Abstract
In recent years, there has been a growing appreciation by regulatory authorities that cannabis‐based medicines can play a useful role in disease therapy. Although often conflagrated by proponents of recreational use, the legislative rescheduling of cannabis‐derived compounds, such as cannabidiol (CBD), has been associated with the steady increase in the pursuit of use of medicinal cannabis. One key driver in this interest has been the scientific demonstration of efficacy and safety of CBD in randomised, placebo‐controlled clinical trials in children and young adults with difficult‐to‐treat epilepsies, which has encouraged increasing numbers of human trials of CBD for other indications and in other populations. The introduction of CBD as the medicine Epidiolex in the United States (in 2018) and as Epidyolex in the European Union (in 2019) as the first cannabis‐derived therapeutic for the treatment of seizures was underpinned by preclinical research performed at the University of Reading. This work was awarded the British Pharmacological Society Sir James Black Award for Contributions to Drug Discovery 2019 and is discussed in the following review article.
Keywords: cannabidiol, Dravet syndrome, epilepsy
Abbreviations
- AED
anti‐epileptic drug
- CBD
cannabidiol
- CBDV
cannabidivarin
- CBG
cannabigerol
- CBPM
cannabis‐based products for medicinal use
- DS
Dravet syndrome
- FDA
Food and Drug Administration
- GPCR
G protein‐coupled receptor
- GPR18
GPCR 18
- GPR55
GPCR 55
- LGS
Lennox–Gastaut syndrome
- MES
maximal electroshock seizure
- PTZ
pentylenetetrazole
- RISE‐SRS
reduced intensity status epilepticus‐spontaneous recurrent seizures
- THC
tetrahydrocannabinol
- THCV
tetrahydrocannabivarin
- TRPA1
transient receptor potential ankyrin 1
- TRPV1
transient receptor potential vanilloid 1
- TRPV2
transient receptor potential vanilloid 2
- TSC
Tuberous Sclerosis Complex
- VDAC1
voltage‐dependent anion‐selective channel protein 1
- VGSC
voltage‐gated sodium channel
1. INITIAL INTEREST IN CANNABIS COMPONENTS FOR TREATMENT OF EPILEPSIES
General interest in the potential therapeutic utility of non‐tetrahydrocannabinol (THC) cannabinoids was pioneered by researchers such as Raphael Mechoulam at the Hebrew University of Jerusalem in Israel and Roger Pertwee, initially at Oxford University and then at Aberdeen University. In 2007, Gary Stephens and Ben Whalley had established an electrophysiology group at the University of Reading within the then new School of Pharmacy; they joined with Claire Williams from the School of Psychology and Clinical Language Sciences to investigate the role of plant‐derived cannabinoids in different acute seizure models. Prior to joining the University of Reading, Ben Whalley, working with Andrew Constanti and Elizabeth Williamson at the London School of Pharmacy, had shown that standardised cannabis extracts lacking THC could inhibit muscarinic receptor agonist‐induced epileptiform‐like activity in ex vivo rat piriform cortical brain slices (Wilkinson et al., 2003). In parallel, Gary Stephens had an interest in the coupling of G protein‐coupled receptors (GPCRs), such as cannabinoid CB1 receptors, to downstream signalling pathways including voltage‐gated calcium channels (Stephens, Canti, Page, & Dolphin, 1998; Stephens & Mochida, 2005; reviewed in Stephens, 2009). Claire Williams had complementary expertise in animal models of disease and, working with Tim Kirkham at Reading, had reported extensively on the effects of plant‐derived phytocannabinoids, endocannabinoids, and exogenous cannabinoids in appetitive behavioural models. This work had demonstrated the role of CB1 receptors in hyperphagia and highlighted the potential to target such receptors in obesity disorders (Williams & Kirkham, 1999; Williams, Rogers, & Kirkham, 1998; reviewed in Kirkham & Williams, 2004).
The common research interest of the team at Reading centred around epilepsy, a range of syndromes characterised by hyperexcitable neural networks in the brain, which clinically manifest as seizures and are associated with a clear unmet clinical need. Worldwide, there are around 65 M people with epilepsy, making it the most prevalent global neurological condition; however, around one third of patients with epilepsy do not respond to current anti‐epileptic drugs (AEDs) and therefore live with uncontrolled seizures (Janmohamed, Brodie, & Kwan, 2019). Despite anecdotal reports of cannabis having therapeutic benefit in epilepsy, there had been little rigorous scientific research between 1970 and 2010 to support any clinical use, in particular with regard to preclinical efficacy, or safety and toxicity data. Our focus, cannabidiol (CBD), was isolated in 1963 (Mechoulam & Shvo, 1963) and first investigated functionally in the early 1970s (Paton & Pertwee, 1972). Amongst a few small‐scale case studies, of particular interest was a study in Brazil suggesting that plant‐derived CBD had potential utility in seizures (Cunha et al., 1980). By contrast, some reports had implicated THC as a pro‐convulsant agent (as discussed in Rosenberg, Tsien, Whalley, & Devinsky, 2015). These findings were further set against the desire to find agents that did not promote the euphoria associated with recreational THC use.
The interest in use of non‐psychoactive cannabinoids was supported by the introduction of Sativex (nabiximols, a 50:50 mixture of the two principal components of Cannabis sativa, THC and CBD) to relieve spasticity and pain associated with multiple sclerosis, representing the first cannabis‐based medicine to be licensed in the United Kingdom. Thus, Stephens, Whalley, and Williams began collaborating with the intention of testing isolated cannabinoid extracts in seizure and epilepsy models. The makers of Sativex, GW Pharmaceuticals, initially partnering with Otsuka Pharmaceuticals, Japan, provided funding in 2007 to conduct preclinical research to assess the therapeutic potential of isolated phytocannabinoids in in vivo models of chemically induced seizures and associated in vitro studies to investigate modes and mechanisms of action. Individual phytocannabinoids, including CBD, were investigated as botanical drug substances.
Initial work using the Mg2+‐free and the 4‐aminopyridine models of epileptiform activity in ex vivo rat hippocampal brain slices demonstrated clear CBD effects on local field potential burst amplitude, duration, and frequency in different cornus ammonis and dentate gyrus regions (Jones et al., 2010). Complementary work investigated the effects of CBD, applied via the intraperitoneal route, in a range of rat models of acute seizure induced by chemicals including pentylenetetrazole (PTZ) (to model generalised seizures) (Jones et al., 2010), pilocarpine (temporal lobe seizures), and penicillin (partial seizures) (Jones et al., 2012). Our initial in vivo work identified CBD as a major phytocannabinoid with the greatest anticonvulsant potential across all of the acute seizure models tested (Figure 1). It was similarly shown that intraperitoneal CBD was anticonvulsant in rat and mouse maximal electroshock seizure (MES) models, and in 6‐Hz psychomotor and corneal kindling models, and that intravenous CBD was also effective in reduced seizure severity in the pilocarpine model (Patra et al., 2019; Figure 1). The latter study also showed that chronic administration of CBD oral solutions attenuated seizure burden and motor co‐morbidities in the rat reduced intensity status epilepticus‐spontaneous recurrent seizures (RISE‐SRS) model of temporal lobe epilepsy (TLE) (Patra et al., 2019), the RISE‐SRS model being developed between researchers at University of Reading and Gavin Woodhall's group at Aston University (Modebadze et al., 2016). From a pharmacological viewpoint, CBD was shown to possess only low, non‐relevant binding affinity at CB1 receptors (Jones et al., 2010); this work was in line with studies by other groups (as reviewed by Pertwee, 2008). These data also supported earlier work in the MES model in mice by Lisa Wallace and co‐workers, who demonstrated that CBD had an anticonvulsant action, independent of effects on CB1 receptors (Wallace, Wiley, Martin, & DeLorenzo, 2001). Following the investigation of CBD in human genetic paediatric epilepsies (described more fully below), we extended our studies to the effects of CBD in corresponding mouse models. In particular, we investigated models of Dravet syndrome (DS), a genetic disease due to a voltage‐gated sodium channel (VGSC) SCN1A gene mutation. It was shown that chronic CBD (100 mg·kg−1 injected subcutaneously twice daily) treatment was able to increase survival in Scn1a −/− mice and prevent premature mortality and improve associated co‐morbidities in Scn1a +/− mice (Patra et al., 2020). Such preclinical work provided a strong association with the clinical data that had emerged following our initial work.
FIGURE 1.
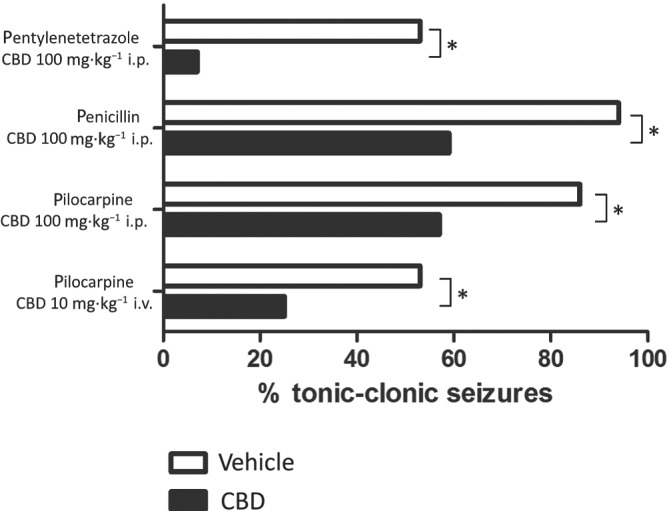
CBD reduces seizure severity in different rat models of chemically induced acute seizure. Intraperitoneal CBD (100 mg·kg−1) caused a significant reduction in seizure severity induced by pentylenetetrazole (80 mg·kg−1 i.p., n = 15; Jones et al., 2010), penicillin 525 IU intracerebroventricular infusion (n = 17–18; Jones et al., 2012), or pilocarpine (380 mg·kg−1 i.p., n = 15; Jones et al., 2010). Intravenous CBD (10 mg·kg−1) caused a significant reduction in seizure severity induced by pilocarpine (380 mg·kg−1 i.v., n = 12–15; Patra et al., 2019). *P < 0.05, nonparametric binomial test
We have also investigated the effects of other phytocannabinoids. These compounds included the THC homologue tetrahydrocannabivarin (THCV), cannabigerol (CBG), and the CBD homologue cannabidivarin (CBDV). We initially showed that THCV had actions analogous to CB1 receptor antagonists on inhibitory neurotransmission at interneuron‐Purkinje cell synapses (Ma, Weston, Whalley, & Stephens, 2008). THCV was also shown to act as a CB1 receptor antagonist in ligand binding and GTPγS binding assays (Dennis, Whalley, & Stephens, 2008; Hill et al., 2010). Interestingly, THCV reduced epileptiform activity induced by Mg2+‐free media in adult rat piriform cortical brain slices and demonstrated anticonvulsant effects against PTZ‐induced generalised seizures in adult rats (Hill et al., 2010). The potential clinical utility of CB1 receptor antagonists was however challenged by the high‐profile withdrawal of the prototypic agent rimonabant. CBG was also shown to lack anticonvulsant effects in the PTZ model of generalised seizures (Hill et al., 2014). More prominently, we also demonstrated that CBDV possessed useful anticonvulsant effects in in vitro and in vivo seizure models (Amada, Yamasaki, Williams, & Whalley, 2013; Hill, Williams, Whalley, & Stephens, 2012). The former study further detailed the suppressive effects of CBDV on expression of specific epilepsy‐related genes. We further demonstrated that the anticonvulsant effects of CBDV‐rich cannabis extracts occurred independently of actions at CB1 receptors (Hill et al., 2013). There are ongoing investigations into the potential clinical utility of CBDV in epilepsy; however, overall preclinical data in in vivo seizure models generated at the University of Reading strongly suggested CBD as a prominent cannabinoid which had clear potential to address the unmet clinical need associated with different forms of epilepsy.
2. THE HUMAN EXPERIENCE
In parallel with efforts within the scientific research community, families of children with difficult‐to‐treat childhood epilepsies were beginning to connect with cannabis growers who were developing previously ‘forgotten’ high‐CBD cannabis strains. The realisation that such strains may possess anti‐seizure effects began to emerge via social and mainstream media. A notable example was the story of Charlotte Figi, which was captured in a memorable 2013 CNN documentary. At that time, Charlotte was a 5‐year‐old American girl who suffered dozens of seizures a day due to intractable DS, a disease associated with average life expectancy of just 8 years. Local producers of cannabis oil in Colorado developed a specialised high CBD/low THC product, later dubbed “Charlotte's Web,” which was able to cause a remarkable reduction in Charlotte's seizure rate (Maa & Figi, 2014). Against a backdrop of Charlotte being a twin to a healthy sister, together with her father being in the US military, the human‐interest documentary helped propel potential use of ‘medical marijuana’ into the public's consciousness. Other growers began to develop high CBD/low THC strains, and several families began to seek access to such strains, some via knowledge of the publications from University of Reading. In particular, the mother of Sam Vogelstein (a child with intractable childhood epilepsy who became Epidiolex patient #1) contacted GW Pharmaceuticals, citing the work of Jones et al. (2010), to request access to CBD. The treatment reportedly had significant efficacy—after 3 days of treatment, Sam was down from dozens of seizures per day to around one seizure per day. This led to the family being permitted by the Food and Drug Administration (FDA) to use CBD under a compassionate use program in California; the success of this program led to the pursuit of wider trials across more epilepsy centres. Thus, one of the foremost US epilepsy clinicians, Orrin Devinsky, Professor of Neurology at NYU Langone School of Medicine, oversaw moves towards the first randomised, controlled human clinical trials of CBD in patients with DS and another devastating paediatric epilepsy, Lennox–Gastaut syndrome (LGS). DS and LGS represent two of the most difficult to treat genetic childhood epilepsy syndromes; as patients with these syndromes have severely reduced lifespans and are commonly resistant to traditional AEDs, they represented a significant unmet need for new therapeutic options. These trials were further facilitated by the FDA decision in 2013 to grant CBD an Orphan Drug designation (i.e., special status to a drug with clear potential treat a rare disease, as defined in the United States as a condition that affects fewer than 200,000 people). Although classified as “rare” orphan diseases, figures from National Organization for Rare Diseases can be used to estimate that there are around 45,000–70,000 patients with DS and LGS in the United States. Such people with epilepsy present with multiple seizure types, commonly resistant to traditional AEDs, as well as developmental delay and numerous co‐morbidities.
From 2014, open‐label trials in patients with treatment‐resistant epilepsy, including DS and LGS, were initiated. Such trials first reported an encouraging 36.5% median reduction in monthly motor seizures (Devinsky et al., 2016). Following successful Phase 2 trials (Devinsky et al., 2017; Devinsky, Patel, Thiele, & GWPCARE1 Part A Study Group, 2018), the first pivotal positive Phase 3 study results for CBD in the treatment of DS and LGS were announced. Each of these clinical studies recruited patients typically with a history of using at least two previous AEDs and displaying at least two uncontrolled seizures weekly during a 4‐week pretreatment baseline. Across the studies, treatment groups displayed a significant ~20 percentage point decrease in seizures compared to placebo. Based on these positive human trials, the FDA approved Epidiolex as the first prescription plant extract cannabinoid medicine in the United States and as the first in a new class of anti‐epileptic medications for the treatment of pharmacoresistant seizures in children and young adults with DS and LGS. Overall, the GWPCARE1 and GWPCARE2 trials demonstrated that an oral suspension of CBD at 10 or 20 mg·kg−1·day−1 two times a day had an acceptable safety profile and that individuals with treatment‐resistant DS achieved a sustained, clinically meaningful reduction in monthly convulsive seizures compared with a placebo (Devinsky et al., 2017; Devinsky, Patel, Thiele, et al., 2018; Miller et al., 2020). Similarly, Phase 3 trials for oral CBD in LGS (GWPCARE3 and GWPCARE 4) were also positive (Devinsky, Patel, Cross, et al., 2018; Thiele et al., 2018). Thus, four major trials were carried out, recruiting a total of 550 (DS, 154; LGS, 396) patients who had previously been unable to control their seizures despite AED polytherapy (see Morano et al., 2020). An open‐label extension trial, GWPCARE5, with 630 subjects (DS, 264; LGS, 366) further supported oral CBD safety and persistence efficacy in DS and LGS patients (Devinsky et al., 2019; Thiele, Bebin, et al., 2019). Prior to Epidiolex being available for prescription, it has been estimated that over 2,000 patients had already been treated through GW's compassionate use program and open‐label extension. Epidiolex became available for prescription in the United States in November 2018. The Drug Enforcement Agency reclassified CBD (containing no more than 0.1% [w/w] residual THC) to Schedule V, indicating proven medical use and low potential for abuse. Similar European Medicine Authority approval for CBD followed in 2019, under the brand name Epidyolex. In the United Kingdom, the medicine became fully available on the NHS in January 2020. Drug scheduling of cannabis in the United Kingdom has been under regular review, and in November 2018, “cannabis‐based products for medicinal use (CBPMs)” were reclassified from Schedule 1 controlled drugs (compounds of no medical value) into Schedule 2 of the Misuse of Drugs Regulations 2001. Most recently, Epidyolex was reclassified as a Schedule 5 drug in the United Kingdom.
During approval, FDA Commissioner Scott Gottlieb stated in a press release: “This approval serves as a reminder that advancing sound development programs that properly evaluate active ingredients contained in marijuana can lead to important medical therapies. And, the FDA is committed to this kind of careful scientific research and drug development.”
3. CBD MECHANISM OF ACTION
A definitive pharmacological target for CBD in epilepsy (and elsewhere) remains elusive, with various reports of pharmacologically relevant affinity at several molecular targets (Gray & Whalley, 2020; Senn, Cannazza, & Biagini, 2020). The best recognised targets include transient receptor potential vanilloid channels and GPCR 18 (GPR18) and GPR55 receptors (Alexander et al., 2019). Work at the University of Reading has contributed to descriptions of CBD activation, and subsequent desensitisation, of transient receptor potential vanilloid 1 (TRPV1), TRPV2, and transient receptor potential ankyrin 1 (TRPA1) (Iannotti et al., 2014). We have also shown that CBD blocks the activation of GPR55 receptors by the endogenous lipid agonist lysophosphatidylinositol in ex vivo brain slices taken from rats displaying spontaneous epileptiform activity (Rosenberg et al., 2018).
Pharmacological blockage/antagonism and/or transgenic technology is good evidence to define mechanism of action. One recent study demonstrates that CBD's anticonvulsant properties were attenuated in TRPV1 knockout mice (Gray, Stott, Jones, Di Marzo, & Whalley, 2020). CBD also acts as allosteric modulator of 5HT1A; however, one specific study has ruled out 5HT1A as a CBD target in seizure control (Pelz, Schoolcraft, Larson, Spring, & Lopez, 2017). CBD was also shown to act as a blocker of VGSCs (Hill et al., 2014), which represents a potential mode of anticonvulsive action in epilepsy. However, another phytocannabinoid, CBG, was shown to share blockade of VGSCs but, unlike CBD, had no clear effect on PTZ‐induced seizures in rats (Hill et al., 2014). CBD has also been shown to retain anti‐seizure effects and improve social deficits and survival in a mouse model of DS (Kaplan, Stella, Catterall, & Westenbroek, 2017; Patra et al., 2020), itself a syndrome associated with a loss‐of‐function mutation to NaV1.1 VGSCs. Moreover, nanomolar CBD was effective in regulating neuronal excitability without effects on VGSCs in a human‐induced pluripotent stem cell‐based model of DS (Sun & Dolmetsch, 2018). Such data suggest that VGSCs were not the primary CBD target in epilepsy; these findings are significant as current AEDs which act as VGSC blockers are known to exacerbate DS.
As described above, CBD has only very low affinity at CB1 receptors; correspondingly, CBD lacks any appreciably coupling to G protein turnover (Jones et al., 2010). There are however reports that CBD can act as a negative allosteric modulator at CB1 receptors (Laprairie, Bagher, Kelly, & Denovan‐Wright, 2015; Straiker, Dvorakova, Zimmowitch, & Mackie, 2018) and also as a partial agonist at CB2 receptors (Tham et al., 2019). Of further mechanistic interest is our recent study which was consistent with CBD ameliorating the pro‐convulsant effect of long‐term THC treatment in rats (Whalley et al., 2018). In this study, we also described a potential mechanism whereby CBD reduced THC‐mediated CB1 receptor activation in a species‐specific way (Figure 2); such CBD actions may reflect a functional reduction in seizures, for example, via negative allosteric modulation of CB1 receptors.
FIGURE 2.
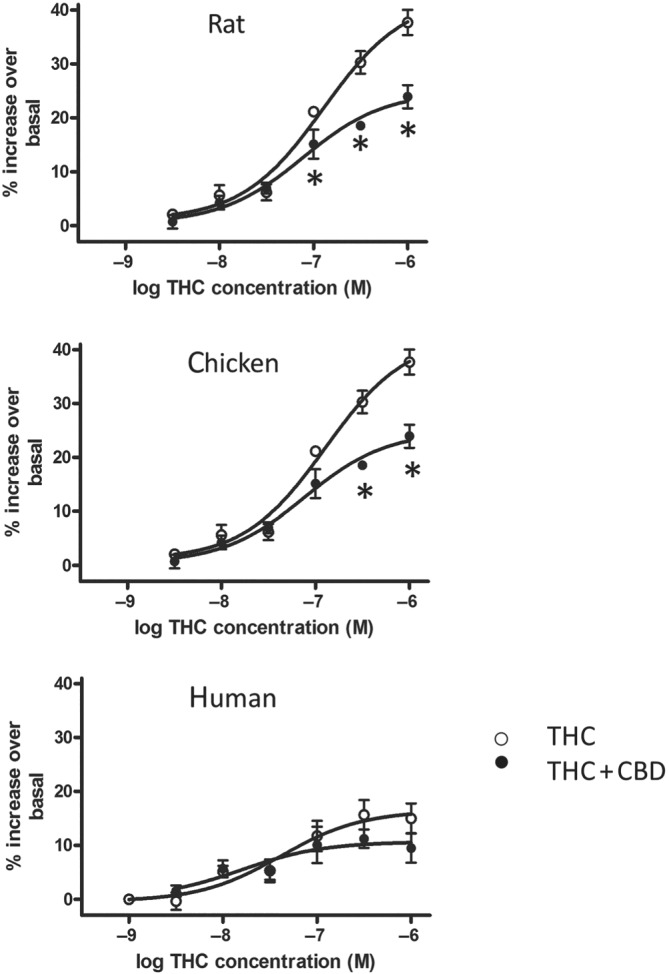
CBD affects THC‐stimulated 35S‐GTPγS turnover in cerebellar tissue in a species‐specific manner. CBD (applied at a THC:CBD 1.08:1 ratio) attenuated THC‐stimulated G protein turnover in rat and chicken, but not human, tissue. Note that in human tissue, THC‐stimulated G protein turnover was of generally lower magnitude. *P < 0.05, ANOVA followed by a Tukey's post hoc test on raw data from three separate experiments in triplicate, preparations from multiple brains (data derived from Whalley et al., 2018)
Several other potential molecular targets of CBD, including classical receptors, ion channels, transporters, and enzymes, have been proposed (reviewed by us in Hill et al., 2012; Ibeas Bih et al., 2015; Turner, Williams, Iversen, & Whalley, 2017). Most prominent amongst these are alternative receptor targets including 5HT1A, adenosine receptor subtypes, and PPARγ nuclear receptors; additional ion channel targets including CaV3 voltage‐gated calcium channels and mitochondrial voltage‐dependent anion‐selective channel protein 1 (VDAC1); adenosine transporters; and, potentially, enzymes involved in the endocannabinoid system, such as fatty acid amide hydrolase. One common CBD effect may be the modulation of downstream levels of calcium as a second messenger (see Ibeas Bih et al., 2015). In this regard, CBD has been proposed to regulate mitochondrial control (potentially via VDAC1) of intracellular calcium levels in hippocampal neurons and to restore homeostasis in pathological situations (Ryan, Drysdale, Lafourcade, Pertwee, & Platt, 2009); such function may contribute to CBD effects in epilepsy. More recently, we and co‐workers have shown that CBD can act on a glycine cleavage system component to inhibit synthesis of the essential amino acid methionine, including in heterozygous Scn1a +/− mice in the DS model (Perry et al., 2020). This work introduces one‐carbon signalling as a further potential target for CBD action.
A potential confounder in some studies is the use of high (~ >10 μM) CBD concentrations. As discussed by us previously, the lipophilic nature of CBD means that results based on higher micromolar concentrations of CBD are likely influenced by insertion of CBD into the plasma membrane (Ibeas Bih et al., 2015). In practice, the poor bioavailability of CBD in vivo (see below) may limit these effects. However, the general lipophilic profile of cannabinoids, including CBD, also means that they have potential to accumulate in fatty tissue and be released over time. In general, more credence is typically placed on CBD effects that occur at low/sub‐micromolar concentrations. Overall, CBD may exhibit a relevant “polypharmacology” profile; there are also consistent reports of anti‐oxidant and anti‐inflammatory properties of CBD which have potential to contribute to anti‐epileptic (and other) effects.
Finally here, the lack of a well‐defined molecular target can lead to speculation about the potential role of metabolites. In humans, CBD is metabolised mainly into 7‐OH‐CBD and the major 7‐COOH‐CBD metabolite. However, work on effects of CBD metabolites is, as yet, still in its infancy.
4. CLINICAL/FUTURE PERSPECTIVES
Over the last few years, there has been an explosion in interest in the use of CBD‐containing foods and supplements, supported by widespread availability of numerous different CBD preparations on the High Street and of further unregulated access via the internet. There have been recent warnings about levels of such consumption from the UK Food Standards Agency and efforts to curtail many unsubstantiated claims of therapeutic benefit. The latter will, of course, require controlled human trials which investigate appropriate formulations of standardised product, delivered at an efficacious dose. The original case of Charlotte Figi described above has several recent parallels in the United Kingdom. The publicity around cases of uncontrolled childhood epilepsies, such as those of Billy Caldwell and Alfie Dingley, were key in the reclassification and increased availability of CBPMs, including those with a CBD component. These moves were no doubt also fuelled by the approval of Epidiolex as a scientifically tested medicine.
From a clinical viewpoint, there are challenges to effective delivery of CBD. Animal studies suggest that CBD oral bioavailability is low (<20%) (Mechoulam, Parker, & Gallily, 2002), with CBD being subject to extensive first‐pass metabolism (Huestis, 2007); however, wide‐ranging human data for CBD bioavailability are currently lacking (Millar et al., 2019). CBD is also associated with low water solubility and variability in pharmacokinetic profiles (Millar, Maguire, Yates, & O'Sullivan, 2020). Therefore, further work is needed to establish therapeutic dosing and most effective route of administration. Clinically, Epidiolex is given as an oral solution; in addition, the CBD‐containing medicine Sativex is administered sublingually to avoid first‐pass metabolism. CBD bioavailability may be improved by intranasal delivery (e.g., of vaporised drug) or even by transdermal delivery (Paudel, Hammell, Agu, Valiveti, & Stinchcomb, 2010). However, of interest for anticonvulsant effects is that CNS levels of CBD were found to be higher via the oral, compared to the inhaled, route; moreover, such exposure correlated with increased behavioural effects (Hložek et al., 2017).
There is a well‐described “entourage effect,” originally posited for the endocannabinoid system by Ben‐Shabat et al. (1998), whereby cannabinoid effects are synergically increased by associated components. Such effects have also been ascribed to CBD and to Epidiolex, which represents a 98% CBD plant extract; however, future controlled human trials will be required to more fully define this effect. Some evidence in support of CBD synergism comes from a recent meta‐analysis of observational clinical studies that concluded patients required ~4‐fold less CBD dose in enriched plant extracts versus purified drug and that adverse effects were also reduced in extracts (Pamplona, da Silva, & Coan, 2018). An additional challenge is to define the nature and contribution of the highly numerous additional components in cannabis.
Epilepsy is a condition prone to a “honeymoon” effect, that is, a reduction of initial drug efficacy with prolonged use, often associated with development of tolerance to AEDs (Avanzini, 2006; Dale et al., 2019). It remains unclear if people with epilepsy will develop similar tolerance to CBD. At present, there is no published evidence for CBD tolerance in other conditions; however, a recent open‐label study reported that around one third of paediatric and adults with different treatment resistance epilepsies showed some tolerance to CBD‐enriched oil in terms of reduced control of mean monthly seizure frequency (although concomitant medication was maintained) (Uliel‐Sibony, Hausman‐Kedem, Fattal‐Valevski, & Kramer, 2020). Follow‐up studies to further investigate CBD tolerance in epilepsy, and its relevance in comparison to AEDs, are thus warranted.
Epidiolex may be given as a monotherapy in the United States in uncontrolled DS and LGS and Epidyolex as an adjunct to standard anti‐epileptic therapies in the European Union. CBD is generally considered a safe drug (Taylor, Gidal, Blakey, Tayo, & Morrison, 2018), in particular, in comparison to standard AEDs (Bergamaschi, Queiroz, Zuardi, & Crippa, 2011; Iffland & Grotenhermen, 2017). Original studies reported that CBD caused some adverse effects, including tiredness, diarrhoea, and changes of appetite/weight (Huestis et al., 2019); however, many such adverse effects occur at doses higher than those used for seizure control. A major CBD effect is inhibition of hepatic drug metabolism leading to recognised, significant increases in the levels of several co‐administered AEDs (Gaston, Bebin, Cutter, Liu, & Szaflarski, 2017). A common standard AED used in conjunction with Epidiolex is clobazam. One report (in a preclinical model) has proposed that an interaction between CBD and clobazam may underlie improved anti‐epileptic profiles (Anderson et al., 2019), potentially via CBD inhibition of CYP2C19, which metabolises clobazam. However, recent open‐label studies have shown that such interactions appear to have no effect on seizure frequency and severity outcomes in the patient population (Gaston et al., 2019; Savage et al., 2019). Isobolographic studies (which are gold standard for pharmacological interactions) for CBD (and 7‐OH‐CBD) and clobazam (and the major metabolite N‐desmethylclobazam) indicated a synergistic action in the MES mouse model of acute generalised seizures that occurred independently of pharmacokinetic interaction (Rana, 2019). A recent meta‐analysis further suggests that CBD has anti‐seizure efficacy independent of clobazam administration (Lattanzi et al., 2020).
With the recent approval of Epidiolex, several other disease states are the subject of intense investigation with regard to CBD use, with many now progressing to human trials. Recent reviews have outlined clinical work on CBD in, amongst others, Parkinson's disease and psychosis, society anxiety disorder, Rett syndrome, schizophrenia, cognitive dysfunction, and ulcerative colitis and Crohn's disease, as well as the potential for CBD to counteract recreational use of cannabis and dependence on opioids (Millar et al., 2019; Pauli, Conroy, Van Den Heuval, & Park, 2020). In particular, positive results from Phase III trials showing that CBD reduced seizures associated with Tuberous Sclerosis Complex (TSC) were reported in the GWPCARE6 study (Thiele, Marsh, et al., 2019); this work was also supported by our mechanistic work on CBD effects in a zebrafish model of TSC (Serra et al., 2019). CBD is also being widely investigated in different forms of cancer (Hinz & Ramer, 2019). This activity strongly suggests that CBD therapeutic use will likely soon extend to other conditions.
Thus, preclinical developmental work on CBD at the University of Reading leading to the introduction of Epidiolex for the treatment of severe childhood epilepsies has been recognised by the British Pharmacological Society, which has awarded Stephens, Whalley, and Williams the 2019 Sir James Black Award for Contributions to Drug Discovery. Much of this initial and ongoing work has been published in the British Journal of Pharmacology, including in different themed issues on cannabinoids, and has been presented at European Workshops on Cannabinoid Research organised by the BPS and several annual BPS Pharmacology meetings. The commitment of the BPS to this area of preclinical work has helped push CBD to the clinic for paediatric epilepsies; it is highly likely that additional areas of therapeutic utility will arise in the next few years for CBD and perhaps other plant‐derived cannabinoids.
4.1. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander et al., 2019).
AUTHOR CONTRIBUTIONS
C.W. and G.S. contributed to preclinical work discussed in this review and wrote the manuscript.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGEMENTS
This work is a review of the preclinical work for which Gary Stephens, Benjamin Whalley, and Claire Williams were awarded the British Pharmacological Society Sir James Black Award for Contributions to Drug Discovery 2019. The work is submitted as an academic review by Claire Williams and Gary Stephens and is our personal opinion on the work and does not reflect any viewpoint of the major funders of the work, GW Pharmaceuticals, who are also the current employers of the award co‐recipient, Benjamin Whalley. We thank and acknowledge GW Pharmaceuticals for their funding of the preclinical work described.
Williams CM, Stephens GJ. Development of cannabidiol as a treatment for severe childhood epilepsies. Br J Pharmacol. 2020;177:5509–5517. 10.1111/bph.15274
REFERENCES
- Alexander, S. P. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Mathie, A. , Peters, J. A. , … CGTP Collaborators . (2019). The Concise Guide to PHARMACOLOGY 2018/19: G protein‐coupled receptors. British Journal of Pharmacology, 176, S21–S141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amada, N. , Yamasaki, Y. , Williams, C. M. , & Whalley, B. J. (2013). Cannabidivarin (CBDV) suppresses pentylenetetrazole (PTZ)‐induced increases in epilepsy‐related gene expression. Peer J, 1, e214 10.7717/peerj.214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, L. L. , Absalom, N. L. , Abelev, S. V. , Low, I. K. , Doohan, P. T. , Martin, L. J. , … Arnold, J. C. (2019). Coadministered cannabidiol and clobazam: Preclinical evidence for both pharmacodynamic and pharmacokinetic interactions. Epilepsia, 60, 2224–2234. 10.1111/epi.16355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avanzini, G. (2006). Is tolerance to antiepileptic drugs clinically relevant? Epilepsia, 47, 1285–1287. 10.1111/j.1528-1167.2006.00616.x [DOI] [PubMed] [Google Scholar]
- Ben‐Shabat, S. , Fride, E. , Sheskin, T. , Tamiri, T. , Rhee, M. H. , Vogel, Z. , … Mechoulam, R. (1998). An entourage effect: Inactive endogenous fatty acid glycerol esters enhance 2‐arachidonoyl‐glycerol cannabinoid activity. European Journal of Pharmacology, 353, 23–31. 10.1016/s0014-2999(98)00392-6 [DOI] [PubMed] [Google Scholar]
- Bergamaschi, M. M. , Queiroz, R. H. , Zuardi, A. W. , & Crippa, J. A. (2011). Safety and side effects of cannabidiol, a Cannabis sativa constituent. Current Drug Safety, 6, 237–249. 10.2174/157488611798280924 [DOI] [PubMed] [Google Scholar]
- Cunha, J. M. , Carlini, E. A. , Pereira, A. E. , Ramos, O. L. , Pimentel, C. , Gagliardi, R. , … Mechoulam, R. (1980). Chronic administration of cannabidiol to healthy volunteers and epileptic patients. Pharmacology, 21, 175–185. 10.1159/000137430 [DOI] [PubMed] [Google Scholar]
- Dale, T. , Downs, J. , Olson, H. , Bergin, A. M. , Smith, S. , & Leonard, H. (2019). Cannabis for refractory epilepsy in children: A review focusing on CDKL5 Deficiency Disorder. Epilepsy Research, 151, 31–39. 10.1016/j.eplepsyres.2019.02.001 [DOI] [PubMed] [Google Scholar]
- Dennis, I. , Whalley, B. J. , & Stephens, G. J. (2008). Effects of Δ9‐tetrahydrocannabivarin on [35S]‐GTPγS binding in mouse brain cerebellum and piriform cortex membranes. British Journal of Pharmacology, 154, 1349–1358. 10.1038/bjp.2008.190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinsky, O. , Cross, J. H. , Laux, L. , Marsh, E. , Miller, I. , Nabbout, R. , … Cannabidiol in Dravet Syndrome Study Group . (2017). Trial of cannabidiol for drug‐resistant seizures in the Dravet syndrome. The New England Journal of Medicine, 376, 2011–2020. 10.1056/NEJMoa1611618 [DOI] [PubMed] [Google Scholar]
- Devinsky, O. , Marsh, E. , Friedman, D. , Thiele, E. , Laux, L. , Sullivan, J. , … Cilio, M. R. (2016). Cannabidiol in patients with treatment‐resistant epilepsy: An open‐label interventional trial. Lancet Neurology, 15, 270–278. 10.1016/S1474-4422(15)00379-8 [DOI] [PubMed] [Google Scholar]
- Devinsky, O. , Nabbout, R. , Miller, I. , Laux, L. , Zolnowska, M. , Wright, S. , … Roberts, C. (2019). Long‐term cannabidiol treatment in patients with Dravet syndrome: An open‐label extension trial. Epilepsia, 60, 294–302. 10.1111/epi.14628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinsky, O. , Patel, A. D. , Cross, J. H. , Villanueva, V. , Wirrell, E. C. , Privitera, M. , … GWPCARE3 Study Group . (2018). Effect of cannabidiol on drop seizures in the Lennox‐Gastaut syndrome. The New England Journal of Medicine, 378, 1888–1897. 10.1056/NEJMoa1714631 [DOI] [PubMed] [Google Scholar]
- Devinsky, O. , Patel, A. D. , Thiele, E. A. , & GWPCARE1 Part A Study Group . (2018). Randomized, dose‐ranging safety trial of cannabidiol in Dravet syndrome. Neurology, 90, e1204–e1211. 10.1212/WNL.0000000000005254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaston, T. E. , Bebin, E. M. , Cutter, G. R. , Ampah, S. B. , Liu, Y. , Grayson, L. P. , … UAB CBD Program . (2019). Drug–drug interactions with cannabidiol (CBD) appear to have no effect on treatment response in an open‐label Expanded Access Program. Epilepsy & Behavior, 98, 201–206. 10.1016/j.yebeh.2019.07.008 [DOI] [PubMed] [Google Scholar]
- Gaston, T. E. , Bebin, E. M. , Cutter, G. R. , Liu, Y. , & Szaflarski, J. P. (2017). Interactions between cannabidiol and commonly used antiepileptic drugs. Epilepsia, 58, 1586–1592. 10.1111/epi.13852 [DOI] [PubMed] [Google Scholar]
- Gray, R. A. , Stott, C. G. , Jones, N. A. , Di Marzo, V. , & Whalley, B. J. (2020). Anticonvulsive properties of cannabidiol in a model of generalized seizure are transient receptor potential vanilloid 1 dependent. Cannabis Cannabinoid Res, 5, 145–149. 10.1089/can.2019.0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray, R. A. , & Whalley, B. J. (2020). The proposed mechanisms of action of CBD in epilepsy. Epileptic Disorders, 22, S10–S15. [DOI] [PubMed] [Google Scholar]
- Hill, A. J. , Jones, N. A. , Smith, I. , Hill, C. L. , Williams, C. M. , Stephens, G. J. , & Whalley, B. J. (2014). Voltage‐gated sodium (NaV) channel blockade by plant cannabinoids does not confer anticonvulsant effects per se. Neuroscience Letters, 30, 269–274. [DOI] [PubMed] [Google Scholar]
- Hill, A. J. , Mercier, M. S. , Hill, T. D. M. , Glyn, S. , Jones, N. , Yamasaki, Y. , … Whalley, B. J. (2012). Cannabidivarin is anticonvulsant in mouse and rat. British Journal of Pharmacology, 167, 1629–1642. 10.1111/j.1476-5381.2012.02207.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill, A. J. , Weston, S. E. , Jones, N. A. , Smith, I. , Bevan, S. A. , Williamson, E. M. , … Whalley, B. J. (2010). Δ9‐Tetrahydrocannabivarin suppresses in vitro epileptiform and in vivo seizure activity in adult rats. Epilepsia, 51, 1522–1532. 10.1111/j.1528-1167.2010.02523.x [DOI] [PubMed] [Google Scholar]
- Hill, A. J. , Williams, C. M. , Whalley, B. J. , & Stephens, G. J. (2012). Phytocannabinoids as novel therapeutic agents in CNS disorders. Pharmacology & Therapeutics, 133, 79–97. 10.1016/j.pharmthera.2011.09.002 [DOI] [PubMed] [Google Scholar]
- Hill, T. D. , Cascio, M. G. , Romano, B. , Duncan, M. , Pertwee, R. G. , Williams, C. M. , … Hill, A. J. (2013). Cannabidivarin‐rich cannabis extracts are anticonvulsant in mouse and rat via a CB1 receptor‐independent mechanism. British Journal of Pharmacology, 170, 679–692. 10.1111/bph.12321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz, B. , & Ramer, R. (2019). Anti‐tumour actions of cannabinoids. British Journal of Pharmacology, 176, 1384–1394. 10.1111/bph.14426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hložek, T. , Uttl, L. , Kadeřábek, L. , Balíková, M. , Lhotková, E. , Horsley, R. R. , … Páleníček, T. (2017). Pharmacokinetic and behavioural profile of THC, CBD, and THC+CBD combination after pulmonary, oral, and subcutaneous administration in rats and confirmation of conversion in vivo of CBD to THC. European Neuropsychopharmacology, 27, 1223–1237. 10.1016/j.euroneuro.2017.10.037 [DOI] [PubMed] [Google Scholar]
- Huestis, M. A. (2007). Human cannabinoid pharmacokinetics. Chemistry & Biodiversity, 4, 1770–1804. 10.1002/cbdv.200790152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huestis, M. A. , Solimini, R. , Pichini, S. , Pacifici, R. , Carlier, J. , & Busardò, F. P. (2019). Cannabidiol adverse effects and toxicity. Current Neuropharmacology, 17, 974–989. 10.2174/1570159X17666190603171901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannotti, F. A. , Hill, C. L. , Leo, A. , Alhusaini, A. , Soubrane, C. , Mazzarella, E. , … Stephens, G. J. (2014). Nonpsychotropic plant cannabinoids, cannabidivarin (CBDV) and cannabidiol (CBD), activate and desensitize transient receptor potential vanilloid 1 (TRPV1) channels in vitro: Potential for the treatment of neuronal hyperexcitability. ACS Chemical Neuroscience, 5, 1131–1141. 10.1021/cn5000524 [DOI] [PubMed] [Google Scholar]
- Ibeas Bih, C. , Chen, T. , Nunn, A. V. , Bazelot, M. , Dallas, M. , & Whalley, B. J. (2015). Molecular targets of cannabidiol in neurological disorders. Neurotherapeutics, 12, 699–730. 10.1007/s13311-015-0377-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iffland, K. , & Grotenhermen, F. (2017). An update on safety and side effects of cannabidiol: A review of clinical data and relevant animal studies. Cannabis Cannabinoid Res, 2, 139–154. 10.1089/can.2016.0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janmohamed, M. , Brodie, M. J. , & Kwan, P. (2019). Pharmacoresistance—Epidemiology, mechanisms, and impact on epilepsy treatment. Neuropharmacology, 168, 107790. [DOI] [PubMed] [Google Scholar]
- Jones, N. A. , Glyn, S. E. , Akiyama, S. , Hill, T. D. , Hill, A. J. , Weston, S. E. , … Williams, C. M. (2012). Cannabidiol exerts anti‐convulsant effects in animal models of temporal lobe and partial seizures. Seizure, 21, 344–352. 10.1016/j.seizure.2012.03.001 [DOI] [PubMed] [Google Scholar]
- Jones, N. A. , Hill, A. J. , Smith, I. , Bevan, S. A. , Williams, C. M. , Whalley, B. J. , & Stephens, G. J. (2010). Cannabidiol displays anti‐epileptiform and anti‐seizure properties in vitro and in vivo. The Journal of Pharmacology and Experimental Therapeutics, 332, 569–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan, J. S. , Stella, N. , Catterall, W. A. , & Westenbroek, R. E. (2017). Cannabidiol attenuates seizures and social deficits in a mouse model of Dravet syndrome. Proc Natl Acad Sci USA, 114, 11229–11234. 10.1073/pnas.1711351114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkham, T. C. , & Williams, C. M. (2004). Endocannabinoid receptor antagonists: Potential for obesity treatment. Treatments in Endocrinology, 3, 345–360. 10.2165/00024677-200403060-00003 [DOI] [PubMed] [Google Scholar]
- Laprairie, R. B. , Bagher, A. M. , Kelly, M. E. , & Denovan‐Wright, E. M. (2015). Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. British Journal of Pharmacology, 172, 4790–4805. 10.1111/bph.13250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattanzi, S. , Trinka, E. , Striano, P. , Zaccara, G. , Del Giovane, C. , Nardone, R. , … Brigo, F. (2020). Cannabidiol efficacy and clobazam status: A systematic review and meta‐analysis. Epilepsia, 61, 1090–1098. 10.1111/epi.16546 [DOI] [PubMed] [Google Scholar]
- Ma, Y. L. , Weston, S. E. , Whalley, B. J. , & Stephens, G. J. (2008). The phytocannabinoid Δ9‐tetrahydrocannabivarin modulates inhibitory neurotransmission in the cerebellum. British Journal of Pharmacology, 154, 204–221. 10.1038/bjp.2008.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maa, E. , & Figi, P. (2014). The case for medical marijuana in epilepsy. Epilepsia, 55, 783–786. 10.1111/epi.12610 [DOI] [PubMed] [Google Scholar]
- Mechoulam, R. , Parker, L. A. , & Gallily, R. (2002). Cannabidiol: An overview of some pharmacological aspects. Journal of Clinical Pharmacology, 42, 11S–19S. 10.1002/j.1552-4604.2002.tb05998.x [DOI] [PubMed] [Google Scholar]
- Mechoulam, R. , & Shvo, Y. (1963). Hashish—I: The structure of cannabidiol. Tetrahedron, 19, 2073–2078. 10.1016/0040-4020(63)85022-x [DOI] [PubMed] [Google Scholar]
- Millar, S. A. , Maguire, R. F. , Yates, A. S. , & O'Sullivan, S. E. (2020). Towards better delivery of cannabidiol (CBD). Pharmaceuticals, 13, E219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar, S. A. , Stone, N. L. , Bellman, Z. D. , Yates, A. S. , England, T. J. , & O'Sullivan, S. E. (2019). A systematic review of cannabidiol dosing in clinical populations. British Journal of Clinical Pharmacology, 85, 1888–1900. 10.1111/bcp.14038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, I. , Scheffer, I. E. , Gunning, B. , Sanchez‐Carpintero, R. , Gil‐Nagel, A. , Scott Perry, M. , … Knappertz, V. (2020). Dose‐ranging effect of adjunctive oral cannabidiol vs placebo on convulsive seizure frequency in Dravet syndrome: A randomized clinical trial. JAMA Neurology, 77, 613–621. 10.1001/jamaneurol.2020.0073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modebadze, T. , Morgan, N. H. , Pérès, I. A. , Hadid, R. D. , Amada, N. , Hill, C. , … Whalley, B. J. (2016). A low mortality, high morbidity reduced intensity status epilepticus (rise) model of epilepsy and epileptogenesis in the rat. PLoS ONE, 11, e0147265 10.1371/journal.pone.0147265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morano, A. , Fanella, M. , Albini, M. , Cifelli, P. , Palma, E. , Giallonardo, A. T. , … di Bonaventura, C. (2020). Cannabinoids in the treatment of epilepsy: Current status and future prospects. Neuropsychiatric Disease and Treatment, 16, 381–396. 10.2147/NDT.S203782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamplona, F. A. , da Silva, R. L. , & Coan, A. C. (2018). Potential clinical benefits of CBD‐rich Cannabis extracts over purified CBD in treatment‐resistant epilepsy: Observational data meta‐analysis. Frontiers in Neurology, 9, 759 10.3389/fneur.2018.00759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton, W. D. , & Pertwee, R. G. (1972). Effect of cannabis and certain of its constituents on pentobarbitone sleeping time and phenazone metabolism. British Journal of Pharmacology, 44, 250–261. [PMC free article] [PubMed] [Google Scholar]
- Patra, P. H. , Barker‐Haliski, M. , White, H. S. , Whalley, B. J. , Glyn, S. , Sandhu, H. , … McNeish, A. (2019). Cannabidiol reduces seizures and associated behavioral comorbidities in a range of animal seizure and epilepsy models. Epilepsia, 60, 303–314. 10.1111/epi.14629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patra, P. H. , Serafeimidou‐Pouliou, E. , Bazelot, M. , Whalley, B. J. , Williams, A. , & McNeish, A. (2020). Cannabidiol (CBD) improves survival and behavioural comorbidities of Dravet syndrome in mice. British Journal of Pharmacology, 177, 2779–2792. 10.1111/bph.15003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paudel, K. S. , Hammell, D. C. , Agu, R. U. , Valiveti, S. , & Stinchcomb, A. L. (2010). Cannabidiol bioavailability after nasal and transdermal application: Effect of permeation enhancers. Drug Development and Industrial Pharmacy, 36, 1088–1097. 10.3109/03639041003657295 [DOI] [PubMed] [Google Scholar]
- Pauli, C. S. , Conroy, M. , Van Den Heuval, B. D. , & Park, S.‐H. (2020). Cannabidiol drugs clinical trial outcomes and adverse effects. Frontiers in Pharmacology, 11, 63 10.3389/fphar.2020.00063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelz, M. C. , Schoolcraft, K. D. , Larson, C. , Spring, M. G. , & Lopez, H. H. (2017). Assessing the role of serotonergic receptors in cannabidiol's anticonvulsant efficacy. Epilepsy & Behavior, 73, 111–118. 10.1016/j.yebeh.2017.04.045 [DOI] [PubMed] [Google Scholar]
- Perry, C. J. , Finch, P. , Muller‐Taubenberger, A. , Leung, K.‐Y. , Warren, E. , Oddy, J. , … Stewart, B. (2020). A new mechanism for cannabidiol in regulating the one‐carbon cycle and methionine levels in Dictyostelium and in mammalian epilepsy models. British Journal of Pharmacology, 177, 912–928. 10.1111/bph.14892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee, R. G. (2008). The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Δ9‐Tetrahydrocannabinol, cannabidiol and Δ9‐tetrahydrocannabivarin. British Journal of Pharmacology, 153, 199–215. 10.1038/sj.bjp.0707442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana, R. R. (2019). Cannabidiol drug‐drug interaction with clobazam in an acute mouse model of generalized seizures. Pharmacology 2019 Edinburgh meeting Abstract LB035.
- Rosenberg, E. , Bazelot, M. , Salah, A. , Chamberland, S. , Nebet, E. , Whalley, B. J. , … Tsien, R. (2018). Cannabidiol (CBD) exerts antiepileptic properties by targeting the LPI‐GPR55 lipid signaling system potentiated by seizures. American Epilepsy Society meeting (Abstract 3.052).
- Rosenberg, E. C. , Tsien, R. W. , Whalley, B. J. , & Devinsky, O. (2015). Cannabinoids and epilepsy. Neurotherapeutics, 12, 747–768. 10.1007/s13311-015-0375-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan, D. , Drysdale, A. J. , Lafourcade, C. , Pertwee, R. G. , & Platt, B. (2009). Cannabidiol targets mitochondria to regulate intracellular Ca2+ levels. The Journal of Neuroscience, 29, 2053–2063. 10.1523/JNEUROSCI.4212-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage, T. E. , Sourbron, J. , Bruno, P. L. , Skirvin, L. A. , Wolper, E. S. , Anagnos, C. J. , & Thiele, E. A. (2019). Efficacy of cannabidiol in subjects with refractory epilepsy relative to concomitant use of clobazam. Epilepsy Research, 160, 106263. [DOI] [PubMed] [Google Scholar]
- Senn, L. , Cannazza, G. , & Biagini, G. (2020). Receptors and channels possibly mediating the effects of phytocannabinoids on seizures and epilepsy. Pharmaceuticals, 13, E174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra, I. , Scheldeman, C. , Bazelot, M. , Whalley, B. J. , Dallas, M. L. , de Witte, P. A. M. , … Williams, C. M. (2019). Cannabidiol modulates phosphorylated rpS6 signalling in a zebrafish model of Tuberous Sclerosis Complex. Behavioural Brain Research, 363, 135–144. 10.1016/j.bbr.2019.01.040 [DOI] [PubMed] [Google Scholar]
- Stephens, G. J. (2009). G‐protein‐coupled‐receptor‐mediated presynaptic inhibition in the cerebellum. Trends in Pharmacological Sciences, 30, 421–430. 10.1016/j.tips.2009.05.008 [DOI] [PubMed] [Google Scholar]
- Stephens, G. J. , Canti, C. , Page, K. M. , & Dolphin, A. C. (1998). Role of domain I of neuronal Ca2+ channel α1 subunits in G protein modulation. The Journal of Physiology, 509, 163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens, G. J. , & Mochida, S. (2005). G protein βγ subunits mediate presynaptic inhibition of transmitter release from rat superior cervical ganglion neurones in culture. The Journal of Physiology, 563, 765–776. 10.1113/jphysiol.2004.080192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straiker, A. , Dvorakova, M. , Zimmowitch, A. , & Mackie, K. (2018). Cannabidiol inhibits endocannabinoids signaling in autaptic hippocampal neurons. Molecular Pharmacology, 94, 743–748. 10.1124/mol.118.111864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, Y. , & Dolmetsch, R. E. (2018). Investigating the therapeutic mechanism of cannabidiol in a human induced pluripotent stem cell (iPSC)‐based model of Dravet syndrome. Cold Spring Harbor Symposia on Quantitative Biology, 83, 185–191. 10.1101/sqb.2018.83.038174 [DOI] [PubMed] [Google Scholar]
- Taylor, L. , Gidal, B. , Blakey, G. , Tayo, B. , & Morrison, G. (2018). Phase I, randomized, double‐blind, placebo‐controlled, single ascending dose, multiple dose, and food effect trial of the safety, tolerability and pharmacokinetics of highly purified cannabidiol in healthy subjects. CNS Drugs, 32, 1053–1067. 10.1007/s40263-018-0578-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tham, M. , Yilmaz, O. , Alaverdashvili, M. , Kelly, M. E. M. , Denovan‐Wright, E. M. , & Laprairie, R. B. (2019). Allosteric and orthosteric pharmacology of cannabidiol and cannabidiol‐dimethylheptyl at the type 1 and type 2 cannabinoid receptors. British Journal of Pharmacology, 176, 1455–1469. 10.1111/bph.14440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele, E. , Marsh, E. , Mazurkiewicz‐Beldzinska, M. , Halford, J. J. , Gunning, B. , Devinsky, O. , … Roberts, C. (2019). Cannabidiol in patients with Lennox‐Gastaut syndrome: Interim analysis of an open‐label extension study. Epilepsia, 60, 419–428. 10.1111/epi.14670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele, E. A. , Bebin, E. M. , Bhathai, H. , Jansen, F. , Kotulska‐Jóźwiak, K. , & Lawson, J. A. (2019). Cannabidiol (CBD) treatment in patients with seizures associated with tuberous sclerosis complex: A randomized, double‐blind, placebo‐controlled phase 3 trial (GWPCARE6). American Epilepsy Society meeting (Abstract 1.293).
- Thiele, E. A. , Marsh, E. D. , French, J. A. , Mazurkiewicz‐Beldzinska, M. , Benbadis, S. R. , Joshi, C. , … GWPCARE4 Study Group . (2018). Cannabidiol in patients with seizures associated with Lennox‐Gastaut syndrome (GWPCARE4): A randomised, double‐blind, placebo‐controlled phase 3 trial. Lancet, 391, 1085–1096. 10.1016/S0140-6736(18)30136-3 [DOI] [PubMed] [Google Scholar]
- Turner, S. E. , Williams, C. M. , Iversen, L. , & Whalley, B. J. (2017). Molecular pharmacology of phytocannabinoids. Progress in the Chemistry of Organic Natural Products, 103, 61–101. 10.1007/978-3-319-45541-9_3 [DOI] [PubMed] [Google Scholar]
- Uliel‐Sibony, S. , Hausman‐Kedem, M. , Fattal‐Valevski, A. , & Kramer, U. (2020). Cannabidiol‐enriched oil in children and adults with treatment‐resistant epilepsy—Does tolerance exist? Brain and Development, [Epub in print]. S0387–7604(20)30183–2 [DOI] [PubMed] [Google Scholar]
- Wallace, M. J. , Wiley, J. L. , Martin, B. R. , & DeLorenzo, R. J. (2001). Assessment of the role of CB1 receptors in cannabinoid anticonvulsant effects. European Journal of Pharmacology, 428, 51–57. 10.1016/s0014-2999(01)01243-2 [DOI] [PubMed] [Google Scholar]
- Whalley, B. J. , Lin, H. , Bell, L. , Hill, T. , Patel, A. , Gray, R. A. , … Stephens, G. J. (2018). Species‐specific susceptibility to cannabis‐induced convulsions. British Journal of Pharmacology, 176, 1506–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson, J. D. , Whalley, B. J. , Baker, D. , Pryce, G. , Constanti, A. , Gibbons, S. , … Williamson, E. M. (2003). Medicinal cannabis: Is Δ9‐tetrahydrocannabinol necessary for all its effects? The Journal of Pharmacy and Pharmacology, 55, 1687–1694. 10.1211/0022357022304 [DOI] [PubMed] [Google Scholar]
- Williams, C. M. , & Kirkham, T. C. (1999). Anandamide induces overeating: Mediation by central cannabinoid (CB1) receptors. Psychopharmacology, 143, 315–317. [DOI] [PubMed] [Google Scholar]
- Williams, C. M. , Rogers, P. J. , & Kirkham, T. C. (1998). Hyperphagia in pre‐fed rats following oral Δ9‐THC. Physiology & Behavior, 65, 343–346. 10.1016/s0031-9384(98)00170-x [DOI] [PubMed] [Google Scholar]


