Abstract
PDE type 5 inhibitors (PDE5Is), such as sildenafil, tadalafil and vardenafil, are a class of drugs used to prolong the physiological effects of NO/cGMP signalling in tissues through the inhibition of cGMP degradation. Although these agents were originally developed for the treatment of hypertension and angina, unanticipated side effects led to advances in the treatment of erectile dysfunction and, later, pulmonary arterial hypertension. In the last decade, accumulating evidence suggests that PDE5Is may confer a wider range of clinical benefits than was previously recognised. This has led to a broader interest in the cardiovascular therapeutic potential of PDE5Is, in conditions such as hypertension, myocardial infarction, stroke, peripheral arterial disease, chronic kidney disease and diabetes mellitus. Here, we review the pharmacological properties and established licensed uses of this class of drug, along with emerging therapeutic developments and possible future indications.
Abbreviations
- 6MWD
6‐min walking distance
- ACEI
ACE inhibitor
- ARB
angiotensin receptor blocker
- BPS
British Pharmacological Society
- CCB
calcium channel blocker
- CKD
chronic kidney disease
- Cmax
maximum plasma/serum concentration
- CTEPH
chronic thromboembolic pulmonary hypertension
- CVD
cardiovascular disease
- CYP2C19
cytochrome P450, family 2, subfamily C, polypeptide 19
- CYP2C9
cytochrome P450, family 2, subfamily C, polypeptide 9
- CYP2D6
cytochrome P450, family 2, subfamily D, polypeptide 6
- CYP3A
cytochrome P450, family 3, subfamily A
- CYP3A4
cytochrome P450, family 3, subfamily A, polypeptide 4
- CYP450
cytochrome P450 enzyme family
- DN
diabetic nephropathy
- ED
erectile dysfunction
- eGFR
estimated GFR
- ERA
endothelin receptor antagonist
- ET
endothelin
- ET‐1
endothelin‐1
- FGR
fetal growth restriction
- GSK3B
glycogen synthase kinase 3 β
- HbA1c
glycated Hb
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- IC50
half maximal inhibitory concentration
- IUPHAR
International Union of Basic and Clinical Pharmacology
- MI
myocardial infarction
- mitoKATP
mitochondrial ATP‐sensitive potassium channels
- NAION
nonarteritic anterior ischaemic optic neuropathy
- Na+/K+‐ATPase
sodium–potassium pump
- NOS1
neuronal NOS
- NOS2
inducible NOS
- NOS3
endothelial NOS
- PAH
pulmonary arterial hypertension
- PDE5I
PDE type 5 inhibitor
- PH
pulmonary hypertension
- PPHN
persistent pulmonary hypertension of the newborn
- RCT
randomised controlled trial
- RHTN
treatment‐resistant hypertension
- RP
Raynaud's phenomenon
- sGC
soluble GC
- SGLT2
sodium–glucose cotransporter 2
- Tmax
time taken to reach the maximum plasma concentration
- T2DM
type 2 diabetes mellitus
- UACR
urinary albumin/creatinine ratio
- V/Q
ventilation/perfusion
1. INTRODUCTION
Few other pharmaceutical agents have had a history as serendipitous as the class of PDE type 5 inhibitors (PDE5Is; Figure 1). Developed over 30 years ago, initially for cardiovascular indications, PDE5Is emerged as a revolutionary treatment for erectile dysfunction (ED), licensed in 1998 (Goldstein, Lue et al., 1998). Further investigation of their effects on pulmonary arterial pressure culminated in their approval for the treatment of pulmonary arterial hypertension (PAH) in 2005 (Galiè, Ghofrani et al., 2005). Early reports of fatal cardiovascular events in patients prescribed a PDE5I delayed wider research into their therapeutic benefits, but it soon became apparent that these adverse events reflected the complex nature of patients initially receiving the drug and their high level of cardiovascular risk, rather than the properties of the drug itself (Cheitlin, Hutter et al., 1999; Giuliano, Jackson et al., 2010). With a growing body of evidence supporting the clinically established safety profile and efficacy of these agents, PDE5Is became available as over‐the‐counter pharmacy medicines in the United Kingdom in early 2018. Now, there is renewed research interest in their therapeutic potential in conditions including cardiovascular disease (CVD), chronic kidney disease (CKD) and diabetes mellitus. This narrative literature review discusses the historical and biochemical basis for the established uses of PDE5Is and presents emerging evidence of their utility in novel indications.
FIGURE 1.
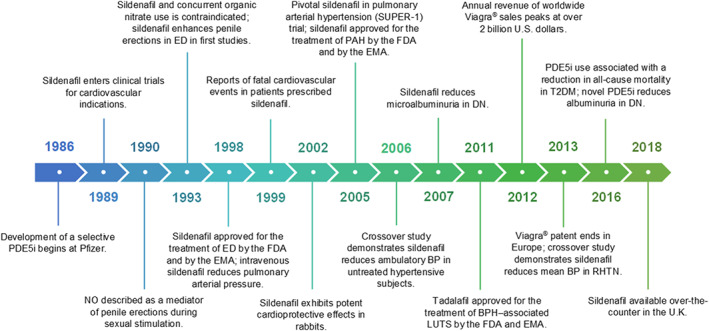
Milestones in the development of PDE type 5 inhibitors (PDE5Is). The figure depicts the key milestones in the development of PDE5Is for clinical indications, from their inception and early clinical trials, to their success in the amelioration of several cardiovascular conditions and finally their availability as an over‐the‐counter medication. BP, blood pressure; BPH, benign prostatic hyperplasia; DN, diabetic nephropathy; ED, erectile dysfunction; EMA, European Medicines Agency; FDA, U.S. Food and Drug Administration; LUTS, lower urinary tract symptoms; NO, nitric oxide; PAH, pulmonary arterial hypertension; PDE5I, PDE type 5 inhibitor; RHTN, treatment‐resistant hypertension; T2DM, type 2 diabetes mellitus
2. METHODS
We conducted a literature search of multiple electronic databases including Medline, Embase, Cochrane Library, Google Scholar and Trip medical database over the period of December 2016 to September 2019. Search terms included, but were not limited to, phosphodiesterase type 5, phosphodiesterase type 5 inhibitors, erectile dysfunction, pulmonary hypertension, lower urinary tract symptoms, cardiovascular disease, blood pressure, stroke, connective tissue disease, chronic kidney disease and diabetes mellitus. One hundred and fifty articles were included in our qualitative synthesis and are referred to in this review.
3. BIOCHEMISTRY
Before discussing the current use of PDE5Is and their new clinical indications, we briefly review the underlying cellular and molecular processes. Where relevant, endogenous ligands and drugs mentioned in the text are hyperlinked directly to The International Union of Basic and Clinical Pharmacology (IUPHAR) /British Pharmacological Society (BPS) Guide to Pharmacology (https://www.guidetopharmacology.org), an online, expert‐curated database with up‐to‐date information on drug targets and the substances that act on them (Harding, Sharman et al., 2018).
Cyclic nucleotide PDEs are a diverse family of enzymes that regulate the cellular levels of the secondary messenger molecules cAMP and cGMP by catalysing the degradation of their phosphodiester bond (Boswell‐Smith, Spina, & Page, 2006). The superfamily of PDE enzymes is classified into 11 major isozyme groups, namely, PDE1–PDE11, each with different substrate specificities: PDE5 together with PDE6 and PDE9 are selective cGMP hydrolases, whereas other PDEs are cAMP‐selective (PDE4, PDE7 and PDE8) or have mixed specificity (PDE1, PDE2, PDE3, PDE10 and PDE11; Boswell‐Smith, Spina, & Page, 2006). Although PDE5 is abundantly distributed in vascular smooth muscle, PDE5 mRNA and isoform expression has also been identified in various human organs, including brain, lung, heart, liver, kidney, bladder, prostate, urethra, penis, uterus and skeletal muscle, where it performs numerous physiological functions mediated by the NO–cGMP pathway (Figure 2; Boswell‐Smith, Spina, & Page, 2006). Due to its widespread tissue distribution and functions, PDE5 has been established as an attractive target for pharmacological intervention to prolong or enhance the effects of processes mediated by cGMP by inhibiting its degradation (Boswell‐Smith, Spina, & Page, 2006).
FIGURE 2.
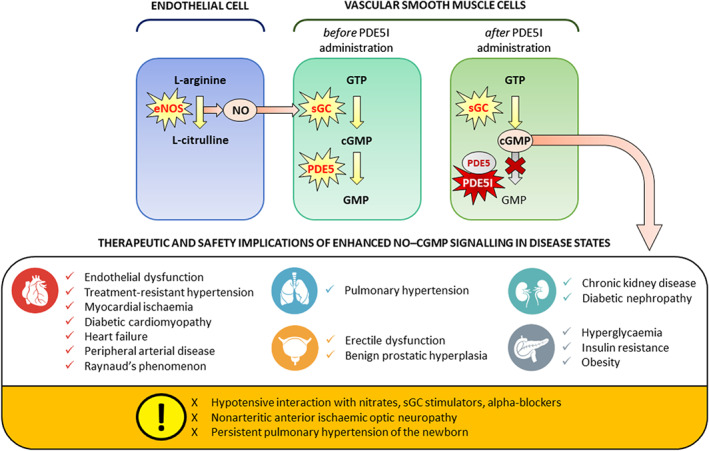
The NO–cGMP signalling pathway in the vasculature. The figure shows the generator cells, for example, vascular endothelial cells, the target cells, for example, vascular smooth muscle cells and biochemical processes involved in NO signalling in the vasculature. NO is produced through the conversion of the substrate l‐arginine to l‐citrulline by endothelial NO synthase (NOS3) in vascular endothelial cells. Subsequently, NO diffuses to neighbouring vascular smooth muscle cells where it activates soluble GC (sGC), which converts guanosine triphosphate (GTP) to cGMP. cGMP is a secondary messenger enacting cellular processes through the regulation of protein‐dependent kinases, for example, PKG, and cGMP‐gated ion channels. The cellular actions of cGMP can be prolonged by PDE type 5 inhibitors (PDE5Is), which prevent its degradation by PDE type 5 (PDE5), resulting in vasodilatory, antioxidative and anti‐proliferative effects in several organ systems and diseases. Icons with permission from © iStock.com/Alex Doubovitsky
cAMP and cGMP play key roles in cell signalling through the activation of intracellular protein kinases which modulate several metabolic processes such as ion conductance, cellular apoptosis and glycogenolysis. In smooth muscle cells, for example, the binding of cGMP to the cGMP‐dependent PKG causes a reduction in intracellular calcium concentrations and, subsequently, relaxation of the smooth muscle layer and vasodilatation (Boswell‐Smith, Spina, & Page, 2006). cGMP is synthesised from GTP by soluble GC (sGC), a heterodimeric enzyme which is itself activated by NO. NO is a cellular signalling molecule implicated in a wide range of regulatory functions, including control of vascular tone, platelet aggregation and cell communication, as well as in the immunological response to infection, chronic inflammation and tumours (Ignarro, 2019). NO is synthesised endogenously from the oxidation of the amino acid l ‐arginine to l ‐citrulline under the effect of NOS, of which there are three isoforms, neuronal NOS (NOS1), inducible NOS (NOS2) and endothelial NOS (NOS3), but can also be derived from the reduction of exogenously administered nitrates (Stuehr & Haque, 2019).
Vascular smooth muscle relaxation is also mediated by the effects of the prostacyclin pathway, whereby arachidonic acid is cleaved by cyclooxygenase to synthesise prostacyclin, a lipid molecule with potent vasodilatory, anti‐proliferative and anti‐platelet effects mediated through interactions with cell surface and nuclear receptors, which ultimately result in increased cAMP production and PKA activation (Mitchell & Kirkby, 2019). Local vascular tone is regulated by an interaction between these relaxing processes and the effects of constrictor actions, including the endothelin (ET) pathway, primarily mediated by secretion of the potent vasoconstrictor endothelin‐1 (ET‐1) from vascular endothelial cells. Cumulative evidence suggests that the ET pathway opposes the NO–cGMP pathway and contributes to the pathogenesis of several cardiovascular and inflammatory diseases. Pharmacological manipulation of this system via inhibition of ET biosynthesis or direct antagonism of ET receptors has generated significant experimental interest and potential therapeutic applications (Dhaun & Webb, 2019).
The vasodilatory properties of the NO–cGMP pathway have been successfully exploited for the management of ischaemic heart disease, whereby exogenous nitrate administration in the form of nitroglycerin reduces peripheral vascular resistance and cardiac preload to improve myocardial perfusion. However, despite its potent anti‐anginal effect, such practice is limited by the rapid development of nitrate tolerance with sustained exogenous administration. In an attempt to circumvent this issue, research interest in the mid‐1980s shifted to the modulation of intracellular cGMP levels and its downstream effectors (Beavo & Reifsnyder, 1990).
4. DEVELOPMENT OF PDE TYPE 5 INHIBITORS
In 1986, Pfizer began preclinical work on the development of a selective PDE5‐inhibitor (PDE5I) to compete with cGMP for access to the PDE5 catalytic site. It was proposed that such an agent could prolong the effects of NO–cGMP signalling in vascular tissue and thus ameliorate conditions associated with NO deficiency, such as angina pectoris. In preclinical studies, the resulting agent — later named sildenafil citrate — demonstrated potent vasodilatory effects and was selected for further development (Terrett, Bell et al., 1996). In 1991, sildenafil entered clinical trials for use in cardiovascular indications. In single‐dose trials, the agent produced reductions in systemic BP in healthy volunteers, as well as modest vasodilatory and anti‐platelet effects when administered intravenously to patients with ischaemic heart disease (Boswell‐Smith, Spina, & Page, 2006; Jackson, Benjamin et al., 1999). Despite these initial promising results, research on the cardiovascular benefits of PDE5I therapy was complicated by the short plasma half‐life of sildenafil of around 4 hr, which would necessitate frequent dosing for the chronic treatment of angina. Development was further compounded by the contraindication of concurrent nitrate use within 24 hr of PDE5I ingestion (48 hr for tadalafil) due to the risk of a significant synergistic vasodilatory interaction between the agents on the systemic vasculature, leading to dangerous levels of hypotension in recipients (Webb, Freestone et al., 1999).
5. THERAPEUTIC APPLICATION OF PDE TYPE 5 INHIBITORS
5.1. Erectile dysfunction (ED)
As multiple‐dose Phase I trials of sildenafil for the treatment of angina continued, healthy volunteers began reporting an unexpected and, in hindsight, fortuitous side effect: penile erections. ED, defined as the inability to attain or maintain a penile erection sufficient for successful vaginal intercourse (Lue, 2000), had, until the 1990s, been a clinically neglected issue, despite its global burden. At the time, therapeutic options for ED were limited to experimental intracavernosal injections of prostacyclin analogues or unlicensed antidepressants and herbal remedies, which had limited effectiveness and potential harms (Goldstein, Lue et al., 1998). A breakthrough in our understanding of this condition came in 1990, when NO was identified as a key neurotransmitter facilitating penile erections. Its release into the penile vasculature at high levels from the endothelium and parasympathetic cavernous nerve terminals during sexual stimulation led to cGMP‐mediated relaxation of corpus cavernosal smooth muscle with subsequent vasocongestion, occlusion of local venous return, and a sustained erection (Ignarro, Bush et al., 1990). By 1993, the future of sildenafil as a treatment for angina looked increasingly uncertain. However, the occurrence of penile erections, linked with a clear mechanistic underpinning, provided the stimulus for the first clinical trials of PDE5Is for the treatment of ED.
The results from the first clinical study investigating PDE5I treatment in ED were unequivocally positive: Through the inhibition of cGMP degradation in the cavernous smooth muscles, sildenafil enhanced and prolonged the vasodilatory response of cGMP within the corpora cavernosa during sexual stimulation in a dose‐dependent manner to facilitate penile erection, without any safety concerns (Boolell, Allen et al., 1996). Later trials would confirm the high response rates, with the benefits of sildenafil treatment extending to ED associated with CVD, neurological disease, diabetes mellitus and radical prostatectomy (Lue, 2000).
In March 1998, sildenafil citrate (Viagra®; Pfizer; New York City, New York, USA) was approved for use in both the United States and the European Union as the first‐line treatment for ED secondary to a broad variety of physical causes (Goldstein, Lue et al., 1998; Ignarro, 2019); within 8 months, more than six million prescriptions of sildenafil were written in an unparalleled maelstrom of publicity (Lue, 2000). The testing and approval of other PDE5Is for the treatment of ED soon followed, culminating in 2003 with U.S. Food and Drug Administration and European Medicines Agency approval of tadalafil (Cialis®; Eli Lilly and Company; Indianapolis, Indiana, USA), the longest acting PDE5I,and vardenafil (Levitra®; Bayer; West Haven, Connecticut, USA), the first PDE5I to be available in both film‐coated and oro‐dispersible tablet forms to facilitate adherence in difficult patient groups. This was followed in 2012 by the introduction of avanafil (Stendra®; Vivus, Incorporated; Mountain View, California, USA), a more PDE5‐selective agent with favourable tolerability. Alternative PDE5Is have also been licensed in some countries: udenafil is a rapid‐onset but long‐acting agent available in South Korea, Russia and the Philippines (Paick, Kim et al., 2008); mirodenafil is a short‐acting PDE5I which has been extensively studied in ED but is currently only marketed in South Korea (Paick, Ahn et al., 2008); lodenafil is another short‐acting agent, which is only available in Brazil (Glina, Toscano et al., 2009). The clinical implications of these pharmacokinetic and pharmacodynamic variations in PDE5Is are explored further in the pharmacology and safety section.
5.2. Pulmonary hypertension
The demonstration of safe, consistent vasodilatation by sildenafil during treatment for ED generated renewed interest in the potential effects of PDE5 inhibition in other vascular beds. Pulmonary hypertension (PH) is a disorder characterised by a progressive increase in pulmonary vascular resistance resulting in right heart failure. Five subclasses of PH have been defined (as per the classification of the 6th World Symposium on Pulmonary Hypertension Task Force), among which Group 1 represents PAH, a term which encompasses a number of heterogeneous conditions sharing common pathophysiological, histological and prognostic features (Simonneau, Montani et al., 2019). Prior to 2005, treatment options for PAH included prostacyclin and prostacyclin analogue therapy, and the non‐selective ET receptor antagonist (ERA) bosentan. Although these drugs were successful in improving prognosis and exercise capacity in individuals with PAH, they were limited by concerns over safety, tolerability and ease of delivery (Simonneau, Montani et al., 2019). However, it should be noted that these issues have since been addressed following the development of novel ERAs. Both ambrisentan, an ET‐A selective agent, and macitentan, a dual ERA with a long half‐life and sustained receptor binding, have demonstrated favourable efficacy and tolerability profiles in the treatment of PAH (Galiè, Olschewski et al., 2008; Pulido, Adzerikho et al., 2013).
Local NO production regulates ventilation/perfusion (V/Q) matching as its production is up‐regulated in response to alveolar distension, leading to redirection of blood flow to well‐ventilated areas of the lung (Ghofrani, Pepke‐Zaba et al., 2004). PDE5 is highly expressed in healthy lung tissue (Corbin, Beasley et al., 2005) and up‐regulation of PDE5 gene expression was first observed in the lungs of rats with PH (Sanchez, De La Monte et al., 1998). Moreover, the weak and relatively non‐selective PDE5Is zaprinast and dipyridamole improved pulmonary pressure in models of PH (Ichinose, Adrie et al., 1998; Ziegler, Ivy et al., 1998). These factors, combined with the prospect of a well‐tolerated oral agent to enhance NO/cGMP‐mediated vasodilatation specifically in areas of poor perfusion through the augmentation of V/Q matching, made PDE5 an ideal target for pharmacological intervention.
The first placebo‐controlled study investigating the effect of intravenous sildenafil in PH patients showed significant reductions in pulmonary pressure and pulmonary vascular resistance (Ghofrani, Osterloh, & Grimminger, 2006). Following several case reports and clinical trials confirming the anti‐pulmonary hypertensive effects of sildenafil (Ghofrani, Osterloh, & Grimminger, 2006), this growing body of evidence culminated in the pivotal Sildenafil Use in Pulmonary HypERtension (SUPER‐1) study in 2005: a large, multinational, randomised controlled trial (RCT) which demonstrated improvements in both functional and haemodynamic outcomes from baseline to week 12 following three times daily sildenafil administration (Galiè, Ghofrani et al., 2005). In a long‐term extension study, improvements were maintained after 3 years of therapy (Rubin, Badesch et al., 2011). Oral sildenafil (Revatio®; Pfizer) was approved for the treatment of PAH in both the United States and the European Union in 2005, followed by tadalafil (Adcirca®; Eli Lilly and Company) in 2009.
There have now been several RCTs demonstrating the clear benefits of sildenafil, tadalafil, and vardenafil in PAH, despite a lack of comparative therapeutic data between the agents and the fact that vardenafil is not currently licensed for treatment. A recent Cochrane systematic review and meta‐analysis of 36 studies involving 2,999 patients concluded that patients receiving PDE5Is were more likely to significantly improve their WHO functional class and 6‐min walking distance (6MWD)—a measure of exercise capacity—and were less likely to die, despite an increase in the incidence of side effects such as headache, flushing, and myalgia (Barnes, Brown et al., 2019). Promisingly, these benefits are seen across the PH Group 1 subclass regardless of PAH aetiology, whether idiopathic or associated with connective tissue disease, congenital heart disease, or infection with human immunodeficiency virus (Barnes, Brown et al., 2019). Recently, the AMBITION study demonstrated the rationale for early intervention and targeting multiple pathways in PAH by showing a significant mortality benefit in treatment‐naïve PAH patients receiving long‐term tadalafil–ambrisentan combination therapy compared to monotherapy with either agent (Galiè, Barberà et al., 2015). This evidence of PDE5I–ERA synergy has been adopted in current European guidelines, which favour the use of PDE5I or ERA monotherapy for low‐risk patients (WHO functional class I–III), escalating to the use of combination therapy or intravenous prostacyclin for those at high risk (WHO functional class IV; Hoeper, McLaughlin et al., 2016).
However, existing evidence is insufficient to establish whether pulmonary vasodilatory therapies are safe and effective in other PH subclasses (Hoeper, McLaughlin et al., 2016). Although there is mixed evidence to support PDE5I therapy in PH secondary to left‐heart disease (Group 2; Hutchings, Anderson et al., 2018), there is no clear benefit for use of the agents in PH due to lung disease and/or hypoxia (Group 3) or thrombotic obstruction (Group 4; Barnes, Brown et al., 2019). Indeed, the results seem conflicting even within the same subclass. In PH Group 5 (secondary to systemic or haematological disease), tadalafil significantly improved haemodynamic parameters in β‐thalassaemia intermedia with PH, whereas a trial of sildenafil in PH secondary to sickle cell disease was terminated as a result of a significant increase in clinical worsening and hospitalisation (Barnes, Brown et al., 2019). Similarly, PDE5Is may be harmful in valvular heart disease, a subtype of PH Group 2 (Barnes, Brown et al., 2019). Therefore, whilst PDE5Is have demonstrated significant disease‐modifying properties in PH Group 1 (PAH), the limited and heterogenous nature of trials in other PH subclasses prevents conclusive interpretation of their risk–benefit profile, thus prohibiting their recommendation for the treatment of PH Groups 2 to 5.
Interestingly, therapeutic NO–cGMP intervention with the sGC stimulator riociguat has shown promise in the treatment of chronic thromboembolic PH (CTEPH), the main Group 4 subtype, by reducing pulmonary artery pressure and improving WHO functional class and 6MWD. Although evidence of mortality benefit is lacking, riociguat is currently recommended as a first‐line therapy in the treatment of CTEPH patients who are unfit for pulmonary endarterectomy (Barnes, Brown et al., 2019; Wardle, Seager et al., 2016). Future research into the therapeutic potential of NO–cGMP intervention in PH Groups 2 to 5 should be sufficiently powered and incorporate long‐term follow‐up of clinical, haemodynamic and mortality parameters. This will be aided by further elucidation of the mechanisms of left‐heart disease PH, borderline mean pulmonary arterial pressure increases and mixed clinical phenotypes.
6. EMERGING THERAPEUTIC USES OF PDE TYPE 5 INHIBITORS
6.1. Cardiovascular disease
CVD now accounts for one third of global mortality—over 90% of these deaths are attributable to three conditions: ischaemic heart disease, stroke and hypertensive heart disease (Joseph, Leong et al., 2017). Research into the cardiovascular benefits of PDE5Is was deferred for several years due to early reports of adverse cardiovascular events in patients prescribed sildenafil (Cheitlin, Hutter et al., 1999). In hindsight, however, this reflected the nature of the patients receiving the drug and long‐term evidence has since established the safety of PDE5Is in both health and CVD (Giuliano, Jackson et al., 2010). Moreover, the demonstration of PDE5 expression in human heart tissue has renewed interest in the use of PDE5Is in this field (Pokreisz, Vandenwijngaert et al., 2009).
6.1.1. Endothelial function
The vascular endothelium, a continuous monolayer of cells lining the entire cardiovascular system, has a key role in a wide range of physiological processes, including regulation of vascular tone and response to inflammation. Endothelial functions are partially mediated by NO–cGMP signalling, deficiency of which is a key feature of endothelial dysfunction (Napoli & Ignarro, 2009), a pro‐thrombotic and pre‐atherosclerotic state which underlies several diseases and is an important adverse modifier of CVD risk and clinical outcomes (Brunner, Cockcroft et al., 2005). As highlighted in a recent review (Schwartz, Jackson et al., 2013), both single‐use and long‐term administration of PDE5Is in patients with high cardiovascular risk have been shown to ameliorate endothelial function parameters, reduce plasma concentrations of ET‐1 and endothelial inflammatory mediators and increase levels of circulating endothelial progenitor cells. Promisingly, several studies have indicated that PDE5I‐mediated improvements in endothelial function may be sustained for weeks to months after discontinuation of long‐term treatment (McLaughlin, Lytvyn et al., 2014), signifying that, in addition to their acute haemodynamic effects, the agents may produce fundamental modifications at a cellular level, which may explain the therapeutic effects of PDE5Is in CVD, CKD and diabetes mellitus, as discussed below. Moreover, limited clinical data suggest that sildenafil provides sustained pharmacological preconditioning of human endothelium which may protect against the adverse effects of ischaemia–reperfusion on vascular function (McLaughlin, Lytvyn et al., 2014).
6.1.2. BP homeostasis
Hypertension is the most important global risk factor for CVD (Olsen, Angell et al., 2016). NO–cGMP signalling is central to the regulation of BP homeostasis in hypertension through its effects on vascular tone, the sympathetic nervous system, and renal salt and water handling (Mergia & Stegbauer, 2016). The vasodilatory effects of PDE5Is provide a rationale for use in systemic arterial hypertension: Acutely, single doses of PDE5Is result in transient reductions of both peripheral and aortic BP, although some variation in effect exists between health and hypertension and between different PDE5Is (Jackson, Benjamin et al., 1999; Kloner, Mitchell, & Emmick, 2003; Mahmud, Hennessy, & Feely, 2001; Pomara, Morelli et al., 2004). In a landmark study, regular sildenafil monotherapy for 16 days significantly reduced ambulatory BP by around 6 mmHg in patients with untreated hypertension compared to placebo (Oliver, Melville, & Webb, 2006). These results were further supported by the demonstration of significant ambulatory BP reductions in hypertensive patients of around 6 mmHg following 28 days of monotherapy with PF‐00489791, a highly specific and long‐acting PDE5I, compared to placebo (Wolk, Smith et al., 2009). In both studies, PDE5I use was safe and well‐tolerated.
Alternative uses for PDE5Is are also being explored in the field of treatment‐resistant hypertension (RHTN). Several studies have demonstrated the safety of PDE5I use in combination with other antihypertensive therapies (Kloner, Brown et al., 2001; Kloner, Mitchell et al., 2003; Nehra, 2009; Pickering, Shepherd et al., 2004). Concomitant administration of organic nitrates and PDE5Is is currently contraindicated due to the acute risk of hypotension (Webb, Freestone et al., 1999), although more recent studies have suggested that the interaction between the two agents is confined to within 6 hr post‐sildenafil administration (Oliver, Kerr, & Webb, 2009; Parker, Bart et al., 2007). Nevertheless, the synergistic hypotensive interaction occurring with concomitant PDE5I and nitrate administration may yet be exploited therapeutically, as demonstrated in a proof of concept study in which sildenafil and isosorbide mononitrate combination therapy in RHTN patients produced an additional BP reduction superior to the two agents individually, suggesting a novel approach for the treatment of RHTN (Oliver, Dear, & Webb, 2010). Recently, a crossover trial showed that acute administration of sildenafil reduced mean BP and improved diastolic dysfunction parameters in patients with RHTN by facilitating a reduction in total peripheral resistance (Quinaglia, de Faria et al., 2013). Notably, exploratory analysis revealed that NOS3 polymorphism between subjects may have played a role in modulating the sildenafil response. PDE5 activation has also been implicated in angiotensin II‐dependent hypertension (Ramseyer, Ortiz et al., 2016) and in experimental models, PDE5 inhibition has been demonstrated to reduce angiotensin II levels and restore the baroreflex in renovascular hypertension (Cavalcanti, Alves et al., 2016; Dias, Rodrigues et al., 2014).
6.1.3. Cardioprotection
The NO–cGMP signalling pathway has been identified as a survival signal in ischaemic heart disease (Burley, Ferdinandy, & Baxter, 2007), facilitating its essential cardioprotective effects via PKG‐dependent phosphorylation of ERK and GSK3B, increased generation of NO by NOS2/3, activation of PKC and opening of mitochondrial ATP‐sensitive potassium channels (mitoKATP), which stabilise the membrane potential to allow ATP synthesis and calcium ion transport (Inserte & Garcia‐Dorado, 2015). Preclinical studies have successfully shown that PDE5Is can enhance this pathway in ischaemia–reperfusion injury and myocardial infarction (MI) by increasing levels of inducible and NOS3 activity in the heart (Das, Xi, & Kukreja, 2005), augmenting mitoKATP opening via PKG‐dependent mechanisms (Ockaili, Salloum et al., 2002) and delaying normalisation of intracellular acidosis (Inserte, Barba et al., 2011). These effects are independent of vasoactive properties, mediated instead through direct myocardial anti‐inflammatory and anti‐apoptotic processes (Das, Xi, & Kukreja, 2005; Salloum, Abbate et al., 2008). These mechanisms of post‐ischaemic protection have been linked to significant reductions in infarct size and cardiomyocyte death in animals, regardless of the timing of PDE5I administration, whether given minutes to weeks in advance of the ischaemic insult (preconditioning; Jamnicki‐Abegg, Weihrauch et al., 2007; Ockaili, Salloum et al., 2002; Salloum, Abbate et al., 2008; Wang, Fisher et al., 2008), or indeed immediately prior to or during coronary reperfusion (postconditioning; Ebner, Ebner et al., 2013; Madhani, Hall et al., 2010; Salloum, Takenoshita et al., 2007). However, there is no experimental evidence on the administration of PDE5I during reperfusion following more than half an hour of ischaemia, which may more accurately reflect clinical practice, associated with delayed patient presentation and longer inter‐hospital transfer times.
Similarly, the rationale for PDE5I‐mediated augmentation of the cGMP–PKG pathway in select cases of MI and heart failure has been fully appraised in a recent review (Hutchings, Anderson et al., 2018). The authors conclude that PDE5Is may ameliorate the clinical condition of patients with heart failure with reduced ejection fraction (HFrEF) through improvements in the pulmonary circulation, cardiac remodelling and diastolic function. In heart failure with preserved ejection fraction (HFpEF), however, these beneficial observations are largely offset by a reduction in cardiac contractility. Therefore, PDE5I use in heart failure may be limited to HFrEF and HPpEF with associated PH or right ventricular systolic impairment, although the underlying signalling pathways for this variation in effect are not well understood. In future studies, careful patient stratification by phenotype is crucial. The cardioprotective effects of PDE5Is are also being investigated in chemotherapy‐induced cardiotoxicity, where adjunctive treatment has been shown to reduce apoptosis and improve left ventricular function without interfering with the chemotherapeutic benefits of doxorubicin (Fisher, Salloum et al., 2005; Koka, Das et al., 2010).
6.1.4. CNS effects
The NO–cGMP signalling pathway is involved also in several physiological and pathological cerebral functions. PDE5 is widely expressed in the CNS in neural and vascular tissue (Zhang, Zhang, & Chopp, 2013), and PDE5Is have been shown to cross the blood–brain barrier to directly affect brain tissue (García‐Barroso, Ricobaraza et al., 2013; Gómez‐Vallejo, Ugarte et al., 2016), perhaps by enhancing angiogenesis and neurogenesis (Zhang, Zhang, & Chopp, 2013), highlighting the potential for therapeutic intervention in both ischaemic stroke and neurodegenerative disorders. However, the few studies investigating these effects in humans are limited by their focus on cerebral blood flow (Pauls, Moynihan et al., 2018), which is not a key surrogate for brain function. Moreover, their findings are inconclusive, and there is no attempt to correlate changes in blood flow to improvements in cognitive function. Therefore, the potential relevance of PDE5I therapy to cerebrovascular disease remains uncertain. Nevertheless, recent developments in our understanding of the neurophysiological roles of NO–cGMP signalling, reviewed elsewhere (Garthwaite, 2019), may yield novel interventional opportunities in stroke and other neurological disorders.
6.1.5. Peripheral circulation
Reports of improvements in walking distance in patients with both limb and buttock claudication following sildenafil therapy support the potential for PDE5I therapy to benefit patients with peripheral arterial disease (Omarjee, Le Pabic et al., 2019). In a similar fashion to the angiogenic potential of PDE5Is demonstrated in experimental stroke models, preclinical studies have demonstrated improvements in vascular perfusion, tissue blood flow, and vascular density with sildenafil (Senthilkumar, Smith et al., 2007) and vardenafil (Sahara, Sata et al., 2010) in a mouse model of unilateral hindlimb ischaemia. Recently, a prospective crossover study of 12 patients reported a significant improvement in maximal walking time with a single oral dose of sildenafil, although this did not impact pain‐free walking time or skin tissue oxygenation during exercise (Omarjee, Le Pabic et al., 2019). Further prospective studies investigating the impact of PDE5I treatment on clinical outcomes are ongoing (Table 3).
TABLE 3.
Ongoing interventional clinical trials of PDE5Is beyond ED and PH
| Drug(s) tested | Sponsor | Target group | Stage of development (status; clinical identifier) | Results of clinical trial/reason for termination |
|---|---|---|---|---|
| Hypertension | ||||
| Sildenafil | University of Edinburgh, UK; Pfizer, USA | Primary hypertension | Phase IV (completed 2005; NCT00479908) | Significant ambulatory BP reduction compared to placebo (Oliver, Melville, & Webb, 2006). |
| PF‐00489791 | Pfizer, USA | Primary hypertension | Phase II (completed 2008; NCT00422461) | Significant ambulatory BP reduction compared to placebo (Wolk, Smith et al., 2009). |
| Sildenafil | University of Campinas, Brazil | RHTN | Not applicable (completed 2012; NCT01392638) | Significant improvement in diastolic function and BP compared to placebo (Quinaglia, de Faria et al., 2013). |
| Tadalafil | University of Campinas, Brazil | Left ventricular function in RHTN | Not applicable (completed 2012; NCT01743911) | Significant improvement in diastolic function compared to placebo. No significant change in BP (Santos, de Faria et al., 2014). |
| Cardiac | ||||
| Tadalafil | New England Research Institutes; NHLBI; Massachusetts General Hospital, USA | HFrEF | Phase III (terminated 2015; NCT01910389) | Terminated by funding agency. |
| Udenafil | Seoul National University Hospital; Dong‐A Pharm, South Korea | HFrEF | Phase III (terminated 2014; NCT01646515) | Substantial benefit was observed in the active treatment group. |
| Sildenafil | Massachusetts General Hospital, USA | HFrEF | Phase III (completed 2006; NCT00309790) | Improved cardiac indices (Malhotra, Dhakal et al., 2016). |
| Sildenafil | University of Milan, Italy | HFpEF | Phase II/III (completed 2009; NCT00781508 and NCT01156636) | Improved cardiac indices and clinical status (Guazzi, Vicenzi et al., 2011). |
| Sildenafil | University of Milan, Italy | HFrEF | Phase II (completed 2010; NCT01185925) | Not reported. |
| Vardenafil | McGuire Research Institute, USA | IRI after cardiac surgery | Phase I (completed 2011; NCT01260285) | Not reported. |
| Sildenafil | Duke University; NHLBI; Pfizer, USA | HFpEF | Phase III (completed 2012; NCT00763867) | No effect on exercise capacity or clinical status (Redfield, Chen et al., 2013). |
| Sildenafil | Rigshospitalet, Denmark | HFpEF after MI | Phase IV (completed 2012; NCT01046838) | No improvement in filling pressure. Significant improvements in cardiac indices, exercise capacity and diastolic BP (Andersen, Ersbøll et al., 2013). |
| Udenafil | Seoul National University Hospital; Dong‐A Pharm, South Korea | HFpEF | Phase III (completed 2013; NCT01599117) | Not reported. |
| Sildenafil | Hospital Ana Nery, Brazil | Unspecified HF | Phase II/III (completed 2013; NCT01936350) | No effect on cardiac indices (Fernandes, Andrade et al., 2015). |
| Tadalafil ± Nesiritide | Mayo Clinic; NCRR; NHLBI, USA | Preclinical HF, expected renal function improvement | Phase I/II (completed 2014; NCT01544998) | Not reported. |
| Sildenafil | University Medical Center Groningen, Netherlands; Pfizer, USA | HFpEF | Phase III (completed 2014; NCT01726049) | No effect on cardiac indices or clinical status (Liu, Hummel et al., 2017). |
| Sildenafil | Pfizer, USA | HFrEF | Phase III (ongoing; NCT01616381 and NCT03460470) | Ongoing. |
| Udenafil | Mezzion Pharma Co. Ltd, South Korea; NHLBI, USA | Congenital heart defects | Phase III (ongoing; NCT02741115) | Ongoing. |
| Sildenafil | University of Calgary, Canada; Pfizer, USA | RHF | Phase III (ongoing; NCT03356353) | Ongoing. |
| Stroke | ||||
| PF‐03049423 | Pfizer, USA | Acute ischaemic stroke | Phase II (terminated 2016; NCT01208233) | Interim analysis demonstrated futility. No reported safety concerns (Di Cesare, Mancuso et al., 2016). |
| Sildenafil | University of Utah, USA | Subacute ischaemic stroke | Phase I (terminated 2017; NCT02628847) | Recruitment failure. |
| Sildenafil | Henry Ford Hospital, USA | Subacute ischaemic stroke | Phase I (terminated 2011; NCT00452582) | Recruitment failure. |
| Tadalafil | Herlev Hospital, Denmark | Cerebral small vessel disease | Phase II (completed 2017; NCT02801032) | Not reported. |
| Sildenafil | University of Oxford, UK | Cerebral small vessel disease | Phase II (ongoing; NCT03855332) | Ongoing. |
| Peripheral circulation | ||||
| Vardenafil | University of Cologne, Germany | Mixed RP | Phase II/III (completed 2010; NCT01291199) | Significant improvement in digital blood flow and clinical symptoms compared to placebo (Caglayan, Axmann et al., 2012). |
| Tadalafil | Sanjay Gandhi Postgraduate Institute of Medical Sciences, India | Treatment‐resistant secondary RP | Phase III (completed 2010; NCT00626665 and NCT01117298) | Significant improvement in clinical symptoms, quality of life, digital ulcer frequency, and healing with add‐on PDE5I therapy compared to placebo (Shenoy, Kumar et al., 2010). |
| PF‐00489791 | Pfizer, USA | Mixed RP | Phase II (completed 2011; NCT01090492) | Not reported. Trials discontinued. |
| Udenafil | Seoul National University Hospital, South Korea | Secondary RP | Phase II/III (completed 2011; NCT01280266) | Significant improvement in digital blood flow compared to amlodipine. Similar efficacy in reducing RP attacks (Lee, Park et al., 2013). |
| Sildenafil | Lille University Hospital, France | Secondary RP | Phase III (completed 2013; NCT01295736) | Frequent sildenafil use for 12 weeks significantly improved healing of digital ulcers secondary to systemic sclerosis compared to placebo (Hachulla, Hatron et al., 2016). |
| Tadalafil | Northwestern University; Eli Lilly and Company, USA | Secondary RP | Not applicable (completed 2014; NCT00822354) | Not reported. |
| Sildenafil | Federal University of São Paulo, Brazil | Secondary RP | Phase III (completed 2016; NCT01347008) | Significant improvement in digital blood flow and symptoms after 8 weeks of treatment compared to placebo (Andrigueti, Ebbing et al., 2017). |
| Sildenafil | Grenoble University Hospital, France; Pfizer, USA | Mixed RP | Phase II/III (completed 2016; NCT02050360) | Non‐significant improvement in clinical symptoms compared to placebo, with substantial heterogeneity in patient response (Roustit, Giai et al., 2018). |
| Sildenafil (topical) | Pontifical Catholic University of Chile, Chile | Secondary RP | Phase I (completed 2016; NCT03027674) | Significant improvement in digital blood flow compared to topical nifedipine cream (Wortsman, Del Barrio‐Díaz et al., 2018). |
| Sildenafil | Angers University Hospital, France | PAD | Phase III (completed 2017; NCT02832570) | Significant improvement in maximal walking time but no significant effects on pain‐free walking time and oxygenation parameters (Omarjee, Le Pabic et al., 2019). |
| Sildenafil | Angers University Hospital, France | PAD | Phase II/III (not yet recruiting; NCT02387450) | Not yet recruiting. |
| Sildenafil | Rennes University Hospital, France | PAD | Phase III (ongoing; NCT03686306) | Ongoing. |
| Chronic kidney disease | ||||
| Sildenafil | University of Guadalajara, Mexico | Diabetic nephropathy | Not applicable. | Significant reduction in microalbuminuria compared to placebo (Grover‐Páez, Rivera et al., 2007). |
| PF‐00489791 | Pfizer, USA | Diabetic nephropathy | Phase II (completed 2010; NCT01200394) | Significant reduction in macroalbuminuria compared to placebo (Scheele, Diamond et al., 2016). |
| Mirodenafil | Asan Medical Center; SK Chemicals, South Korea | CKD | Phase I (completed 2011; NCT01232010) | Not reported. |
| Sildenafil | University of Alabama at Birmingham, USA | Mixed CKD requiring arteriovenous fistula surgery | Not applicable (completed 2018; NCT02414204) | Not reported. |
| Tadalafil | New England Research Institutes; NHLBI; Massachusetts General Hospital, USA | CKD and AKI in HF | Phase III (completed 2018; NCT01960153) | Not reported. |
| Diabetes mellitus and metabolic syndrome | ||||
| Tadalafil | Sahlgrenska University Hospital, Sweden | Hyperglycaemia | Phase I (terminated 2015; NCT01238224) | Durability of study medications could not be guaranteed after the expiry date. |
| Sildenafil | University of Roma La Sapienza, Italy | Diabetic cardiomyopathy | Phase IV (completed 2009; NCT00692237) | Significant improvement in cardiac kinetic and metabolic indices compared to placebo (Giannetta, Isidori et al., 2012). |
| Sildenafil | Vanderbilt University; NHLBI; Pfizer, USA | Metabolic syndrome | Phase I/II (completed 2013; NCT01334554) | Not reported. |
| Tadalafil | Massachusetts General Hospital, USA | Metabolic syndrome | Phase III (completed 2013; NCT01444651) | No improvement in insulin resistance compared to placebo. Potential favourable effects on β‐cell compensation (Ho, Arora et al., 2014). |
| Vardenafil | Modena Local Health Unit, Italy | Endothelial dysfunction in T2DM | Phase II (completed 2014; NCT02219646) | Significant improvement in endothelial parameters and hypogonadism in men compared with placebo (Santi, Granata et al., 2016). |
| Tadalafil | University of Roma La Sapienza, Italy | Metabolic syndrome | Phase IV (completed 2015; NCT02554045) | Not reported. |
| Tadalafil | University of Guadalajara, Mexico | Metabolic syndrome | Phase IV (completed 2015; NCT02595684) | Not reported. |
| Sildenafil | Vanderbilt University, USA | Hyperglycaemia | Phase IV (completed 2016; NCT01409993 and NCT02129725) | Significant improvement in insulin sensitivity, endothelial parameters, and urine albuminuria after 3 months of PDE5I therapy. Sub‐study investigating insulin signalling in skeletal muscle not yet reported (Ramirez, Nian et al., 2015). |
| Sildenafil + leucine ± metformin | NuSirt Biopharma, USA | Metabolic syndrome | Phase II (completed 2018; NCT03364335) | Not reported. |
| Tadalafil | University of Roma La Sapienza, Italy | Diabetic cardiomyopathy | Phase IV (completed 2019; NCT01803828) | Not reported. |
| Tadalafil | Göteborg University, Sweden | Insulin resistance in T2DM | Phase II (ongoing; NCT02601989) | Ongoing. |
| Tadalafil | University Center for Health Science, Mexico | Metabolic syndrome | Phase III (ongoing; NCT03905018) | Ongoing. |
Note. Mode of drug administration is oral unless otherwise specified.
Abbreviations: AKI, acute kidney injury; CKD, chronic kidney disease; ED, erectile dysfunction; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; IRI, ischaemia reperfusion injury; MI, myocardial infarction; NCRR, National Center for Research Resources; NHLBI, National Heart, Lung, and Blood Institute; PAD, peripheral arterial disease; PDE5I, PDE type 5 inhibitor; PH, pulmonary hypertension; RHF, right heart failure; RHTN, treatment‐resistant hypertension; RP, Raynaud's phenomenon; T2DM, type 2 diabetes mellitus.
The vasodilatory and endothelial effects of PDE5Is have also shown promise in the treatment of Raynaud's phenomenon (RP). RP is a condition characterised by episodic cold‐ or stress‐induced ischaemic vasospastic episodes affecting the digital arteries and arterioles leading to ulcerations and necrosis and is either idiopathic (primary RP) or due to connective tissue disease (secondary RP). A recent meta‐analysis of six RCTs that included 244 patients with secondary RP, predominantly due to scleroderma, found that PDE5I therapy moderately reduced the daily frequency and duration of vasospastic attacks and promoted visible healing of ulcers (Roustit, Blaise et al., 2013) and a prospective crossover study suggests that these beneficial effects also extend to primary RP (Caglayan, Huntgeburth et al., 2006). These findings suggest that PDE5I therapy may have an equivalent effect on vasospastic attack frequency to first‐line treatment with calcium channel blockers (CCBs) and is more successful at reducing attack frequency and promoting ulcer healing than ERAs, an effective and approved treatment for reducing digital ulceration in scleroderma but which has inconsistent or minimal effects on other clinical parameters and is of uncertain benefit in primary RP (Roustit, Blaise et al., 2013). Moreover, recent interventional trials (Table 3) have demonstrated a significant improvement in digital blood flow in patients with secondary RP following the use of both oral and topical sildenafil preparations (Andrigueti, Ebbing et al., 2017; Wortsman, Del Barrio‐Díaz et al., 2018), and a recent RCT showed that chronic treatment with sildenafil improved healing of digital ulcers secondary to systemic sclerosis (Hachulla, Hatron et al., 2016).
Although there has been little research into the therapeutic efficacy of PDE5Is in primary RP, subgroup analysis of a recent RCT investigating chronic vardenafil use in 53 adults with mixed RP revealed an increased efficacy in patients with primary RP (Caglayan, Axmann et al., 2012). However, significant clinical improvement was not replicated in a series of n‐of‐1 trials of increasing sildenafil dosage in 38 patients with mixed RP (68% of whom had primary RP), which demonstrated a markedly heterogenous response (Roustit, Giai et al., 2018). Therefore, although PDE5I therapy may represent a promising intervention for improving the microcirculation and clinical outcomes of patients with RP, further research with clearer stratification of RP causality, direct comparison with oral CCBs, and standardised tools for assessment of digital blood flow is necessary to clarify the efficacy and safety of these agents and determine which group of patients may benefit most from treatment.
6.2. Chronic kidney disease
CKD is a global public health problem characterised by a reduced GFR and/or evidence of kidney damage (Levin, Stevens et al., 2013). High BP and diabetes mellitus are major contributors to the development of CKD and both are associated with an increased risk of CVD. In addition, CKD is a major independent risk factor for incident CVD, which remains the main cause of death in these patients (Go, Chertow et al., 2004). The incidence and prevalence of CKD is rising, and its progressive clinical course is characterised by high rates of morbidity and mortality (Hoerger, Simpson et al., 2015).
The past decade has seen substantial progress in understanding the pathogenesis of CKD, with compelling evidence to suggest that the NO–cGMP pathway plays an important role in the maintenance of renal perfusion and glomerular filtration and that CKD is a state of systemic NO deficiency (Brown, Dhaun et al., 2014). Recently, the administration of sildenafil in a 5/6 renal ablation model has been shown to suppress vascular smooth muscle cell proliferation and inflammation and, subsequently, prevent progression of histological and functional damage in the remaining glomeruli (Tapia, Sanchez‐Lozada et al., 2012). Moreover, preclinical studies have indicated the potential for PDE5Is to reverse podocyte dysfunction and normalise proteinuria, independently of BP (Fang, Radovits et al., 2012; Hall, Rowell et al., 2014).
Although the first clinical trial to evaluate the potential therapeutic benefits of PDE5Is in CKD demonstrated that chronic sildenafil monotherapy significantly reduced microalbuminuria in patients with diabetic nephropathy (DN; Grover‐Páez, Rivera, & Ortíz, 2007), there were a number of methodological limitations which prohibited conclusive interpretation of the results (Brown, Dhaun et al., 2014). In order to address these issues, Pfizer launched an RCT investigating the use of PF‐00489791 for the treatment of overt DN (Scheele, Diamond et al., 2016). The inclusion criteria dictated a history of at least 3 months of persistent, overt macroalbuminuria—defined as a urinary albumin/creatinine ratio (UACR) of over 300 mg·g−1 — despite treatment with ACE inhibitor (ACEI) or angiotensin receptor blocker (ARB) in combination with antihypertensive and antidiabetic medications. Eligible participants had an estimated GFR (eGFR) of 25–59 ml·min−1 per 1.73 m2 (CKD stage G3 or G4) and were randomly assigned to receive either oral PF‐00489791 therapy or placebo once daily for 12 weeks. Remarkably, in the context of treatment‐resistant macroalbuminuria, the PDE5I group demonstrated a significant reduction in albuminuria as early as the third week of therapy. At the end of the 12‐week treatment period, patients receiving PF‐00489791 had a significant, BP‐independent, 16% reduction in UACR compared with placebo. However, this reduction in UACR was reversible and was non‐significant at 16 weeks, 4 weeks after the end of treatment. Although there was no difference in eGFR between treatment and placebo groups throughout the treatment period, this would be unlikely within the short timeframe. Interestingly, there was no initial short‐term fall in eGFR with PDE5I as is usually observed with ACEI/ARB treatment.
These encouraging results suggest that enhancing NO–cGMP signalling confers renoprotective effects and improvements in kidney function, possibly as a result of both haemodynamic and intrarenal anti‐inflammatory, anti‐proliferative and antioxidant mechanisms (Brown et al., 2014). However, no ongoing interventional studies on the long‐term benefits of PDE5I therapy in renal diseases could be identified (Table 3). We eagerly await the results of two recently completed studies evaluating the use of PDE5Is in renal disease to determine whether further research is merited (NCT02414204 and NCT01960153).
6.3. Diabetes mellitus
Type 2 diabetes mellitus (T2DM) is characterised by hyperglycaemia and results from a combination of peripheral insulin resistance and progressive pancreatic β‐cell dysfunction. These pro‐inflammatory states confer higher rates of incident hypertension and cardiovascular risk. Indeed, CVD accounts for two‐thirds of deaths in diabetic patients, with renal sequelae compounding the high rates of morbidity and mortality of this condition (Wang, Hess et al., 2016). It is well recognised that achieving tight glycaemic control must be combined with appropriate prevention and management of CVD and renal disease in order to maximise positive outcomes. Recent data suggest the potential for PDE5Is to modulate the macrovascular, microvascular and metabolic complications associated with diabetes mellitus.
6.3.1. Macrovascular effects
In a landmark study, a population of 5,956 men diagnosed with T2DM were monitored over a median period of 7 years to determine the effect of PDE5I use on their overall survival (Anderson, Hutchings et al., 2016). Compared to a random sample of non‐users from the same background population, men prescribed a PDE5I had a significant 31% lower risk of all‐cause mortality. This reduction in mortality remained statistically significant after adjusting for known risk modifiers including age, eGFR, smoking status, prior history of CVD or hypertension, known MI, systolic BP, lipid profile, number of prescriptions and Townsend deprivation index, as well as use of cardioprotective agents such as statins, metformin, aspirin and ß‐adrenoceptor antagonists (beta‐blockers), although life course socio‐economic position, compliance and doctor selection practices could not be adjusted for. Significant reductions were also observed in age‐adjusted incidence and mortality rates of acute MI in patients with a history of PDE5I use when compared to subjects never prescribed this class of drug. Notably, ED—the most common indication for PDE5I use—is a strong predictor of subsequent ischaemic heart disease (Gazzaruso, Solerte et al., 2008), suggesting that the potential protective effects of PDE5Is on cardiac tissue and vasculature may offset the pre‐existing additional CVD risk associated with this condition. Moreover, in an RCT of 59 T2DM men with diabetic cardiomyopathy, 3 months of sildenafil treatment led to significant improvements in cardiac indices compared to placebo (Giannetta, Isidori et al., 2012). Notably, these men were also on optimal anti‐hyperglycaemic, ACEI/ARB, ß‐adrenoceptor antagonists and CCB therapies, highlighting the additional therapeutic potential of PDE5Is in the management of diabetic complications.
Although these are promising findings, the exact mechanism by which PDE5Is may improve survival in diabetic patients is unclear but may pertain to the ability of PDE5Is to ameliorate, or prevent the development of, cardiovascular and renal pathology in both diabetic and non‐diabetic subjects.
6.3.2. Microvascular effects
The cardioprotective effects of PDE5Is may also extend to the diabetic myocardium. T2DM is associated with higher levels of inflammation and oxidative stress in the heart, leading to myocardial injury and worse outcomes compared to healthy heart tissue (Ray, Cannon et al., 2007). Also, the diabetic myocardium is more susceptible to ischaemia–reperfusion injury and is resistant to several cardioprotective modalities (Miki, Itoh et al., 2012). With this background, it is promising that, in T2DM mice exposed to ischaemia–reperfusion events, chronic tadalafil treatment significantly reduced infarct size, improved glucose metabolism, and ameliorated inflammatory and oxidative profiles in the diabetic myocardium (Koka, Das et al., 2013; Koka, Xi, & Kukreja, 2012; Varma, Das et al., 2012). Moreover, an RCT of 54 male patients with T2DM recently demonstrated significant, sustained improvements in endothelial parameters following chronic vardenafil use (Santi, Granata et al., 2016). Interestingly, this was also accompanied by restoration of testosterone levels in men with hypogonadism as early as 1 week into the treatment protocol, unrelated to erectile function scoring and, therefore, possibly independent of sexual activity.
6.3.3. Metabolic effects
Clinical trials have suggested that PDE5Is may influence glucose metabolism. In addition to the reductions in albuminuria seen in studies evaluating the renoprotective effects of these agents, daily PDE5I therapy for 1–4 months produced significant decreases in mean glycated Hb (HbA1c) of 0.3–0.59% from baseline (Grover‐Páez, Rivera et al., 2007; Scheele, Diamond et al., 2016), representing a reduction of hyperglycaemia comparable to that observed with established oral antidiabetic agents administered for longer treatment periods (0.5–1.25%; Sherifali, Nerenberg et al., 2010). Given the linear association between HbA1c and macrovascular disease, with an estimated 11–16% increase in cardiovascular events for every 11 mmol·mol−1 (1%) rise in HbA1c (Wang, Hess et al., 2016), these findings may have important implications for the management of insulin resistance in diabetes and obesity.
Insulin has recognised vasodilatory properties mediated by the up‐regulation of NOS3 expression and activity (Kuboki, Jiang et al., 2000). Insulin resistance has been shown to inhibit this pathway by reducing endothelial NO bioavailability (Wang, Hess et al., 2016). This disruption of NO–cGMP signalling and the resulting endothelial dysfunction has been shown to precede the development of inflammation and insulin resistance across muscle, liver and adipose tissue in mice with diet‐induced obesity (Kim, Pham et al., 2008; Rizzo, Maloney et al., 2010; Tateya, Rizzo et al., 2011). PDE5Is have proven anti‐inflammatory effects in diabetes‐induced endothelial dysfunction (Schwartz, Jackson et al., 2013) and recent evidence suggests that PDE5Is have additional direct effects on glucose metabolism: Significant improvements in insulin sensitivity and glucose uptake have been demonstrated in the muscles of mice fed a high‐fat diet following 12‐week sildenafil treatment, suggesting that PDE5I therapy may improve energy balance and prevent insulin resistance (Ayala, Bracy et al., 2007). Similar improvements in glucose uptake with PDE5I use have also been reported in experimental animal (Sabatini, Sgrò et al., 2011; Zhang et al., 2010) and human (Jansson, Murdolo et al., 2010) studies, further suggesting a link between NO–cGMP signalling and insulin action. Although insulin treatment does not induce NO production in insulin resistance (Salt, Morrow et al., 2003), chronic sildenafil treatment has been shown to enhance NOS3 activity in insulin resistance conditions (Mammi, Pastore et al., 2011), suggesting potential for sildenafil to improve vascular function in diabetes mellitus. Finally, others have demonstrated that sildenafil may promote adipogenesis via PKG‐mediated up‐regulation of lipid accumulation and insulin sensitivity in adipocytes (Zhang, Ji et al., 2010). Indeed, two RCTs have recently reported an improvement in oral disposition index (a composite measure of insulin sensitivity and secretion) in patients with the metabolic syndrome following long‐term PDE5I therapy, reflecting an overall improvement in β‐cell function (Ramirez, Nian et al., 2015; Santi, Granata et al., 2016).
Therefore, PDE5I treatment may affect outcomes in patients with diabetes mellitus through reversal of both vascular dysfunction, which is associated with increased CVD risk, and metabolic impairment. Collectively, the available data provide a strong rationale for further research into the therapeutic targeting of the NO–cGMP pathway for the management of T2DM and obesity, although the promise shown in recent studies with ERAs (Dhaun & Webb, 2019) and sodium–glucose cotransporter 2 (SGLT2) inhibitors (Perkovic, Jardine et al., 2019) in patients with T2DM and renal disease serves to make this a competitive field, to the benefit of future patients.
7. PHARMACOLOGY AND SAFETY PROFILE OF PDE TYPE 5 INHIBITORS
Although all PDE5Is employ the same mechanism of action and exhibit similar therapeutic efficacy and safety (Jannini, Isidori et al., 2009), they are distinguished on the basis of their pharmacokinetic and pharmacodynamic properties (Hatzimouratidis & Hatzichristou, 2005).
7.1. Pharmacokinetics
The primary pharmacokinetic variables which determine PDE5I administration are bioavailability, maximum plasma concentration after an oral dose (C max), time to achieve maximum plasma concentration (T max) and terminal half‐life (t ½; Table 1). All marketed PDE5Is have a high volume of distribution into the vascular space, tissues and binding by extravascular proteins such as albumin (Mehrotra, Gupta et al., 2007). Although comparative bioavailability data between the agents are lacking, this can be estimated by quantifying the area under the plasma drug concentration‐time curve (AUC). Notably, vardenafil has a significantly lower AUC than sildenafil and tadalafil, which may explain its comparatively lower C max. However, it is not possible to correlate these kinetics with the clinical dosing regimens of the other agents from the information that is publicly available.
TABLE 1.
PDE5I pharmacokinetic and pharmacodynamic comparison
| PDE5I | Pharmacokinetics | Pharmacodynamics | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Dose (mg) | T max (hr) | t ½ (hr) | C max (ng·ml−1) | AUC (ng × hr·ml−1) | MW (g·mol−1) | MF | IC50 for PDE5 (nmol·L−1) | PDE selectivity | |
| Sildenafil | 100 | 0.95 | 3.98 | 514 | 1,670 | 475 | C22H30N6O4S | 3.7 | Low activity against PDE6; very low activity against PDE1. |
| Tadalafil | 20 | 2 | 17.5 | 378 | 8,066 | 389 | C22H19N3O4 | 1.8 | Low activity against PDE11; very low activity against PDE6. |
| Vardenafil FCT | 20 | 0.66 | 3.9 | 20.9 | 74.5 | 488 | C23H32N6O4S | 0.091 | Low activity against PDE6; very low activity against PDE1. |
| Avanafil | 200 | 0.75 | 5.1 | 2,920 | 8,490 | 484 | C23H26CIN7O3 | 5.2 | Highly selective for PDE5. |
| Udenafil | 200 | 0.76 | 9.88 | 1,137 | 7,898 | 517 | C25H36N6O4S | 8.25 | Comparable to sildenafil for PDE5. |
| Mirodenafil | 100 | 1.4 | 2.5 | 2,989 | 7,907 | 532 | C26H37N5O5S | 0.33 | Comparable to sildenafil for PDE5. |
| Lodenafil | 160 | 1.2 | 2.4 | 157 | 530 | 1,035 | C47H62N12O11S2 | 0.015 | Low activity against PDE1 and PDE6. |
| PF‐00489791 | 20 | 1.1–3.5 | 11.9–15.7 | 1,570–1,630 | N/A | 477 | C20H28N8O4S | 0.71 | Highly selective for PDE5. |
Abbreviations: AUC, area under the plasma drug concentration‐time curve; C max, maximum plasma serum concentration; FCT, film‐coated tablet; IC50, half maximal inhibitory concentration; MF, molecular formula; PDE5I, PDE type 5 inhibitor; t ½, terminal plasma half‐life; T max, time taken to reach the maximum plasma concentration.
Sildenafil, udenafil, vardenafil and avanafil have similar T max values and thus comparable onset of action after oral administration (30–60 min), with the latter two having the fastest onset: Vardenafil, in both formulations, acts within 30 min of intake whereas avanafil is the only PDE5I approved for use 15–30 min before sexual intercourse in the treatment of ED. In comparison, mirodenafil and lodenafil have slightly higher T max values, with limited published data suggesting that these have a similar onset of action to sildenafil, whereas tadalafil has the highest T max, reaching peak plasma concentration after 30–120 min (Hatzimouratidis, Salonia et al., 2016).
Tadalafil and udenafil have the highest t ½ of marketed PDE5Is, although it is unclear whether this is due to either delayed absorption or degradation. Clinically, this translates to a longer duration of therapeutic effect (up to 36 hr for tadalafil and 12 hr for udenafil). Tadalafil is uniquely unaffected by dietary intake, enabling once daily dosing such as that required for the management of lower urinary tract symptoms in benign prostatic hyperplasia—an indication for which tadalafil was licensed in 2011 and which is further explored in a recent review (Andersson, 2018). Avanafil has the shortest duration of action (up to 6 hr), with sildenafil, vardenafil, mirodenafil and lodenafil lasting slightly longer (up to 10–12 hr; Hatzimouratidis, Salonia et al., 2016).
PDE5Is are rapidly metabolised by the cytochrome P450 enzyme family (CYP450) in the liver, chiefly through the CYP3A system with contributions from CYP2C9, CYP2C19, and CYP2D6 pathways (Mehrotra, Gupta et al., 2007), and systemic clearance of sildenafil and vardenafil, but not tadalafil, is reduced by mild and moderate hepatic impairment (Muirhead, Wilner et al., 2002). Both sildenafil and vardenafil have active metabolites which significantly contribute to their pharmacological activity (Mehrotra, Gupta et al., 2007). Although PDE5Is are largely faecally excreted, undergoing minimal renal clearance due to their extensive tubular reabsorption, the secondary effects of renal impairment may affect the clearance of sildenafil through alterations in protein binding or inflammatory protein synthesis (Muirhead, Wilner et al., 2002). Therefore, patients with severe hepatic or renal impairment should be prescribed a lower starting dose of PDE5Is and titrated up as appropriate.
7.2. Pharmacodynamics
The available PDE5Is differ in their affinity and selectivity for the PDE5 isoform, with consequent effects on their tolerability and safety profile. The biochemical affinity of the various agents is quantified by the half maximal inhibitory concentration (IC50), which represents the concentration of the agent that is required to inhibit 50% of PDE5 activity in vitro. Affinity is an inverse measure of potency, such that an agent with a higher IC50 requires a lower dose to reach therapeutic effect. Selectivity refers to the ability of a PDE5I to preferentially bind PDE5 over other PDEs (Table 1). The cross‐reactivity of PDE5Is has important implications for the tolerability of the agent as the ubiquitous presence of PDEs in the body means that non‐selective inhibition may result in wide‐ranging systemic effects.
All agents are generally well‐tolerated (Table 2) and share similar transient, mild side effects, such as facial flushing, headache, dyspepsia, nasal congestion and dizziness. Tadalafil has also been associated with myalgia and back pain in up to 6% of patients. However, sildenafil and, to a lesser extent, vardenafil have demonstrated cross‐reactivity with PDE1 and PDE6. PDE6 is highly expressed in retinal photoreceptors, where its inhibition causes transient blue/green colour vision disturbances which coincide with peak plasma sildenafil concentrations (Hatzimouratidis & Hatzichristou, 2005). PDE5Is also show some cross‐reactivity with PDE1. PDE1 is highly expressed in vascular smooth muscle, where its inhibition results in vasodilatation and BP reduction, as demonstrated recently in rats with novel PDE1‐inhibitors (Laursen, Beck et al., 2017). It has been suggested that the higher incidence of facial flushing following sildenafil and vardenafil administration, compared with tadalafil and avanafil, may be the result of greater PDE1 cross‐reactivity (Kouvelas, Goulas et al., 2009). Finally, tadalafil has been shown to inhibit the activity of PDE11, an isozyme which is expressed in the mammalian testes and prostate, although no impact on reproductive function has been reported with PDE5I use (Hatzimouratidis & Hatzichristou, 2005). Indeed, a large meta‐analysis suggests that chronic PDE5I use may have a beneficial effect on spermatogenesis, although the improvements with tadalafil are less than would be expected given its long duration of action (Tan, Liu et al., 2017). Although quantitative isozyme affinity and selectivity data for udenafil, mirodenafil and lodenafil are not publicly available, these are said to have a comparable selectivity profile to sildenafil, which may explain their similar side effect profile. In comparison, avanafil and PF‐00489791 are newer agents with improved PDE5 selectivity and seemingly favourable tolerability (Table 2), particularly with respect to visual disturbances (Wang, Burnett et al., 2012).
TABLE 2.
Reported PDE5I side effect incidence
| PDE5I | Observed incidence of side effect (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Headache | Facial flushing | Dyspepsia | Rhino‐sinusitis | Back pain | Myalgia | Abnormal vision | Red eye | Chest discomfort | Peripheral oedema | |
| Sildenafil | 19 | 14 | 9 | 5 | 0 | 0 | 6 | 0 | 0 | 0 |
| Tadalafil | 21 | 5 | 17 | 5 | 9 | 7 | 0 | 0 | 0 | 0 |
| Vardenafil | 16 | 12 | 4 | 10 | 0 | 0 | <2 | 0 | 0 | 0 |
| Avanafil | 7–8 | 3–5 | 2 | 2 | <2 | 0 | 0 | 0 | 0 | 0 |
| Udenafil | 2–9 | 11–23 | 0 | 4–7 | 0 | 0 | 0 | 4–7 | 0–5 | 0 |
| Mirodenafil | 8–11 | 10–16 | 3 | 0 | 0 | 0 | 0 | 3–4 | 0–3 | 0 |
| Lodenafil | 15–22 | 5–6 | 5–22 | 5–11 | 0 | 0 | 5–6 | 0 | 0 | 0 |
| PF‐00489791 | 5–21 | 0 | 3–15 | 0 | 0–12 | 0 | 0 | 0 | 0 | 1–9 |
Note. Data sourced from Glina, Toscano et al. (2009); Hatzimouratidis and Hatzichristou (2005); Limin, Johnsen, & Hellstrom (2010); Paick, Ahn et al. (2008); Paick, Kim et al. (2008); Scheele et al. (2016); Wang, Burnett et al. (2012); and Wolk, Smith et al. (2009).
Abbreviation: PDE5I, PDE type 5 inhibitor.
In theory, agents with improved PDE5 selectivity may achieve comparable efficacy with minimal systemic consequences by limiting PDE1/PDE6 cross‐reactivity. However, the highly selective agents PF‐00489791 and PF‐03049423 have recently been withdrawn from several clinical trials investigating their use in various cardiovascular indications (https://adisinsight.springer.com/drugs/800025495), despite a lack of safety concerns and the former's previously discussed efficacy in hypertension and CKD. Although PF‐03049423 was not found to be effective in improving outcomes following ischaemic stroke (Di Cesare, Mancuso et al., 2016), the rationale behind the withdrawal of PF‐00489791 is not publicly available. Similarly, research into KD027/SLx‐2101, a PDE5I which was uniquely converted into a long‐acting active metabolite and had been in the final stages of development for the treatment of hypertension (NCT00562549 and NCT00562614; Hatzimouratidis & Hatzichristou, 2008), has also been suspended following the closure of its sponsor company (Professor I. Wilkinson, personal communication, September 16, 2019). In combination with the recent surge in registered trials of sildenafil and tadalafil in several cardiovascular conditions (Table 3), these developments indicate a market shift towards re‐purposing already established PDE5Is. Although the reasons for this are unclear, it is possible that these novel PDE5Is may not have achieved adequate therapeutic efficacy for their development to be financially sustainable given recent advancements in the field of cardiovascular therapeutics, particularly with regard to the extensive and evolving therapeutic profile of SGLT2‐inhibitors.
7.3. Drug interactions
Although concomitant use of PDE5Is with nitrates, sGC stimulators and α‐adrenoreceptor antagonists is contraindicated due to the risk of a potent hypotensive interaction (Webb, Freestone et al., 1999), PDE5Is do not have synergistic effects on BP with other antihypertensive agents, such as ACEIs, ARBs, beta‐blockers, CCBs, or thiazide diuretics (Kloner, Mitchell et al., 2003; Webb, Freestone et al., 1999).
Potent inhibitors of the CYP450 system (particularly CYP3A4), including several antiretroviral protease inhibitors, have been shown to modify the pharmacokinetics of PDE5Is. Whilst coadministration has not been associated with changes in the safety or tolerability of either agent (Muirhead, Wulff et al., 2000), cautious use of a lower PDE5I dose is still recommended for patients receiving agents with CYP450 inhibitory properties (Baxter & Preston, 2019).
Lastly, following several years of uncertainty as to whether PDE5Is were associated with changes in cardiac repolarisation, a well‐designed study in healthy males demonstrated that the small increases in corrected QT interval which may be observed at higher doses of the agents are clinically insignificant (Morganroth, Ilson et al., 2004). In fact, an increasing body of experimental evidence suggests that PDE5Is may exert antiarrhythmic effects on ischaemic myocardium through the modulation of voltage‐gated calcium channels, Na+/K+‐ATPases and β‐adrenoceptor signalling (Hutchings, Anderson et al., 2018).
7.4. Nonarteritic anterior ischaemic optic neuropathy
There is still some uncertainty surrounding the safety profile of PDE5Is. In particular, there have been numerous case reports and case series of nonarteritic anterior ischaemic optic neuropathy (NAION) following use of the agents. NAION, an optic neuropathy precipitated by a decrease in perfusion pressure in the prelaminar region of the optic nerve causing ischaemic infarction and sudden, painless vision loss, is a rare condition, but the leading acquired optic neuropathy in adults over 50 years old, with an annual incidence of 2–10 cases per 100,000 in this population. Several experimental and epidemiological studies have failed to establish a definitive effect of PDE5Is on the ocular circulation or on the risk of NAION (Pomeranz, 2016), which is more likely in the presence of cardiovascular risk factors such as ED. However, important insights into this association have recently been provided by two prospective trials using a case–crossover study design, which controls for the effects of recall bias and participant characteristics by defining the day of NAION onset and the day before as the risk period and matching this with control periods in the preceding weeks. The first, a study of 64 definite or probable cases of NAION, found a twofold increase in the risk of NAION within five half‐lives of PDE5I use, extending to 2 days after sildenafil or vardenafil use and 5 days after tadalafil use (Campbell, Walker et al., 2015). These results were echoed by a second crossover trial which identified 279 men with confirmed NAION and revealed that intermittent exposure to a PDE5I within the preceding month also increased the baseline risk of developing NAION twofold (Flahavan, Li et al., 2017). These findings translate to an annual contribution of approximately three additional NAION cases per 100,000 men 50 years and older with weekly PDE5I use. Consequently, PDE5Is are contraindicated in patients with previous NAION or with a hereditary eye disease.
7.5. Persistent pulmonary hypertension of the newborn
Concerns have been raised about the safety profile of PDE5Is in the treatment of fetal growth restriction (FGR). Despite generally promising results in preclinical studies and small clinical trials, in July 2018, the Dutch arm of the international Sildenafil TheRapy In Dismal prognosis Early‐onset fetal growth Restriction (STRIDER) series of RCTs was prematurely terminated after interim data suggested an increased risk of persistent pulmonary hypertension of the newborn (PPHN) and neonatal mortality in the intervention group (Groom, Ganzevoort et al., 2018). In the sildenafil group, 17 cases of PPHN were recorded in 64 babies (27%) compared to three of 58 babies (5%) in the placebo group, in the absence of any survival benefit. In the United Kingdom and the New Zealand and Australia STRIDER trials, there was no observable difference in clinically relevant pathological or histological findings between the sildenafil and placebo groups and no significant amelioration of neonatal survival until term age (Sharp, Cornforth et al., 2019). Although the planned meta‐analysis of individual patient data from these trials is eagerly anticipated, it is uncertain whether PDE5Is will have a therapeutic role in pregnancies complicated by FGR.
8. CONCLUSIONS
The proven clinical efficacy and excellent safety profile of PDE5Is in the management of ED and PAH has led to their introduction as over‐the‐counter medications as well as prompting significant research interest in the use of these agents for the enhancement of the actions of cGMP in a range of vascular and non‐vascular conditions. In addition to their acute haemodynamic effects, PDE5Is have demonstrated potent anti‐inflammatory, antioxidant, anti‐proliferative and metabolic properties, largely attributable to modulation of the NO–cGMP signalling pathway. A wide array of experimental data suggests a protective effect of PDE5Is in several major organ systems and therapeutic potential in hypertension, chronic kidney disease and diabetes mellitus. In particular, the recent association of long‐term PDE5I treatment with a reduction in all‐cause mortality merits a broader evaluation of PDE5Is. Ultimately, establishing the optimal use and long‐term safety of these agents in CVD will depend on the implementation of larger RCTs, particularly with other comparative treatments, which may be facilitated by the development of novel, longer acting, and more selective PDE5Is.
8.1. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander, et al.,2019 2019).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGEMENTS
The work of the authors is supported by a British Heart Foundation (BHF) Centre of Research Excellence Award (RE/08/001/23904). N.D. has been supported by a BHF Intermediate Clinical Research Fellowship (FS/13/30/29994). T.F. is supported by an MRC Clinical Research Training Fellowship (MR/R017840/1).
Tzoumas N, Farrah TE, Dhaun N, Webb DJ. Established and emerging therapeutic uses of PDE type 5 inhibitors in cardiovascular disease. Br J Pharmacol. 2020;177:5467–5488. 10.1111/bph.14920
REFERENCES
- Alexander, S. P. , Fabbro, D. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , … CGTP collaboratories (2019). The concise guide to pharmacology 2019/20: Enzymes. British Journal of Pharmacology, 176, S297–S396. 10.1111/bph.14572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen, M. J. , Ersbøll, M. , Axelsson, A. , Gustafsson, F. , Hassager, C. , Køber, L. , … Møller, J. E. (2013). Sildenafil and diastolic dysfunction after acute myocardial infarction in patients with preserved ejection fraction. Circulation, 127, 1200–1208. 10.1161/CIRCULATIONAHA.112.000056 [DOI] [PubMed] [Google Scholar]
- Anderson, S. G. , Hutchings, D. C. , Woodward, M. , Rahimi, K. , Rutter, M. K. , Kirby, M. , … Heald, A. H. (2016). Phosphodiesterase type‐5 inhibitor use in type 2 diabetes is associated with a reduction in all‐cause mortality. Heart, 102, 1750–1756. 10.1136/heartjnl-2015-309223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson, K. E. (2018). PDE5 inhibitors—Pharmacology and clinical applications 20 years after sildenafil discovery. British Journal of Pharmacology, 175, 2554–2565. 10.1111/bph.14205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrigueti, F. , Ebbing, P. , Arismendi, M. , & Kayser, C. (2017). Evaluation of the effect of sildenafil on the microvascular blood flow in patients with systemic sclerosis: A randomised, double‐blind, placebo‐controlled study. Clinical and Experimental Rheumatology, 35, S151–S158. [PubMed] [Google Scholar]
- Ayala, J. E. , Bracy, D. P. , Julien, B. M. , Rottman, J. N. , Fueger, P. T. , & Wasserman, D. H. (2007). Chronic treatment with sildenafil improves energy balance and insulin action in high fat–fed conscious mice. Diabetes, 56, 1025–1033. [DOI] [PubMed] [Google Scholar]
- Barnes, H. , Brown, Z. , Burns, A. , & Williams, T. (2019). Phosphodiesterase 5 inhibitors for pulmonary hypertension. Cochrane Database of Systematic Reviews, CD012621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter, K. , & Preston, C. L. (2019). Stockley's drug interactions. [online] London: Pharmaceutical Press. Retrieved from http://www.new.medicinescomplete.com. Last modified 04‐Jul‐2018. Accessed 01‐Oct‐2019.
- Beavo, J. A. , & Reifsnyder, D. H. (1990). Primary sequence of cyclic nucleotide phosphodiesterase isozymes and the design of selective inhibitors. Trends in Pharmacological Sciences, 11, 150–155. 10.1016/0165-6147(90)90066-H [DOI] [PubMed] [Google Scholar]
- Boolell, M. , Allen, M. , Ballard, S. , Gepi‐Attee, S. , Muirhead, G. , Naylor, A. , … Gingell, C. (1996). Sildenafil: An orally active type 5 cyclic GMP‐specific phosphodiesterase inhibitor for the treatment of penile erectile dysfunction. International Journal of Impotence Research, 8, 47–52. [PubMed] [Google Scholar]
- Boswell‐Smith, V. , Spina, D. , & Page, C. P. (2006). Phosphodiesterase inhibitors. British Journal of Pharmacology, 147, S252–S257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, K. E. , Dhaun, N. , Goddard, J. , & Webb, D. J. (2014). Potential therapeutic role of phosphodiesterase type 5 inhibition in hypertension and chronic kidney disease. Hypertension, 63, 5–11. [DOI] [PubMed] [Google Scholar]
- Brunner, H. , Cockcroft, J. R. , Deanfield, J. , Donald, A. , Ferrannini, E. , Halcox, J. , … Natali, A. (2005). Endothelial function and dysfunction. Part II: Association with Cardiovascular Risk Factors and Diseases. A Statement by the Working Group on Endothelins and Endothelial Factors of the European Society of Hypertension. Journal of Hypertension, 23, 233–246. 10.1097/00004872-200502000-00001 [DOI] [PubMed] [Google Scholar]
- Burley, D. S. , Ferdinandy, P. , & Baxter, G. F. (2007). Cyclic GMP and protein kinase‐G in myocardial ischaemia‐reperfusion: Opportunities and obstacles for survival signaling. British Journal of Pharmacology, 152, 855–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caglayan, E. , Axmann, S. , Hellmich, M. , Moinzadeh, P. , & Rosenkranz, S. (2012). Vardenafil for the treatment of Raynaud phenomenon: A randomized, double‐blind, placebo‐controlled crossover study. Archives of Internal Medicine, 172, 1182–1184. [DOI] [PubMed] [Google Scholar]
- Caglayan, E. , Huntgeburth, M. , Karasch, T. , Weihrauch, J. , Hunzelmann, N. , Krieg, T. , … Rosenkranz, S. (2006). Phosphodiesterase type 5 inhibition is a novel therapeutic option in Raynaud disease. Archives of Internal Medicine, 166, 231–233. [DOI] [PubMed] [Google Scholar]
- Campbell, U. B. , Walker, A. M. , Gaffney, M. , Petronis, K. R. , Creanga, D. , Quinn, S. , … Reynolds, R. F. (2015). Acute nonarteritic anterior ischemic optic neuropathy and exposure to phosphodiesterase type 5 inhibitors. The Journal of Sexual Medicine, 12, 139–151. 10.1111/jsm.12726 [DOI] [PubMed] [Google Scholar]
- Cheitlin, M. D. , Hutter, A. M. , Brindis, R. G. , Ganz, P. , Kaul, S. , Russell, R. O. , … Faxon, D. P. (1999). Use of sildenafil (Viagra) in patients with cardiovascular disease. Journal of the American College of Cardiology, 33, 273–282. 10.1016/s0735-1097(98)00656-1 [DOI] [PubMed] [Google Scholar]
- Clerin, V. , Gale, J. , & Tamimi, N. (2014). Use of a tetrasubstituted pyrazolo[4,3‐d]pyrimidine compound for treating diabetic nephropathy. World Intellectual Property Organization Patent No. WO2014064566A1. Retrieved from https://patents.google.com/patent/WO2014064566A1/en. Last modified 01‐May‐2019. Accessed 01‐Oct‐2019.
- Corbin, J. D. , Beasley, A. , Blount, M. A. , & Francis, S. H. (2005). High lung PDE5: A strong basis for treating pulmonary hypertension with PDE5 inhibitors. Biochemical and Biophysical Research Communications, 334, 930–938. [DOI] [PubMed] [Google Scholar]
- Das, A. , Xi, L. , & Kukreja, R. C. (2005). Phosphodiesterase‐5 inhibitor sildenafil preconditions adult cardiac myocytes against necrosis and apoptosis. Essential role of nitric oxide signaling. The Journal of Biological Chemistry, 280, 12944–12955. 10.1074/jbc.M404706200 [DOI] [PubMed] [Google Scholar]
- Dhaun, N. , & Webb, D. J. (2019). Endothelins in cardiovascular biology and therapeutics. Nature Reviews. Cardiology, 16, 491–502. 10.1038/s41569-019-0176-3 [DOI] [PubMed] [Google Scholar]
- Di Cesare, F. , Mancuso, J. , Woodward, P. , Bednar, M. M. , Loudon, P. T. , & Group, A. S. S (2016). Phosphodiesterase‐5 inhibitor PF‐03049423 effect on stroke recovery: A double‐blind, placebo‐controlled randomized clinical trial. Journal of Stroke and Cerebrovascular Diseases, 25, 642–649. 10.1016/j.jstrokecerebrovasdis.2015.11.026 [DOI] [PubMed] [Google Scholar]
- Dias, A. T. , Rodrigues, B. P. , Porto, M. L. , Gava, A. L. , Balarini, C. M. , Freitas, F. P. , … Pereira, T. M. (2014). Sildenafil ameliorates oxidative stress and DNA damage in the stenotic kidneys in mice with renovascular hypertension. Journal of Translational Medicine, 12, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner, B. , Ebner, A. , Reetz, A. , Böhme, S. , Schauer, A. , Strasser, R. H. , & Weinbrenner, C. (2013). Pharmacological postconditioning by bolus injection of phosphodiesterase‐5 inhibitors vardenafil and sildenafil. Molecular and Cellular Biochemistry, 379, 43–49. 10.1007/s11010-013-1625-7 [DOI] [PubMed] [Google Scholar]
- Fang, L. , Radovits, T. , Szabó, G. , Mózes, M. M. , Rosivall, L. , & Kökény, G. (2012). Selective phosphodiesterase‐5 (PDE‐5) inhibitor vardenafil ameliorates renal damage in type 1 diabetic rats by restoring cyclic 3′, 5′ guanosine monophosphate (cGMP) level in podocytes. Nephrology, Dialysis, Transplantation, 28, 1751–1761. [DOI] [PubMed] [Google Scholar]
- Fernandes, A. M. S. , Andrade, A. C. , Barroso, N. D. , Borges, I. C. , Carvalho‐Andrade, D. , Rodrigues, E. S. J. , … Aras, R. J. (2015). The immediate effect of sildenafil on right ventricular function in patients with heart failure measured by cardiac magnetic resonance: A randomized control trial. PLoS ONE, 10, e0119623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher, P. W. , Salloum, F. , Das, A. , Hyder, H. , & Kukreja, R. C. (2005). Phosphodiesterase‐5 inhibition with sildenafil attenuates cardiomyocyte apoptosis and left ventricular dysfunction in a chronic model of doxorubicin cardiotoxicity. Circulation, 111, 1601–1610. 10.1161/01.CIR.0000160359.49478.C2 [DOI] [PubMed] [Google Scholar]
- Flahavan, E. M. , Li, H. , Gupte‐Singh, K. , Rizk, R. T. , Ruff, D. D. , Francis, J. L. , & Kinchen, K. S. (2017). Prospective case‐crossover study investigating the possible association between nonarteritic anterior ischemic optic neuropathy and phosphodiesterase type 5 inhibitor exposure. Urology, 105, 76–84. [DOI] [PubMed] [Google Scholar]
- Galiè, N. , Barberà, J. A. , Frost, A. E. , Ghofrani, H. A. , Hoeper, M. M. , McLaughlin, V. V. , … Rubin, L. J. (2015). Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. The New England Journal of Medicine, 373, 834–844. 10.1056/NEJMoa1413687 [DOI] [PubMed] [Google Scholar]
- Galiè, N. , Ghofrani, H. A. , Torbicki, A. , Barst, R. J. , Rubin, L. J. , Badesch, D. , … The Sildenafil Use in Pulmonary Arterial Hypertension Study (SUPER) Study Group (2005). Sildenafil citrate therapy for pulmonary arterial hypertension. The New England Journal of Medicine, 353, 2148–2157. 10.1056/NEJMoa050010 [DOI] [PubMed] [Google Scholar]
- Galiè, N. , Olschewski, H. , Oudiz, R. J. , Torres, F. , Frost, A. , Ghofrani, H. A. , … Roecker, E. B. (2008). Ambrisentan for the treatment of pulmonary arterial hypertension: Results of the ambrisentan in pulmonary arterial hypertension, randomized, double‐blind, placebo‐controlled, multicenter, efficacy (ARIES) study 1 and 2. Circulation, 117, 3010–3019. 10.1161/CIRCULATIONAHA.107.742510 [DOI] [PubMed] [Google Scholar]
- García‐Barroso, C. , Ricobaraza, A. , Pascual‐Lucas, M. , Unceta, N. , Rico, A. J. , Goicolea, M. A. , … Franco, R. (2013). Tadalafil crosses the blood–brain barrier and reverses cognitive dysfunction in a mouse model of AD. Neuropharmacology, 64, 114–123. 10.1016/j.neuropharm.2012.06.052 [DOI] [PubMed] [Google Scholar]
- Garthwaite, J. (2019). NO as a multimodal transmitter in the brain: Discovery and current status. British Journal of Pharmacology, 176, 197–211. 10.1111/bph.14532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaruso, C. , Solerte, S. B. , Pujia, A. , Coppola, A. , Vezzoli, M. , Salvucci, F. , … Garzaniti, A. (2008). Erectile dysfunction as a predictor of cardiovascular events and death in diabetic patients with angiographically proven asymptomatic coronary artery disease. Journal of the American College of Cardiology, 51, 2040–2044. 10.1016/j.jacc.2007.10.069 [DOI] [PubMed] [Google Scholar]
- Ghofrani, H. A. , Osterloh, I. H. , & Grimminger, F. (2006). Sildenafil: From angina to erectile dysfunction to pulmonary hypertension and beyond. Nature Reviews. Drug Discovery, 5, 689–702. 10.1038/nrd2030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghofrani, H. A. , Pepke‐Zaba, J. , Barbera, J. A. , Channick, R. , Keogh, A. M. , Gomez‐Sanchez, M. A. , … Grimminger, F. (2004). Nitric oxide pathway and phosphodiesterase inhibitors in pulmonary arterial hypertension. Journal of the American College of Cardiology, 43, S68–S72. [DOI] [PubMed] [Google Scholar]
- Giannetta, E. , Isidori, A. M. , Galea, N. , Carbone, I. , Mandosi, E. , Vizza, C. D. , … Lenzi, A. (2012). Chronic inhibition of cyclic GMP phosphodiesterase 5A improves diabetic cardiomyopathy: A randomized, controlled clinical trial using magnetic resonance imaging with myocardial tagging. Circulation, 125, 2323–2333. 10.1161/CIRCULATIONAHA.111.063412 [DOI] [PubMed] [Google Scholar]
- Giuliano, F. , Jackson, G. , Montorsi, F. , Martin‐Morales, A. , & Raillard, P. (2010). Safety of sildenafil citrate: Review of 67 double‐blind placebo‐controlled trials and the postmarketing safety database. International Journal of Clinical Practice. Supplement, 64, 240–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glina, S. , Toscano, I. , Gomatzky, C. , De Góes, P. M. , Júnior, A. N. , de Almeida Claro, J. F. , & Pagani, E. (2009). Efficacy and tolerability of lodenafil carbonate for oral therapy in erectile dysfunction: A phase II clinical trial. The Journal of Sexual Medicine, 6, 553–557. 10.1111/j.1743-6109.2008.01079.x [DOI] [PubMed] [Google Scholar]
- Go, A. S. , Chertow, G. M. , Fan, D. , McCulloch, C. E. , & Hsu, C.‐y. (2004). Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. The New England Journal of Medicine, 351, 1296–1305. 10.1056/NEJMoa041031 [DOI] [PubMed] [Google Scholar]
- Goldstein, I. , Lue, T. F. , Padma‐Nathan, H. , Rosen, R. C. , Steers, W. D. , & Wicker, P. A. (1998). Oral sildenafil in the treatment of erectile dysfunction. The New England Journal of Medicine, 338, 1397–1404. 10.1056/NEJM199805143382001 [DOI] [PubMed] [Google Scholar]
- Gómez‐Vallejo, V. , Ugarte, A. , García‐Barroso, C. , Cuadrado‐Tejedor, M. , Szczupak, B. , Dopeso‐Reyes, I. G. , … Oyarzabal, J. (2016). Pharmacokinetic investigation of sildenafil using positron emission tomography and determination of its effect on cerebrospinal fluid cGMP levels. Journal of Neurochemistry, 136, 403–415. [DOI] [PubMed] [Google Scholar]
- Groom, K. , Ganzevoort, W. , Alfirevic, Z. , Lim, K. , Papageorghiou, A. , & the STRIDER Consortium (2018). Clinicians should stop prescribing sildenafil for fetal growth restriction (FGR): Comment from the STRIDER Consortium. Ultrasound in Obstetrics & Gynecology, 52, 295–296. [DOI] [PubMed] [Google Scholar]
- Grover‐Páez, F. , Rivera, G. V. , & Ortíz, R. G. (2007). Sildenafil citrate diminishes microalbuminuria and the percentage of A1c in male patients with type 2 diabetes. Diabetes Research and Clinical Practice, 78, 136–140. 10.1016/j.diabres.2007.02.006 [DOI] [PubMed] [Google Scholar]
- Guazzi, M. , Vicenzi, M. , Arena, R. , & Guazzi, M. D. (2011). Pulmonary hypertension in heart failure with preserved ejection fraction. Circulation, 124, 164–174. 10.1161/CIRCULATIONAHA.110.983866 [DOI] [PubMed] [Google Scholar]
- Hachulla, E. , Hatron, P.‐Y. , Carpentier, P. , Agard, C. , Chatelus, E. , Jego, P. , … Michon‐Pasturel, U. (2016). Efficacy of sildenafil on ischaemic digital ulcer healing in systemic sclerosis: The placebo‐controlled SEDUCE study. Annals of the Rheumatic Diseases, 75, 1009–1015. 10.1136/annrheumdis-2014-207001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, G. , Rowell, J. , Farinelli, F. , Gbadegesin, R. A. , Lavin, P. , Wu, G. , … Jiang, R. (2014). Phosphodiesterase 5 inhibition ameliorates angiontensin II‐induced podocyte dysmotility via the protein kinase G‐mediated downregulation of TRPC6 activity. American Journal of Physiology. Renal Physiology, 306, F1442–F1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR (2018). The IUPHAR/BPS guide to pharmacology in 2018: Updates and expansion to encompass the new guide to immunopharmacology. Nucleic Acids Research, 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzimouratidis, K. , & Hatzichristou, D. G. (2005). A comparative review of the options for treatment of erectile dysfunction. Drugs, 65, 1621–1650. 10.2165/00003495-200565120-00003 [DOI] [PubMed] [Google Scholar]
- Hatzimouratidis, K. , & Hatzichristou, D. G. (2008). Looking to the future for erectile dysfunction therapies. Drugs, 68, 231–250. [DOI] [PubMed] [Google Scholar]
- Hatzimouratidis, K. , Salonia, A. , Adaikan, G. , Buvat, J. , Carrier, S. , El‐Meliegy, A. , … Khera, M. (2016). Pharmacotherapy for erectile dysfunction: recommendations from the Fourth International Consultation for Sexual Medicine (ICSM 2015). The Journal of Sexual Medicine, 13, 465–488. [DOI] [PubMed] [Google Scholar]
- Ho, J. E. , Arora, P. , Walford, G. A. , Ghorbani, A. , Guanaga, D. P. , Dhakal, B. P. , … Newton‐Cheh, C. (2014). Effect of phosphodiesterase inhibition on insulin resistance in obese individuals. Journal of the American Heart Association, 3, e001001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeper, M. M. , McLaughlin, V. V. , Al Dalaan, A. M. , Satoh, T. , & Galiè, N. (2016). Treatment of pulmonary hypertension. The Lancet Respiratory Medicine, 4, 323–336. [DOI] [PubMed] [Google Scholar]
- Hoerger, T. J. , Simpson, S. A. , Yarnoff, B. O. , Pavkov, M. E. , Burrows, N. R. , Saydah, S. H. , … Zhuo, X. (2015). The future burden of CKD in the United States: A simulation model for the CDC CKD initiative. American Journal of Kidney Diseases, 65, 403–411. 10.1053/j.ajkd.2014.09.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchings, D. C. , Anderson, S. G. , Caldwell, J. L. , & Trafford, A. W. (2018). Phosphodiesterase‐5 inhibitors and the heart: Compound cardioprotection? Heart, 104, 1244–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinose, F. , Adrie, C. , Hurford, W. E. , Bloch, K. D. , & Zapol, W. M. (1998). Selective pulmonary vasodilation induced by aerosolized zaprinast. Anesthesiology, 88, 410–416. [DOI] [PubMed] [Google Scholar]
- Ignarro, L. J. (2019). Nitric oxide is not just blowing in the wind. British Journal of Pharmacology, 176, 131–134. 10.1111/bph.14540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignarro, L. J. , Bush, P. A. , Buga, G. M. , Wood, K. S. , Fukuto, J. M. , & Rajfer, J. (1990). Nitric oxide and cyclic GMP formation upon electrical field stimulation cause relaxation of corpus cavernosum smooth muscle. Biochemical and Biophysical Research Communications, 170, 843–850. [DOI] [PubMed] [Google Scholar]
- Inserte, J. , Barba, I. , Poncelas‐Nozal, M. , Hernando, V. , Agulló, L. , Ruiz‐Meana, M. , & Garcia‐Dorado, D. (2011). cGMP/PKG pathway mediates myocardial postconditioning protection in rat hearts by delaying normalization of intracellular acidosis during reperfusion. Journal of Molecular and Cellular Cardiology, 50, 903–909. [DOI] [PubMed] [Google Scholar]
- Inserte, J. , & Garcia‐Dorado, D. (2015). The cGMP/PKG pathway as a common mediator of cardioprotection: Translatability and mechanism. British Journal of Pharmacology, 172, 1996–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson, G. , Benjamin, N. , Jackson, N. , & Allen, M. J. (1999). Effects of sildenafil citrate on human hemodynamics. The American Journal of Cardiology, 83, 13–20. [DOI] [PubMed] [Google Scholar]
- Jamnicki‐Abegg, M. , Weihrauch, D. , Chiari, P. C. , Krolikowski, J. G. , Pagel, P. S. , Warltier, D. C. , & Kersten, J. R. (2007). Diabetes abolishes sildenafil‐induced cGMP‐dependent protein kinase‐I expression and cardioprotection. Journal of Cardiovascular Pharmacology, 50, 670–676. 10.1097/FJC.0b013e318157fd5b [DOI] [PubMed] [Google Scholar]
- Jannini, E. A. , Isidori, A. M. , Gravina, G. L. , Aversa, A. , Balercia, G. , Bocchio, M. , … Lenzi, A. (2009). The ENDOTRIAL study: A spontaneous, open‐label, randomized, multicenter, crossover study on the efficacy of sildenafil, tadalafil, and vardenafil in the treatment of erectile dysfunction. The Journal of Sexual Medicine, 6, 2547–2560. [DOI] [PubMed] [Google Scholar]
- Jansson, P.‐A. , Murdolo, G. , Sjögren, L. , Nyström, B. , Sjöstrand, M. , Strindberg, L. , & Lönnroth, P. (2010). Tadalafil increases muscle capillary recruitment and forearm glucose uptake in women with type 2 diabetes. Diabetologia, 53, 2205–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph, P. , Leong, D. , McKee, M. , Anand, S. S. , Schwalm, J.‐D. , Teo, K. , … Yusuf, S. (2017). Reducing the global burden of cardiovascular disease, part 1: The epidemiology and risk factors. Circulation Research, 121, 677–694. [DOI] [PubMed] [Google Scholar]
- Kim, F. , Pham, M. , Maloney, E. , Rizzo, N. O. , Morton, G. J. , Wisse, B. E. , … Schwartz, M. W. (2008). Vascular inflammation, insulin resistance, and reduced nitric oxide production precede the onset of peripheral insulin resistance. Arteriosclerosis, Thrombosis, and Vascular Biology, 28, 1982–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloner, R. A. , Brown, M. , Prisant, L. M. , & Collins, M. (2001). Effect of sildenafil in patients with erectile dysfunction taking antihypertensive therapy. American Journal of Hypertension, 14, 70–73. 10.1016/s0895-7061(00)01177-8 [DOI] [PubMed] [Google Scholar]
- Kloner, R. A. , Mitchell, M. , & Emmick, J. T. (2003). Cardiovascular effects of tadalafil in patients on common antihypertensive therapies. The American Journal of Cardiology, 92, 47–57. [DOI] [PubMed] [Google Scholar]
- Koka, S. , Das, A. , Salloum, F. N. , & Kukreja, R. C. (2013). Phosphodiesterase‐5 inhibitor tadalafil attenuates oxidative stress and protects against myocardial ischemia/reperfusion injury in type 2 diabetic mice. Free Radical Biology & Medicine, 60, 80–88. [DOI] [PubMed] [Google Scholar]
- Koka, S. , Das, A. , Zhu, S.‐G. , Durrant, D. , Xi, L. , & Kukreja, R. C. (2010). Long‐acting phosphodiesterase‐5 inhibitor tadalafil attenuates doxorubicin‐induced cardiomyopathy without interfering with chemotherapeutic effect. The Journal of Pharmacology and Experimental Therapeutics, 334, 1023–1030. 10.1124/jpet.110.170191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koka, S. , Xi, L. , & Kukreja, R. C. (2012). Chronic treatment with long acting phosphodiesterase‐5 inhibitor tadalafil alters proteomic changes associated with cytoskeletal rearrangement and redox regulation in type 2 diabetic hearts. Basic Research in Cardiology, 107, 249. [DOI] [PubMed] [Google Scholar]
- Kouvelas, D. , Goulas, A. , Papazisis, G. , Sardeli, C. , & Pourzitaki, C. (2009). PDE5 inhibitors: in vitro and in vivo pharmacological profile. Current Pharmaceutical Design, 15, 3464–3475. [DOI] [PubMed] [Google Scholar]
- Kuboki, K. , Jiang, Z. Y. , Takahara, N. , Ha, S. W. , Igarashi, M. , Yamauchi, T. , … King, G. L. (2000). Regulation of endothelial constitutive nitric oxide synthase gene expression in endothelial cells and in vivo. Circulation, 101, 676–681. [DOI] [PubMed] [Google Scholar]
- Laursen, M. , Beck, L. , Kehler, J. , Christoffersen, C. T. , Bundgaard, C. , Mogensen, S. , … Simonsen, U. (2017). Novel selective PDE type 1 inhibitors cause vasodilatation and lower blood pressure in rats. British Journal of Pharmacology, 174, 2563–2575. 10.1111/bph.13868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, E. Y. , Park, J. K. , Lee, W. , Kim, Y. K. , Park, C. S.‐Y. , Giles, J. T. , … Lee, E. B. (2013). Head‐to‐head comparison of udenafil vs amlodipine in the treatment of secondary Raynaud's phenomenon: A double‐blind, randomized, cross‐over study. Rheumatology, 53, 658–664. [DOI] [PubMed] [Google Scholar]
- Levin, A. , Stevens, P. E. , Bilous, R. W. , Coresh, J. , De Francisco, A. L. , De Jong, P. E. , … Lamb, E. J. (2013). Kidney disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney International. Supplement, 3, 1–150. [Google Scholar]
- Limin, M. , Johnsen, N. , & Hellstrom, W. J. (2010). Avanafil, a new rapid‐onset phosphodiesterase 5 inhibitor for the treatment of erectile dysfunction. Expert Opinion on Investigational Drugs, 19, 1427–1437. [DOI] [PubMed] [Google Scholar]
- Liu, L. C. Y. , Hummel, Y. M. , van der Meer, P. , Berger, R. M. F. , Damman, K. , van Veldhuisen, D. J. , … Hoendermis, E. S. (2017). Effects of sildenafil on cardiac structure and function, cardiopulmonary exercise testing and health‐related quality of life measures in heart failure patients with preserved ejection fraction and pulmonary hypertension. European Journal of Heart Failure, 19, 116–125. 10.1002/ejhf.662 [DOI] [PubMed] [Google Scholar]
- Lue, T. F. (2000). Erectile dysfunction. The New England Journal of Medicine, 342, 1802–1813. 10.1056/NEJM200006153422407 [DOI] [PubMed] [Google Scholar]
- Madhani, M. , Hall, A. R. , Cuello, F. , Charles, R. L. , Burgoyne, J. R. , Fuller, W. , … Eaton, P. (2010). Phospholemman Ser69 phosphorylation contributes to sildenafil‐induced cardioprotection against reperfusion injury. American Journal of Physiology. Heart and Circulatory Physiology, 299, H827–H836. 10.1152/ajpheart.00129.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmud, A. , Hennessy, M. , & Feely, J. (2001). Effect of sildenafil on blood pressure and arterial wave reflection in treated hypertensive men. Journal of Human Hypertension, 15, 707–713. 10.1038/sj.jhh.1001244 [DOI] [PubMed] [Google Scholar]
- Malhotra, R. , Dhakal, B. P. , Eisman, A. S. , Pappagianopoulos, P. P. , Dress, A. , Weiner, R. B. , … Lewis, G. D. (2016). Pulmonary vascular distensibility predicts pulmonary hypertension severity, exercise capacity, and survival in heart failure. Circulation. Heart Failure, 9, e003011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammi, C. , Pastore, D. , Lombardo, M. F. , Ferrelli, F. , Caprio, M. , Consoli, C. , … Federici, M. (2011). Sildenafil reduces insulin‐resistance in human endothelial cells. PLoS ONE, 6, e14542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin, K. , Lytvyn, Y. , Luca, M. C. , Liuni, A. , Gori, T. , & Parker, J. D. (2014). Repeated daily dosing with sildenafil provides sustained protection from endothelial dysfunction caused by ischemia and reperfusion: A human in vivo study. American Journal of Physiology. Heart and Circulatory Physiology, 307, H888–H894. 10.1152/ajpheart.00215.2014 [DOI] [PubMed] [Google Scholar]
- Mehrotra, N. , Gupta, M. , Kovar, A. , & Meibohm, B. (2007). The role of pharmacokinetics and pharmacodynamics in phosphodiesterase‐5 inhibitor therapy. International Journal of Impotence Research, 19, 253–264. [DOI] [PubMed] [Google Scholar]
- Mergia, E. , & Stegbauer, J. (2016). Role of phosphodiesterase 5 and cyclic GMP in hypertension. Current Hypertension Reports, 18, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki, T. , Itoh, T. , Sunaga, D. , & Miura, T. (2012). Effects of diabetes on myocardial infarct size and cardioprotection by preconditioning and postconditioning. Cardiovascular Diabetology, 11, 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell, J. A. , & Kirkby, N. S. (2019). Eicosanoids, prostacyclin and cyclooxygenase in the cardiovascular system. British Journal of Pharmacology, 176, 1038–1050. 10.1111/bph.14167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morganroth, J. , Ilson, B. E. , Shaddinger, B. C. , Dabiri, G. A. , Patel, B. R. , Boyle, D. A. , … Montague, T. H. (2004). Evaluation of vardenafil and sildenafil on cardiac repolarization. The American Journal of Cardiology, 93, 1378–1383. [DOI] [PubMed] [Google Scholar]
- Muirhead, G. J. , Wilner, K. , Colburn, W. , Haug‐Pihale, G. , & Rouviex, B. (2002). The effects of age and renal and hepatic impairment on the pharmacokinetics of sildenafil. British Journal of Clinical Pharmacology, 53, 21S–30S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muirhead, G. J. , Wulff, M. B. , Fielding, A. , Kleinermans, D. , & Buss, N. (2000). Pharmacokinetic interactions between sildenafil and saquinavir/ritonavir. British Journal of Clinical Pharmacology, 50, 99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli, C. , & Ignarro, L. J. (2009). Nitric oxide and pathogenic mechanisms involved in the development of vascular diseases. Archives of Pharmacal Research, 32, 1103–1108. [DOI] [PubMed] [Google Scholar]
- Nehra, A. (2009). Erectile dysfunction and cardiovascular disease: Efficacy and safety of phosphodiesterase type 5 inhibitors in men with both conditions. Mayo Clinic Proceedings, 84, 139–148. 10.1016/S0025-6196(11)60822-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ockaili, R. , Salloum, F. , Hawkins, J. , & Kukreja, R. C. (2002). Sildenafil (Viagra) induces powerful cardioprotective effect via opening of mitochondrial K ATP channels in rabbits. American Journal of Physiology. Heart and Circulatory Physiology, 283, H1263–H1269. [DOI] [PubMed] [Google Scholar]
- Cavalcanti, C. D. O. , Alves, R. R. , de Oliveira, A. L. , de Campos Cruz, J. , França‐Silva, M. d. S. , de Andrade Braga, V. , & de Moura Balarini, C. (2016). Inhibition of PDE5 restores depressed baroreflex sensitivity in renovascular hypertensive rats. Frontiers in Physiology, 7, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver, J. J. , Dear, J. W. , & Webb, D. J. (2010). Clinical potential of combined organic nitrate and phosphodiesterase type 5 inhibitor in treatment‐resistant hypertension. Hypertension, 56, 62–67. 10.1161/HYPERTENSIONAHA.109.147686 [DOI] [PubMed] [Google Scholar]
- Oliver, J. J. , Kerr, D. M. , & Webb, D. J. (2009). Time‐dependent interactions of the hypotensive effects of sildenafil citrate and sublingual glyceryl trinitrate. British Journal of Clinical Pharmacology, 67, 403–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver, J. J. , Melville, V. P. , & Webb, D. J. (2006). Effect of regular phosphodiesterase type 5 inhibition in hypertension. Hypertension, 48, 622–627. 10.1161/01.HYP.0000239816.13007.c9 [DOI] [PubMed] [Google Scholar]
- Olsen, M. H. , Angell, S. Y. , Asma, S. , Boutouyrie, P. , Burger, D. , Chirinos, J. A. , … Hering, D. (2016). A call to action and a lifecourse strategy to address the global burden of raised blood pressure on current and future generations: The Lancet Commission on hypertension. Lancet, 388, 2665–2712. [DOI] [PubMed] [Google Scholar]
- Omarjee, L. , Le Pabic, E. , Custaud, M.‐A. , Fontaine, C. , Locher, C. , Renault, A. , … Mahé, G. (2019). Effects of sildenafil on maximum walking time in patients with arterial claudication: The ARTERIOFIL study. Vascular Pharmacology, 118‐119, 106563. [DOI] [PubMed] [Google Scholar]
- Paick, J. S. , Ahn, T. Y. , Choi, H. K. , Chung, W. S. , Kim, J. J. , Kim, S. C. , … Moon, K. H. (2008). Efficacy and safety of mirodenafil, a new oral phosphodiesterase type 5 inhibitor, for treatment of erectile dysfunction. The Journal of Sexual Medicine, 5, 2672–2680. 10.1111/j.1743-6109.2008.00945.x [DOI] [PubMed] [Google Scholar]
- Paick, J. S. , Kim, S. W. , Yang, D. Y. , Kim, J. J. , Lee, S. W. , Ahn, T. Y. , … Kim, S. C. (2008). The efficacy and safety of udenafil, a new selective phosphodiesterase type 5 inhibitor, in patients with erectile dysfunction. The Journal of Sexual Medicine, 5, 946–953. 10.1111/j.1743-6109.2007.00723.x [DOI] [PubMed] [Google Scholar]
- Parker, J. D. , Bart, B. A. , Webb, D. J. , Koren, M. J. , Siegel, R. L. , Wang, H. , … Glue, P. (2007). Safety of intravenous nitroglycerin after administration of sildenafil citrate to men with coronary artery disease: A double‐blind, placebo‐controlled, randomized, crossover trial. Critical Care Medicine, 35, 1863–1868. 10.1097/01.CCM.0000269371.70738.30 [DOI] [PubMed] [Google Scholar]
- Pauls, M. M. , Moynihan, B. , Barrick, T. R. , Kruuse, C. , Madigan, J. B. , Hainsworth, A. H. , & Isaacs, J. D. (2018). The effect of phosphodiesterase‐5 inhibitors on cerebral blood flow in humans: A systematic review. Journal of Cerebral Blood Flow & Metabolism, 38, 189–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkovic, V. , Jardine, M. J. , Neal, B. , Bompoint, S. , Heerspink, H. J. , Charytan, D. M. , … Bull, S. (2019). Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. The New England Journal of Medicine, 380, 2295–2306. 10.1056/NEJMoa1811744 [DOI] [PubMed] [Google Scholar]
- Pickering, T. G. , Shepherd, A. M. , Puddey, I. , Glasser, D. B. , Orazem, J. , Sherman, N. , & Mancia, G. (2004). Sildenafil citrate for erectile dysfunction in men receiving multiple antihypertensive agents: A randomized controlled trial. American Journal of Hypertension, 17, 1135–1142. 10.1016/j.amjhyper.2004.07.004 [DOI] [PubMed] [Google Scholar]
- Pokreisz, P. , Vandenwijngaert, S. , Bito, V. , Van den Bergh, A. , Lenaerts, I. , Busch, C. , … Biesmans, L. (2009). Ventricular phosphodiesterase‐5 expression is increased in patients with advanced heart failure and contributes to adverse ventricular remodeling after myocardial infarction in mice. Circulation, 119, 408–416. 10.1161/CIRCULATIONAHA.108.822072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomara, G. , Morelli, G. , Pomara, S. , Taddei, S. , Ghiadoni, L. , Dinelli, N. , … Salvetti, A. (2004). Cardiovascular parameter changes in patients with erectile dysfunction using PDE‐5 inhibitors: A study with sildenafil and vardenafil. Journal of Andrology, 25, 625–629. 10.1002/j.1939-4640.2004.tb02833.x [DOI] [PubMed] [Google Scholar]
- Pomeranz, H. D. (2016). The relationship between phosphodiesterase‐5 inhibitors and nonarteritic anterior ischemic optic neuropathy. Journal of Neuro‐Ophthalmology, 36, 193–196. [DOI] [PubMed] [Google Scholar]
- Pulido, T. , Adzerikho, I. , Channick, R. N. , Delcroix, M. , Galiè, N. , Ghofrani, H. A. , … Simonneau, G. (2013). Macitentan and morbidity and mortality in pulmonary arterial hypertension. The New England Journal of Medicine, 369, 809–818. 10.1056/NEJMoa1213917 [DOI] [PubMed] [Google Scholar]
- Quinaglia, T. , de Faria, A. P. C. , Fontana, V. , Barbaro, N. R. , Sabbatini, A. R. , Sertório, J. T. , … Moreno, H. (2013). Acute cardiac and hemodynamic effects of sildenafil on resistant hypertension. European Journal of Clinical Pharmacology, 69, 2027–2036. 10.1007/s00228-013-1571-z [DOI] [PubMed] [Google Scholar]
- Ramirez, C. E. , Nian, H. , Yu, C. , Gamboa, J. L. , Luther, J. M. , Brown, N. J. , & Shibao, C. A. (2015). Treatment with sildenafil improves insulin sensitivity in prediabetes: A randomized, controlled trial. The Journal of Clinical Endocrinology and Metabolism, 100, 4533–4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramseyer, V. D. , Ortiz, P. A. , Carretero, O. A. , & Garvin, J. L. (2016). Angiotensin II‐mediated hypertension impairs nitric oxide‐induced NKCC2 inhibition in thick ascending limbs. American Journal of Physiology. Renal Physiology, 310, F748–F754. 10.1152/ajprenal.00473.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray, K. K. , Cannon, C. P. , Morrow, D. A. , Kirtane, A. J. , Buros, J. , Rifai, N. , … Braunwald, E. (2007). Synergistic relationship between hyperglycaemia and inflammation with respect to clinical outcomes in non‐ST‐elevation acute coronary syndromes: Analyses from OPUS‐TIMI 16 and TACTICS‐TIMI 18. European Heart Journal, 28, 806–813. 10.1093/eurheartj/ehm010 [DOI] [PubMed] [Google Scholar]
- Redfield, M. M. , Chen, H. H. , Borlaug, B. A. , Semigran, M. J. , Lee, K. L. , Lewis, G. , … Trial, R. E. L. A. X. (2013). Effect of phosphodiesterase‐5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: A randomized clinical trial. JAMA, 309, 1268–1277. 10.1001/jama.2013.2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo, N. O. , Maloney, E. , Pham, M. , Luttrell, I. , Wessells, H. , Tateya, S. , … Kim, F. (2010). Reduced NO‐cGMP signaling contributes to vascular inflammation and insulin resistance induced by high‐fat feeding. Arteriosclerosis, Thrombosis, and Vascular Biology, 30, 758–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roustit, M. , Blaise, S. , Allanore, Y. , Carpentier, P. H. , Caglayan, E. , & Cracowski, J.‐L. (2013). Phosphodiesterase‐5 inhibitors for the treatment of secondary Raynaud's phenomenon: Systematic review and meta‐analysis of randomised trials. Annals of the Rheumatic Diseases, 72, 1696–1699. 10.1136/annrheumdis-2012-202836 [DOI] [PubMed] [Google Scholar]
- Roustit, M. , Giai, J. , Gaget, O. , Khouri, C. , Mouhib, M. , Lotito, A. , … Cracowski, J.‐L. (2018). On‐demand sildenafil as a treatment for Raynaud phenomenon: A series of n‐of‐1 trials. Annals of Internal Medicine, 169, 694–703. 10.7326/M18-0517 [DOI] [PubMed] [Google Scholar]
- Rubin, L. J. , Badesch, D. B. , Fleming, T. R. , Galiè, N. , Simonneau, G. , Ghofrani, H. A. , … McLaughlin, V. V. (2011). Long‐term treatment with sildenafil citrate in pulmonary arterial hypertension: The SUPER‐2 study. Chest, 140, 1274–1283. [DOI] [PubMed] [Google Scholar]
- Sabatini, S. , Sgrò, P. , Duranti, G. , Ceci, R. , & Di Luigi, L. (2011). Tadalafil alters energy metabolism in C2C12 skeletal muscle cells. Acta Biochimica Polonica, 58, 237–241. [PubMed] [Google Scholar]
- Sahara, M. , Sata, M. , Morita, T. , Nakajima, T. , Hirata, Y. , & Nagai, R. (2010). A phosphodiesterase‐5 inhibitor vardenafil enhances angiogenesis through a protein kinase G–dependent hypoxia‐inducible factor‐1/vascular endothelial growth factor pathway. Arteriosclerosis, Thrombosis, and Vascular Biology, 30, 1315–1324. [DOI] [PubMed] [Google Scholar]
- Salloum, F. N. , Abbate, A. , Das, A. , Houser, J.‐E. , Mudrick, C. A. , Qureshi, I. Z. , … Prabhakar, S. (2008). Sildenafil (Viagra) attenuates ischemic cardiomyopathy and improves left ventricular function in mice. American Journal of Physiology. Heart and Circulatory Physiology, 294, H1398–H1406. [DOI] [PubMed] [Google Scholar]
- Salloum, F. N. , Takenoshita, Y. , Ockaili, R. A. , Daoud, V. P. , Chou, E. , & Yoshida, K.‐i., & Kukreja, R. C. (2007). Sildenafil and vardenafil but not nitroglycerin limit myocardial infarction through opening of mitochondrial K ATP channels when administered at reperfusion following ischemia in rabbits. Journal of Molecular and Cellular Cardiology, 42, 453–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salt, I. P. , Morrow, V. A. , Brandie, F. M. , Connell, J. M. , & Petrie, J. R. (2003). High glucose inhibits insulin‐stimulated nitric oxide production without reducing endothelial nitric‐oxide synthase Ser1177 phosphorylation in human aortic endothelial cells. The Journal of Biological Chemistry, 278, 18791–18797. 10.1074/jbc.M210618200 [DOI] [PubMed] [Google Scholar]
- Sanchez, L. S. , De La Monte, S. M. , Filippov, G. , Jones, R. C. , Zapol, W. M. , & Bloch, K. D. (1998). Cyclic‐GMP‐binding, cyclic‐GMP‐specific phosphodiesterase (PDE5) gene expression is regulated during rat pulmonary development. Pediatric Research, 43, 163–168. 10.1203/00006450-199802000-00002 [DOI] [PubMed] [Google Scholar]
- Santi, D. , Granata, A. R. M. , Guidi, A. , Pignatti, E. , Trenti, T. , Roli, L. , … Simoni, M. (2016). Six months of daily treatment with vardenafil improves parameters of endothelial inflammation and of hypogonadism in male patients with type 2 diabetes and erectile dysfunction: A randomized, double‐blind, prospective trial. 174, 513. [DOI] [PubMed] [Google Scholar]
- Santos, R. C. , de Faria, A. P. C. , Barbaro, N. R. , Modolo, R. , Ferreira‐Melo, S. E. , Matos‐Souza, J. R. , … Moreno, H. (2014). Tadalafil‐induced improvement in left ventricular diastolic function in resistant hypertension. European Journal of Clinical Pharmacology, 70, 147–154. [DOI] [PubMed] [Google Scholar]
- Scheele, W. , Diamond, S. , Gale, J. , Clerin, V. , Tamimi, N. , Le, V. , … Rolph, T. (2016). Phosphodiesterase type 5 inhibition reduces albuminuria in subjects with overt diabetic nephropathy. J Am Soc Nephrol, 27, 3459–3468. 10.1681/ASN.2015050473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz, B. G. , Jackson, G. , Stecher, V. J. , Campoli‐Richards, D. M. , & Kloner, R. A. (2013). Phosphodiesterase type 5 inhibitors improve endothelial function and may benefit cardiovascular conditions. The American Journal of Medicine, 126, 192–199. 10.1016/j.amjmed.2012.08.015 [DOI] [PubMed] [Google Scholar]
- Senthilkumar, A. , Smith, R. D. , Khitha, J. , Arora, N. , Veerareddy, S. , Langston, W. , … Patel, R. P. (2007). Sildenafil promotes ischemia‐induced angiogenesis through a PKG‐dependent pathway. Arteriosclerosis, Thrombosis, and Vascular Biology, 27, 1947–1954. [DOI] [PubMed] [Google Scholar]
- Sharp, A. , Cornforth, C. , Jackson, R. , Harrold, J. , Turner, M. A. , Kenny, L. , … Alfirevic, Z. (2019). Mortality in the UK STRIDER trial of sildenafil therapy for the treatment of severe early‐onset fetal growth restriction. Lancet Child Adolesc Health, 3, e2–e3. 10.1016/S2352-4642(19)30020-3 [DOI] [PubMed] [Google Scholar]
- Shenoy, P. D. , Kumar, S. , Jha, L. K. , Choudhary, S. K. , Singh, U. , Misra, R. , & Agarwal, V. (2010). Efficacy of tadalafil in secondary Raynaud's phenomenon resistant to vasodilator therapy: A double‐blind randomized cross‐over trial. Rheumatology, 49, 2420–2428. 10.1093/rheumatology/keq291 [DOI] [PubMed] [Google Scholar]
- Sherifali, D. , Nerenberg, K. , Pullenayegum, E. , Cheng, J. E. , & Gerstein, H. C. (2010). The effect of oral antidiabetic agents on A1C levels. Diabetes Care, 33, 1859–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonneau, G. , Montani, D. , Celermajer, D. S. , Denton, C. P. , Gatzoulis, M. A. , Krowka, M. , … Souza, R. (2019). Haemodynamic definitions and updated clinical classification of pulmonary hypertension. The European Respiratory Journal, 53. 1801913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuehr, D. J. , & Haque, M. M. (2019). Nitric oxide synthase enzymology in the 20 years after the Nobel Prize. British Journal of Pharmacology, 176, 177–188. 10.1111/bph.14533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan, P. , Liu, L. , Wei, S. , Tang, Z. , Yang, L. , & Wei, Q. (2017). The effect of oral phosphodiesterase‐5 inhibitors on sperm parameters: A meta‐analysis and systematic review. Urology, 105, 54–61. 10.1016/j.urology.2017.02.032 [DOI] [PubMed] [Google Scholar]
- Tapia, E. , Sanchez‐Lozada, L. G. , Soto, V. , Manrique, A. M. , Ortiz‐Vega, K. M. , Santamaría, J. , … Pedraza‐Chaverri, J. (2012). Sildenafil treatment prevents glomerular hypertension and hyperfiltration in rats with renal ablation. Kidney & Blood Pressure Research, 35, 273–280. 10.1159/000334952 [DOI] [PubMed] [Google Scholar]
- Tateya, S. , Rizzo, N. O. , Handa, P. , Cheng, A. M. , Morgan‐Stevenson, V. , Daum, G. , … Kim, F. (2011). Endothelial NO/cGMP/VASP signaling attenuates Kupffer cell activation and hepatic insulin resistance induced by high‐fat feeding. Diabetes, 60, 2792–2801. 10.2337/db11-0255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrett, N. K. , Bell, A. S. , Brown, D. , & Ellis, P. (1996). Sildenafil (VIAGRA™), a potent and selective inhibitor of type 5 cGMP phosphodiesterase with utility for the treatment of male erectile dysfunction. Bioorganic & Medicinal Chemistry Letters, 6, 1819–1824. [Google Scholar]
- Varma, A. , Das, A. , Hoke, N. N. , Durrant, D. E. , Salloum, F. N. , & Kukreja, R. C. (2012). Anti‐inflammatory and cardioprotective effects of tadalafil in diabetic mice. PLoS ONE, 7, e45243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, C. C. L. , Hess, C. N. , Hiatt, W. R. , & Goldfine, A. B. (2016). Clinical update: Cardiovascular disease in diabetes mellitus. Circulation, 133, 2459–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, R. , Burnett, A. L. , Heller, W. H. , Omori, K. , Kotera, J. , Kikkawa, K. , … Peterson, C. A. (2012). Selectivity of avanafil, a PDE5 inhibitor for the treatment of erectile dysfunction: Implications for clinical safety and improved tolerability. The Journal of Sexual Medicine, 9, 2122–2129. [DOI] [PubMed] [Google Scholar]
- Wang, X. , Fisher, P. W. , Xi, L. , & Kukreja, R. C. (2008). Essential role of mitochondrial Ca2+‐activated and ATP‐sensitive K+ channels in sildenafil‐induced late cardioprotection. Journal of Molecular and Cellular Cardiology, 44, 105–113. 10.1016/j.yjmcc.2007.10.006 [DOI] [PubMed] [Google Scholar]
- Wardle, A. J. , Seager, M. J. , Wardle, R. , Tulloh, R. M. R. , & Gibbs, J. S. R. (2016). Guanylate cyclase stimulators for pulmonary hypertension. Cochrane Database of Systematic Reviews, CD011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb, D. J. , Freestone, S. , Allen, M. J. , & Muirhead, G. J. (1999). Sildenafil citrate and blood‐pressure–lowering drugs: Results of drug interaction studies with an organic nitrate and a calcium antagonist. The American Journal of Cardiology, 83, 21–28. [DOI] [PubMed] [Google Scholar]
- Wolk, R. , Smith, W. B. , Neutel, J. M. , Rubino, J. , Xuan, D. , Mancuso, J. , … Pressler, M. L. (2009). Blood pressure lowering effects of a new long‐acting inhibitor of phosphodiesterase 5 in patients with mild to moderate hypertension. Hypertension, 53, 1091–1097. [DOI] [PubMed] [Google Scholar]
- Wortsman, X. , Del Barrio‐Díaz, P. , Meza‐Romero, R. , Poehls‐Risco, C. , & Vera‐Kellet, C. (2018). Nifedipine cream versus sildenafil cream for patients with secondary Raynaud phenomenon: A randomized, double‐blind, controlled pilot study. Journal of the American Academy of Dermatology, 78, 189–190. [DOI] [PubMed] [Google Scholar]
- Zhang, R. L. , Zhang, Z. G. , & Chopp, M. (2013). Targeting nitric oxide in the subacute restorative treatment of ischemic stroke. Expert Opinion on Investigational Drugs, 22, 843–851. 10.1517/13543784.2013.793672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X. , Ji, J. , Yan, G. , Wu, J. , Sun, X. , Shen, J. , … Wang, H. (2010). Sildenafil promotes adipogenesis through a PKG pathway. Biochemical and Biophysical Research Communications, 396, 1054–1059. [DOI] [PubMed] [Google Scholar]
- Ziegler, J. W. , Ivy, D. D. , Wiggins, J. W. , Kinsella, J. P. , Clarke, W. R. , & Abman, S. H. (1998). Effects of dipyridamole and inhaled nitric oxide in pediatric patients with pulmonary hypertension. American Journal of Respiratory and Critical Care Medicine, 158, 1388–1395. 10.1164/ajrccm.158.5.9710117 [DOI] [PubMed] [Google Scholar]


