Supplemental Digital Content is available in the text.
Keywords: Estimated fetal weight, Fetal growth, Growth charts, Reference charts, Small-for-gestational-age birth
Abstract
Background:
Fetal growth standards (prescriptive charts derived from low-risk pregnancies) are theoretically better tools to monitor fetal growth than conventional references. We examined how modifying chart inclusion criteria influenced the resulting curves.
Methods:
We summarized estimated fetal weight (EFW) distributions from a hospital’s routine 32-week ultrasound in all nonanomalous singleton fetuses (reference) and in those without maternal–fetal conditions affecting fetal growth (standard). We calculated EFWs for the 3rd, 5th, 10th, and 50th percentiles, and the proportion of fetuses each chart classified as small for gestational age.
Results:
Of 2309 fetuses in our reference, 690 (30%) met the standard’s inclusion criteria. There were no meaningful differences between the EFW distributions of the reference and standard curves (50th percentile: 1989 g reference vs. 1968 g standard; 10th percentile: 1711 g reference vs. 1710 g standard), or the proportion of small for gestational age fetuses (both 9.9%).
Conclusions:
In our study, there was little practical difference between a fetal growth reference and standard for detecting small infants.
Birthweight- and estimated fetal weight-for-gestational-age charts have long been used as the cornerstone of fetal growth assessment.1,2 Fetuses with a weight below a certain statistical threshold of the population, usually the 10th percentile (or less frequently, the 5th or 3rd), are classified as small for gestational age (SGA), and considered to be at increased risk of adverse outcomes due to fetal growth restriction.3
Although the statistical thresholds to define high-risk fetuses have been standardized by convention, the characteristics of the study populations that the percentiles are derived from can vary considerably. Historically, charts tended to include most births in the population, including those with suboptimal fetal growth conditions.4 Such charts are known as reference charts, which are descriptive charts of how fetuses actually grow. In the past 6 years, three new methodologically rigorous fetal growth charts have been published: the World Health Organization (WHO) and INTERGROWTH-21st charts derived from multijurisdictional international populations,5–7 and the US National Institute for Child Health and Development (NICHD) fetal growth charts derived from multistate US populations.8 These charts were derived from selected populations of healthy, low-risk pregnancies, producing prescriptive charts of how fetuses ought to grow (known as standards). From a theoretical perspective, charts that reflect the growth of fetuses under optimal conditions should be better for monitoring fetal growth and identifying fetuses whose growth is failing. Thus, their status as standards, rather than references, is viewed as an important reason for adopting the new charts.9,10
Yet, introducing the new charts into clinical practice has proven challenging. Concerns have been raised that the charts do not always seem to “fit” real-world populations.11,12 For example, in a US cohort from New Mexico with an SGA rate at birth of 13.6%, the NICHD fetal growth standard only identified 6.5% as such (vs. 12.2% using the conventional chart of Hadlock).11 Likewise, in another US cohort with a 13.2% rate of SGA births, the INTERGROWTH-21st chart only identified 3.2% of pregnancies as being below the 10th percentile.12 It has been suggested such differences are due to the fact that these are standards, as opposed to references.9,10 It has also been speculated that cut-points other than the conventional 10th percentile may be needed on these new standards.10 Understanding the practical differences between fetal growth standards and references is critical for successfully implementing the new charts into real-world settings.
In this report, we examined how restricting the study population of fetal weight-for-gestational-age charts to exclude characteristics linked with poor fetal growth influences the weight thresholds and proportion of infants classified as SGA based on conventional statistical thresholds.
METHODS
Our study population was drawn from singleton births delivering at the Royal Victoria Hospital, in Montreal, Canada, 2002–2006. We obtained data from the McGill Obstetrical and Neonatal Database, which contains highly detailed clinical information abstracted from the maternal and newborn medical records. This study was approved by the Royal Victoria Hospital Research Ethics Board. Until 2006, the hospital had a policy of conducting a routine ultrasound at 32–33 weeks, providing estimated fetal weight measurements from a general obstetrical population. In practice, the scan occurred anytime between 31 and 34 weeks due to scheduling issues; for this study, we restricted analyses to fetuses with a scan between 32 + 0 and 32 + 6 weeks. Estimated fetal weight (g) was calculated using Hadlock’s formula combining abdominal circumference, head circumference, and femur length.13
Our reference was created from the 32-week estimated fetal weights of all pregnancies resulting in nonanomalous live births. Our standard was created from the 32-week estimated fetal weights of all nonanomalous live births conceived without assisted reproductive technology with an estimate of gestational age based on the last menstrual period that agreed with early ultrasound estimates, born to nonsmoking mothers 18–34.9 years of age at booking (<14 weeks), with a prepregnancy body mass index (BMI) 18.5–29.9 kg/m2 with no documented alcohol or illicit substance consumption, history of obstetrical complications (repeated pregnancy losses, prior stillbirth, neonatal death, preterm birth, growth restricted infant, low birthweight infant, congenital anomaly, preeclampsia), hypertensive disorders of pregnancy (including pre-existing hypertension), prepregnancy or gestational diabetes, maternal anemia (hemoglobin <100 g/L), or evidence of sexually transmitted diseases (HIV/AIDS, syphilis, gonorrhea, genital herpes), and not residing a single-parent household. These exclusion criteria were based primarily on those of the INTERGROWTH-21st study.14
We plotted the smoothed distribution of estimated fetal weight in each of the reference and standard populations using univariate kernel density estimation. We calculated the weight thresholds corresponding to the 3rd, 5th, 10th, and 50th percentiles with 95% confidence intervals, and tabulated the proportion of fetuses identified as SGA (<10th, <5th, and <3rd percentile) by each of the reference and the standard when applied to the entire population. We focused on SGA infants because management of fetal overgrowth is based on absolute weights (>4500 and >5000 g) rather than weight percentiles.15
RESULTS
There were 2984 nonanomalous singleton fetuses at the Royal Victoria Hospital with a prenatal ultrasound between 32 + 0 and 32 + 6 weeks’ gestation. We excluded 675 pregnancies (22.6%) with missing prepregnancy BMI information, leaving 2309 fetuses for inclusion. There was no meaningful difference in the estimated fetal weight distribution of women with and without available BMI (medians and interquartile ranges of 1989 g [1839, 2152] and 1986 g [1828, 2158], respectively).
Of the 2309 fetuses in our reference, 1619 (70%) had one or more characteristic that excluded them from the standard, leaving 690 fetuses (30%) eligible for our standard. The most common reasons for exclusion were: maternal age ≥35 years (n = 681, 29.5%), single parent household (n = 428, 18.5%), and uncertain gestational age estimate (n = 358, 15.5%).
As shown in the Figure and Table, the estimated fetal weight distributions obtained from the reference and the standard were highly similar. The 50th percentile weights differed by only 21 g, while the 10th percentiles were within 1 g. As a result, the proportion of infants identified as SGA by the standard was also virtually identical to the proportion classified by the reference (9.9% vs. 9.9% using a 10th percentile cutoff, 3.0% vs. 3.0% using a 3rd percentile cutoff). Even when comparing the estimated fetal weights of fetuses included in the standard with those of only fetuses excluded from the standard, differences between distributions were minor (eFigure 1; http://links.lww.com/EDE/B739).
TABLE.
Percentile Weights and Small-for-gestational-age Births Among 2309 Fetuses With a Routine 32-week Ultrasound at the Royal Victoria Hospital, Montreal, Canada
| Reference | Standard | |
|---|---|---|
| N (percent of total population) | 2,309 (100) | 690 (29.9) |
| 50th percentile estimated fetal weight [95% CI] | 1,989 [1,976, 2,002] | 1,968 [1,950, 1,993] |
| 10th percentile estimated fetal weight (g) [95% CI] | 1,711 [1,694, 1,726] | 1,710 [1,679, 1,728] |
| 5th percentile estimated fetal weight (g) [95% CI] | 1,624 [1,605, 1,643] | 1,624 [1,592, 1,656] |
| 3rd percentile weight (g) [95% CI] | 1,569 [1,539, 1,595] | 1,572 [1,523, 1,618] |
| N (%) < 10th | 228 (9.9) | 228 (9.9) |
| N (%) < 5th | 115 (5.0) | 116 (5.0) |
| N (%) < 3rd | 69 (3.0) | 70 (3.0) |
CI, confidence interval.
Figure.
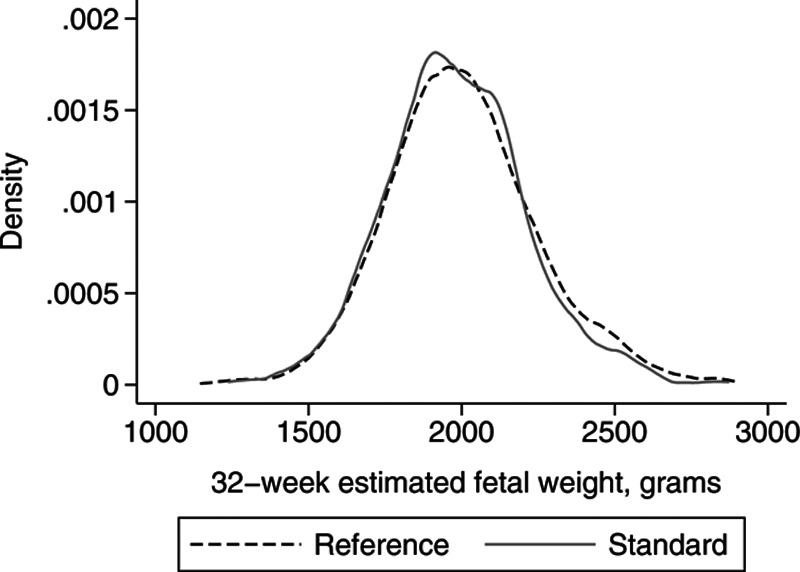
Estimated fetal weight distributions at 32 weeks of reference (all nonanomalous singletons) vs. standard (nonanomalous singletons with no maternal or fetal conditions associated with suboptimal fetal growth) populations, Royal Victoria Hospital, Montreal, Canada.
DISCUSSION
We found that the fetal weight thresholds to define SGA birth in our reference population were nearly identical to those in our standard population. The philosophical merits of assessing fetal growth using a standard rather than a reference is one of the driving arguments for adoption of the newly developed fetal growth charts from INTERGROWTH-21st, WHO, and NICHD.9,10,16 Our work shows that from a practical perspective, the philosophical differences underlying the creation of standards versus references have minimal differences in the resulting fetal growth charts. Our work suggests that observed differences between these new standards and locally derived reference charts currently in use11,12 are likely not due to differences in their inclusion criteria, but rather, to true differences in fetal size distributions between their source populations.
We excluded a large fraction of the cohort (70%) from our standard population due to the presence of one or more risk factors linked with suboptimal fetal growth. This rate is close to the exclusion rate of 65% in the INTERGROWTH-21st study,6 supporting the validity of our standard. Our finding, that exclusion of pregnancies with suboptimal fetal growth conditions makes little practical difference for the resulting chart percentiles, is consistent with the WHO fetal growth study, which found that including pregnancies with complications such as preeclampsia, hypertension, and gestational diabetes, did not change the distribution as described by percentiles, compared to excluding these pregnancies.16 In addition, a recent French cohort study of 14,607 pregnancies showed that mean femur length and abdominal circumference biometric z-scores were very similar before and after restricting the cohort to the INTERGROWTH-21st exclusion criteria,17 and a Norwegian study found that birthweight and other neonatal outcomes were not meaningfully different in infants who met versus did not meet the INTERGROWTH-21st exclusion criteria.18
Strength of this study was our access to a large number of ultrasounds performed at the same gestational week in a general obstetrical population, enabling comparisons of crude fetal weight distributions free from statistical smoothing to account for gestational age differences. However, our standard population did not have identical exclusion criteria as previous standard populations (e.g., adverse environmental conditions), so although unlikely, it is possible that findings might be different using different standard exclusion criteria.
Our findings do not necessarily argue against the adoption of the new fetal growth standards. However, they do suggest that the primary benefit of the new standards is their role in standardizing the assessment of fetal growth nationally and internationally, rather than an improved ability to identify suboptimal growth per se. For example, use of the same chart enables comparisons of SGA rates across jurisdictions for surveillance purposes, and facilitates multi-site research studies wishing to use “SGA” as an inclusion criteria (e.g., studies evaluating the management or treatment of fetal growth restriction) or an outcome. Nevertheless, our findings suggest that the primary aim of fetal growth charts, to identify fetuses at increased risk of adverse outcomes due to growth restriction, will not be meaningfully affected by the decision to use a reference or a standard.
Supplementary Material
Footnotes
This research was funded by a Canada Research Chair in Perinatal Population Health (J.A.H.). J.L. holds a University of British Columbia Clinical Investigator Program award.
The authors report no conflicts of interest.
Supplemental digital content is available through direct URL citations in the HTML and PDF versions of this article (www.epidem.com).
Computing code to replicate results is available from the corresponding author. Data cannot be shared by the authors to due restrictions in the data access agreement; individuals seeking access should contact the Division of Maternal Fetal Medicine, Department of Obstetrics and Gynecology, Royal Victoria Hospital, Montreal, Quebec, Canada.
REFERENCES
- 1.Hadlock FP, Harrist RB, Martinez-Poyer J. In utero analysis of fetal growth: a sonographic weight standard. Radiology. 1991;181:129–133. [DOI] [PubMed] [Google Scholar]
- 2.Lubchenco LO, Hansman C, Dressler M, Boyd E. Intrauterine growth as estimated from liveborn birth-weight data at 24 to 42 weeks of gestation. Pediatrics. 1963;32:793–800. [PubMed] [Google Scholar]
- 3.McIntire DD, Bloom SL, Casey BM, Leveno KJ. Birth weight in relation to morbidity and mortality among newborn infants. N Engl J Med. 1999;340:1234–1238. [DOI] [PubMed] [Google Scholar]
- 4.Ioannou C, Talbot K, Ohuma E, et al. Systematic review of methodology used in ultrasound studies aimed at creating charts of fetal size. BJOG. 2012;119:1425–1439. [DOI] [PubMed] [Google Scholar]
- 5.Kiserud T, Piaggio G, Carroli G, et al. The World Health Organization fetal growth charts: a Multinational Longitudinal Study of Ultrasound Biometric Measurements and Estimated Fetal Weight. PLoS Med. 2017;14:e1002220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Papageorghiou AT, Ohuma EO, Altman DG, et al. International Fetal and Newborn Growth Consortium for the 21st Century (INTERGROWTH-21st). International standards for fetal growth based on serial ultrasound measurements: the Fetal Growth Longitudinal Study of the INTERGROWTH-21st Project. Lancet. 2014;384:869–879. [DOI] [PubMed] [Google Scholar]
- 7.Stirnemann J, Villar J, Salomon LJ, et al. International estimated fetal weight standards of the INTERGROWTH-21(st) Project. Ultrasound Obstet Gynecol. 2017;49:478–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buck Louis GM, Grewal J, Albert PS, et al. Racial/ethnic standards for fetal growth: the NICHD Fetal Growth Studies. Am J Obstet Gynecol. 2015;213:449.e1–449.e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Papageorghiou AT, Kennedy SH, Salomon LJ, et al. International Fetal and Newborn Growth Consortium for the 21(st) Century (INTERGROWTH-21(st)). The INTERGROWTH-21st fetal growth standards: toward the global integration of pregnancy and pediatric care. Am J Obstet Gynecol. 2018;218(2S)S630–S640. [DOI] [PubMed] [Google Scholar]
- 10.Grantz KL, Hediger ML, Liu D, Buck Louis GM. Fetal growth standards: the NICHD fetal growth study approach in context with INTERGROWTH-21st and the World Health Organization Multicentre Growth Reference Study. Am J Obstet Gynecol. 2018;218(2S)S641–S655.e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blue NR, Beddow ME, Savabi M, Katukuri VR, Chao CR. Comparing the Hadlock fetal growth standard to the Eunice Kennedy Shriver National Institute of Child Health and Human Development racial/ethnic standard for the prediction of neonatal morbidity and small for gestational age. Am J Obstet Gynecol. 2018;219:474.e1–474.e12. [DOI] [PubMed] [Google Scholar]
- 12.Odibo AO, Nwabuobi C, Odibo L, Leavitt K, Obican S, Tuuli MG. Customized fetal growth standard compared with the INTERGROWTH-21st century standard at predicting small-for-gestational-age neonates. Acta Obstet Gynecol Scand. 2018;97:1381–1387. [DOI] [PubMed] [Google Scholar]
- 13.Hadlock FP, Harrist RB, Carpenter RJ, Deter RL, Park SK. Sonographic estimation of fetal weight. The value of femur length in addition to head and abdomen measurements. Radiology. 1984;150:535–540. [DOI] [PubMed] [Google Scholar]
- 14.Villar J, Altman DG, Purwar M, et al. International Fetal and Newborn Growth Consortium for the 21st Century. The objectives, design and implementation of the INTERGROWTH-21st Project. BJOG. 2013;120suppl 29–26, v. [DOI] [PubMed] [Google Scholar]
- 15.Kiserud T, Benachi A, Hecher K, et al. The World Health Organization fetal growth charts: concept, findings, interpretation, and application. Am J Obstet Gynecol. 2018;218(2S)S619–S629. [DOI] [PubMed] [Google Scholar]
- 16.American College of Obstetrics and Gynecology. ACOG practice bulletin 216: macrosomia. Obstet Gynecol. 2020;135:e18–e35. [DOI] [PubMed] [Google Scholar]
- 17.Heude B, Le Guern M, Forhan A, et al. Are selection criteria for healthy pregnancies responsible for the gap between fetal growth in the French national Elfe birth cohort and the Intergrowth-21st fetal growth standards?. Paediatr Perinat Epidemiol. 2019;33:47–56. [DOI] [PubMed] [Google Scholar]
- 18.Sletner L, Kiserud T, Vangen S, Nakstad B, Jenum AK. Effects of applying universal growth standards in a Scandinavian multi-ethnic population. Acta Obstet Gynecol Scand. 2018;97:168–179. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


