gerber R, Barbaz BJ, Martin LL, Neale R, Williams M, Liebman JM. Antagonism of L-5-hydroxytryptophan-induced head twitching in rats by lisuride: a mixed 5-hydroxytryptamine agonist-antagonist? Neurosci Lett. 1985;60(2):207–213. [DOI] [PubMed] [Google Scholar]
- 13. Halberstadt AL, Geyer MA. Characterization of the head-twitch response induced by hallucinogens in mice: detection of the behavior based on the dynamics of head movement. Psychopharmacology (Berl). 2013;227(4):727–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ouagazzal A, Grottick AJ, Moreau J, Higgins GA. Effect of LSD on prepulse inhibition and spontaneous behavior in the rat. A pharmacological analysis and comparison between two rat strains. Neuropsychopharmacology. 2001;25(4):565–575. [DOI] [PubMed] [Google Scholar]
- 15. Wing LL, Tapson GS, Geyer MA. 5HT-2 mediation of acute behavioral effects of hallucinogens in rats. Psychopharmacology (Berl). 1990;100(3):417–425. [DOI] [PubMed] [Google Scholar]
- 16. Krebs-Thomson K, Paulus MP, Geyer MA. Effects of hallucinogens on locomotor and investigatory activity and patterns: influence of 5-HT2A and 5-HT2C receptors. Neuropsychopharmacology. 1998;18(5):339–351. [DOI] [PubMed] [Google Scholar]
- 17. Adams LM, Geyer MA. Patterns of exploration in rats distinguish lisuride from lysergic acid diethylamide. Pharmacol Biochem Behav. 1985;23(3):461–468. [DOI] [PubMed] [Google Scholar]
- 18. Body S, Kheramin S, Ho MY, Miranda F, Bradshaw CM, Szabadi E. Effects of a 5-HT2 receptor agonist, DOI (2,5-dimethoxy-4-iodoamphetamine), and antagonist, ketanserin, on the performance of rats on a free-operant timing schedule. Behav Pharmacol. 2003;14(8):599–607. [DOI] [PubMed] [Google Scholar]
- 19. Asgari K, Body S, Bak VK, et al. Effects of 5-HT2A receptor stimulation on the discrimination of durations by rats. Behav Pharmacol. 2006;17(1):51–59. [DOI] [PubMed] [Google Scholar]
- 20. Halberstadt AL, Sindhunata IS, Scheffers K, et al. Effect of 5-HT2A and 5-HT2C receptors on temporal discrimination by mice. Neuropharmacology. 2016;107:364–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vollenweider FX, Vollenweider-Scherpenhuyzen MF, Bäbler A, Vogel H, Hell D. Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. Neuroreport. 1998;9(17):3897–3902. [DOI] [PubMed] [Google Scholar]
- 22. Preller KH, Herdener M, Pokorny T, et al. The fabric of meaning and subjective effects in LSD-induced states depend on serotonin 2a receptor activation. Curr Biol. 2017;27(3):451–457. [DOI] [PubMed] [Google Scholar]
- 23. Preller KH, Burt JB, Ji JL, et al. Changes in global and thalamic brain connectivity in LSD-induced altered states of consciousness are attributable to the 5-HT2A receptor. Elife. 2018;7:1–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kraehenmann R, Pokorny D, Vollenweider L, et al. Dreamlike effects of LSD on waking imagery in humans depend on serotonin 2A receptor activation. Psychopharmacology (Berl). 2017;234(13):2031–2046. [DOI] [PubMed] [Google Scholar]
- 25. Madsen MK, Fisher PM, Burmester D, et al. Psychedelic effects of psilocybin correlate with serotonin 2A receptor occupancy and plasma psilocin levels. Neuropsychopharmacology. 2019;44(7):1328–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sommer IEC, Diederen KMJ, Blom JD, et al. Auditory verbal hallucinations predominantly activate the right inferior frontal area. Brain. 2008;131(12):3169–3177. [DOI] [PubMed] [Google Scholar]
- 27. Jardri R, Pouchet A, Pins D, Thomas P. Cortical activations during auditory verbal hallucinations in schizophrenia: a coordinate-based meta-analysis. Am J Psychiatry. 2011;168(1):73–81. [DOI] [PubMed] [Google Scholar]
- 28. Jardri R, Thomas P, Delmaire C, Delion P, Pins D. The neurodynamic organization of modality-dependent hallucinations. Cereb Cortex. 2013;23(5):1108–1117. [DOI] [PubMed] [Google Scholar]
- 29. Leroy A, Foucher JR, Pins D, et al. fMRI capture of auditory hallucinations: validation of the two-steps method. Hum Brain Mapp. 2017;38(10):4966–4979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zmigrod L, Garrison JR, Carr J, Simons JS. The neural mechanisms of hallucinations: a quantitative meta-analysis of neuroimaging studies. Neurosci Biobehav Rev. 2016;69:113–123. [DOI] [PubMed] [Google Scholar]
- 31. Lefebvre S, Demeulemeester M, Leroy A, et al. Network dynamics during the different stages of hallucinations in schizophrenia. Hum Brain Mapp. 2016;37(7):2571–2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Carhart-Harris RL, Muthukumaraswamy S, Roseman L, et al. Neural correlates of the LSD experience revealed by multimodal neuroimaging. Proc Natl Acad Sci U S A. 2016;113(17):4853–4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kometer M, Cahn BR, Andel D, Carter OL, Vollenweider FX. The 5-HT2A/1A agonist psilocybin disrupts modal object completion associated with visual hallucinations. Biol Psychiatry. 2011;69(5):399–406. [DOI] [PubMed] [Google Scholar]
- 34. Kometer M, Vollenweider FX. Serotonergic hallucinogen-induced visual perceptual alterations. In: Halberstadt AL, Vollenweider FX, Nichols DE, eds. Behavioral Neurobiology of Psychedelic Drugs. Vol 36 Berlin: Springer; 2016:257–282. [DOI] [PubMed] [Google Scholar]
- 35. Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102(27):9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Smith SM, Fox PT, Miller KL, et al. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci U S A. 2009;106(31):13040–13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fox MD, Zhang D, Snyder AZ, Raichle ME. The global signal and observed anticorrelated resting state brain networks. J Neurophysiol. 2009;101(6):3270–3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Carbonell F, Bellec P, Shmuel A. Quantification of the impact of a confounding variable on functional connectivity confirms anti-correlated networks in the resting-state. Neuroimage. 2014;86:343–353. [DOI] [PubMed] [Google Scholar]
- 39. Raichle ME. The brain’s default mode network. Annu Rev Neurosci. 2015;38:433–447. [DOI] [PubMed] [Google Scholar]
- 40. Zhou Y, Friston KJ, Zeidman P, Chen J, Li S, Razi A. The hierarchical organization of the default, dorsal attention and salience networks in adolescents and young adults. Cereb Cortex. 2018;28(2):726–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Alderson-Day B, Diederen K, Fernyhough C, et al. Auditory hallucinations and the brain’s resting-state networks: findings and methodological observations. Schizophr Bull. 2016;42(5):1110–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Williamson P. Are anticorrelated networks in the brain relevant to schizophrenia? Schizophr Bull. 2007;33(4):994–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011;15(10):483–506. [DOI] [PubMed] [Google Scholar]
- 44. Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214(5–6):655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kucyi A, Daitch A, Raccah O, et al. Electrophysiological dynamics of antagonistic brain networks reflect attentional fluctuations. Nat Commun. 2020;11(1):325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Doll A, Sorg C, Manoliu A, et al. Shifted intrinsic connectivity of central executive and salience network in borderline personality disorder. Front Hum Neurosci. 2013;7:727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bolton TAW, Wotruba D, Buechler R, et al. Triple network model dynamically revisited: lower salience network state switching in pre-psychosis. Front Physiol. 2020;11:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang J, Wang Y, Wu X, et al. Shared and specific functional connectivity alterations in unmedicated bipolar and major depressive disorders based on the triple-network model. Brain Imaging Behav. 2020;14(1):186–199. [DOI] [PubMed] [Google Scholar]
- 49. Weng Y, Qi R, Zhang L, et al. Disturbed effective connectivity patterns in an intrinsic triple network model are associated with posttraumatic stress disorder. Neurol Sci. 2019;40(2):339–349. [DOI] [PubMed] [Google Scholar]
- 50. Fan J, Zhong M, Gan J, et al. Altered connectivity within and between the default mode, central executive, and salience networks in obsessive-compulsive disorder. J Affect Disord. 2017;223:106–114. [DOI] [PubMed] [Google Scholar]
- 51. Palaniyappan L, Liddle PF. Does the salience network play a cardinal role in psychosis? An emerging hypothesis of insular dysfunction. J Psychiatry Neurosci. 2012;37(1):17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Carhart-Harris RL, Erritzoe D, Williams T, et al. Neural correlates of the psychedelic state as determined by fMRI studies with psilocybin. Proc Natl Acad Sci U S A. 2012;109(6):2138–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Carhart-Harris RL, Leech R, Erritzoe D, et al. Functional connectivity measures after psilocybin inform a novel hypothesis of early psychosis. Schizophr Bull. 2013;39(6):1343–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Preller KH, Duerler P, Burt JB, et al. Psilocybin induces time-dependent changes in global functional connectivity. Biol Psychiatry. 2020;88(2):197–207. [DOI] [PubMed] [Google Scholar]
- 55. Barnett L, Muthukumaraswamy SD, Carhart-Harris RL, Seth AK. Decreased directed functional connectivity in the psychedelic state. Neuroimage. 2020;209:116462. [DOI] [PubMed] [Google Scholar]
- 56. Carlsson M, Carlsson A. Schizophrenia: a subcortical neurotransmitter imbalance syndrome? Schizophr Bull. 1990;16(3):425–432. [DOI] [PubMed] [Google Scholar]
- 57. Anticevic A, Cole MW, Repovs G, et al. Characterizing thalamo-cortical disturbances in schizophrenia and bipolar illness. Cereb Cortex. 2014;24(12):3116–3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Murray JD, Anticevic A. Toward understanding thalamocortical dysfunction in schizophrenia through computational models of neural circuit dynamics. Schizophr Res. 2017;180:70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Giraldo-Chica M, Woodward ND. Review of thalamocortical resting-state fMRI studies in schizophrenia. Schizophr Res. 2017;180:58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Preller KH, Razi A, Zeidman P, Stämpfli P, Friston KJ, Vollenweider FX. Effective connectivity changes in LSD-induced altered states of consciousness in humans. Proc Natl Acad Sci U S A. 2019;116(7):2743–2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Müller F, Lenz C, Dolder P, et al. Increased thalamic resting-state connectivity as a core driver of LSD-induced hallucinations. Acta Psychiatr Scand. 2017;136(6):648–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bauer SM, Schanda H, Karakula H, et al. Culture and the prevalence of hallucinations in schizophrenia. Compr Psychiatry. 2011;52(3):319–325. [DOI] [PubMed] [Google Scholar]
- 63. Waters F, Collerton D, Ffytche DH, et al. Visual hallucinations in the psychosis spectrum and comparative information from neurodegenerative disorders and eye disease. Schizophr Bull. 2014;40(Suppl. 4):233–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Jablensky A, Sartorius N, Ernberg G, et al. Schizophrenia: manifestations, incidence and course in different cultures. A World Health Organization ten-country study. Psychol Med Monogr Suppl. 1992;20:1–97. [DOI] [PubMed] [Google Scholar]
- 65. McCarthy-Jones S, Smailes D, Corvin A, et al. Occurrence and co-occurrence of hallucinations by modality in schizophrenia-spectrum disorders. Psychiatry Res. 2017;252:154–160. [DOI] [PubMed] [Google Scholar]
- 66. Clark ML, Waters F, Vatskalis TM, Jablensky A. On the interconnectedness and prognostic value of visual and auditory hallucinations in first-episode psychosis. Eur Psychiatry. 2017;41:122–128. [DOI] [PubMed] [Google Scholar]
- 67. Oorschot M, Lataster T, Thewissen V, et al. Symptomatic remission in psychosis and real-life functioning. Br J Psychiatry. 2012;201(3):215–220. [DOI] [PubMed] [Google Scholar]
- 68. Mueser KT, Bellack AS, Brady EU. Hallucinations in schizophrenia. Acta Psychiatr Scand. 1990;82(1):26–29. [DOI] [PubMed] [Google Scholar]
- 69. Montagnese M. et al. A Review of Multimodal Hallucinations: Categorization, Assessment, Theoretical Perspectives, and Clinical Recommendations, Schizophrenia Bulletin, 2020. doi:10.1093/schbul/sbaa101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Dudley R, Aynsworth C, Cheetham R, McCarthy-Jones S, Collerton D. Prevalence and characteristics of multi-modal hallucinations in people with psychosis who experience visual hallucinations. Psychiatry Res. 2018;269:25–30. [DOI] [PubMed] [Google Scholar]
- 71. Lim A, Hoek HW, Deen ML, Blom JD; GROUP Investigators Prevalence and classification of hallucinations in multiple sensory modalities in schizophrenia spectrum disorders. Schizophr Res. 2016;176(2–3):493–499. [DOI] [PubMed] [Google Scholar]
- 72. Llorca PM, Pereira B, Jardri R, et al. Hallucinations in schizophrenia and Parkinson’s disease: an analysis of sensory modalities involved and the repercussion on patients. Sci Rep. 2016;6:38152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hoffman RE, Varanko M. “Seeing voices”: fused visual/auditory verbal hallucinations reported by three persons with schizophrenia-spectrum disorder. Acta Psychiatr Scand. 2006;114(4):290–2; discussion 292. [DOI] [PubMed] [Google Scholar]
- 74. Masters REL, Houston J The Varieties of Psychedelic Experience. New York, NY: Holt, Rinehart and Winston; 1966. [Google Scholar]
- 75. Timmermann C, Roseman L, Schartner M, et al. Neural correlates of the DMT experience assessed with multivariate EEG. Sci Rep. 2019;9(1):16324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Strassmann R. DMT: The Spirit Molecule. A Doctor’s Revolutionary Research into the Biology of near-Death and Mystical Experience. Rochester, VT: Park Street Press; 2001. [Google Scholar]
- 77. Studerus E, Kometer M, Hasler F, Vollenweider FX. Acute, subacute and long-term subjective effects of psilocybin in healthy humans : a pooled analysis of experimental studies. J Psychopharmacol. 2011;25(11):1434–1452. [DOI] [PubMed] [Google Scholar]
- 78. Kluver H. Mescal and Mechanisms of Hallucination. Chicago, IL: University of Chicago Press; 1966. [Google Scholar]
- 79. Luke DP, Terhune DB. The induction of synaesthesia with chemical agents: a systematic review. Front Psychol. 2013;4:753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Shanon B. The Antipodes of the Mind: Charting the Phenomenology of the Ayahuasca Experience. New York, NY: Oxford University Press; 2002. [Google Scholar]
- 81. Shulgin A, Shulgin A TIHKAL (Tryptamines I Have Known and Loved): The Continuation. Berkeley, CA: Transform Press; 1997. [Google Scholar]
- 82. Siegel R, Jarvik M. Drug-induced hallucinations in animals and man. In: Siegel R, West L, eds. Hallucinations: Behavior, Experience and Theory. New York, NY: John Wiley; 1975:81–161. [Google Scholar]
- 83. Nayani TH, David AS. The auditory hallucination: a phenomenological survey. Psychol Med. 1996;26(1):177–189. [DOI] [PubMed] [Google Scholar]
- 84. Handest P, Klimpke C, Raballo A, Larøi F. From thoughts to voices: understanding the development of auditory hallucinations in schizophrenia. Rev Philos Psychol. 2016;7(3):595–610. [Google Scholar]
- 85. Raballo A, Larøi F. Murmurs of thought: phenomenology of hallucinatory consciousness in impending psychosis. Psychosis. 2011;3(2):163–166. [Google Scholar]
- 86. Chadwick PD, Birchwood M. Omnipotence of voices : a cognitive approach to auditory hallucinations. Br J Psychiatry. 1994;164:190–201. [DOI] [PubMed] [Google Scholar]
- 87. Griffiths R, Richards W, Johnson M, McCann U, Jesse R. Mystical-type experiences occasioned by psilocybin mediate the attribution of personal meaning and spiritual significance 14 months later. J Psychopharmacol. 2008;22(6):621–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Barrett FS, Griffiths RR. Classic hallucinogens and mystical experiences: phenomenology and neural correlates. Curr Top Behav Neurosci. 2018;36:393–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Huxley A. The Doors of Perception and Heaven and Hell. London: Harper & Brothers; 1954. [Google Scholar]
- 90. Woods A, Jones N, Bernini M, et al. Interdisciplinary approaches to the phenomenology of auditory verbal hallucinations. Schizophr Bull. 2014;40(Suppl. 4):S246–S254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Waters F, Allen P, Aleman A, et al. Auditory hallucinations in schizophrenia and nonschizophrenia populations: a review and integrated model of cognitive mechanisms. Schizophr Bull. 2012;38(4):683–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Fortier M. Sense of reality, metacognition and culture in schizophrenic and drug-induced hallucinations. In: Proust J, Fortier M, eds. Metacognitive Diversity: An Interdisciplinary Approach. Oxford: Oxford University Press; 2018:343–379. [Google Scholar]
- 93. Sanz C, Tagliazucchi E. The experience elicited by hallucinogens presents the highest similarity to dreaming within a large database of psychoactive substance reports. Front Neurosci. 2018;12:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Krebs TS, Johansen PØ. Psychedelics and mental health: a population study. PLoS One. 2013;8(8):e63972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Nelson B, Sass LA. The phenomenology of the psychotic break and Huxley’s trip: substance use and the onset of psychosis. Psychopathology. 2008;41(6):346–355. [DOI] [PubMed] [Google Scholar]
- 96. Winkelman MJ. An ontology of psychedelic entity experiences in evolutionary psychology and neurophenomenology. J Psychedelic Stud. 2018;2(1):5–23. [Google Scholar]
- 97. Dobkin de Rios M. Cultural persona in drug-induced altered states of consciousness. In: Fitzgerald TK, ed. Social and Cultural Identity. Athens: University of Georgia Press; 1974:18–19. [Google Scholar]
- 98. Furst PT. Hallucinogens and Culture. Brand: Chandler Sharp Pub; 1976. [Google Scholar]
- 99. Hernandez F. De Historia Plantarum Novae Hispaniae. Madrid: Ibarra; 1790. [Google Scholar]
- 100. Marquez ME. Los Tunebo: Una Cosmogonia Recolombiana. Medellin: Editorial Copymundo; 1979. [Google Scholar]
- 101. Myerhoff BG. Peyote Hunt: The Sacred Journey of the Huichol Indians. Ithaca, NY: Cornell University Press; 1974. [Google Scholar]
- 102. Polo de Ondegardo J. Informaciones acerca de la religio y gobierno de los Incas. In: Urteaga HH, ed. Coleccion de Libros y Documentos Referentes a La Historia Del Peru. Lima: Sanmarti y Cia; 1916:whole book. [Google Scholar]
- 103. de Sahagun B. Historia General de Las Cosas de Nueva Espana: Libro IX. Mexico City: Editorial Pedro Robredo; 1939. [Google Scholar]
- 104. Schultes RE. Virola as an orally administered hallucinogen. Bot Mus Lealf Harv Univ. 1969;22(6):229–240. [Google Scholar]
- 105. Wasson RG. Le champignon sacré au Mexique contemporain. In: Heim R, Wasson RG, eds. Les Champignons Hallucinogènes Du Mexique. Paris: Editions du Museum; 1958:45–100. [Google Scholar]
- 106. Wright R. Mysteries of the Jaguar Shamans of the Northwest Amazon. Lincoln, NE: University of Nebraska Press; 2013. [Google Scholar]
- 107. Reichel-Dolmatoff G. The Shaman and the Jaguar: A Study of Narcotic Drugs among the Indians of Colombia. Philadelphia, PA: Temple University Press; 1975. [Google Scholar]
- 108. Wassen SH. The anthropological outlook for Amerindian medicinal plants. In: Swain T, ed. Plants in the Development of Modern Medicine. Cambridge, MA: Harvard University Press; 1972:1–65. [Google Scholar]
- 109. Kirchhoff P. Food-gathering tribes of the Venezuelan Llanos. In: Steward JH, ed. Handbook of South American Indians. Vol 4. Washington, DC: Smithsonian Institution; 1948:445–468. [Google Scholar]
- 110. Lumholtz C. Tarahumari dances and plant worship. Scribner’s Mag 16. 1894;16:438–456. [Google Scholar]
- 111. Kopenawa D, Albert B The Falling Sky: Words of a Yanomami Shaman. London: Belknap Press of Harvard University Press; 2013. [Google Scholar]
- 112. Tezozomoc F. Cronica Mexicana. Mexico City: Jose M. Vigil; 1878. [Google Scholar]
- 113. Wallace AF. Cultural determinants of response to hallucinatory experince. AMA Arch Gen Psychiatry. 1959;1:58–69. [DOI] [PubMed] [Google Scholar]
- 114. Mooney J. The mescal plant and ceremony. Ther Gaz. 1896;12:7–11. [Google Scholar]
- 115. Levi-Strauss C. Structural Anthropology, Vol 2 Chicago, Illinois: University of Chicago Press; 1976. [Google Scholar]
- 116. Brown MF. From the hero’s bones: three aguaruna hallucinogens and their uses. In: Ford RI, ed. The Nature and Status of Ethnobotany. Ann-Arbor, Michigan: University of Michigan Museum of Anthropology; 1978:118–136. [Google Scholar]
- 117. Langdon EJ. Yage among the Siona: cultural patternsin visions. In: Browman D, Schwarz R, eds. Spirits, Shamans and Stars. Perspectives from South America. La Haye: De Gruyter Mouton; 1979:63–80. [Google Scholar]
- 118. Reichel-Dolmatoff G. The cultural context of an aboriginal hallucinogen: Banisteriopis Caapi. In: Furst PT, ed. Flesh of the Gods: The Ritual Use of Hallucinogens. Prospect Heights, Illinois: Waveland Press, Inc.; 1972:84–113. [Google Scholar]
- 119. Dupuis D. Apprendre a voir l’ invisible. De la pedagogie visionnaire dans un centre chamanique d’ Amazonie peruvienne. Cah d’ Anthropol Soc. 2019;17:20–42. (“Images visionnaires”). [Google Scholar]
- 120. Dupuis D. The socialization of hallucinations. Cultural priors, social interactions and contextual factors in the use of ayahuasca. Transcult Psychiatry. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Noorani T, Alderson-day B. Spotlight commentary : REBUS and the anarchic brain. Neurosci Conscious. 2020;6(1):1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Hartogsohn I. Set and setting, psychedelics and the placebo response: an extra-pharmacological perspective on psychopharmacology. J Psychopharmacol. 2016;30(12):1259–1267. [DOI] [PubMed] [Google Scholar]
- 123. Studerus E, Gamma A, Kometer M, Vollenweider FX. Prediction of psilocybin response in healthy volunteers. PLoS One. 2012;7(2):e30800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Kaelen M, Giribaldi B, Raine J, et al. The hidden therapist: evidence for a central role of music in psychedelic therapy. Psychopharmacology (Berl). 2018;235(2):505–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Carhart-Harris RL, Roseman L, Haijen E, et al. Psychedelics and the essential importance of context. J Psychopharmacol. 2018;32(7):725–731. [DOI] [PubMed] [Google Scholar]
- 126. Johnson M, Richards W, Griffiths R. Human hallucinogen research: guidelines for safety. J Psychopharmacol. 2008;22(6):603–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Nuevo R, Chatterji S, Verdes E, Naidoo N, Arango C, Ayuso-Mateos JL. The continuum of psychotic symptoms in the general population: a cross-national study. Schizophr Bull. 2012;38(3):475–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Luhrmann TM, Padmavati R, Tharoor H, Osei A. Differences in voice-hearing experiences of people with psychosis in the U.S.A., India and Ghana: interview-based study. Br J Psychiatry. 2015;206(1):41–44. [DOI] [PubMed] [Google Scholar]
- 129. Laroi F, Luhrmann TM, Bell V, et al. Culture and hallucinations: overview and future directions. Schizophr Bull. 2014;40(Suppl. 4):213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Dupuis D. Learning to control voices: comparing voice control ability during psychosis and in ritual use of ayahuasca in the Peruvian amazon. In: Alderson-Day B, Fernyhough C, Woods A. eds. Voices in Psychosis. Interdisciplinary Perspectives. Oxford: Oxford University Press; 2020. [PubMed] [Google Scholar]
- 131. Montague PR, Dolan RJ, Friston KJ, Dayan P. Computational psychiatry. Trends Cogn Sci. 2012;16(1):72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Chater N, Tenenbaum JB, Yuille A. Probabilistic models of cognition: conceptual foundations. Trends Cogn Sci. 2006;10(7):287–291. [DOI] [PubMed] [Google Scholar]
- 133. Clark A. Whatever next? Predictive brains, situated agents, and the future of cognitive science. Behav Brain Sci. 2013;36(3):181–204. [DOI] [PubMed] [Google Scholar]
- 134. Friston KJ, Stephan KE, Montague R, Dolan RJ. Computational psychiatry: the brain as a phantastic organ. Lancet Psychiatry. 2014;1(2):148–158. [DOI] [PubMed] [Google Scholar]
- 135. Friston K. Hierarchical models in the brain. PLoS Comput Biol. 2008;4(11):e1000211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Spratling MW. A review of predictive coding algorithms. Brain Cogn. 2017;112:92–97. [DOI] [PubMed] [Google Scholar]
- 137. Kalman RE. A new approach to linear filtering and prediction problems. J Basic Eng. 1960;82(Series D):35–45. [Google Scholar]
- 138. Wolpert DM, Ghahramani Z. Computational principles of movement neuroscience. Nat Neurosci. 2000;3 Suppl:1212–1217. [DOI] [PubMed] [Google Scholar]
- 139. Friston K, Kiebel S. Predictive coding under the free-energy principle. Philos Trans R Soc Lond B Biol Sci. 2009;364(1521):1211–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Friston K, FitzGerald T, Rigoli F, Schwartenbeck P, Pezzulo G. Active inference: a process theory. Neural Comput. 2017;29(1):1–49. [DOI] [PubMed] [Google Scholar]
- 141. Tschantz A, Seth AK, Buckley CL. Learning action-oriented models through active inference. PLoS Comput Biol. 2020;16(4):e1007805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Corlett PR, Frith CD, Fletcher PC. From drugs to deprivation: a Bayesian framework for understanding models of psychosis. Psychopharmacology (Berl). 2009;206(4):515–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Friston KJ. Hallucinations and perceptual inference. Behav Brain Sci. 2005;28(6):764–766. [Google Scholar]
- 144. Benrimoh D, Parr T, Vincent P, Adams RA, Friston K. Active inference and auditory hallucinations. Comput Psychiatr. 2018;2:183–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Corlett PR, Horga G, Fletcher PC, Alderson-Day B, Schmack K, Powers AR 3rd. Hallucinations and strong priors. Trends Cogn Sci. 2019;23(2):114–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Powers AR, Mathys C, Corlett PR. Pavlovian conditioning-induced hallucinations result from overweighting of perceptual priors. Science. 2017;357(6351):596–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Carhart-Harris RL, Friston KJ. REBUS and the anarchic brain: toward a unified model of the brain action of psychedelics. Pharmacol Rev. 2019;71(3):316–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Sterzer P, Adams RA, Fletcher P, et al. The predictive coding account of psychosis. Biol Psychiatry. 2018; 84(9):634–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Jardri R, Denève S. Circular inferences in schizophrenia. Brain. 2013;136(Pt 11):3227–3241. [DOI] [PubMed] [Google Scholar]
- 150. Leptourgos P, Denève S, Jardri R. Can circular inference relate the neuropathological and behavioral aspects of schizophrenia? Curr Opin Neurobiol. 2017;46:154–161. [DOI] [PubMed] [Google Scholar]
- 151. Leptourgos P. Dynamical circular inference in the general population and the psychosis spectrum: insights from perceptual decision making. 2018. [Google Scholar]
- 152. Jardri R, Duverne S, Litvinova AS, Denève S. Experimental evidence for circular inference in schizophrenia. Nat Commun. 2017;8:14218. [DOI] [PMC free article] [PubMed] [Google Scholar]