Abstract
Motor dysfunction in youth at clinical high risk (CHR) for psychosis is thought to reflect abnormal neurodevelopment within cortical-subcortical motor circuits and may be important for understanding clinical trajectories of CHR individuals. However, to date, our perspective of brain-behavior relationships has been informed solely by cross-sectional correlational studies linking behavior in the lab to brain structure or respective resting-state network connectivity. Here, we assess movement dysfunction from 2 perspectives: study 1 investigates the longitudinal progression of handwriting variability and positive symptoms in a sample of 91 CHR and healthy controls during a 12-month follow-up and study 2 involves a multiband functional magnetic resonance imaging task exploring the relationship between power grip force stability and motor network brain activation in a subset of participants. In study 1, we found that greater handwriting variability was a stable feature of CHR participants who experienced worse symptom progression. Study 2 results showed that CHR individuals had greater variability in their grip force and greater variability was related to decreased activation in the associative cortico-striatal network compared to controls. Motor variability may be a stable marker of vulnerability for psychosis risk and possible indicator of a vulnerable cortico-striatal brain network functioning in CHR participants, although the effects of antipsychotic medication should be considered.
Keywords: clinical high risk, motor control, variability, handwriting, power grip, longitudinal, functional magnetic resonance imaging
Introduction
Prominent theories suggest that the development of psychosis is due to an already vulnerable and over-reactive dopaminergic system leading to impaired cortical-striatal network functioning.1 These theoretical approaches have led researchers toward investigating motor behavior and motor brain networks as critical components for understanding the pathophysiology of psychosis.1–4 A large body of work suggests that movement dysfunction during young adulthood may be associated with vulnerability for developing a psychotic disorder.5–8 Seminal studies using live observation and home videos have noted that for people who later go on to develop psychosis, movement dysfunction is observable from birth and is present in those at high risk for the disorder.9–11 Recent longitudinal and prospective research of motor dysfunction has bolstered the need for assessing motor dysfunction during the clinical high risk (CHR) period as it may inform risk predictions for conversion and different illness trajectories.12–15
Intact motor control relies on the complex coordination of neural, cognitive, muscular, and skeletal components: noise within this system is characterized by greater variability (ie, motor dysfunction).16 Past research examining motor variability from multiple perspectives in at-risk and schizophrenia populations highlight the use of instrumental measures that may detect more subtle spontaneous movement dysfunction independent of antipsychotic medication.12,17–21 One such paradigm involves assessing smoothness in handwriting. Digitizing tablets allow researchers to capture handwriting kinematics of pen movement variability such as the normalized jerk.13,22–24 CHR individuals tend to show greater normalized jerk compared with healthy controls13; however, longitudinal investigations are needed to determine whether greater handwriting variability may help parse CHR participants based on symptom course.
The power grip, which incorporates the force of the fingers and palm to hold a drinking glass, lift a barbell, or use a hammer, is another important paradigm.25,26 Greater variability and poor grip strength have been associated with aging,27 declines in cognitive performance, and overall health in people with schizophrenia.25,28 In schizophrenia patients, poor sensorimotor control may also be evident with greater variation during isometric forces (the steady-state phase of a grip) and occurs independent of medication.29 In studies involving power grip, task difficulty is often modulated so that the task mimics different real-world demands.30–32 Another point to consider, few tasks that test motor control in CHR samples have been designed to see how repeated trials impact variability; it may be important to consider how motor control deteriorates over the course of a task as this may reveal insights into how the cortico-striatal system manages performance over time.33,34
Movement variability observed in the lab has been associated with abnormal brain structure and functional connectivity in the motor brain network (comprising cortico-striatal and cortico-cerebellar circuits).12,35–38 Interestingly, greater grip strength can result in greater force variability and brain activation within the cortico-striatal network,39,40 although people with schizophrenia do not show the expected increase in brain activity in somatosensory areas with greater levels of force.41 There is little research in task-based functional neuroimaging examining motor function during the CHR period, limiting our understanding of brain-behavior relationships in cortico-striatal and cortico-cerebellar brain regions.42 Grip force stability experiments are easily administered in a magnetic resonance environment and research in healthy individuals point toward the involvement of cortico-striatal and cortico-cerebellar brain networks,31,32,40,43,44 providing a powerful method for examining motor behavior and motor network associations prior to the onset of psychosis.
Here, we present 2 studies investigating movement variability from a sample of 116 right-handed CHR (n = 60) and healthy control (n = 56) young adults. In study 1, we examine a group of 91 participants (n = 45 CHR and n = 46 healthy control) who returned for a 12-month follow-up assessment. The CHR group was separated into groups based on either a stable/improved or worsening positive symptom progression. We examined baseline and follow-up differences in handwriting kinematics between CHR clinical subgroups. In study 2, a subsample of 37 participants (n = 18 CHR and n = 19 healthy control), participants completed a functional magnetic resonance imaging (fMRI) power grip paradigm. The fMRI task modulated grip difficulty under 2 different conditions corresponding to a light and hard grip strength. In theory, normalized jerk handwriting kinematics may be considered analogous—and are thought to share neurobiological mechanisms in schizophrenia patients—to force stability paradigms.45 Thus, the 2 studies were conceptualized as complementary approaches to understanding motor variability mechanisms. In study 1, based on the research showing movement dysfunction in people who later develop psychosis,7,10,12,46 we hypothesized that greater pen movement variability at baseline would be worse in participants with worsening symptom progression compared with those who remain stable/improved after 1 year. In study 2, we hypothesized that the CHR group would show greater force instability in both conditions during the task, force instability would increase over the course of the task, and CHR individuals would show lower brain activation within the motor network related to worse performance on the task.
Methods and Materials
Participants
Data for both studies were obtained from a total of 116 right-handed adolescent and young adults (n = 60 CHR and n = 56 healthy controls) between 12 and 24 years of age who consented to participate in a longitudinal study at the Adolescent Development and Preventive Treatment (ADAPT) research program. The Structured Interview for Psychosis-Risk Syndromes (SIPS) was administered to diagnose a psychosis-risk syndrome within the past 2 months or rule out the presence of a syndrome in healthy controls.47 The Structured Clinical Interview for Axis-I DSM-IV Disorders (SCID) was administered to determine the presence of a psychotic disorder diagnosis.48 The protocol and informed consent procedures were approved by the University Institutional Review Board.
Study 1
Sample Characteristics
Study 1 included 91 participants (n = 45 CHR and n = 46 healthy controls) who completed both clinical and handwriting assessments at baseline and follow-up, thus representing a 22% attrition rate for the entire sample. CHR participants were separated into 2 groups based on the symptom progression of the SIPS positive symptom domain at a 12-month follow-up clinical interview. Worsening illness progression was defined as an increase of 1 point on any of the positive symptom dimension scales of the SIPS. The groups were defined by a stable/improved psychosis-risk syndrome (CHR-S/I) or worsening illness progression (CHR-W). Twenty-nine (63.04%) of the 45 CHR participants with a follow-up assessment over the 12-month period experienced worsening illness progression. Three of the CHR-W participants (6.6%) converted to a psychotic disorder within 12 months of their baseline assessment (see table 1).
Table 1.
Demographic Characteristics of Study 1
| Study 1 Sample | |||||
|---|---|---|---|---|---|
| CHR-S/I | CHR-W | Healthy Control | Statistic | P ≤ | |
| Age | 18.2 (2.01) | 18.6 (1.99) | 18.6 (2.24) | F(2, 88) = 0.23 | NS |
| Sex | |||||
| Male | 6 | 22 | 18 | ||
| Female | 10 | 7 | 28 | ||
| Total | 16 | 29 | 46 | X2 = 10.92 | .004 |
| Education (years) | 12.3 (1.89) | 12.6 (2.28) | 12.1 (2.07) | t(114) = 1.27 | NS |
| Parent education | 15.6 (2.50) | 16.0 (2.10) | 15.8 (2.61) | t(114) = 0.77 | NS |
Note: NS indicates P > .05. Mean (SD). CHR-S/I refers to the stable/improved group and CHR-W refers to the CHR participants with a worsened symptom progression.
Handwriting.
Handwriting kinematics was acquired using Neuroscript MoveAlyzer software (http://www.neuroscript.net) installed on a Fujitsu Lifebook T901 tablet computer with a non-inking pen. Participants drew 8 concentric circles continuously in a clockwise direction within a 2-cm boundary line using their right hand over 3 separate trials. A measure of pen movement smoothness, average normalized jerk (ANJ), was extracted from MoveAlyzer software. More details are described in the supplemental material and elsewhere.13,14,22,23,49
Statistical Analysis
One-way analysis of variance (ANOVA) and chi-squared tests were run in R (v.3.6.1) and used to compare continuous and categorical demographic data, respectively. A repeated-measures ANOVA was run to confirm positive symptom changes between CHR subgroups across time points.
ANJ was averaged across trials. In order to assess changes to ANJ, a 3 × 2 repeated measures analysis of variance (ANOVA) with group (CHR-S/I and CHR-W and healthy control participants) as a between-subjects factor and timepoint (baseline and follow-up) as a within-subject factor was run. Post hoc testing between groups involved Tukey honest significant difference (HSD) correction for multiple comparisons using the Multcomp package.
Study 2
Sample Characteristics
A total of 41 right-handed adolescent and young adult CHR (n = 20) and healthy control (n = 21) participants were recruited opportunistically during and following the longitudinal data collection to complete the fMRI power grip task for study 2. Four participants (n = 2 CHR and n = 2 healthy controls) were excluded from the analysis because of technical errors during the task leaving a total of 37 participants (n = 18 CHR and n = 19 healthy controls). See table 2.
Table 2.
Demographic Characteristics of Study 2
| Study 2 Sample | ||||
|---|---|---|---|---|
| CHR | Healthy Control | Statistic | P ≤ | |
| Age | 20.86 (1.54) | 21.6 (1.90) | t(35) = 1.29 | NS |
| Sex | ||||
| Male | 13 | 8 | ||
| Female | 5 | 11 | ||
| Total | 18 | 19 | X2 = 2.30 | NS |
| Education (years) | 13.56 (1.38) | 14.37 (1.54) | t(35) = 1.69 | NS |
| Parent education | 16.61 (1.91) | 15.79 (3.03) | t(35) = 0.98 | NS |
| SIPS symptoms | ||||
| Positive | 12.11 (4.6) | 0.42 (0.9) | t(18.24) = 10.59 | .001 |
| Negative | 14.39 (6.9) | 0.47 (0.77) | t(17.40) = 8.51 | .001 |
| Grip strength (Kgs) | 37.29 (9.46) | 35.17 (10.23) | t(35) = 0.65 | NS |
Note: NS indicates P > .05. Mean (SD).
Power Grip Force Stability fMRI Task
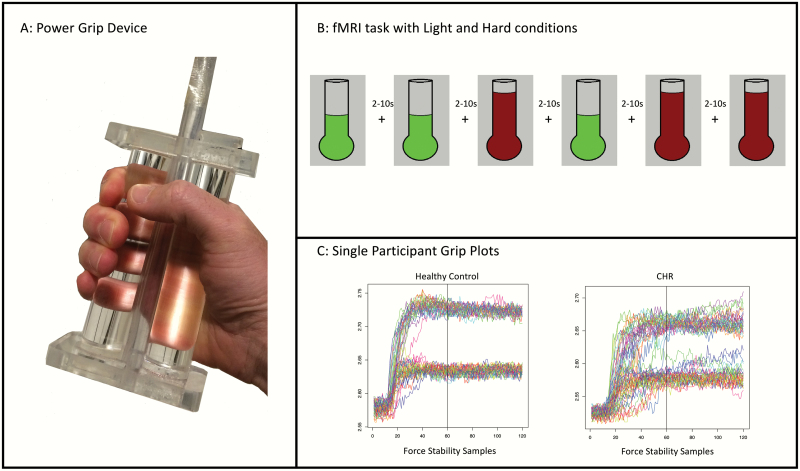
The force stability device is pressure sensitive and built from 2 polymer rods that clamp down on a compressible, polyvinyl air tube. The participant held the device in their right hand while viewing a squeeze gauge with a line indicating how much force should be applied. Visual feedback was presented throughout the trial for 2 different conditions, light and hard, with a total of 30 trials per condition over 3 blocks of trials with 20 trials per block (see figure 1). Grip trials were separated by rest trials lasting between 2 and 10 seconds; rest trials were jittered and randomly presented based on a Gaussian distribution of time intervals. Handgrip strength was quantified by the average reading of 3 trials of maximum grip using a force dynamometer, following the scanning procedure, in order to rule out a potential covariate between groups.
Fig. 1.
Power grip device, paradigm, and raw subject data. Panel A shows the custom grip device. Panel B shows an example of the task paradigm (light [green] and hard [red] conditions). Panel C shows 2 examples of a single participant power grip functional magnetic resonance imaging (fMRI) experiment.
The behavioral data for the grip force stability task were processed according to similar procedures as past studies.12,17,18,37 Based on visual inspection of the data, the initial 3 seconds of the trial were removed in the analysis to focus on the steady-state phase of isometric contraction (see figure 1). A coefficient of variation (CV), a unitless measurement of variability, for each trial and participant was calculated by dividing the standard deviation by the mean amplitude of the force waveform. A linear coefficient measuring the change in CV across the 30 trials (larger values indicate worsened performance) was calculated for each condition to examine the change in variability over the course of the task.
The fMRI data were acquired on a Siemens Magnetom TIM Trio (3T) MRI scanner with a 32-channel head coil. fMRI data analysis was carried out using the FMRIB Software Library 5.0.10 (FSL) FMRI Expert Analysis Tool v6.0 (FEAT). Four fixed-effects models comparing grip > rest were defined at the second level to examine brain activation as a function of an average across blocks of trials (ie, block 1 = block 2 = block 3) or as a linear change in activation across blocks (ie, block 1 < block 2 < block 3) for each condition separately. See supplemental material.
Statistical Analysis
Independent samples t-tests and chi-squared tests were used to compare continuous and categorical demographic data, respectively.
Comparison of force stability (ie, CV) between CHR and healthy controls involved a 2 × 3 × 2 repeated measures ANOVA with group as a between subject’s factor and block and condition as within-subject factors. A 2 × 2 repeated-measures ANOVA was also used to examine the change in force stability (ie, CV linear coefficient) between group and condition.
Group comparisons of brain activation were conducted in FEAT for each second level model. Results used the standard threshold, z > 3.1, PFWE < .05. Effect size maps were created with fslmaths for exploratory analysis of whole brain group comparisons and thresholded at d > 0.5.50
Region of interest (ROI) analysis involved anatomical masks of bilateral caudate, putamen, thalamus, whole cerebellum, supplementary motor area, and primary and secondary motor cortex from the Harvard-Oxford and Montreal Neurologic Institute (MNI) structural atlases in FSL. Mean brain activation in each ROI was extracted using group-level whole brain cope images in Featquery. A series of 4 repeated measures ANOVAs were used to examine between-group differences within ROI for each second level model separately. For each ANOVA, interaction and main effects used a Bonferroni-corrected α < .0125 to control for multiple comparisons followed by Tukey HSD correction in post hoc tests.
Brain-behavior relationships within motor network ROIs were assessed using a linear mixed model procedure (lmer package) to test the interaction between group, brain region, and CV. P-values were obtained using the car package. The average CV for each condition was used as a predictor; in models 3 and 4, the CV change in slope was used as a predictor according to the second level models defined above. For each linear mixed model, interaction and main effects used a Bonferroni-corrected α < .0125 to control for multiple comparisons followed by Tukey HSD correction in post hoc tests.
Results
Study 1
Sample Characteristics
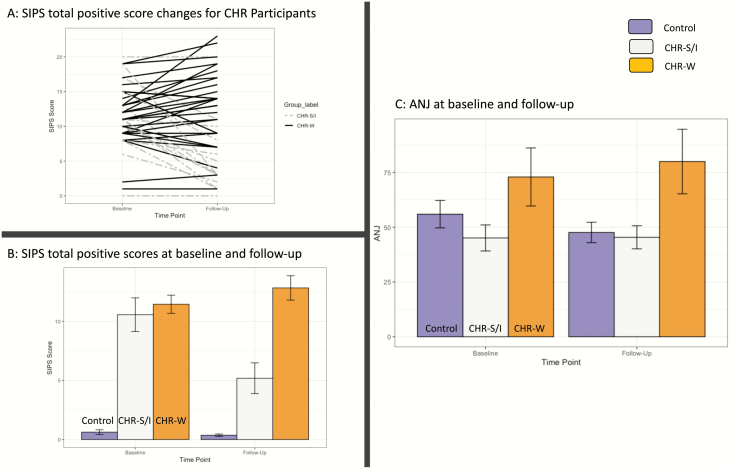
There were no group differences between CHR subgroups and healthy controls in terms of age, years of education, or parent education; however, CHR subgroups and healthy controls differed with regard to sex. A total of 11 CHR participants were prescribed antipsychotic medication (4 CHR-W at baseline, and 1 CHR-S/I and 8 CHR-W at follow-up). Consistent with what would be expected given the grouping strategy, there was a significant group by timepoint interaction in positive symptom change between CHR subgroups F(1,43) = 35.08, P < .001, ηp2 = 0.45. At baseline, CHR subgroups did not differ t(43) = 0.59, P = .55. At follow-up, CHR-S/I had significantly lower positive symptoms compared with CHR-W t(43) = 4.51, P < .001, d = 1.37. See figure 2.
Fig. 2.
Study 1 results. Panel (A) shows SIPS total positive symptom trajectories from baseline to follow-up for each CHR participant, separated by clinical outcome subgroup. Panel (B) shows the mean Structured Interview for Psychosis-Risk Syndromes (SIPS) positive total scores at baseline and follow-up. Panel (C) shows the average normalized jerk across the 3 groups. Error bars represent standard error.
Handwriting Variability
There was not a significant group × timepoint interaction between CHR-S/I, CHR-W, and healthy controls F(2,88) = 0.79, P = .45 or main effect of timepoint F(1,88) = 0.13, P = .72. There was a significant main effect of group F(2,88) = 3.68, P = .03, ηp2 =0 .08. Tukey HSD post hoc tests collapsed across timepoints revealed that CHR-W showed an elevated ANJ compared with CHR-S/I (t = 2.79, P = .01, d = 0.85) and healthy controls (t = 2.87, P = .01, d = 0.87). There were no differences between CHR-S/I and healthy controls (t = 0.62, P = .53). See figure 2C.
Study 2
Sample Characteristics
There were no group differences in terms of age, sex, years of education, or parent education. Two CHR participants were currently taking antipsychotic medication at the time of scanning. There were no group differences in handgrip strength quantified using a force dynamometer. See table 2.
Power Grip Force Stability Behavior Comparisons
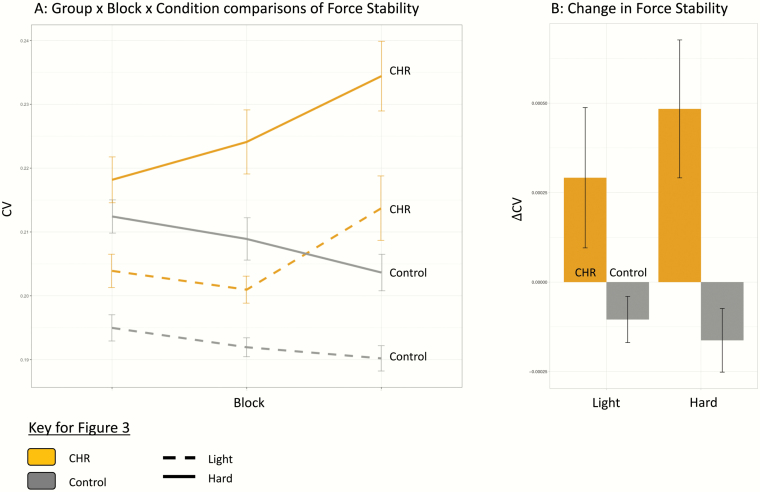
Initial comparison of grip force stability did not reveal a significant group × block × condition interaction, F(2,70) = 1.058, P = .35; however, there was a significant group × block interaction, F(2,70) = 4.76, P = .01, ηp2 = 0.12. A main effect of group showed that the CHR had a higher CV compared with healthy controls across conditions, F(1,35) = 5.96, P = .02, ηp2 = 0.15. As expected, a significant main effect for condition indicated that the hard condition resulted in a larger CV compared with the light condition, F(1,35) = 26.84, P < .001, ηp2 = 0.43. See figure 3A.
Fig. 3.
Force stability behavior comparisons. Panel (A) shows the outcome of a group × block × condition ANOVA predicting force instability (CV). Panel (B) shows group differences in the linear slope coefficient of CV (ΔCV). Error bars represent standard error.
With respect to change in force stability over the course of the task, a group × condition ANOVA predicting the linear slope coefficient of CV did not show a significant interaction. A significant main effect of group showed that on average, the CHR participants showed worsening performance over the course of task, F(1,35) = 8.60, P = .006, ηp2 = 0.19, characterized by a positive CV slope; in contrast, the controls showed a negative slope indicating improved performance. See figure 3B.
Whole Brain Comparisons
There were no significant group differences in whole brain comparisons for any of the second level models. Exploratory analysis of effect sizes for whole brain group comparisons revealed medium to large effect sizes corresponding to lower BOLD signal in motor regions, including the caudate, putamen, cerebellum, primary motor, and somatosensory cortical regions in the CHR group compared with healthy controls. See supplemental material.
Motor Network ROI Comparisons
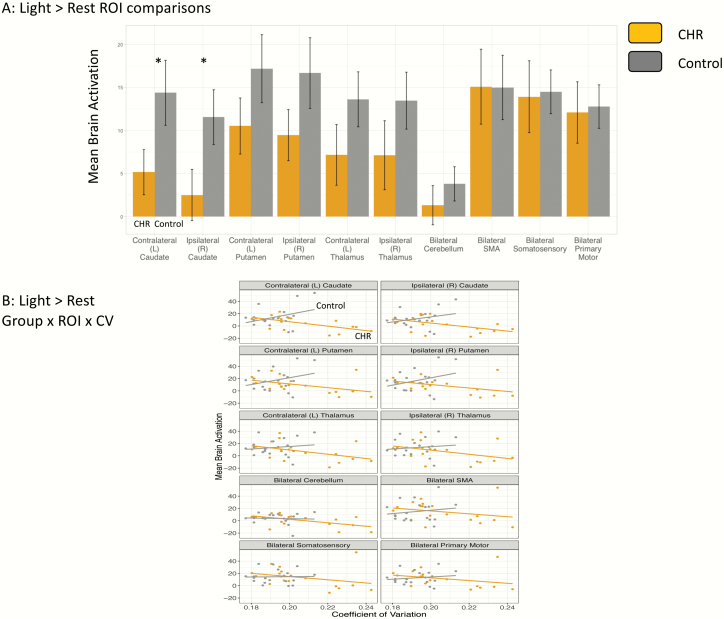
There was a significant group × ROI interaction in the light > rest: average across blocks comparison, F(9,315) = 2.75, P = .004, ηp2 = 0.04. Post hoc testing showed that the CHR group had lower brain activation in the left (t(35) = 2.08, P = .04, d = 0.58) and right caudate (t(35) = 2.05, P = .04, d = 0.57). There were no significant group × ROI interactions or group differences in any of the other models. See figure 4A.
Fig. 4.
Group differences in brain activation and brain-behavior relationships in the motor network. Panel (A) shows mean brain activation in each of the motor network regions of interest (ROI) for the light > rest: average across blocks comparison between groups. Panel (B) shows the relationship between brain activation in each ROI and the coefficient of variation. Error bars represent standard error.
Brain and Behavior Relationships
Four linear mixed models were conducted separately to test the relationships between brain activation and CV in motor network ROIs. There was a trend level group × ROI × CV interaction following multiple comparison correction for Light > rest: Averaged Across Conditions F(9,297) = 2.13, P = .027. Further analysis showed a trend level group × CV interaction within the left caudate t(35) = 2.13, P = .04, d = 0.60 but no interactions for any of the other ROIs. Within the CHR group, left caudate activation was negatively associated with CV r(16) = −0.62, P = .006, while in the controls, there was a nonsignificant positive relationship r(17) = .35, P = .14. There were no significant group × ROI × CV interactions in any of the other models. See figure 4B.
Discussion
Here, we present 2 studies that highlight important features of motor variability in youth at risk for psychosis. Study 1 showed that a measure of handwriting smoothness is a stable marker of motor variability and remains elevated in CHR participants with a worsening positive symptom course. Study 2 is the first fMRI study of power grip force stability in CHR youth. As hypothesized—consistent with past studies of force stability in psychosis spectrum populations12,17,19,20,37,51–53—the CHR group displayed greater force instability compared with controls as well as a linear increase in variability over the course of the task. For CHR individuals, the power grip task elicited less brain activation in the caudate compared with controls. Poor performance on the motor task was moderately correlated with less brain activation in the left contralateral caudate in the CHR group. Interestingly, controls showed the opposite pattern, suggesting that motor dysfunction in the CHR group is associated with abnormal striatal functioning.
Parsing the clinical heterogeneity of CHR youth is critically important for staging interventions aimed at symptom reduction. Study 1 suggested that handwriting variability may be higher in individuals at baseline and 12-month follow-up, providing evidence for motor variability as a stable feature of elevated risk for psychosis, consistent with recent longitudinal research showing that fine motor control is a stable and impaired feature of psychosis risk from childhood to adolescence.54,55 Assessing motor abnormalities is also helpful for determining different vulnerability subtypes in CHR youth; future research should consider the addition of motor performance in risk prediction models of psychosis.5,12,56 The results of study 1 should be approached with some caution; antipsychotic medication may have had a small effect on the magnitude of group differences in handwriting variability; and follow-up studies are needed to examine medication-free or naïve samples to better understand the mechanisms underlying this variability (see supplemental material).
Experimental and computational models of grip force stability in schizophrenia suggest that impaired motor inhibition produces greater variability in patient groups compared with healthy controls, perhaps suggesting an inefficient system of modulating sensory-motor commands.29 Effort, motivation, and fatigue may have played a role in the CHR group’s performance on the power grip task, which is consistent with schizophrenia patients who show a poor sense of intended effort during isometric pinch grip.57 It is interesting that force grip variability increased over the course of the task in CHR participants but not healthy controls, suggesting that in the CHR participants, inefficiency in either motor control or a sense of effort declines, producing greater variability. We may posit a guess from study 2 that physical exertion interacts with neural vulnerabilities during the CHR period: as physical stress is repeated, systems responding to motor commands become quickly overtaxed.58,59
ROI analysis elicited a specific decrease in the BOLD signal in the caudate for CHR participants during the power grip task compared with healthy controls. The caudate is a part of the associative cortico-striatal loop, and BOLD signal tends to increase in the caudate when motor control requires higher-order cognitive processes, such as attention and selection of upcoming motor actions.60 Examining cognitive correlates of motor performance and striatal activation may be an important follow-up to these results.55 Exploratory analysis using effect size maps also noted medium to large effect size group differences in the caudate, putamen, primary motor and sensory cortices, and cerebellar brain regions across both light and hard conditions as well as cortical activation in frontal, temporal, and occipital cortices, providing preliminary evidence for aberrant motor network functioning in the CHR group.
We observed a negative relationship between brain activation and force stability in the CHR group, whereas the control group showed a positive relationship. It is interesting that the same or even larger effects were not observed in the hard condition, which previous research suggests that we would expect in increased production of force variability and motor brain network activation.31,44However, researchers have also found that cortical motor regions (ie, primary and somatosensory motor cortices) as well as the cerebellum show a linear increase in activation with larger levels of force, whereas the striatum shows a nonlinear relationship with force level.30,39 It is possible that the hard condition produced too much intra-individual variability in brain activation, limiting our ability to detect differences at the group level. Further work varying the level of force amplitude toward the lower ranges may produce more informative results in terms of cortico-striatal and cortico-cerebellar activation.
There are several limitations to the current study. First, the sample sizes in both studies could be improved; a larger sample size would help determine predictors for psychosis and whole brain group differences. Second, an investigation of the associations between motor variables would help in our understanding of shared mechanisms of variability in handwriting and power grip. Large multisite CHR consortia utilizing neuroimaging (eg, Psychosis Risk Outcome Network [PRONET]) and mechanistically informed behavioral testing (eg, Computerized Assessment for Psychosis Risk [CAPR]) would be an invaluable step toward further clarifying mechanisms and translating motor markers to clinically relevant applications. Motor variability is associated with risk for worsening course of CHR symptoms and abnormal motor network function prior to the onset of psychosis, and future longitudinal studies are needed to examine ANJ and power grip as predictors of transition to psychosis.
Funding
This work was supported by a Brain and Behavior Research Foundation NARSAD Young Investigator Grant, as the Donald and Janet Boardman Family Investigator to J.A.B. Further support for this work came from the National Institutes of Health Grants (R01MH094650, R21/R33MH103231, and R21MH110374 to V.A.M.).
Supplementary Material
Acknowledgment
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Grace AA, Gomes FV. The circuitry of dopamine system regulation and its disruption in schizophrenia: insights into treatment and prevention. Schizophr Bull. 2019; 45(1): 148– 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Robbins TW. The case of frontostriatal dysfunction in schizophrenia. Schizophr Bull. 1990;16(3):391–402. [DOI] [PubMed] [Google Scholar]
- 3. Andreasen NC, Paradiso S, O’Leary DS. “Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr Bull. 1998;24(2):203–218. [DOI] [PubMed] [Google Scholar]
- 4. Picard H, Amado I, Mouchet-Mages S, Olié JP, Krebs MO. The role of the cerebellum in schizophrenia: an update of clinical, cognitive, and functional evidences. Schizophr Bull. 2008;34(1):155–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van Harten PN, Walther S, Kent JS, Sponheim SR, Mittal VA. The clinical and prognostic value of motor abnormalities in psychosis, and the importance of instrumental assessment. Neurosci Biobehav Rev. 2017;80:476–487. [DOI] [PubMed] [Google Scholar]
- 6. Hirjak D, Meyer-Lindenberg A, Kubera KM, Thomann PA, Wolf RC. Motor dysfunction as research domain in the period preceding manifest schizophrenia: a systematic review. Neurosci Biobehav Rev. 2018;87:87–105. [DOI] [PubMed] [Google Scholar]
- 7. Mittal VA, Neumann C, Saczawa M, Walker EF. Longitudinal progression of movement abnormalities in relation to psychotic symptoms in adolescents at high risk of schizophrenia. Arch Gen Psychiatry. 2008;65(2):165–171. [DOI] [PubMed] [Google Scholar]
- 8. Mittal VA, Bernard JA, Northoff G. What can different motor circuits tell us about psychosis? An RDoC perspective. Schizophr Bull. 2017;43(5):949–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fish B, Hagin R. Visual-motor disorders in infants at risk for schizophrenia. Arch Gen Psychiatry. 1972;27(5):594–598. [DOI] [PubMed] [Google Scholar]
- 10. Walker EF, Savoie T, Davis D. Neuromotor precursors of schizophrenia. Schizophr Bull. 1994;20(3):441–451. [DOI] [PubMed] [Google Scholar]
- 11. Filatova S, Koivumaa-Honkanen H, Khandaker GM, et al. Early motor developmental milestones and schizotypy in the Northern Finland Birth Cohort study 1966. Schizophr Bull. 2018;44(5):1151–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dean DJ, Walther S, Bernard JA, Mittal VA. Motor clusters reveal differences in risk for psychosis, cognitive functioning, and thalamocortical connectivity: evidence for vulnerability subtypes. Clin Psychol Sci. 2018;6(5):721–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dean DJ, Teulings HL, Caligiuri M, Mittal VA. Handwriting analysis indicates spontaneous dyskinesias in neuroleptic naïve adolescents at high risk for psychosis. J Vis Exp. 2013;( 81): e50852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dean DJ, Orr JM, Newberry RE, Mittal VA. Motor behavior reflects reduced hemispheric asymmetry in the psychosis risk period. Schizophr Res. 2016;170(1):137–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Callaway DA, Perkins DO, Woods SW, Liu L, Addington J. Movement abnormalities predict transitioning to psychosis in individuals at clinical high risk for psychosis. Schizophr Res. 2014;159(2-3):263–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vieluf S, Temprado JJ, Berton E, Jirsa VK, Sleimen-Malkoun R. Effects of task and age on the magnitude and structure of force fluctuations: insights into underlying neuro-behavioral processes. BMC Neurosci. 2015;16:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Caligiuri MP, Lohr JB. A disturbance in the control of muscle force in neuroleptic-naive schizophrenic patients. Biol Psychiatry. 1994;35(2):104–111. [DOI] [PubMed] [Google Scholar]
- 18. Mittal VA, Dean DJ, Pelletier A, Caligiuri M. Associations between spontaneous movement abnormalities and psychotic-like experiences in the general population. Schizophr Res. 2011;132(2-3):194–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cortese L, Caligiuri MP, Malla AK, Manchanda R, Takhar J, Haricharan R. Relationship of neuromotor disturbances to psychosis symptoms in first-episode neuroleptic-naive schizophrenia patients. Schizophr Res. 2005;75(1):65–75. [DOI] [PubMed] [Google Scholar]
- 20. Purdon SE, Woodward ND, Flor-Henry P. Asymmetrical hand force persistence and neuroleptic treatment in schizophrenia. J Int Neuropsychol Soc. 2001;7(5):606–614. [DOI] [PubMed] [Google Scholar]
- 21. Pappa S, Dazzan P. Spontaneous movement disorders in antipsychotic-naive patients with first-episode psychoses: a systematic review. Psychol Med. 2009;39(7):1065–1076. [DOI] [PubMed] [Google Scholar]
- 22. Caligiuri MP, Teulings HL, Dean CE, Niculescu AB, Lohr J. Handwriting movement analyses for monitoring drug-induced motor side effects in schizophrenia patients treated with risperidone. Hum Mov Sci. 2009;28(5):633–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Caligiuri MP, Teulings HL, Dean CE, Niculescu AB 3rd, Lohr JB. Handwriting movement kinematics for quantifying extrapyramidal side effects in patients treated with atypical antipsychotics. Psychiatry Res. 2010;177(1-2):77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kent JS, Disner SG, Van Voorhis AC, Urošević S, Caligiuri MP, Sponheim SR. Exploring the relationship of transdiagnostic mood and psychosis symptom domains with motor dysfunction. Neuropsychobiology 2019:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. King MTC. Toward a greater underdstanding of the brain processes underlying handgrip and handgrip fatigue [thesis]. University of Cape Town;2016.
- 26. King M, Rauch HG, Stein DJ, Brooks SJ. The handyman’s brain: a neuroimaging meta-analysis describing the similarities and differences between grip type and pattern in humans. Neuroimage 2014;102 (Pt 2):923–937. [DOI] [PubMed] [Google Scholar]
- 27. Vaillancourt DE, Newell KM. Changing complexity in human behavior and physiology through aging and disease. Neurobiol Aging. 2002;23(1):1–11. [DOI] [PubMed] [Google Scholar]
- 28. Firth J, Stubbs B, Vancampfort D, et al. Grip strength is associated with cognitive performance in schizophrenia and the general population: a UK biobank study of 476559 participants. Schizophr Bull. 2018; 44(4): 728– 736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Teremetz M, Amado I, Bendjemaa N, Krebs MO, Lindberg PG, Maier MA. Deficient grip force control in schizophrenia: behavioral and modeling evidence for altered motor inhibition and motor noise. PLoS One. 2014;9(11):e111853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Keisker B, Hepp-Reymond MC, Blickenstorfer A, Meyer M, Kollias SS. Differential force scaling of fine-graded power grip force in the sensorimotor network. Hum Brain Mapp. 2009;30(8):2453–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cramer SC, Weisskoff RM, Schaechter JD, et al. Motor cortex activation is related to force of squeezing. Hum Brain Mapp. 2002;16(4):197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Noble JW, Eng JJ, Kokotilo KJ, Boyd LA. Aging effects on the control of grip force magnitude: an fMRI study. Exp Gerontol. 2011;46(6):453–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gupta T, Dean DJ, Kelley NJ, Bernard JA, Ristanovic I, Mittal VA. Cerebellar transcranial direct current stimulation improves procedural learning in nonclinical psychosis: a double-blind crossover study. Schizophr Bull. 2018;44(6):1373–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dean DJ, Bernard JA, Orr JM, et al. Cerebellar morphology and procedural learning impairment in neuroleptic-naive youth at ultrahigh risk of psychosis. Clin Psychol Sci. 2014;2(2):152–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dean DJ, Mittal VA. Spontaneous Parkinsonisms and striatal impairment in neuroleptic free youth at ultrahigh risk for psychosis. NPJ Schizophr. 2015;1(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mittal VA, Dean DJ, Bernard JA, et al. Neurological soft signs predict abnormal cerebellar-thalamic tract development and negative symptoms in adolescents at high risk for psychosis: a longitudinal perspective. Schizophr Bull. 2014;40(6):1204–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mittal VA, Orr JM, Turner JA, et al. Striatal abnormalities and spontaneous dyskinesias in non-clinical psychosis. Schizophr Res. 2013;151(1-3):141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Walther S, Stegmayer K, Federspiel A, Bohlhalter S, Wiest R, Viher PV. Aberrant hyperconnectivity in the motor system at rest is linked to motor abnormalities in schizophrenia spectrum disorders. Schizophr Bull. 2017;43(5):982–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Spraker MB, Yu H, Corcos DM, Vaillancourt DE. Role of individual basal ganglia nuclei in force amplitude generation. J Neurophysiol. 2007;98(2):821–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ehrsson HH, Fagergren E, Forssberg H. Differential fronto-parietal activation depending on force used in a precision grip task: an fMRI study. J Neurophysiol. 2001;85(6):2613–2623. [DOI] [PubMed] [Google Scholar]
- 41. Martinelli C, Rigoli F, Shergill SS. Aberrant force processing in schizophrenia. Schizophr Bull. 2017; 43(2): 417– 424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Broome MR, Matthiasson P, Fusar-Poli P, et al. Neural correlates of movement generation in the ‘at-risk mental state’. Acta Psychiatr Scand. 2010;122(4):295–301. [DOI] [PubMed] [Google Scholar]
- 43. Kuhtz-Buschbeck JP, Ehrsson HH, Forssberg H. Human brain activity in the control of fine static precision grip forces: an fMRI study. Eur J Neurosci. 2001;14(2):382–390. [DOI] [PubMed] [Google Scholar]
- 44. Kuhtz-Buschbeck JP, Gilster R, Wolff S, Ulmer S, Siebner H, Jansen O. Brain activity is similar during precision and power gripping with light force: an fMRI study. Neuroimage 2008;40(4):1469–1481. [DOI] [PubMed] [Google Scholar]
- 45. Caligiuri MP, Teulings HL, Filoteo JV, Song D, Lohr JB. Quantitative measurement of handwriting in the assessment of drug-induced Parkinsonism. Hum Mov Sci. 2006;25(4-5):510–522. [DOI] [PubMed] [Google Scholar]
- 46. Mittal VA, Walker EF, Bearden CE, et al. Markers of basal ganglia dysfunction and conversion to psychosis: neurocognitive deficits and dyskinesias in the prodromal period. Biol Psychiatry. 2010;68(1):93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Miller TJ, McGlashan TH, Rosen JL, et al. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull. 2003;29(4):703–715. [DOI] [PubMed] [Google Scholar]
- 48. First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition, January 1995 FINAL. SCID-I/P Version 2.0). New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 1995. [Google Scholar]
- 49. Teulings HL, Contreras-Vidal JL, Stelmach GE, Adler CH. Parkinsonism reduces coordination of fingers, wrist, and arm in fine motor control. Exp Neurol. 1997;146(1):159–170. [DOI] [PubMed] [Google Scholar]
- 50. Reddan MC, Lindquist MA, Wager TD. Effect size estimation in neuroimaging. JAMA Psychiatry. 2017;74(3):207–208. [DOI] [PubMed] [Google Scholar]
- 51. Mittal VA, Smolen A, Dean DJ, Pelletier AL, Lunsford-Avery J, Smith A. BDNF Val66Met and spontaneous dyskinesias in non-clinical psychosis. Schizophr Res. 2012;140(1–3): 65– 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Koning JP, Kahn RS, Tenback DE, van Schelven LJ, van Harten PN. Movement disorders in nonpsychotic siblings of patients with nonaffective psychosis. Psychiatry Res. 2011;188(1):133–137. [DOI] [PubMed] [Google Scholar]
- 53. Willems AE, Sommer IEC, Tenback DE, Koning JPF, van Harten PN. Instrumental measurements of spontaneous dyskinesia and schizotypy in subjects with auditory verbal hallucinations and healthy controls. Psychiatry Res. 2016;244:24–27. [DOI] [PubMed] [Google Scholar]
- 54. Dickson H, Roberts RE, To M, Wild K, Loh M, Laurens KR. Adolescent trajectories of fine motor and coordination skills and risk for schizophrenia. Schizophr Res. 2020;215: 263–269. [DOI] [PubMed] [Google Scholar]
- 55. Dickson H, Laurens KR, Cullen AE, Hodgins S. Meta-analyses of cognitive and motor function in youth aged 16 years and younger who subsequently develop schizophrenia. Psychol Med. 2012;42(4):743–755. [DOI] [PubMed] [Google Scholar]
- 56. Mittal VA, Walther S. As motor system pathophysiology returns to the forefront of psychosis research, clinical implications should hold center stage. Schizophr Bull. 2019; 45(3): 495– 497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lafargue G, Franck N, Sirigu A. Sense of motor effort in patients with schizophrenia. Cortex. 2006;42(5):711–719. [DOI] [PubMed] [Google Scholar]
- 58. Thompson JL, Pogue-Geile MF, Grace AA. Developmental pathology, dopamine, and stress: a model for the age of onset of schizophrenia symptoms. Schizophr Bull. 2004;30(4): 875–900. [DOI] [PubMed] [Google Scholar]
- 59. Pruessner M, Iyer SN, Faridi K, Joober R, Malla AK. Stress and protective factors in individuals at ultra-high risk for psychosis, first episode psychosis and healthy controls. Schizophr Res. 2011;129(1):29–35. [DOI] [PubMed] [Google Scholar]
- 60. Lehéricy S, Bardinet E, Tremblay L, et al. Motor control in basal ganglia circuits using fMRI and brain atlas approaches. Cereb Cortex. 2006;16(2):149–161. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.