Abstract
Background
Psychotic-like experiences (PLE) are present in nonclinical populations, yet their association with brain structural variation, especially markers of early neurodevelopment, is poorly understood. We tested the hypothesis that cortical surface gyrification, a putative marker of early brain development, is associated with PLE in healthy subjects.
Methods
We analyzed gyrification from 3 Tesla MRI scans (using CAT12 software) and PLE (positive, negative, and depressive symptom dimensions derived from the Community Assessment of Psychic Experiences, CAPE) in 103 healthy participants (49 females, mean age 29.13 ± 9.37 years). A subsample of 63 individuals completed tasks from the Wechsler Adult Intelligence Scale and Controlled Oral Word Association Test. Estimated IQ and a composite neuropsychological score were used to explore mediation pathways via cognition.
Results
Positive PLE distress was negatively associated with gyrification of the left precuneus. PLE depression dimension showed a negative association with gyrification in the right supramarginal and temporal region. There was no significant mediating effect of cognition on these associations.
Conclusion
Our results support a neurobiological psychosis spectrum, for the first time linking an early developmental imaging marker (rather than volume) to dimensional subclinical psychotic symptoms. While schizophrenia risk, neurodevelopment, and cognitive function might share genetic risk factors, additional mediation analyses did not confirm a mediating effect of cognition on the gyrification-psychopathology correlation.
Keywords: cognitive function, endophenotype, neurodevelopment, subclinical, magnetic resonance imaging (MRI)
Introduction
Schizophrenia is associated with core cognitive deficits predictive of risk for illness onset,1 treatment response, and recovery.2,3 Hallmark dysfunctions consistently include general intellectual ability4 and domains of attention, working memory, and verbal fluency.5 While gradual changes in cognitive, perceptual, and negative symptoms mark the prodromal phase in the ultra-high risk (UHR) state, performances in these domains are also reduced in non-afflicted first-degree relatives6,7 and healthy adults endorsing psychosis phenotypes including schizotypy and psychotic-like experiences (PLE).8,9 Previously, we reported positive correlations between psychosis proneness in healthy adults and gray matter (GM) volumes in the precuneus, inferior, and parietal cortical areas.10 GM, white matter, and functional abnormalities in fronto-parieto-temporal network areas,11,12 parahippocampal, and cingulate gyri are frequently reported in schizophrenia.13,14 Cortical and subcortical alterations in prefrontal network GM volume15,16 and functional connectivity between frontal, temporal, hippocampal, and striatal regions across the psychosis continuum17–20 are evident, yet somewhat inconclusive regarding directionality. Modinos et al21 detected GM volume increases in the precuneus and anterior cingulate cortex in high schizotypy as well as the medial posterior cingulate areas in high positive PLE. Another study did not support regional prefrontal GM reductions associated with schizophrenia in twins and relatives of patients, suggesting that deficits in prefrontal executive function, rather than GM variation, are attributable to genetic liability for schizophrenia.22
Altogether these findings demonstrate that disease-stage and genetic risk profile account for overlap and discrepancies in functional and cortical variation, especially in prefrontal and precuneus regions. Neurobiological correlates of polygenic risk for psychotic disorders and cognitive disturbance support accumulating evidence for 2 neurodevelopmentally meaningful endophenotypes.23–26 The shared variance between polygenetic risk for schizophrenia and cognition-related pathways in a causal mediation model suggests that cognitive disturbance lies upstream to schizophrenia liability and not vice versa.27 This is further supported by putative pathways involving, eg, calcium signaling associated with executive function in schizophrenia.28
A growing body of research recognizes cortical gyrification as a neurobiological marker of early genetic and environmental modulation in cortical surface morphology in schizophrenia. Signifying the degree of cortical folding that peaks during early neurodevelopment, gyrification has been strongly implicated as an early endophenotype in psychopathology.29 Increased spatial resolution is achieved by quantifying the local gyrification of individual surface vertices.30 A recent study using vertex-wise local gyrification index (GI),31 for instance, has shown an association with polygenic risk indicating an early neurodevelopmental disturbance in schizophrenia.32 Compared with cortical thickness, morphometry of cortical gyrification might be less susceptible to heterogeneous illness-related effects.33 This can aid to delineate etiological phenomena across groups of varying phenotype expression without confounds of acute neuroanatomical changes in schizophrenia34 and antipsychotic treatment thereof.35 Thus, gyrification provides a novel approach to map differential phenotype correlates,36,37 which are continuously expressed in the general population.38 Case-control studies of gyrification in schizophrenia have pointed to prefrontal and temporal alterations, but have not always been consistent.37,39–41 While psychotic phenomena such as auditory hallucinations have been linked to cortical folding abnormalities in schizophrenia patients,42 studies of cortical folding in nonclinical subjects are rare.43 Hence, there is a paucity in the studies linking gyrification to subclinical phenomena, such as PLE, that form part of the psychosis spectrum. These mostly transitory PLE38 feature positive (delusional, hallucinatory, and dissociative experiences) and negative (affective flattening, avolition, and social withdrawal) subclinical phenomena corresponding to the typical dimensions of schizophrenia spectrum disorders.44,45 Recently, a study using local GI found a significant role of the persistence of psychotic experiences during a 2-year follow-up period on gyrification reduction in the left temporal gyrus and brain volume in left occipital and right prefrontal brain regions,23 thus replicating morphological findings in regions implicated in schizophrenia. Negative associations of cortical volume and local GI in orbitofrontal, parietal, and temporal regions were driven by the interaction of polygenic risk score and psychotic experiences. However, these symptoms were not differentiated by dimensionality or quality, such as PLE frequency or symptom-related distress accounting for cortical variation in relevant areas, including left precuneus and right inferior temporal pole.46
Despite some initial volume-based morphometric studies, it is unclear whether more specific morphometric markers related to core processes such as early development/cortical gyrification are related to different dimensions of PLE (positive, negative, and depressive) and cognitive function. Our aims were, therefore, 2-fold: our primary objective, based on previous GM volumetric studies of PLE, was to test the hypothesis that variation in cortical surface morphology is associated with different dimensions of subclinical PLE in healthy nonclinical individuals. Guided by previous voxel-based morphometry (VBM) findings,10 we expected associations between psychosis proneness (assessed by CAPE) and gyrification in prefrontal, superior parietal, and precuneus regions. Secondly, we tested the hypothesis that PLE-associated gyrification is mediated by cognitive function in this nonclinical cohort. This hypothesis was based on the findings in the clinical spectrum, showing close relations between cognition and clinical outcomes across high-risk, first-episode, and multi-episode patients47,48 and cognition pathways mediating some genetic risk on schizophrenia in a recent study.27
Methods
Subjects
We included 103 healthy participants (49 males, 54 females; mean age = 29.13 years, SD = 9.37) recruited from the local community. We obtained written informed consent from each participant for the study protocol approved by the local Ethics Committee of the Jena University Medical School and in line with the Declaration of Helsinki. The sample is based on a previously published community sample, which was enlarged subsequently.10 Mean laterality index for the overall sample was 73.78 (SD = 36.38) right-handedness.49 Subjects were recruited from the local community by advertising (press releases and word of mouth) and were compensated for study participation. They first underwent telephone screening and subsequent screening in person to assess the inclusion and exclusion criteria. A semi-structured interview was used to screen subjects for the absence of current or previous psychiatric disorders, including substance abuse or dependence, psychiatric or psychological treatment, intake of psychopharmacotherapy, or first-degree family liability for psychotic disorders. Subjects were also excluded if any neurological disorders, untreated major chronic or acute organic medical conditions, history of traumatic brain injury/loss of consciousness, or intellectual disability/ learning impairment (IQ < 80) were present. Next, all subjects underwent screening about lifetime history of psychiatric and general medical health care, and illicit substance and alcohol use. These screening questions were a requirement for subsequent scanning to ensure the inclusion of healthy volunteers from the general population only.
CAPE Assessment
Clinically meaningful levels of psychosis risk can be detected in healthy individuals using self-report measures, such as the 42-item Community Assessment of Psychic Experiences (CAPE).50 The CAPE is widely used to assess lifetime prevalence of PLE in the general population whilst available in multiple languages. These strengths were recently demonstrated in a meta-analysis45 and a cross-cultural study,51 and it may be cost-effectively employed in non-specialized settings to examine traits associated with psychosis proneness.52,53 Including positive (CAPE-pos, 20 items) and negative (CAPE-neg, 14 items) subscales, the CAPE provides a comprehensive and reliable45 self-report measure of the dichotomous symptom dimensions reflecting both frequency and distress related to psychosis-prone traits. Additionally, we also explored the depressive symptom (CAPE-dep) subdimension, which consists of an 8-item scale from the 3-factor model.44
Neuropsychological Assessment
In a subsample of 63 healthy subjects (28 females; mean age 30.32 years, SD = 10.47), we assessed cognitive performance using multiple subtests of the German Wechsler Intelligenztest für Erwachsene (WIE),54 the German adaptation of Wechsler Adult Intelligence Scale (WAIS-III)55 neuropsychological testing battery, and the Controlled Oral Word Association Test (COWAT).56 A general estimate of intelligence (IQ) was obtained from a German-language multiple-choice vocabulary test (Mehrfachwahl-Wortschatz-Intelligenztest, MWT-B).57 The MWT-B provides a resourceful approximation of crystallized intelligence.58 The combination of cognitive tasks typically utilized in clinical schizophrenia25,59 and UHR samples60 from the 2 extensive test batteries included Letter-Number Sequencing task (LNS), Digit Symbol Coding task (DSCT) of the WAIS-III, Letters FAS, and Animals of the COWAT (table 1).
Table 1.
Demographic and Cognitive Sample Characteristics of 103 Healthy Adults
| Variable | N | Mean | SD | Skewness | Kurtosis |
|---|---|---|---|---|---|
| Age | 103 | 29.13 | 9.37 | 1.80 | 2.68 |
| Female (%) | 49 (47.60%) | ||||
| IQ (MWT-B estimate) | 103 | 105.28 | 12.08 | 1.30 | 1.20 |
| Neuropsychological assessment | |||||
| Age | 63 | 30.32 | 10.47 | 1.52 | 1.48 |
| Female (%) | 28 (44.40%) | ||||
| IQ (MWT-B estimate) | 63 | 108.70 | 13.42 | 0.94 | −0.10 |
| MWT-B Score | 63 | 29.22 | 3.48 | −0.03 | −0.53 |
| WAIS-III | |||||
| Arithmetic | 63 | 16.41 | 3.78 | −0.48 | −0.78 |
| Digit symbol coding task | 63 | 83.63 | 14.93 | −0.04 | −0.48 |
| Matrix reasoning | 63 | 20.89 | 3.45 | −0.72 | −0.21 |
| Digit span | 63 | 19.03 | 3.69 | 0.05 | −0.73 |
| Information | 63 | 19.92 | 5.53 | −0.77 | −0.43 |
| Letter-number sequencing | 63 | 13.17 | 2.55 | −0.44 | −0.51 |
| COWAT | |||||
| Letters FAS | 63 | 39.84 | 12.08 | 0.27 | −0.71 |
| Animals | 63 | 25.83 | 6.32 | 0.20 | −0.15 |
Note: Of 103 healthy adults, 63 participants also completed neuropsychological tasks from the Wechsler Adult Intelligence Scale (WAIS-III) and Controlled Oral Word Association Test (COWAT). MWT-B, Mehrfachwahl-Wortschatz-Intelligenztest; SD, standard deviation.
MRI Acquisition and Surface-Based Morphometric Analysis
We obtained high-resolution T1-weighted scans using a 3 Tesla Siemens Tim Trio scanner (Siemens) with standard quadrature head coil and MPRAGE sequence for all subjects. Images were visually inspected followed by automated data quality check with homogeneity bias correction and tissue segmentation of images, followed by surface-based morphometry (SBM) analysis conducted using the CAT12 toolbox, v12.5 r1363 (Christian Gaser, Structural Brain Mapping Group, Jena University Hospital; http://www.neuro.uni-jena.de/cat12/) within SPM12 v7219 (Statistical Parametric Mapping, Wellcome Trust Centre for Neuroimaging; http://www.fil.ion.ucl.ac.uk/spm/software/spm12/) for Matlab R2017a (The MathWorks, Inc.). This novel pipeline allows for the computation of surface-based parameters based on, eg, the mean curvature. Images were smoothed using a Gaussian kernel with 20-mm full width at half maximum, as recommended for vertex-wise gyrification in the CAT12 user manual (http://www.neuro.uni-jena.de/cat12/CAT12-Manual.pdf). All subjects passed both the visual quality inspection and the CAT12 data quality checks. Together, all scans from 103 participants reached a weighted average (IQR) of 86.01% (range 82.32%–86.55%) corresponding to a quality grade B.
Statistical Analysis
For statistical analysis of CAPE-gyrification associations (N = 103), we applied general linear models (GLM) implemented in SPM12 and the CAT12 toolbox using CAPE subscale scores as predictors for local gyrification, while covarying for age and sex nuisance in the vertex-wise analysis (i.e. multiple linear regression models). We applied familywise error (FWE) cluster-level correction at P < .05 (with initial P < .001 uncorrected peak-level thresholding) for significance testing.61 Secondly, in the subsample of n = 63 subjects, we examined the relationship between neuropsychological predictors and CAPE outcome variables. Due to previously reported negative relationships between cognitive measures and psychosis proneness,8 we carried out 1-tailed partial correlations using Statistical Package for the Social Sciences (SPSS, Version 25, IBM Corp.). Finally, in this subsample, we explored mediating effect IQ and cognition on mean extracted predicted values in anatomical regions-of-interest (ROI) from the primary GLM analysis (ie, CAPE-gyrification association) as predictors and CAPE as outcome variables using the ordinary least squares (OLS) regression analysis implemented in PROCESS Version 3.362 for SPSS. Model coefficients P-values were adjusted with the false discovery rate (FDR)63 correction for multiple comparisons using R.64 For mediation model predictors, we used mean gyrification estimates across Desikan-Killiany atlas regions.65
Results
PLE Measures
In our whole sample, subjects scored on CAPE-pos dimension with mean frequency 1.24 (SD =0.18, range 1.00–2.15, kurtosis = 5.80, skewness = 1.87) and mean distress 1.64 (SD = 0.50, range 1.00–3.13, kurtosis = −0.10, skewness = 0.45), CAPE-neg dimension with mean frequency 1.72 (SD = 0.43, range 1.07–3.14, kurtosis = 1.10, skewness = 0.97) and distress mean 1.88 (SD = 0.65, range 1.00–3.50, kurtosis = −0.61, skewness = 0.49), and CAPE-dep scale with mean frequency 1.69 (SD = 0.40, range 1.13–3.88, kurtosis = 7.99, skewness = 2.05) and distress mean 2.02 (SD = 0.67, range 1.00–3.83, kurtosis = 0.10, skewness = 0.52).
Cortical Gyrification and PLE
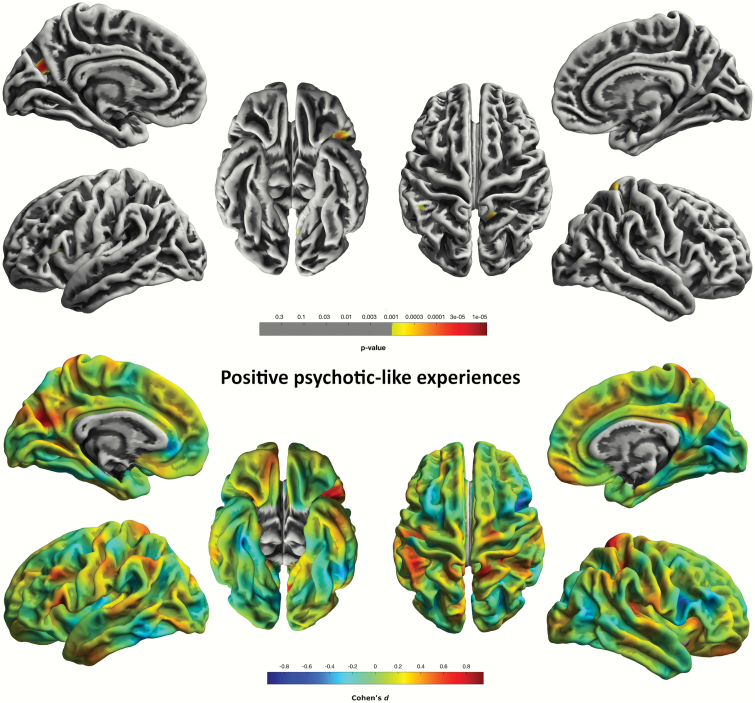
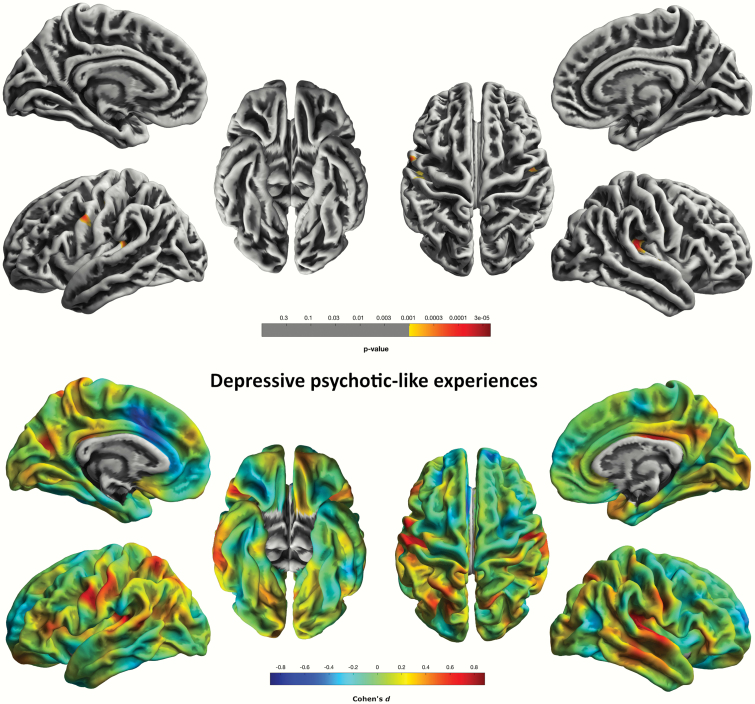
For the CAPE-pos scale, we found cluster-level significant (P = .015, FWE-corr.) effects in a cluster comprised of 178 vertices in the precuneus/cuneus region of the left hemisphere. We also found a trend-level effect for this scale in a cluster comprising 112 vertices in the left pars triangularis extending from the inferior prefrontal lobe to the pars opercularis region in the left middle frontal region (P = .080, FWE-corr.) (figure 1). CAPE-neg was associated with gyrification in the right posterior and isthmus cingulate area; however, these correlations were not significant at the chosen P < .05 FWE-correction level. GLM with CAPE-dep yielded negative associations with gyrification spanning supramarginal to superior temporal gyrus (STG) regions (P = .001, FWE-corr.) (figure 2; supplementary table 2). All results significant at the P < .05 FWE-threshold concerned CAPE PLE-associated distress levels.
Fig. 1.
Logarithmic P-value maps of significant negative correlations of cortical gyrification and CAPE-pos scale in 103 healthy individuals (P < 0.001, uncorrected, for display purposes) (top). Cohen’s d maps of effect sizes for uncorrected correlations of gyrification with the CAPE-pos scale in 103 healthy individuals (bottom).
Fig. 2.
Logarithmic P-value maps of significant negative correlations of cortical gyrification and CAPE-dep scale in 103 healthy individuals (P < 0.001, uncorrected, for display purposes) (top). Cohen’s d maps of effect sizes for uncorrected correlations of gyrification with the CAPE-dep scale in 103 healthy individuals (bottom).
Neuropsychological Findings
Nonparametric and where appropriate parametric correlational analyses between individual raw scores of each neuropsychological subtest and CAPE frequency and distress scores were conducted to explore the relationship between cognitive and PLE phenotype variables. Correlation coefficients from partial 1-tailed correlation analyses controlled for age and sex are shown in table 2.
Table 2.
One-Tailed Partial (Covariates Age and Sex) Spearman’s (rs) and Pearson’s (r; in Italics) Correlation Coefficients for Neuropsychological Subtest Raw Scores and IQ Estimated by MWT-B and Global Neuropsychological Composite Scores Correlated With Dimensional Community Assessment of Psychic Experiences (CAPE) Scales With Uncorrected (P) and False Discovery Rate Adjusted (Padj) Significance Levels
| Positive Dimension | Negative Dimension | Depressive Dimension | |||||
|---|---|---|---|---|---|---|---|
| Frequency | Distress | Frequency | Distress | Frequency | Distress | ||
| DSCT | r/r s | −.132 | −.113 | .027 | −.013 | −.207 | − .118 |
| P (Padj) | .156 (.478) | .194 (.478) | .418 (.478) | .461 (.493) | .055 (.374) | .182 (.478) | |
| Arithmetic | r s | −.399 | −.055 | −.193 | .060 | −.036 | −.058 |
| P (Padj) | .001 (.066) | .337 (.478) | .068 (.374) | .322 (.478) | .391 (.478) | .327 (.478) | |
| Matrix reasoning | r s | −.039 | .052 | .027 | −.007 | .023 | −.159 |
| P (Padj) | .383 (.478) | .344 (.478) | .420 (.478) | .480 (.493) | .430 (.481) | .110 (.433) | |
| Digit span | r/r s | −.063 | .034 | −.267 | .084 | −.031 | .048 |
| P (Padj) | .314 (.478) | .398 (.478) | .019 (.314) | .260 (.478) | .407 (.478) | .358 (.478) | |
| Information | r s | −.217 | .156 | −.211 | .228 | .042 | .008 |
| P (Padj) | .047 (.374) | .114 (.433) | .052 (.374) | .039 (.374) | .375 (.478) | .475 (.493) | |
| LNS | r s | −.001 | .124 | −.058 | .056 | .057 | −.100 |
| P (Padj) | .496 (.496) | .171 (.478) | .329 (.478) | .335 (.478) | .332 (.478) | .221 (.478) | |
| Animals | r/r s | −.183 | −.005 | −.154 | .070 | .030 | − .060 |
| P (Padj) | .079 (.374) | .486 (.493) | .118 (.433) | .295 (.478) | .409 (.478) | .323 (.478) | |
| Letters FAS | r/r s | −.139 | .037 | −.186 | −.007 | −.074 | − .090 |
| P (Padj) | .143 (.478) | .390 (.478) | .076 (.374) | .478 (.493) | .285 (.478) | .245 (.478) | |
| MWT-B | r s | −.279 | .057 | −.044 | .181 | .083 | −.050 |
| P (Padj) | .015 (.314) | .331 (.478) | .369 (.478) | .082 (.374) | .262 (.478) | .352 (.478) | |
| Global cognitive performance | r s | −.225 | .038 | −.178 | .136 | .010 | −.083 |
| P (Padj) | .041 (.374) | .385 (.478) | .085 (.374) | .148 (.478) | .469 (.493) | .261 (.478) | |
| IQ | r s | −.279 | .057 | −.044 | .181 | .083 | −.050 |
| P (Padj) | .015 (.314) | .331 (.478) | .369 (.478) | .082 (.374) | .262 (.478) | .352 (.478) |
Note: Significant (P < 0.05) correlations are bold. DSCT, Digit Symbol Coding Task; LNS, Letter-Number-Sequencing task; MWT-B, Mehrfachwahl-Wortschatz-Intelligenztest.
Mediation of ROI-Associated PLE via IQ and Cognition
Using the extracted mean predicted gyrification values of the 3 ROI identified in the primary analysis of gyrification (left precuneus, right STG, and the FWE-trend-level sig. left inferior prefrontal cluster), we conducted mediation analyses to predict PLE distress levels. A global neuropsychological performance measure was computed from z-transformed raw scores added together to obtain a single composite score per participant. In separate models, global cognitive measure and MWT-B IQ estimate were entered as mediators. Global cognitive performance significantly predicted MWT-B estimated IQ [F(1,61) = 31.16, P < .001, R2 = 0.34]. There was no significant mediating effect of either estimated IQ or global cognitive performance in the prediction of dimensional PLE distress in the subsample. This is seen in the inclusion of null values in 10 000 bootstrap-sampled confidence intervals of indirect effect coefficients in supplementary table S1.
Discussion
This study tested 2 hypotheses in healthy individuals with varying levels of PLE. First, we tested the effect of PLE on gyrification. Subsequently, we tested the individual explanatory contribution of cognitive performance in brain regions significantly associated with PLE. Both neural and cognitive variables were considered as endophenotypes with some shared genetic variance. In this study, we provide a first evidence of subtle neurodevelopmental variations in cortical areas linked to subclinical psychotic symptoms in nonclinical healthy subjects.
Unlike previous studies on PLE, which used volume-based imaging markers (VBM or cortical thickness), our gyrification approach relates PLE more specifically to the variation of a neurodevelopmental marker. Previous animal and human studies have shown cortical gyrification to result primarily from complex neurodevelopmental processes, beginning at week 16 of gestation and extending into early childhood.66 Between ages 2 and 6, the cortical folding organization reaches a peak29 terminating into a considerably stable marker after adolescence with little variation across the lifespan.67 A recent study of gyrification in manifest schizophrenia compared with healthy controls found convergence between regions of structural GM alterations and cortical thickness; however, threshold-significant gyrification results were more distinctive.37 In line with these findings, we report PLE gyrification effects in the prefrontal and temporal regions. Past studies focused on gyrification patterns in clinical psychotic disorders39,68 but investigations within the subclinical spectrum of psychotic symptoms found across general population cohorts are lacking. A few studies that focused on dimensional PLE were almost exclusively limited to VBM10 and cortical thickness,69 leading to a lack of findings to infer on neurodevelopmental alterations within the wider psychosis continuum. In the present sample, a subclinical positive psychosis phenotype correlated negatively with gyrification, a marker linked to perinatal and early neurodevelopment.
Higher PLE distress in the CAPE-pos dimension was associated with reduced gyrification in the inferior frontal gyrus of the prefrontal lobe at the subthreshold FWE-corrected significance level. Similar to psychotic samples, positive subclinical psychotic signs are associated with prefrontal cortical organization, underlining their relevance in a dimensional psychosis spectrum. Discriminating gyrification correlates between bipolar disorder I and schizophrenia showed some specificity of alterations in anterior medial prefrontal and orbitofrontal cortices for schizophrenia compared with controls. While hypergyrification in regions of affective processing was unique in bipolar disorder, regions associated with cognition were pronounced in both diagnostic phenotypes with some anatomical divergence.40 This raises the question of whether prefrontal correlates of positive psychotic symptoms are a widespread trend at both nonclinical and transdiagnostic levels. Regulatory changes of the dopaminergic and glutamatergic systems linked to typical neurocognitive symptoms of schizophrenia70,71 and specifically prefrontal variation72–74 perhaps also translate to prefrontal morphological and functional signatures in individuals with increased positive PLE. In contrast to positive prefrontal effects (gyrification increase) reported in schizophrenia,37 gyrification patterns in the left inferior frontal gyrus showed a trend for a negative association in our finding. This discrepancy may be reflective of the fluctuations in endophenotype effects across the psychosis spectrum spanning from minor subclinical symptoms in the general population, over increased symptom frequency in high-risk subjects, to those individuals developing schizophrenia spectrum disorders. In order to further support this interpretation, larger nonclinical and clinical samples would have to be combined to test linear vs. nonlinear relationships across such a spectrum.
Prefrontal structural variation within the psychosis spectrum,75 extending to psychosis proneness signified by PLE, is robust and associated with neurodevelopmental processes,76 such as synaptic pruning aberrances.77 A previous study reported impairments on selective domains such as verbal knowledge and working memory but not processing speed to be associated with PLE.7 While none of our results survived FDR-corrections, the trends might suggest heterogeneity dependent on scales, dimensions, and cognitive domain. Both estimated IQ and the global cognition scale comprising all individual tasks showed low to medium (uncorrected) negative correlations with the frequency of positive PLE. Together with previous findings of cognition mediating the genetic risk of schizophrenia, ie, cognitive dysfunction preceding schizophrenia-liability,27,78 this may suggest that increased cognitive performance achieves neuroprotective effects in the presence of PLE. This notion is supported by the positive effects of increased cognitive reserve in first-episode psychosis patients on global function and negative symptoms in a 2-year follow up.79 The positive association of general intelligence80 and working memory with regional cortical gyrification81 further corroborates the functional findings of a parieto-frontal-integration model underlying intelligence variation.82 Altered prefrontal development might impact on the functional integrity of such networks, thus leading to changes in cognitive function. This neural-behavioral framework established in nonclinical populations may be extended for cognitive reserve and compensatory capabilities in at-risk mental health states. Volumetric integrity of these network nodes, ie, frontal, temporal, and parietal regions, is also featured in UHR subjects resilient against the transition to psychosis over a 6-year period.83
We found evidence for the negative effects of depressive symptoms on gyrification in the right STG and supramarginal regions. Left-sided STG also showed GM increase associated with low-level depressive symptoms in another healthy sample.84 Convergence of decreased functional activity between psychotic disorders and major depressive disorder (MDD) highlights the critical role of the STG within the salience network in major psychiatric diagnoses. Another study also investigated cortical folding in MDD patients based on the whole-cortex mean curvature presented here.85 Within the patient group, clinical outcomes such as symptom severity were negatively associated with gyrification in parietal, occipitotemporal, and prefrontal cortices. However, the group comparison showed that MDD is associated with right STG hypergyrification pointing toward heightened vulnerability. Here, the endorsement of subclinical depressive states was associated with reduced gyrification of the right STG, which together with hypergyrification in diagnosed MDD proposes plastic alterations associated with the dopaminergic salience system and its role in cognitive interpretative processes ensuing over the course of illness.86,87 These and our observations in the depressive spectrum, as well as STG-associations in schizotypy and schizophrenia,88 may also indicate the absence of psychopathological and/or trait specificity, which in turn may be a result of symptom overlap.
There was a notable specificity for the PLE distress scale among the present results. The utility of CAPE as a screening tool for prodromal phenomena in clinical and non-specialized early treatment settings is particularly owed to its distinction of frequency and distress experienced due to PLE.89,90 High positive PLE levels, if perceived distressful, may, therefore, tap into higher psychopathological risk burden, supported by the covariance of positive and depressive symptoms.91 Another study differentiating risk variants demonstrated that the relationship between PLE and subjective distress experienced due to PLE is moderated by the levels of trait schizotypy.92 Dimensionality and psychosis specificity of the chosen scales may explain discrepancies in the directionality of precuneus GM volume in schizotypy,10,93 which is further factored into positive and negative psychosis-prone traits with differential cognitive outcomes.94 Moving along the spectrum, negative clinical outcomes such as imminent transition to psychosis are accompanied by intensified tissue loss and cortical thinning,95–97 notably in the precuneus, parietal, and temporal regions.98 In agreement with notions of dynamic neurobiology,99 our findings map long-term cortical effects associated with subjective negatively perceived PLE, which are not attributed to disease progression but instead to vulnerability. Contrary to significant GM volume findings,10 the absence of cortical gyrification alterations for the CAPE-neg dimension suggests that the surrogate neurodevelopmental surface parameter is not sensitive to the effects of clinical avolition, affective flattening, and social anhedonia reflecting transdiagnostic features of psychosis spectrum disorders.
While we did not find a mediating effect of either estimated IQ or global neuropsychological performance, they showed low to medium (uncorrected) correlations with the frequency of positive PLE. We confined our analysis to ROI correlated with PLE distress levels but propose that PLE frequency may be of greater importance in these models. This conservative approach, together with variation in sample sizes, may cause underestimation of true mediator effects, constituting one of the main limitations of the present study. Siddi et al8 showed that differences in neuropsychological performance between high and low schizotypy individuals were predominantly small (attention, visuospatial working memory, learning, short-term visual, and long-term memory) to medium (verbal working memory) sized. A post hoc sensitivity analysis with an 85% power criterion showed that the size of the subsample included in the present mediation analysis was only sufficient to detect medium effects (ƒ2 = 0.18) of additional cognitive predictors. This might also explain our lack of significant findings (after multiple comparison correction) for CAPE-cognition associations. Future efforts should, therefore, achieve wider PLE variance and compare metrics of local GI with mean curvature. Additionally, weaknesses regarding the present IQ estimate needs to be pointed out. IQ estimation based on the MWT-B and educational level are not independent,58 and global estimates from comprehensive neuropsychological batteries could increase robustness. Also, other cognitive tests such as those tapping into visuospatial and motor skills, which were not included in our test battery despite showing heritability in schizophrenia,100 might be useful for future studies. The cross-sectional design allows for inferences about the natural state of cortical gyrification at an average age of about 30 years when the influence of time-invariant subclinical psychotic traits reaches a peak,101 but not across the lifespan.67 Using the gyrification metric as a proxy measure of early genetic influence on cytoarchitecture, its stability may be tested in PLE combined with cumulative risk burden and clinical trait-state markers. Longitudinal designs with increased PLE variability are required to address such time variants and effects of PLE heterogeneity. Despite limited understanding of cytoarchitectural mechanisms involved in gyrification in the psychosis spectrum, this study together with previous neuroimaging research suggests that differences in PLE dimensionality correspond to distinctive genetically determined neurobiological characteristics.
Besides previously mentioned limitations, our explicit investigation of the subclinical spectrum warrants some considerations. To our knowledge, few studies including different patient groups across the psychosis spectrum, eg, schizotypal personality disorder,41 exist, which calls for further research in the subclinical range. Here, we operationalized CAPE symptom dimensions derived by a 3-factor solution. However, further partitioning of subscales resulting in more PLE phenotypes in exchange for lower indices of construct validity102 offers an alternative research avenue to these dimensions. Future studies might also consider white matter changes related to PLE, which have been linked to symptom dimensions in schizophrenia.103,104
In conclusion, we report evidence for a relationship between variation in cortical gyrification and subclinical psychosis phenotypes in the nonclinical spectrum. If cognition influences psychotic pathogenesis, interactions between cognitive and neurobiological endophenotype levels may be associated with attenuated PLE on the clinical spectrum end.
Supplementary Material
Acknowledgment
All authors declare no conflict of interest.
Funding
This study was supported by a research grant of the University Medical Center Giessen and Marburg (UKGM) (grant 11/2017 MR to I.N. and grant 05/2018 MR to Sarah Grezellschak and I.N.) and a Junior Scientist Grant of the Friedrich-Schiller-University of Jena (DRM, 21007087, to I.N.).
References
- 1. Sánchez-Gutiérrez T, Rodríguez-Toscano E, Llorente C, et al. Neuropsychological, clinical and environmental predictors of severe mental disorders in offspring of patients with schizophrenia [published online ahead of print July 16, 2019]. Eur Arch Psychiatry Clin Neurosci. doi:10.1007/s00406-019-01044-7 [DOI] [PubMed] [Google Scholar]
- 2. Torgalsbøen AK, Mohn C, Rishovd Rund B. Neurocognitive predictors of remission of symptoms and social and role functioning in the early course of first-episode schizophrenia. Psychiatry Res. 2014;216(1):1–5. [DOI] [PubMed] [Google Scholar]
- 3. Kurtz MM. Neurocognition as a predictor of response to evidence-based psychosocial interventions in schizophrenia: what is the state of the evidence? Clin Psychol Rev. 2011;31(4):663–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gur RE, Nimgaonkar VL, Almasy L, et al. Neurocognitive endophenotypes in a multiplex multigenerational family study of schizophrenia. Am J Psychiatry. 2007;164(5):813–819. [DOI] [PubMed] [Google Scholar]
- 5. Mesholam-Gately RI, Giuliano AJ, Goff KP, Faraone SV, Seidman LJ. Neurocognition in first-episode schizophrenia: a meta-analytic review. Neuropsychology 2009;23(3):315–336. [DOI] [PubMed] [Google Scholar]
- 6. Üçok A, Direk N, Koyuncu A, et al. Cognitive deficits in clinical and familial high risk groups for psychosis are common as in first episode schizophrenia. Schizophr Res. 2013;151(1-3):265–269. [DOI] [PubMed] [Google Scholar]
- 7. Mollon J, David AS, Morgan C, et al. Psychotic experiences and neuropsychological functioning in a population-based sample. JAMA Psychiatry. 2016;73(2):129–138. [DOI] [PubMed] [Google Scholar]
- 8. Siddi S, Petretto DR, Preti A. Neuropsychological correlates of schizotypy: a systematic review and meta-analysis of cross-sectional studies. Cogn Neuropsychiatry. 2017;22(3):186–212. [DOI] [PubMed] [Google Scholar]
- 9. Grau A, Ruiz de Azúa S, Díez Á, et al. Relationship between subclinical psychotic symptoms and cognitive performance in the general population (English Ed). Rev Psiquiatr y Salud Ment. 2016;9(2):78–86. [DOI] [PubMed] [Google Scholar]
- 10. Nenadic I, Lorenz C, Langbein K, et al. Brain structural correlates of schizotypy and psychosis proneness in a non-clinical healthy volunteer sample. Schizophr Res. 2015;168(1-2):37–43. [DOI] [PubMed] [Google Scholar]
- 11. Goldstein KE, Koenigsberg HW, Rimsky LS, et al. White matter abnormalities in schizophrenia and schizotypal personality disorder. Schizophr Bull. 2014;41(1):300–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wolter S, Goya-Maldonado R, Richter A, Gruber O, Chahine G. Disruptions in the left frontoparietal network underlie resting state endophenotypic markers in schizophrenia. Hum Brain Mapp. 2016;38(4):1741–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wojtalik JA, Smith MJ, Keshavan MS, Eack SM. A systematic and meta-analytic review of neural correlates of functional outcome in schizophrenia. Schizophr Bull. 2017;43(6):1329–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chang M, Womer FY, Bai C, et al. Voxel-based morphometry in individuals at genetic high risk for schizophrenia and patients with schizophrenia during their first episode of psychosis. PLoS One. 2016;11(10):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ettinger U, Schmechtig A, Toulopoulou T, et al. Prefrontal and striatal volumes in monozygotic twins concordant and discordant for schizophrenia. Schizophr Bull. 2012;38(1):192–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ettinger U, Williams SC, Meisenzahl EM, Möller HJ, Kumari V, Koutsouleris N. Association between brain structure and psychometric schizotypy in healthy individuals. World J Biol Psychiatry. 2012;13(7):544–549. [DOI] [PubMed] [Google Scholar]
- 17. Falkenberg I, Valli I, Raffin M, et al. Pattern of activation during delayed matching to sample task predicts functional outcome in people at ultra high risk for psychosis. Schizophr Res. 2017;181:86–93. [DOI] [PubMed] [Google Scholar]
- 18. Waltmann M, O’Daly O, Egerton A, et al. Multi-echo fMRI, resting-state connectivity, and high psychometric schizotypy. Neuroimage Clin. 2019;21:101603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang Y, Yan C, Yin DZ, et al. Neurobiological changes of schizotypy: Evidence from both volume-based morphometric analysis and resting-state functional connectivity. Schizophr Bull. 2015;41(Suppl 2):S444–S454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhu Y, Tang Y, Zhang T, et al. Reduced functional connectivity between bilateral precuneus and contralateral parahippocampus in schizotypal personality disorder. BMC Psychiatry. 2017;17(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Modinos G, Mechelli A, Ormel J, Groenewold NA, Aleman A, McGuire PK. Schizotypy and brain structure: a voxel-based morphometry study. Psychol Med. 2010;40(9): 1423–1431. [DOI] [PubMed] [Google Scholar]
- 22. Owens SF, Picchioni MM, Ettinger U, et al. Prefrontal deviations in function but not volume are putative endophenotypes for schizophrenia. Brain 2012;135(7):2231–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fonville L, Drakesmith M, Zammit S, Lewis G, Jones DK, David AS. MRI Indices of Cortical Development in Young People With Psychotic Experiences: Influence of Genetic Risk and Persistence of Symptoms. Schizophr Bull. 2019;45(1):169–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lencz T, Knowles E, Davies G, et al. Molecular genetic evidence for overlap between general cognitive ability and risk for schizophrenia: a report from the Cognitive Genomics consorTium (COGENT). Mol Psychiatry. 2014;19(2):168–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Goldberg X, Alemany S, Rosa A, et al. Substantial genetic link between IQ and working memory: implications for molecular genetic studies on schizophrenia. The European Twin Study of Schizophrenia (EUTwinsS). Am J Med Genet B Neuropsychiatr Genet. 2013;162(4):413–418. [DOI] [PubMed] [Google Scholar]
- 26. Hauser M, Zhang JP, Sheridan EM, et al. Neuropsychological test performance to enhance identification of subjects at clinical high risk for psychosis and to be most promising for predictive algorithms for conversion to psychosis: a meta-analysis. J Clin Psychiatry. 2017;78(1):e28–e40. [DOI] [PubMed] [Google Scholar]
- 27. Toulopoulou T, Zhang X, Cherny S, et al. Polygenic risk score increases schizophrenia liability through cognition-relevant pathways. Brain 2019;142(2):471–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kirchner SK, Ozkan S, Musil R, et al. Polygenic analysis suggests the involvement of calcium signaling in executive function in schizophrenia patients. Eur Arch Psychiatry Clin Neurosci. 2020;270(4):425–431. [DOI] [PubMed] [Google Scholar]
- 29. Li G, Wang L, Shi F, et al. Mapping longitudinal development of local cortical gyrification in infants from birth to 2 years of age. J Neurosci. 2014;34(12):4228–4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Luders E, Thompson PM, Narr KL, Toga AW, Jancke L, Gaser C. A curvature-based approach to estimate local gyrification on the cortical surface. Neuroimage. 2006;29(4):1224–1230. [DOI] [PubMed] [Google Scholar]
- 31. Schaer M, Cuadra MB, Schmansky N, Fischl B, Thiran J-P, Eliez S. How to measure cortical folding from MR images: a step-by-step tutorial to compute local gyrification index. J Vis Exp. 2012;(59):e3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu B, Tao Y, Yu C, et al. Polygenic risk for schizophrenia influences cortical gyrification in 2 independent general populations. Schizophr Bull. 2016;43(3):673–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cannon TD, Chung Y, He G, et al. Progressive reduction in cortical thickness as psychosis develops: a multisite longitudinal neuroimaging study of youth at elevated clinical risk. Biol Psychiatry. 2015;77(2):147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jacomb I, Stanton C, Vasudevan R, et al. C-reactive protein: higher during acute psychotic episodes and related to cortical thickness in schizophrenia and healthy controls. Front Immunol. 2018;9:2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sugihara G, Oishi N, Son S, Kubota M, Takahashi H, Murai T. Distinct patterns of cerebral cortical thinning in schizophrenia: a neuroimaging data-driven approach. Schizophr Bull. 2017;43(4):900–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Besteher B, Gaser C, Spalthoff R, Nenadić I. Associations between urban upbringing and cortical thickness and gyrification. J Psychiatr Res. 2017;95:114–120. [DOI] [PubMed] [Google Scholar]
- 37. Spalthoff R, Gaser C, Nenadić I. Altered gyrification in schizophrenia and its relation to other morphometric markers. Schizophr Res. 2018;202:195–202. [DOI] [PubMed] [Google Scholar]
- 38. Linscott RJ, van Os J. An updated and conservative systematic review and meta-analysis of epidemiological evidence on psychotic experiences in children and adults: on the pathway from proneness to persistence to dimensional expression across mental disorders. Psychol Med. 2013;43(6):1133–1149. [DOI] [PubMed] [Google Scholar]
- 39. Palaniyappan L, Mallikarjun P, Joseph V, White TP, Liddle PF. Folding of the prefrontal cortex in schizophrenia: regional differences in gyrification. Biol Psychiatry. 2011;69(10):974–979. [DOI] [PubMed] [Google Scholar]
- 40. Nenadic I, Maitra R, Dietzek M, et al. Prefrontal gyrification in psychotic bipolar I disorder vs. schizophrenia. J Affect Disord. 2015;185:104–107. [DOI] [PubMed] [Google Scholar]
- 41. Matsuda Y, Ohi K. Cortical gyrification in schizophrenia: current perspectives. Neuropsychiatr Dis Treat. 2018;14:1861–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cachia A, Paillère-Martinot ML, Galinowski A, et al. Cortical folding abnormalities in schizophrenia patients with resistant auditory hallucinations. Neuroimage 2008;39(3):927–935. [DOI] [PubMed] [Google Scholar]
- 43. Garrison JR, Fernyhough C, McCarthy-Jones S, Simons JS, Sommer IEC. Paracingulate sulcus morphology and hallucinations in clinical and nonclinical groups. Schizophr Bull. 2019;45(4):733–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Stefanis NC, Hanssen M, Smirnis NK, et al. Evidence that three dimensions of psychosis have a distribution in the general population. Psychol Med. 2002;32(2):347–358. [DOI] [PubMed] [Google Scholar]
- 45. Mark W, Toulopoulou T. Psychometric properties of “community assessment of psychic experiences”: review and meta-analyses. Schizophr Bull. 2016;42(1):34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Meller T., Schmitt S., Ettinger U., et al. Brain structural correlates of schizotypal signs and subclinical schizophrenia nuclear symptoms in healthy individuals [published online ahead of print June 24, 2020]. Psychol. Med. 1–10. doi: 10.1017/S0033291720002044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bolt LK, Amminger GP, Farhall J, et al. Neurocognition as a predictor of transition to psychotic disorder and functional outcomes in ultra-high risk participants: findings from the NEURAPRO randomized clinical trial. Schizophr Res. 2019;206:67–74. [DOI] [PubMed] [Google Scholar]
- 48. Liu Y, Wang G, Jin H, et al. Cognitive deficits in subjects at risk for psychosis, first-episode and chronic schizophrenia patients. Psychiatry Res. 2019;274:235–242. [DOI] [PubMed] [Google Scholar]
- 49. Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 1971;9(1):97–113. [DOI] [PubMed] [Google Scholar]
- 50. Mossaheb N, Becker J, Schaefer MR, et al. The community assessment of psychic experience (CAPE) questionnaire as a screening-instrument in the detection of individuals at ultra-high risk for psychosis. Schizophr Res. 2012;141(2-3):210–214. [DOI] [PubMed] [Google Scholar]
- 51. Vermeiden M, Janssens M, Thewissen V, et al. Cultural differences in positive psychotic experiences assessed with the Community Assessment of Psychic Experiences-42 (CAPE-42): a comparison of student populations in the Netherlands, Nigeria and Norway. BMC Psychiatry. 2019;19(1):244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Konings M, Bak M, Hanssen M, van Os J, Krabbendam L. Validity and reliability of the CAPE: a self-report instrument for the measurement of psychotic experiences in the general population. Acta Psychiatr Scand. 2006; 114(1):55–61. [DOI] [PubMed] [Google Scholar]
- 53. Schloegelhofer M, Klier CM, Becker J, et al. The Community Assessment of Psychic Experience (CAPE) questionnaire as a screening-instrument in the detection of individuals at ultra-high risk for psychosis. Schizophr Res. 2012;141(2–3):210–214. [DOI] [PubMed] [Google Scholar]
- 54. von Aster M, Neubauer A, Horn R. Wechsler Intelligenztest Für Erwachsene (WIE). Deutschsprachige Bearbeitung Und Adaptation Des WAlS-III von David Wechsler. Frankfurt Main, Germany: Harcourt Test Services; 2006. [Google Scholar]
- 55. Wechsler D, Coalson D, Raiford S. Wechsler Adult Intelligence Scale. 4th ed. San Antonio, TX: The Psychological Corporation; 2008. [Google Scholar]
- 56. Benton A, Hamsher K, Sivan A. Multilingual Aphasia Examination. Iowa City, IA: AJA Associates; 1994. [Google Scholar]
- 57. Lehrl S. Mehrfachwahl-Wortschatz-Intelligenztest MWT-B. Balingen: Spitta Verlag; 2005. [Google Scholar]
- 58. Satzger W, Fessmann H, Engel RR. Liefern HAWIE-R , WST und MWT-B vergleichbare IQ-Werte? Zeitschrift für Differentielle und Diagnostische Psychologie. 2002;23(2):159–170. [Google Scholar]
- 59. Dickinson D, Goldberg TE, Gold JM, Elvevåg B, Weinberger DR. Cognitive factor structure and invariance in people with schizophrenia, their unaffected siblings, and controls. Schizophr Bull. 2011;37(6):1157–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Fusar-Poli P, Deste G, Smieskova R, et al. Cognitive functioning in prodromal psychosis: a meta-analysis. Arch Gen Psychiatry. 2012;69(6):562–571. [DOI] [PubMed] [Google Scholar]
- 61. Nichols T, Hayasaka S. Controlling the familywise error rate in functional neuroimaging: a comparative review. Stat Methods Med Res. 2003;12(5):419–446. [DOI] [PubMed] [Google Scholar]
- 62. Hayes AF. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. 2nd ed. New York, NY: Guilford Press; 2018. [Google Scholar]
- 63. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B. 1995;57(1):289–300. [Google Scholar]
- 64.R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2020. https://www.R-project.org/ [Google Scholar]
- 65. Desikan RS, Ségonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006;31(3):968–980. [DOI] [PubMed] [Google Scholar]
- 66. Zilles K, Palomero-Gallagher N, Amunts K. Development of cortical folding during evolution and ontogeny. Trends Neurosci. 2013;36(5):275–284. [DOI] [PubMed] [Google Scholar]
- 67. Cao B, Mwangi B, Passos IC, et al. Lifespan gyrification trajectories of human brain in healthy individuals and patients with major psychiatric disorders. Sci Rep. 2017;7(1):511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Palaniyappan L, Park B, Balain V, Dangi R, Liddle P. Abnormalities in structural covariance of cortical gyrification in schizophrenia. Brain Struct Funct. 2015;220(4):2059–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Córdova-Palomera A, Alemany S, Falcón C, et al. Cortical thickness correlates of psychotic experiences: examining the effect of season of birth using a genetically informative design. J Psychiatr Res. 2014;56:144–149. [DOI] [PubMed] [Google Scholar]
- 70. Simpson E, Kellendonk C, Kandel ER. Cognitive symptoms of schizophrenia. Neuron 2010;65(5):585–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kambeitz J, Abi-Dargham A, Kapur S, Howes OD. Alterations in cortical and extrastriatal subcortical dopamine function in schizophrenia: systematic review and meta-analysis of imaging studies. Br J Psychiatry. 2014;204(6):420–429. [DOI] [PubMed] [Google Scholar]
- 72. Barnett JH, Xu K, Heron J, Goldman D, Jones PB. Cognitive effects of genetic variation in monoamine neurotransmitter systems: a population-based study of COMT, MAOA, and 5HTTLPR. Am J Med Genet B Neuropsychiatr Genet. 2011;156(2):158–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Bosia M, Pigoni A, Pirovano A, et al. COMT and STH polymorphisms interaction on cognition in schizophrenia. Neurol Sci. 2015;36(2):215–220. [DOI] [PubMed] [Google Scholar]
- 74. Thomas EHX, Bozaoglu K, Rossell SL, Gurvich C. The influence of the glutamatergic system on cognition in schizophrenia: a systematic review. Neurosci Biobehav Rev. 2017;77:369–387. [DOI] [PubMed] [Google Scholar]
- 75. Jirsaraie RJ, Sheffield JM, Barch DM. Neural correlates of global and specific cognitive deficits in schizophrenia. Schizophr Res. 2018;201:237–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Bora E, Murray RM. Meta-analysis of cognitive deficits in ultra-high risk to psychosis and first-episode psychosis: do the cognitive deficits progress over, or after, the onset of psychosis? Schizophr Bull. 2014;40(4):744–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Selemon LD, Zecevic N. Schizophrenia: a tale of two critical periods for prefrontal cortical development. Transl Psychiatry. 2015;5:e623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Toulopoulou T, Van Haren N, Zhang X, et al. Reciprocal causation models of cognitive vs volumetric cerebral intermediate phenotypes for schizophrenia in a pan-European twin cohort. Mol Psychiatry. 2015;20(11):1386–1396. [DOI] [PubMed] [Google Scholar]
- 79. Amoretti S, Bernardo M, Bonnin CM, et al. The impact of cognitive reserve in the outcome of first-episode psychoses: 2-year follow-up study. Eur Neuropsychopharmacol. 2016;26(10):1638–1648. [DOI] [PubMed] [Google Scholar]
- 80. Gregory MD, Kippenhan JS, Dickinson D, et al. Regional variations in brain gyrification are associated with general cognitive ability in humans. Curr Biol. 2016;26(10):1301–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Green S, Blackmon K, Thesen T, et al. Parieto-frontal gyrification and working memory in healthy adults. Brain Imaging Behav. 2018;12(2):303–308. [DOI] [PubMed] [Google Scholar]
- 82. Vakhtin AA, Ryman SG, Flores RA, Jung RE. Functional brain networks contributing to the parieto-frontal integration theory of intelligence. Neuroimage 2014;103:349–354. [DOI] [PubMed] [Google Scholar]
- 83. de Wit S, Wierenga LM, Oranje B, et al. Brain development in adolescents at ultra-high risk for psychosis: longitudinal changes related to resilience. Neuroimage Clin. 2016;12:542–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Besteher B, Gaser C, Langbein K, Dietzek M, Sauer H, Nenadić I. Effects of subclinical depression, anxiety and somatization on brain structure in healthy subjects. J Affect Disord. 2017;215:111–117. [DOI] [PubMed] [Google Scholar]
- 85. Schmitgen MM, Depping MS, Bach C, et al. Aberrant cortical neurodevelopment in major depressive disorder. J Affect Disord. 2019;243:340–347. [DOI] [PubMed] [Google Scholar]
- 86. Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry. 2003;160(1):13–23. [DOI] [PubMed] [Google Scholar]
- 87. Müller VI, Cieslik EC, Kellermann TS, Eickhoff SB. Crossmodal emotional integration in major depression. Soc Cogn Affect Neurosci. 2014;9(6):839–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Takahashi T, Suzuki M, Zhou S, Tanino R, Nakamura K. A follow-up MRI study of the superior temporal subregions in schizotypal disorder and first-episode schizophrenia. Schizophr Res. 2010;119(1–3):65–74. [DOI] [PubMed] [Google Scholar]
- 89. Sisti D, Rocchi MB, Siddi S, et al. Preoccupation and distress are relevant dimensions in delusional beliefs. Compr Psychiatry. 2012;53(7):1039–1043. [DOI] [PubMed] [Google Scholar]
- 90. Yung AR, Buckby JA, Cotton SM, et al. Psychotic-like experiences in nonpsychotic help-seekers: associations with distress, depression, and disability. Schizophr Bull. 2006;32(2):352–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Brañas A, Barrigón ML, Lahera G, Canal-Rivero M, Ruiz-Veguilla M. Influence of depressive symptoms on distress related to positive psychotic-like experiences in women. Psychiatry Res. 2017;258:469–475. [DOI] [PubMed] [Google Scholar]
- 92. Kline E, Wilson C, Ereshefsky S, et al. Schizotypy, psychotic-like experiences and distress: an interaction model. Psychiatry Res. 2012;200(2-3):647–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Modinos G, Egerton A, McLaughlin A, et al. Neuroanatomical changes in people with high schizotypy : relationship to glutamate levels. Psychol Med. 2019;48(11):1880–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Sahakyan L, Kwapil TR. Positive schizotypy and negative schizotypy are associated with differential patterns of episodic memory impairment. Schizophr Res Cogn. 2016;5:35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Dietsche B, Kircher T, Falkenberg I. Structural brain changes in schizophrenia at different stages of the illness: a selective review of longitudinal magnetic resonance imaging studies. Aust N Z J Psychiatry. 2017;51(5):500–508. [DOI] [PubMed] [Google Scholar]
- 96. Ziermans TB, Schothorst PF, Schnack HG, et al. Progressive structural brain changes during development of psychosis. Schizophr Bull. 2012;38(3):519–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Cannon TD. Brain biomarkers of vulnerability and progression to psychosis. Schizophr Bull. 2016;42Suppl 1:S127–S132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Borgwardt SJ, McGuire PK, Aston J, et al. Structural brain abnormalities in individuals with an at-risk mental state who later develop psychosis. Br J Psychiatry Suppl. 2007;51:s69–s75. [DOI] [PubMed] [Google Scholar]
- 99. Palaniyappan L, Das T, Dempster K. The neurobiology of transition to psychosis: clearing the cache. J Psychiatry Neurosci. 2017;42(5):294–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Blokland GAM, Mesholam-Gately RI, Toulopoulou T, et al. ; GENUS Consortium Heritability of neuropsychological measures in schizophrenia and nonpsychiatric populations: a systematic review and meta-analysis. Schizophr Bull. 2017;43(4):788–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Rössler W, Hengartner MP, Ajdacic-Gross V, Haker H, Angst J. Deconstructing sub-clinical psychosis into latent-state and trait variables over a 30-year time span. Schizophr Res. 2013;150(1):197–204. [DOI] [PubMed] [Google Scholar]
- 102. Schlier B, Jaya ES, Moritz S, Lincoln TM. The community assessment of psychic experiences measures nine clusters of psychosis-like experiences: a validation of the German version of the CAPE. Schizophr Res. 2015;169(1-3): 274–279. [DOI] [PubMed] [Google Scholar]
- 103. Ohtani T, Bouix S, Hosokawa T, et al. Abnormalities in white matter connections between orbitofrontal cortex and anterior cingulate cortex and their associations with negative symptoms in schizophrenia: a DTI study. Schizophr Res. 2014;157(1-3):190–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Makris N, Seidman LJ, Ahern T, et al. White matter volume abnormalities and associations with symptomatology in schizophrenia. Psychiatry Res Neuroimaging. 2010;183(1):21–29. doi: 10.1016/j.pscychresns.2010.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.