Abstract
Infertility rates for both females and males have increased continuously in recent years. Currently, effective treatments for male infertility with defined mechanisms or targets are still lacking. G protein‐coupled receptors (GPCRs) are the largest class of drug targets, but their functions and the implications for the therapeutic development for male infertility largely remain elusive. Nevertheless, recent studies have shown that several members of the GPCR superfamily play crucial roles in the maintenance of ion–water homeostasis of the epididymis, development of the efferent ductules, formation of the blood–epididymal barrier and maturation of sperm. Knowledge of the functions, genetic variations and working mechanisms of such GPCRs, along with the drugs and ligands relevant to their specific functions, provide future directions and a great arsenal for new developments in the treatment of male infertility.
Keywords: ADGRG2, AT2 receptor, epididymis, G protein‐coupled receptor (GPCR), LGR4, male infertility
Abbreviations
- ADGRG2
adhesion G protein‐coupled receptor G2
- Ang I
angiotensin I
- Ang II
angiotensin II
- AQP 9
aquaporin 9
- BMs
basement membranes
- CBAVD
congenital bilateral absence of the vas deferens
- CFTR
cystic fibrosis transmembrane conductance regulator
- GPER
G protein‐coupled estrogen receptor 1
- GSK3‐β
glycogen synthase kinase 3 beta
- HE6
human epididymal gene product 6
- IPF
idiopathic pulmonary fibrosis
- LGR4
leucine‐rich repeat containing G protein‐coupled receptor 4
- PAMs
positive allosteric modulators
- RAS
renin–angiotensin system
- tACE
angiotensin‐converting enzyme specific to the testes
1. INTRODUCTION
The infertility rate in humans has continued to increase in recent years and has become a significant social burden (Krausz & Riera‐Escamilla, 2018; Winters & Walsh, 2014). Currently, infertility ranks as the third most common public health concern, after cancer and cardiovascular disease. Problems in males and females contribute equally to the increasing infertility rate, and nearly 7% of the male population has fertility problems (Krausz & Riera‐Escamilla, 2018; Winters & Walsh, 2014). However, few effective treatments are available for male infertility with defined mechanisms. It is now recognized that defects in sperm production, decrease of sperm motility and inability of sperm to interact with the oocyte, all contribute to male infertility (Aitken, 2006; Elzanaty, Richthoff, Malm, & Giwercman, 2002).
After spermatogenesis in the testis, the spermatozoa are morphologically complete but immotile and unable to fertilize an oocyte. They must travel through the efferent ductules and the epididymis to acquire the ability to move, capacitate and migrate through the female tract and finally fertilize an oocyte. The efferent ductules are small, coiled tubules that convey sperm from the testis to the epididymis. In mammals, efferent ductules begin with several discrete wide‐lumen ducts that eventually merge into highly convoluted tubules with a narrow lumen (Hess, 2015; Joseph, Shur, & Hess, 2011). The efferent ductule epithelium contains ciliated cells with long motile cilia and non‐ciliated cells with microvillus brush borders (Hess, 2015; Joseph et al., 2011) (Figure 1). It is now known that the major function of the efferent ductules is reabsorption of luminal fluid, which increases the concentration of sperm before they enter the epididymis (Clulow, Jones, Hansen, & Man, 1998; Hess, 2000; Hess & Nakai, 2000).
FIGURE 1.
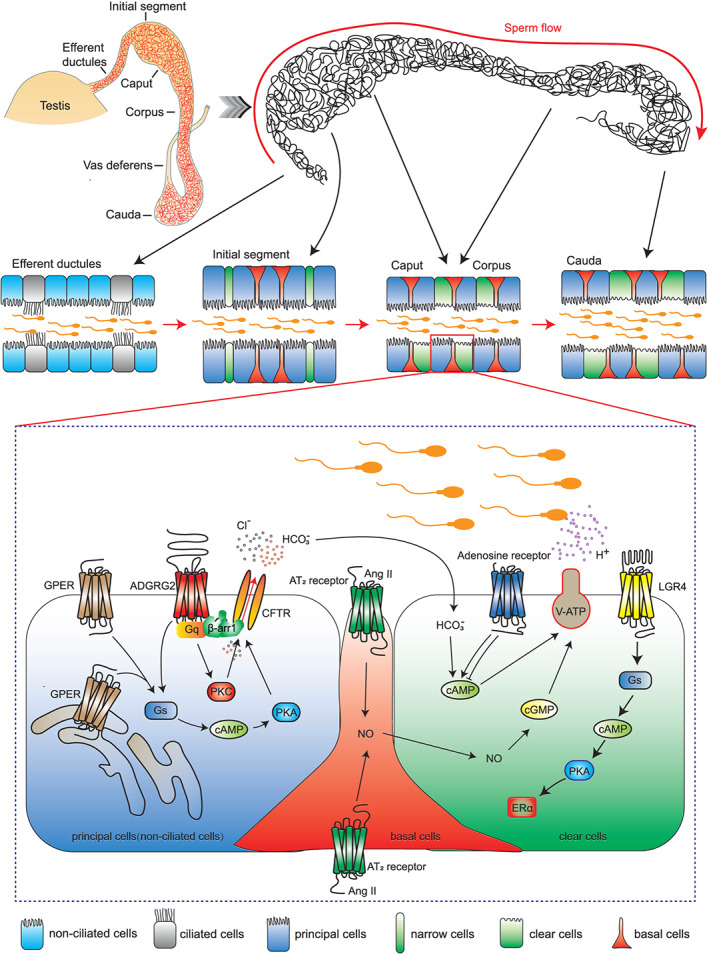
Diagram showing GPCR signalling and functions in the epididymis and efferent ductules. Above: The efferent ductules are a series of tubules that connect the rete testis to the epididymis. The epithelia of the efferent ductules are mainly composed of two cell types, ciliated cells and non‐ciliated cells. The epididymis is composed of one highly convoluted tubule. The epididymis is segmented morphologically and functionally into following distinct regions: the initial segment (not existing in human epididymis), the caput, the corpus, and the cauda. Each part consists of several cell types, including principal cells, narrow cells, clear cells, and basal cells. Inset: GPER activates cAMP‐CFTR‐chloride transportation to maintain the osmotic pressure of the perfusion solution. ADGRG2 is located exclusively on the apical membrane in non‐ciliated cells. ADGRG2/β‐arrestin1/Gq/CFTR forms a supercomplex that maintains pH and chloride anion homeostasis. AT2 receptors are specifically detected in basal cells and are essential for the proton‐secretion function of the epididymal lumen through activation of the NO‐cGMP pathway. Different members of the adenosine receptor family have opposite effects on the contractility of the epididymis. LGR4 activates Gs to increase intracellular cAMP levels, which promote ERα expression
The mammalian epididymis is an exceedingly long, convoluted ductal system connecting the efferent ductules with the vas deferens. Functionally, the epididymis creates an ideal environment to promote the functional transformation of spermatozoa and their later storage before ejaculation. The epididymis is segmented into four functionally distinct segments: the initial segment (not existing in human epididymis), the caput, the corpus, and the cauda (Abou‐Haila & Fain‐Maurel, 1984; W. Zhou, De Iuliis, Dun, & Nixon, 2018) (Figure 1). The initial segment, together with the upstream efferent ductules, is responsible for the resorption of the testicular fluid that enters the duct, resulting in a pronounced concentration of the luminal spermatozoa (Abe, Takano, & Ito, 1984). The caput epididymis is highly active in protein synthesis and hormone secretion and plays important roles in sperm maturation. The sperm passing through this region begin to obtain the ability to swim in a progressive manner and to recognize an oocyte (Aitken et al., 2007; Chevrier & Dacheux, 1992). The functional maturation of the sperm continues in the corpus epididymis and reaches full activity in the distal caudal segment. The caudal segment contains a relatively large lumen, and its surrounding epithelial cells have strong absorptive activity (Hermo, Dworkin, & Oko, 1988). There are four main cell types in the epithelium of the epididymal lumen, namely, narrow cells, clear cells, principal cells, and basal cells. Each cell type has different functions involved in the establishment and regulation of a unique luminal environment (Cornwall, 2009; Shum, Da Silva, Brown, & Breton, 2009).
In general, an appropriate microenvironment established by the efferent ductules and epididymis is required for sperm to undergo maturation and acquire progressive motility and the ability to fertilize oocyte during their transit. To date, the exact molecular mechanism involved in maintaining the effective microenvironment in the efferent ductules and epididymis remains to be defined, which creates significant obstacles to developing effective treatments for male infertility. Therefore, there is an urgent need to understand the regulatory mechanisms in the efferent ductules and epididymis involved in both physiological and pathological processes, and to use this knowledge to provide potential drug targets for developing effective therapies.
G protein‐coupled receptors (GPCRs), also called seven‐transmembrane receptors, are a group of important drug targets, accounting for approximately one third of all clinically marketed drugs (Hauser et al., 2018; Santos et al., 2017). Although the roles of GPCRs in cardiovascular disease, neuronal disease, diabetes, and many other diseases have been extensively investigated (Desimine et al., 2018; Dong et al., 2017; Hauser, Attwood, Rask‐Andersen, Schioth, & Gloriam, 2017; Kim et al., 2020; Lammermann & Kastenmuller, 2019; T. Li et al., 2018; Liu et al., 2017; Srivastava, Gupta, Gupta, & Shukla, 2015), there is very much less knowledge of the functions of GPCRs in the efferent ductules and epididymis.
GPCRs, by definition, carry out their selective functions through coupling to the different G protein subtypes or to arrestins (Bridges & Lindsley, 2008; Cahill et al., 2017; Gilman, 1987; Pierce, Premont, & Lefkowitz, 2002; Ritter & Hall, 2009). In general, the binding of ligands (such as hormones, neurotransmitters, or sensory stimuli) induces conformational changes in the transmembrane and intracellular domains of the receptor, thereby allowing interactions with heterotrimeric G proteins or with arrestins (Weis & Kobilka, 2018; F. Yang et al., 2018). For G protein signalling, activated GPCRs act as guanine nucleotide exchange factors (GEFs) for the α subunits of heterotrimeric G proteins, catalyzing the release of GDP and the binding of GTP for G protein activation. Activation of different G proteins, including Gs/o, Gi, G12/13 or Gq subtypes, affect various cellular processes through different downstream effectors, such as adenylyl cyclase (AC), RhoGEF, or phospholipase C (PLC) (Flock et al., 2015; Flock et al., 2017; Furness et al., 2016; Gilman, 1987; Isogai et al., 2016; Pierce et al., 2002; Ritter & Hall, 2009; Sounier et al., 2015; Venkatakrishnan et al., 2016). The activated GPCRs are also phosphorylated by the GPCR kinases (GRKs), a family of protein kinases that phosphorylate specific serine/threonine residues of GPCRs. Receptor phosphorylation leads to arrestin recruitment and activation (Homan & Tesmer, 2014; Komolov et al., 2017; Premont & Gainetdinov, 2007; Reiter & Lefkowitz, 2006; F. Yang et al., 2015). Activated arrestins not only desensitize receptors but also mediate a second wave of signalling independent of G proteins (Desimine et al., 2018; Dong et al., 2017; Kumari et al., 2016; Lefkowitz & Shenoy, 2005; Liu et al., 2017; Reiter & Lefkowitz, 2006; Shukla et al., 2014; W. Wang, Qiao, & Li, 2018; F. Yang et al., 2015; F. Yang et al., 2018). Even a single type of GPCR can initiate a broad range of physiological processes through arrestin engagement by scaffolding different downstream effectors (Hara et al., 2011; Liu et al., 2017; Luttrell et al., 1999; Miller et al., 2000; Peterson & Luttrell, 2017; Srivastava et al., 2015; Tobin, Butcher, & Kong, 2008; Xiao et al., 2007; F. Yang et al., 2015; F. Yang et al., 2018). However, the exact roles of the G protein subtype or arrestins downstream of the GPCRs in the epididymis remain undefined.
At present, there are no U.S. Food and Drug Administration (FDA)‐approved drugs targeting the GPCRs in the efferent ductules or epididymis, for the treatment of male infertility. In contrast, there are more than 470 GPCR‐targeted drugs for therapies treating other diseases in clinical markets (Hauser et al., 2018). Nevertheless, recent research has elucidated the expression patterns and functions of several important GPCRs in the efferent ductules and epididymis, such as the adhesion G protein‐coupled receptor G2 (ADGRG2), angiotensin AT2 receptors and the leucine‐rich repeat containing G protein‐coupled receptor 4 (LGR4), and has successfully developed the corresponding ligands to regulate their functions, generating the possibility of developing treatments for male infertility (Figure 1). Here, we review the progress in our knowledge of GPCRs in epididymis and efferent ductules and suggest potential therapeutic approaches by targeting these GPCRs for male infertility.
2. FUNCTION OF ADGRG2 IN FLUID REABSORPTION AND EPIDIDYMIS DEVELOPMENT
Few GPCRs have tissue‐specific distributions in male reproductive systems. The receptor protein ADGRG2, also called GPR64 or human epididymal gene product 6 (HE6), has attracted substantial attention for its relatively specific expression and essential function in male reproductive systems. It is highly expressed in the efferent ductules and the proximal epididymis (Table 1) (Kirchhoff, Osterhoff, & Samalecos, 2008; Obermann et al., 2003). Expression of ADGRG2 has also been detected in parathyroid, prostate, fibroblasts, neuronal and adipose tissue, as well as various types of cancers (Ahn et al., 2019; Balenga et al., 2017; Hamann et al., 2015; Suchy et al., 2020; Xie et al., 2020). Further studies have confirmed the functional importance of ADGRG2 in male fertility. The human and mouse ADGRG2/Adgrg2 gene is localized on chromosome X (Obermann et al., 2003). Adgrg2 −/Y mice exhibit reduced sperm numbers, decreased sperm motility, and increased number of spermatozoa with deficient heads or angulated flagella (Davies et al., 2004). Moreover, dysfunction in the fluid resorption of the efferent ductules is observed, which might eventually lead to the above‐mentioned phenotypes in Adgrg2 −/Y mice (Table 1) (Davies et al., 2004; Gottwald, Davies, Fritsch, & Habenicht, 2006; Zhang et al., 2018).
TABLE 1.
GPCRs with known functions in epididymis or efferent ductules
| Receptor name | Family name | Expression | Function | Signalling effectors | References |
|---|---|---|---|---|---|
| ADGRG2 (GPR64) | Adhesion Class GPCRs | Efferent ductules; proximal epididymis (non‐ciliated cells; principal cells) | Fluid reabsorption; possibly sperm maturation | Gs; Gi; Gq; G12/13; β‐arrestin 1; β‐arrestin 2 | Davies et al. (2004), Demberg, Rothemund, Schoneberg, and Liebscher (2015), Peeters et al. (2015), Zhang et al. (2018), and Azimzadeh, Talamantez‐Lyburn, Chang, Inoue, and Balenga (2019) |
| AT2 receptor | Angiotensin receptors | Basal cells | Proton secretion | Unknown | Krege et al. (1995), Esther et al. (1996), and Shum et al. (2008) |
| LGR4 (GPR48) | Class A orphans | Epithelial cells in the reproductive organs | Epithelial–mesenchymal interactions | Gs, Gq, Wnt | Mendive et al. (2006), Gao et al. (2006), Li et al. (2010), Carmon, Gong, Lin, Thomas, and Liu (2011), and Luo et al. (2016) |
| GPER (GPR30) | G protein‐coupled estrogen receptor | Testis; spermatozoa; prostate; efferent ductules; epididymis | Formation of blood–epididymal barrier and regulation of osmotic pressure | Gs; β‐arrestin 2 | Filardo, Quinn, Bland, and Frackelton (2000), Katleba, Legacki, Conley, and Berger (2015), and Cao et al. (2017) |
| Adenosine receptor | Adenosine receptors | Epididymis | Contractility of the epididymis | Gs; Gi; Gq; β‐arrestin | Haynes, Alexander, and Hill (1998b), Geldenhuys, Hanif, Yun, and Nayeem (2017), and Santiago et al. (2020) |
ADGRG2/GPR64 knockout (KO) results in down‐regulation of epididymis‐specific expression of cystatin, lipocalins, β‐defensins, Adam28, Crisp1, and Enpp2 among others, which have been shown to be closely related to sperm maturation (Davies et al., 2007). For example, cystatin‐related epididymal spermatogenic (CERS) subgroup members are part of an amyloid matrix and associated with extracellular vesicles in the mouse epididymal lumen and may play a functional role in sperm maturation through coordinating interactions between the luminal fluid and spermatozoa (Whelly et al., 2016). Lipocalin 2 modulates sperm maturation through binding to membrane phosphatidylethanolamine to induce lipid raft movement in a PKA‐dependent manner (Watanabe et al., 2014). Lipocalin 6 is involved in preventing calcium overload and premature acrosome reaction of sperm (Yin et al., 2018). β‐defensin 22 enables spermatozoa to undergo the process of fertilization through its heparin binding activity (Diao, Yu, Sun, Zhang, & Tanphaichitr, 2011), and β‐defensin 15 helps with sperm motility and fertility (Y. Zhao et al., 2011). The transcriptional change of these proteins helps to explain the phenotypes in GPR64 KO mice including reduced and defective sperms (Davies et al., 2004).
ADGRG2 belongs to the adhesion GPCR (aGPCR) subfamily, and all members of this family share a very large N‐terminal domain (Fredriksson, Lagerstrom, Lundin, & Schioth, 2003; Hu et al., 2014; Hamann et al., 2015; Kishore & Hall, 2017; Liebscher, Schoneberg, & Promel, 2013; Paavola, Stephenson, Ritter, Alter, & Hall, 2011; Paavola & Hall, 2012; Sun et al., 2013; X. J. Wang et al., 2014). Another feature of aGPCRs is the presence of the GPCR proteolytic site (GPS) at the GPCR Autoproteolysis‐INducing (GAIN) domain (Arac et al., 2012), which auto‐cleaves aGPCRs into an N‐terminal fragment (NTF) and a C‐terminal fragment (CTF) for most of its family members (Arac et al., 2012; de Graaf, Nijmeijer, Wolf, & Ernst, 2016; Hamann et al., 2015). Many members of this family have been shown to function through G protein coupling, and the NTF has been shown to inhibit the constitutive G protein‐coupling activity of its CTF in some members (Demberg et al., 2015; Folts, Giera, Li, & Piao, 2019; Hamann et al., 2015; Hu et al., 2014; Kishore, Purcell, Nassiri‐Toosi, & Hall, 2016; Purcell & Hall, 2018; Sun et al., 2013; X. J. Wang et al., 2014; Zhang et al., 2018). Activation of aGPCRs could be induced through (1) ligand binding (R. Luo et al., 2014; Paavola, Sidik, Zuchero, Eckart, & Talbot, 2014; Petersen et al., 2015), (2) mechanostimulation (Hilbig et al., 2018; Scholz et al., 2015; Wilde et al., 2016), or (3) removing the NTF through autoproteolysis (Demberg et al., 2015; Liebscher et al., 2014; Okajima, Kudo, & Yokota, 2010; Paavola et al., 2011; Ward et al., 2011). Many aGPCRs have been shown to contain a tethered agonist sequence in the N‐terminal region between the cleavage site and the first transmembrane domain. The exposed N‐terminal‐tethered agonist sequence after auto‐cleavage could act as a certain type of endogenous agonists of aGPCR families. Derived peptides from this tethered agonist sequence of aGPCRs can be used as agonists in vitro and in vivo (Balenga et al., 2017; Brown et al., 2017; Demberg et al., 2015; Demberg et al., 2017; Liebscher et al., 2014; Muller et al., 2015; Stoveken, Hajduczok, Xu, & Tall, 2015; Suchy et al., 2020; Wilde et al., 2016).
The signal transduction of ADGRG2 has been extensively investigated. A suspected Gs or Gq coupling was initially proposed in Xenopus melanophores (Foord, Jupe, & Holbrook, 2002). The coupling of ADGRG2 to the calcium‐sensing CaS receptor, G12/13, Gs, and Gq was then confirmed (Balenga et al., 2017; Demberg et al., 2015; Peeters et al., 2015). Through generation of ADGRG2 mutants that lack NTF, a constitutive β‐arrestin coupling activity and constitutive internalization of ADGRG2 were observed, and GRKs and dynamin were shown to mediate the constitutive internalization of this specific ADGRG2 mutant (Azimzadeh et al., 2019). Parallel to these observations, our study showed that in cells overexpressing either full‐length ADGRG2 or the ADGRG2‐CTF, significant constitutive Gs or Gq coupling activity was observed (Zhang et al., 2018). These studies suggested that ADGRG2‐mediated Gs or Gq signalling may play important roles in the regulation of fluid resorption in the efferent ductules and epididymis (Figure 1). However, the exact functions of G protein subtypes in maintaining the microenvironment of the efferent ductules or epididymis are still unknown, and the downstream effectors involved in controlling the luminal ion/water homeostasis balance in these tissues also remain elusive. Interestingly, immunostaining assays revealed specific expression of ADGRG2 on the apical membrane only in non‐ciliated cells (in the efferent ductules) and principal cells (in the epididymis), not in ciliated cells (Kirchhoff et al., 2008; Obermann et al., 2003). The non‐ciliated cells in efferent ductules are frequently referred as principal cells in the epididymis (Burkett, Schulte, & Spicer, 1987). Cellular expression specificity of ADGRG2 suggests a cell type‐specific function of ADGRG2 in the regulation of ion/water homeostasis in the efferent ductules and epididymis. The specific expression pattern of ADGRG2 allowed us to develop a non‐ciliated cell‐specific labelling technique by exploiting the promoter of the ADGRG2 gene. Using this newly developed method, we successfully isolated non‐ciliated cells and showed that a diminished constitutive chloride current was the cause of the unbalanced pH state in the efferent ductules and dysfunction in fluid resorption in Adgrg2 −/Y mice (Zhang et al., 2018).
Further analysis combining Gq −/+ and Adgrg2 −/Y mouse models, pharmacological intervention and cell labelling techniques demonstrated that ADGRG2 regulated Cl− and pH homeostasis through Gq‐dependent coupling between the receptor and the anion channel cystic fibrosis transmembrane conductance regulator (CFTR) (Figure 1) (Zhang et al., 2018). CFTR and ADGRG2 co‐localize at the apical membrane of non‐ciliated cells, along with selective high expression of Gq in the same cells. Through coupling to Gq, ADGRG2 maintains the basic CFTR outward‐rectifying current, which is required for fluid resorption and sperm maturation (Figure 1) (Zhang et al., 2018). Importantly, whereas disruption of β‐arrestin2 has no significant effects on the fluid resorption function, β‐arrestin 1 deficiency impaired pH and Cl− homeostasis in the efferent ductules and initial segment of the epididymis (Zhang et al., 2018). Further investigation confirmed the coexistence of ADGRG2, CFTR, β‐arrestin 1, and Gq in the same protein complex (Figure 1), while β‐arrestin 1 deficiency abolished the colocalization of ADGRG2 and CFTR on the apical membrane. These data suggested that the ADGRG2/β‐arrestin 1/ Gq /CFTR supercomplex localizes at the apical membrane of non‐ciliated cells and functions as a regional signalling hub, controlling fluid reabsorption and maintaining pH and Cl− homeostasis in the efferent ductules and initial segment of the epididymis (Figure 1) (Zhang et al., 2018). The ADGRG2/CFTR interaction in the epididymis represents yet another example of the functional divergence between the two β‐arrestin isoforms, already established in several other tissues and organs (Lymperopoulos, 2018; Lymperopoulos et al., 2019; Srivastava et al., 2015). For example, in the heart, β‐arrestins 1 and 2 were initially thought of as functionally interchangeable but actually exerted diametrically opposite effects in the mammalian myocardium. β‐arrestin 1 exerts overall detrimental effects on the heart; in contrast, β‐arrestin 2 is overall beneficial for the myocardium (Lymperopoulos et al., 2019).
Consistent with our findings that inhibition of ADGRG2 or Gq activity caused fluid resorption dysfunction, recent clinical studies have revealed that multiple ADGRG2 mutations are associated with male infertility. For example, p.Glu516Ter, p.Leu668ArgfsTer21, p.Arg814Ter, or p.Lys818Ter results in the absence or truncation of the seven‐transmembrane domain, which might abolish receptor coupling to downstream Gq and Gs proteins and β‐arrestins, and eventually leads to male infertility (Figure 2a, Table 2) (Khan et al., 2018; Patat et al., 2016; Yuan et al., 2019). However, it is worth noting the potential CTF‐independent functions of aGPCRs. For example, Gpr126 st49 mutant zebrafish still express a functional fragment of NTF but no CTF (Patra et al., 2013). Therefore, these ADGRG2 mutations might preserve some of the ADGRG2 functions and knock‐in models of these mutations are needed for detailed characterization of these disease‐associated ADGRG2 mutations. Moreover, the p.Cys949AlafsTer81 frameshift mutation, the missense p.Lys990Glu, and p.Arg1008Gln mutations produce a protein with an affected C‐terminal domain of ADGRG2, which may affect the expression level of the receptor or G protein/arrestin‐mediated signalling (Figure 2a, Table 2) (Patat et al., 2016; B. Yang et al., 2017; Yuan et al., 2019). Therefore, different ADGRG2 mutations may cause similar male infertility phenotypes, through quite different cellular signalling mechanisms.
FIGURE 2.
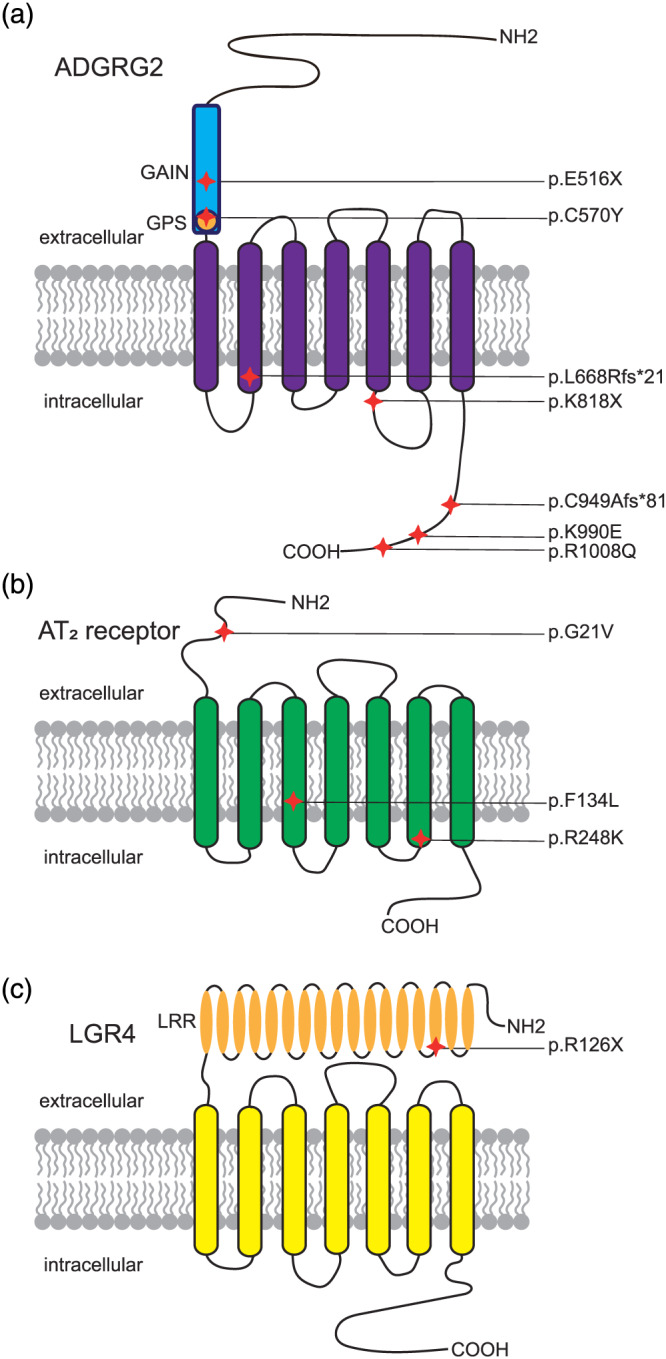
GPCR mutations associated with disease. The approximate positions of different mutations are indicated in the structures of ADGRG2 (A), AT2 receptor (B), and LGR4 (C).. Abbreviations: GAIN domain: GPCR Autoproteolysis‐INducing domain; GPS, G protein‐coupled receptor proteolytic site; LRR, leucine‐rich repeats
TABLE 2.
Disease‐related SNP analysis in GPCRs
| GPCR | dbSNP rs# cluster id | dbSNP allele change | Protein residue change | Amino acid pos | Accession disease names | References |
|---|---|---|---|---|---|---|
| ADGRG2 (GPR64) Xp22.13 | rs879255540 | ‐>T | Glu [E]>* | 516 | Congenital bilateral absence of vas deferens | Patat et al. (2016) |
| G > A | Cys [C] > Tyr [Y] | 570 | Congenital bilateral absence of vas deferens | Yang et al. (2017) | ||
| rs879255539 | CTGTG > AGA | Leu [L] > Arg [R] | 668 | Congenital bilateral absence of vas deferens | Patat et al. (2016) | |
| C > T | p.Arg [R]>* | 814 | Obstructive azoospermia | Khan et al. (2018) | ||
| A > T | p.Lys [K]>* | 818 | Congenital absence of vas deferens | Yuan et al. (2019) | ||
| rs879255538 | T>‐ | Cys [C] > Ala [A] | 949 | Congenital bilateral absence of vas deferens | Patat et al. (2016) | |
| A > G | p.Lys [K] > Glu [E] | 990 | Congenital bilateral absence of vas deferens | Yang et al. (2017) | ||
| G > A | p.Arg [R] > Gln [Q] | 1008 | Congenital absence of vas deferens | Yuan et al. (2019) | ||
| AT2 receptorXq23 | rs121917810 | G > T | Gly [G] > Val [V] | 21 | X‐linked mental retardation | Vervoort et al. (2002), Bienvenu, (2003), and Ylisaukko‐oja et al. (2004) |
| T>‐ | Phe [F] > Leu [L] | 134 | Not specified | Piton, Redin, & Mandel, (2013) | ||
| rs5191 | G > A | Arg [R] > Lys [K] | 248 | Not specified | Bean, Tinker, da Silva, & Hegde, (2013) and Wang et al. (2006) | |
| LGR4 (GPR48) 11p14.1 | rs587777005 | C > T | Arg [R]>* | 126 | Low bone mineral density | Styrkarsdottir et al. (2013) |
Notably, the human ADGRG2 mutations mentioned above are clinically associated with congenital bilateral absence of the vas deferens (CBAVD). In general, CBAVD involves a complete or partial absence of the Wolffian duct derivatives. In most cases of CBAVD, it is generally presumed that the genital tract abnormality is developed by a progressive atrophy related to abnormal electrolyte ion balance and dysfunction of fluid homeostasis in the male excurrent ducts, rather than agenesis. This model is supported by the link between CBAVD and mutations of the gene encoding the chloride channel CFTR (Patat et al., 2016). In our recent report, we have demonstrated a functional coupling between ADGRG2 and CFTR that serves as the key event in maintenance of Cl− and pH homeostasis in efferent ductules and epididymis, and a persistent dysfunction may finally cause progressive atrophy of the efferent/epididymis ductules (Zhang et al., 2018). Thus, impairment of the ADGRG2/CFTR coupling may directly relate to the CBAVD in male infertility patients. It is worth noting that the infertile patients are usually identified as adults, whereas the animal models normally have a shorter life span. This could explain the fact that ADGRG2 KO mice did not develop CBVAD in their life time. For an ADGRG2‐targeted therapy, as a treatment for male infertility, a systematic screening for male sterility genes, identification of the genetic mutations in ADGRG2 or CFTR, as well as genetic or pharmacological intervention in the early stage of a male patient carrying the mutations could be considered.
ADGRG2 show increased constitutive activity in the absence of the NTF (Demberg et al., 2015; Liebscher et al., 2015). The ADGRG2‐CTF is able to constitutively activate the CFTR current in transfected HEK293 cells (Zhang et al., 2018). Therefore, further investigation is needed to determine whether constitutive ADGRG2 activity is sufficient to maintain the microenvironment of the epididymis and efferent ductules or whether an endogenous ADGRG2 ligand is required in this process. It is worth noting that a 15‐amino acid peptide derived from the N‐terminus of the ADGRG2‐CTF was shown to activate ADGRG2 with low affinity (Table 3) (Balenga et al., 2017; Demberg et al., 2015; Demberg et al., 2017). Further modification of ADGRG2 ligands derived from this peptide might increase the activity of certain ADGRG2 mutants and exhibit therapeutic potential. Of note, the peptide‐based agonists often show non‐selectivity toward aGPCR members; hence, structural analysis of a peptide agonist‐bound aGPCR is necessary to elucidate the detailed activation mechanisms of aGPCRs (Demberg et al., 2017). Alternatively, we have also shown that activation of AT2 receptors in the efferent ductules can restore the dysfunction in fluid resorption in isolated efferent ductules, derived from Adgrg2 −/Y mice (Zhang et al., 2018). Thus, further investigation is warranted to determine whether specific therapeutic methods such as treatment with a selective agonist need to be developed for different ADGRG2 mutants or whether a more general rescue approach, such as activation of AT2 receptors, is sufficient to treat patients carrying ADGRG2 mutations.
TABLE 3.
Potential therapeutic ligands targeting to GPCRs in epididymis
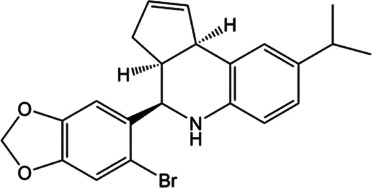
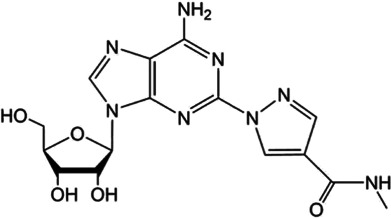
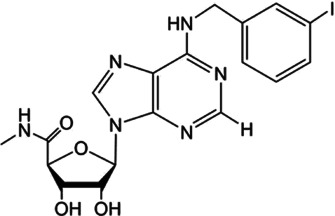
| Receptor | Ligand | Structure (or sequence) | Mode of action | Highest status | References |
|---|---|---|---|---|---|
| ADGRG2 | Tethered peptide agonist | TSFGILLDLSRTSLP | Agonist | Demberg et al. (2015) and Balenga et al. (2017) | |
| AT2 receptor | Angiotensin II (Ang II) | Asp1‐Arg2‐Val3‐Tyr4‐Ile5‐His6‐Pro7‐Phe8 | Agonist | Clinic | Guimond, Hallberg, Gallo‐Payet, and Wallinder (2014) and Hallberg, Sumners, Steckelings, and Hallberg (2018) |
| Saralasin | [Sar1,Val5,Ala8]Ang II | Agonist | Clinic | Guimond et al. (2014) and Hallberg et al. (2018) | |
| Sarile | [Sar1,Ile8]Ang II | Agonist | Clinic | Guimond et al. (2014) and Hallberg et al. (2018) | |
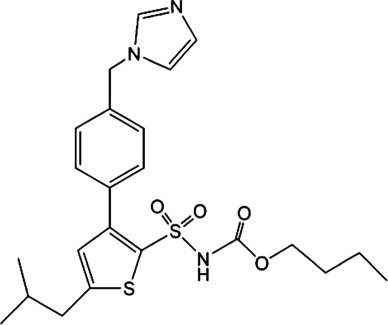
| MP‐157 | No structural formula is disclosed | Agonist | Phase 1 | Hallberg et al. (2018) | |
| C21/M24 |
|
Agonist | Phase 2 | Hallberg et al. (2018) | |
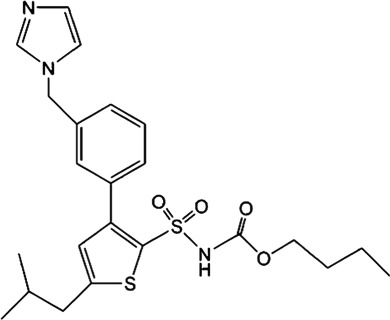
| C38/M132 |
|
Antagonist | Hallberg et al. (2018) | ||
| LGR4 | R‐spondins | R‐spondin1‐4(RSPO1‐4) | Agonist | Carmon et al. (2011), de Lau et al. (2011), and Glinka et al. (2011) | |
| Norrin | MRKHVLAASFSMLSLLVIMGDTDSKTDSSFIMDSDPRRCMRHHYVDSISHPLYKCSSKMVLLARCEGHCSQASRSEPLVSFSTVLKQPFRSSCHCCRPQTSKLKALRLRCSGGMRLTATYRYILSCHCEECNS | Agonist | Deng et al. (2013) | ||
| TNFSF11(RANKL) | Tumor necrosis factor (TNF) superfamily member 11 | Agonist | Luo et al. (2016) | ||
| GPER | G‐1 |
|
Agonist | Bologa et al. (2006) | |
| G15 |
|
Antagonist | Dennis et al. (2009) and Dennis et al. (2011) | ||
| G36 |
|
Antagonist | Dennis et al. (2011) | ||
| A1AR | Trabodenoson (INO‐8875) |
|
Partial agonist | Phase 3 | Jacobson, Tosh, Jain, and Gao (2019) |
| A2AAR | Regadenoson |
|
Agonist | Jacobson et al. (2019) | |
| A3AR | IB‐MECA |
|
Agonist | Phase 3 | Jacobson et al. (2019) |
3. ENDOGENOUS ANGIOTENSIN SYSTEM AND AT2 RECEPTORS IN EPIDIDYMIS
The epididymal lumen and efferent ductules contain a complete local renin–angiotensin system (RAS) including renin, angiotensin I (Ang I), and angiotensin II (Ang II) in the seminal fluid, the angiotensin‐converting enzyme specific to the testes (tACE), and angiotensin AT1 and AT2 receptors in the basal cells of the epididymis (Leung, Chan, Fu, Zhou, & Wong, 1997; Leung & Sernia, 2003; Saez, Legare, Laflamme, & Sullivan, 2004; Speth, Daubert, & Grove, 1999; Strittmatter, Thiele, De Souza, & Snyder, 1985; Wong & Uchendu, 1990; W. Zhao, Leung, Chew, Chan, & Wong, 1996). Importantly, Ang II in the epididymal lumen is mainly produced through the cleavage of Ang I by tACE (Langford, Zhou, Russell, Wilcox, & Bernstein, 1993; Sibony, Segretain, & Gasc, 1994). Deficiency in tACE leads to male infertility through impairing the function but not the production of sperm, implying that the RAS plays an important role in sperm maturation (Esther et al., 1996; Hagaman et al., 1998; Krege et al., 1995).
AT1 and AT2 receptors have been found in a radio‐ligand binding assay to be expressed in the epididymal lumen. In particular, AT2 receptors were specifically detected in basal cells and were required for the proton‐secretion function of the epididymal lumen (Figures 1 and 2b, Table 1) (Shum et al., 2008). Unexpectedly, AT2 receptors were absent in clear cells, which regulated proton secretion. Further studies showed that AT2 receptors also activated the NO‐cGMP pathway in response to Ang II stimulation in basal cells (Figure 1). NO produced by basal cells quickly diffuses to clear cells, activating soluble guanylate cyclase. Then, the elevation of the cGMP concentration mediated by guanylate cyclase triggers the apical accumulation of V‐ATPase in the microvilli, ultimately leading to increased proton secretion (Figure 1) (Shum et al., 2008). This model is consistent with the essential role of Ang II production and the requirement for tACE in the maintenance of the proper luminal ion/water environment and sperm maturation. Thus, a delicate signalling network between basal cells and adjacent clear cells modulated by the AT2 receptor may contribute to the finely tuned microenvironment of the luminal space of the epididymis.
Interestingly, male infertility may result from dysfunction in the proton balance in the efferent ductules without significant impairment of AT2 receptor function, suggesting that an AT2 receptor‐targeted treatment may have therapeutic potential. In our recent study, applying 100‐nM Ang II restored pH homeostasis and fluid reabsorption in efferent ductules derived from Adgrg2 −/Y mice. This restoration was blocked specifically by PD123319, an antagonist of AT2 receptors, but not by an AT1 receptor antagonist (Zhang et al., 2018). Therefore, specific agonists of AT2 receptors could be considered as therapeutic drugs to treat male infertility associated with a significant impairment in the pH balance in the efferent ductules or epididymis. AT2 receptor agonist treatment might also be applied to male infertility caused by deficits in the ionic exchangers NHE3 and DRA, that modulate pH homeostasis in the efferent ductules and epididymis (Lee, Finnigan‐Bunick, Bahr, & Bunick, 2001; Q. Zhou et al., 2001; Zhang et al., 2018).
For AT2 receptors, both peptide‐based agonists and low MW non‐peptide agonists have been developed, which have therapeutic potential to treat several human diseases (Table 3) (Bennion, Steckelings, & Sumners, 2018; Hallberg et al., 2018). Sarile and saralasin are two peptide agonists of AT2 receptors that have been approved by the FDA to treat hypertension and used in the clinic for a short period (Table 3) (Guimond et al., 2014; Hallberg et al., 2018). These peptides block AT1 receptors but activate AT2 receptors. Currently, it remains unknown whether the blockade of AT1 receptors activity is critical for the normal function of the efferent ductules or epididymis. Therefore, the application of these two peptides for the treatment of sperm obstruction in male infertility requires further evaluation. Recently, β‐Pro7AngIII was reported to show high selectivity for the activation of AT2 receptors, with no significant effect on AT1 receptors (Hallberg et al., 2018), providing an alternative choice for peptide‐based AT2 receptor activation therapy in male infertility. Low MW non‐peptide compounds have also been developed as agonists of AT2 receptors for clinical treatment. For example, MP‐157 was used as an AT2 receptor agonist for cardiovascular disease treatment in a Phase 1 clinical trial, whereas C21/M24 was tested in a Phase 2 exploration of idiopathic pulmonary fibrosis (IPF) (Table 3) (Hallberg et al., 2018). Testing these low MW compounds or their derivatives will be of great interest for developing treatment for male infertility, related to impaired pH homeostasis in the efferent ductules or epididymis. Meanwhile, as AT2 receptors are widely expressed and play important roles in many tissues such as heart, brain, adrenal glands, uterine myometrium, ovarian follicles, kidney, and pancreas, the side effects of AT2 receptor agonists have to be considered (de Gasparo & Siragy, 1999; Leung, 2001; Verdonk, Danser, & van Esch, 2012). Local application of AT2 receptor agonists can reduce systemic side effects caused by conventional oral or injection administration. Moreover, appropriate therapeutic dosage should also be considered.
4. LGR4: AN ESSENTIAL GPCR FOR EPIDIDYMAL DEVELOPMENT
LGR4, also called G protein‐coupled receptor 48 (GPR48), is a member of the LGR subgroup of the rhodopsin‐like GPCR superfamily, which derives its name from a large extracellular domain consisting of multiple leucine‐rich repeats (Figure 2c). LGR4 is widely expressed in human and mouse tissues, with the highest expression levels in the epidermis and hair follicles of the skin, pancreatic islet cells and epithelial cells in the male and female reproductive organs (Van Schoore, Mendive, Pochet, & Vassart, 2005; Yi et al., 2013).
LRG4 plays an important role in postnatal epididymal development in mice. In Lgr4 KO mice, the epididymal tubule, especially the caput region, fails to elongate and convolute, and the resulting duct is surrounded by a thick condensation of mesenchymal cells. This abnormal cellular organization suggests that LGR4 is important for epithelial–mesenchymal interactions (Table 1) (Mendive et al., 2006). Furthermore, the expression levels of the oestrogen receptor α (ERα) and the androgen receptor (NR3C4) are dramatically reduced in the epididymis of male Lgr4 KO mice, which in turn leads to decreased expression of Na+‐K+‐ATPase, the Na+/ H+ exchanger NHE3 and aquaporin 9 (AQP 9) (X. Y. Li et al., 2010). LRG4 up‐regulates ERα expression via the cAMP/PKA signalling pathway (Figure 1). Downstream of the LRG4‐cAMP‐PKA pathway, CREB binds to a Cre motif in the ERα promoter and activates its expression (X. Y. Li et al., 2010).
The pivotal role of LGR4 in the epididymis is further supported by a Lgr4 hypomorphic mutant mouse line (Lgr4 Gt) that was developed through gene‐trap insertional mutagenesis. Short and dilated epididymal tubules are detected in homozygous Lgr4 Gt/Gt mice, which have only one tenth of the normal Lgr4 expression level. Moreover, multi‐lamination and distortion of the basement membranes (BMs) are observed in the caput region, and the initial segment is completely lost (Hoshii et al., 2007). Lgr4 KO or hypomorphic mice also show deficits in the testes and efferent ductules (Qian et al., 2013), which together with the epididymal defects eventually lead to male infertility in mice.
Overexpressed LGR4 has been found to activate heterotrimeric Gs proteins to elevate intracellular cAMP levels (Gao et al., 2006). Moreover, R‐spondins and norrin were identified as LGR4 ligands that could bind LGR4 and stimulate the Wnt signalling pathway (Table 3) (Carmon et al., 2011; de Lau et al., 2011; Deng et al., 2013; Glinka et al., 2011). Recently, tumor necrosis factor (TNF) superfamily member 11 (TNFSF11, also known as RANKL) was identified as a novel LGR4 ligand (Table 3) (J. Luo et al., 2016). TNFRSF11A (also called RANK) was considered to be the sole receptor for TNFSF11 until LGR4 was found to compete with RANK and suppress canonical RANK signalling. TNFSF11 binds to LGR4 and subsequently activates the Gq and glycogen synthase kinase 3 beta (GSK3‐β) signalling pathway (J. Luo et al., 2016). Unlike LRG4/cAMP/PKA pathway, the specific role of R‐spondin/LGR4/Wnt or TNFSF11/LRG4/GSK3‐β pathway in male reproduction has not been characterized yet. Nevertheless, RNAseq revealed that mRNAs encoding R‐spondins, norrin, and TNFSF11 are expressed in human epididymis epithelial cells, albeit at low levels (Browne, Yang, Leir, Eggener, & Harris, 2016).
5. COMPLEX FUNCTIONS OF GPER IN THE EPIDIDYMIS
The G protein‐coupled estrogen receptor 1 (GPER), also known as G protein‐coupled receptor 30 (GPR30), was first identified as a receptor that induced MAP kinase (ERK1/2) activation by binding to estrogen (Carmeci, Thompson, Ring, Francke, & Weigel, 1997; Filardo et al., 2000; O'Dowd et al., 1998; Prossnitz, Arterburn, & Sklar, 2007). Compounds such as the GPER antagonist fulvestrant (ICI 182780) and the GPER agonist G‐1 can also modulate GPER to induce rapid non‐genomic cellular responses (Bologa et al., 2006; Lucas, Royer, Siu, Lazari, & Porto, 2010; Revankar, Cimino, Sklar, Arterburn, & Prossnitz, 2005). Unlike the other members of the GPCR family that mainly reside on the plasma membrane, GPER is broadly localized on the endoplasmic reticulum and nuclear envelope, as well as the plasma membrane (Figure 1) (Funakoshi, Yanai, Shinoda, Kawano, & Mizukami, 2006; Prossnitz et al., 2007; Revankar et al., 2005; Thomas, Pang, Filardo, & Dong, 2005).
GPER has been detected in many male reproductive structures, such as the testes (Cassault‐Meyer, Gress, Seralini, & Galeraud‐Denis, 2014; Gautier et al., 2016; Lucas et al., 2010), spermatozoa (Arkoun et al., 2014; Cassault‐Meyer et al., 2014; Gautier et al., 2016), and prostate (Rago, Romeo, Giordano, Ferraro, & Carpino, 2016). It has also been found in the efferent ductules and epididymis (Cao et al., 2017; Hess et al., 2011; Katleba et al., 2015; Krejcirova et al., 2018; Lu et al., 2016; Malivindi, Aquila, & Rago, 2018; Martinez‐Traverso & Pearl, 2015; Menad et al., 2017; Pereira et al., 2014; Rago et al., 2018), indicating that GPER may play important roles in sperm maturation, protection and storage (Table 1). For instance, in the corpus epididymis of postnatal pigs, GPER participates in sperm maturation by affecting the formation of the blood–epididymal barrier (Katleba et al., 2015). In the caudal epididymal epithelium in immature rats, GPER induces a pathway involved in cAMP‐CFTR‐chloride secretion to regulate osmotic pressure in response to a perfusion solution and thus affects sperm motility (Figure 1) (Cao et al., 2017).
In addition, the relative abundance of GPER in the efferent ductules and each part of the epididymis, the cellular localization of GPER and the molecular weight of the protein differ depending on the species, developmental stage and physiological cycle studied (Krege et al., 1995; Krejcirova et al., 2018; Lu et al., 2016; Pereira et al., 2014). Therefore, the role of GPER in the efferent ductules and epididymis appears to be complex. The first GPER‐specific agonist, G‐1, has been identified through virtual and biomolecular screening (Table 3) (Bologa et al., 2006). Based on the synthesis of the G‐1 analogue as well as additional screening, two GPER‐selective antagonists, G15 and G36, were also identified, both of which inhibit estrogen‐ and G‐1‐stimulated cell proliferation in vivo (Table 3) (Dennis et al., 2009; Dennis et al., 2011). Recently, a series of indole–thiazole derivatives were identified as new GPER agonists (O'Dea, Sondergard, Sweeney, & Arnatt, 2018). These newly identified agonists and antagonists provide very useful tools for further evaluation of the therapeutic potential of GPER in treating male infertility, given the potential complex function of GPER in male systems. Overall, the evaluation of GPER as a drug target in male infertility requires further investigation, and the new compounds identified for specific regulation of GPER activity will certainly accelerate this assessment.
6. TWO ADENOSINE RECEPTORS WITH OPPOSITE FUNCTIONS IN THE EPIDIDYMIS
Adenosine receptors consist of four members, namely, A1, A2A, A2B and A3, and the corresponding genes are ADORA1, ADORA2A, ADORA2B, and ADORA3. Adenosine receptors couple to different G proteins that trigger the activation of distinct intracellular signalling pathways. The A1 and A3 adenosine receptors are coupled to Gi/o proteins, and the activation of these receptors inhibits cAMP production. In contrast, A2A and A2B adenosine receptors are coupled to Gs/olf proteins, and the activation of these receptors results in increased cAMP production (Chen, Lee, & Chern, 2014). Besides Gs and Gi coupling that modulates cAMP levels, Gq coupling with PLC and subsequent IP3 and Ca2+ modulation downstream of adenosine receptors has also been described (Santiago et al., 2020). Moreover, the A1 adenosine receptor can also signal via interaction with β‐arrestins, which leads to the activation of ERK1/2 (Table 1) (Geldenhuys et al., 2017). Most adenosine receptors have been suggested to be present in the epididymis (Table 1) (Haynes et al., 1998b; Minelli, Miscetti, Allegrucci, & Mezzasoma, 1995).
The A1 and A2 adenosine receptors regulate the contractility of the vas deferens and epididymis (Table 1) (Brownhill, Hourani, & Kitchen, 1996; Haynes et al., 1998b; Haynes, Alexander, & Hill, 1998a). Interestingly, it seems that the A1 and A2 receptors have opposite effects on the contractility of the epididymis, with the A1 receptors enhancing contractility, whereas A2 receptors inhibit contractility (Haynes et al., 1998b). This phenomenon might be explained by the difference in their G protein‐coupling selectivity (van Galen, Stiles, Michaels, & Jacobson, 1992). In the epididymis, A2 adenosine receptors increase intracellular cAMP levels (Haynes et al., 1998b), consistent with the generally accepted view that A2 adenosine receptors are coupled to Gs protein and activate AC to increase intracellular cAMP levels (Figure 1) (Chen et al., 2014; Fredholm et al., 1994; Santiago et al., 2020). Further investigation showed that the A2A receptor mediates potassium channel activation through PKA and PKG in rat epididymal smooth muscle (Haynes, 2000). This result is consistent with the finding that A2 receptor activation stimulated cAMP‐dependent PKA, which in turn modulated potassium channel activity in arterial or skeletal muscles (Barrett‐Jolley, Comtois, Davies, Stanfield, & Standen, 1996; Kleppisch & Nelson, 1995).
Besides important roles in the contractility of epididymis, adenosine receptors also actively participate in spermatogenesis, capacitation, and acrosome reaction (Bellezza & Minelli, 2017). The number of capacitated spermatozoa incubated in human tubal fluid was significantly reduced in Adora1 −/− spermatozoa, and the difference between Adora1 +/+ and Adora1 −/− mouse spermatozoa is mainly in the time necessary to reach the maximum percentage of capacitation (Minelli et al., 2004). Meanwhile, the spermatozoa of Adora1 −/− mice are less prone to acrosome reaction (Minelli et al., 2004; Minelli, Bellezza, Collodel, & Fredholm, 2008). Furthermore, a significant reduction of the number of pups produced by Adora1 −/− male mice suggests that A1 adenosine receptors must be fully operative to accomplish the optimal degree of capacitation and thereby fertilization (Minelli et al., 2004). Polydeoxyribonucleotide (PDRN), an agonist of A2A receptors, significantly restored spermatogenic function in varicocele rats and testicular torsion rats (Minutoli et al., 2012; Minutoli et al., 2015).
Adenosine (and its precursor ATP) has been used for several decades to treat cardiac arrhythmias through activating A1 adenosine receptors (Szentmiklosi et al., 2015). Adenosine is also the gold‐standard agent to create maximum coronary hyperemia through activating A2A adenosine receptors (McGeoch & Oldroyd, 2008). However, given that adenosine can activate the range of adenosine receptors, it inevitably produces some undesirable adverse effects. To avoid non‐specific global adverse reactions, selective agonists of A1, A2A, and A3 adenosine receptors have been developed, some of which are currently undergoing clinical trials (Jacobson et al., 2019). For example, the A1 receptor partial agonist trabodenoson (INO‐8875) was tested for the treatment of glaucoma and ocular hypertension, but it failed in a Phase 3 trial because its primary endpoint was not achieved (Table 3) (Jacobson et al., 2019). The moderately selective A2A adenosine receptor agonist regadenoson was first approved as a pharmacological stress agent in 2008 and is currently being tested in various clinical trials for cardiovascular treatment and diagnosis (Table 3) (Jacobson et al., 2019). The moderately selective A3 adenosine receptor agonist IB‐MECA (CF101, piclidenoson) is being tested in a Phase 3 clinical trial for the treatment of autoimmune anti‐inflammatory diseases (Table 3) (Jacobson et al., 2019).
An important limitation of adenosine receptor agonists is agonist‐induced desensitization (Mundell & Kelly, 2011). The application of either partial agonists or positive allosteric modulators (PAMs) may circumvent desensitization and improve therapies. Currently, only adenosine and regadenoson are approved for human use (Jackson et al., 2018). However, many adenosine receptor agonists and PAMs (such as the A1 adenosine receptor PAM benzoylthiophenes) are being tested in humans, and it is of great interest to test the effects of these compounds on the regulation of epididymis functions and the treatment of male infertility.
7. FUTURE QUESTIONS AND PERSPECTIVES
Numerous GPCRs are expressed in the efferent ductules and epididymis, which consist of various cell types. In the present review, we focus on the role of ADGRG2, AT2 receptors, LGR4, GPER and the adenosine receptors in the epididymis. Other GPCRs such as bradykinin receptors and Frizzled receptors have also been shown to be expressed in the epididymis (Belleannée et al., 2009; K. Wang et al., 2015). Moreover, RNAseq suggested that many other GPCRs, including ADGRF1 (GPR110), ADGRG1 (GPR56), GPRC5C, GPR107, GPR108, GPR125, GPR137, and GPR160 are expressed in the epididymis (Browne et al., 2016). The precise role of these GPCRs in the epididymis awaits further investigation and will be reviewed in the future.
At present, the following questions remain. (1) Which GPCRs are expressed in a particular cell type? (2) How do these GPCRs contribute to the development and normal physiological functions of the epididymis and efferent ductules? (3) Can any of these GPCRs functionally compensate for each other? (4) If so, is it possible to activate an alternative GPCR in the epididymis or efferent ductules to rescue the dysfunction of a particular GPCR, such as in cases of infertility caused by ADGRG2 mutations? (5) Is there crosstalk between different GPCRs or between GPCRs and other membrane proteins in specific cell types? (6) Are endogenous ligands of the GPCRs in epididymis and efferent ductules constantly produced in the local environment to actively regulate specific physiological processes of epididymis development and sperm maturation? (7) Do second messengers downstream of GPCRs, such as cAMP and calcium, have distinct functions in different types of cells in the epididymis and efferent ductules, and how are they regulated by different GPCRs? (8) Are location bias (signalling compartments) and effector bias important for the regulation of different GPCRs expressed in the epididymis and efferent ductules? (9) What are the endogenous ligands for ADGRG2, AT2 receptor, GPER, and LGR4 in the local male fertility system? (10) Do FDA‐approved drugs targeted to GPCRs with known functions in the epididymis, such as AT2 receptor and adenosine receptors, have beneficial effects on male fertility? (11) Are there regional drug delivery systems that can target specific GPCRs in the epididymis to decrease the side effects of GPCR ligands? To answer these questions, a systematic investigation of the GPCR expression in epididymis and efferent ductules by transcriptional analysis and the single cell sequencing, utilization of the conditional knock mice driven by the specific epididymis or efferent ductule marker Cre, combined with the molecular and cellular approaches to delineate the mechanism underlying the specific GPCR functions in male infertility and the usage of the biochemical approach and the proteomics and metabolomics to identify the endogenous ligands for specific GPCR such as the ADGRG2, will lay an important foundation for evaluation of these GPCRs as potential therapeutic targets for male infertility treatment. Moreover, use of the specific known chemical ligands for these GPCRs, together with selective drug delivery methods and assessment of the effects of these ligands in male infertility mice models, will provide further information for drug development toward these GPCRs.
8. CONCLUSIONS
Male infertility rates have continuously increased in recent years, and few effective treatments with known targets and defined mechanisms exist. Recently, the identification of mutations in specific GPCR superfamily members related to male infertility and the increased understanding of the detailed molecular mechanisms involving these GPCRs in the regulation of sperm maturation and homeostasis of the microenvironments of the epididymis and efferent ductules have provided new clues on the potential development of therapies to treat male infertility, given that these receptors account for almost 1/3 of current clinical drug targets.
In addition to ADGRG2 and AT2 receptors, GPCR superfamily members such as LGR4, GPER, and adenosine receptors are known to play important roles in the regulation of postnatal epididymal development, the formation of the blood–epididymal barrier, the maintenance of osmotic pressure in a perfusion solution, and the contractility of the epididymis (Table 1). The repertoire of the physiological roles of these GPCRs and other uncharacterized GPCRs, as well as further detailed studies of these receptor connecting to male infertility development, provide entirely novel therapeutic opportunities for the treatment of male infertility.
Currently, a variety of low MW compounds, peptide ligands and endogenous ligands have been found or developed to target AT2 receptors, LGR4, GPER, and adenosine receptors (Table 3). It is worth noting that several such compounds or ligands have been approved by the FDA for the treatment of diseases other than male infertility. Therefore, there is great interest in testing these ligands and compounds in male infertility animal models to examine their therapeutic potential. It is also worth noting that endogenous or high‐affinity ligands involved in the regulation of ADGRG2 have not been identified. Such tools are greatly needed to understand the function of ADGRG2 in male fertility and evaluate the potential role of ADGRG2 as a therapeutic target in male infertility.
Only a small number of the signalling pathways downstream of GPCRs have been characterized in detail in the efferent ductules and epididymis, and these pathways have shown unique signalling properties, although they sometimes share signal‐transducing effectors (Figure 1). For example, both ADGRG2 and GPER have been shown to couple to Gs in the epididymis; however, they exhibit distinct subcellular microdomain biases in their signalling. ADGRG2 forms a signal transduction complex with β‐arrestin 1, Gq, and CFTR on the apical membrane, whereas GPER forms a complex with Gs at the endoplasmic reticulum, nuclear envelope, and plasma membrane (Figure 1). Therefore, even when sharing effectors, the location bias of each GPCR may determine its detailed specific functions in the epididymis and efferent ductules. This possibility raises the question of whether activation of an alternative GPCR in the epididymis or efferent ductules will be able to restore the dysfunction of a particular GPCR, such as in cases of infertility caused by the ADGRG2 mutations.
Collectively, the complex signalling of GPCR members in the epididymis and the specific physiological roles of these GPCRs that contribute to male fertility are worthy of further detailed investigation. In addition, the prospect of using their ligands highlights new opportunities for the development of treatments for male infertility.
8.1. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in the IUPHAR/BPS Guide to PHARMACOLOGY (http://www.guidetopharmacology.org) and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander, Christopoulos, et al., 2019; Alexander, Cidlowski, et al., 2019; Alexander, Fabbro, et al., 2019; Alexander, Mathie, et al., 2019).
CONFLICT OF INTEREST
The authors declare no conflicts of interests.
ACKNOWLEDGEMENTS
We acknowledge support from the National Key Basic Research Program of China (2018YFC1003600 to J. S., Z. X., W. X. and X.Y.), the National Natural Science Foundation of China (81773704 to J. S., 81771001 to Z. X., and 81901548 to D. Z.), the Shandong Provincial Natural Science Foundation (ZR2019BC078 to D. Z.), the Shandong Provincial Medicine and Health Science Technology Development Program (2017WS692 to D. Z.), the National Science Fund for Distinguished Young Scholars (81825022 to J. S.), the Fundamental Research Funds of Shandong University (2018JC025 to Z. X.), and the Program for Changjiang Scholars and Innovative Research Team in University (IRT13028).
Zhang D, Wang Y, Lin H, et al. Function and therapeutic potential of G protein‐coupled receptors in epididymis. Br J Pharmacol. 2020;177:5489–5508. 10.1111/bph.15252
Daolai Zhang and Yanfei Wang contributed equally to this work.
Contributor Information
Jin‐Peng Sun, Email: sunjinpeng@sdu.edu.cn.
Zhigang Xu, Email: xuzg@sdu.edu.cn.
REFERENCE
- Abe, K. , Takano, H. , & Ito, T. (1984). Microvasculature of the mouse epididymis, with special reference to fenestrated capillaries localized in the initial segment. The Anatomical Record, 209(2), 209–218. 10.1002/ar.1092090208 [DOI] [PubMed] [Google Scholar]
- Abou‐Haila, A. , & Fain‐Maurel, M. A. (1984). Regional differences of the proximal part of mouse epididymis: Morphological and histochemical characterization. The Anatomical Record, 209(2), 197–208. 10.1002/ar.1092090207 [DOI] [PubMed] [Google Scholar]
- Ahn, J. I. , Yoo, J. Y. , Kim, T. H. , Kim, Y. I. , Broaddus, R. R. , Ahn, J. Y. , … Jeong, J. W. (2019). G‐protein coupled receptor 64 (GPR64) acts as a tumor suppressor in endometrial cancer. BMC Cancer, 19(1), 810 10.1186/s12885-019-5998-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitken, R. J. (2006). Sperm function tests and fertility. International Journal of Andrology, 29(1), 69–75discussion 105‐108. 10.1111/j.1365-2605.2005.00630.x [DOI] [PubMed] [Google Scholar]
- Aitken, R. J. , Nixon, B. , Lin, M. , Koppers, A. J. , Lee, Y. H. , & Baker, M. A. (2007). Proteomic changes in mammalian spermatozoa during epididymal maturation. Asian Journal of Andrology, 9(4), 554–564. 10.1111/j.1745-7262.2007.00280.x [DOI] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Mathie, A. , Peters, J. A. , … Davies, J. A. (2019). THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: G protein‐coupled receptors. British Journal of Pharmacology, 176(Suppl 1), S21–S141. 10.1111/bph.14748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Cidlowski, J. A. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , … Davies, J. A. (2019). The concise guide to pharmacology 2019/20: Nuclear hormone receptors. British Journal of Pharmacology, 176(Suppl 1), S229–S246. 10.1111/bph.14750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , … Davies, J. A. (2019). The concise guide to pharmacology 2019/20: Enzymes. British Journal of Pharmacology, 176(Suppl 1), S297–S396. 10.1111/bph.14752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Mathie, A. , Peters, J. A. , Veale, E. L. , Striessnig, J. , Kelly, E. , … Davies, J. A. (2019). The concise guide to pharmacology 2019/20: Ion channels. British Journal of Pharmacology, 176(Suppl 1), S142–S228. 10.1111/bph.14749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arac, D. , Boucard, A. A. , Bolliger, M. F. , Nguyen, J. , Soltis, S. M. , Sudhof, T. C. , & Brunger, A. T. (2012). A novel evolutionarily conserved domain of cell‐adhesion GPCRs mediates autoproteolysis. The EMBO Journal, 31(6), 1364–1378. 10.1038/emboj.2012.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arkoun, B. , Gautier, C. , Delalande, C. , Barrier‐Battut, I. , Guenon, I. , Goux, D. , & Bouraima‐Lelong, H. (2014). Stallion spermatozoa: putative target of estrogens; presence of the estrogen receptors ESR1, ESR2 and identification of the estrogen‐membrane receptor GPER. General and Comparative Endocrinology, 200, 35–43. 10.1016/j.ygcen.2014.02.016 [DOI] [PubMed] [Google Scholar]
- Azimzadeh, P. , Talamantez‐Lyburn, S. C. , Chang, K. T. , Inoue, A. , & Balenga, N. (2019). Spatial regulation of GPR64/ADGRG2 signaling by β‐arrestins and GPCR kinases. Annals of the New York Academy of Sciences, 1456(1), 26–43. 10.1111/nyas.14227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balenga, N. , Azimzadeh, P. , Hogue, J. A. , Staats, P. N. , Shi, Y. , Koh, J. , … Olson, J. A. Jr. (2017). Orphan adhesion GPCR GPR64/ADGRG2 is overexpressed in parathyroid tumors and attenuates calcium‐sensing receptor‐mediated signaling. Journal of Bone and Mineral Research, 32(3), 654–666. 10.1002/jbmr.3023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett‐Jolley, R. , Comtois, A. , Davies, N. W. , Stanfield, P. R. , & Standen, N. B. (1996). Effect of adenosine and intracellular GTP on KATP channels of mammalian skeletal muscle. The Journal of Membrane Biology, 152(2), 111–116. 10.1007/s002329900090 [DOI] [PubMed] [Google Scholar]
- Bean, L. J. H. , Tinker, S. W. , da Silva, C. , & Hegde, M. R. (2013). Free the Data: One Laboratory's Approach to Knowledge‐Based Genomic Variant Classification and Preparation for EMR Integration of Genomic Data. Human Mutation, 34(9), 1183–1188. 10.1002/humu.22364 [DOI] [PubMed] [Google Scholar]
- Belleannée, C. , Da Silva, N. , Shum, W. W. , Marsolais, M. , Laprade, R. , Brown, D. , & Breton, S. (2009). Segmental expression of the bradykinin type 2 receptor in rat efferent ducts and epididymis and its role in the regulation of aquaporin 9. Biology of Reproduction, 80(1), 134–143. 10.1095/biolreprod.108.070797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellezza, I. , & Minelli, A. (2017). Adenosine in sperm physiology. Molecular Aspects of Medicine, 55, 102–109. 10.1016/j.mam.2016.11.009 [DOI] [PubMed] [Google Scholar]
- Bennion, D. M. , Steckelings, U. M. , & Sumners, C. (2018). Neuroprotection via AT2 receptor agonists in ischemic stroke. Clinical Science (London, England), 132(10), 1055–1067. 10.1042/CS20171549 [DOI] [PubMed] [Google Scholar]
- Bienvenu, T. (2003). Rare polymorphic variants of the AGTR2 gene in boys with non‐specific mental retardation. Journal of Medical Genetics, 40(5), 357–359. 10.1136/jmg.40.5.357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bologa, C. G. , Revankar, C. M. , Young, S. M. , Edwards, B. S. , Arterburn, J. B. , Kiselyov, A. S. , … Prossnitz, E. R. (2006). Virtual and biomolecular screening converge on a selective agonist for GPR30. Nature Chemical Biology, 2(4), 207–212. 10.1038/nchembio775 [DOI] [PubMed] [Google Scholar]
- Bridges, T. M. , & Lindsley, C. W. (2008). G‐protein‐coupled receptors: from classical modes of modulation to allosteric mechanisms. ACS Chemical Biology, 3(9), 530–541. 10.1021/cb800116f [DOI] [PubMed] [Google Scholar]
- Brown, K. , Filuta, A. , Ludwig, M. G. , Seuwen, K. , Jaros, J. , Vidal, S. , … Bridges, J. P. (2017). Epithelial Gpr116 regulates pulmonary alveolar homeostasis via Gq/11 signaling. JCI Insight, 2(11), e93700 10.1172/jci.insight.93700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne, J. A. , Yang, R. , Leir, S. H. , Eggener, S. E. , & Harris, A. (2016). Expression profiles of human epididymis epithelial cells reveal the functional diversity of caput, corpus and cauda regions. Molecular Human Reproduction, 22(2), 69–82. 10.1093/molehr/gav066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownhill, V. R. , Hourani, S. M. , & Kitchen, I. (1996). Differential distribution of adenosine A2 receptors in the epididymal and prostatic portions of the rat vas deferens. European Journal of Pharmacology, 303(1‐2), 87–90. [DOI] [PubMed] [Google Scholar]
- Burkett, B. N. , Schulte, B. A. , & Spicer, S. S. (1987). Histochemical evaluation of glycoconjugates in the male reproductive tract with lectin‐horseradish peroxidase conjugates: I. Staining of principal cells and spermatozoa in the mouse. The American Journal of Anatomy, 178(1), 11–22. 10.1002/aja.1001780103 [DOI] [PubMed] [Google Scholar]
- Cahill, T. J. 3rd , Thomsen, A. R. , Tarrasch, J. T. , Plouffe, B. , Nguyen, A. H. , Yang, F. , … Lefkowitz, R. J. (2017). Distinct conformations of GPCR‐beta‐arrestin complexes mediate desensitization, signaling, and endocytosis. Proceedings of the National Academy of Sciences of the United States of America, 114(10), 2562–2567. 10.1073/pnas.1701529114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, X. , Huang, J. , Zhang, G. , Zuo, W. , Lan, C. , Sun, Q. , … Zhou, W. L. (2017). Functional expression of G protein‐coupled receptor 30 in immature rat epididymal epithelium. Cell Biology International, 41(2), 134–146. 10.1002/cbin.10709 [DOI] [PubMed] [Google Scholar]
- Carmeci, C. , Thompson, D. A. , Ring, H. Z. , Francke, U. , & Weigel, R. J. (1997). Identification of a gene (GPR30) with homology to the G‐protein‐coupled receptor superfamily associated with estrogen receptor expression in breast cancer. Genomics, 45(3), 607–617. 10.1006/geno.1997.4972 [DOI] [PubMed] [Google Scholar]
- Carmon, K. S. , Gong, X. , Lin, Q. , Thomas, A. , & Liu, Q. (2011). R‐spondins function as ligands of the orphan receptors LGR4 and LGR5 to regulate Wnt/beta‐catenin signaling. Proceedings of the National Academy of Sciences of the United States of America, 108(28), 11452–11457. 10.1073/pnas.1106083108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassault‐Meyer, E. , Gress, S. , Seralini, G. E. , & Galeraud‐Denis, I. (2014). An acute exposure to glyphosate‐based herbicide alters aromatase levels in testis and sperm nuclear quality. Environmental Toxicology and Pharmacology, 38(1), 131–140. 10.1016/j.etap.2014.05.007 [DOI] [PubMed] [Google Scholar]
- Chen, J. F. , Lee, C. F. , & Chern, Y. (2014). Adenosine receptor neurobiology: Overview. International Review of Neurobiology, 119, 1–49. 10.1016/B978-0-12-801022-8.00001-5 [DOI] [PubMed] [Google Scholar]
- Chevrier, C. , & Dacheux, J. L. (1992). Evolution of the flagellar waveform of ram spermatozoa in relation to the degree of epididymal maturation. Cell Motility and the Cytoskeleton, 23(1), 8–18. 10.1002/cm.970230103 [DOI] [PubMed] [Google Scholar]
- Clulow, J. , Jones, R. C. , Hansen, L. A. , & Man, S. Y. (1998). Fluid and electrolyte reabsorption in the ductuli efferentes testis. Journal of Reproduction and Fertility. Supplement, 53, 1–14. [PubMed] [Google Scholar]
- Cornwall, G. A. (2009). New insights into epididymal biology and function. Human Reproduction Update, 15(2), 213–227. 10.1093/humupd/dmn055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies, B. , Baumann, C. , Kirchhoff, C. , Ivell, R. , Nubbemeyer, R. , Habenicht, U. F. , … Gottwald, U. (2004). Targeted deletion of the epididymal receptor HE6 results in fluid dysregulation and male infertility. Molecular and Cellular Biology, 24(19), 8642–8648. 10.1128/MCB.24.19.8642-8648.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies, B. , Behnen, M. , Cappallo‐Obermann, H. , Spiess, A. N. , Theuring, F. , & Kirchhoff, C. (2007). Novel epididymis‐specific mRNAs downregulated by HE6/Gpr64 receptor gene disruption. Molecular Reproduction and Development, 74(5), 539–553. 10.1002/mrd.20636 [DOI] [PubMed] [Google Scholar]
- Demberg, L. M. , Rothemund, S. , Schoneberg, T. , & Liebscher, I. (2015). Identification of the tethered peptide agonist of the adhesion G protein‐coupled receptor GPR64/ADGRG2. Biochemical and Biophysical Research Communications, 464(3), 743–747. 10.1016/j.bbrc.2015.07.020 [DOI] [PubMed] [Google Scholar]
- Demberg, L. M. , Winkler, J. , Wilde, C. , Simon, K. U. , Schon, J. , Rothemund, S. , … Liebscher, I. (2017). Activation of adhesion G protein‐coupled receptors: Agonist specificity of stachel sequence‐derived peptides. The Journal of Biological Chemistry, 292(11), 4383–4394. 10.1074/jbc.M116.763656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, C. , Reddy, P. , Cheng, Y. , Luo, C. W. , Hsiao, C. L. , & Hsueh, A. J. (2013). Multi‐functional norrin is a ligand for the LGR4 receptor. Journal of Cell Science, 126(Pt 9), 2060–2068. 10.1242/jcs.123471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis, M. K. , Burai, R. , Ramesh, C. , Petrie, W. K. , Alcon, S. N. , Nayak, T. K. , … Prossnitz, E. R. (2009). In vivo effects of a GPR30 antagonist. Nature Chemical Biology, 5(6), 421–427. 10.1038/nchembio.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis, M. K. , Field, A. S. , Burai, R. , Ramesh, C. , Petrie, W. K. , Bologa, C. G. , … Prossnitz, E. R. (2011). Identification of a GPER/GPR30 antagonist with improved estrogen receptor counterselectivity. The Journal of Steroid Biochemistry and Molecular Biology, 127(3‐5), 358–366. 10.1016/j.jsbmb.2011.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimine, V. L. , McCrink, K. A. , Parker, B. M. , Wertz, S. L. , Maning, J. , & Lymperopoulos, A. (2018). Biased agonism/antagonism of cardiovascular GPCRs for heart failure therapy. International Review of Cell and Molecular Biology, 339, 41–61. 10.1016/bs.ircmb.2018.02.007 [DOI] [PubMed] [Google Scholar]
- Diao, H. , Yu, H. G. , Sun, F. , Zhang, Y. L. , & Tanphaichitr, N. (2011). Rat recombinant beta‐defensin 22 is a heparin‐binding protein with antimicrobial activity. Asian Journal of Andrology, 13(2), 305–311. 10.1038/aja.2010.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, J. H. , Wang, Y. J. , Cui, M. , Wang, X. J. , Zheng, W. S. , Ma, M. L. , … Sun, J. P. (2017). Adaptive activation of a stress response pathway improves learning and memory through Gs and beta‐arrestin‐1‐regulated lactate metabolism. Biological Psychiatry, 81(8), 654–670. 10.1016/j.biopsych.2016.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzanaty, S. , Richthoff, J. , Malm, J. , & Giwercman, A. (2002). The impact of epididymal and accessory sex gland function on sperm motility. Human Reproduction, 17(11), 2904–2911. 10.1093/humrep/17.11.2904 [DOI] [PubMed] [Google Scholar]
- Esther, C. R. Jr. , Howard, T. E. , Marino, E. M. , Goddard, J. M. , Capecchi, M. R. , & Bernstein, K. E. (1996). Mice lacking angiotensin‐converting enzyme have low blood pressure, renal pathology, and reduced male fertility. Laboratory Investigation, 74(5), 953–965. [PubMed] [Google Scholar]
- Filardo, E. J. , Quinn, J. A. , Bland, K. I. , & Frackelton, A. R. Jr. (2000). Estrogen‐induced activation of Erk‐1 and Erk‐2 requires the G protein‐coupled receptor homolog, GPR30, and occurs via trans‐activation of the epidermal growth factor receptor through release of HB‐EGF. Molecular Endocrinology, 14(10), 1649–1660. 10.1210/mend.14.10.0532 [DOI] [PubMed] [Google Scholar]
- Flock, T. , Hauser, A. S. , Lund, N. , Gloriam, D. E. , Balaji, S. , & Babu, M. M. (2017). Selectivity determinants of GPCR‐G‐protein binding. Nature, 545(7654), 317–322. 10.1038/nature22070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flock, T. , Ravarani, C. N. J. , Sun, D. , Venkatakrishnan, A. J. , Kayikci, M. , Tate, C. G. , … Babu, M. M. (2015). Universal allosteric mechanism for Galpha activation by GPCRs. Nature, 524(7564), 173–179. 10.1038/nature14663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folts, C. J. , Giera, S. , Li, T. , & Piao, X. (2019). Adhesion G protein‐coupled receptors as drug targets for neurological diseases. Trends in Pharmacological Sciences, 40(4), 278–293. 10.1016/j.tips.2019.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foord, S. M. , Jupe, S. , & Holbrook, J. (2002). Bioinformatics and type II G‐protein‐coupled receptors. Biochemical Society Transactions, 30(4), 473–479. 10.1042/bst0300473 [DOI] [PubMed] [Google Scholar]
- Fredholm, B. B. , Abbracchio, M. P. , Burnstock, G. , Daly, J. W. , Harden, T. K. , Jacobson, K. A. , … Williams, M. (1994). Nomenclature and classification of purinoceptors. Pharmacological Reviews, 46(2), 143–156. [PMC free article] [PubMed] [Google Scholar]
- Fredriksson, R. , Lagerstrom, M. C. , Lundin, L. G. , & Schioth, H. B. (2003). The G‐protein‐coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Molecular Pharmacology, 63(6), 1256–1272. 10.1124/mol.63.6.1256 [DOI] [PubMed] [Google Scholar]
- Funakoshi, T. , Yanai, A. , Shinoda, K. , Kawano, M. M. , & Mizukami, Y. (2006). G protein‐coupled receptor 30 is an estrogen receptor in the plasma membrane. Biochemical and Biophysical Research Communications, 346(3), 904–910. 10.1016/j.bbrc.2006.05.191 [DOI] [PubMed] [Google Scholar]
- Furness, S. G. B. , Liang, Y. L. , Nowell, C. J. , Halls, M. L. , Wookey, P. J. , Dal Maso, E. , … Sexton, P. M. (2016). Ligand‐dependent modulation of G protein conformation alters drug efficacy. Cell, 167(3), 739–749e711. 10.1016/j.cell.2016.09.021 [DOI] [PubMed] [Google Scholar]
- van Galen, P. J. , Stiles, G. L. , Michaels, G. , & Jacobson, K. A. (1992). Adenosine A1 and A2 receptors: Structure–function relationships. Medicinal Research Reviews, 12(5), 423–471. 10.1002/med.2610120502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, Y. , Kitagawa, K. , Shimada, M. , Uchida, C. , Hattori, T. , Oda, T. , & Kitagawa, M. (2006). Generation of a constitutively active mutant of human GPR48/LGR4, a G‐protein‐coupled receptor. Hokkaido Igaku Zasshi, 81(2), 101–105. 107, 109 [PubMed] [Google Scholar]
- de Gasparo, M. , & Siragy, H. M. (1999). The AT2 receptor: Fact, fancy and fantasy. Regulatory Peptides, 81(1‐3), 11–24. 10.1016/s0167-0115(99)00023-3 [DOI] [PubMed] [Google Scholar]
- Gautier, C. , Barrier‐Battut, I. , Guenon, I. , Goux, D. , Delalande, C. , & Bouraima‐Lelong, H. (2016). Implication of the estrogen receptors GPER, ESR1, ESR2 in post‐testicular maturations of equine spermatozoa. General and Comparative Endocrinology, 233, 100–108. 10.1016/j.ygcen.2016.05.022 [DOI] [PubMed] [Google Scholar]
- Geldenhuys, W. J. , Hanif, A. , Yun, J. , & Nayeem, M. A. (2017). Exploring adenosine receptor ligands: Potential role in the treatment of cardiovascular diseases. Molecules, 22(6), 917 10.3390/molecules22060917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman, A. G. (1987). G proteins: Transducers of receptor‐generated signals. Annual Review of Biochemistry, 56, 615–649. 10.1146/annurev.bi.56.070187.003151 [DOI] [PubMed] [Google Scholar]
- Glinka, A. , Dolde, C. , Kirsch, N. , Huang, Y. L. , Kazanskaya, O. , Ingelfinger, D. , … Niehrs, C. (2011). LGR4 and LGR5 are R‐spondin receptors mediating Wnt/beta‐catenin and Wnt/PCP signalling. EMBO Reports, 12(10), 1055–1061. 10.1038/embor.2011.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottwald, U. , Davies, B. , Fritsch, M. , & Habenicht, U. F. (2006). New approaches for male fertility control: HE6 as an example of a putative target. Molecular and Cellular Endocrinology, 250(1‐2), 49–57. 10.1016/j.mce.2005.12.024 [DOI] [PubMed] [Google Scholar]
- de Graaf, C. , Nijmeijer, S. , Wolf, S. , & Ernst, O. P. (2016). 7TM domain structure of adhesion GPCRs. Handbook of Experimental Pharmacology, 234, 43–66. 10.1007/978-3-319-41523-9_3 [DOI] [PubMed] [Google Scholar]
- Guimond, M. O. , Hallberg, M. , Gallo‐Payet, N. , & Wallinder, C. (2014). Saralasin and sarile are AT2 receptor agonists. ACS Medicinal Chemistry Letters, 5(10), 1129–1132. 10.1021/ml500278g [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagaman, J. R. , Moyer, J. S. , Bachman, E. S. , Sibony, M. , Magyar, P. L. , Welch, J. E. , … O'Brien, D. A. (1998). Angiotensin‐converting enzyme and male fertility. Proceedings of the National Academy of Sciences of the United States of America, 95(5), 2552–2557. 10.1073/pnas.95.5.2552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallberg, M. , Sumners, C. , Steckelings, U. M. , & Hallberg, A. (2018). Small‐molecule AT2 receptor agonists. Medicinal Research Reviews, 38(2), 602–624. 10.1002/med.21449 [DOI] [PubMed] [Google Scholar]
- Hamann, J. , Aust, G. , Arac, D. , Engel, F. B. , Formstone, C. , Fredriksson, R. , … Schioth, H. B. (2015). International union of basic and clinical pharmacology. XCIV. Adhesion G protein‐coupled receptors. Pharmacological Reviews, 67(2), 338–367. 10.1124/pr.114.009647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara, M. R. , Kovacs, J. J. , Whalen, E. J. , Rajagopal, S. , Strachan, R. T. , Grant, W. , … Lefkowitz, R. J. (2011). A stress response pathway regulates DNA damage through beta2‐adrenoreceptors and beta‐arrestin‐1. Nature, 477(7364), 349–353. 10.1038/nature10368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser, A. S. , Attwood, M. M. , Rask‐Andersen, M. , Schioth, H. B. , & Gloriam, D. E. (2017). Trends in GPCR drug discovery: New agents, targets and indications. Nature Reviews. Drug Discovery, 16(12), 829–842. 10.1038/nrd.2017.178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser, A. S. , Chavali, S. , Masuho, I. , Jahn, L. J. , Martemyanov, K. A. , Gloriam, D. E. , & Babu, M. M. (2018). Pharmacogenomics of GPCR drug targets. Cell, 172(1‐2), 41–54e19. 10.1016/j.cell.2017.11.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes, J. M. (2000). A(2A) adenosine receptor mediated potassium channel activation in rat epididymal smooth muscle. British Journal of Pharmacology, 130(3), 685–691. 10.1038/sj.bjp.0703323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes, J. M. , Alexander, S. P. , & Hill, S. J. (1998a). A1 adenosine receptor modulation of electrically‐evoked contractions in the bisected vas deferens and cauda epididymis of the guinea‐pig. British Journal of Pharmacology, 124(5), 964–970. 10.1038/sj.bjp.0701909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes, J. M. , Alexander, S. P. , & Hill, S. J. (1998b). A1 and A2 adenosine receptor modulation of contractility in the cauda epididymis of the guinea‐pig. British Journal of Pharmacology, 125(3), 570–576. 10.1038/sj.bjp.0702095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermo, L. , Dworkin, J. , & Oko, R. (1988). Role of epithelial clear cells of the rat epididymis in the disposal of the contents of cytoplasmic droplets detached from spermatozoa. The American Journal of Anatomy, 183(2), 107–124. 10.1002/aja.1001830202 [DOI] [PubMed] [Google Scholar]
- Hess, R. A. (2000). Oestrogen in fluid transport in efferent ducts of the male reproductive tract. Reviews of Reproduction, 5(2), 84–92. 10.1530/ror.0.0050084 [DOI] [PubMed] [Google Scholar]
- Hess, R. A. (2015). Small tubules, surprising discoveries: From efferent ductules in the turkey to the discovery that estrogen receptor alpha is essential for fertility in the male. Animal Reproduction, 12(1), 7–23. [PMC free article] [PubMed] [Google Scholar]
- Hess, R. A. , Fernandes, S. A. , Gomes, G. R. , Oliveira, C. A. , Lazari, M. F. , & Porto, C. S. (2011). Estrogen and its receptors in efferent ductules and epididymis. Journal of Andrology, 32(6), 600–613. 10.2164/jandrol.110.012872 [DOI] [PubMed] [Google Scholar]
- Hess, R. A. , & Nakai, M. (2000). Histopathology of the male reproductive system induced by the fungicide benomyl. Histology and Histopathology, 15(1), 207–224. 10.14670/HH-15.207 [DOI] [PubMed] [Google Scholar]
- Hilbig, D. , Sittig, D. , Hoffmann, F. , Rothemund, S. , Warmt, E. , Quaas, M. , … Aust, G. (2018). Mechano‐Dependent Phosphorylation of the PDZ‐binding motif of CD97/ADGRE5 Modulates Cellular Detachment. Cell Reports, 24(8), 1986–1995. 10.1016/j.celrep.2018.07.071 [DOI] [PubMed] [Google Scholar]
- Homan, K. T. , & Tesmer, J. J. (2014). Structural insights into G protein‐coupled receptor kinase function. Current Opinion in Cell Biology, 27, 25–31. 10.1016/j.ceb.2013.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshii, T. , Takeo, T. , Nakagata, N. , Takeya, M. , Araki, K. , & Yamamura, K. (2007). LGR4 regulates the postnatal development and integrity of male reproductive tracts in mice. Biology of Reproduction, 76(2), 303–313. 10.1095/biolreprod.106.054619 [DOI] [PubMed] [Google Scholar]
- Hu, Q. X. , Dong, J. H. , Du, H. B. , Zhang, D. L. , Ren, H. Z. , Ma, M. L. , … Sun, J. P. (2014). Constitutive Gαi coupling activity of very large G protein‐coupled receptor 1 (VLGR1) and its regulation by PDZD7 protein. The Journal of Biological Chemistry, 289(35), 24215–24225. 10.1074/jbc.M114.549816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isogai, S. , Deupi, X. , Opitz, C. , Heydenreich, F. M. , Tsai, C. J. , Brueckner, F. , … Grzesiek, S. (2016). Backbone NMR reveals allosteric signal transduction networks in the beta1‐adrenergic receptor. Nature, 530(7589), 237–241. 10.1038/nature16577 [DOI] [PubMed] [Google Scholar]
- Jackson, S. , Weingart, J. , Nduom, E. K. , Harfi, T. T. , George, R. T. , McAreavey, D. , … Grossman, S. A. (2018). The effect of an adenosine A2A agonist on intra‐tumoral concentrations of temozolomide in patients with recurrent glioblastoma. Fluids Barriers CNS, 15(1), 2 10.1186/s12987-017-0088-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson, K. A. , Tosh, D. K. , Jain, S. , & Gao, Z. G. (2019). Historical and current adenosine receptor agonists in preclinical and clinical development. Frontiers in Cellular Neuroscience, 13, 124 10.3389/fncel.2019.00124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph, A. , Shur, B. D. , & Hess, R. A. (2011). Estrogen, efferent ductules, and the epididymis. Biology of Reproduction, 84(2), 207–217. 10.1095/biolreprod.110.087353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katleba, K. D. , Legacki, E. L. , Conley, A. J. , & Berger, T. (2015). Steroid regulation of early postnatal development in the corpus epididymidis of pigs. The Journal of Endocrinology, 225(3), 125–134. 10.1530/JOE-15-0001 [DOI] [PubMed] [Google Scholar]
- Khan, M. J. , Pollock, N. , Jiang, H. , Castro, C. , Nazli, R. , Ahmed, J. , … Yatsenko, A. N. (2018). X‐linked ADGRG2 mutation and obstructive azoospermia in a large Pakistani family. Scientific Reports, 8(1), 16280 10.1038/s41598-018-34262-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J. , Grotegut, C. A. , Wisler, J. W. , Mao, L. , Rosenberg, P. B. , Rockman, H. A. , & Lefkowitz, R. J. (2020). The beta‐arrestin‐biased beta‐adrenergic receptor blocker carvedilol enhances skeletal muscle contractility. Proceedings of the National Academy of Sciences of the United States of America, 117(22), 12435–12443. 10.1073/pnas.1920310117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhoff, C. , Osterhoff, C. , & Samalecos, A. (2008). HE6/GPR64 adhesion receptor co‐localizes with apical and subapical F‐actin scaffold in male excurrent duct epithelia. Reproduction, 136(2), 235–245. 10.1530/REP-08-0078 [DOI] [PubMed] [Google Scholar]
- Kishore, A. , & Hall, R. A. (2017). Disease‐associated extracellular loop mutations in the adhesion G protein‐coupled receptor G1 (ADGRG1; GPR56) differentially regulate downstream signaling. The Journal of Biological Chemistry, 292(23), 9711–9720. 10.1074/jbc.M117.780551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishore, A. , Purcell, R. H. , Nassiri‐Toosi, Z. , & Hall, R. A. (2016). Stalk‐dependent and stalk‐independent signaling by the adhesion G protein‐coupled receptors GPR56 (ADGRG1) and BAI1 (ADGRB1). The Journal of Biological Chemistry, 291(7), 3385–3394. 10.1074/jbc.M115.689349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleppisch, T. , & Nelson, M. T. (1995). Adenosine activates ATP‐sensitive potassium channels in arterial myocytes via A2 receptors and cAMP‐dependent protein kinase. Proceedings of the National Academy of Sciences of the United States of America, 92(26), 12441–12445. 10.1073/pnas.92.26.12441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komolov, K. E. , Du, Y. , Duc, N. M. , Betz, R. M. , Rodrigues, J. , Leib, R. D. , … Benovic, J. L. (2017). Structural and Functional Analysis of a beta2‐Adrenergic Receptor Complex with GRK5. Cell, 169(3), 407–421e416. 10.1016/j.cell.2017.03.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krausz, C. , & Riera‐Escamilla, A. (2018). Genetics of male infertility. Nature Reviews. Urology, 15(6), 369–384. 10.1038/s41585-018-0003-3 [DOI] [PubMed] [Google Scholar]
- Krege, J. H. , John, S. W. , Langenbach, L. L. , Hodgin, J. B. , Hagaman, J. R. , Bachman, E. S. , … Smithies, O. (1995). Male‐female differences in fertility and blood pressure in ACE‐deficient mice. Nature, 375(6527), 146–148. 10.1038/375146a0 [DOI] [PubMed] [Google Scholar]
- Krejcirova, R. , Manasova, M. , Sommerova, V. , Langhamerova, E. , Rajmon, R. , & Manaskova‐Postlerova, P. (2018). G protein‐coupled estrogen receptor (GPER) in adult boar testes, epididymis and spermatozoa during epididymal maturation. International Journal of Biological Macromolecules, 116, 113–119. 10.1016/j.ijbiomac.2018.05.015 [DOI] [PubMed] [Google Scholar]
- Kumari, P. , Srivastava, A. , Banerjee, R. , Ghosh, E. , Gupta, P. , Ranjan, R. , … Shukla, A. K. (2016). Functional competence of a partially engaged GPCR‐beta‐arrestin complex. Nature Communications, 7, 13416 10.1038/ncomms13416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammermann, T. , & Kastenmuller, W. (2019). Concepts of GPCR‐controlled navigation in the immune system. Immunological Reviews, 289(1), 205–231. 10.1111/imr.12752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford, K. G. , Zhou, Y. , Russell, L. D. , Wilcox, J. N. , & Bernstein, K. E. (1993). Regulated expression of testis angiotensin‐converting enzyme during spermatogenesis in mice. Biology of Reproduction, 48(6), 1210–1218. 10.1095/biolreprod48.6.1210 [DOI] [PubMed] [Google Scholar]
- de Lau, W. , Barker, N. , Low, T. Y. , Koo, B. K. , Li, V. S. , Teunissen, H. , … Clevers, H. (2011). Lgr5 homologues associate with Wnt receptors and mediate R‐spondin signalling. Nature, 476(7360), 293–297. 10.1038/nature10337 [DOI] [PubMed] [Google Scholar]
- Lee, K. H. , Finnigan‐Bunick, C. , Bahr, J. , & Bunick, D. (2001). Estrogen regulation of ion transporter messenger RNA levels in mouse efferent ductules are mediated differentially through estrogen receptor (ER) alpha and ER beta. Biology of Reproduction, 65(5), 1534–1541. 10.1095/biolreprod65.5.1534 [DOI] [PubMed] [Google Scholar]
- Lefkowitz, R. J. , & Shenoy, S. K. (2005). Transduction of receptor signals by beta‐arrestins. Science, 308(5721), 512–517. 10.1126/science.1109237 [DOI] [PubMed] [Google Scholar]
- Leung, P. S. (2001). Local renin‐angiotensin system in the pancreas: The significance of changes by chronic hypoxia and acute pancreatitis. Journal of the Pancreas: JOP, 2(1), 3–8. [PubMed] [Google Scholar]
- Leung, P. S. , Chan, H. C. , Fu, L. X. , Zhou, W. L. , & Wong, P. Y. (1997). Angiotensin II receptors, AT1 and AT2 in the rat epididymis. Immunocytochemical and electrophysiological studies. Biochimica et Biophysica Acta, 1357(1), 65–72. 10.1016/s0167-4889(97)00015-3 [DOI] [PubMed] [Google Scholar]
- Leung, P. S. , & Sernia, C. (2003). The renin‐angiotensin system and male reproduction: New functions for old hormones. Journal of Molecular Endocrinology, 30(3), 263–270. 10.1677/jme.0.0300263 [DOI] [PubMed] [Google Scholar]
- Li, T. , Yu, B. , Liu, Z. , Li, J. , Ma, M. , Wang, Y. , … Kong, W. (2018). Homocysteine directly interacts and activates the angiotensin II type I receptor to aggravate vascular injury. Nature Communications, 9(1), 11 10.1038/s41467-017-02401-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X. Y. , Lu, Y. , Sun, H. Y. , Wang, J. Q. , Yang, J. , Zhang, H. J. , … Ning, G. (2010). G protein‐coupled receptor 48 upregulates estrogen receptor alpha expression via cAMP/PKA signaling in the male reproductive tract. Development, 137(1), 151–157. 10.1242/dev.040659 [DOI] [PubMed] [Google Scholar]
- Liebscher, I. , Schon, J. , Petersen, S. C. , Fischer, L. , Auerbach, N. , Demberg, L. M. , … Schoneberg, T. (2014). A tethered agonist within the ectodomain activates the adhesion G protein‐coupled receptors GPR126 and GPR133. Cell Reports, 9(6), 2018–2026. 10.1016/j.celrep.2014.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebscher, I. , Schon, J. , Petersen, S. C. , Fischer, L. , Auerbach, N. , Demberg, L. M. , … Schoneberg, T. (2015). A tethered agonist within the ectodomain activates the adhesion G protein‐coupled receptors GPR126 and GPR133. Cell Reports, 10(6), 1021 10.1016/j.celrep.2015.01.065 [DOI] [PubMed] [Google Scholar]
- Liebscher, I. , Schoneberg, T. , & Promel, S. (2013). Progress in demystification of adhesion G protein‐coupled receptors. Biological Chemistry, 394(8), 937–950. 10.1515/hsz-2013-0109 [DOI] [PubMed] [Google Scholar]
- Liu, C. H. , Gong, Z. , Liang, Z. L. , Liu, Z. X. , Yang, F. , Sun, Y. J. , … Sun, J. P. (2017). Arrestin‐biased AT1R agonism induces acute catecholamine secretion through TRPC3 coupling. Nature Communications, 8, 14335 10.1038/ncomms14335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, P. , Wang, F. , Song, X. , Liu, Y. , Zhang, K. , & Cao, N. (2016). Relative abundance of G protein‐coupled receptor 30 and localization in testis and epididymis of sheep at different developmental stages. Animal Reproduction Science, 175, 10–17. 10.1016/j.anireprosci.2016.10.002 [DOI] [PubMed] [Google Scholar]
- Lucas, T. F. , Royer, C. , Siu, E. R. , Lazari, M. F. , & Porto, C. S. (2010). Expression and signaling of G protein‐coupled estrogen receptor 1 (GPER) in rat sertoli cells. Biology of Reproduction, 83(2), 307–317. 10.1095/biolreprod.110.084160 [DOI] [PubMed] [Google Scholar]
- Luo, J. , Yang, Z. , Ma, Y. , Yue, Z. , Lin, H. , Qu, G. , … Liu, M. (2016). LGR4 is a receptor for RANKL and negatively regulates osteoclast differentiation and bone resorption. Nature Medicine, 22(5), 539–546. 10.1038/nm.4076 [DOI] [PubMed] [Google Scholar]
- Luo, R. , Jeong, S. J. , Yang, A. , Wen, M. , Saslowsky, D. E. , Lencer, W. I. , … Piao, X. (2014). Mechanism for adhesion G protein‐coupled receptor GPR56‐mediated RhoA activation induced by collagen III stimulation. PLoS ONE, 9(6), e100043 10.1371/journal.pone.0100043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttrell, L. M. , Ferguson, S. S. , Daaka, Y. , Miller, W. E. , Maudsley, S. , Della Rocca, G. J. , … Lefkowitz, R. J. (1999). Beta‐arrestin‐dependent formation of beta2 adrenergic receptor‐Src protein kinase complexes. Science, 283(5402), 655–661. 10.1126/science.283.5402.655 [DOI] [PubMed] [Google Scholar]
- Lymperopoulos, A. (2018). Arrestins in the cardiovascular system: An update. Progress in Molecular Biology and Translational Science, 159, 27–57. 10.1016/bs.pmbts.2018.07.003 [DOI] [PubMed] [Google Scholar]
- Lymperopoulos, A. , Wertz, S. L. , Pollard, C. M. , Desimine, V. L. , Maning, J. , & McCrink, K. A. (2019). Not all arrestins are created equal: Therapeutic implications of the functional diversity of the beta‐arrestins in the heart. World Journal of Cardiology, 11(2), 47–56. 10.4330/wjc.v11.i2.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malivindi, R. , Aquila, S. , & Rago, V. (2018). Immunolocalization of G protein‐coupled estrogen receptor in the pig epididymis. Anatomical Record (Hoboken), 301(8), 1467–1473. 10.1002/ar.23837 [DOI] [PubMed] [Google Scholar]
- Martinez‐Traverso, G. B. , & Pearl, C. A. (2015). Immunolocalization of G protein‐coupled estrogen receptor in the rat epididymis. Reproductive Biology and Endocrinology, 13, 48 10.1186/s12958-015-0042-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeoch, R. J. , & Oldroyd, K. G. (2008). Pharmacological options for inducing maximal hyperaemia during studies of coronary physiology. Catheterization and Cardiovascular Interventions, 71(2), 198–204. 10.1002/ccd.21307 [DOI] [PubMed] [Google Scholar]
- Menad, R. , Fernini, M. , Smai, S. , Bonnet, X. , Gernigon‐Spychalowicz, T. , Moudilou, E. , … Exbrayat, J. M. (2017). GPER1 in sand rat epididymis: Effects of seasonal variations, castration and efferent ducts ligation. Animal Reproduction Science, 183, 9–20. 10.1016/j.anireprosci.2017.06.012 [DOI] [PubMed] [Google Scholar]
- Mendive, F. , Laurent, P. , Van Schoore, G. , Skarnes, W. , Pochet, R. , & Vassart, G. (2006). Defective postnatal development of the male reproductive tract in LGR4 knockout mice. Developmental Biology, 290(2), 421–434. 10.1016/j.ydbio.2005.11.043 [DOI] [PubMed] [Google Scholar]
- Miller, W. E. , Maudsley, S. , Ahn, S. , Khan, K. D. , Luttrell, L. M. , & Lefkowitz, R. J. (2000). beta‐arrestin1 interacts with the catalytic domain of the tyrosine kinase c‐SRC. Role of beta‐arrestin1‐dependent targeting of c‐SRC in receptor endocytosis. The Journal of Biological Chemistry, 275(15), 11312–11319. 10.1074/jbc.275.15.11312 [DOI] [PubMed] [Google Scholar]
- Minelli, A. , Bellezza, I. , Collodel, G. , & Fredholm, B. B. (2008). Promiscuous coupling and involvement of protein kinase C and extracellular signal‐regulated kinase 1/2 in the adenosine A1 receptor signalling in mammalian spermatozoa. Biochemical Pharmacology, 75(4), 931–941. 10.1016/j.bcp.2007.10.024 [DOI] [PubMed] [Google Scholar]
- Minelli, A. , Liguori, L. , Bellazza, I. , Mannucci, R. , Johansson, B. , & Fredholm, B. B. (2004). Involvement of A1 adenosine receptors in the acquisition of fertilizing capacity. Journal of Andrology, 25(2), 286–292. 10.1002/j.1939-4640.2004.tb02789.x [DOI] [PubMed] [Google Scholar]
- Minelli, A. , Miscetti, P. , Allegrucci, C. , & Mezzasoma, I. (1995). Evidence of A1 adenosine receptor on epidydimal bovine spermatozoa. Archives of Biochemistry and Biophysics, 322(1), 272–276. 10.1006/abbi.1995.1462 [DOI] [PubMed] [Google Scholar]
- Minutoli, L. , Antonuccio, P. , Squadrito, F. , Bitto, A. , Nicotina, P. A. , Fazzari, C. , … Altavilla, D. (2012). Effects of polydeoxyribonucleotide on the histological damage and the altered spermatogenesis induced by testicular ischaemia and reperfusion in rats. International Journal of Andrology, 35(2), 133–144. 10.1111/j.1365-2605.2011.01194.x [DOI] [PubMed] [Google Scholar]
- Minutoli, L. , Arena, S. , Antonuccio, P. , Romeo, C. , Bitto, A. , Magno, C. , … Marini, H. (2015). Role of inhibitors of apoptosis proteins in testicular function and male fertility: Effects of polydeoxyribonucleotide administration in experimental varicocele. BioMed Research International, 2015, 248976 10.1155/2015/248976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller, A. , Winkler, J. , Fiedler, F. , Sastradihardja, T. , Binder, C. , Schnabel, R. , … Promel, S. (2015). Oriented cell division in the C. elegans embryo is coordinated by G‐protein signaling dependent on the adhesion GPCR LAT‐1. PLoS Genetics, 11(10), e1005624 10.1371/journal.pgen.1005624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundell, S. , & Kelly, E. (2011). Adenosine receptor desensitization and trafficking. Biochimica et Biophysica Acta, 1808(5), 1319–1328. 10.1016/j.bbamem.2010.06.007 [DOI] [PubMed] [Google Scholar]
- Obermann, H. , Samalecos, A. , Osterhoff, C. , Schroder, B. , Heller, R. , & Kirchhoff, C. (2003). HE6, a two‐subunit heptahelical receptor associated with apical membranes of efferent and epididymal duct epithelia. Molecular Reproduction and Development, 64(1), 13–26. 10.1002/mrd.10220 [DOI] [PubMed] [Google Scholar]
- O'Dea, A. , Sondergard, C. , Sweeney, P. , & Arnatt, C. K. (2018). A series of indole‐thiazole derivatives act as GPER agonists and inhibit breast cancer cell growth. ACS Medicinal Chemistry Letters, 9(9), 901–906. 10.1021/acsmedchemlett.8b00212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dowd, B. F. , Nguyen, T. , Marchese, A. , Cheng, R. , Lynch, K. R. , Heng, H. H. , … George, S. R. (1998). Discovery of three novel G‐protein‐coupled receptor genes. Genomics, 47(2), 310–313. 10.1006/geno.1998.5095 [DOI] [PubMed] [Google Scholar]
- Okajima, D. , Kudo, G. , & Yokota, H. (2010). Brain‐specific angiogenesis inhibitor 2 (BAI2) may be activated by proteolytic processing. Journal of Receptor and Signal Transduction Research, 30(3), 143–153. 10.3109/10799891003671139 [DOI] [PubMed] [Google Scholar]
- Paavola, K. J. , & Hall, R. A. (2012). Adhesion G protein‐coupled receptors: Signaling, pharmacology, and mechanisms of activation. Molecular Pharmacology, 82(5), 777–783. 10.1124/mol.112.080309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paavola, K. J. , Sidik, H. , Zuchero, J. B. , Eckart, M. , & Talbot, W. S. (2014). Type IV collagen is an activating ligand for the adhesion G protein‐coupled receptor GPR126. Science Signaling, 7(338), ra76 10.1126/scisignal.2005347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paavola, K. J. , Stephenson, J. R. , Ritter, S. L. , Alter, S. P. , & Hall, R. A. (2011). The N terminus of the adhesion G protein‐coupled receptor GPR56 controls receptor signaling activity. The Journal of Biological Chemistry, 286(33), 28914–28921. 10.1074/jbc.M111.247973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patat, O. , Pagin, A. , Siegfried, A. , Mitchell, V. , Chassaing, N. , Faguer, S. , … Bieth, E. (2016). Truncating mutations in the adhesion G protein‐coupled receptor G2 gene ADGRG2 cause an X‐linked congenital bilateral absence of vas deferens. American Journal of Human Genetics, 99(2), 437–442. 10.1016/j.ajhg.2016.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patra, C. , van Amerongen, M. J. , Ghosh, S. , Ricciardi, F. , Sajjad, A. , Novoyatleva, T. , … Engel, F. B. (2013). Organ‐specific function of adhesion G protein‐coupled receptor GPR126 is domain‐dependent. Proceedings of the National Academy of Sciences of the United States of America, 110(42), 16898–16903. 10.1073/pnas.1304837110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters, M. C. , Fokkelman, M. , Boogaard, B. , Egerod, K. L. , van de Water, B. , IJzerman, A. P. , & Schwartz, T. W. (2015). The adhesion G protein‐coupled receptor G2 (ADGRG2/GPR64) constitutively activates SRE and NFkappaB and is involved in cell adhesion and migration. Cellular Signalling, 27(12), 2579–2588. 10.1016/j.cellsig.2015.08.015 [DOI] [PubMed] [Google Scholar]
- Pereira, M. F. , Fernandes, S. A. , Nascimento, A. R. , Siu, E. R. , Hess, R. A. , Oliveira, C. A. , … Lazari, M. F. (2014). Effects of the oestrogen receptor antagonist Fulvestrant on expression of genes that affect organization of the epididymal epithelium. Andrology, 2(4), 559–571. 10.1111/j.2047-2927.2014.00219.x [DOI] [PubMed] [Google Scholar]
- Petersen, S. C. , Luo, R. , Liebscher, I. , Giera, S. , Jeong, S. J. , Mogha, A. , … Monk, K. R. (2015). The adhesion GPCR GPR126 has distinct, domain‐dependent functions in Schwann cell development mediated by interaction with laminin‐211. Neuron, 85(4), 755–769. 10.1016/j.neuron.2014.12.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson, Y. K. , & Luttrell, L. M. (2017). The diverse roles of arrestin scaffolds in G protein‐coupled receptor signaling. Pharmacological Reviews, 69(3), 256–297. 10.1124/pr.116.013367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce, K. L. , Premont, R. T. , & Lefkowitz, R. J. (2002). Seven‐transmembrane receptors. Nature Reviews. Molecular Cell Biology, 3(9), 639–650. 10.1038/nrm908 [DOI] [PubMed] [Google Scholar]
- Piton A., Redin C., & Mandel J‐L.. (2013). XLID‐Causing Mutations and Associated Genes Challenged in Light of Data From Large‐Scale Human Exome Sequencing. The American Journal of Human Genetics, 93(2), 368–383. 10.1016/j.ajhg.2013.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premont, R. T. , & Gainetdinov, R. R. (2007). Physiological roles of G protein‐coupled receptor kinases and arrestins. Annual Review of Physiology, 69, 511–534. 10.1146/annurev.physiol.69.022405.154731 [DOI] [PubMed] [Google Scholar]
- Prossnitz, E. R. , Arterburn, J. B. , & Sklar, L. A. (2007). GPR30: A G protein‐coupled receptor for estrogen. Molecular and Cellular Endocrinology, 265‐266, 138–142. 10.1016/j.mce.2006.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell, R. H. , & Hall, R. A. (2018). Adhesion G protein‐coupled receptors as drug targets. Annual Review of Pharmacology and Toxicology, 58, 429–449. 10.1146/annurev-pharmtox-010617-052933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian, Y. , Liu, S. , Guan, Y. , Pan, H. , Guan, X. , Qiu, Z. , … Li, D. (2013). Lgr4‐mediated Wnt/beta‐catenin signaling in peritubular myoid cells is essential for spermatogenesis. Development, 140(8), 1751–1761. 10.1242/dev.093641 [DOI] [PubMed] [Google Scholar]
- Rago, V. , Romeo, F. , Giordano, F. , Ferraro, A. , & Carpino, A. (2016). Identification of the G protein‐coupled estrogen receptor (GPER) in human prostate: Expression site of the estrogen receptor in the benign and neoplastic gland. Andrology, 4(1), 121–127. 10.1111/andr.12131 [DOI] [PubMed] [Google Scholar]
- Rago, V. , Romeo, F. , Giordano, F. , Malivindi, R. , Pezzi, V. , Casaburi, I. , & Carpino, A. (2018). Expression of oestrogen receptors (GPER, ESR1, ESR2) in human ductuli efferentes and proximal epididymis. Andrology, 6(1), 192–198. 10.1111/andr.12443 [DOI] [PubMed] [Google Scholar]
- Reiter, E. , & Lefkowitz, R. J. (2006). GRKs and beta‐arrestins: Roles in receptor silencing, trafficking and signaling. Trends in Endocrinology and Metabolism, 17(4), 159–165. 10.1016/j.tem.2006.03.008 [DOI] [PubMed] [Google Scholar]
- Revankar, C. M. , Cimino, D. F. , Sklar, L. A. , Arterburn, J. B. , & Prossnitz, E. R. (2005). A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science, 307(5715), 1625–1630. 10.1126/science.1106943 [DOI] [PubMed] [Google Scholar]
- Ritter, S. L. , & Hall, R. A. (2009). Fine‐tuning of GPCR activity by receptor‐interacting proteins. Nature Reviews. Molecular Cell Biology, 10(12), 819–830. 10.1038/nrm2803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez, F. , Legare, C. , Laflamme, J. , & Sullivan, R. (2004). Vasectomy‐dependent dysregulation of a local renin‐angiotensin system in the epididymis of the cynomolgus monkey (Macaca fascicularis). Journal of Andrology, 25(5), 784–796. 10.1002/j.1939-4640.2004.tb02857.x [DOI] [PubMed] [Google Scholar]
- Santiago, A. R. , Madeira, M. H. , Boia, R. , Aires, I. D. , Rodrigues‐Neves, A. C. , Santos, P. F. , & Ambrosio, A. F. (2020). Keep an eye on adenosine: Its role in retinal inflammation. Pharmacology & Therapeutics, 210, 107513 10.1016/j.pharmthera.2020.107513 [DOI] [PubMed] [Google Scholar]
- Santos, R. , Ursu, O. , Gaulton, A. , Bento, A. P. , Donadi, R. S. , Bologa, C. G. , … Overington, J. P. (2017). A comprehensive map of molecular drug targets. Nature Reviews. Drug Discovery, 16(1), 19–34. 10.1038/nrd.2016.230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz, N. , Gehring, J. , Guan, C. , Ljaschenko, D. , Fischer, R. , Lakshmanan, V. , … Langenhan, T. (2015). The adhesion GPCR latrophilin/CIRL shapes mechanosensation. Cell Reports, 11(6), 866–874. 10.1016/j.celrep.2015.04.008 [DOI] [PubMed] [Google Scholar]
- Shukla, A. K. , Westfield, G. H. , Xiao, K. , Reis, R. I. , Huang, L. Y. , Tripathi‐Shukla, P. , … Lefkowitz, R. J. (2014). Visualization of arrestin recruitment by a G‐protein‐coupled receptor. Nature, 512(7513), 218–222. 10.1038/nature13430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shum, W. W. , Da Silva, N. , Brown, D. , & Breton, S. (2009). Regulation of luminal acidification in the male reproductive tract via cell‐cell crosstalk. The Journal of Experimental Biology, 212(Pt 11), 1753–1761. 10.1242/jeb.027284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shum, W. W. , Da Silva, N. , McKee, M. , Smith, P. J. , Brown, D. , & Breton, S. (2008). Transepithelial projections from basal cells are luminal sensors in pseudostratified epithelia. Cell, 135(6), 1108–1117. 10.1016/j.cell.2008.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibony, M. , Segretain, D. , & Gasc, J. M. (1994). Angiotensin‐converting enzyme in murine testis: Step‐specific expression of the germinal isoform during spermiogenesis. Biology of Reproduction, 50(5), 1015–1026. 10.1095/biolreprod50.5.1015 [DOI] [PubMed] [Google Scholar]
- Sounier, R. , Mas, C. , Steyaert, J. , Laeremans, T. , Manglik, A. , Huang, W. , … Granier, S. (2015). Propagation of conformational changes during mu‐opioid receptor activation. Nature, 524(7565), 375–378. 10.1038/nature14680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speth, R. C. , Daubert, D. L. , & Grove, K. L. (1999). Angiotensin II: A reproductive hormone too? Regulatory Peptides, 79(1), 25–40. 10.1016/s0167-0115(98)00141-4 [DOI] [PubMed] [Google Scholar]
- Srivastava, A. , Gupta, B. , Gupta, C. , & Shukla, A. K. (2015). Emerging functional divergence of beta‐arrestin isoforms in GPCR function. Trends in Endocrinology and Metabolism, 26(11), 628–642. 10.1016/j.tem.2015.09.001 [DOI] [PubMed] [Google Scholar]
- Stoveken, H. M. , Hajduczok, A. G. , Xu, L. , & Tall, G. G. (2015). Adhesion G protein‐coupled receptors are activated by exposure of a cryptic tethered agonist. Proceedings of the National Academy of Sciences of the United States of America, 112(19), 6194–6199. 10.1073/pnas.1421785112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter, S. M. , Thiele, E. A. , De Souza, E. B. , & Snyder, S. H. (1985). Angiotensin‐converting enzyme in the testis and epididymis: Differential development and pituitary regulation of isozymes. Endocrinology, 117(4), 1374–1379. 10.1210/endo-117-4-1374 [DOI] [PubMed] [Google Scholar]
- Styrkarsdottir, U. , Thorleifsson, G. , Sulem, P. , Gudbjartsson, D. F. , Sigurdsson, A. , Jonasdottir, A. , … Stefansson, K. (2013). Nonsense mutation in the LGR4 gene is associated with several human diseases and other traits. Nature, 497(7450), 517–520. 10.1038/nature12124 [DOI] [PubMed] [Google Scholar]
- Suchy, T. , Zieschang, C. , Popkova, Y. , Kaczmarek, I. , Weiner, J. , Liebing, A. D. , … Thor, D. (2020). The repertoire of adhesion G protein‐coupled receptors in adipocytes and their functional relevance. International Journal of Obesity. 10.1038/s41366-020-0570-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, J. P. , Li, R. , Ren, H. Z. , Xu, A. T. , Yu, X. , & Xu, Z. G. (2013). The very large G protein coupled receptor (Vlgr1) in hair cells. Journal of Molecular Neuroscience, 50(1), 204–214. 10.1007/s12031-012-9911-5 [DOI] [PubMed] [Google Scholar]
- Szentmiklosi, A. J. , Galajda, Z. , Cseppento, A. , Gesztelyi, R. , Susan, Z. , Hegyi, B. , & Nanasi, P. P. (2015). The Janus face of adenosine: Antiarrhythmic and proarrhythmic actions. Current Pharmaceutical Design, 21(8), 965–976. 10.2174/1381612820666141029100346 [DOI] [PubMed] [Google Scholar]
- Thomas, P. , Pang, Y. , Filardo, E. J. , & Dong, J. (2005). Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology, 146(2), 624–632. 10.1210/en.2004-1064 [DOI] [PubMed] [Google Scholar]
- Tobin, A. B. , Butcher, A. J. , & Kong, K. C. (2008). Location, location, location… site‐specific GPCR phosphorylation offers a mechanism for cell‐type‐specific signalling. Trends in Pharmacological Sciences, 29(8), 413–420. 10.1016/j.tips.2008.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Schoore, G. , Mendive, F. , Pochet, R. , & Vassart, G. (2005). Expression pattern of the orphan receptor LGR4/GPR48 gene in the mouse. Histochemistry and Cell Biology, 124(1), 35–50. 10.1007/s00418-005-0002-3 [DOI] [PubMed] [Google Scholar]
- Venkatakrishnan, A. J. , Deupi, X. , Lebon, G. , Heydenreich, F. M. , Flock, T. , Miljus, T. , … Babu, M. M. (2016). Diverse activation pathways in class A GPCRs converge near the G‐protein‐coupling region. Nature, 536(7617), 484–487. 10.1038/nature19107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdonk, K. , Danser, A. H. , & van Esch, J. H. (2012). Angiotensin II type 2 receptor agonists: Where should they be applied? Expert Opinion on Investigational Drugs, 21(4), 501–513. 10.1517/13543784.2012.664131 [DOI] [PubMed] [Google Scholar]
- Vervoort, V. S. , Beachem, M. A. , Edwards, P. S. , Ladd, S. , Miller, K. E. , de Mollerat, X. , … Srivastava, A. K. (2002). AGTR2 mutations in X‐linked mental retardation. Science, 296(5577), 2401–2403. 10.1126/science.1072191 [DOI] [PubMed] [Google Scholar]
- Wang, K. , Li, N. , Yeung, C. H. , Cooper, T. G. , Liu, X. X. , Liu, J. , … Liu, F. J. (2015). Comparison of gene expression of the oncogenic Wnt/beta‐catenin signaling pathway components in the mouse and human epididymis. Asian Journal of Andrology, 17(6), 1006–1011. 10.4103/1008-682X.157396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L. , Wang, X. , Laird, N. , Zuckerman, B. , Stubblefield, P. , & Xu, X. (2006). Polymorphism in Maternal LRP8 Gene Is Associated with Fetal Growth. The American Journal of Human Genetics, 78(5), 770–777. 10.1086/503712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, W. , Qiao, Y. , & Li, Z. (2018). New insights into modes of GPCR activation. Trends in Pharmacological Sciences, 39(4), 367–386. 10.1016/j.tips.2018.01.001 [DOI] [PubMed] [Google Scholar]
- Wang, X. J. , Zhang, D. L. , Xu, Z. G. , Ma, M. L. , Wang, W. B. , Li, L. L. , … Sun, J. P. (2014). Understanding cadherin EGF LAG seven‐pass G‐type receptors. Journal of Neurochemistry, 131(6), 699–711. 10.1111/jnc.12955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward, Y. , Lake, R. , Yin, J. J. , Heger, C. D. , Raffeld, M. , Goldsmith, P. K. , … Kelly, K. (2011). LPA receptor heterodimerizes with CD97 to amplify LPA‐initiated RHO‐dependent signaling and invasion in prostate cancer cells. Cancer Research, 71(23), 7301–7311. 10.1158/0008-5472.CAN-11-2381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe, H. , Takeo, T. , Tojo, H. , Sakoh, K. , Berger, T. , Nakagata, N. , … Kondoh, G. (2014). Lipocalin 2 binds to membrane phosphatidylethanolamine to induce lipid raft movement in a PKA‐dependent manner and modulates sperm maturation. Development, 141(10), 2157–2164. 10.1242/dev.105148 [DOI] [PubMed] [Google Scholar]
- Weis, W. I. , & Kobilka, B. K. (2018). The molecular basis of G protein‐coupled receptor activation. Annual Review of Biochemistry, 87, 897–919. 10.1146/annurev-biochem-060614-033910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelly, S. , Muthusubramanian, A. , Powell, J. , Johnson, S. , Hastert, M. C. , & Cornwall, G. A. (2016). Cystatin‐related epididymal spermatogenic subgroup members are part of an amyloid matrix and associated with extracellular vesicles in the mouse epididymal lumen. Molecular Human Reproduction, 22(11), 729–744. 10.1093/molehr/gaw049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde, C. , Fischer, L. , Lede, V. , Kirchberger, J. , Rothemund, S. , Schoneberg, T. , & Liebscher, I. (2016). The constitutive activity of the adhesion GPCR GPR114/ADGRG5 is mediated by its tethered agonist. The FASEB Journal, 30(2), 666–673. 10.1096/fj.15-276220 [DOI] [PubMed] [Google Scholar]
- Winters, B. R. , & Walsh, T. J. (2014). The epidemiology of male infertility. The Urologic Clinics of North America, 41(1), 195–204. 10.1016/j.ucl.2013.08.006 [DOI] [PubMed] [Google Scholar]
- Wong, P. Y. , & Uchendu, C. N. (1990). The role of angiotensin‐converting enzyme in the rat epididymis. The Journal of Endocrinology, 125(3), 457–465. 10.1677/joe.0.1250457 [DOI] [PubMed] [Google Scholar]
- Xiao, K. , McClatchy, D. B. , Shukla, A. K. , Zhao, Y. , Chen, M. , Shenoy, S. K. , … Lefkowitz, R. J. (2007). Functional specialization of beta‐arrestin interactions revealed by proteomic analysis. Proceedings of the National Academy of Sciences of the United States of America, 104(29), 12011–12016. 10.1073/pnas.0704849104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, T. , Tang, Y. , Luo, R. , Zhang, X. , Wu, S. , Gu, Y. , … Hu, F. (2020). GPR64 promotes cAMP pathway in tumor aggressiveness in sparsely granulated growth hormone cell adenomas. Endocrine, 68(3), 629–639. 10.1007/s12020-020-02263-y [DOI] [PubMed] [Google Scholar]
- Yang, B. , Wang, J. , Zhang, W. , Pan, H. , Li, T. , Liu, B. , … Wang, B. (2017). Pathogenic role of ADGRG2 in CBAVD patients replicated in Chinese population. Andrology, 5(5), 954–957. 10.1111/andr.12407 [DOI] [PubMed] [Google Scholar]
- Yang, F. , Xiao, P. , Qu, C. X. , Liu, Q. , Wang, L. Y. , Liu, Z. X. , … Wang, J. (2018). Allosteric mechanisms underlie GPCR signaling to SH3‐domain proteins through arrestin. Nature Chemical Biology, 14(9), 876–886. 10.1038/s41589-018-0115-3 [DOI] [PubMed] [Google Scholar]
- Yang, F. , Yu, X. , Liu, C. , Qu, C. X. , Gong, Z. , Liu, H. D. , … Sun, J. P. (2015). Phospho‐selective mechanisms of arrestin conformations and functions revealed by unnatural amino acid incorporation and (19)F‐NMR. Nature Communications, 6, 8202 10.1038/ncomms9202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi, J. , Xiong, W. , Gong, X. , Bellister, S. , Ellis, L. M. , & Liu, Q. (2013). Analysis of LGR4 receptor distribution in human and mouse tissues. PLoS ONE, 8(10), e78144 10.1371/journal.pone.0078144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin, Q. , Shen, J. , Wan, X. , Liu, Q. , Zhou, Y. , & Zhang, Y. (2018). Impaired sperm maturation in conditional Lcn6 knockout mice. Biology of Reproduction, 98(1), 28–41. 10.1093/biolre/iox128 [DOI] [PubMed] [Google Scholar]
- Ylisaukko‐oja, T. , Rehnstrom, K. , Vanhala, R. , Tengstrom, C. , Lahdetie, J. , & Jarvela, I. (2004). Identification of two AGTR2 mutations in male patients with non‐syndromic mental retardation. Human Genetics, 114(2), 211–213. 10.1007/s00439-003-1048-8 [DOI] [PubMed] [Google Scholar]
- Yuan, P. , Liang, Z. K. , Liang, H. , Zheng, L. Y. , Li, D. , Li, J. , … Wang, W. J. (2019). Expanding the phenotypic and genetic spectrum of Chinese patients with congenital absence of vas deferens bearing CFTR and ADGRG2 alleles. Andrology, 7(3), 329–340. 10.1111/andr.12592 [DOI] [PubMed] [Google Scholar]
- Zhang, D. L. , Sun, Y. J. , Ma, M. L. , Wang, Y. J. , Lin, H. , Li, R. R. , … Sun, J. P. (2018). Gq activity‐ and beta‐arrestin‐1 scaffolding‐mediated ADGRG2/CFTR coupling are required for male fertility. eLife, 7, e33432 10.7554/eLife.33432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, W. , Leung, P. Y. , Chew, S. B. , Chan, H. C. , & Wong, P. Y. (1996). Localization and distribution of angiotensin II in the rat epididymis. The Journal of Endocrinology, 149(2), 217–222. 10.1677/joe.0.1490217 [DOI] [PubMed] [Google Scholar]
- Zhao, Y. , Diao, H. , Ni, Z. , Hu, S. , Yu, H. , & Zhang, Y. (2011). The epididymis‐specific antimicrobial peptide beta‐defensin 15 is required for sperm motility and male fertility in the rat (Rattus norvegicus). Cellular and Molecular Life Sciences, 68(4), 697–708. 10.1007/s00018-010-0478-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, Q. , Clarke, L. , Nie, R. , Carnes, K. , Lai, L. W. , Lien, Y. H. , … Hess, R. A. (2001). Estrogen action and male fertility: roles of the sodium/hydrogen exchanger‐3 and fluid reabsorption in reproductive tract function. Proceedings of the National Academy of Sciences of the United States of America, 98(24), 14132–14137. 10.1073/pnas.241245898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, W. , De Iuliis, G. N. , Dun, M. D. , & Nixon, B. (2018). Characteristics of the epididymal luminal environment responsible for sperm maturation and storage. Frontiers in Endocrinology (Lausanne), 9, 59 10.3389/fendo.2018.00059 [DOI] [PMC free article] [PubMed] [Google Scholar]