Abstract
Although slow gastric emptying (gastroparesis) is a well-known complication of chronic hyperglycemia in diabetes mellitus (DM), it recently has become clear that rapid gastric emptying also is a frequent and important diabetic complication. In contrast, acute hyperglycemia causes slow gastric emptying, and acute hypoglycemia causes rapid gastric emptying. Rapid gastric emptying is frequent in T2DM; however, it may also occur in T1DM, particularly in the early stages of the disease, but may persist even into late stages. Recent studies suggest that usually, the stomach restricts the emptying of nutrients to 1–4 kcals/min. This restriction is due to the action of the gastric ‘braking’ hormones such as GLP-1, leptin, and amylin acting via the gastric inhibitory vagal motor circuit (GIVMC). Disruption of this braking system leads to rapid gastric emptying. Acute hyperglycemia also slows gastric emptying by stimulating the GIVMC, while acute hypoglycemia causes rapid gastric emptying by stimulating the gastric excitatory vagal motor circuit (GEVMC). In contrast, chronic hyperglycemia causes rapid gastric emptying by inducing oxidative stress in the stomach wall that disrupts inhibitory neuromuscular transmission and increases the contractility of the smooth muscle, while chronic hyperglycemia may also cause slow gastric emptying via severe inflammatory stress caused by proinflammatory macrophages and reduce contractility of the smooth muscle. There is a bidirectional relationship between blood glucose and gastric emptying. Thus, rapid gastric emptying may lead to a sizeable postprandial spike, and slow gastric emptying may blunt it. Postprandial hyperglycemia is involved in the development, progression, and complications of DM. Correction of fast gastric emptying involves agents that activate GIVMC and the use of gastric ‘braking’ hormones or their analogs. Recognition and treatment of rapid gastric emptying may contribute to better management of postprandial hyperglycemia and prevention of some diabetic complications.
Keywords: Gastric emptying, Rapid gastric emptying, Diabetes mellitus, Pathophysiology, postprandial Hyperglycemia, Hypoglycemia
1. Introduction
The stomach is responsible for the intake of food, its blenderization to form chyme (semiliquid food), and provision of highly regulated timely caloric load to the intestines. The intestinal nutrient load determines 1) glucose levels in the blood and 2) utilization of the blood glucose by secretion of incretins and subsequent secretion of insulin and suppression of glucagon secretion. Thus, gastric emptying plays a central role in postprandial glycemia.
Rapid gastric emptying may manifest itself widely from severe gastrointestinal symptoms of ‘dumping syndrome,’ to milder and even asymptomatic forms. Moreover, the effect of gastric emptying on blood glucose levels depends on multiple factors including the size, content, and timing of meals, the rate of glucose absorption into the blood, release of intestinal hormones such as incretins, and the release of insulin. Thus, fast gastric emptying may be associated with (1) reactive hypoglycemia,42 (2) amelioration of hyperglycemia in obese T2DM by bariatric surgery,29 and (3) severe postprandial hyperglycemia due to insufficiency of incretins or insulin.
Blood glucose levels have a complicated bidirectional relationship with gastric emptying rate. On the one hand, the rate of gastric emptying is a crucial determinant of postprandial glycemia because it influences the timing and a load of nutrients delivered to the intestine. The intestinal nutrient load affects both glucose absorption and the release of incretin hormones. Blood glucose and incretin hormones regulate insulin and glucagon secretion, which regulate blood glucose levels. Moreover, small changes in the rate of gastric emptying may cause considerable variability in blood glucose levels. Postprandial hyperglycemia and glucose variability contribute importantly to the pathogenesis of T2DM and its complications and have important implications for patient management.43
On the other hand, acute hyperglycemia and chronic hyperglycemia in DM cause a spectrum of changes in gastric emptying (Fig. 1). Acute hyperglycemia causes transient slowing of gastric emptying, while acute hypoglycemia causes rapid gastric emptying. Originally, ‘diabetic gastroparesis’ was described in a case report of a patient with persistent (at baseline) slow gastric emptying that was thought to be a complication of untreated T1DM32; subsequent studies have revealed that milder forms of delayed gastric emptying are present in one-third to one-half of patients with long-standing T1DM or T2DM.5,6,50 Although there have been sporadic reports of rapid gastric emptying in DM, this important diabetic complication has been mostly ignored. Nevertheless, it is now clear that rapid gastric emptying is a significant complication of DM.5,6,47 By causing fast gastric emptying, chronic hyperglycemia augments postprandial glucose and worsens DM.
Fig. 1.
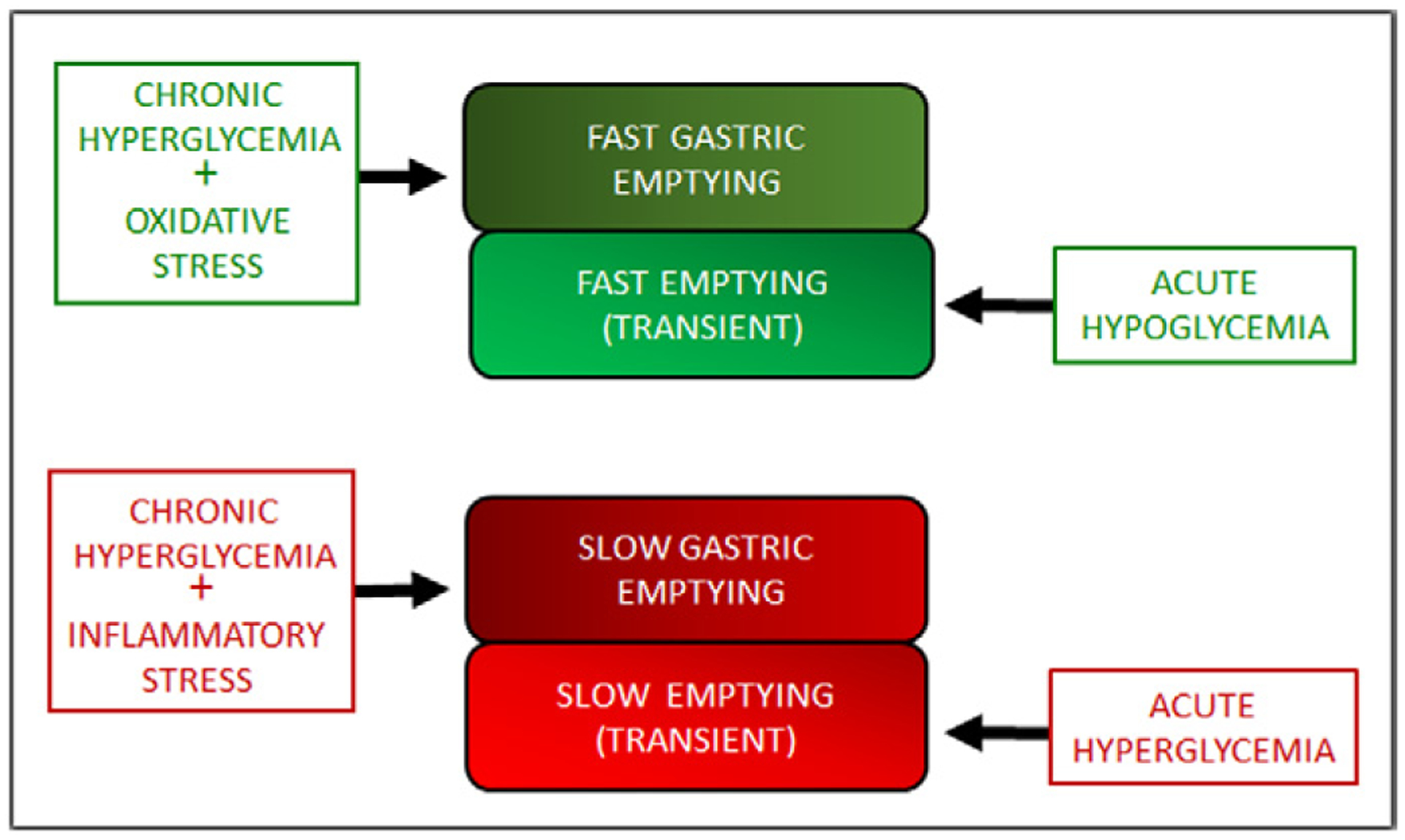
Spectrum of gastric emptying abnormalities in acute hyperglycemia and hypoglycemia and in chronic hyperglycemia associated with diabetes mellitus. Acute hypoglycemia is associated with transient rapid gastric emptying and acute hyperglycemia is associated with transient slow gastric emptying. On the other hand, chronic hyperglycemia may be associated with either basal rapid gastric emptying or slow gastric emptying.
The purpose of this review is to summarize the pathogenesis of rapid gastric emptying caused by acute hypoglycemia and chronic hyperglycemia and discuss the prevalence, clinical impact and rational therapeutic considerations based on the underlying pathophysiology of rapid gastric emptying.
2. Regulation of gastric emptying
Gastric emptying in the digestive (prandial) and inter-digestive (fasting) periods is highly regulated. Physical characteristics of food and its caloric density are important regulators of gastric emptying, which is mediated by anatomic segments of the stomach that perform different motor functions. The gastric fundus and proximal corpus form the ‘pressure pump,’ and the distal corpus and proximal antrum comprise the ‘peristaltic pump,’ and the distal antrum and pylorus are the ‘grinder/filter.’ Decreased motor activity of the pressure and peristaltic pumps slows gastric emptying, while inadequate relaxation (accommodation) and increased contractile activity of the peristaltic pump result in rapid gastric emptying.9,25 Current concepts suggest that the motor activity of the gastric pumps is regulated by the gastric inhibitory vagal motor circuit (GIVMC), whose stimulation slows gastric emptying, and by the gastric excitatory vagal motor circuit (GEVMC), whose stimulation hastens gastric emptying. These circuits include vagal afferents, interneurons in the nucleus tractus solitarius (NTS), vagal motor preganglionic neurons in the DMV, vagal efferent fibers, postganglionic neurons in the gastric myenteric plexus and their projections on the smooth muscle cells.25
During the digestive period (lasting 2–3 h after a quick meal), gastric emptying of chyme (semiliquid food processed by the stomach) delivered into the duodenum is usually limited to a caloric value of 4 kcals/min.60 During this period, the stomach holds back all large food particles and indigestible food residues.25 Neurohormonal regulation of gastro-duodenal motility is responsible for the tight control of gastric emptying. The so-called gastric ‘breaking’ hormones released from the intestine and pancreas play a critical role in the management of gastric emptying in the digestive period.59 These hormones slow gastric emptying primarily by stimulating the GIVMC at various levels. They also cause early satiety by acting on the pro-opiomelanocortin (POMC) neurons in the NTS.63 In contrast, long-term reduced food intake and increased energy expenditure are mediated by the POMC neurons in the hypothalamus.25 Table 1 lists some of the braking hormones. Out of these, GLP-1, leptin, and amylin have received much attention.
Table 1.
Gastric ‘braking’ hormones.
|
Glucagon-like peptide (GLP-1) is a hormone and a neurotransmitter. As a hormone, it is produced by L cells in the intestine where glucose and bile salts act to stimulate its release.1 As a neurotransmitter, it is present in the pre-proglucagon (PPG) cells in the NTS and POMC neurons in the NTS and the hypothalamus. In the NTS, it is an important part of the GIVMC, and its stimulation causes slow gastric emptying and transient satiety.63 GLP-1 also stimulates insulin secretion and inhibits glucagon secretion (incretin effect). The enzyme dipeptidyl-peptidase IV (DPP-IV) rapidly degrades GLP-1. Although GLP-1 deficiency has not been documented to cause T2DM, GLP-1 analogs and inhibitors of PPD-IV are useful in the treatment of T2DM. Leptin is primarily produced from the fat white cells (adipokine leptin) but is also produced in the stomach (gastric leptin).13 Effective action of adipokine leptin is to reduce food intake via the POMC neurons in the hypothalamus, and that of the gastric leptin may be on the GIVMC and the POMC neurons in the NTS.63 Congenital deficiency of leptin or its receptors in humans or animals produces a phenotype with hyperglycemia due to 1) loss of slow gastric emptying leading to fast gastric emptying, resulting in a high intestinal glucose load, and 2) gain of insulin resistance by increased glucagon secretion. Amylin (pancreatic amyloid polypeptide) is co-secreted with insulin by the beta cells. It inhibits glucagon secretion and causes slow gastric emptying by its actions in the NTS and brain stem likely similar to other gastric ‘braking’ hormones. Amylin deficiency may be associated with nonobese NIDDM.26 Loss of function of the gastric braking hormones results in fast gastric emptying of nutrients and postprandial hyperglycemia associated with T2DM. Genetically leptin-deficient, ob/ob mice or leptin receptor-deficient, Leprdb/db mice additionally have loss of satiety signals and energy consumption resulting in weight gain and obesity. These mutants are extensively used models of obese T2DM.19
After the digestive period is over, in the inter-digestive period, gastric emptying is designed to expel food-residues by forceful migrating motor complexes. Hormones ghrelin and motilin activate the migrating motor complexes. These hormones act by inhibiting GIVMC and stimulating the GEVMC to accelerate gastric emptying.25
3. Determination of gastric emptying rate
The gastric emptying rate is experimentally determined to be the time taken when only 50% of the ingested meal remains in the stomach. Gastric emptying tests have utilized different test meals and techniques. Scintigraphic estimation of the gastric residue of liquid or digestible solid or a mixed standardized-meals labeled with 99 m technetium sulfur colloid is considered the gold standard for determining gastric emptying in the digestive period. Bharucha and colleagues6 assessed gastric emptying by scintigraphy using a 300-kcal mixed meal containing 99mTc sulfur labeled egg and established the normal values as 11–39% at 1 h, 40–76% at 2 h, 84–98% at 4 h. Values below the normal range at 1 or 2 h defined rapid gastric emptying and values above the normal range at 2 or 4 h defined slow gastric emptying.
Currently, the gastric emptying breath test using the stable isotope, 13C octanoic acid or 13C-Spirulina platensis labeled standard meal has gained favor. In this test, the subject ingests a 13C labeled standardized meal, and breath samples are collected at baseline and at different time intervals for 4 h. Labeled carbon dioxide is measured using mass spectrometry in each breath sample. T50(t1/2) of 13C excretion is calculated using the Wagner-Nelson method.55 The results from this method compare well with scintigraphy and allow for calculation of a caloric rate of gastric emptying of both liquid and solid meals.55 The rate of gastric emptying shows a low intraindividual variability but a large interindividual variability. Using a standardized semisolid meal of 65 g powdered potato, 20 g glucose, constituted with 200 mL water and one egg yolk (368.5 kcal, 61.4 g carbohydrate, 7.4 g protein, and 8.9 g fat), the rate of gastric emptying ranged from 2.2 to 3.9 (mean 2.3 ± 0.1) kcal/min in young, healthy controls.61 In T2DM, the gastric emptying ranged from 1.6 to 4.7 (mean 2.8 ± 0.1) kcal/min and 1.6–3.3 (mean 3 ± 0.1) kcals/min in age-matched older subjects.61
Normal range gastric emptying in the healthy subjects is considered between 10th–90th percentile of the observed T ½ values. In a 13C-Spirulina gastric emptying breath test using 223 kcal (19.2 g carbohydrates, 12 g protein, and 10.9 g fat) the 10th–90th percentile range for t ½ in healthy subjects was 50–92 min. Hence, values <50 and >92 min reflect rapid and delayed gastric emptying, respectively.5,6,8 Determination of tlag, t15, and t30 may be particularly useful in the assessment of fast gastric emptying.7
In the USA, the 13C-Spirulina gastric emptying breath test (GEBT) has been approved by the FDA and is commercially available (Cairn Diagnostics (Brentwood, TN)). This kit includes detailed instructions for the standard food labeled with 13C-Spirulina and collection of breath samples. After the test, the samples are mailed to Cairn Diagnostics for determination of the gastric emptying rates. This development has determined the gastric emptying studies suitable for everyday use.
The acetaminophen absorption test measures the emptying rate of liquids only, whereas the wireless capsule (Smart Capsule) provides information on the gastric emptying of food residue in the inter-digestive period. However, the Smart Capsule does not provide information on the emptying of nutrients in the digestive period. For gastric emptying of nutrients, emptying of liquid and digestible solids during the early periods should be used.
4. Transient rapid gastric emptying in acute hypoglycemia
Several studies have reported that acute hyperglycemia causes slow gastric emptying in the fasting state or during postprandial hyperglycemia in healthy or diabetic subjects.50 In contrast, acute hypoglycemia causes counter-regulatory fast gastric emptying in healthy or diabetic subjects.40 ‘Iatrogenic hypoglycemia’ is a common and serious complication associated with insulin treatment in T1DM or T2DM with slow gastric emptying. Delayed gastric emptying reduces the postprandial glucose spike, and failure to reduce the dose and adjust the timing of insulin administration may lead to hypoglycemia40 and severe neurologic damage.18
4.1. Pathogenesis
In contrast to acute hyperglycemia that acts to slow gastric emptying by stimulating the GIVMC,64 acute hypoglycemia causes rapid gastric emptying by activating the GEVMC to the gastric antrum (Fig. 2). GABA neurons exert a complex inhibitory and excitatory effect on the adjoining neurons.3 Lamy and colleagues35 have shown that hypoglycemia stimulates GLUT2 positive GABA neurons in the NTS that activate the GEVMC and sympathoadrenal pathways. Activation of GEVMC leads to fast gastric emptying and stimulation of the sympathoadrenal pathway, triggering counter-regulatory responses, including secretion of glucagon. The effect of hypoglycemia is mediated by reduced intracellular glucose metabolism, increased AMP-activated protein kinase activity, and closure of leak channels. Glucose is the primary fuel for the neurons. Hypoglycemia is associated with the activation of counter-regulatory hormonal and neuroendocrine responses in an attempt to restore glucose to safe levels. However, recurrent hypoglycemia impairs these protective mechanisms, resulting in a potentially life-threatening condition known as a hypoglycemia-associated autonomic failure (HAAF)18 and ‘Impaired awareness of hypoglycemia’ (IAH).40 Pathogenesis of HAAF is not well understood; it may be due to the upregulation of the enzyme glucose kinase.
Fig. 2.
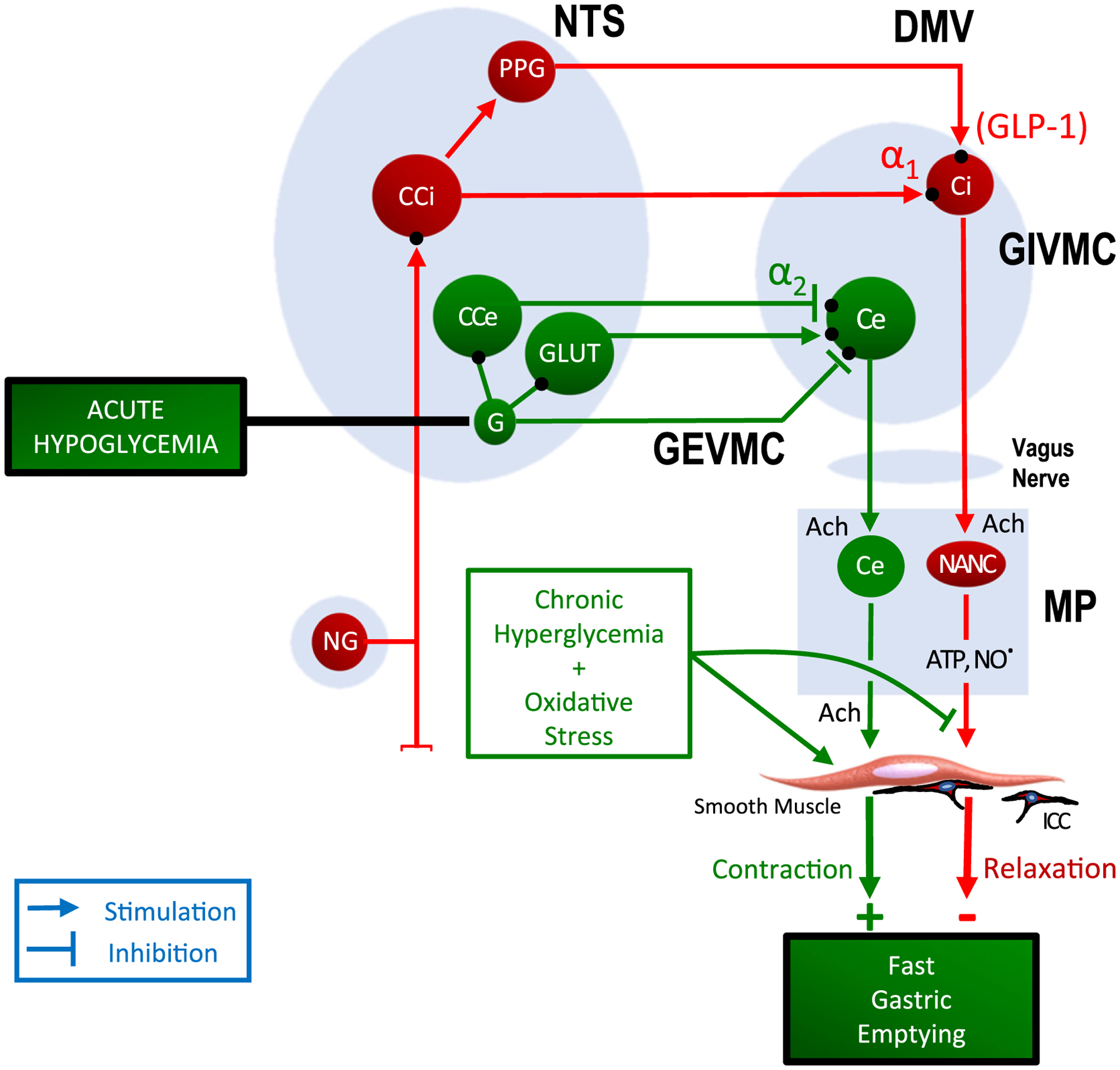
Sites of action of acute hypoglycemia and chronic hyperglycemia causing rapid gastric emptying. Acute hypoglycemia causes fast gastric emptying by stimulating the gastric excitatory vagal motor circuit (GEVMC) (shown in green). The GEVMC includes glutaminergic (GLUT) neurons and catecholaminergic neuros of the gastric excitatory pathway (CCe) located in the nucleus tractus solitarius (NTS). NTS-GLUT neurons project on to the cholinergic neurons of the gastric excitatory pathway (Ce) that are located in the dorsal motor nucleus of the vagus nerve (DMV). The DMV-Ce neurons send preganglionic vagal fibers to postganglionic cholinergic neurons of the gastric excitatory pathway (Ce) located in the myenteric plexus in the gastric wall. The MP-Ce neurons release the excitatory neurotransmitter acetylcholine and cause contraction of the smooth muscle. The effect of acute hypoglycemia on GEVMC is mediated by GABA interneurons in the NTS. Stimulation of the GEVMC to gastric pumps leads to increase gastric emptying.25 In contrast, chronic hyperglycemia causes rapid gastric emptying by suppression of the gastric inhibitory vagal circuit, thus decreasing inhibitory neuromusclular transmission and increasing contractility of smooth muscle.
5. Rapid gastric emptying in chronic hyperglycemia in DM
Chronic hyperglycemia in diabetes mellitus (DM) may be associated with either fast or slow gastric emptying. Clinicians and investigators traditionally have focused primarily on slow gastric emptying and gastroparesis, although there have been many sporadic reports of rapid gastric emptying of liquids and solid meals in long-standing DM. Cases of rapid gastric emptying are often overlooked or included in the control group with normal gastric emptying as shown by. Nowak and colleagues.47
5.1. Pathogenesis
In contrast to the slow gastric emptying that is associated with decreased contractility, rapid gastric emptying in DM is associated with increased contractility of the gastric fundus and proximal corpus (pressure pump) as well as the distal corpus and proximal antrum (peristaltic pump).47,9,25
In the digestive period, gastric emptying is slowed by the action of the gastric ‘braking’ hormones’ that are released from the intestines. Loss of function of the gastric ‘braking’ hormones such as GLP-1, leptin and amylin cause fast gastric emptying as well as other unrelated abnormalities including hyperglycemia. Therefore, the cause and effect relationship of hyperglycemia and fast gastric emptying have not been established. However, recent studies in animals have defined the biochemical abnormalities and mechanism of their action in the pathogenesis of fast gastric emptying in chronic hyperglycemia.
5.1.1. Biochemical abnormalities
The biochemical abnormalities in rapid gastric emptying include hyperglycemia-associatedoxidative stress.24 Chronic hyperglycemia has been shown to be associated with epigenetic changes in microRNAs that can mediate further oxidative stress. For example, downregulation of miR25 and upregulation of NOX4 leads to an incease in superoxide dismutase (SOD), an enzyme that may increase hydrogen peroxide (H2O2) production.38 Oxidative stress may cause rapid gastric emptying by affecting the functions of nerves and hormones, ICC and smooth muscles.
5.1.2. Neural abnormalities
In the early stages of DM development in nonobese diabetic (NOD) mice with rapid gastric emptying, there was loss of inhibitory purinergic and nitrergic transmission that may adversely impact the contractility of both the pressure and peristaltic pumps of the stomach. The loss of purinergic transmission may be due to 1) oxidative stress-associated mitochondrial damage with loss of ATP production; 2) reduced activity of glucokinase due to persistent high glucose load, which also depresses ATP synthesis; 3) impaired intracellular transport of purinergic neurotransmitter-containing vesicles for exocytosis at the varicosity membrane; and 4) loss of inhibitory neurons. Similarly, loss of nitrergic NMT may be due to 1) loss of nitrergic neurons; 2) translational downregulation and metabolic alteration in the enzyme nNOSα; 3) impaired intracellular transport of nNOSα to the varicosity membrane for release of the nitrergic neurotransmitter; and 4) elevated levels of ROS in diabetic oxidative stress causing accelerated scavenging of the neurotransmitter nitric oxide.28
5.1.3. Increased interstitial cells of cajal (ICC)
Recently, Hayashi and colleagues27 suggested that rapid gastric emptying in Leprdb/db mice was due to gain in the function of the c-kit and ICC due to oxidative stress. Hyperplasia of undefined ICC occurred despite reduced signaling of the insulin-like growth factor 1-dependent Kit ligand that typically sustains ICC. Oxidative stress may activate p38 MAPK1 and MAPK3 that stabilizes the transcription factor ETSV1, leading to upregulation of the c-kit and gain in the number of ICC.27 The increased ICC number may enhance slow waves and cause enhanced smooth muscle contractility. However, in another model of an increased number of undefined ICC [Kit (V558Delta)/+ mice], spontaneous electrical activity and post-junctional neural responses in the stomach are reportedly normal.34
5.1.4. Increased Smooth Muscle Contractility
Smooth muscle is the final mediator of gastric motor function. Several studies have reported that gastric smooth muscle from hyperglycemic Leprdb/db mice exhibits enhanced contractility in response to excitatory stimuli.27,38 Similar changes in gastric smooth muscles were reported in Akita mice and other animal models of T1DM.33,38 Moreover, hyperglycemic NOD mice demonstrate depolarization of smooth muscle membrane potential and attenuation of non-adrenergic non-cholinergic inhibitory neurotransmission, consistent with enhanced smooth muscle excitability.28
The hypercontractile nature of smooth muscle may be due to genetic and epigenetic changes in actively multiplying muscle cells, such as those reacting to oxidative stress. Oxidative stress results in downregulation of miR-133a, which is associated with upregulation of RhoA/ROCK, which decreases myosin light chain phosphatase (MLCP) activity and enhances myosin light chain kinase (MLCK) activity and smooth muscle contractility.38 Other factors may also contribute to the hypercontractility of smooth muscles. High glucose levels cause an opening of L-type calcium channels in vascular smooth muscles.33,48 The loss of nitrergic inhibitory neurotransmission may also upregulate Rho/ROCK signaling and increase smooth muscle contractility. Increased contractility in the smooth muscle of the gastric fundus and antrum is expected to accelerate gastric emptying in vivo.25,27 These changes are different from those implicated in the pathogenesis of slow gastric emptying in DM.17
5.2. Clinical implications of rapid gastric emptying
5.2.1. Symptoms of rapid gastric emptying
Rapid gastric emptying in diabetes may be associated with early ‘dumping syndrome’ manifested by post-prandial nausea, bloating, light-headedness, flushing, palpitations, and abdominal pain, often with cramps, borborygmi, and diarrhea.4 However, most patients with rapid gastric emptying demonstrable by scintigraphy have no upper gastrointestinal symptoms. Nearly two-thirds of patients with poorly controlled T2DM were asymptomatic yet had abnormal gastric emptying. Moreover, any symptoms, when present, correlate poorly with the rate of gastric emptying.50
5.2.2. Prevalence of rapid gastric emptying
Gastric consequences of long-standing DM are geneally attributed to slow gastric emptying. However, many reports of rapid gastric emptying of liquids and solid meals have been described. Nowak et al.47 investigated solid phase gastric emptying scintiscans using in vivo labeled chicken liver in complicated insulin-dependent diabetics. When compared to healthy controls, the mean values were not different, but emptying rates in diabetic patients were highly variable. A careful analysis of the data revealed that emptying rates were slow in 62% and rapid in 38% of patients. This study did not recognize cases with normal gastric emptying. However, recent studies of patients with poorly controlled, advanced T1DM have found normal gastric emptying in 42%, delayed gastric emptying in 30%, and rapid gastric emptying in 22% of the cases.6 In another study of poorly controlled T2DM (glycosylated hemoglobin, >9%) gastric emptying was normal in 33%, delayed in 47%, and accelerated in 20% of cases.8 About one-fifth of poorly controlled, long standing T1DM or T2DM diabetic subjects have rapid gastric emptying (Fig. 3).6,8 A recent study has reported that gastric emptying was faster in well-controlled T2DM than appropriate controls.61
Fig. 3.
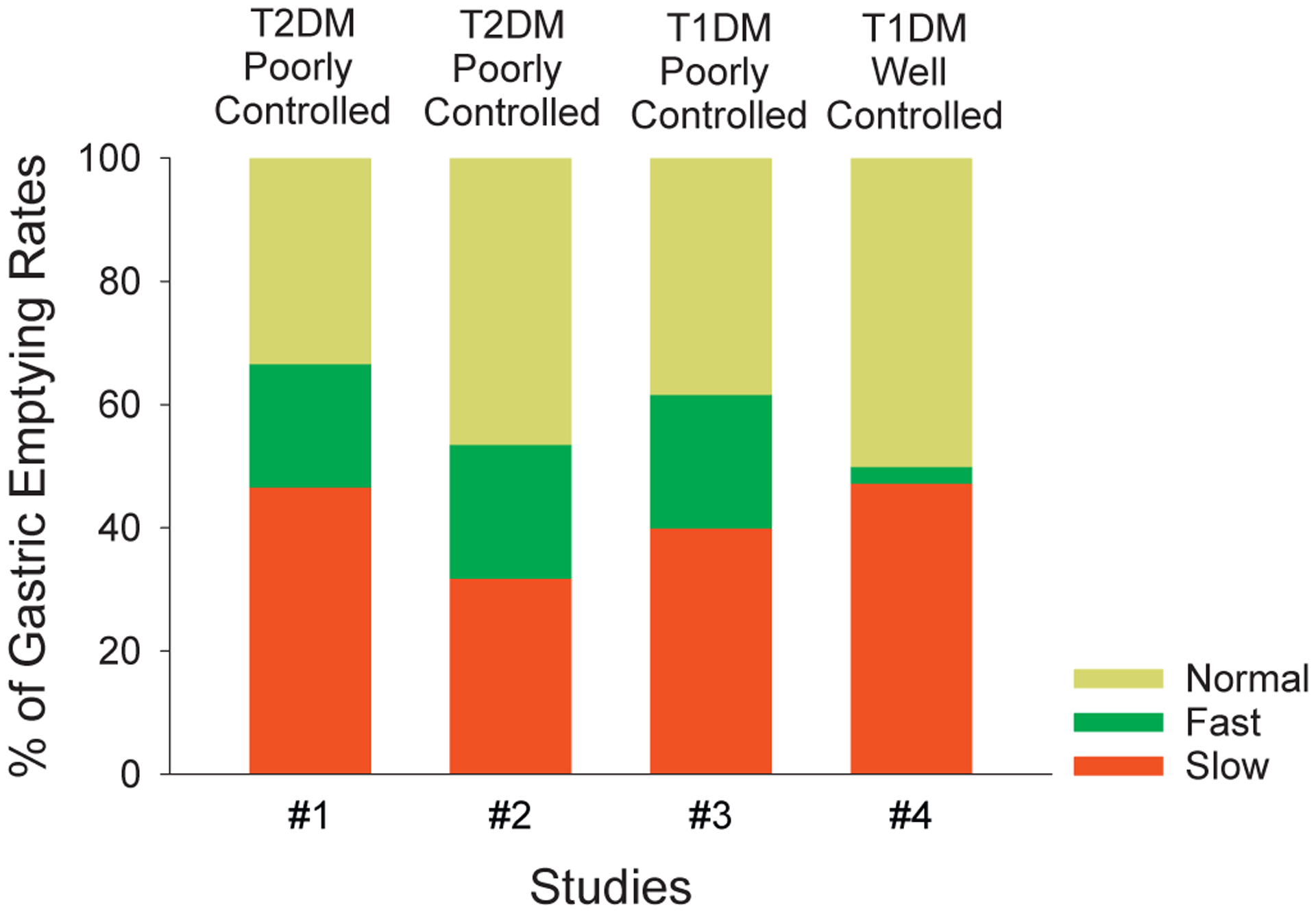
Effects of intensive control of glycemia on the prevalence of rapid gastric emptying. Prevalence of slow, rapid and normal gastric emptying in poorly controlled T2DM (#1), mixed T1DM and T2DM (#2), and T1DM (#3); and well controlled T1DM (#4). Note that prevalence of slow gastric emptying was similar in all poorly controlled studies of T1DM or T2DM. Moreover, these rates were not significantly different from that in the well-controlled T1DM study. The prevalence of rapid gastric emptying was also similar (20%–22%) in all the poorly controlled groups. However, in the well-controlled T1DM study there was significant reduction (to 5%) in the rapid gastric emptying (p < 0.001). #1 This study includes 30 patients with poorly controlled T2DM8 #2 This study includes information on 69 patients with T2DM6 #3This study includes information on 60 patients with poorly controlled T1DM6 #4 This study includes 74 patients with well-controlled cases with TIDM.5
Studies in animal models of T1DM and T2DM revealed that the prevalence of rapid gastric emptying in DM depends on the stage of the disease. A longitudinal study that serially examined gastric emptying in NOD mice (model of T1DM) showed that in the early stages of development of hyperglycemia, almost all animals exhibited rapid gastric emptying, but on follow-up, only a small proportion of mice had slow gastric emptying.16 A recent study in leptin deficient Leprdb/db mice showed that these mice have accelerated gastric emptying.27 It has also been reported that the basal gastric emptying rate in young Lepob/ob mice was significantly faster than in older Lepob/ob mice.2 These observations are consistent with the concept that rapid gastric emptying may occur in the early stages of DM. Interestingly, rapid gastric emptying has been found to be common in adolescents with DM.49
Limited information is available on the natural history of gastric emptying in DM. In a study on 20 patients with different types of DM, gastric emptying remained unchanged over a 12 year period.31 A subsequent follow-up of 8 of these patients over 25 years also failed to show any change in gastric emptying.15
5.3. Rapid gastric emptying and development and progression of DM
Rapid gastric emptying is associated with a large increase in blood glucose at 2-h during oral glucose tolerance test and progression to type 2 diabetes.39 Moreover, “early” type 2 diabetes has been associated with more rapid gastric emptying.52 Rapid gastric emptying has also been observed more frequently in certain ethnic groups and disease states such as morbid obesity that are known to be predisposed to type 2 diabetes.14,51 These studies show that rapid gastric emptying occurs in early stages of hyperglycemia in T2DM. These studies, however, do not make clear whether rapid gastric emptying or hyperglycemia is the primary abnormality. Studies in NOD mice showed that loss of inhibitory transmission was seen only after the establishment of hyperglycemia, but not before.28 These observations suggest that it is the early chronic hyperglycemia that may be the cause of accelerated gastric emptying.
It has been reported that even in subjects with normal glucose tolerance test, a low oral glucose disposition index may predict the development of future diabetes above and beyond fasting and 2 h glucose levels.58 The oral disposition index is the product of the early insulin secretory response during an oral glucose tolerance test and insulin sensitivity. Moreover, in subjects with normal glucose tolerance test, rapid gastric emptying is associated with decrease in early insulin secretory response and the disposition index, suggesting that rapid gastric emptying may predispose to development of T2DM.41 Further studies are needed to test this interesting possibility.
5.4. Reversibility of rapid gastric emptying
Whether the changes due to chronic hyperglycemia and oxidative stress on fast gastric emptying are reversible is not known. However, data on gastric emptying from an extension of the Diabetes Control and Complications Trial (DCCT), the Epidemiology of Diabetic Interventions and Complications (EDIC) study is of considerable interest in this regard. This multicenter study included 74 patients with T1DM with intensive control of hyperglycemia over 20 years. Gastric emptying was assessed using the 13C-spirulina breath test. This study found that in these patients with T1DM, tight control of blood sugar is associated with slow gastric emptying in 47%, normal gastric emptying in 48% and rapid gastric emptying in only 5% of the cases.5 As summarized in Fig. 3, the prevalence of slow gastric emptying in the intensively treated cases was similar to that reported in poorly controlled T1DM or T2DM. These data indicate that the complication of slow gastric emptying does not change over time and is not responsive to treatment of hyperglycemia. However, the prevalence of fast gastric emptying was significantly lower than in other studies with poorly controlled T1DM or T2DM (Fig. 3).5,6,8 The differential response of slow gastric emptying and fast gastric emptying is reminiscent of the observation that reasonable control of hyperglycemia was associated with improvement in the microvascular but not the macrovascular complications of DM.21 Interestingly, smooth muscle hypercontractility is common to both fast gastric emptying and microvascular complications. Further studies are needed to test this hypothesis.
5.5. Effect of rapid gastric emptying on postprandial hyperglycemia and associated diabetic complications
Rapid gastric emptying increases basal blood sugar as well as glycemic variability including the postprandial hyperglycemia. Basal hyperglycemia is the main contributor to HbA1c levels, however postprandial hyperglycemia also contributes significantly to the HbA1c levels. The postprandial glucose level is also dependent upon the characteristics of the food, processing in the stomach, and delivery into the small bowel. The rate of duodenal exposure to nutrients, such as glucose, determines intestinal absorption as well as secretion of the intestinal ‘breaking’ hormones such as incretins, amylin and leptin.59 Other things being equal, the rate of gastric emptying determines the rate of duodenal exposure to glucose and the postprandial hyperglycemia. Studies in T2DM and healthy subjects have shown that gastric emptying has a significant impact on the early glycemic response.30 After a standardized meal, increments in blood glucose were closely related to gastric emptying.61 Gastric emptying is a major determinant of postprandial glycemia to a glucose drink or a high carbohydrate solid meal,50 and may contribute an almost one-third rise in postprandial glucose.30
Rapid gastric emptying contributes to HbA1c levels, but it specifically contributes to postprandial hyperglycemia. At lower HbA1c levels, postprandial glucose is a prominent contributor to HbA1c levels.57 Treatment studies have shown that the improvement in HbA1c levels with intensive insulin therapy is associated with improvement in microvascular but not macrovascular complications in T1DM.46 However, postprandial hyperglycemia and other causes of blood glucose variability (GV), including episodes of iatrogenic hypoglycemia and the counterregulatory hyperglycemia associated with intensive insulin treatment,40 may be independent risk factors for microvascular, as well as macrovascular complications including coronary artery disease. Availability of continuous glucose monitoring (CGM) is helping to define the pathogenesis and the role of glucose variability on complications of DM including macrovascular complications.12,20,21,57
6. Therapeutic implications of rapid gastric emptying
Since fast gastric emptying is one of the main determinants of the postprandial hyperglycemic spike, recognition and management of rapid gastric emptying may be helpful in the control of glycemia and prevention of diabetic complications. A variety of maneuvers that slow gastric emptying is successful in the treatment of postprandial hyperglycemia in T2DM. Suitability and effectiveness of these maneuvers may depend on the stage of the disease, dietary habits including carbohydrate intake and underlying pathophysiology of hyperglycemia. Therefore, their use may require individualization and personalized treatment. A detailed discussion of these treatments is outside the scope of this review. However, we list here some classes of treatments that exert at least part of their action by slowing gastric emptying and have been effective in the treatment of T2DM. Even small changes in gastric emptying may have a significant impact on postprandial hyperglycemia. For example, in T2DM subjects, slowing of gastric emptying even in those with gastric emptying within the normal range may show improvement in postprandial hyperglycemia.44,54 Many agents that slow gastric emptying also have other effects such as stimulation of insulin secretion and inhibition of glucagon secretion as well as suppression of appetite and reduction in food intake (e.g., leptin). Some of the methods that affect fast gastric emptying to cause slow gastric emptying are listed below.
1). Pre-prandial nutrient preloads
Pre-prandial preload of nutrients such as whey proteins ingested 15 min before a meal can attenuate postprandial glycemic excursions. Whey proteins are rich in branched-chain amino acids that have a robust insulinotropic effect45 and also stimulate the release of incretins, and slow gastric emptying. Although whey proteins also stimulate glucagon release, the net effect is to lower postprandial glucose excursion.60 Nutrient preload has been shown to improve the efficacy of the dipeptidyl peptidase-4 (DPP-4) inhibitor vildagliptin to increase incretin concentrations, slow gastric emptying, and lower postprandial glycemia in type 2 diabetes.62 It is important to point out that pre-load with materials that enter the duodenum in the inter-digestive period may not affect the rate of emptying of nutrients in the digestive period. The effect of the preload of whey protein is sustained over 4 weeks.37
2). Pharmacological agents
Pharmacological agents used in the treatment of T2DM that exert at least a part of their beneficial effect by countering rapid gastric emptying include the following.
Metformin is a commonly used first-line treatment for new cases of T2DM. Metformin may act in part by slowing gastric emptying in T2DM leading to lowering of postprandial glucose and triglycerides.56
- Incretin based agents
- GLP-1 receptor agonists such as exenatide exert part of their effect by slowing gastric emptying via GLP-1 receptor mediated stimulation of GIVMC. They also enhance insulin secretion and depress glucagon secretion. Exenatide slows gastric emptying10 and reduces postprandial hyperglycemiac not only in T2DM patients with rapid gastric emptying but also in those with normal gastric emptying but not in those with delayed gastric emptying.36
- Dipeptidyl peptidase-4 inhibitors (DPP4i) suppress the break-down of the endogenous GLP-1 and thus enhance the availability of GLP-1 to stimulates insulin secretion, inhibit glucagon secretion and also stimulate GIVMC to slow gastric emptying.22
Amylin is co-released with insulin from beta cells. Pramlintide is injectable amylin. It slows gastric emptying and causes satiety. The exact site of action of amylin is not currently known. However, it inhibits glucagon secretion.26
Leptin is secreted primarily from brown fat cells. However, leptin is also secreted by the chief cell in the stomach.13 Congenital deficiency of leptin is a model for obese T2DM. A few cases of human congenital leptin deficiency have been reported. Interestingly these cases have responded to recombinant leptin.19
α-glucosidase inhibitors such as acarbose act to delay intestinal absorption of monosaccharides such as glucose. α-glucosidase inhibitors also increase GLP-1 secretion and GLP-1 mediated slowing of gastric emptying, and stimulate insulin secretion and inhibit glucagon secretion, thereby reducing postprandial hyperglycemia. α-glucosidase inhibitors may be of particular importance in patients who consume high carbohydrate diets.23
-
Control of glycemia with intensive insulin therapy
There is some suggestion that intensive control of glycemia in T1DM is associated with a reduced complication of fast gastric emptying but not slow gastric emptying5,6,8 (Fig. 3). Therefore, control of glycemia may be useful in the reversal of fast gastric emptying.
-
Control of oxidative stress and reducing gastric smooth muscle contractility
Since oxidative stress and increased contractility of gastric muscle is responsible for fast gastric emptying,38 countering oxidative stress11 and reducing the smooth muscle excitability53 may provide new targets for drugs that may act to reverse rapid gastric emptying.
7. Conclusions
This review reveals that: 1) rapid gastric emptying is now established as a frequent, independent complication of DM. 2) Iatrogenic hypoglycemia is a serious complication of insulin therapy in DM. Acute hypoglycemia causes transient rapid gastric emptying by activating the gastric excitatory vagal motor circuit and the sympathoadrenal axis that attempts to reverse hypoglycemia and to alarm the patient of the danger. 3) Chronic hyperglycemia causes lasting rapid gastric emptying by inducing oxidative stress to suppress nitrergic neurotransmission and increase contractility of the gastric smooth muscles via genetic and epigenetic modifications. 4) Rapid gastric emptying is seen in the early stages of the disease and is equally frequent in T1DM and T2DM and may reverse with control of hyperglycemia. 5) While chronic hyperglycemia causes rapid gastric emptying, rapid gastric emptying leads to postprandial hyperglycemia. 6) Postprandial hyperglycemia contributes to overall glycemic state as measured by HbA1c and to glycemic variability as determined by continuous glucose monitoring. HbA1c levels are an important determinant of microvascular complications of diabetes mellitus. 7) Postprandial hyperglycemia and glycemic variability are independent risk factors for diabetic complications. 8) Control of rapid gastric emptying may have important implications in the therapy of T2DM. Currently used treatments of T2DM that act, at least in part, by slowing gastric emptying include preload with whey protein, and use of gastric ‘braking’ hormones such as incretin-based agents and amylin. 9) Determination of the gastric emptying status of diabetic subjects, particularly T2DM, using the recently available gastric emptying breath test that is performed in the clinic, may advance the personalized management of DM.
Funding
Funded by William S. Middleton Award (RKG), BX002806 (RKG), and BX001790 (MPS) from the U.S. Department of Veterans Affairs, Biomedical Laboratory Research and Development Service.
Footnotes
Declaration of competing interest: Authors declare no conflict of interest.
References
- 1.Albaugh VL, Banan B, Antoun J, Xiong Y, Guo Y, Ping J, et al. Role of bile acids and GLP-1 in mediating the metabolic improvements of bariatric surgery. Gastroenterology 2019;156 (1041–1051.e1044). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asakawa A, Inui A, Ueno N, Makino S, Uemoto M, Fujino MA, et al. Ob/ob mice as a model of delayed gastric emptying. J Diabetes Complications 2003;17:27–8. [DOI] [PubMed] [Google Scholar]
- 3.Babic T, Browning KN, Travagli RA. Differential organization of excitatory and inhibitory synapses within the rat dorsal vagal complex. Am J Physiol Gastrointest Liver Physiol 2011;300:G21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berg P, McCallum R. Dumping syndrome: a review of the current concepts of pathophysiology, diagnosis, and treatment. Dig Dis Sci 2016;61:11–8. [DOI] [PubMed] [Google Scholar]
- 5.Bharucha AE, Batey-Schaefer B, Cleary PA, Murray JA, Cowie C, Lorenzi G, et al. Delayed gastric emptying is associated with early and long-term hyperglycemia in type 1 diabetes mellitus. Gastroenterology 2015;149:330–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bharucha AE, Camilleri M, Forstrom LA, Zinsmeister AR. Relationship between clinical features and gastric emptying disturbances in diabetes mellitus. Clin Endocrinol (Oxf) 2009;70:415–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bharucha AE, Camilleri M, Veil E, Burton D, Zinsmeister AR. Comprehensive assessment of gastric emptying with a stable isotope breath test. Neurogastroenterol Motil 2013;25:e60–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bharucha AE, Kudva Y, Basu A, Camilleri M, Low PA, Vella A, et al. Relationship between glycemic control and gastric emptying in poorly controlled type 2 diabetes. Clin Gastroenterol Hepatol 2015;13 (466–476.e461). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bharucha AE, Manduca A, Lake DS, Fidler J, Edwards P, Grimm RC, et al. Gastric motor disturbances in patients with idiopathic rapid gastric emptying. Neurogastroenterol Motil 2011;23:617–e252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhavsar S, Mudaliar S, Cherrington A. Evolution of exenatide as a diabetes therapeutic. Curr Diabetes Rev 2013;9:161–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bigagli E, Lodovici M. Circulating oxidative stress biomarkers in clinical studies on type 2 diabetes and its complications. Oxid Med Cell Longev 2019;2019, 5953685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brownlee M, Hirsch IB. Glycemic variability: a hemoglobin A1c-independent risk factor for diabetic complications. Jama 2006;295:1707–8. [DOI] [PubMed] [Google Scholar]
- 13.Cammisotto P, Bendayan M. A review on gastric leptin: the exocrine secretion of a gastric hormone. Anat Cell Biol 2012;45:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cardoso-Junior A, Coelho LG, Savassi-Rocha PR, Vignolo MC, Abrantes MM, de Almeida AM, et al. Gastric emptying of solids and semi-solids in morbidly obese and non-obese subjects: an assessment using the 13C-octanoic acid and 13C-acetic acid breath tests. Obes Surg 2007;17:236–41. [DOI] [PubMed] [Google Scholar]
- 15.Chang J, Russo A, Bound M, Rayner CK, Jones KL, Horowitz M. A 25-year longitudinal evaluation of gastric emptying in diabetes. Diabetes Care 2012;35:2594–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi KM, Zhu J, Stoltz GJ, Vernino S, Camilleri M, Szurszewski JH, et al. Determination of gastric emptying in nonobese diabetic mice. Am J Physiol Gastrointest Liver Physiol 2007;293:G1039–45. [DOI] [PubMed] [Google Scholar]
- 17.Cipriani G, Gibbons SJ, Miller KE, Yang DS, Terhaar ML, Eisenman ST, et al. Change in populations of macrophages promotes development of delayed gastric emptying in mice. Gastroenterology 2018;154 (2122–2136.e2112). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cryer PE. Hypoglycemia in diabetes: pathophysiological mechanisms and diurnal variation. Prog Brain Res 2006;153:361–5. [DOI] [PubMed] [Google Scholar]
- 19.D’Souza AM, Neumann UH, Glavas MM, Kieffer TJ. The glucoregulatory actions of leptin. Mol Metab 2017;6:1052–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DECODE. Glucose tolerance and cardiovascular mortality: comparison of fasting and 2-hour diagnostic criteria. Arch Intern Med 2001;161:397–405. [DOI] [PubMed] [Google Scholar]
- 21.Fullerton B, Jeitler K, Seitz M, Horvath K, Berghold A, Siebenhofer A. Intensive glucose control versus conventional glucose control for type 1 diabetes mellitus. Cochrane Database Syst Rev 2014(2), Cd009122. 10.1002/14651858.CD009122.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gallwitz B Clinical use of DPP-4 inhibitors. Front Endocrinol (Lausanne) 2019;10:389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao X, Cai X, Yang W, Chen Y, Han X, Ji L. Meta-analysis and critical review on the efficacy and safety of alpha-glucosidase inhibitors in Asian and non-Asian populations. J Diabetes Investig 2018;9:321–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res 2010;107:1058–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goyal RK, Guo Y, Mashimo H. Advances in the physiology of gastric emptying. Neurogastroenterol Motil 2019;31, e13546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hay DL, Chen S, Lutz TA, Parkes DG, Roth JD. Amylin: pharmacology, physiology, and clinical potential. Pharmacol Rev 2015;67:564–600. [DOI] [PubMed] [Google Scholar]
- 27.Hayashi Y, Toyomasu Y, Saravanaperumal SA, Bardsley MR, Smestad JA, Lorincz A, et al. Hyperglycemia increases interstitial cells of cajal via MAPK1 and MAPK3 signaling to ETV1 and KIT, leading to rapid gastric emptying. Gastroenterology 2017;153 (521–535.e520). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He XD, Guo YM, Goyal RK. Effect of hyperglycemia on purinergic and nitrergic inhibitory neuromuscular transmission in the antrum of the stomach: implications for fast gastric emptying. Front Med (Lausanne) 2018;5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holst JJ, Madsbad S, Bojsen-Moller KN, Svane MS, Jorgensen NB, Dirksen C, et al. Mechanisms in bariatric surgery: gut hormones, diabetes resolution, and weight loss. Surg Obes Relat Dis 2018;14:708–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horowitz M, Edelbroek MA, Wishart JM, Straathof JW. Relationship between oral glucose tolerance and gastric emptying in normal healthy subjects. Diabetologia 1993;36:857–62. [DOI] [PubMed] [Google Scholar]
- 31.Jones KL, Russo A, Berry MK, Stevens JE, Wishart JM, Horowitz M. A longitudinal study of gastric emptying and upper gastrointestinal symptoms in patients with diabetes mellitus. Am J Med 2002;113:449–55. [DOI] [PubMed] [Google Scholar]
- 32.Kassander P Asymptomatic gastric retention in diabetics (gastroparesis diabeticorum). Ann Intern Med 1958;48:797–812. [DOI] [PubMed] [Google Scholar]
- 33.Kato T, Imaeda K, Okayama N, Yamada K, Mizuno T, Kimura R, et al. Alteration of the responses of gastric smooth muscle to endothelin in streptozotocin-induced diabetic rats. J Smooth Muscle Res 2007;43:191–9. [DOI] [PubMed] [Google Scholar]
- 34.Kwon JG, Hwang SJ, Hennig GW, Bayguinov Y, McCann C, Chen H, et al. Changes in the structure and function of ICC networks in ICC hyperplasia and gastrointestinal stromal tumors. Gastroenterology 2009;136:630–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lamy CM, Sanno H, Labouebe G, Picard A, Magnan C, Chatton JY, et al. Hypoglycemia-activated GLUT2 neurons of the nucleus tractus solitarius stimulate vagal activity and glucagon secretion. Cell Metab 2014;19:527–38. [DOI] [PubMed] [Google Scholar]
- 36.Lu JM. The role of glucagon-like peptide-1receptor agonists in type 2 diabetes in Asia . Adv Ther 2019;36:798–805. [DOI] [PubMed] [Google Scholar]
- 37.Ma J, Jesudason DR, Stevens JE, Keogh JB, Jones KL, Clifton PM, et al. Sustained effects of a protein ‘preload’ on glycaemia and gastric emptying over 4 weeks in patients with type 2 diabetes: a randomized clinical trial. Diabetes Res Clin Pract 2015;108: e31–4. [DOI] [PubMed] [Google Scholar]
- 38.Mahavadi S, Sriwai W, Manion O, Grider JR, Murthy KS. Diabetes-induced oxidative stress mediates upregulation of RhoA/Rho kinase pathway and hypercontractility of gastric smooth muscle. PLoS One 2017;12, e0178574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marathe CS, Horowitz M, Trahair LG, Wishart JM, Bound M, Lange K, et al. Relationships of early and late glycemic responses with gastric emptying during an oral glucose tolerance test. J Clin Endocrinol Metab 2015;100:3565–71. [DOI] [PubMed] [Google Scholar]
- 40.Marathe CS, Marathe JA, Rayner CK, Kar P, Jones KL, Horowitz M. Hypoglycaemia and gastric emptying. Diabetes Obes Metab 2019;21:491–8. [DOI] [PubMed] [Google Scholar]
- 41.Marathe CS, Rayner CK, Lange K, Bound M, Wishart J, Jones KL, et al. Relationships of the early insulin secretory response and oral disposition index with gastric emptying in subjects with normal glucose tolerance. Physiol Rep 2017;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Middleton SJ, Balan K. Idiopathic accelerated gastric emptying presenting in adults with post-prandial diarrhea and reactive hypoglycemia: a case series. J Med Case Reports 2012;6:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mihai BM, Mihai C, Cijevschi-Prelipcean C, Grigorescu ED, Dranga M, Drug V, et al. Bidirectional relationship between gastric emptying and plasma glucose control in normoglycemic individuals and diabetic patients. J Diabetes Res 2018;2018:1736959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Monnier L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA(1c). Diabetes Care 2003;26:881–5. [DOI] [PubMed] [Google Scholar]
- 45.Mortensen LS, Holmer-Jensen J, Hartvigsen ML, Jensen VK, Astrup A, de Vrese M, et al. Effects of different fractions of whey protein on postprandial lipid and hormone responses in type 2 diabetes. Eur J Clin Nutr 2012;66:799–805. [DOI] [PubMed] [Google Scholar]
- 46.Nathan DM. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care 2014;37:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nowak TV, Johnson CP, Kalbfleisch JH, Roza AM, Wood CM, Weisbruch JP, et al. Highly variable gastric emptying in patients with insulin dependent diabetes mellitus. Gut 1995;37:23–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nystoriak MA, Nieves-Cintron M, Patriarchi T, Buonarati OR, Prada MP, Morotti S, et al. Ser1928 phosphorylation by PKA stimulates the L-type Ca2+ channel CaV1.2 and vasoconstriction during acute hyperglycemia and diabetes. Sci Signal 2017;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perano SJ, Rayner CK, Kritas S, Horowitz M, Donaghue K, Mpundu-Kaambwa C, et al. Gastric emptying is more rapid in adolescents with type 1 diabetes and impacts on postprandial glycemia. J Clin Endocrinol Metab 2015;100:2248–53. [DOI] [PubMed] [Google Scholar]
- 50.Phillips LK, Deane AM, Jones KL, Rayner CK, Horowitz M. Gastric emptying and glycaemia in health and diabetes mellitus. Nat Rev Endocrinol 2015;11:112–28. [DOI] [PubMed] [Google Scholar]
- 51.Phillips WT, Schwartz JG, McMahan CA. Rapid gastric emptying in patients with early non-insulin-dependent diabetes mellitus. N Engl J Med 1991;324:130–1. [DOI] [PubMed] [Google Scholar]
- 52.Phillips WT, Schwartz JG, McMahan CA. Rapid gastric emptying of an oral glucose solution in type 2 diabetic patients. J Nucl Med 1992;33:1496–500. [PubMed] [Google Scholar]
- 53.Porter KE, Riches K. Vascular smooth muscle as a target for novel therapeutics. Curr Diab Rep 2015;15:72. [DOI] [PubMed] [Google Scholar]
- 54.Riddle M, Umpierrez G, DiGenio A, Zhou R, Rosenstock J. Contributions of basal and postprandial hyperglycemia over a wide range of A1C levels before and after treatment intensification in type 2 diabetes. Diabetes Care 2011;34:2508–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sanaka M, Nakada K, Nosaka C, Kuyama Y. The Wagner-Nelson method makes the [13C]-breath test comparable to radioscintigraphy in measuring gastric emptying of a solid/liquid mixed meal in humans. Clin Exp Pharmacol Physiol 2007;34: 641–4. [DOI] [PubMed] [Google Scholar]
- 56.Sato D, Morino K, Nakagawa F, Murata K, Sekine O, Beppu F, et al. Acute effect of metformin on postprandial hypertriglyceridemia through delayed gastric emptying. Int J Mol Sci 2017;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Suh S, Kim JH. Glycemic variability: how do we measure it and why is it important? Diabetes Metab J 2015;39:273–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Utzschneider KM, Prigeon RL, Faulenbach MV, Tong J, Carr DB, Boyko EJ, et al. Oral disposition index predicts the development of future diabetes above and beyond fasting and 2-h glucose levels. Diabetes Care 2009;32:335–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vinik A, Nakave A, Chuecos MdPS. A break in the brake mechanism in diabetes: a cause of postprandial hyperglycemia. Diabetes Care 2008;31:2410–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Watson LE, Phillips LK, Wu T, Bound MJ, Checklin H, Grivell J, et al. Differentiating the effects of whey protein and guar gum preloads on postprandial glycemia in type 2 diabetes. Clin Nutr 2018 10.1016/j.clnu.2018.12.014. [DOI] [PubMed] [Google Scholar]
- 61.Watson LE, Xie C, Wang X, Li Z, Phillips LK, Sun Z, et al. Gastric emptying in patients with well-controlled type 2 diabetes compared with young and older control subjects without diabetes. J Clin Endocrinol Metab 2019;104:3311–9. [DOI] [PubMed] [Google Scholar]
- 62.Wu T, Little TJ, Bound MJ, Borg M, Zhang X, Deacon CF, et al. A protein preload enhances the glucose-lowering efficacy of vildagliptin in type 2 diabetes. Diabetes Care 2016;39:511–7. [DOI] [PubMed] [Google Scholar]
- 63.Zhan C, Zhou J, Feng Q, Zhang JE, Lin S, Bao J, et al. Acute and long-term suppression of feeding behavior by POMC neurons in the brainstem and hypothalamus, respectively. J Neurosci 2013;33:3624–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou SY, Lu Y, Song I, Owyang C. Inhibition of gastric motility by hyperglycemia is mediated by nodose ganglia KATP channels. Am J Physiol Gastrointest Liver Physiol 2011;300:G394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]


