Abstract
Nitric oxide (NO) is a versatile molecule that plays key roles in the development and survival of mammalian species by endowing brain neuronal networks with the ability to make continual adjustments to function in response to moment‐to‐moment changes in physiological input. Here, we summarize the progress in the field and argue that NO‐synthetizing neurons and NO signalling in the brain provide a core hub for integrating sensory‐ and homeostatic‐related cues, control key bodily functions, and provide a potential target for new therapeutic opportunities against several neuroendocrine and behavioural abnormalities.
Abbreviations
- AOB
accessory olfactory bulb
- ARH
arcuate nucleus
- CNG
cyclic nucleotide‐gated channels
- DMH
dorsomedial hypothalamic nucleus
- eNOS, NOS3
endothelial NOS
- GnRH
gonadotropin‐releasing hormone
- IC
inferior colliculus
- iNOS, NOS2
inducible NOS
- LHA
lateral hypothalamic area
- L‐NMMA
NG‐methyl‐L‐arginine
- MePO
median preoptic nucleus
- nNOS, NOS1
neuronal NOS
- NPY
neuropeptide Y
- OB
olfactory bulb
- OVLT
organum vasculosum of the lamina terminalis
- PBP
parabrachial pigmented nucleus
- PV
paraventricular nucleus of the thalamus
- PVH
paraventricular hypothalamic nucleus
- RLi
rostral linear nucleus
- sGC
soluble guanylate cyclase
- SNc
substantia nigra pars compacta
- VMH
ventromedial hypothalamic nucleus
- VTA
ventral tegmental area
1. INTRODUCTION
Nitric oxide (NO), originally referred to as endothelium relaxing factor (Furchgott & Zawadzki, 1980; Ignarro, Byrns, Buga, & Wood, 1987; R. M. J. Palmer, Ferrige, & Moncada, 1987), is a biological intracellular messenger that unlike established neurotransmitters can diffuse across cell biological membranes, including those of the CNS (Garthwaite, 2016). Even though NO was discovered approximately 40 years ago, it still remains the subject of active research. Exogenous NO was known, since 1977, to stimulate soluble guanylate cyclase (sGC) activity (Arnold, Mittal, Katsuki, & Murad, 1977; Miki, Kawabe, & Kuriyama, 1977), producing cGMP, which is a second messenger with a broad range of functions in the CNS. Endogenous NO was found to form in the brain in response to the activation of the NMDA subtype of glutamate receptors at a much later date than its initial identification (Garthwaite, Charles, & Chess‐Williams, 1988). Brain‐derived NO is mainly produced by the neuronal form of NOS (nNOS or NOS1) through a reaction that converts L‐arginine and oxygen into citrulline and NO (Figure 1; Bredt, Glatt, et al., 1991; Bredt, Hwang, et al., 1991). The enzyme nNOS is actually one of the three homologous isoforms of NOS, with the other two being the endothelial NOS (eNOS or NOS3) and inducible NOS (iNOS or NOS2). The broad distribution of the NOS isoforms and thus the ability of NO to affect various different cell targets across the CNS support a role for this molecule in a wide range of physiological functions, including the hypothalamic control of reproduction and energy homeostasis (Chachlaki, Garthwaite, & Prevot, 2017). In line with the importance of NO as a key signalling molecule, NO, as well as downstream targets of its pathway, have been the target of pharmacological research concerning numerous brain disorders (Garthwaite, 2010; Ghasemi, Mayasi, Hannoun, Eslami, & Carandang, 2018; Pradhan, Bertels, & Akerman, 2018; Shim, Shuman, & Duncan, 2016; Virarkar, Alappat, Bradford, & Awad, 2013). In this review, we will go through the main signalling molecules of the NO pathway and detail the anatomical distribution of NO‐synthetizing neurons and their diverse phenotype in the brain, and we will present some of the key functions of this distinctive neurotransmitter in the CNS.
FIGURE 1.
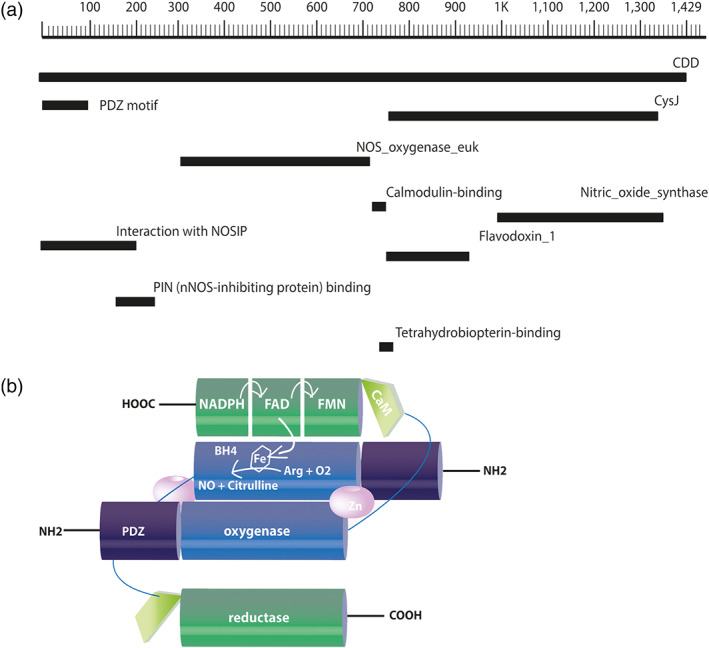
Schematic representation of the primary structure and the dimeric conformation of mouse neuronal NOS. (a) The primary structure of nNOS contains 1,434 amino acids in its chain and has a predicted molecular weight of 160.8 kDa. Amino acids are numbered in the form of a scale, and regions encoding structural domains and cofactor‐binding sites are shown in black bars according to their position on the chain of the nNOS. (b) The oxygenase/haem domain (blue) is connected to the reductase domain (green), consisting of the CysJ, flavodoxinJ, and ferrodoxin domains, by a flexible linker, containing a CaM‐binding sequence (light green). The FAD‐containing domain uses NADPH as an electron source. The FMN‐binding domain shuttles electrons from NADPH/FAD to the haem group of the oxygenase domain. The binding of CaM to NOS promotes the electron transfer from the FMN domain of one monomer to the haem domain of the other monomer. Arg, arginine; BH4, (6R)‐tetrahydrobiopterin; CaM, calmodulin; FAD, ferrodoxin; FMN, flavodoxin (flavin mononucleotide)
2. NO SYNTHASE SIGNALLING IN THE BRAIN
2.1. NOS dimerization: A key step for the activation of the enzyme and the subsequent production of NO
As mentioned above, there are three NOS isoforms that are responsible for the enzymic formation of NO from L‐arginine. Among these isoforms, nNOS and eNOS are constitutively expressed and activated by an increase in the intracellular concentration of free Ca2+ (Mayer, Schmidt, Humbert, & Böhme, 1989). Each NOS monomer contains two domains: an N‐terminal oxygenase domain and a C‐terminal reductase domain. The process of NO formation involves both of these domains, as the reductase domain supplies the electrons needed for the NOS reaction, which takes place in the oxygenase domain (Crane et al., 1998; Stuehr, 1997). Interestingly, NOS requires homodimerization, because this electron transfer only occurs between the reductase domain of one subunit to the haem in the oxygenase domain of the adjacent subunit of the dimer (Figure 1b; Panda, Ghosh, & Stuehr, 2001; Siddhanta et al., 1998). Dimerization not only enables the electron transfer but also results in the creation of high‐affinity binding sites for (6R)‐tetrahydrobiopterin (BH4; sapropterin) and arginine in the oxygenase domain (Bendall, Douglas, McNeill, Channon, & Crabtree, 2013). Thus, the process of enzymic NO production involves the following: (a) the oxidation of L‐arginine in the N‐terminal oxygenase domain (containing a P450‐type haem and binding sites for the BH4 and the substrates L‐arginine and molecular oxygen) and (b) reduction of O2 via the transfer of NADPH‐derived electrons from the flavin‐containing C‐terminal reductase domain to the catalytic haem site (Crane et al., 1998; Stuehr, 1997).
This feature of NOS has important consequences because it implies that NOS monomers will not catalyse NO production. Actually, the destabilization of the nNOS homodimer renders the protein more flexible and/or disordered and eventually triggers the degradation of the enzyme (Bender, Demady, & Osawa, 2000; Dunbar et al., 2004). Similarly, removing the ability of nNOS to dimerize renders the enzyme inactive (Hallmark, Phung, & Black, 1999).
Hence, the maintenance of physiological NO levels is based upon the formation of NOS homodimers, while their disruption is thought to be accompanied by an increased production of ROS, usually as superoxide anion (Tejero, Shiva, & Gladwin, 2018), which can be harmful (Lin & Beal, 2006). However, the ROS also exert important signalling functions in various tissues (Dröge, 2002), including the brain (Iadecola, 2004). The expression of mutated NOS monomers have been shown to cause dominant‐negative effects (De Seranno et al., 2010; Lee, Robinson, & Michel, 1995; Phung & Black, 1999). Interestingly, posttranslational modifications of eNOS, such as S‐nitrosylation, have been reported to block the formation of the eNOS dimer, resulting in the loss of NO synthesis activity (Ravi, Brennan, Levic, Ross, & Black, 2004). Alterations in eNOS dimerization are linked to endothelial dysfunction in aged vessels, hypertension (Yang, Huang, Kaley, & Sun, 2009), and the autonomic control of heart rate (Paton, Kasparov, & Paterson, 2002; Wong, Polson, Murphy, Paton, & Kasparov, 2002). The importance of nNOS dimerization is also evident in studies linking changes in the presence of nNOS homodimers with the appearance of metabolic disorders, such as diabetes and obesity (Gangula, Maner, Micci, Garfield, & Pasricha, 2007; Lajoix et al., 2004; Mezghenna et al., 2011; Showkat Ali et al., 2012). Recently, a study suggested that disruption of nNOS dimerization may contribute to the development of Alzheimer's disease because of the impaired NO synthesis and hence the lack of NO‐mediated neuroprotective effects (Kwon et al., 2016). This raises the possibility that alterations in the levels of nNOS dimerization might be involved in the development of several neurodegenerative disorders.
2.2. The “canonical” pathway of NO signal mediation
As mentioned above, NO is produced from its precursor L‐arginine by three major isoforms of NOS (i.e., nNOS, eNOS, and iNOS). Both nNOS and eNOS are constitutively expressed and activated by Ca2+/calmodulin‐dependent signalling, whereas iNOS is inducibly activated, independently of Ca2+ levels. Classically, the effect of intracellular NO signalling is mediated by the sGC/cGMP pathway and activated sGC will lead to the production of cGMP from GTP (as described in the next section). In turn, cGMP will interact with three main groups of proteins, mediating its downstream effects: PKGs, PDEs, and cGMP‐gated cation channels (Azevedo et al., 2014). This cascade of events is referred to as the “canonical” NO pathway. Additionally, NO can induce chemical reactions, without the involvement of any enzymes, leading to posttranslational modifications of protein targets. This action of NO, referred to as the “non‐canonical” NO pathway, will be analysed later on.
2.2.1. NO activates the formation of cGMP upon stimulation of guanylate cyclase
In neurons, nNOS is physically associated with the NMDA receptor via its PDZ domains and the assembly of a ternary complex involving the scaffolding protein, postsynaptic density 95 (PSD‐95; Figure 2a; Brenman et al., 1996; Christopherson, Hillier, Lim, & Bredt, 1999). The Ca2+ influx through activated NMDA receptors is largely responsible for the stimulation of nNOS (Garthwaite et al., 1988), although other mechanisms for increasing cytoplasmic [Ca2+], such as voltage‐gated Ca2+‐channels or the release of Ca2+ from internal stores, can also be involved (Daniel, Levenes, & Crépel, 2016). This rise in intracellular Ca2+ results in its binding to calmodulin, creating a Ca2+‐calmodulin complex that can directly activate the constitutive isoforms of NOS (Toda & Okamura, 2003). This leads to NO production as long as Ca2+ levels are high. Once NO is released, it diffuses rapidly and stimulates the formation of cGMP by sGC (Garthwaite, 2016). NO‐activated GC contains a haem group, which acts as the ligand binding site, and a transduction domain (Hobbs, 1997). Even though this haem group is of the type used in haemoglobin to bind O2, NO‐activated GC exhibits a marked preference for NO, initiating NO signalling even in the presence of a >10,000‐fold excess of O2 (Garthwaite, 2008). Upon the binding of NO to the haem group, a conformational change occurs because of the displacement of the histidine group, leading to the activation of the enzyme (Ignarro, Ross, & Tillisch, 1991), which can now convert GTP to cGMP. This initial step of NO binding to sGC (1:1 stoichiometry of NO to sGC) leads, according to more recent studies, to a moderate activation state of the enzyme, forming a stable haem complex because of the slow dissociation rate of the NO (i.e., NO acts as a long‐lasting partial agonist). Addition of excess NO (e.g., acute NO production) further stimulates sGC to a high activity form that only persists in the presence of excess NO, the removal of which will result to a rapid return to the low activity state (i.e., NO acts as a transient full agonist; Cary, Winger, & Marletta, 2005; Russwurm & Koesling, 2004). Physiological activation and deactivation of sGC has been discussed in recent comprehensive reviews (Derbyshire & Marletta, 2012; Horst & Marletta, 2018).
FIGURE 2.
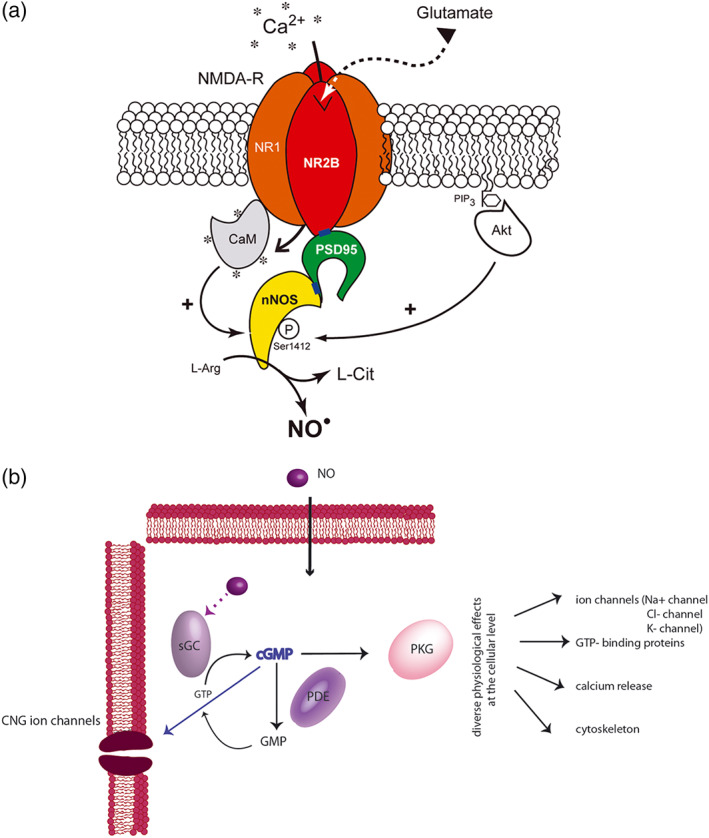
Schematic representation of the NO/cGMP signalling pathway. (a) The translocation of nNOS from the cytosol to the membrane, its physical interaction with the NR2B subunit of the NMDA receptor (NMDA‐R) via PDZ domains (blue rectangles) involves the postsynaptic density 95 (PSD‐95) scaffolding protein and the assembly of a ternary complex nNOS/PSD‐95/NMDA receptor. Binding of glutamate to the NMDA receptor allows Ca+2 entry into the neuron. Ca+2 influx activates nNOS α through calmodulin (CaM) binding leading to the production of NO, which is formed enzymically from L‐arginine (L‐Arg) in equimolar amounts with L‐citrulline (L‐Cit). In parallel, membrane‐tethered nNOS is also subjected to posttranscriptional modifications (such as phosphorylation via Akt) that modulates its catalytic activity (Adak et al., 2001; Guerra et al., 2019; Rameau et al., 2007). (b) NO is a highly soluble and membrane permeable neurotransmitter. Upon binding to NO‐sensitive guanylate cyclase, NO induces a conformational change resulting in the activation of the enzyme and the subsequent conversion of GTP to cGMP. The newly produced cGMP can interact with various intracellular proteins, including cGMP‐binding PDE, cGMP‐gated cation channels (CNGs), and PKG, triggering the phosphorylation of many different substrates. The NO/cGMP pathway is thus implicated in multiple distinct physiological processes such as cytoskeletal organization, Ca2+ release from intracellular stores, and differentiation/proliferation of vascular smooth muscle
2.2.2. PDEs: Key members of the NO signalling pathway
PDEs are of particular importance, since they are responsible for catalysing the hydrolysis of the 3′ cyclic phosphate bond of cAMP or cGMP, thus modulating the duration and intensity of the intracellular response of downstream targets to cGMP and cAMP (see Azevedo et al., 2014). Among the 11 known PDE families, 1, 2, 3, 5, 6, 9, 10, and 11 are those with the highest affinity for cGMP, of which PDE 5, 6, and 9 are referred to as cGMP‐specific PDEs, whereas PDE 4, 7, and 8 are considered to be cAMP specific (Garthwaite, 2008). The selectivity for either cAMP or cGMP may be determined by what is known as the “glutamine switch.” According to this hypothesis, a glutamine residue in the binding pocket of the PDEs is constrained by neighbouring residues to a position favouring selectivity for either cAMP or cGMP. In PDEs with the ability to hydrolyse both cyclic nucleotides, this glutamine residue can rotate freely (K. Y. J. Zhang et al., 2004). Thus far, studies have described 21 different gene products from the 11 PDE families (Azevedo et al., 2014). Nevertheless, theoretically, alternative splicing events taking place in these genes could raise that number to more than 100 different mRNA products, the majority of which could be translated into functional proteins (for review, see Beavo, 1995; Omori & Kotera, 2007). Apart from this “genetic” variation in the expression of the PDEs, posttranslational modifications and distinct biochemical mechanisms (i.e., phosphorylation, protein–protein interactions, and allosteric binding of cAMP or cGMP) serve to further reinforce the complexity of these proteins and could affect several distinct PDE families within a single cell or cell type (Castro, Verde, Cooper, & Fischmeister, 2006; D. Palmer & Maurice, 2000). As each PDE protein has distinct hydrolysis rates and enzyme kinetics, their combinatorial expression could further increase the complexity of their action, which ultimately determines the amounts of second messengers available to act on downstream targets. The cGMP‐specific PDEs behave in ways described by Michaelis–Menten kinetics, allowing for the extensive study of the changes in the PDE activity according to the GC activity and time. PDE5 is one of the most studied members of the PDE family. The general idea proposed is that the activity of PDE5 in its resting state is very low but increases as the cellular cGMP concentration rises (Kass, Takimoto, Nagayama, & Champion, 2007). cGMP can accumulate to significant concentrations before being hydrolysed by the PDEs, whose activity may return to its basal state, quite slowly (Garthwaite, 2010).
2.2.3. cGMP‐dependent PKG and ion channels
PKG activation is probably the most widespread mechanism employed by cGMP to mediate its downstream signalling effects (see Francis, Busch, & Corbin, 2010). PKG is a serine/threonine‐specific PK that phosphorylates a number of biologically important downstream molecules, such as small G proteins (RhoA) and even members of the cGMP pathway, such as PDEs and ion channels (see Francis et al., 2010). Its various ultimate effects include alterations of their activity, function, and subcellular localization, as well as cell differentiation and proliferation. PKG is encoded by two genes in mammalian species, Prkg1 and Prkg2, resulting in the production of PKG type I (PKG‐I) and type II (PKG‐II) respectively (Hofmann, 2005). PKG‐I and ‐II are homodimeric proteins that share the same structure, which involves an N‐terminal domain, a regulatory domain that contains two non‐identical cGMP‐binding sites and a kinase domain.
In addition to the ion channels mentioned above, whose activity is being indirectly altered by cGMP‐induced PKG phosphorylation, the activity of cyclic nucleotide gated (CNG) and hyperpolarization‐activated cyclic nucleotide‐gated (HCN) channels can be directly regulated by cGMP binding (Biel, 2009). CNG channels are composed of two subunits, the α and β subunits, which are encoded by four (CNGA1–4) and two (CNGB1–2) genes respectively. The presence of the β subunit is not only a prerequisite for the CNG channel to be functional but is also a modifying factor, altering the physiological characteristics of the CNG channels, as well as its membrane targeting. HCN channels consist of four identical or non‐identical subunits encoded by four genes (HCN1–4), all of which are expressed in the brain. They are voltage‐gated cation channels that open following hyperpolarizing membrane potentials. In contrast to CNG channels that are more sensitive to cGMP over cAMP, HCN channels are 100 times less sensitive to cGMP (compared to cAMP). Thus, in the CNS, HCN functioning is mostly indirectly affected via the PKG action, rather than directly modified by cGMP binding (Craven & Zagotta, 2006).
2.3. The “non‐canonical” pathway of NO signal mediation: The example of S‐nitrosylation
So far, we have presented the mode of action of NO through the canonical pathway, which involves the activation of sGC and the subsequent generation of cGMP. There are, however, several other ways in which NO can affect its downstream targets. These involve NO‐induced posttranslational modifications of various target proteins, of which three are known so far: thiol nitrosation (also called S‐nitrosylation), S‐glutathionylation, and tyrosine nitration (see Benhar, Forrester, & Stamler, 2009; Hess, Matsumoto, Kim, Marshall, & Stamler, 2005; Nakamura et al., 2013). Here, we will briefly mention S‐nitrosylation, as evidence suggests that this posttranslational modification is a determining mechanism of NO signal transmission in the brain (Nakamura et al., 2013), including the hypothalamus (Fioramonti et al., 2013).
The term S‐nitrosylation describes the incorporation of a nitroso group at a reactive cysteine thiol, forming a nitrosothiol group (SNO; Hess et al., 2005). This posttranslational modification is strictly regulated in time and space, following rules that increase both the selectivity and the specificity of this reaction. Specifically, proteins that reside in proximity to NOS isoforms and that interact or colocalize with them are believed to be more prone to S‐nitrosylation (Derakhshan, Hao, & Gross, 2007). This subcellular topology‐related specificity has been observed on several occasions, as for example in the case of Hsp90, the scaffolding protein of eNOS, which acts as an activator under physiological conditions. Following its S‐nitrosylation at a cysteine residue located in the region of the protein interacting with eNOS, hsp90 is no longer able to promote the activity of eNOS but acts thereafter as a negative regulator of NO production (Martínez‐Ruiz et al., 2005). NMDA receptors, key regulators of nNOS activity via the mediation of the Ca2+ flux, have also been suggested as substrate for S‐nitrosylation. Specifically, in vitro studies have shown that the NR2A subunit of the NMDA receptor is S‐nitrosylated at a single cysteine residue (identified as Cys399), an modification that de‐activates the receptor due to the negative regulation of its regulatory and agonist‐binding domains (Choi et al., 2000). Additional in vitro studies also suggest that NMDA‐mediated production of NO may physiologically regulate targeting of PSD‐95 to synapse via competitive cysteine modifications, including S‐nitrosylation (Ho et al., 2011). At the inhibitory synapse, the S‐nitrosylation of gephyrin, the principal scaffolding protein of the GABAergic synapse, has been proposed to regulate the plasticity of postsynaptic GABAergic sites (Dejanovic & Schwarz, 2014). S‐nitrosylation has been also identified as an important mediator of dendritic growth and axonal retraction and has been implicated in adult neurogenesis via the negative regulation of the transcriptional activity of MEF2, a pro‐survival transcription factor also known to control synapse formation and dendritic remodelling (Nott, Watson, Robinson, Crepaldi, & Riccio, 2008; Okamoto & Lipton, 2015; Stroissnigg et al., 2007). Most importantly, the S‐nitrosylation of the NO receptor, sGC, has been claimed to be a mechanism for its desensitization, thus directly modulating the NO response (Sayed, Baskaran, Ma, van den Akker, & Beuve, 2007).
In the hypothalamus, S‐nitrosylation of key proteins has been proposed to be involved in the modulation of NO‐sGC‐mediated glucose sensing in the hypothalamus (Fioramonti et al., 2013; C. Zhou & Routh, 2018). NO has also been implicated in the regulation of glutamate metabolism in the CNS through the selective nNOS‐dependent S‐nitrosylation of proteins controlling glutamate transport and metabolism (Raju et al., 2015). A balance between the S‐nitrosylation and the denitrosylation of proteins participating in the coordination of glutamate metabolism would result in a transient inhibition/activation of these targets, important for their functional regulation and consequently for the control of glutamate concentrations (Benhar et al., 2009; Qu et al., 2012; Raju et al., 2015).
More recently, a study has raised the intriguing hypothesis that signalling by NO (and potentially other gasotransmitters) could be a general strategy for interspecies communication through epigenetic modification of the host proteomes (Seth et al., 2019). More specifically, microbial NO by causing the S‐nitrosylation of Argonaute proteins was demonstrated to regulate the host miRNA machinery, regulating thus gene expression. This is a major finding because it implicates resident microbiota (naturally residing in all mammals) in the control of bodily functions. Thus, the intake of dietary sources of NO in mammals, by increasing S‐nitrosylation of targets, could have physiological consequences during the early developmental stages, shaping gene expression and proteome identity (Seth et al., 2019).
S‐nitrosylation, akin to phosphorylation, is increasingly being recognized as being a potential mechanism of regulation of signal transduction pathways and cellular functions under physiological conditions, with its deregulation being linked to pathophysiological conditions and abnormal cell function (see Foster, McMahon, & Stamler, 2003). Aberrant S‐nytrosylation has indeed been shown to affect protein folding, mitochondrial integrity, synaptic function, apoptosis, and autophagy and could play a crucial role in the pathogenesis of brain diseases (see Nakamura et al., 2013).
3. ANATOMICAL DISTRIBUTION AND PHENOTYPIC DIVERSITY OF NOS NEURONS IN THE BRAIN
3.1. Distribution of nNOS‐expressing cells
The distribution of nNOS expression in the CNS has been mainly assessed through in situ hybridization (Figure 3), immunocytochemistry using antibodies specifically directed against the nNOS protein and histochemical studies probing NADPH‐diaphorase activity. In the brain, immunoreactivity for nNOS has only been visualized in the cell bodies, dendrites, and axons of neurons (de Vente et al., 1998; Gotti, Sica, Viglietti‐Panzica, & Panzica, 2005; Schmidt et al., 1992; Vincent & Kimura, 1992).
FIGURE 3.
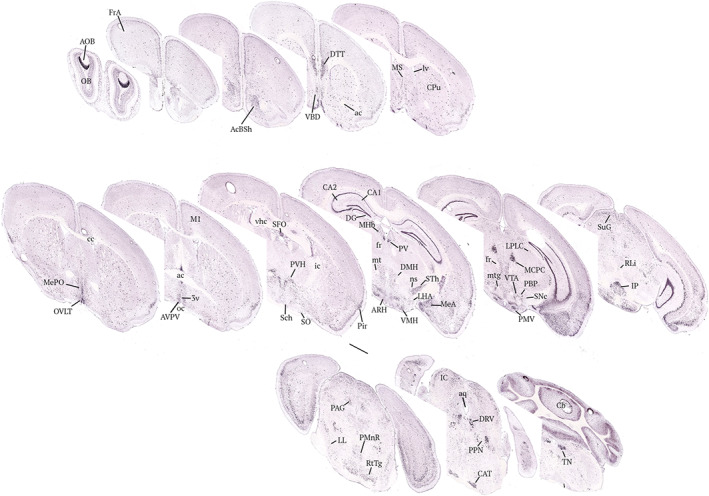
Neuroanatomical distribution of Nos1 transcripts in the mouse brain. Nos1‐expessing cells appear as dark dots; the brain slices have been counterstained with Nissl (pink labelling). Images have been captured from the Allen Brain atlas (https://mouse.brain‐map.org/gene/show/17892). ac, anterior commissure; AcSh, accumbens nucleus, shell region; AOB, accessory olfactory bulb; aq, aqueduct; ARH, arcuate nucleus of the hypothalamus; AVPV, anteroventral periventricular nucleus; CA1, field CA1 of the hippocampus; CA2, field CA2 of the hippoampus; CAT, nucleus of the central acoustic tract; Cb, cerebellar vermis; cc, corpus callosum; CPu, caudate putamen (striatum); DG, dentate gyrus; DMH, dorsomedial nucleus of the hypothalamus; DTT, dorsal tenia tract; EPI, external plexiform layer of the olfactory bulb; fr, fasciculus retroflexus; FrA, frontal association cortex; ic, internal capsule; IC, inferior colliculus; IP, interpeduncular nucleus; LHA, lateral area of the hypothalamus; LL, lateral lemniscus; LPLC, lateral posterior nucleus of the thalamus; lv, lateral ventricle; M1, primary motor cortex; MCPC, magnocellular nucleus of the posterior commissure; MeA, medial amygdala; MePO, median preoptic nucleus; MHb, medial habenular nucleus; MS, medial septal nucleus; mt, mammillary tract; ns, nigrostriatal bundle; OB, olfactory bulb; oc, optic chiasm; OVLT, organum vasculosum lamina terminalis; PAG, periaquesuctal grey; PBP, parabrachial pigmented nucleus; Pir, piriform cortex; PMnR, paramedial raphe nucleus; PMV, perimamillary nucleus; PPN, pediculopontine nucleus; PV, paraventricular nucleus of the thalamus; PVH, paraventriculat nucleus of the hypothalamus; RLi, rostral linear nucleus; RM, retromamillary nucleus; RtTG, reticulotegmental nucleus of the pons; Sch, suprachiasmatic nucleus; SFO, subfornical organ; SNc, substantia nigra pars compacta; SO, supraoptic nucleus; STh, subthalamic nucleus; SuG, superficial grey layer; TN, tegmental nucleus; VBD, nucleus of the ventricular limb of the diagonal band; Vhc, ventral hippocampal commissure; VTA, ventral tegmental area. Scale bar, 1 mm
Neurons expressing NOS are distributed in numerous structures of the brain throughout its rostro‐caudal extent (Gotti et al., 2005) but are particularly abundant in the hypothalamus (Chachlaki, Malone, et al., 2017; Reis et al., 2018; Yamada, Emson, & Hökfelt, 1996) and in brain structures processing sensory information (Figure 3), such as the olfactory bulb (OB) and the inferior colliculus (IC; Endoh, Maiese, & Wagner, 1994; Fujimoto, Konno, Watanabe, & Jinno, 2017; Vincent & Kimura, 1992), which is the principal midbrain centre of the auditory pathway (nucleus of the central acoustic tract; Oliver & Cant, 2018). More specifically, nNOS‐immunoreactive neurons are seen in the neocortex, the hippocampus (field CA1 of the hippocampus, CA3, and dentate gyrus), and several thalamic nuclei, with particularly intense signals observed in the regions of the lateral posterior nucleus of the thalamus, the paraventricular thalamic nucleus (PV), and the medial habenular nucleus. They are also seen in most lateral and medial septal nucleus and the caudoputamen of the striatum, as well as in the nucleus of the solitary tract in the brainstem and in the cerebellum (Gotti et al., 2005). A large population of nNOS neurons also reside in the interpeduncular nucleus, and a smaller population is diffusely distributed throughout the substantia nigra pars compacta (SNc) and in the parabrachial pigmented nucleus (PBP) and rostral linear nucleus (RLi) of the ventral tegmental area (VTA; Paul et al., 2018). Particularly, dense populations of nNOS‐expressing neurons are seen in the organum vasculosum of the lamina terminalis (OVLT) in the preoptic region and the subfornical organ below the ventral hippocampal commissure (Chachlaki, Malone, et al., 2017; Rodrigo et al., 1997) and the area postrema in the brainstem (Rodrigo et al., 1997). These are three sensory circumventricular organs in which neurons reside, outside the blood–brain barrier (Langlet, Mullier, Bouret, Prevot, & Dehouck, 2013). In the hypothalamus, nNOS‐immunoreactive neurons are particularly abundant in the median preoptic nucleus (MePO), which lies just above the OVLT, and the ventrolateral part of the ventromedial hypothalamic nucleus (VMH). Immunoreactivity for nNOS is also seen in hypothalamic neurons in the anteroventral periventricular nucleus, the posterior periventricular nucleus, the lateral hypothalamic area (LHA), the PV hypothalamic nucleus (PVH), the supraoptic nucleus, the dorsomedial hypothalamic nucleus (DMH), the LHA, the arcuate nucleus (ARH), and the ventral premamillary nucleus, where their distribution is sparser (Chachlaki, Malone, et al., 2017). In the caudal hypothalamus, immunoreactivity for nNOS is also detected in the supramammillary nucleus, a region just dorsal to the mammillary body (Pedersen et al., 2017; Yamada et al., 1996).
3.2. Phenotypic heterogeneity of nNOS neurons
Phenotypic heterogeneity of nNOS cells has been found in several regions of the brain. In the hippocampus, nNOS is expressed in a large population of GABAergic neurons (Fuentealba et al., 2008; Tricoire et al., 2010, 2011). Most of these originate from the medial ganglionic eminence during embryogenesis and are known to co‐express paravalbumin and somatostatin (Tricoire et al., 2011). However, in the hippocampus, the distinct subpopulation of nNOS interneurons also expressing cholecystokinin, vasoactive intestinal peptide, reelin, and the calcium‐binding protein calretinin appears to originate from the caudal ganglionic eminence (Tricoire et al., 2011). In contrast, in the region of the neocortex, nNOS‐expressing cells, which also mainly originate from the medial ganglionic eminence, are only a small minority of the total neocortical GABAergic neuronal population (Tomioka, Sakimura, & Yanagawa, 2015) with all neocortical nNOS neurons co‐expressing somatostatin and neuropeptide Y (NPY) and approximately half of them expressing calretinin (Jaglin, Hjerling‐Leffler, Fishell, & Batista‐Brito, 2012). While the number of nNOS neurons in the neocortex is small, their effects on the neocortical network through NO release might be significant, as they are highly ramified, and project long distances rostro‐caudally and medio‐laterally, connecting neocortical areas up to 6–8 mm apart (J.‐L. Li et al., 2005).
Unlike the hippocampus and the neocortex, in the hypothalamus, the vast majority of nNOS neurons appear to be glutamatergic in nature (Chachlaki, Malone, et al., 2017). In the preoptic region, nNOS‐expressing glutamatergic cells constitute the main population of the NO‐synthesizing neurons. For example, in the OVLT/MePO and the anteroventral periventricular nucleus, almost all nNOS neurons have Vglut2 promoter activity (Chachlaki, Malone, et al., 2017). The prevalence of glutamatergic signalling in those nNOS neurons, which are otherwise known to express the NMDA glutamatergic receptor (d'Anglemont de Tassigny et al., 2007), suggest that the glutamate‐mediated calcium‐dependent activation of nNOS via the influx of Ca2+ through the NMDA receptor could occur locally through autaptic or ultrashort loops, although they may also be modulated by glutamatergic inputs from other brain regions. In the caudal hypothalamus, nNOS neurons have also been found to be mostly glutamatergic (e.g., in the supramammillary nucleus; Pedersen et al., 2017), whereas in the tuberal region of the hypothalamus, the phenotypic identity of nNOS‐expressing neurons is more diverse. In the DMH and VMH, the majority of NO‐synthesizing neurons exhibit Vglut2 promoter activity, while in the ARH, all the nNOS‐expressing cells have Vgat promoter activity. This signifies that in contrast to the glutamatergic nNOS population of VMH and DMH, the nNOS‐expressing cells residing in the ARH are GABA neurons (Chachlaki, Malone, et al., 2017; Marshall, Desroziers, McLennan, & Campbell, 2017). Another feature of the ARH is that, unlike most of the other hypothalamic nuclei, where nNOS expression is steady throughout postnatal development, nNOS expression is acquired only after birth, during the late infantile period (Chachlaki, Malone, et al., 2017). Equally intriguing are the changes in nNOS expression during postnatal development in the suprachiasmatic nucleus, where nNOS expression is found at birth but vanishes before weaning (Chachlaki, Malone, et al., 2017).
In the midbrain, while nNOS‐expressing neurons in the PBP part of the VTA and the SNc appear to be GABAergic, nNOS neuron in the RLi of the VTA have been shown to be mostly glutamatergic (Paul et al., 2018; X. Yu et al., 2019). Interestingly, cell‐type specific virus‐based anterograde tracing in mice has shown that PBP and SNc GABAergic neurons expressing nNOS do not make any detectable projection outside their residing area and thus appear to be interneurons (Paul et al., 2018). In contrast, the glutamatergic nNOS‐expressing neurons of the RLi have been shown to project to a number of regions, including the LHA, the ventral pallidum, and the median raphe nucleus (Paul et al., 2018). Interestingly, a study from 2019 showed that the chemogenic activation of the subset of glutamatergic neurons of the VTA expressing nNOS projecting to the LHA has wake‐promoting effects, while the activation of the GABAergic neurons of the VTA‐PBN expressing nNOS did not induce either sleep or wakefulness (X. Yu et al., 2019). Although these findings demonstrate that in addition to its contribution to goal‐ and reward‐directed behaviours (Morales & Margolis, 2017), the VTA has a role in regulating arousal, it did not show whether NO signalling in VTA neurons expressing nNOS is actually involved in this process.
In the IC, another structure of the midbrain in which the majority of the neurons expressing nNOS appear to be glutamatergic (Fujimoto et al., 2017), the pattern of nNOS expression has been shown to vary markedly according to its different subdivisions. The neurons of the dorsal and lateral cortices densely express nNOS throughout their somata and dendrites, like most nNOS neurons in the brain. However, the neurons of the central nucleus (a region long thought to be devoid of nNOS) have been shown to express nNOS in a discrete punctuated pattern that can easily be missed at low magnification (Olthof, Gartside, & Rees, 2019). The use of multiple immunofluorescent labelling revealed that these nNOS puncta are actually morphologically associated with PSD‐95, the NMDA receptor, and the sGC at the postsynaptic site of functional glutamatergic synapses (Olthof et al., 2019). This study raises the intriguing possibility that the expression of nNOS, and by extension NO signalling, may have gone unnoticed in other brain regions.
3.3. Distribution of eNOS‐ and iNOS‐expressing cells
Although eNOS is almost exclusively expressed in vascular endothelial cells in the brain (Blackshaw et al., 2003; Garthwaite, 2008), some discrete neuronal populations have also been reported to express eNOS (Tabansky et al., 2018). In contrast, the expression of iNOS in the healthy brain is less conspicuous; yet data suggest that iNOS is expressed in physiological conditions (Bechade, Colasse, Diana, Rouault, & Bessis, 2014) and that loss of iNOS function may affect brain maturation and behaviour (Bechade, Pascual, Triller, & Bessis, 2011; Chen, Majde, & Krueger, 2003). One study using Cre‐mediated expression of reporter genes reports on transient expression of iNOS promoter activity in the hypothalamus, thalamus, hippocampus, the piriform cortex, and the amygdaloid nuclei during postnatal development (Bechade et al., 2014). This iNOS promoter activity has been shown to be restricted to neurons in the healthy brain, whereas inflammatory processes are seen to induce its expression in microglia, but not in neurons (Bechade et al., 2014). In the mature brain, even though iNOS activity has been shown to have beneficial effects when the brain gets injured (Sinz et al., 1999), iNOS is usually associated with pathophysiological situations and will not be further considered here.
4. A KEY ROLE FOR NO IN MODULATING NEURONAL CIRCUIT ACTIVITY INVOLVED IN PHYSIOLOGICAL, BEHAVIOURAL, AND COGNITIVE FUNCTIONS?
4.1. Role of nNOS‐derived NO in the processing of sensory information
4.1.1. The olfactory system
Cells expressing nNOS are found in the olfactory epithelium, where NO is thought to play a prominent role in the activity‐dependent establishment of connections in both developing and regenerating olfactory neurons (Jane Roskams, Bredt, Dawson, & Ronnett, 1994). The expression of nNOS in the olfactory epithelium is down‐regulated shortly after birth (Jane Roskams et al., 1994). However, NADPH diaphorase activity is still strongly expressed in mature sensory olfactory neurons during postnatal life (Kishimoto, Keverne, Hardwick, & Emson, 1993; Kulkarni, Getchell, & Getchell, 1994). In the adult olfactory epithelium, mature sensory olfactory neurons have been shown to express eNOS (Brunert et al., 2009). Although neurogenesis appears not to be affected in the olfactory epithelium of eNOS‐null mice (Brunert et al., 2009), in vitro studies suggest that NO levels may regulate cell proliferation and neuronal differentiation in this epithelium in adults (Sülz et al., 2009). The exposure of olfactory sensory neurons to odours induces an increase in the cGMP concentrations in the entire sensory olfactory neuron, from cilia‐dendrite to the axon terminus growth cone, which is an effect that is blocked by NOS inhibitors (Breer & Shepherd, 1993; Pietrobon et al., 2011). The cGMP levels can also be affected by cAMP levels and, conversely, the interplay of PDEs hydrolysing both second messengers (Pietrobon et al., 2011). In sensory olfactory neurons, the main target of cGMP and cAMP is the CNG channel (Podda & Grassi, 2014). CNG channels are highly expressed on the ciliary membrane of olfactory neurons and, as they are highly permeable to calcium ions, their activation provides a pathway for increasing intracellular Ca2+ concentration, a first step in many cellular processes, including the activation of NOS activity (Pietrobon et al., 2011). Conversely, CNG channels have also been described to be directly activated by NO via a cyclic nucleotide‐independent mechanism apparently involving S‐nitrosylation and the regulation of channel gating (Broillet, 2000; Broillet & Firestein, 1996).
In the OB, nNOS neurons are abundant in the main OB and at even higher density in the accessory OB (AOB; Bredt, Glatt, et al., 1991). In the main OB, which receives input from the main olfactory epithelium, nNOS is found in bundles of nerve fibres reaching individual glomeruli, a portion of the population of the periglomerular cells, occasional larger cells scattered in different layers, and a dense fibre network around the granule cell bodies (Vincent & Kimura, 1992). NO signalling has been shown to promote key structural and functional changes in the functional circuitry of the OB at birth underlying the formation of indelible memories in newborns that are required for the recognition of its own mother (Kendrick et al., 1997). NO‐mediated cGMP production and the potentiation of glutamate release were shown to be involved in these brain plasticity changes underlying memory formation in the OB (Hopkins, Steinbusch, Ittersum, & De Vente, 2018; Kendrick et al., 1997). However, at the level of the OB, the NO signalling pathway has been shown not to be critical for memory recall; thus, it might not be involved in odour perception per se (Kendrick et al., 1997).
In the AOB, nNOS neurons are confined almost exclusively to the granule cells, with scattered large cells in the granule layer (Vincent & Kimura, 1992). The input from the AOB is from the vomeronasal organ, which in mammals, including humans, is the birth place of gonadotropin‐releasing hormone (GnRH) neurons that migrate from the nose to brain during embryogenesis (Casoni et al., 2016) and is believed to be sensitive to pheromone‐like molecules important in mating and kin‐recognition behaviour later on in life (Buck, 2000; Dulac & Torello, 2003). Like in the main OB at birth, NO signalling in the AOB is thought to be involved in the formation of pheromone‐specific olfactory memories in sexually mature animals (Okere, Kaba, & Higuchi, 1996). Odours perceived by the vomeronasal organ/AOB have recently been shown to activate kisspeptin neurons in the preoptic region that regulate GnRH‐dependent ovulation and control lordosis behaviour in female mice via the activation of NO release in downstream hypothalamic nNOS‐expressing neurons (Hellier et al., 2018).
In humans, decreased sense of smell (i.e., hyposmia) or lack of smell (i.e., anosmia) can be the result of either a CNS dysfunction of the olfactory signalling cascades or the presence of disturbances in the upper airways (Landis & Lacroix, 2009). Intriguingly, levels of nasal NO (the main source being the epithelium of the paranasal sinuses) have been shown to correlate positively with olfactory discrimination and identification (Gupta, Drusch, Landis, & Hummel, 2013). This reflects the CNS aspects of olfactory processing (Hedner, Larsson, Arnold, Zucco, & Hummel, 2010). Treatment of patients suffering from acquired and congenital hyposmia with a non‐selective PDE inhibitor (theophylline) was shown to correct smell loss in association with an increase in nasal cGMP and cAMP levels (Henkin, Abdelmeguid, & Knöppel, 2016a; Henkin, Abdelmeguid, & Knöppel, 2016b; Henkin, Schultz, & Minnick‐Poppe, 2012), which was found to be decreased in the nasal mucus of patients with olfactory dysfunction (Henkin & Velicu, 2008). Routine testing of nasal NO levels may thus provide valuable clinical information for the diagnosis of olfactory function (Landis & Lacroix, 2009). Interestingly, olfactory dysfunction has been proposed as a sensitive indicator of cognitive deterioration and is considered to be an early marker for the presence of neurodegenerative diseases, such as Alzheimer's and Parkinson's diseases (Devanand, 2016; Schapira, Chaudhuri, & Jenner, 2017). It is also an indicator of mental disorders, such as depression and schizophrenia (Croy & Hummel, 2017) and diabetes (Zaghloul, Pallayova, Al‐Nuaimi, Hovis, & Taheri, 2018), and is the hallmark of the congenital reproductive disorder termed Kallmann syndrome (Boehm et al., 2015). Whether NO signalling plays a role in the link between olfactory dysfunction and these neurological disorders needs to be investigated.
4.1.2. The auditory system
The presence of NOS has been described in both the peripheral and the central auditory system; the sensory component of the cochlear tissue, the organ of Corti, consists of both sensory hair cells and supporting cells, spiralling down the cochlear duct. NO was shown to regulate cochlear blood flow and modulate presynaptic transmitter release from inner hair cells via the glutamate/NMDA pathway (Reuss & Riemann, 2000). Actually, NO production was shown to occur in several types of cells in the guinea pig cochlea (including neurons and blood vessels), using the NO‐sensitive dye, 4,5‐diaminofluorescein, thus suggesting that NO may play an important role in the inner ear (see Takumida & Anniko, 2002). Further studies have demonstrated the presence of NOS expression, with the predominant isoform being the nNOS, in many of these cellular populations of the cochlea nuclei including the hair cells, the organ of Corti, and the spiral ganglion neurons (SGNs; Fessenden, Altschuler, Seasholtz, & Schacht, 1999; Shen, Harada, Nakazawa, & Yamashita, 2005; Vyas, Wu, Jimenez, Glowatzki, & Fuchs, 2019). The auditory transmission will continue in a more central level, with the axons of the SGNs (i.e., auditory nerve) transmitting the auditory information in the superior olivary complex, from where the signal will be transferred to the superior colliculus (midbrain). From the midbrain, the signal will be eventually transferred to the thalamus, at the level of the medial geniculate body, before reaching its final destination, the auditory cortex. All of these key brain regions contain, as mentioned above (see Figure 3), a large population of NOS1 expressing neurons, as well as other components of the canonical NO pathway (e.g., sGC, cGMP, and PDEs; Fessenden et al., 1999; Southam & Garthwaite, 1993), further supporting the role of NO in the processing of auditory information.
Hearing deficits can have various origins like noise trauma, head trauma, ototoxicity, or genetic origins. In the cochlea, increased NO production by ill‐defined sources (e.g., loud sound stress) is thought to contribute to hearing disorders. Recently, NO‐derived free radicals were implicated in the cochlear pathophysiology of noise‐induced hearing loss in the guinea pig (Han, Shi, & Nuttall, 2018). This was not the first time that hearing loss has been correlated with the formation of peroxynitrates (generated by the formation of superoxide and NO) or other reactive nitrogen species (RNS) deriving from NO (Jiang, Talaska, Schacht, & Sha, 2007; Yamashita, Jiang, Schacht, & Miller, 2004). The presence of these destructive species actually constitutes the primary evidence that excessive NO production in the cochlear is ototoxic in a non‐cGMP‐dependent manner.
Even though mechanistic studies demonstrating the actual involvement of NO signalling in the pathophysiology of hearing loss in the inner ear are missing (Heinrich & Helling, 2012), in the brain, studies have shown that the inhibition of NOS activity impairs the electrocortical arousal response induced by sound stimulation in awake, freely moving animals (Bagetta, Iannone, Del Duca, & Nisticò, 1993). The nNOS neurons in the IC, the key midbrain site involved in processing virtually all ascending auditory information (Oliver & Cant, 2018), appear to be the neuronal population underlying this process (Iannone, Del Duca, Granato, Rispoli, & Nisticò, 1996; Olthof et al., 2019). Indeed, the microinfusion of NMDA‐receptor and NOS inhibitors in the IC has been shown to inhibit sound‐evoked electrocortical desynchronization, as measured by EEG in Iannone et al. (1996), whereas the microinjection of the NOS inhibitor in the other relay stations of the acoustic pathway, such as the geniculatus medialis, lemniscus lateralis, and olivaris superior nuclei, was seen to have no effect (Nistico, Bagetta, Iannone, & Duca, 1994). In addition, findings from 2019 have shown that both the local delivery of the nNOS inhibitor NG‐methyl‐L‐arginine (L‐NMMA) and the sGC inhibitor 1H‐[1,2,4]oxadiazolo[4,3‐a]quinolaxine‐1‐one (ODQ) blunts the NMDA‐evoked increase in sound‐driven activity of neurons in the IC using in vivo electrophysiological recordings (Olthof et al., 2019). In humans, hearing loss, which is sometimes associated with anosmia, constitutes a “red flag” for the diagnosis of syndromic forms of congenital hypogonadotropic hypogonadism, such as the Kallmann syndrome (Boehm et al., 2015), and is increasingly recognized as a risk factor for developing dementia (Bowl & Dawson, 2018). Genes for several components of the NO/cGMP pathway have been found located in human deafness loci (GUCY, NOS1, NOS2, and NOS3; Shen, Scheffer, Kwan, & Corey, 2015), however, and although effects on cochlear function have been reported in mice when NO pathway related genes have been knocked out (Labbé, Bloch, Schick, & Michel, 2016; Möhrle et al., 2017), no direct confirmation of the existence of mutations in NO‐related genes exists. The putative involvement of NOS1 and genes in the NO signalling pathway in the genetic architecture of this common sensory loss needs further investigation.
4.1.3. The nociceptive system
NO signalling is involved in the acute and chronic state of pain, mediating its effects in both the peripheral and the CNS. Increased glutamate‐evoked NO levels have been associated with central sensitization in the nociceptive pathway (i.e., increased activity and responses to pain; Jin, Chen, Cao, Li, & Pan, 2011), including in the brain (Pradhan et al., 2018). Endogenous processes underlining the establishment of the brain hypersensitivity are still unknown, yet migraine could occur from increased mechanosensitivity, or sensitization, of nociceptive neurons that innervate the intracranial meninges.
Elevated plasma levels of NO metabolites and increased exhaled NO gas have been found in spontaneous migraine attacks (Sarchielli, Alberti, Codini, Floridi, & Gallai, 2000). NO donors or PDE inhibitors have been also extensively used to induce a headache, mimicking human conditions of migraine (for review, see Ashina, Hansen, Á Dunga, & Olesen, 2017). In parallel, the sGC inhibitor (ODQ) has been efficiently used to block chronic migraine‐associated pain in the presence or absence of exogenous NO stimulation (Ben Aissa et al., 2017), supporting the notion that aberrant NO levels, arising from an increased activity of the endogenous NOS synthases, can act through the sGC/cGMP pathway to induce or maintain migraine.
Considering NO is broadly implicated in the causative mechanisms of the pathophysiology of migraine, targeting the production of NO by administering NOS inhibitors has been a very active part of research. The use of non‐specific NOS inhibitors (i.e., L‐NMMA) in clinical studies has been efficient against migraine attacks (Ashina, Lassen, Bendtsen, Jensen, & Olesen, 1999; Lassen, Ashina, Christiansen, Ulrich, & Olesen, 1997). The mechanisms behind the action of the NOS inhibitors seem to differ according to the type of headache in question and are not clearly understood. In acute migraine, administration of a NO donor was shown to activate the MAPK pathway, inducing ERK phosphorylation that in turn contributes to the elaboration of proinflammatory nociceptive molecules within the intracranial meninges (X. Zhang, Kainz, Zhao, Strassman, & Levy, 2013). Other studies demonstrated that increased expression of nNOS around the trigeminal ganglion cells could result in overproduction of NO, which in turn stimulates the release of CGRP, and other migraine‐relevant neuropeptides (Dieterle, Fischer, Link, Neuhuber, & Messlinger, 2010; Edvinsson, Mulder, Goadsby, & Uddman, 1998). In vitro studies using trigeminal ganglion primary cultures have also demonstrated the ability of CGRP to increase iNOS activity in glial cells, promoting NO production, suggesting a main role of neuro–glia interactions involving the NO pathway, in the underlying pathology of migraine (J. Li, Vause, & Durham, 2008). Less possible seems the notion that NO could directly modulate the neuronal activity of trigeminal neurons, resulting in increased discharge firing (Koulchitsky, Fischer, De Col, Schlechtweg, & Messlinger, 2004). Overall, while NO donors have some functions independent of CGRP, their function in migraine may strongly relate to promoting CGRP production and release, adding to the CGRP‐influenced pathways in migraine.
Polymorphism of NOS could affect production of NO, which may be connected with migraine. The associations between a single nucleotide polymorphism of NOS and migraine have been widely studied. However, studies evaluating the association between nNOS polymorphism and migraine were limited, with most of the studies reporting relationships between single nucleotide polymorphisms of iNOS, eNOS genes (Dong, Wang, Dong, Hu, & Zhao, 2018).
4.2. Role of hypothalamic NO in sexual maturation and fertility
The hypothalamus is the single most important integrator of vegetative and endocrine regulation of the body. Accordingly, it controls diverse processes, such as cardiovascular function, sleep, metabolism, stress, thermoregulation, water and electrolyte balance, and reproduction. In the hypothalamus, specialized neuronal populations sense moment‐to‐moment changes in blood osmolality and circulating levels of hormones and nutrients, and they relay this information to downstream neuronal populations within neuronal circuits regulating body homeostasis (Elmquist, Coppari, Balthasar, Ichinose, & Lowell, 2005; Gizowski & Bourque, 2017) and species survival (Hill & Elias, 2018; Manfredi‐Lozano, Roa, & Tena‐Sempere, 2018). The latter function is orchestrated by the hypothalamic neuronal population in the preoptic region that expresses GnRH, which is the neuropeptide controlling sexual maturation and fertility (Herbison, 2015; Moenter, 2017; Prevot, 2015). GnRH is a decapeptide released by the GnRH nerve terminals into pituitary portal blood circulation in the median eminence of the hypothalamus. From there, GnRH will travel to the anterior pituitary where it will stimulate the synthesis and secretion of the gonadotropins luteinizing hormone and follicle stimulating hormone. These gonadotropin hormones, as indicated by their names, act on the gonads (i.e., the testes and ovaries) to control the production of sperm and eggs and the secretion of sex steroids.
4.2.1. nNOS activity in neurons
Infertility is the most striking phenotype found in the genetic mouse model lacking the catalytic haem‐binding domain encoded by exon 6 of nNOS (Gyurko, Leupen, & Huang, 2002). The strong reproductive phenotype displayed by these knockout mice resembles that of congenital hypogonadotropic hypogonadism in humans (Boehm et al., 2015).
GnRH neuronal activity is controlled by a dynamic array of internal and external signals involving various different neuropeptides, hormones, and neurotransmitters, including NO (Chachlaki, Garthwaite, & Prevot, 2017). Although studies published in 2018 suggest that a proportion of hypothalamic GnRH neurons may show nNOS immunoreactivity in certain mammalian species (Bedenbaugh et al., 2018), nNOS immunoreactivity is not detected in adult GnRH neurons in rats (Herbison, Simonian, Norris, & Emson, 1996) or mice (Figure 4; Chachlaki, Malone, et al., 2017; Clasadonte, Poulain, Beauvillain, & Prevot, 2008; Hanchate et al., 2012). Interestingly, nNOS neurons are morphologically and functionally associated with GnRH neuronal cell bodies and dendrites in the MePO/OVLT (Chachlaki, Malone, et al., 2017). In addition to the glutamatergic NMDA receptor (d'Anglemont de Tassigny et al., 2007), these nNOS neurons also express receptors for gonadal steroids, including the oestrogen receptor α (Chachlaki, Malone, et al., 2017), receptors for key circulating metabolic signals, such as leptin (Bellefontaine et al., 2014; Donato, Frazão, Fukuda, Vianna, & Elias, 2010), and receptors for the mandatory neuropeptide controlling ovulation, kisspeptin (Hanchate et al., 2012). All have been shown to modulate nNOS activity (see Chachlaki, Garthwaite, & Prevot, 2017). MePO/OVLT nNOS neurons are thus well poised to play a role in the rapid integration and transmission of both gonadal and metabolic signals to GnRH neurons (Chachlaki, Garthwaite, & Prevot, 2017). Electrophysiological studies have revealed that the endogenous production of NO by nNOS exerts a potent inhibitory effect on GnRH spontaneous neuronal activity by causing changes in membrane properties in the GnRH neuron requiring sGC activity and potentially involving potassium conductance (Clasadonte et al., 2008). The activity of nNOS appears to be coordinated across the population of neurons in the MePO/OVLT by homeostatic blood‐borne signals (e.g., oestrogens and leptin) or by transsynaptic inputs (e.g., kisspeptin and glutamate). The mathematical modelling of the regulation of nNOS activity during the oestrous cycle suggests that the NO concentration of these neurons builds up to levels capable of influencing neighbouring GnRH neurons and coordinating their activity (Bellefontaine et al., 2014; Chachlaki, Garthwaite, & Prevot, 2017). Coordinated GnRH neuronal activity is required to promote meaningful episodes of GnRH release into the pituitary portal blood circulation and thus elicit gonadotropin release from the pituitary gland (Chachlaki, Garthwaite, & Prevot, 2017).
FIGURE 4.

Representative images showing nNOS and GnRH immunoreactive neurons in the preoptic region in mice. GnRH neuronal cell bodies (arrows, red) and processes (red) morphologically interact with nNOS neurons (white) in the median preoptic nucleus (MePO). However, GnRH immunoreactivity does not colocalize with nNOS immunoreactivity. OVLT, organum vasculosum of the lamina terminalis; 3V, third ventricle. Scale bar = 100 μm (25 μm in insets). Adapted from Chachlaki, Malone, et al. (2017) with permission
The modulation of GnRH neuronal function by neuronal NO has been shown to occur as early as the infantile period, in rodents and humans alike, during the first postnatal activation of the hypothalamic–pituitary–gonadal axis that is termed minipuberty (Kuiri‐Hänninen, Sankilampi, & Dunkel, 2014; Prevot, 2015). Over the second week of life in mice, when nNOS activity increases in the preoptic region (Messina et al., 2016), NO interacts with the transcription factor CAAT/enhancer‐binding protein‐β (C/EBPβ, which is encoded by Cepbp) to repress the activity of the Gnrh promoter (Belsham & Mellon, 2000). Importantly, the levels of Cepbp expression are tightly controlled by the increased expression of miR155 (which represses Cepbp) during minipuberty (Messina et al., 2016) to enable the GnRH‐fuelled run‐up to puberty, that is, the first ovulation or the appearance of spermatozoa in the vas deferens, which occurs at approximately 6 weeks of age in mice and after 8–9 years of dormancy in humans (Howard & Dunkel, 2018; Prevot, 2015).
In addition to its role in the GnRH neuroendocrine axis, several studies have implicated NO in sexual behaviour (González‐Flores & Etgen, 2004; Mani et al., 1994). Findings published in 2018 demonstrated that hypothalamic NO signalling could indeed be an essential mechanism downstream of GnRH and kisspeptin neurons in governing mate preference and lordosis respectively (Hellier et al., 2018). In particular, using advanced tracing genetic tools, the authors found that the nNOS neuronal population in the VMH plays an important role in the kisspeptin neuron‐mediated lordosis behaviour (Hellier et al., 2018). In humans, kisspeptin is also shown to be involved in sexual and emotional processing (Comninos et al., 2017, 2018). However, whether the underlying molecular mechanisms involve hypothalamic NO signalling is not known (Mills, Dhillo, & Comninos, 2018).
4.2.2. eNOS activity in vascular endothelial cells
In the projection field of neuroendocrine GnRH neurons, the median eminence of the hypothalamus, GnRH axon terminals engage in communication processes with the fenestrated endothelial cells of the pituitary blood vessels, where they release their neurohormone, and the processes of a specific type of glial cells composing the floor of the third ventricle named tanycytes (Prevot et al., 2018). While fenestrated endothelial cells of the median eminence regulate the outgrowth of neuropilin‐1‐expressing GnRH neuroendocrine terminals towards the pericapillary space through the ovarian‐steroid‐dependent release of semaphorin 3A (Giacobini et al., 2014), they induce retraction of the tanycytic processes engulfing GnRH nerve terminals via the release of NO (De Seranno et al., 2004), thus helping position the GnRH nerve terminals next to the capillaries to facilitate delivery of secreted neurohormone (Figure 5a) and regulate reproductive function (Clasadonte & Prevot, 2017). Median eminence vascular endothelial cells synthetize NO via eNOS; the expression and activity of eNOS is tightly regulated by circulating oestrogens during the oestrous cycle (Figure 5b; De Seranno et al., 2010; Knauf et al., 2001; Prevot et al., 1999). Endothelial NO causes the remodelling of the actin cytoskeleton in tanycytes (De Seranno et al., 2004, 2010) and promotes the neurosecretion of GnRH (Knauf et al., 2001; Prevot et al., 1999) that requires cGMP production and also involves COX activity and synthesis of PGE2 (Figure 5b; De Seranno et al., 2004, 2010; Prevot et al., 1999).
FIGURE 5.
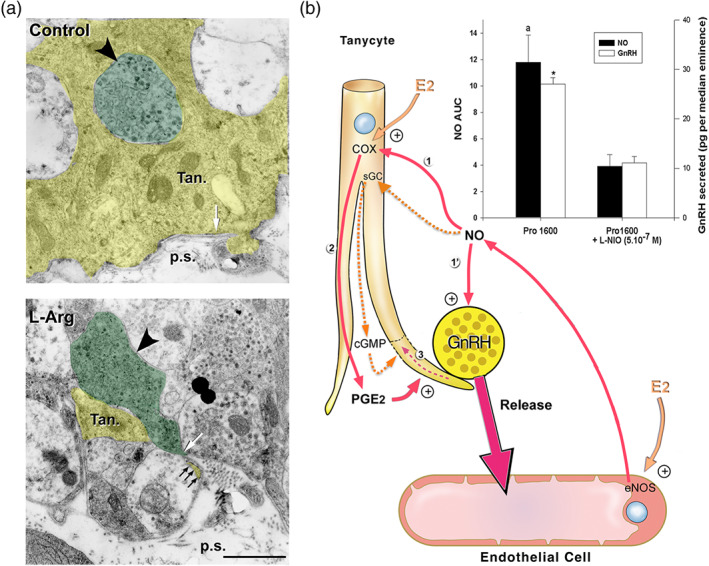
Involvement of eNOS and endothelial NO signalling in oestrous‐cycle‐mediated brain plasticity and neurosecretion in the neuroendocrine hypothalamus. (a) Activation of endogenous NO secretion in endothelial cells of the median eminence promotes tanycytic process retraction (yellow, Tan) that allows GnRH nerve terminals (green, big arrowhead) to form direct neurovascular junctions in isolated median eminence explants. Living median eminence explants were incubated for 30 min in the presence or absence of the NO precursor, L‐arginine (L‐Arg, 500 mM). Under basal unstimulated conditions (control), the GnRH axonal endings were separated from the pericapillary space (p.s.; delineated by the parenchymatous basal lamina, white arrow). Most of the nerve endings were enwrapped by a single tanycytic end‐foot (top panel, Tan). Retraction of tanycytic processes (white arrowhead) and formation of neurovascular junctions by GnRH nerve terminals that directly contact the pericapillary space (bottom panel, white arrow) were detected upon treatment with L‐Arg. Scale bar, 1 μm. From De Seranno et al. (2004) with permission. (b) Schematic representation of endothelial–glial–neuronal interactions involved in the control of GnRH neurohormone secretion in the hypothalamus. Endothelial–neuronal interactions at the level of the median eminence (the termination field of GnRH neurons in the hypothalamus) involves the production of NO by endothelial cells of fenestrated capillaries of the portal blood vessels. Upon its secretion, NO diffuses from its source, where it not only stimulates the release of GnRH from the neighbouring GnRH neuroendocrine terminals (1′; Knauf et al., 2001) but also promotes their access to the blood stream by inducing cytoarchitectural changes in tanycytic end‐feet (1–3; De Seranno et al., 2004). Importantly, on the afternoon of proestrus (when oestrogen levels are at their highest), the preovulatory GnRH/NO release is blocked with L‐N 5‐(1‐iminoethyl)ornithine (L‐NIO), an NOS inhibitor selective for eNOS at 0.5 μM (bar graph; * and a, significantly different from treated samples, P < .05: AUC; NO levels have been measured by amperometry during a 30‐min period in living median eminence explants; Knauf et al., 2001). Downstream effectors of endothelial NO‐meditated plasticity in tanycytes were shown to be both soluble GC (sGC) and COX (1). Note: Whether NO activates COX activity directly or indirectly is unknown (Garthwaite & Boulton, 1995); NO from endogenous sources stimulates PGE2 production via COX in a cGMP‐independent manner (Salvemini et al., 1995), alternatively NO could somehow prevent the auto‐inactivation of COX (Smith, Marnett, & DeWitt, 1991). Oestrogens are likely to be the key humoral factors involved in the orchestration of the endothelia‐to‐glia communication that allows GnRH neurons to directly contact the pituitary portal blood vessels on the day of proestrus (De Seranno et al., 2010). Oestrogens treatment up‐regulates COX expression while leaving unchanged the expression of sGC. In addition, COX products and PGE2 in particular (2) induce acute remodelling of actin cytoskeleton in tanycytes and cause cytoplasm retraction within tanycytic processes and end‐feet (3). In parallel, oestrogens stimulate endothelial NOS (eNOS) expression in median eminence endothelial cells. Both tanycytes and endothelial cells express oestrogen receptors in vitro (De Seranno et al., 2010) and in vivo (Giacobini et al., 2014; Parkash et al., 2015). Adapted from Prevot (2002) and Prevot et al. (2010) with permission
On a broader perspective, the aforementioned vasculo–glio–neuronal processes, which are likely to occur in other brain structures (Garthwaite et al., 2006), suggest the intriguing possibility that microvascular endothelial cells participate in signal processing in the brain.
4.3. Role of hypothalamic NO signalling in body homeostasis
Even though the engineering of advanced genetic tools have enabled the selective manipulation of the expression of genes in neurons expressing the Nos1 promoter, while hypothalamic nNOS neurons are known to be involved in the control of vital homeostatic functions, such as the regulation of thirst (Oka, Ye, & Zuker, 2015; Zimmerman et al., 2016), and the integration of key peripheral metabolic signals, such as leptin (Leshan, Greenwald‐Yarnell, Patterson, Gonzalez, & Myers, 2012; Sutton, Myers, & Olson, 2016), only sparse information has been accumulated regarding the actual role of NO signalling in these processes. The only striking genetic evidence that nNOS activity plays a role in the control of energy homeostasis is the fact that mice lacking both Nos1 and leptin (Lep) gene expression (Nos1 −/−; Lep ob/ob mice) weigh approximately 20 g less than their Nos1 +/+; Lep ob/ob littermates (Bellefontaine et al., 2014). Interestingly, this study has shown also that NO signalling in the preoptic region, which is increasingly recognized to play an active role in the control of energy homeostasis (Yu, Cheng, et al., 2018; Yu, François, Huesing, & Münzberg, 2018), facilitates leptin's action on reproduction by relaying this key circulating metabolic signal to GnRH neurons (Bellefontaine et al., 2014). Within the hypothalamus, the ARH melanocortinergic neurons governing appetite project into the PVH, which is the brain region that mediates the majority of hypothalamic output to control both feeding and energy expenditure (Sutton et al., 2014). The pharmacogenetic activation of the Nos1 neurons in the PVH projecting to the hindbrain and spinal cord has been shown to suppress feeding (Sutton et al., 2014). Findings published in 2018 suggest that, in the PVH, both NO signalling and nNOS activity may indeed play an active role in modulating appetite by regulating the effects on food intake and locomotor activity caused by the orexigenic peptide NPY released by ARH neurons (Péterfi et al., 2018). Interestingly, the intra‐PVH administration of the selective nNOS inhibitor Nω‐propyl‐L‐arginine was shown to block the NPY‐induced feeding and increase ambulatory activity in mice (Péterfi et al., 2018). Another 2018 study supports that GABAergic nNOS neurons of the ARH and the DMH could also be a crutial leptin‐sensing population (Rupp et al., 2018).
Hypothalamic NO is also involved in the control of glucose homeostasis in the context of the powerful neuroendocrine and autonomic counterregulatory mechanisms set in motion by the organism to protect the brain from hypoglycaemia (Faber et al., 2018). These protective mechanisms, known as the counterregulatory response to hypoglycaemia, require the production of NO from neuronal processes and/or neurons expressing nNOS in the VMH (Fioramonti et al., 2010; Murphy, Fakira, Song, Beuve, & Routh, 2009) are fully reproduced by the optogenetic photo‐activation of the fibres of nNOS‐expressing VMH neurons in the anterior bed nucleus of the stria terminalis. In parallel, intriguing studies suggest that hypothalamic NO can also modulate glucose metabolism via a mode of communication involving the gut–brain axis and the bioactive peptide present in the gut, apelin, which acts both on peripheral tissues and in the brain (Fournel et al., 2017; Knauf, Abot, Wemelle, & Cani, 2019; Rastelli, Cani, & Knauf, 2019).
4.4. Role of NO in cortical brain functions
nNOS‐released NO is involved in numerous physiological processes regulating neurovascular communication (Du, Stern, & Filosa, 2015; Garthwaite, 2008; Mapelli et al., 2017) and neuroplasticity, including aspects of presynaptic neurotransmitter release on both glutamatergic and GABAergic systems (Garthwaite, 2008; Garthwaite & Boulton, 1995). Additionally, NO signalling can affect the availability and size of the readily releasable pool, vesicle recycling, and activity of ion channels (Hardingham, Dachtler, & Fox, 2013). By affecting synaptic plasticity and activity, NO may actually play a homeostatic role in maintaining the balance between excitation and inhibition. NO‐producing interneurons in the neocortex have been shown to convey lateral inhibition to neighbouring columns and thus may be involved in shaping the spatiotemporal dynamics of the cortical network's activity (Shlosberg, Buskila, Abu‐Ghanem, & Amitai, 2012). During postnatal development, the NO released by nNOS‐expressing GABA interneurons in the hippocampus is thought to be involved in the stabilization of synchronous network activity by reducing GABAergic and glutamatergic synaptic transmission (Ben‐Ari, Gaiarsa, Tyzio, & Khazipov, 2007; Cserép et al., 2011). From its potential involvement in shaping neuronal circuits, it is tempting to speculate that NO signalling plays a role in higher level cognitive functions, such as learning, memory, cognition, and language, in addition to playing a possible role in sleep (Wisor, Gerashchenko, & Kilduff, 2011) or wakefulness (Pedersen et al., 2017; X. Yu et al., 2019). Being expressed specifically during mid‐fetal development of the human neocortical areas involved in speech, language, and cognition (i.e., the frontal operculum and the anterior cingulate cortex), nNOS appears to be a key enzyme involved in the assembly of these neuronal networks and their respective maturation and function (Funk & Kwan, 2014; Gally, Montague, Reeke, & Edelman, 1990; Montague & Sejnowski, 1994). In line with this possibility is the fact that in the absence of neuronal NO, defective brain development results in impaired cognitive performance, as reported in a Nos1‐deficient mouse model. In this model, it was found that Nos1 deficiency results in the failure of mice to perform basic cognitive behavioural tests and that the mice exhibited abnormal social behaviour towards their littermates (Weitzdoerfer et al., 2004). In addition, nNOS‐derived NO has been found to play a role in depression‐ and anxiety‐like behaviours and fear conditioning in mice (L.‐P. Li et al., 2018; J. Zhang et al., 2010; Q.‐G. Zhou et al., 2011) and is involved in several psychiatric disorders, such as autism (Colvin & Kwan, 2014; Kwan et al., 2012), schizophrenia (Freudenberg, Alttoa, & Reif, 2015; Hallak, Maia‐de‐Oliveira, Abrao, & Al, 2013), and mood disorders (Ghasemi, Claunch, & Niu, 2019) in humans. It may also play a role in neurodegenerative disorders, including Alzheimer's disease (Domek‐Łopacińska & Strosznajder, 2010). Finally, association studies have identified nNOS as genetic risk factor for some of these disorders (Freudenberg et al., 2015; O'Donovan et al., 2008; van Ewijk et al., 2017). Hence, NO therapy has been hailed as a putative treatment in several of these disorders. For example, the treatment of 20 patients with schizophrenia (aged 19–40 years) with an NO donor (sodium nitroprusside) has been shown to rapidly (within 4 hr) improve symptoms for up to 4 weeks post‐infusion (Hallak et al., 2013), reinforcing the notion that the glutamate–NO–cGMP signalling pathway has an important role in the pathophysiology of schizophrenia (Shim et al., 2016). Furthermore, considering that all the PDEs are expressed in the CNS, this gene family is a particularly attractive source of new targets for the treatment of psychiatric and neurodegenerative disorders via the NO signalling pathway (Hollas, Aissa, Lee, Gordon‐Blake, & Thatcher, 2019; Maurice et al., 2014; Prickaerts, Heckman, & Blokland, 2017).
5. OUTLOOK
From the numerous examples detailed in this review, it is obvious that in the brain, NO, which is a versatile signalling molecule, plays important roles in the development and survival of mammalian species. NO provides brain neuronal networks with the ability to adapt to continuously changing physiological conditions. However, although considerable efforts have been made to better understand the involvement of neurons expressing nNOS in the neuronal circuits underlying the brain's control of bodily functions using advanced genetic tools, such as optogenetics (Kim, Adhikari, & Deisseroth, 2017) and chemogenetics (Atasoy & Sternson, 2017), that enable the time‐controlled manipulation of select populations of neurons in discrete brain areas in vivo, the actual causal role of NO signalling in these processes remains largely unexplored. Equally understudied is the putative involvement of NOS1 in the genetic architecture of disease, including neurodevelopmental disorders leading to congenital hypogonadotropic hypogonadism, sensory deficits, and mental illness. Finally, a number of studies now indicate that inhaled NO may have not only an important role in treating pulmonary hypertension of paediatric and adult patients with respiratory and cardiac failure (Bhatraju, Crawford, Hall, & Lang, 2015) but also in treating mental health problems that present themselves during infancy and adolescence. It also may prevent the persistence of these problems through adulthood (Charriaut‐Marlangue et al., 2013; Hallak et al., 2013; Patton & Viner, 2016). Intriguingly, recent studies show that nitrate (NO3 −) and nitrite (NO2 −), which were previously thought to be inert products of endogenous NO metabolism, can be recycled in vivo to form bioactive NO via a NOS‐independent pathway in tissues (Lundberg, Weitzberg, & Gladwin, 2008), including the brain (Jung et al., 2006). These anions can enter the circulation through dietary intake (Weitzberg & Lundberg, 2013) and have increasingly recognized therapeutic potential in reducing blood pressure and thereby improving cardiovascular health (Gee & Ahluwalia, 2016). Investigating the contribution of dietary nitrate and nitrite as cheap and effective treatment options in brain disorders involving the NO‐cGMP signalling pathway will thus be of utmost interest in the future.
5.1. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018) and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander, Cidlowski et al., 2017 ; Alexander, Fabbro et al., 2017; Alexander, Kelly et al., 2017; Alexander, Peters et al., 2017 ; Alexander, Striessnig et al., 2017).
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGEMENTS
The author's work is supported by the Agence Nationale de la Recherche Grant ANR‐17‐CE16‐0015‐01 to V.P. and LabEx DISTALZ and EGID to V.P. and the Fédération Hospital‐Universitaire “1000 days for health” to K.C.
Chachlaki K, Prevot V. Nitric oxide signalling in the brain and its control of bodily functions. Br J Pharmacol. 2020;177:5437–5458. 10.1111/bph.14800
REFERENCES
- Adak, S. , Santolini, J. , Tikunova, S. , Wang, Q. , Johnson, J. D. , & Stuehr, D. J. (2001). Neuronal nitric-oxide synthase mutant (Ser‐1412 → Asp) demonstrates surprising connections between heme reduction, NO complex formation, and catalysis. Journal of Biological Chemistry, 276, 1244‐1252. 10.1074/jbc.M006857200 [DOI] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Cidlowski, J. A. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … CGTP Collaborators (2017). The Concise Guide to PHARMACOLOGY 2017/18: Nuclear hormone receptors. British Journal of Pharmacology, 174, S208–S224. 10.1111/bph.13880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … CGTP Collaborators (2017). The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. British Journal of Pharmacology, 174, S272–S359. 10.1111/bph.13877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , Harding, S. D. , … CGTP Collaborators (2017). The Concise Guide to PHARMACOLOGY 2017/18: Other proteins. British Journal of Pharmacology, 174, S1–S16. 10.1111/bph.13882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Peters, J. A. , Kelly, E. , Marrion, N. V. , Faccenda, E. , Harding, S. D. , … CGTP Collaborators (2017). The Concise Guide to PHARMACOLOGY 2017/18: Ligand‐gated ion channels. British Journal of Pharmacology, 174, S130–S159. 10.1111/bph.13879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Striessnig, J. , Kelly, E. , Marrion, N. V. , Peters, J. A. , Faccenda, E. , … CGTP Collaborators (2017). THE CONCISE GUIDE TO PHARMACOLOGY 2017/18: Voltage‐gated ion channels. British Journal of Pharmacology, 174, S160–S194. 10.1111/bph.13884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold, W. P. , Mittal, C. K. , Katsuki, S. , & Murad, F. (1977). Nitric oxide activates guanylate cyclase and increases guanosine 3′:5′‐cyclic monophosphate levels in various tissue preparations. Proceedings of the National Academy of Sciences of the United States of America, 74, 3203–3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashina, M. , Hansen, J. M. , Á Dunga, B. O. , & Olesen, J. (2017). Human models of migraine—Short‐term pain for long‐term gain. Nature Reviews. Neurology, 13, 713. [DOI] [PubMed] [Google Scholar]
- Ashina, M. , Lassen, L. H. , Bendtsen, L. , Jensen, R. , & Olesen, J. (1999). Effect of inhibition of nitric oxide synthase on chronic tension‐type headache: A randomised crossover trial. Lancet, 353, 287–289. [DOI] [PubMed] [Google Scholar]
- Atasoy, D. , & Sternson, S. M. (2017). Chemogenetic tools for causal cellular and neuronal biology. Physiological Reviews, 98, 391–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo, M. F. , Faucz, F. R. , Bimpaki, E. , Horvath, A. , Levy, I. , de Alexandre, R. B. , … Stratakis, C. A. (2014). Clinical and molecular genetics of the phosphodiesterases (PDEs). Endocrine Reviews, 35, 195–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagetta, G. , Iannone, M. , Del Duca, C. , & Nisticò, G. (1993). Inhibition by N omega‐nitro‐L‐arginine methyl ester of the electrocortical arousal response in rats. British Journal of Pharmacology, 108, 858–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beavo, J. A. (1995). Cyclic nucleotide phosphodiesterases: Functional implications of multiple isoforms. Physiological Reviews, 75, 725–748. [DOI] [PubMed] [Google Scholar]
- Bechade, C. , Colasse, S. , Diana, M. A. , Rouault, M. , & Bessis, A. (2014). NOS2 expression is restricted to neurons in the healthy brain but is triggered in microglia upon inflammation. Glia, 62, 956–963. [DOI] [PubMed] [Google Scholar]
- Bechade, C. , Pascual, O. , Triller, A. , & Bessis, A. (2011). Nitric oxide regulates astrocyte maturation in the hippocampus: Involvement of NOS2. Molecular and Cellular Neurosciences, 46, 762–769. [DOI] [PubMed] [Google Scholar]
- Bedenbaugh, M. N. , McCosh, R. B. , Lopez, J. A. , Connors, J. M. , Goodman, R. L. , & Hileman, S. M. (2018). Neuroanatomical relationship of neuronal nitric oxide synthase to gonadotropin‐releasing hormone and kisspeptin neurons in adult female sheep and primates. Neuroendocrinology, 107, 218–227. [DOI] [PubMed] [Google Scholar]
- Bellefontaine, N. , Chachlaki, K. , Parkash, J. , Vanacker, C. , Colledge, W. , d'Anglemont de Tassigny, X. , … Prevot, V. (2014). Leptin‐dependent neuronal NO signaling in the preoptic hypothalamus facilitates reproduction. The Journal of Clinical Investigation, 124, 2550–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsham, D. D. , & Mellon, P. L. (2000). Transcription factors Oct‐1 and C/EBPβ (CCAAT/enhancer‐binding protein‐β) are involved in the glutamate/nitric oxide/cyclic‐guanosine 5′‐monophosphate‐mediated repression of gonadotropin‐releasing hormone gene expression. Molecular Endocrinology, 14, 212–228. [DOI] [PubMed] [Google Scholar]
- Ben Aissa, M. , Tipton, A. F. , Bertels, Z. , Gandhi, R. , Moye, L. S. , Novack, M. , … Pradhan, A. A. (2017). Soluble guanylyl cyclase is a critical regulator of migraine‐associated pain. Cephalalgia, 38, 1471–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben‐Ari, Y. , Gaiarsa, J.‐L. , Tyzio, R. , & Khazipov, R. (2007). GABA: A pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiological Reviews, 87, 1215–1284. [DOI] [PubMed] [Google Scholar]
- Bendall, J. K. , Douglas, G. , McNeill, E. , Channon, K. M. , & Crabtree, M. J. (2013). Tetrahydrobiopterin in cardiovascular health and disease. Antioxidants & Redox Signaling, 20, 3040–3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender, A. T. , Demady, D. R. , & Osawa, Y. (2000). Ubiquitination of neuronal nitric‐oxide synthase in vitro and in vivo. The Journal of Biological Chemistry, 275, 17407–17411. [DOI] [PubMed] [Google Scholar]
- Benhar, M. , Forrester, M. T. , & Stamler, J. S. (2009). Protein denitrosylation: Enzymatic mechanisms and cellular functions. Nature Reviews. Molecular Cell Biology, 10, 721–732. [DOI] [PubMed] [Google Scholar]
- Bhatraju, P. , Crawford, J. , Hall, M. , & Lang, J. D. Jr. (2015). Inhaled nitric oxide: Current clinical concepts. Nitric Oxide, 50, 114–128. [DOI] [PubMed] [Google Scholar]
- Biel, M. (2009). Cyclic nucleotide‐regulated cation channels. The Journal of Biological Chemistry, 284, 9017–9021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackshaw, S. , Eliasson, M. J. , Sawa, A. , Watkins, C. C. , Krug, D. , Gupta, A. , … Snyder, S. H. (2003). Species, strain and developmental variations in hippocampal neuronal and endothelial nitric oxide synthase clarify discrepancies in nitric oxide‐dependent synaptic plasticity. Neuroscience, 119, 979–990. [DOI] [PubMed] [Google Scholar]
- Boehm, U. , Bouloux, P.‐M. , Dattani, M. T. , de Roux, N. , Dodé, C. , Dunkel, L. , … Young, J. (2015). European consensus statement on congenital hypogonadotropic hypogonadism—Pathogenesis, diagnosis and treatment. Nature Reviews. Endocrinology, 11, 547. [DOI] [PubMed] [Google Scholar]
- Bowl, M.R. , & Dawson, S.J. (2018). Age‐related hearing loss. Cold Spring Harb. Perspect. Med. [DOI] [PMC free article] [PubMed]
- Bredt, D. S. , Glatt, C. E. , Hwang, P. M. , Fotuhi, M. , Dawson, T. M. , & Snyder, S. H. (1991). Nitric oxide synthase protein and mRNA are discretely localized in neuronal populations of the mammalian CNS together with NADPH diaphorase. Neuron, 7, 615–624. [DOI] [PubMed] [Google Scholar]
- Bredt, D. S. , Hwang, P. M. , Glatt, C. E. , Lowenstein, C. , Reed, R. R. , & Snyder, S. H. (1991). Cloned and expressed nitric oxide synthase structurally resembles cytochrome P‐450 reductase. Nature, 351, 714–718. [DOI] [PubMed] [Google Scholar]
- Breer, H. , & Shepherd, G. M. (1993). Implications of the NO/cGMP system for olfaction. Trends in Neurosciences, 16, 5–9. [DOI] [PubMed] [Google Scholar]
- Brenman, J. E. , Chao, D. S. , Gee, S. H. , McGee, A. W. , Craven, S. E. , Santillano, D. R. , … Bredt, D. S. (1996). Interaction of nitric oxide synthase with the postsynaptic density protein PSD‐95 and α1‐syntrophin mediated by PDZ domains. Cell, 84, 757–767. [DOI] [PubMed] [Google Scholar]
- Broillet, M.‐C. (2000). A single intracellular cysteine residue is responsible for the activation of the olfactory cyclic nucleotide‐gated channel by NO. The Journal of Biological Chemistry, 275, 15135–15141. [DOI] [PubMed] [Google Scholar]
- Broillet, M.‐C. , & Firestein, S. (1996). Direct activation of the olfactory cyclic nucleotide–gated channel through modification of sulfhydryl groups by NO compounds. Neuron, 16, 377–385. [DOI] [PubMed] [Google Scholar]
- Brunert, D. , Kurtenbach, S. , Isik, S. , Benecke, H. , Gisselmann, G. , Schuhmann, W. , … Wetzel, C. H. (2009). Odorant‐dependent generation of nitric oxide in mammalian olfactory sensory neurons. PLoS ONE, 4, e5499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck, L. B. (2000). The molecular architecture of odor and pheromone sensing in mammals. Cell, 100, 611–618. [DOI] [PubMed] [Google Scholar]
- Cary, S. P. L. , Winger, J. A. , & Marletta, M. A. (2005). Tonic and acute nitric oxide signaling through soluble guanylate cyclase is mediated by nonheme nitric oxide, ATP, and GTP. Proceedings of the National Academy of Sciences of the United States of America, 102, 13064–13069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casoni, F. , Malone, S. A. , Belle, M. , Luzzati, F. , Collier, F. , Allet, C. , … Giacobini, P. (2016). Development of the neurons controlling fertility in humans: New insights from 3D imaging and transparent fetal brains. Development, 143, 3969 LP–3981. [DOI] [PubMed] [Google Scholar]
- Castro, L. R. V. , Verde, I. , Cooper, D. M. F. , & Fischmeister, R. (2006). Cyclic guanosine monophosphate compartmentation in rat cardiac myocytes. Circulation, 113, 2221–2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chachlaki, K. , Garthwaite, J. , & Prevot, V. (2017). The gentle art of saying NO: How nitric oxide gets things done in the hypothalamus. Nature Reviews. Endocrinology, 13, 521–535. [DOI] [PubMed] [Google Scholar]
- Chachlaki, K. , Malone, S. A. , Qualls‐Creekmore, E. , Hrabovszky, E. , Münzberg, H. , Giacobini, P. , … Prevot, V. (2017). Phenotyping of nNOS neurons in the postnatal and adult female mouse hypothalamus. The Journal of Comparative Neurology, 525, 3177–3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charriaut‐Marlangue, C. , Bonnin, P. , Pham, H. , Loron, G. , Leger, P.‐L. , Gressens, P. , … Baud, O. (2013). Nitric oxide signaling in the brain: A new target for inhaled nitric oxide? Annals of Neurology, 73, 442–448. [DOI] [PubMed] [Google Scholar]
- Chen, L. , Majde, J. A. , & Krueger, J. M. (2003). Spontaneous sleep in mice with targeted disruptions of neuronal or inducible nitric oxide synthase genes. Brain Research, 973, 214–222. [DOI] [PubMed] [Google Scholar]
- Choi, Y.‐B. , Tenneti, L. , Le, D. A. , Ortiz, J. , Bai, G. , Chen, H.‐S. V. , & Lipton, S. A. (2000). Molecular basis of NMDA receptor‐coupled ion channel modulation by S‐nitrosylation. Nature Neuroscience, 3, 15–21. [DOI] [PubMed] [Google Scholar]
- Christopherson, K. S. , Hillier, B. J. , Lim, W. A. , & Bredt, D. S. (1999). PSD‐95 assembles a ternary complex with the N‐methyl‐D‐aspartic acid receptor and a bivalent neuronal NO synthase PDZ domain. Journal of Biological Chemistry, 274, 27467–27473. [DOI] [PubMed] [Google Scholar]
- Clasadonte, J. , Poulain, P. , Beauvillain, J. C. , & Prevot, V. (2008). Activation of neuronal nitric oxide release inhibits spontaneous firing in adult gonadotropin‐releasing hormone neurons: A possible local synchronizing signal. Endocrinology, 149, 587–596. [DOI] [PubMed] [Google Scholar]
- Clasadonte, J. , & Prevot, V. (2017). The special relationship: Glia–neuron interactions in the neuroendocrine hypothalamus. Nature Reviews. Endocrinology, 14, 25. [DOI] [PubMed] [Google Scholar]
- Colvin, S. M. , & Kwan, K. Y. (2014). Dysregulated nitric oxide signaling as a candidate mechanism of fragile X syndrome and other neuropsychiatric disorders. Frontiers in Genetics, 5, 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comninos, A. N. , Demetriou, L. , Wall, M. B. , Shah, A. J. , Clarke, S. A. , Narayanaswamy, S. , … Dhillo, W. S. (2018). Modulations of human resting brain connectivity by kisspeptin enhance sexual and emotional functions. JCI Insight, 3. pii: 121958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comninos, A. N. , Wall, M. B. , Demetriou, L. , Shah, A. J. , Clarke, S. A. , Narayanaswamy, S. , … Dhillo, W. S. (2017). Kisspeptin modulates sexual and emotional brain processing in humans. The Journal of Clinical Investigation, 127, 709–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane, B. R. , Arvai, A. S. , Ghosh, D. K. , Wu, C. , Getzoff, E. D. , Stuehr, D. J. , & Tainer, J. A. (1998). Structure of nitric oxide synthase oxygenase dimer with pterin and substrate. Science (80‐.), 279, 2121 LP–2126. [DOI] [PubMed] [Google Scholar]
- Craven, K. B. , & Zagotta, W. N. (2006). CNG and HCN channels: Two peas, one pod. Annual Review of Physiology, 68, 375–401. [DOI] [PubMed] [Google Scholar]
- Croy, I. , & Hummel, T. (2017). Olfaction as a marker for depression. Journal of Neurology, 264, 631–638. [DOI] [PubMed] [Google Scholar]
- Cserép, C. , Szőnyi, A. , Veres, J. M. , Németh, B. , Szabadits, E. , de Vente, J. , … Nyiri, G. (2011). Nitric oxide signaling modulates synaptic transmission during early postnatal development. Cerebral Cortex, 21, 2065–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Anglemont de Tassigny, X. , Campagne, C. , Dehouck, B. , Leroy, D. , Holstein, G. R. , Beauvillain, J.‐C. , … Prevot, V. (2007). Coupling of neuronal nitric oxide synthase to NMDA receptors via postsynaptic density‐95 depends on estrogen and contributes to the central control of adult female reproduction. The Journal of Neuroscience, 27, 6103–6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel, H. , Levenes, C. , & Crépel, F. (2016). Cellular mechanisms of cerebellar LTD. Trends in Neurosciences, 21, 401–407. [DOI] [PubMed] [Google Scholar]
- De Seranno, S. , d'Anglemont de Tassigny, X. , Estrella, C. , Loyens, A. , Kasparov, S. , Leroy, D. , … Prevot, V. (2010). Role of estradiol in the dynamic control of tanycyte plasticity mediated by vascular endothelial cells in the median eminence. Endocrinology, 151, 1760–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Seranno, S. , Estrella, C. , Loyens, A. , Cornea, A. , Ojeda, S. R. , Beauvillain, J.‐C. , & Prevot, V. (2004). Vascular endothelial cells promote acute plasticity in ependymoglial cells of the neuroendocrine brain. The Journal of Neuroscience, 24, 10353–10363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejanovic, B. , & Schwarz, G. (2014). Neuronal nitric oxide synthase‐dependent S‐nitrosylation of gephyrin regulates gephyrin clustering at GABAergic synapses. The Journal of Neuroscience, 34, 7763–7768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derakhshan, B. , Hao, G. , & Gross, S. S. (2007). Balancing reactivity against selectivity: The evolution of protein S‐nitrosylation as an effector of cell signaling by nitric oxide. Cardiovascular Research, 75, 210–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derbyshire, E. R. , & Marletta, M. A. (2012). Structure and regulation of soluble guanylate cyclase. Annual Review of Biochemistry, 81, 533–559. [DOI] [PubMed] [Google Scholar]
- Devanand, D. P. (2016). Olfactory identification deficits, cognitive decline, and dementia in older adults. The American Journal of Geriatric Psychiatry, 24, 1151–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieterle, A. , Fischer, M. J. M. , Link, A. S. , Neuhuber, W. L. , & Messlinger, K. (2010). Increase in CGRP‐ and nNOS‐immunoreactive neurons in the rat trigeminal ganglion after infusion of an NO donor. Cephalalgia, 31, 31–42. [DOI] [PubMed] [Google Scholar]
- Domek‐Łopacińska, K. U. , & Strosznajder, J. B. (2010). Cyclic GMP and nitric oxide synthase in aging and Alzheimer's disease. Molecular Neurobiology, 41, 129–137. [DOI] [PubMed] [Google Scholar]
- Donato, J. , Frazão, R. , Fukuda, M. , Vianna, C. R. , & Elias, C. F. (2010). Leptin induces phosphorylation of neuronal nitric oxide synthase in defined hypothalamic neurons. Endocrinology, 151, 5415–5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, H. , Wang, Z. H. , Dong, B. , Hu, Y. N. , & Zhao, H. Y. (2018). Endothelial nitric oxide synthase (−786T>C) polymorphism and migraine susceptibility: A meta‐analysis. Medicine (Baltimore), 97, e12241–e12241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dröge, W. (2002). Free radicals in the physiological control of cell function. Physiological Reviews, 82, 47–95. [DOI] [PubMed] [Google Scholar]
- Du, W. , Stern, J. E. , & Filosa, J. A. (2015). Neuronal‐derived nitric oxide and somatodendritically released vasopressin regulate neurovascular coupling in the rat hypothalamic supraoptic nucleus. The Journal of Neuroscience, 35, 5330–5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulac, C. , & Torello, A. T. (2003). Molecular detection of pheromone signals in mammals: From genes to behaviour. Nature Reviews. Neuroscience, 4, 551–562. [DOI] [PubMed] [Google Scholar]
- Dunbar, A. Y. , Kamada, Y. , Jenkins, G. J. , Lowe, E. R. , Billecke, S. S. , & Osawa, Y. (2004). Ubiquitination and degradation of neuronal nitric‐oxide synthase in vitro: Dimer stabilization protects the enzyme from proteolysis. Molecular Pharmacology, 66, 964 LP–969. [DOI] [PubMed] [Google Scholar]
- Edvinsson, L. , Mulder, H. , Goadsby, P. J. , & Uddman, R. (1998). Calcitonin gene‐related peptide and nitric oxide in the trigeminal ganglion: Cerebral vasodilatation from trigeminal nerve stimulation involves mainly calcitonin gene‐related peptide. Journal of the Autonomic Nervous System, 70, 15–22. [DOI] [PubMed] [Google Scholar]
- Elmquist, J. K. , Coppari, R. , Balthasar, N. , Ichinose, M. , & Lowell, B. B. (2005). Identifying hypothalamic pathways controlling food intake, body weight, and glucose homeostasis. The Journal of Comparative Neurology, 493, 63–71. [DOI] [PubMed] [Google Scholar]
- Endoh, M. , Maiese, K. , & Wagner, J. A. (1994). Expression of the neural form of nitric oxide synthase by CA1 hippocampal neurons and other central nervous system neurons. Neuroscience, 63, 679–689. [DOI] [PubMed] [Google Scholar]
- van Ewijk, H. , Bralten, J. , van Duin, E. D. A. , Hakobjan, M. , Buitelaar, J. K. , Heslenfeld, D. J. , … Franke, B. (2017). Female‐specific association of NOS1 genotype with white matter microstructure in ADHD patients and controls. Journal of Child Psychology and Psychiatry, 58, 958–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber, C. L. , Matsen, M. E. , Velasco, K. R. , Damian, V. , Phan, B. A. , Adam, D. , … Morton, G. J. (2018). Distinct neuronal projections from the hypothalamic ventromedial nucleus mediate glycemic and behavioral effects. Diabetes, 67, 2518 LP–2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fessenden, J. D. , Altschuler, R. A. , Seasholtz, A. F. , & Schacht, J. (1999). Nitric oxide/cyclic guanosine monophosphate pathway in the peripheral and central auditory system of the rat. The Journal of Comparative Neurology, 404, 52–63. [PubMed] [Google Scholar]
- Fioramonti, X. , Deak, A. , Deshpande, S. , Carneiro, L. , Zhou, C. , Sayed, N. , … Routh, V. H. (2013). Hypothalamic S‐nitrosylation contributes to the counter‐regulatory response impairment following recurrent hypoglycemia. PLoS ONE, 8, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fioramonti, X. , Marsollier, N. , Song, Z. , Fakira, K. A. , Patel, R. M. , Brown, S. , … Routh, V. H. (2010). Ventromedial hypothalamic nitric oxide production is necessary for hypoglycemia detection and counterregulation. Diabetes, 59, 519–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster, M. W. , McMahon, T. J. , & Stamler, J. S. (2003). S‐nitrosylation in health and disease. Trends in Molecular Medicine, 9, 160–168. [DOI] [PubMed] [Google Scholar]
- Fournel, A. , Drougard, A. , Duparc, T. , Marlin, A. , Brierley, S. M. , Castro, J. , … Knauf, C. (2017). Apelin targets gut contraction to control glucose metabolism via the brain. Gut, 66, 258 LP–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis, S. H. , Busch, J. L. , & Corbin, J. D. (2010). cGMP‐dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacological Reviews, 62, 525–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudenberg, F. , Alttoa, A. , & Reif, A. (2015). Neuronal nitric oxide synthase (NOS1) and its adaptor, NOS1AP, as a genetic risk factors for psychiatric disorders. Genes, Brain, and Behavior, 14, 46–63. [DOI] [PubMed] [Google Scholar]
- Fuentealba, P. , Begum, R. , Capogna, M. , Jinno, S. , Márton, L. F. , Csicsvari, J. , … Klausberger, T. (2008). Ivy cells: A population of nitric‐oxide‐producing, slow‐spiking GABAergic neurons and their involvement in hippocampal network activity. Neuron, 57, 917–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto, H. , Konno, K. , Watanabe, M. , & Jinno, S. (2017). Late postnatal shifts of parvalbumin and nitric oxide synthase expression within the GABAergic and glutamatergic phenotypes of inferior colliculus neurons. The Journal of Comparative Neurology, 525, 868–884. [DOI] [PubMed] [Google Scholar]
- Funk, O. H. , & Kwan, K. Y. (2014). Nitric oxide signaling in the development and evolution of language and cognitive circuits. Neuroscience Research, 86, 77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott, R. F. , & Zawadzki, J. V. (1980). The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature, 288, 373–376. [DOI] [PubMed] [Google Scholar]
- Gally, J. A. , Montague, P. R. , Reeke, G. N. Jr. , & Edelman, G. M. (1990). The NO hypothesis: Possible effects of a short‐lived, rapidly diffusible signal in the development and function of the nervous system. Proceedings of the National Academy of Sciences of the United States of America, 87, 3547–3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangula, P. R. R. , Maner, W. L. , Micci, M.‐A. , Garfield, R. E. , & Pasricha, P. J. (2007). Diabetes induces sex‐dependent changes in neuronal nitric oxide synthase dimerization and function in the rat gastric antrum. American Journal of Physiology. Gastrointestinal and Liver Physiology, 292, G725–G733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthwaite, G. , Bartus, K. , Malcolm, D. , Goodwin, D. , Kollb‐Sielecka, M. , Dooldeniya, C. , & Garthwaite, J. (2006). Signaling from blood vessels to CNS axons through nitric oxide. The Journal of Neuroscience, 26, 7730 LP–7740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthwaite, J. (2008). Concepts of neural nitric oxide‐mediated transmission. The European Journal of Neuroscience, 27, 2783–2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthwaite, J. (2010). New insight into the functioning of nitric oxide‐receptive guanylyl cyclase: Physiological and pharmacological implications. Molecular and Cellular Biochemistry, 334, 221–232. [DOI] [PubMed] [Google Scholar]
- Garthwaite, J. (2016). From synaptically localized to volume transmission by nitric oxide. The Journal of Physiology, 594(1), 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthwaite, J. , & Boulton, C. L. (1995). Nitric oxide signaling in the central nervous system. Annual Review of Physiology, 57, 683–706. [DOI] [PubMed] [Google Scholar]
- Garthwaite, J. , Charles, S. L. , & Chess‐Williams, R. (1988). Endothelium‐derived relaxing factor release on activation of NMDA receptors suggests role as intercellular messenger in the brain. Nature, 336, 385–388. [DOI] [PubMed] [Google Scholar]
- Gee, L. C. , & Ahluwalia, A. (2016). Dietary nitrate lowers blood pressure: Epidemiological, pre‐clinical experimental and clinical trial evidence. Current Hypertension Reports, 18, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemi, M. , Claunch, J. , & Niu, K. (2019). Pathologic role of nitrergic neurotransmission in mood disorders. Progress in Neurobiology, 173, 54–87. [DOI] [PubMed] [Google Scholar]
- Ghasemi, M. , Mayasi, Y. , Hannoun, A. , Eslami, S. M. , & Carandang, R. (2018). Nitric oxide and mitochondrial function in neurological diseases. Neuroscience, 376, 48–71. [DOI] [PubMed] [Google Scholar]
- Giacobini, P. , Parkash, J. , Campagne, C. , Messina, A. , Casoni, F. , Vanacker, C. , … Prevot, V. (2014). Brain endothelial cells control fertility through ovarian‐steroid–dependent release of semaphorin 3A. PLoS Biology, 12, e1001808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gizowski, C. , & Bourque, C. W. (2017). The neural basis of homeostatic and anticipatory thirst. Nature Reviews. Nephrology, 14, 11. [DOI] [PubMed] [Google Scholar]
- González‐Flores, O. , & Etgen, A. M. (2004). The nitric oxide pathway participates in estrous behavior induced by progesterone and some of its ring A‐reduced metabolites. Hormones and Behavior, 45, 50–57. [DOI] [PubMed] [Google Scholar]
- Gotti, S. , Sica, M. , Viglietti‐Panzica, C. , & Panzica, G. (2005). Distribution of nitric oxide synthase immunoreactivity in the mouse brain. Microscopy Research and Technique, 68, 13–35. [DOI] [PubMed] [Google Scholar]
- Guerra, D. D. , Bok, R. , Vyas, V. , Orlicky, D. J. , Lorca, R. A. , & Hurt, K. J. (2019). Akt phosphorylation of neuronal nitric oxide synthase regulates gastrointestinal motility in mouseileum. Proceedings of the National Academy of Sciences of the United States of America. 10.1073/pnas.1905902116 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta, N. , Drusch, J. , Landis, B. N. , & Hummel, T. (2013). Nasal nitric oxide levels do not allow for discrimination between olfactory loss due to various etiologies. Laryngoscope, 123, 311–314. [DOI] [PubMed] [Google Scholar]
- Gyurko, R. , Leupen, S. , & Huang, P. L. (2002). Deletion of exon 6 of the neuronal nitric oxide synthase gene in mice results in hypogonadism and infertility. Endocrinology, 143, 2767–2774. [DOI] [PubMed] [Google Scholar]
- Hallak, J. , Maia‐de‐Oliveira, J. , Abrao, J. , & Al, E. (2013). Rapid improvement of acute schizophrenia symptoms after intravenous sodium nitroprusside: A randomized, double‐blind, placebo‐controlled trial. JAMA Psychiatry, 70, 668–676. [DOI] [PubMed] [Google Scholar]
- Hallmark, O. G. , Phung, Y. T. , & Black, S. M. (1999). Chimeric forms of neuronal nitric oxide synthase identify different regions of the reductase domain that are essential for dimerization and activity. DNA and Cell Biology, 18, 397–407. [DOI] [PubMed] [Google Scholar]
- Han, W.‐J. , Shi, X.‐R. , & Nuttall, A. (2018). Distribution and change of peroxynitrite in the guinea pig cochlea following noise exposure. Biomedical Reports, 9, 135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanchate, N. K. , Parkash, J. , Bellefontaine, N. , Mazur, D. , Colledge, W. H. , d'Anglemont de Tassigny, X. , & Prevot, V. (2012). Kisspeptin‐GPR54 signaling in mouse NO‐synthesizing neurons participates in the hypothalamic control of ovulation. The Journal of Neuroscience, 32, 932–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR (2018). The IUPHAR/BPS guide to PHARMACOLOGY in 2018: Updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Research, 46, D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham, N. , Dachtler, J. , & Fox, K. (2013). The role of nitric oxide in pre‐synaptic plasticity and homeostasis. Frontiers in Cellular Neuroscience, 7, 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedner, M. , Larsson, M. , Arnold, N. , Zucco, G. M. , & Hummel, T. (2010). Cognitive factors in odor detection, odor discrimination, and odor identification tasks. Journal of Clinical and Experimental Neuropsychology, 32, 1062–1067. [DOI] [PubMed] [Google Scholar]
- Heinrich, U.‐R. , & Helling, K. (2012). Nitric oxide—A versatile key player in cochlear function and hearing disorders. Nitric Oxide, 27, 106–116. [DOI] [PubMed] [Google Scholar]
- Hellier, V. , Brock, O. , Candlish, M. , Desroziers, E. , Aoki, M. , Mayer, C. , … Bakker, J. (2018). Female sexual behavior in mice is controlled by kisspeptin neurons. Nature Communications, 9, 400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkin, R. I. , Abdelmeguid, M. , & Knöppel, A. B. (2016a). Initiation of smell function in patients with congenital hyposmia. American Journal of Otolaryngology, 37, 175–181. [DOI] [PubMed] [Google Scholar]
- Henkin, R. I. , Abdelmeguid, M. , & Knöppel, A. B. (2016b). On the mechanism of smell loss in patients with Type II congenital hyposmia. American Journal of Otolaryngology, 37, 436–441. [DOI] [PubMed] [Google Scholar]
- Henkin, R. I. , Schultz, M. , & Minnick‐Poppe, L. (2012). Intranasal theophylline treatment of hyposmia and hypogeusia: A pilot studyintranasal theophylline treatment. Archives of Otolaryngology‐‐Head & Neck Surgery, 138, 1064–1070. [DOI] [PubMed] [Google Scholar]
- Henkin, R. I. , & Velicu, I. (2008). cAMP and cGMP in nasal mucus related to severity of smell loss in patients with smell dysfunction. Clinical and Investigative Medicine, 31, 78–84. [DOI] [PubMed] [Google Scholar]
- Herbison, A. E. (2015). Chapter 11—Physiology of the adult gonadotropin‐releasing hormone neuronal network. T.M. Plant, and A.J.B.T.‐K. and N.P. of R. In Zeleznik F. E. (Ed.), (pp. 399–467). San Diego: Academic Press. [Google Scholar]
- Herbison, A. E. , Simonian, S. X. , Norris, P. J. , & Emson, P. C. (1996). Relationship of neuronal nitric oxide synthase immunoreactivity to GnRH neurons in the ovariectomized and intact female rat. Journal of Neuroendocrinology, 8, 73–82. [DOI] [PubMed] [Google Scholar]
- Hess, D. T. , Matsumoto, A. , Kim, S.‐O. , Marshall, H. E. , & Stamler, J. S. (2005). Protein S‐nitrosylation: Purview and parameters. Nature Reviews. Molecular Cell Biology, 6, 150–166. [DOI] [PubMed] [Google Scholar]
- Hill, J. W. , & Elias, C. F. (2018). Neuroanatomical framework of the metabolic control of reproduction. Physiological Reviews, 98, 2349–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho, G. P. , Selvakumar, B. , Mukai, J. , Hester, L. D. , Wang, Y. , Gogos, J. A. , & Snyder, S. H. (2011). S‐nitrosylation and S‐palmitoylation reciprocally regulate synaptic targeting of PSD‐95. Neuron, 71, 131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs, A. J. (1997). Soluble guanylate cyclase: The forgotten sibling. Trends in Pharmacological Sciences, 18, 484–491. [DOI] [PubMed] [Google Scholar]
- Hofmann, F. (2005). The biology of cyclic GMP‐dependent protein kinases. The Journal of Biological Chemistry, 280, 1–4. [DOI] [PubMed] [Google Scholar]
- Hollas, M. A. , Aissa, M. B. , Lee, S. H. , Gordon‐Blake, J. M. , & Thatcher, G. R. J. (2019). Pharmacological manipulation of cGMP and NO/cGMP in CNS drug discovery. Nitric Oxide, 82, 59–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins, D. A. , Steinbusch, H. W. M. , Ittersum, M. M.‐V. , & De Vente, J. (2018). Nitric oxide synthase, cGMP, and NO‐mediated cGMP production in the olfactory bulb of the rat. The Journal of Comparative Neurology, 375, 641–658. [DOI] [PubMed] [Google Scholar]
- Horst, B. G. , & Marletta, M. A. (2018). Physiological activation and deactivation of soluble guanylate cyclase. Nitric Oxide, 77, 65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard, S. R. , & Dunkel, L. (2018). The genetic basis of delayed puberty. Neuroendocrinology, 106, 283–291. [DOI] [PubMed] [Google Scholar]
- Iadecola, C. (2004). Neurovascular regulation in the normal brain and in Alzheimer's disease. Nature Reviews. Neuroscience, 5, 347–360. [DOI] [PubMed] [Google Scholar]
- Iannone, M. , Del Duca, C. , Granato, T. , Rispoli, V. , & Nisticò, G. (1996). Sound‐evoked electrocortical desynchronization is inhibited by N(ω)‐nitro‐L‐arginine methyl ester microinfused into the inferior colliculi in rats. Electroencephalography and Clinical Neurophysiology, 99(1), 57–62. [DOI] [PubMed] [Google Scholar]
- Ignarro, L. J. , Byrns, R. E. , Buga, G. M. , & Wood, K. S. (1987). Endothelium‐derived relaxing factor from pulmonary artery and vein possesses pharmacologic and chemical properties identical to those of nitric oxide radical. Circulation Research, 61, 866 LP–879. [DOI] [PubMed] [Google Scholar]
- Ignarro, L. J. , Ross, G. , & Tillisch, J. (1991). Pharmacology of endothelium‐derived nitric oxide and nitrovasodilators. The Western Journal of Medicine, 154, 51–62. [PMC free article] [PubMed] [Google Scholar]
- Jaglin, X. , Hjerling‐Leffler, J. , Fishell, G. , & Batista‐Brito, R. (2012). The origin of neocortical nitric oxide synthase‐expressing inhibitory neurons. Frontiers in Neural Circuits, 6, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jane Roskams, A. , Bredt, D. S. , Dawson, T. M. , & Ronnett, G. V. (1994). Nitric oxide mediates the formation of synaptic connections in developing and regenerating olfactory receptor neurons. Neuron, 13, 289–299. [DOI] [PubMed] [Google Scholar]
- Jiang, H. , Talaska, A. E. , Schacht, J. , & Sha, S.‐H. (2007). Oxidative imbalance in the aging inner ear. Neurobiology of Aging, 28, 1605–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin, X.‐G. , Chen, S.‐R. , Cao, X.‐H. , Li, L. , & Pan, H.‐L. (2011). Nitric oxide inhibits nociceptive transmission by differentially regulating glutamate and glycine release to spinal dorsal horn neurons. The Journal of Biological Chemistry, 286, 33190–33202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung, K. H. , Chu, K. , Ko, S. Y. , Lee, S. T. , Sinn, D. I. , Park, D. K. , … Roh, J. K. (2006). Early intravenous infusion of sodium nitrite protects brain against in vivo ischemia‐reperfusion injury. Stroke, 37, 2744–2750. [DOI] [PubMed] [Google Scholar]
- Kass, D. A. , Takimoto, E. , Nagayama, T. , & Champion, H. C. (2007). Phosphodiesterase regulation of nitric oxide signaling. Cardiovascular Research, 75, 303 LP–314. [DOI] [PubMed] [Google Scholar]
- Kendrick, K. M. , Guevara‐Guzman, R. , Zorrilla, J. , Hinton, M. R. , Broad, K. D. , Mimmack, M. , & Ohkura, S. (1997). Formation of olfactory memories mediated by nitric oxide. Nature, 388, 670. [DOI] [PubMed] [Google Scholar]
- Kim, C. K. , Adhikari, A. , & Deisseroth, K. (2017). Integration of optogenetics with complementary methodologies in systems neuroscience. Nature Reviews. Neuroscience, 18, 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto, J. , Keverne, E. , Hardwick, J. , & Emson, P. (1993). Localization of nitric oxide synthase in the mouse olfactory and vomeronasal system: A histochemical, immunological and in situ hybridization study. The European Journal of Neuroscience, 5, 1684–1694. [DOI] [PubMed] [Google Scholar]
- Knauf, C. , Abot, A. , Wemelle, E. , & Cani, P. D. (2019). Targeting the enteric nervous system to treat metabolic disorders? “Enterosynes” as therapeutic gut factors. Neuroendocrinology. 10.1159/000500602 [DOI] [PubMed] [Google Scholar]
- Knauf, C. , Prevot, V. , Stefano, G. B. , Mortreux, G. , Beauvillain, J. C. , & Croix, D. (2001). Evidence for a spontaneous nitric oxide release from the rat median eminence: Influence on gonadotropin‐releasing hormone release. Endocrinology, 142, 2343–2350. [DOI] [PubMed] [Google Scholar]
- Koulchitsky, S. , Fischer, M. J. M. , De Col, R. , Schlechtweg, P. M. , & Messlinger, K. (2004). Biphasic response to nitric oxide of spinal trigeminal neurons with meningeal input in rat—Possible implications for the pathophysiology of headaches. Journal of Neurophysiology, 92, 1320–1328. [DOI] [PubMed] [Google Scholar]
- Kuiri‐Hänninen, T. , Sankilampi, U. , & Dunkel, L. (2014). Activation of the hypothalamic‐pituitary‐gonadal axis in infancy: Minipuberty. Hormone Research in Pædiatrics, 82, 73–80. [DOI] [PubMed] [Google Scholar]
- Kulkarni, A. P. , Getchell, T. V. , & Getchell, M. L. (1994). Neuronal nitric oxide synthase is localized in extrinsic nerves regulating perireceptor processes in the chemosensory nasal mucosae of rats and humans. The Journal of Comparative Neurology, 345, 125–138. [DOI] [PubMed] [Google Scholar]
- Kwan, K. Y. , Lam, M. M. S. , Johnson, M. B. , Dube, U. , Shim, S. , Rašin, M.‐R. , … Sestan, N. (2012). Species‐dependent posttranscriptional regulation of NOS1 by FMRP in the developing cerebral cortex. Cell, 149, 899–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon, K. J. , Park, J.‐H. , Jo, I. , Song, K.‐H. , Han, J.‐S. , Park, S. H. , … Cho, D. H. (2016). Disruption of neuronal nitric oxide synthase dimerization contributes to the development of Alzheimer's disease: Involvement of cyclin‐dependent kinase 5‐mediated phosphorylation of neuronal nitric oxide synthase at Ser293. Neurochemistry International, 99, 52–61. [DOI] [PubMed] [Google Scholar]
- Labbé, D. , Bloch, W. , Schick, B. , & Michel, O. (2016). Hearing impairment, cochlear morphology, and peroxynitrite (ONOO−) formation in adult and aging NOS II knockout mice. Acta Oto‐Laryngologica, 136, 991–998. [DOI] [PubMed] [Google Scholar]
- Lajoix, A.‐D. , Pugnière, M. , Roquet, F. , Mani, J.‐C. , Dietz, S. , Linck, N. , … Gross, R. (2004). Changes in the dimeric state of neuronal nitric oxide synthase affect the kinetics of secretagogue‐induced insulin response. Diabetes, 53, 1467 LP–1474. [DOI] [PubMed] [Google Scholar]
- Landis, B. N. , & Lacroix, J.‐S. (2009). Olfactory function and nasal nitric oxide. Current Opinion in Otolaryngology & Head and Neck Surgery, 17, 18–22. [DOI] [PubMed] [Google Scholar]
- Langlet, F. , Mullier, A. , Bouret, S. G. , Prevot, V. , & Dehouck, B. (2013). Tanycyte‐like cells form a blood‐cerebrospinal fluid barrier in the circumventricular organs of the mouse brain. The Journal of Comparative Neurology, 521, 3389–3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassen, L. , Ashina, M. , Christiansen, I. , Ulrich, V. , & Olesen, J. (1997). Nitric oxide synthase inhibition in migraine. Lancet, 349, 401–402. [DOI] [PubMed] [Google Scholar]
- Lee, C. M. , Robinson, L. J. , & Michel, T. (1995). Oligomerization of endothelial nitric oxide synthase: Evidence for a dominant negative effect of truncation mutants. The Journal of Biological Chemistry, 270, 27403–27406. [DOI] [PubMed] [Google Scholar]
- Leshan, R. L. , Greenwald‐Yarnell, M. , Patterson, C. M. , Gonzalez, I. E. , & Myers, M. G. (2012). Leptin action through hypothalamic nitric oxide synthase‐1‐expressing neurons controls energy balance. Nature Medicine, 18, 820–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J. , Vause, C. V. , & Durham, P. L. (2008). Calcitonin gene‐related peptide stimulation of nitric oxide synthesis and release from trigeminal ganglion glial cells. Brain Research, 1196, 22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J.‐L. , Wu, S.‐X. , Tomioka, R. , Okamoto, K. , Nakamura, K. , Kaneko, T. , & Mizuno, N. (2005). Efferent and afferent connections of GABAergic neurons in the supratrigeminal and the intertrigeminal regions: An immunohistochemical tract‐tracing study in the GAD67‐GFP knock‐in mouse. Neuroscience Research, 51, 81–91. [DOI] [PubMed] [Google Scholar]
- Li, L.‐P. , Dustrude, E. T. , Haulcomb, M. M. , Abreu, A. R. , Fitz, S. D. , Johnson, P. L. , … Shekhar, A. (2018). PSD95 and nNOS interaction as a novel molecular target to modulate conditioned fear: Relevance to PTSD. Translational Psychiatry, 8, 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, M. T. , & Beal, M. F. (2006). Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature, 443, 787–795. [DOI] [PubMed] [Google Scholar]
- Lundberg, J. O. , Weitzberg, E. , & Gladwin, M. T. (2008). The nitrate–nitrite–nitric oxide pathway in physiology and therapeutics. Nature Reviews. Drug Discovery, 7, 156. [DOI] [PubMed] [Google Scholar]
- Manfredi‐Lozano, M. , Roa, J. , & Tena‐Sempere, M. (2018). Connecting metabolism and gonadal function: Novel central neuropeptide pathways involved in the metabolic control of puberty and fertility. Frontiers in Neuroendocrinology, 48, 37–49. [DOI] [PubMed] [Google Scholar]
- Mani, S. K. , Allen, J. M. , Rettori, V. , McCann, S. M. , O'Malley, B. W. , & Clark, J. H. (1994). Nitric oxide mediates sexual behavior in female rats. Proceedings of the National Academy of Sciences of the United States of America, 91, 6468–6472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mapelli, L. , Gagliano, G. , Soda, T. , Laforenza, U. , Moccia, F. , & D'Angelo, E. U. (2017). Granular layer neurons control cerebellar neurovascular coupling through an NMDA receptor/NO‐dependent system. The Journal of neuroscience, 37, 1340 LP–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall, C. J. , Desroziers, E. , McLennan, T. , & Campbell, R. E. (2017). Defining subpopulations of arcuate nucleus GABA neurons in male, female, and prenatally androgenized female mice. Neuroendocrinology, 105, 157–169. [DOI] [PubMed] [Google Scholar]
- Martínez‐Ruiz, A. , Villanueva, L. , González de Orduña, C. , López‐Ferrer, D. , Higueras, M. Á. , Tarín, C. , … Lamas, S. (2005). S‐nitrosylation of Hsp90 promotes the inhibition of its ATPase and endothelial nitric oxide synthase regulatory activities. Proceedings of the National Academy of Sciences of the United States of America, 102, 8525–8530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice, D. H. , Ke, H. , Ahmad, F. , Wang, Y. , Chung, J. , & Manganiello, V. C. (2014). Advances in targeting cyclic nucleotide phosphodiesterases. Nature Reviews. Drug Discovery, 13, 290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer, B. , Schmidt, K. , Humbert, P. , & Böhme, E. (1989). Biosynthesis of endothelium‐derived relaxing factor: A cytosolic enzyme in porcine aortic endothelial cells Ca2+‐dependently converts L‐arginine into an activator of soluble guanylyl cyclase. Biochemical and Biophysical Research Communications, 164, 678–685. [DOI] [PubMed] [Google Scholar]
- Messina, A. , Langlet, F. , Chachlaki, K. , Roa, J. , Rasika, S. , Jouy, N. , … Prevot, V. (2016). A microRNA switch regulates the rise in hypothalamic GnRH production before puberty. Nature Neuroscience, 19, 835–844. [DOI] [PubMed] [Google Scholar]
- Mezghenna, K. , Pomiès, P. , Chalançon, A. , Castex, F. , Leroy, J. , Niclauss, N. , … Lajoix, A. D. (2011). Increased neuronal nitric oxide synthase dimerisation is involved in rat and human pancreatic β cell hyperactivity in obesity. Diabetologia, 54, 2856. [DOI] [PubMed] [Google Scholar]
- Miki, N. , Kawabe, Y. , & Kuriyama, K. (1977). Activation of cerebral guanylate cyclase by nitric oxide. Biochemical and Biophysical Research Communications, 75, 851–856. [DOI] [PubMed] [Google Scholar]
- Mills, E. G. A. , Dhillo, W. S. , & Comninos, A. N. (2018). Kisspeptin and the control of emotions, mood and reproductive behaviour. The Journal of Endocrinology, 239, R1–R12. [DOI] [PubMed] [Google Scholar]
- Moenter, S. M. (2017). GnRH neurons on LSD: A year of rejecting hypotheses that may have made Karl Popper proud. Endocrinology, 159, 199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möhrle, D. , Reimann, K. , Wolter, S. , Wolters, M. , Varakina, K. , Mergia, E. , … Rüttiger, L. (2017). NO‐sensitive guanylate cyclase isoforms NO‐GC1 and NO‐GC2 contribute to noise‐induced inner hair cell synaptopathy. Molecular Pharmacology, 92, 375 LP–388. [DOI] [PubMed] [Google Scholar]
- Montague, P. R. , & Sejnowski, T. J. (1994). The predictive brain: Temporal coincidence and temporal order in synaptic learning mechanisms. Learning & Memory, 1, 1–33. [PubMed] [Google Scholar]
- Morales, M. , & Margolis, E. B. (2017). Ventral tegmental area: Cellular heterogeneity, connectivity and behaviour. Nature Reviews. Neuroscience, 18, 73. [DOI] [PubMed] [Google Scholar]
- Murphy, B. A. , Fakira, K. A. , Song, Z. , Beuve, A. , & Routh, V. H. (2009). AMP‐activated protein kinase and nitric oxide regulate the glucose sensitivity of ventromedial hypothalamic glucose‐inhibited neurons. American Journal of Physiology‐Cell Physiology, 297, C750–C758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura, T. , Tu, S. , Akhtar, M. W. , Sunico, C. R. , Okamoto, S. , & Lipton, S. A. (2013). Aberrant protein S‐nitrosylation in neurodegenerative diseases. Neuron, 78, 596–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nistico, G. , Bagetta, G. , Iannone, M. , & Duca, C. D. E. L. (1994). Evidence that nitric oxide is involved in the control of electrocortical arousal. Annals of the New York Academy of Sciences, 738, 191–200. [DOI] [PubMed] [Google Scholar]
- Nott, A. , Watson, P. M. , Robinson, J. D. , Crepaldi, L. , & Riccio, A. (2008). S‐nitrosylation of histone deacetylase 2 induces chromatin remodelling in neurons. Nature, 455, 411–415. [DOI] [PubMed] [Google Scholar]
- O'Donovan, M. C. , Craddock, N. , Norton, N. , Williams, H. , Peirce, T. , Moskvina, V. , … Molecular Genetics of Schizophrenia Collaboration (2008). Identification of loci associated with schizophrenia by genome‐wide association and follow‐up. Nature Genetics, 40, 1053–1055. [DOI] [PubMed] [Google Scholar]
- Oka, Y. , Ye, M. , & Zuker, C. S. (2015). Thirst driving and suppressing signals encoded by distinct neural populations in the brain. Nature, 520, 349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto, S. , & Lipton, S. a. (2015). S‐Nitrosylation in neurogenesis and neuronal development. Biochimica et Biophysica Acta ‐ General Subjects, 1850, 1588–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okere, C. O. O. , Kaba, H. , & Higuchi, T. (1996). Formation of an olfactory recognition memory in mice: Reassessment of the role of nitric oxide. Neuroscience, 71, 349–354. [DOI] [PubMed] [Google Scholar]
- Oliver, D. L. , & Cant, N. B. (2018). In Winer J. A., & Schreiner C. E. (Eds.), Neuronal organization in the inferior colliculus BT—The inferior colliculus (pp. 69–114). New York, NY: Springer New York. [Google Scholar]
- Olthof, B. M. J. , Gartside, S. E. , & Rees, A. (2019). Puncta of neuronal nitric oxide synthase (nNOS) mediate NMDA receptor signaling in the auditory midbrain. The Journal of Neuroscience, 39, 876 LP–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omori, K. , & Kotera, J. (2007). Overview of PDEs and their regulation. Circulation Research, 100, 309–327. [DOI] [PubMed] [Google Scholar]
- Palmer, D. , & Maurice, D. H. (2000). Dual expression and differential regulation of phosphodiesterase 3A and phosphodiesterase 3B in human vascular smooth muscle: Implications for phosphodiesterase 3 inhibition in human cardiovascular tissues. Molecular Pharmacology, 58, 247–252. [DOI] [PubMed] [Google Scholar]
- Palmer, R. M. J. , Ferrige, A. G. , & Moncada, S. (1987). Nitric oxide release accounts for the biological activity of endothelium‐derived relaxing factor. Nature, 327, 524–526. [DOI] [PubMed] [Google Scholar]
- Panda, K. , Ghosh, S. , & Stuehr, D. J. (2001). Calmodulin activates intersubunit electron transfer in the neuronal nitric‐oxide synthase dimer. The Journal of Biological Chemistry, 276, 23349–23356. [DOI] [PubMed] [Google Scholar]
- Parkash, J. , Messina, A. , Langlet, F. , Cimino, I. , Loyens, A. , Mazur, D. , … Giacobini, P. (2015). Semaphorin7A regulates neuroglial plasticity in the adult hypothalamic median eminence. Nature Communications, 6, 6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton, J. F. R. , Kasparov, S. , & Paterson, D. J. (2002). Nitric oxide and autonomic control of heart rate: A question of specificity. Trends in Neurosciences, 25, 626–631. [DOI] [PubMed] [Google Scholar]
- Patton, G. C. , & Viner, R. (2016). Pubertal transitions in health. Lancet, 369, 1130–1139. [DOI] [PubMed] [Google Scholar]
- Paul, E. J. , Kalk, E. , Tossell, K. , Irvine, E. E. , Franks, N. P. , Wisden, W. , … Ungless, M. A. (2018). nNOS‐expressing neurons in the ventral tegmental area and substantia nigra pars compacta. Eneuro, 5, ENEURO.0381‐18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen, N. P. , Ferrari, L. , Venner, A. , Wang, J. L. , Abbott, S. B. G. , Vujovic, N. , … Fuller, P. M. (2017). Supramammillary glutamate neurons are a key node of the arousal system. Nature Communications, 8, 1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Péterfi, Z. , Farkas, I. , Denis, R. G. P. , Farkas, E. , Uchigashima, M. , Füzesi, T. , … Fekete, C. (2018). Endocannabinoid and nitric oxide systems of the hypothalamic paraventricular nucleus mediate effects of NPY on energy expenditure. Molecular Metabolism, 18, 120–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phung, Y. T. , & Black, S. M. (1999). Use of chimeric forms of neuronal nitric‐oxide synthase as dominant negative mutants. IUBMB Life, 48, 333–338. [DOI] [PubMed] [Google Scholar]
- Pietrobon, M. , Zamparo, I. , Maritan, M. , Franchi, S. A. , Pozzan, T. , & Lodovichi, C. (2011). Interplay among cGMP, cAMP, and Ca2+ in living olfactory sensory neurons in vitro and in vivo. The Journal of Neuroscience, 31, 8395 LP–8405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podda, M. V. , & Grassi, C. (2014). New perspectives in cyclic nucleotide‐mediated functions in the CNS: The emerging role of cyclic nucleotide‐gated (CNG) channels. Pflügers Archiv : European Journal of Physiology, 466, 1241–1257. [DOI] [PubMed] [Google Scholar]
- Pradhan, A. A. , Bertels, Z. , & Akerman, S. (2018). Targeted nitric oxide synthase inhibitors for migraine. Neurotherapeutics, 15, 391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevot, V. (2002). Glial–neuronal–endothelial interactions are involved in the control of GnRH secretion. Journal of Neuroendocrinology, 14, 247–255. [DOI] [PubMed] [Google Scholar]
- Prevot, V. (2015). Chapter 30—Puberty in mice and rats In Zeleznik F. E. (Ed.), Knobil and Neill's physiology of reproduction, T.M. Plant, and A.J.B.T.‐K. and N.P. of R. (pp. 1395–1439). San Diego: Academic Press. [Google Scholar]
- Prevot, V. , Croix, D. , Rialas, C. M. , Poulain, P. , Fricchione, G. L. , Stefano, G. B. , & Beauvillain, J. C. (1999). Estradiol coupling to endothelial nitric oxide stimulates gonadotropin‐releasing hormone release from rat median eminence via a membrane receptor. Endocrinology, 140, 652–659. [DOI] [PubMed] [Google Scholar]
- Prevot, V. , Dehouck, B. , Sharif, A. , Ciofi, P. , Giacobini, P. , & Clasadonte, J. (2018). The versatile tanycyte: A hypothalamic integrator of reproduction and energy metabolism. Endocrine Reviews, 39, 333–368. [DOI] [PubMed] [Google Scholar]
- Prevot, V. , Hanchate, N. K. , Bellefontaine, N. , Sharif, A. , Parkash, J. , Estrella, C. , … Baroncini, M. (2010). Function‐related structural plasticity of the GnRH system: A role for neuronal–glial–endothelial interactions. Frontiers in Neuroendocrinology, 31, 241–258. [DOI] [PubMed] [Google Scholar]
- Prickaerts, J. , Heckman, P. R. A. , & Blokland, A. (2017). Investigational phosphodiesterase inhibitors in phase I and phase II clinical trials for Alzheimer's disease. Expert Opinion on Investigational Drugs, 26, 1033–1048. [DOI] [PubMed] [Google Scholar]
- Qu, Z. W. , Miao, W. Y. , Hu, S. Q. , Li, C. , Zhuo, X. L. , Zong, Y. Y. , … Zhang, G. Y. (2012). N‐methyl‐D‐aspartate receptor‐dependent denitrosylation of neuronal nitric oxide synthase increase the enzyme activity. PLoS ONE, 7, e52788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju, K. , Doulias, P. , Evans, P. , Krizman, E. N. , Jackson, J. G. , Horyn, O. , … Ischiropoulos, H. (2015). Regulation of brain glutamate metabolism by nitric oxide and S‐nitrosylation. Science, 8, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rameau, G. A. , Tukey, D. S. , Garcin‐Hosfield, E. D. , Titcombe, R. F. , Misra, C. , Khatri, L ., … Ziff, E. B. (2007). Biphasic coupling of neuronal nitric oxide synthase phosphorylation to the NMDA receptor regulates AMPA receptor trafficking and neuronal cell death. The Journal of Neuroscience, 27, 3445‐3455. 10.1074/10.1523/JNEUROSCI.4799-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastelli, M. , Cani, P. D. , & Knauf, C. (2019). The gut microbiome influences host endocrine functions. Endocrine Reviews, 40, 1271‐1284 [DOI] [PubMed] [Google Scholar]
- Ravi, K. , Brennan, L. A. , Levic, S. , Ross, P. A. , & Black, S. M. (2004). S‐nitrosylation of endothelial nitric oxide synthase is associated with monomerization and decreased enzyme activity. Proceedings of the National Academy of Sciences of the United States of America, 101, 2619 LP–2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis, M. E. M. D. , Araújo, L. T. F. , de Andrade, W. M. G. , Resende, N. D. S. , Lima, R. R. M. , Nascimento, E. S. D. Jr. , … Cavalcante, J. C. (2018). Distribution of nitric oxide synthase in the rock cavy (Kerodon rupestris) brain I: The diencephalon. Brain Research, 1685, 60–78. [DOI] [PubMed] [Google Scholar]
- Reuss, S. , & Riemann, R. (2000). Distribution and projections of nitric oxide synthase neurons in the rodent superior olivary complex. Microscopy Research and Technique, 51, 318–329. [DOI] [PubMed] [Google Scholar]
- Rodrigo, J. , Riveros‐Moreno, V. , Bentura, M. L. , Uttenthal, L. O. , Higgs, E. A. , Fernandez, A. P. , … Martínez‐Murillo, R. (1997). Subcellular localization of nitric oxide synthase in the cerebral ventricular system, subfornical organ, area postrema, and blood vessels of the rat brain. The Journal of Comparative Neurology, 378, 522–534. [DOI] [PubMed] [Google Scholar]
- Rupp, A. C. , Allison, M. B. , Jones, J. C. , Patterson, C. M. , Faber, C. L. , Bozadjieva, N. , … Myers, M. G. Jr . (2018). Specific sub populations of hypothalamic leptin receptor‐expressing neurons mediate the effects of early developmental leptin receptor deletion on energy balance. Molecular Metabolism, 14, 130‐138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russwurm, M. , & Koesling, D. (2004). NO activation of guanylyl cyclase. The EMBO Journal, 23, 4443–4450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvemini, D. , Manning, P. T. , Zweifel, B. S. , Seibert, K. , Connor, J. , Currie, M. G. , … Masferrer, J. L. (1995). Dual inhibition of nitric oxide and prostaglandin production contributes to the antiinflammatory properties of nitric oxide synthase inhibitors. The Journal of Clinical Investigation, 96, 301–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarchielli, P. , Alberti, A. , Codini, M. , Floridi, A. , & Gallai, V. (2000). Nitric oxide metabolites, prostaglandins and trigeminal vasoactive peptides in internal jugular vein blood during spontaneous migraine attacks. Cephalalgia, 20, 907–918. [DOI] [PubMed] [Google Scholar]
- Sayed, N. , Baskaran, P. , Ma, X. , van den Akker, F. , & Beuve, A. (2007). Desensitization of soluble guanylyl cyclase, the NO receptor, by S‐nitrosylation. Proceedings of the National Academy of Sciences of the United States of America, 104, 12312–12317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapira, A. H. V. , Chaudhuri, K. R. , & Jenner, P. (2017). Non‐motor features of Parkinson disease. Nature Reviews. Neuroscience, 18, 435. [DOI] [PubMed] [Google Scholar]
- Schmidt, H. H. , Gagne, G. D. , Nakane, M. , Pollock, J. S. , Miller, M. F. , & Murad, F. (1992). Mapping of neural nitric oxide synthase in the rat suggests frequent co‐localization with NADPH diaphorase but not with soluble guanylyl cyclase, and novel paraneural functions for nitrinergic signal transduction. The Journal of Histochemistry and Cytochemistry, 40, 1439–1456. [DOI] [PubMed] [Google Scholar]
- Seth, P. , Hsieh, P. N. , Jamal, S. , Wang, L. , Gygi, S. P. , Jain, M. K. , … Stamler, J. S. (2019). Regulation of microRNA machinery and development by interspecies S‐nitrosylation. Cell, 176, 1014–1025.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, J. , Harada, N. , Nakazawa, H. , & Yamashita, T. (2005). Involvement of the nitric oxide–cyclic GMP pathway and neuronal nitric oxide synthase in ATP‐induced Ca2+ signalling in cochlear inner hair cells. The European Journal of Neuroscience, 21, 2912–2922. [DOI] [PubMed] [Google Scholar]
- Shen, J. , Scheffer, D. I. , Kwan, K. Y. , & Corey, D. P. (2015). SHIELD: An integrative gene expression database for inner ear research. Database, 2015, bav071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim, S. , Shuman, M. , & Duncan, E. (2016). An emerging role of cGMP in the treatment of schizophrenia: A review. Schizophrenia Research, 170, 226–231. [DOI] [PubMed] [Google Scholar]
- Shlosberg, D. , Buskila, Y. , Abu‐Ghanem, Y. , & Amitai, Y. (2012). Spatiotemporal alterations of cortical network activity by selective loss of NOS‐expressing interneurons. Frontiers in Neural Circuits, 6, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Showkat Ali, M. , Tiscareno‐Grejada, I. , Locovei, S. , Smiley, R. , Collins, T. , Sarosiek, J. , & McCallum, R. (2012). Gender and estradiol as major factors in the expression and dimerization of nNOSα in rats with experimental diabetic gastroparesis. Digestive Diseases and Sciences, 57, 2814–2825. [DOI] [PubMed] [Google Scholar]
- Siddhanta, U. , Presta, A. , Fan, B. , Wolan, D. , Rousseau, D. L. , & Stuehr, D. J. (1998). Domain swapping in inducible nitric‐oxide synthase: Electron transfer occurs between flavin and heme groups located on adjacent subunits in the dimer. The Journal of Biological Chemistry, 273, 18950–18958. [DOI] [PubMed] [Google Scholar]
- Sinz, E. H. , Kochanek, P. M. , Dixon, C. E. , Clark, R. S. , Carcillo, J. A. , Schiding, J. K. , … Billiar, T. R. (1999). Inducible nitric oxide synthase is an endogenous neuroprotectant after traumatic brain injury in rats and mice. The Journal of Clinical Investigation, 104, 647–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, W. L. , Marnett, L. J. , & DeWitt, D. L. (1991). Prostaglandin and thromboxane biosynthesis. Pharmacology & Therapeutics, 49, 153–179. [DOI] [PubMed] [Google Scholar]
- Southam, E. , & Garthwaite, J. (1993). The nitric oxide‐cyclic GMP signalling pathway in rat brain. Neuropharmacology, 32, 1267–1277. [DOI] [PubMed] [Google Scholar]
- Stroissnigg, H. , Trancikova, A. , Descovich, L. , Fuhrmann, J. , Kutschera, W. , Kostan, J. , … Propst, F. (2007). S‐nitrosylation of microtubule‐associated protein 1B mediates nitric‐oxide‐induced axon retraction. Nature Cell Biology, 9, 1035–1045. [DOI] [PubMed] [Google Scholar]
- Stuehr, D. J. (1997). Structure‐function aspects in the nitric oxide synthases. Annual Review of Pharmacology and Toxicology, 37, 339–359. [DOI] [PubMed] [Google Scholar]
- Sülz, L. , Astorga, G. , Bellette, B. , Iturriaga, R. , Mackay‐Sim, A. , & Bacigalupo, J. (2009). Nitric oxide regulates neurogenesis in adult olfactory epithelium in vitro. Nitric Oxide, 20, 238–252. [DOI] [PubMed] [Google Scholar]
- Sutton, A. K. , Myers, M. G. , & Olson, D. P. (2016). The role of PVH circuits in leptin action and energy balance. Annual Review of Physiology, 78, 207–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton, A. K. , Pei, H. , Burnett, K. H. , Myers, M. G. , Rhodes, C. J. , & Olson, D. P. (2014). Control of food intake and energy expenditure by Nos1 neurons of the paraventricular hypothalamus. The Journal of Neuroscience, 34, 15306–15318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabansky, I. , Liang, Y. , Frankfurt, M. , Daniels, M. A. , Harrigan, M. , Stern, S. , … Pfaff, D. W. (2018). Molecular profiling of reticular gigantocellularis neurons indicates that eNOS modulates environmentally dependent levels of arousal. Proceedings of the National Academy of Sciences of the United States of America, 115, E6900–E6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takumida, M. , & Anniko, M. (2002). Nitric oxide in the inner ear. Current Opinion in Neurology, 15, 11–15. [DOI] [PubMed] [Google Scholar]
- Tejero, J. , Shiva, S. , & Gladwin, M. T. (2018). Sources of vascular nitric oxide and reactive oxygen species and their regulation. Physiological Reviews, 99, 311–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda, N. , & Okamura, T. (2003). The pharmacology of nitric oxide in the peripheral nervous system of blood vessels. Pharmacological Reviews, 55, 271–324. [DOI] [PubMed] [Google Scholar]
- Tomioka, R. , Sakimura, K. , & Yanagawa, Y. (2015). Corticofugal GABAergic projection neurons in the mouse frontal cortex. Frontiers in Neuroanatomy, 9, 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricoire, L. , Pelkey, K. A. , Daw, M. I. , Sousa, V. H. , Miyoshi, G. , Jeffries, B. , … McBain, C. J. (2010). Common origins of hippocampal ivy and nitric oxide synthase expressing neurogliaform cells. The Journal of Neuroscience, 30, 2165–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricoire, L. , Pelkey, K. A. , Erkkila, B. E. , Jeffries, B. W. , Yuan, X. , & McBain, C. J. (2011). A blueprint for the spatiotemporal origins of mouse hippocampal interneuron diversity. The Journal of Neuroscience, 31, 10948–10970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vente, J. , Hopkins, D. , Markerink‐van Ittersum, M. , Emson, P. , Schmidt, H. H. H. , & Steinbusch, H. W. (1998). Distribution of nitric oxide synthase and nitric oxide‐receptive, cyclic GMP‐producing structures in the rat brain. Neuroscience, 87, 207–241. [DOI] [PubMed] [Google Scholar]
- Vincent, S. R. , & Kimura, H. (1992). Histochemical mapping of nitric oxide synthase in the rat brain. Neuroscience, 46, 755–784. [DOI] [PubMed] [Google Scholar]
- Virarkar, M. , Alappat, L. , Bradford, P. G. , & Awad, A. B. (2013). L‐arginine and nitric oxide in CNS function and neurodegenerative diseases. Critical Reviews in Food Science and Nutrition, 53, 1157–1167. [DOI] [PubMed] [Google Scholar]
- Vyas, P. , Wu, J. S. , Jimenez, A. , Glowatzki, E. , & Fuchs, P. A. (2019). Characterization of transgenic mouse lines for labeling type I and type II afferent neurons in the cochlea. Scientific Reports, 9, 5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzberg, E. , & Lundberg, J. O. (2013). Novel aspects of dietary nitrate and human health. Annual Review of Nutrition, 33, 129–159. [DOI] [PubMed] [Google Scholar]
- Weitzdoerfer, R. , Hoeger, H. , Engidawork, E. , Engelmann, M. , Singewald, N. , Lubec, G. , … Lubec, B. (2004). Neuronal nitric oxide synthase knock‐out mice show impaired cognitive performance. Nitric Oxide, 10, 130–140. [DOI] [PubMed] [Google Scholar]
- Wisor, J. P. , Gerashchenko, D. , & Kilduff, T. S. (2011). Sleep‐active neuronal nitric oxidesynthase‐positive cells of the cerebral cortex: a local regulator of sleep? Current Topics in Medicinal Chemistry, 11(19), 2483‐2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, L.‐F. , Polson, J. W. , Murphy, D. , Paton, J. F. R. , & Kasparov, S. (2002). Genetic and pharmacological dissection of pathways involved in the angiotensin II‐mediated depression of baroreflex function. The FASEB Journal, 16, 1595–1601. [DOI] [PubMed] [Google Scholar]
- Yamada, K. , Emson, P. , & Hökfelt, T. (1996). Immunohistochemical mapping of nitric oxide synthase in the rat hypothalamus and colocalization with neuropeptides. Journal of Chemical Neuroanatomy, 10, 295–316. [DOI] [PubMed] [Google Scholar]
- Yamashita, D. , Jiang, H.‐Y. , Schacht, J. , & Miller, J. M. (2004). Delayed production of free radicals following noise exposure. Brain Research, 1019, 201–209. [DOI] [PubMed] [Google Scholar]
- Yang, Y.‐M. , Huang, A. , Kaley, G. , & Sun, D. (2009). eNOS uncoupling and endothelial dysfunction in aged vessels. American Journal of Physiology. Heart and Circulatory Physiology, 297, H1829–H1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, S. , Cheng, H. , François, M. , Qualls‐Creekmore, E. , Huesing, C. , He, Y. , … Münzberg, H. (2018). Preoptic leptin signaling modulates energy balance independent of body temperature regulation. eLife, 7, e33505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, S. , François, M. , Huesing, C. , & Münzberg, H. (2018). The hypothalamic preoptic area and body weight control. Neuroendocrinology, 106, 187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, X. , Li, W. , Ma, Y. , Tossell, K. , Harris, J. J. , Harding, E. C. , … Wisden, W. (2019). GABA and glutamate neurons in the VTA regulate sleep and wakefulness. Nature Neuroscience, 22, 106–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaghloul, H. , Pallayova, M. , Al‐Nuaimi, O. , Hovis, K. R. , & Taheri, S. (2018). Association between diabetes mellitus and olfactory dysfunction: current perspectives and future directions. Diabetic Medicine, 35, 41–52. [DOI] [PubMed] [Google Scholar]
- Zhang, J. , Huang, X.‐Y. , Ye, M.‐L. , Luo, C.‐X. , Wu, H.‐Y. , Hu, Y. , … Zhu, D. Y. (2010). Neuronal nitric oxide synthase alteration accounts for the role of 5‐HT1A receptor in modulating anxiety‐related behaviors. The Journal of Neuroscience, 30, 2433 LP–2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, K. Y. J. , Card, G. L. , Suzuki, Y. , Artis, D. R. , Fong, D. , Gillette, S. , … Bollag, G. (2004). A glutamine switch mechanism for nucleotide selectivity by phosphodiesterases. Molecular Cell, 15, 279–286. [DOI] [PubMed] [Google Scholar]
- Zhang, X. , Kainz, V. , Zhao, J. , Strassman, A. M. , & Levy, D. (2013). Vascular extracellular signal‐regulated kinase mediates migraine‐related sensitization of meningeal nociceptors. Annals of Neurology, 73, 741–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, C. , & Routh, V. H. (2018). Thioredoxin‐1 overexpression in the ventromedial nucleus of the hypothalamus preserves the counterregulatory response to hypoglycemia during type 1 diabetes in male rats. Diabetes, 67, 120–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, Q.‐G. , Zhu, L.‐J. , Chen, C. , Wu, H.‐Y. , Luo, C.‐X. , Chang, L. , & Zhu, D. Y. (2011). Hippocampal neuronal nitric oxide synthase mediates the stress‐related depressive behaviors of glucocorticoids by downregulating glucocorticoid receptor. The Journal of Neuroscience, 31, 7579 LP–7590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman, C. A. , Lin, Y.‐C. , Leib, D. E. , Guo, L. , Huey, E. L. , Daly, G. E. , … Knight, Z. A. (2016). Thirst neurons anticipate the homeostatic consequences of eating and drinking. Nature, 537, 680. [DOI] [PMC free article] [PubMed] [Google Scholar]


