Abstract
Lipins are eukaryotic proteins with functions in lipid synthesis and the homeostatic control of energy balance. They execute these functions by acting as phosphatidate phosphatase enzymes in the cytoplasm and by changing gene expression after translocation into the cell nucleus, in particular under fasting conditions. Here, we asked whether nuclear translocation and the enzymatic activity of Drosophila Lipin serve essential functions and how gene expression changes, under both fed and fasting conditions, when nuclear translocation is impaired. To address these questions, we created a Lipin null mutant, a mutant expressing Lipin lacking a nuclear localization signal (LipinΔNLS), and a mutant expressing enzymatically dead Lipin. Our data support the conclusion that the enzymatic but not nuclear gene regulatory activity of Lipin is essential for survival. Notably, adult LipinΔNLS flies were not only viable but also exhibited improved life expectancy. In contrast, they were highly susceptible to starvation. Both the improved life expectancy in the fed state and the decreased survival in the fasting state correlated with changes in metabolic gene expression. Moreover, increased life expectancy of fed flies was associated with a decreased metabolic rate. Interestingly, in addition to metabolic genes, genes involved in feeding behavior and the immune response were misregulated in LipinΔNLS flies. Altogether, our data suggest that the nuclear activity of Lipin influences the genomic response to nutrient availability with effects on life expectancy and starvation resistance. Thus, nutritional or therapeutic approaches that aim at lowering nuclear translocation of lipins in humans may be worth exploring.
Keywords: genomic starvation response, energy metabolism, metabolic health, feeding behavior, immune response
INTRODUCTION
Organisms adjust their metabolism to nutrient availability and, when nutrients become scarce, rely on energy stores in the form of glycogen and triacylglycerols (TAGs). Adjustments require regulation of the balance between the synthesis and breakdown of storage molecules and are associated with changes in feeding behavior (1, 2). A linchpin in this regulatory system is provided by lipins, a group of proteins with dual functions in lipid synthesis and the genomic control of energy metabolism. Lipins, which are conserved among all eukaryotes, can convert phosphatidic acid into diacylglycerol as phosphatidate phosphatases (PAPs) (3–5). This activity is required for TAG synthesis and the synthesis of membrane phospholipids (6). In addition, lipins can translocate into the cell nucleus to participate in gene regulation (7–11). The nuclear translocation of both mammalian lipin 1 and the single Lipin orthologue of Drosophila occurs under fasting conditions and is controlled by the nutrient-sensitive target of rapamycin complex 1 (TORC1) pathway (12, 13).
While the mechanism by which lipins participate in gene regulation has been explored to some extent, little is known about the genomic changes that depend on the nuclear translocation of lipins. ChIP experiments indicate that the yeast lipin orthologue Smp2 and mammalian lipin 1 associate with the promoter regions of specific genes, suggesting a function as transcriptional coregulators (14, 15). However, at least some of the genomic effects of mouse lipin 1 depend on its PAP activity and the effect of the protein on the nuclear abundance of the transcription factor SREBP1 (13). Genes responding to lipins have been identified through an overexpression approach in mice (15), in relation to glucose feeding in Caenorhabditis elegans (16), and through a candidate gene approach in yeast (14).
Similar to mice lacking lipin 1 (4), Drosophila larvae that express reduced amounts of Lipin exhibit a severe underdevelopment of the fat tissue and reduced TAG stores. However, Drosophila Lipin is also broadly expressed outside the fat body, the insect equivalent of vertebrate adipose tissue (17). While the lack of energy reserves that accompanies the loss of fat body prevents the successful metamorphosis of Lipin mutant larvae into adult flies, it is unknown if functions of Lipin in other tissues are equally required for survival and the development of adult flies. Here, we address this question by rescue experiments using a Lipin null mutant. Furthermore, we ask whether a functional PAP motif of lipin and the nuclear translocation signal (NLS) are essential for survival under fed and fasting conditions. The successful creation of a CRISPR/Cas9-generated Lipin mutant without the NLS allowed us to address how interference with nuclear translocation affects gene expression under both fed and fasting conditions. Our data show that Lipin and its PAP activity both inside and outside the fat body are essential for survival, whereas the NLS appears nonessential. Interference with the nuclear translocation of the protein under both fed or fasting conditions leads to substantial changes in the expression of genes involved in energy homeostasis and feeding behavior and of genes involved in the immune response in the fasting state. Notably, as long as sufficient nutrients are available, nuclear functions of Lipin are not only dispensable, but interference with the nuclear entry of Lipin is even beneficial for survival. This observation suggests that a better understanding of nutrients that influence the nuclear entry of lipins may shed light on unhealthy, life-shortening dietary conditions. Ultimately, nuclear translocation may prove to be a property of lipins that can be utilized as a target for specific therapeutic interventions. In summary, our data substantiate the critical role that Lipin has in the metabolic adaptation to starvation and identify gene-regulatory functions in the fed state that have an effect on health and life expectancy.
MATERIALS AND METHODS
Fly stocks
Flies carrying r4-gal4 were provided by Jae Park (18), and flies carrying Df(2R)Exel7095 (#7860), tub-GAL4 (#5138), or UAS-2xeGFP (#6874) were obtained from the Bloomington Drosophila Stock Center. The generation of a UAS-LipinWT transgenic fly line has been described previously (12).
Mutagenesis
CRISPR/Cas9 mutagenesis was used to create an in-frame deletion of the 18-bp DNA segment 5′-AAGAAGCGGCGCAAGAAG-3′, which encodes the NLS of the Lipin protein (amino acid positions 276–281). To introduce the NLS deletion, we used as a repair template a single-stranded oligonucleotide of the sequence 5′- GCGTCTCCGCCGAAGGCAAATCACCGCCGCCGGCGCTGCCCAATGAGCTGCTTGAAGAGTTCTTGCGCTGGGCATTCTTCTTCATTTGCGAGGTTTTGCTCTTGGACACCTCCTTGGTGGCTTCGCT-3′ (Integrated DNA Technologies). This oligonucleotide corresponds to lower-strand genomic DNA extending 36 bp upstream and 91 bp downstream of the NLS but lacks the NLS itself. To construct the vector for Cas9 guide RNA expression, a protospacer of the sequence 5′-CGACTTCTTGCGCCGCTTCT-3′ was cloned into pCFD3 (Addgene). A guide RNA expression vector and mutagenic repair template were injected into embryos expressing Cas9 in the germline (Bloomington injection stock #54591, y[1] M{w[+mC]=nos-Cas9.P}ZH-2A w[*]; Bestgene Inc.). Balanced stocks were established for individual mutagenized chromosomes and screened by PCR and DNA sequencing for the presence of the NLS deletion using forward and reverse primers of the sequences 5′-CGCTGGACAACCAAAGCAAA-3′ and 5′-CTCCGTGTCGCTGAAGAAGT-3′, respectively.
The LipinKO mutant was created by CRISPR/Cas9 mutagenesis using a guide RNA targeting the region immediately downstream of the Lipin start codon. A protospacer of the sequence 5′-CAGACCAAAGATGAATAGCC-3′ was cloned into pCFD3 (Addgene), and the resulting guide RNA expression vector was injected into embryos expressing Cas9 in the germline (yw;;nos-Cas9(III-attP2; Bestgene Inc.). Frame-shift mutations resulting from erroneous nonhomologous end joining were identified by screening flies carrying mutagenized chromosomes by PCR and DNA sequencing.
A C-to-G nucleotide exchange leading to an amino acid exchange (D812E) in the catalytic motif of Lipin (LipinD812E) was introduced by ends-in gene targeting (19). A 6-kb fragment of the Lipin gene was amplified from Bac-Clone #RP98-9N11 (BACPAC Resources Center) using PCR primers 5′-GCTGCGGCCGCGTTGCTATGGCTGTGGCCAC-3′ and 5′-GACTGGGTACCCACCAGCGCCGTCTCCAGCTC-3′ and cloned into the KpnI and NotI sites of pBluescriptKSII. The C-to-G nucleotide exchange was then introduced using primers of the sequence 5′-GGTGGTGATCTCGGAGATTGACGGCACCATCA-3′ and 5′-GCCATTCAGCCGTACGACTAGGTTAGGC-3′ using a Change-IT Multiple Mutation Site Directed Mutagenesis Kit (Affymetrix/USB). A recognition site for the I-SceI homing meganuclease was introduced by PCR using primers 5′-CATCGAACCAGGTATTACCCAGTTATCCCTAGGCGGTCGAACTCCTCGTCCGAGGGTGGT-3′ and 5′-GCTGCGGCCGCGTTGCTATGGCTGTGGCCAC-3′. The resulting product was cut at NotI and SexAI sites introduced through the primers at the 5′- and 3′-ends, respectively, and used to replace the corresponding fragment in the original mutagenized pBluescript construct. Finally, the modified Lipin DNA was excised from the vector and ligated into the NotI and KpnI sites of targeting vector P[TV2] to create a donor construct for injection into Drosophila embryos (Bestgene Inc.). A mutant stock was established following the published protocol for ends-in targeting (19).
Rescue experiments
To determine whether Lipin null mutants can be efficiently rescued by fat-body or ubiquitous expression of wild-type Lipin, we carried out crosses to create animals of the genotypes LipinKO/Df(2R)Exel7095; r4-GAL4/UAS-LipinWT and LipinKO/Df(2R)Exel7095; tub-GAL4/UAS-LipinWT. Survival of these animals was compared with sibling control animals from the same vials carrying one copy of the Lipin wild-type allele plus GAL4 driver and UAS responder or one copy of the Lipin wild-type allele plus GAL4 driver or UAS responder. Pupae were collected daily and genotyped. Further development was monitored daily. Eclosing adults were isolated and crossed to flies of the opposite sex to determine fertility. To determine whether r4-GAL4 expression was indeed specific to the fat body as previously reported (18), we crossed r4-GAL4 flies with flies carrying UAS-2xeGFP. Fluorescent microscopy confirmed strong expression in the larval and adult fat bodies that was maintained until at least 5 weeks after eclosion. In addition, GFP expression was observed in the adult hindgut and Malpighian tubules. Thus, the tissue specificity of the r4-GAL4 driver is not quite as strict as assumed in previous studies.
Lipin immunostaining, starvation, and longevity experiments
Larvae of the genotypes LipinΔNLS/Df(2R)Exel7095 and Lipin/Df(2R)Exel7095 were reared on standard food. Feeding third-instar larvae were removed from the food, transferred to 1% agar starvation plates, and kept in an incubator at 25°C for about 16 h. Fat bodies dissected from the starved larvae and fed control larvae were stained with an affinity-purified antibody directed against Lipin and DAPI to visualize DNA as previously described (12).
To examine the starvation resistance of LipinΔNLS/Df(2R)Exel7095 and control flies carrying the wild-type Lipin allele of the injection stock over Df(2R)Exel7095, newly eclosed flies were collected over 24 h and kept for 3 days on standard food. Flies were then separated by sex, and groups of 25 flies were transferred into starvation vials with water-saturated fly plugs at the bottom (Genesee Scientific). Survival was monitored daily, and dead flies were removed. Flies were regularly transferred to fresh vials with water-saturated plugs to avoid feeding on mold or, in the case of the females, eggs deposited onto the plugs. Experiments were carried out in biological triplicates.
To compare the longevity of LipinΔNLS/Df(2R)Exel7095 flies to the longevity of control flies, we proceeded as for the starvation experiments but kept flies on standard food instead of starving them. Flies were transferred to fresh food every 3–4 days, and survival was monitored daily. Experiments were carried out in biological triplicates.
Triglyceride assays and lipid staining
TAG was measured using a colorimetric assay (20). Briefly, flies were collected as for the starvation assays, and samples were obtained by pooling and homogenizing 10 male or 7 female flies of the same genotype in 500 µl PBS containing 0.05% Tween 20. After setting aside aliquots for protein measurements (Bradford Protein Assay Kit #23200; Thermo Fisher Scientific), homogenates were heated to 70°C for 10 min to inactivate enzymes released from the cells. For the measurement of free glycerol, 80 µl of each sample was mixed with 500 µl PBS containing 0.05% Tween 20, and for the measurement of glycerol released from TAG, another 80 µl was mixed with Triglyceride Reagent (T2449; Sigma-Aldrich). The samples were then incubated at 37°C for 1 h. After mixing 150 µl of the sample with 600 µl of Free Glycerol Reagent (F6428; Sigma-Aldrich) and incubation at 37°C for 5 min, absorbances were measured at 540 nm using polystyrene standard cuvettes and compared with a standard curve derived from Glycerol Standard Solutions (G7793; Sigma-Aldrich). Means for males were derived from seven (LipinΔNLS) or nine (control) biological replicates; means for females were derived from three (LipinΔNLS) or four (control) biological replicates. Fat body from wandering third-instar larvae was stained with Bodipy 493/503 (Invitrogen/Molecular Probes) to visualize fat droplets as described previously (12).
RNA-seq
For RNA-seq analyses, freshly eclosed male and female flies of the genotype LipinΔNLS/Df(2R)Exel7095 and control flies of the genotype Lipin/Df(2R)Exel7095, carrying the wild-type Lipin allele of the CRISPR/Cas9 injection stock, were collected and kept on regular food for 3 days. Male and female flies were then separated and placed in groups of 15 and 10, respectively, in food-containing vials or starvation vials containing only a source of water. After 24 h under continued feeding or fasting conditions the flies were processed for RNA-seq. RNA was extracted from three biological replicates for each combination of genotype, sex, and condition (fed, starved). Flies were snap-frozen in liquid nitrogen and transferred into Trizol Reagent (Invitrogen) for homogenization and RNA extraction. RNA was further purified using a Qiagen RNeasy Mini Kit, and its quality was determined using an Agilent TapeStation. Oligo-dT-based library preparation and RNA-seq were carried out at the Functional Genomics Facility of the University of Chicago. This was followed by bioinformatics analysis at the University of Chicago’s Center for Research Informatics. Illumina RNA-seq was done using a NovaSEQ 6000 sequencer performing 60M 100-bp paired-end reads (30M clusters) per sample.
After data normalization, differential expression analyses were performed using the R/Bioconductor software package limma (21). Reads were aligned to the Drosophila melanogaster assembly BDGP6.22 (Ensembl release 98). The following eight comparisons were made (supplemental Tables S4–S11): wild-type males, fed versus starved; wild-type males, fed versus mutant males, fed; wild-type males, starved versus mutant males, starved; mutant males, fed versus mutant males, starved; wild-type females, fed versus starved; wild-type females, fed versus mutant females, fed; wild-type females, starved versus mutant females, starved; mutant females, fed versus mutant females, starved. Differentially expressed genes were identified by controlling their Benjamini-Hochberg adjusted P values under a false discovery rate cutoff of 0.05 and a base 2 log fold change of 1.5. Overrepresentation among differentially expressed genes of gene ontology (GO) terms was analyzed using the Bioconductor clusterProfiler package (22). If not indicated otherwise, the tissue-specific gene expression data presented in Tables 1 and 2 and supplemental Tables S1–S3 were derived from the Drosophila gene expression atlases FlyAtlas 1 and 2 (23, 24).
TABLE 1.
Genes with changed expression in LipinΔNLS mutant in fed condition
| FlyBase ID (FBgn) | Gene Name | Fold Change | Annotation | |
| Male/Female | Tissue | |||
| GO: fatty acid synthase activity/fatty acid desaturation | ||||
| 0042627 | FASN2 | +1.8/+1.9 | FB, OE | Fatty acid synthase 2 |
| 0040001 | FASN3 | +1.5/+1.3 | FB, OE | Fatty acid synthase 3 |
| 0037762 | eloF | +1.9/+1.5 | FB, OE | Fatty acid elongase F |
| 0051523 | CG31523 | +1.5/+1.4 | DS, FB | Fatty Acid elongase |
| 0039030 | CG6660 | +1.8/+1.6 | FB | Fatty acid elongase |
| 0038983 | CG5326 | +1.5/+1.4 | DS | Fatty acid elongase |
| 0050008 | CG30008 | nc/+1.9 | FB | Fatty acid elongase |
| 0039755 | CG15531 | +1.6/+1.7 | FB | Stearoyl-CoA 9-desaturase |
| Lipid metabolism, other | ||||
| 0023495 | Lip3 | +5.7/+3.4 | MG | Lipase 3 |
| 0032264 | Lip4 | −1.7/nc | DS, FB | Lipase 4 |
| 0038795 | CG4335 | −1.8/nc | FB | Trimethyllysine dioxygenase |
| 0038730 | CG6300 | +3.3/+6.6 | DS | Acyl-CoA synthetase |
| 0034552 | CG17999 | nc/+1.7 | HG | Acyl-CoA synthetase |
| 0032402 | CG14945 | nc/-1.5 | NS, MT, FB | Phosphatidylinositol-specific phospholipase C |
| Carbohydrate metabolism | ||||
| 0261575 | tobi | +7.2/+9.5 | FB, MG | α-Glucosidase |
| GO: serine-type peptidase (see supplemental Table S1) | ||||
| GO: oxidoreductase activity (see supplemental Table S1) | ||||
| GO: transmembrane receptor activity | ||||
| 0052475 | mthl8 | +2.4/+5.7 | DS, FB, MT | Methuselah-like 8 |
| 0052255 | Gr64f | −2.3/nd | PB | Gustatory receptor 64f, sugar-sensing neurons |
| Other | ||||
| 0039298 | to | −2.6/+1.4 | DS | Takeout feeding regulator |
| 0034636 | tiwaz | +2.0/nc | NS | Adult feeding behavior |
| 0039840 | pHCL-2/hodor | nc/+1.8 | DS | Intestinal feeding regulator |
For a complete list of genes, see supplemental Table S1. DS, digestive system; FB, fat body; HG, hindgut; MG, midgut; MT, Malpighian tubule; nc, not changed; nd, not detected. NS, nervous system; OC, ocelli; OE, oenocyte; PB, proboscis; nc, not changed; nd, not detected.
TABLE 2.
Genes showing changed starvation response in the LipinΔNLS mutant
| FlyBase ID (FBgn) | Gene Name | Fold Change | Annotation | ||
| Tissue | |||||
| Genes showing blunted starvation response | |||||
| Lipid metabolism | Males | ||||
| 0038795 | CG4335 | −2.2 > −1.5 | FB | Trimethyllysine dioxygenase; carnitine synthesis | |
| 0030575 | CG5321 | −1.6 > −1.1 | FB, MT | γ-Butyrobetaine dioxygenase; carnitine synthesis | |
| 0283427 | FASN1 | −1.5 > −1.2 | FB | Fatty acid synthase 1 | |
| 0032264 | Lip4 | −1.6 > −1.3 | FB, DS | Lipase 4 | |
| Females | |||||
| 0040813 | Nplp2 | −1.7 > −1.2 | FB, DS | Dietary lipid assimilation | |
| 0034552 | CG17999 | +1.6 > 1.0 | HG | Long-chain-fatty-acid-CoA synthetase | |
| 0027601 | pdgy | +1.6 > +1.1 | DS | Acyl-CoA synthetase | |
| 0031703 | CG12512 | +1.6 > +1.2 | DS, MT, FB | Long-chain-fatty-acid-CoA synthetase | |
| 0037996 | CG4830 | −23.4 > −9.0 | MG | Long-chain-fatty-acid-CoA synthetase | |
| 0033246 | ACC | +1.7 > +1.3 | FB, DS | Acetyl-CoA carboxylase | |
| 0031976 | CG7367 | +4.0 > +1.9 | FB, AH | Predicted lipase | |
| 0033999 | CG8093 | +18.3 > +9.5 | MG | Predicted lipase | |
| 0039471 | CG6295 | −8.9 > −3.9 | MG | Predicted lipase | |
| 0267339 | p38c | −1.8 > +1.1 | DS | MAP kinase; intestinal lipid homeostasis | |
| 0033216 | CG1946 | −1.6 > +1.1 | MG | Acylglycerol O-acyltransferase | |
| Males/Females | |||||
| 0023495 | Lip3 | +4.3 > +3.2 +31.9 > +16.2 | MG | Lipase 3 | |
| Carbohydrate metabolism | Males | ||||
| 0036862 | Gbs-76A | −1.5 > −1.2 | FB, DS, NS | Protein phosphatase 1 regulatory subunit | |
| 0035083 | CG9485 | −1.9 > −1.4 | FB, DS | Glycogen debranching enzyme | |
| Females | |||||
| 0016122 | Acer | −1.5 > −1.1 | FB, DS | Glycogen storage; starvation resistance | |
| 0027621 | Pfrx | +1.6 > +1.1 | FB, DS | 6-phosphofructo-2-kinase; activation of glycolysis | |
| 0003074 | Pgi | +1.5 > +1.2 | UBI | Phosphoglucose isomerase; glycolytic enzyme | |
| Mitochondrial pathways | Females | ||||
| 0013469 | klu | +1.7 > +1.1 | MG, NS | Klumpfuss transcription factor; citrate synthase activity | |
| Proteases (see supplemental Table S2) | |||||
| Immune response genes (see supplemental Table S2) | |||||
| Other | Females | ||||
| 0038914 | fit | −66.3 > −18.2 | FB | Protein-specific satiety hormone | |
| 0039152 | Root | −2.9 > −1.5 | NS | Sensory functions, including taste | |
| Genes showing enhanced starvation response | |||||
| Lipid metabolism | Males | ||||
| 0036449 | bmm | +2.7 < +4.0 | FB, DS | Brummer lipase | |
| 0029831 | CG5966 | +2.1 < +3.9 | FB, DS | Lipase | |
| 0039474 | CG6283 | +3.6 < +5.0 | MG | Lipase | |
| 0038068 | CG11600 | −3.8 < −8.1 | MAG | Lipase | |
| 0039184 | CG6432 | −2.5 < −4.5 | FB | Acyl-CoA synthetase | |
| 0052072 | Elo68α | −8.4 < −34.2 | MG, MGS | Very-long-chain fatty acid elongation | |
| 0037534 | CG2781 | −2.2 < −2.9 | FB, DS | Very-long-chain fatty acid elongation | |
| 0037765 | CG9458 | −2.0 < −2.7 | FB | Very-long-chain fatty acid elongation | |
| 0037764 | CG9459 | −1.9 < −3.0 | FB | Very-long-chain fatty acid elongation | |
| 0037763 | CG16904 | −1.8 < −2.4 | FB | Very-long-chain fatty acid elongation | |
| 0050008 | CG30008 | −1.8 < −2.3 | FB | Very-long-chain fatty acid elongation | |
| 0001128 | Gpdh1 | −1.8 < −2.3 | DS, FB | Glycerol-3-phosphate dehydrogenase, cytosolic | |
| Immune response (see supplemental Table S3) | |||||
| Other | |||||
| 0261560 | Thor | +2.8 < +5.3 | FB, DS, MT | eIF4E-binding protein translational regulation | |
| 0000053 | Gart | +4.9 <+12.3 | FB, DS | Purine synthesis | |
For a complete list of genes, see Supplemental Tables S2 and S3. AH, adult head; DS, digestive system; FB, fat body; HG, hindgut; NS, nervous system; MAG, male accessory gland; MG, midgut; MGS, male genital system; MT, Malpighian tubule; UBI, ubiquitous.
Metabolic rate measurements
Metabolic rate was measured as volume CO2 (µl/min) produced per fly using open-flow respirometry. A minimum of seven independent fly cohorts were measured for each genotype and sex. Flies were kept on standard food for 1–2 weeks after eclosure and then separated by sex into groups of seven flies that were transferred to fresh standard food. Flies were allowed to recover from the CO2 anesthesia for 24 h prior to being transferred to respirometry chambers (25). Respirometry chambers were made by modifying 5.0 ml plastic Luer lock syringe barrels with plastic mesh and glass wool to prevent animal escape. The resulting headspace of each chamber was 1.25 ml. A total of eight chambers (one baseline and seven animal chambers) were measured during each respirometry run. All measurements were performed at the same time of the day at 25°C in the dark. During measurements, CO2 and water-free air was pushed through each chamber at a flow rate of 50 ml/min. A multiplexer (Sable Systems) was used to divert the excurrent air from each chamber to a LI-COR CO2 analyzer for measuring CO2 (ppm) production. During each respirometry run, each chamber of seven flies was measured for 7.5 min per hour; baseline was measured for 3.75 min at the top and bottom of every hour. Each set of flies was measured for a minimum of 6 h. Expedata (Sable Systems) was used to control the multiplexer and record data every 5 s. Expedata was also used to run baseline corrections, calculate volume CO2 per fly, and filter data through the use of a nadir function (26). The nadir function selected the lowest continuous values of volume CO2 for a span of 50 s per chamber per hour. The resulting filtered data were analyzed in R using the car package. CO2 production by the flies was stable for at least 4 h of measurement. The values for the first hour of measurements were used for statistical analysis, as they were the lowest recorded. CO2 production was compared between strains and sexes using a two-way ANOVA. In addition to the significant difference detected between strains (P = 0.021), metabolic rates were also significantly different between sexes (P = 0.023).
RESULTS
Lipin is an essential gene with vital functions outside the fat body
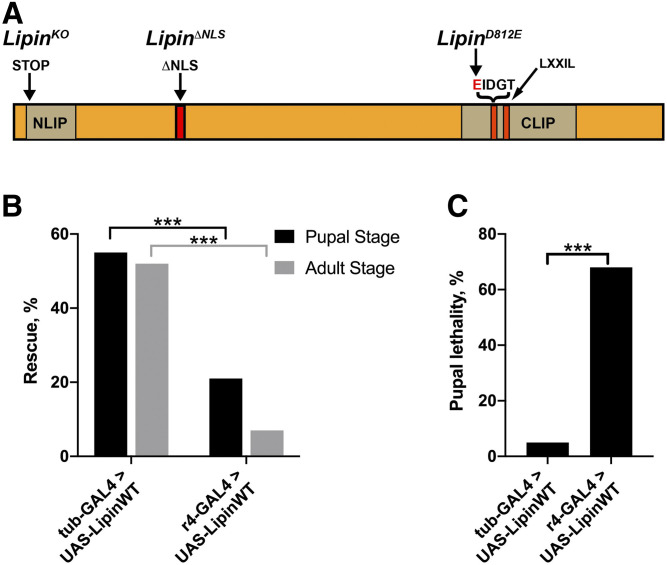
We used CRISPR/Cas9 mutagenesis to create a bona fide null allele of Lipin, LipinKO. LipinKO carries a frame-shift mutation immediately after the start codon, introducing an early stop codon and resulting in a nucleotide sequence encoding a 29-amino acid random peptide (Fig. 1A). Consistent with the prediction that LipinKO is a null allele, animals homozygous for LipinKO, or carrying the allele over the deficiency Df(2R)Exel7095 that uncovers the Lipin locus, are not viable, as indicated by the absence of homozygous larvae, pupae, or adults. Having this mutant available, we asked whether Lipin is required for viability in tissues other than the fat body. Such a requirement is suggested by the broad expression pattern of Lipin, which is not only expressed in the fat body but also in many other tissues, including the gut, the Malpighian tubules, the brain, and the endocrine ring gland (17). We used the r4-GAL4 driver to express wild-type Lipin in the larval fat body in a LipinKO mutant background. Animals carrying this driver exhibit robust GAL4 expression in the larval and adult fat bodies as well as expression in the adult hindgut and Malpighian tubules (see Materials and Methods). For comparison, we attempted a rescue by ubiquitous expression of a wild-type Lipin transgene driven by the tub-GAL4 driver. Expression of Lipin driven by both the r4 and tub-GAL4 driver was sufficient to rescue LipinKO mutants to adulthood. However, while the ubiquitous expression of Lipin led to the rescue of more than half of the mutant animals to the pupal and adult stages, only 21% of the animals expressing Lipin in the fat body were rescued to the pupal stage, and a mere 7% were rescued to the adult stage (Fig. 1B). Animals rescued by fat-body expression, but not by ubiquitous expression, showed substantial pupal lethality (Fig. 1C). Moreover, many (53%) of the adults rescued by fat-body expression died during or shortly after eclosion, whereas none of the adults rescued by ubiquitous expression died at that time. Male flies rescued by ubiquitous expression had a median life span of 61 days (n = 18) and female flies of 47 days (n = 14). The longest-lived male died after 104 days, and the longest-lived female died after 66 days. In striking contrast, none of the flies that had been rescued by fat-body expression, and had not already died at eclosion, lived for more than 14 days. Although flies rescued by ubiquitous expression appeared much healthier and longer lived, flies rescued by either ubiquitous or fat-body expression were infertile. Together, these data strongly suggest that Lipin has vital functions outside the fat body and that proper Lipin expression is particularly critical for fertility.
Fig. 1.
A: Structure of the Drosophila Lipin protein and mutations created for this study. The DIDGT motif mutated to EIDGT in LipinD812E signifies the catalytic motif, and the LXXIL motif signifies the transcriptional coregulator motif. The N-terminal and C-terminal NLIP and CLIP domains are evolutionarily conserved domains shared between lipins from different organisms (4). B: Ubiquitous expression of wild-type Lipin rescues Lipin-null mutants more efficiently than expression that is largely restricted to the fat body. C: Animals rescued by fat-body expression of Lipin exhibit significantly increased pupal lethality compared with animals rescued by ubiquitous expression. Wild-type Lipin was expressed from a UAS-LipinWT transgene in a LipinKO/Df(2R)Exel7095 background using the ubiquitous tub-GAL4 driver or the fat-body r4-GAL4 driver. ***P < 0.0001 (Fisher’s exact test); tub rescue, n = 77; r4 rescue, n = 222.
Lipin’s PAP motif, but not its NLS, is required for the development and viability of adult flies
Next, we asked whether Lipin’s PAP activity and/or nuclear functions of the protein are required for development and survival. To generate a mutant that lacks Lipin’s PAP activity, we used ends-in gene targeting to introduce a single amino acid exchange into Lipin’s catalytic DIDGT motif at position 812, changing it to EIDGT (19) (Fig. 1A). The D-to-E exchange in the motif leads to a complete loss of PAP activity (15). Animals homozygous for the LipinD812E allele developed into first-instar larvae, but none of these larvae reached the second larval instar. The animals could be rescued from the lethality by expressing wild-type Lipin from a transgene in the LipinD812E/LipinD812E background, confirming that the observed lethality was indeed caused by the lack of PAP activity normally provided by Lipin. Most homozygous larvae (83%; n = 75) were able to survive beyond the 24 h that it normally takes to reach the next stage, but all of them died within 60 h as first-instar larvae. This clearly demonstrates that Lipin’s PAP activity is required for development beyond the embryonic and early larval stages. It seems likely that it is also required for embryogenesis because Lipin is a maternally expressed gene and Lipin protein can be detected in the oocyte cytoplasm (17). This suggests that maternal Lipin rescues homozygous mutants through embryogenesis.
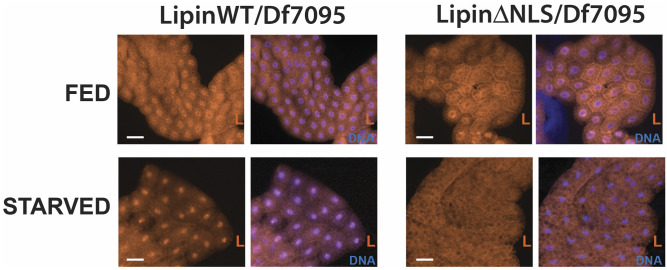
To create a mutant that is defective in carrying out nuclear functions of Lipin, we used CRISPR/Cas9 mutagenesis to introduce an 18-bp deletion into Lipin that removes the protein’s NLS (Fig. 1A). We opted to remove the NLS rather than introducing point mutations into the transcriptional coregulator motif because the latter had been shown to also interfere with the enzymatic activity of the protein (15). We were successful in isolating a LipinΔNLS allele and found that, in contrast to the LipinKO and LipinD812E mutants, LipinΔNLS mutants were viable. To avoid homozygosity for other loci on the mutagenized chromosome, we used animals carrying one copy of LipinΔNLS and control animals carrying one copy of the wild-type Lipin allele of the CRISPR injection stock for all phenotypic characterizations reported here. We accomplished hemizygosity by using a previously characterized deficiency chromosome, Df(2R)Exel7095 (17). To confirm that the mutant protein is excluded from the nucleus, we examined the intracellular distribution of LipinΔNLS and wild-type Lipin under conditions that promote nuclear translocation of Lipin. Nuclear translocation of Lipin is observed when signaling through the nutrient-sensitive TORC1 signaling pathway is attenuated (12). To accomplish this, we subjected prewandering third-instar mutant and wild-type control larvae to starvation conditions. As expected, starvation caused nuclear translocation of Lipin in the fat body of control larvae. In contrast, we did not observe nuclear translocation of LipinΔNLS under these conditions (Fig. 2). These data imply that LipinΔNLS mutants are deficient in carrying out functions that Lipin normally has in the cell nucleus, including gene-regulatory functions.
Fig. 2.
Starvation causes nuclear translocation of wild-type Lipin, but not LipinΔNLS. Feeding third-instar larvae of the indicated genotypes were removed from the food and subjected to starvation conditions. Fat body dissected from starved larvae and fed control larvae was stained with an antibody against Lipin (L) and DAPI to visualize DNA. Scale bars: 50 μm.
LipinΔNLS flies have an improved life expectancy
LipinΔNLS mutant flies were not only viable but also did not show any obvious phenotypic deviations from wild-type flies. Although the absence of Lipin’s NLS did not appear to have major detrimental effects on survival and fecundity, we decided to examine these animals more closely by asking whether they had the same life expectancy as wild-type control flies. To determine whether this was the case, we compared the survival of LipinΔNLS/Df(2R)Exel7095 flies to that of LipinWT/Df(2R)Exel7095 control flies (Fig. 3). Males and female flies were kept separately on standard food, and deaths were monitored regularly until all flies had died. The median life span of both male and female LipinΔNLS flies was significantly increased, an effect that was most pronounced in the females with an increase of 24 days, which amounts to an impressive 34% jump in life expectancy. Females also showed a significant increase in maximum life span by 14 days, whereas the maximum life span in males was not significantly changed (Fig. 3). These data suggest that nuclear functions of Lipin are not only not required but may even be detrimental when flies are raised on a standard diet.
Fig. 3.
Flies that rely exclusively on LipinΔNLS have improved life expectancy but are highly susceptible to starvation. Male and female flies were kept separately on a regular or water-only diet, and survival was monitored daily. P values were determined using the log-rank test.
LipinΔNLS flies have increased susceptibility to starvation
In stark contrast to fed flies, LipinΔNLS flies subjected to starvation showed significantly decreased survival (Fig. 3). LipinΔNLS/Df(2R)Exel7095 and LipinWT/Df(2R)Exel7095 control flies were separated by sex and transferred to vials containing a source of water but no food. Survival was monitored daily, and dead animals were removed. Both LipinΔNLS males and females showed significantly reduced maximum and median life spans. The median life span of males was reduced from 9 to 3 days, and for females the median life span was reduced from 7 to 6 days. The maximum life span for both males and females was reduced from 10 to 6 days. Almost 40% of control females were still alive on day 7 of starvation after all LipinΔNLS females had died. These data suggest that Lipin’s ability to enter the nucleus is of critical importance for physiological adaptation to nutrient deprivation.
Fat stores are not reduced in LipinΔNLS flies
Reduced survival of LipinΔNLS flies under starvation conditions could be due to reduced fat stores of these animals. Therefore, we measured TAG levels of mutant and control flies (Fig. 4A). TAG levels proved to be not reduced in the mutants. On the contrary, they appeared slightly elevated, although differences were not statistically significant. Staining with the lipid dye Bodipy confirmed that the fat body of LipinΔNLS larvae contained normally sized fat droplets, while indicating slightly elevated rather than decreased levels of neutral lipids. This strongly suggests that the nuclear function of Lipin, but not its function in TAG synthesis, is responsible for adaptation to starvation conditions.
Fig. 4.
LipinΔNLS animals do not have reduced fat stores. A: The ratio of total TAG to protein was determined for whole male and female flies of the indicated genotypes. Differences were not statistically significant. B: Fat body of third-instar larvae of the indicated genotypes was stained with Bodipy to visualize fat droplets. Scale bar: 50 μm.
Genes involved in energy homeostasis, feeding behavior, and the immune response are misregulated in fed and food-deprived LipinΔNLS flies
Together, our results suggested that nuclear functions of Lipin are less important when nutrients are plentiful but essential under conditions of nutrient deprivation. Because lipins can act as transcriptional coregulators (14, 15), it seemed likely that nuclear Lipin brings about changes in gene expression that promote adaptation to nutrient deprivation. To test this hypothesis, we carried out RNA-seq analyses with LipinΔNLS and wild-type control flies that had been kept on a standard diet or starved for 24 h. RNA was extracted from male and female flies separately, and mRNA was used for library preparation.
Consistent with the observed differences in life expectancy, many genes were differentially expressed in normally fed LipinΔNLS and Lipin wild-type flies (supplemental Tables S4, S5). About half of the 223 genes that showed an at least 1.5-fold difference in expression in males showed reduced expression in the mutant and the other half increased expression. In females, a similar number of genes (n = 233) were differentially expressed. However, 76% of these genes showed increased expression in the mutant. Overall, sex differences were extensive, with the expression of only 43 genes changing significantly at least 1.5-fold in both males and females. When differentially expressed genes were extracted regardless of sex, genes associated with the gene annotation term fatty acid synthase activity were enriched (Table 1, supplemental Table S1). Increased expression in the mutant was found for two genes encoding the fatty acid synthases FASN2 and FASN3 and five genes encoding putative fatty acid elongases. FASN2, FASN3, and the fatty acid elongase eloF are expressed in adult fat body and oenocytes (24, 27–29). The oenocytes are hepatocyte-like cells in Drosophila with functions in lipid metabolism, cuticular hydrocarbon synthesis, and pheromone production (30, 31). Reduced expression of FASN2, FASN3, and eloF specifically in the oenocytes has been shown to affect hydrocarbon synthesis with consequent effects on desiccation resistance and courtship behavior (27–29, 32). In addition to eloF, four other elongases were affected by Lipin that show expression in fat body and the digestive system.
Other genes associated with lipid metabolism that were misregulated in the Lipin mutant include a trimethyllysine dioxygenase gene encoding an enzyme required for carnitine synthesis. Carnitine serves as a carrier molecule for the transport of long-chain fatty acids into mitochondria, a rate-limiting step in fatty acid β-oxidation. Downregulation of this gene specifically in LipinΔNLS males suggests that β-oxidation is negatively affected in these flies.
Taken together, the data suggest that fatty acid metabolism is shifted from β-oxidation to lipogenesis in LipinΔNLS flies. Thus, when nutrients including fats are plentiful, it seems to be an important function of wild-type Lipin to suppress the de novo synthesis of fatty acids and to promote the use of fatty acids for energy production. At the same time, Lipin appears to impede the use of stored carbohydrates for energy production. In both males and females, one of the most strongly upregulated genes in LipinΔNLS was target of brain insulin (tobi), which encodes an α-glucosidase involved in glycogenolysis (Table 1). Tobi is specifically activated when nutritional sugar is low (33).
Another group of genes that were identified as enriched in the sex-independent analysis consisted of genes encoding serine-type proteases (supplemental Table S1). Half of these proteases that respond to Lipin are specifically expressed in the digestive system. These data suggest that Lipin, which shows both nuclear and cytoplasmic expression in the digestive system (17), plays an important role in the gut in controlling the balance of enzymes involved in the digestion of nutritional protein.
Specifically in male flies, genes differentially expressed in LipinΔNLS and wild-type were enriched for oxidoreductases. Six broadly expressed cytochrome P450 genes showed reduced expression in LipinΔNLS, and several genes annotated as encoding prolyl 4-hydroxylases, which are specifically expressed in the male accessory gland, showed increased expression (supplemental Table S1). The latter suggests a role of Lipin in male fertility, a conclusion that is supported by our observation that rescued LipinKO mutants remain infertile. Interestingly, the expression of certain cytochrome P450 genes is also changed in females, among them Cyp4g1, which encodes an oenocyte-specific ω-hydroxylase that regulates TAG composition (30).
Finally, again specifically in males, genes encoding transmembrane receptors showed enrichment (Table 1, supplemental Table S1). The gene encoding one of these receptors, methuselah-like 8 (mthl8), also showed strong upregulation in females. Interestingly, mthl8 is also upregulated when the transcription factor Cabut is reduced, which is involved in nutrient sensing and rapidly induced by sugar feeding (34). In addition, three gustatory receptors showed altered expression in LipinΔNLS males. Gustatory receptor GR64f is expressed in sugar-sensing neurons of the proboscis and functions as a coreceptor for the detection of sugars in the food (35). Sugar-sensing neurons also mediate fatty acid taste, specifically through the activation of the phospholipase C pathway (36). Therefore, it is interesting to note that phospholipase C encoded by CG14945 is altered in females. Together, these data suggest an involvement of Lipin in the control of responses to nutritional sugars and, possibly, fatty acids. A role in the control of feeding behavior is further supported by the altered expression of several other genes such as tiwaz in males, which encodes an adult feeding regulator that negatively regulates meal size (37); the takeout feeding regulator in both males and females (38, 39); and the intestinal feeding regulator hodor in females (40) (Table 1).
The diminished starvation resistance of LipinΔNLS flies suggested that genes that normally respond to starvation do not do this, or to a lesser degree, in the mutant. As expected, a large number of genes showed a significant 1.5-fold or larger change in expression upon starvation in both males (n = 992) and females (n = 495) (supplemental Tables S6, S7). Not surprisingly, GO analysis revealed that metabolic genes, including genes involved in lipid and carbohydrate metabolism, were enriched among these genes. To test the prediction that the starvation response of some of the genes depended on the presence of wild-type Lipin, we filtered for genes that showed an at least 50% reduction of the starvation response in the LipinΔNLS mutant. This led to the identification of 141 genes in females and 96 genes in males that showed a blunted response to starvation. Many of these genes were immune response genes or involved in energy metabolism (Table 2, supplemental Table S2).
Interestingly, LipinΔNLS flies showed a blunted response of genes encoding enzymes that produce key intermediates of fatty acid metabolism, acyl-CoAs and malonyl-CoA, and that are normally upregulated during starvation. Three acyl-CoA synthetases involved in fatty acid activation that normally show increased expression during starvation in females showed no or reduced increases in the LipinΔNLS mutant. One of them, pdgy, localizes to mitochondria, and fatty acid β-oxidation is reduced in pdgy mutants, suggesting that acyl-CoAs produced by pdgy are primarily used for energy production (41). In another study, pdgy mutants were shown to have decreased starvation resistance (42). Thus, at least some of the reduced starvation resistance of LipinΔNLS flies may be due to the impaired activation of pdgy and the other acyl-CoA synthetases. In females, starvation also activated the gene encoding acetyl-CoA carboxylase (ACC), which catalyzes the rate-limiting step in fatty acid synthesis. In males, ACC was significantly increased as well, but below the 1.5-fold threshold applied here. ACC is the single gene encoding acetyl-CoA carboxylase in Drosophila. Knockdown of ACC in the fat body reduces TAG storage, which is consistent with its role in producing malonyl-CoA for the de novo synthesis of fatty acids (43). Malonyl-CoA is also known to block the carnitine shuttle that transports fatty acids into mitochondria for β-oxidation. Dampening of the carnitine shuttle is also suggested by Lipin-dependent downregulation of two genes in males encoding enzymes required for carnitine synthesis, trimethyllysine dioxygenase and γ-butyrobetaine dioxygenase. This suggests that Lipin contributes to a downregulation of the carnitine shuttle during long-term starvation in both males and females.
Another lipid metabolic gene that was downregulated in response to starvation, but less so in LipinΔNLS males, was FASN1. FASN1 is a broadly expressed fatty acid synthase that is strongly expressed in the fat body and required for the accumulation of normal TAG stores (24, 27). In addition to FASN1, the starvation response of several lipases was altered in LipinΔNLS flies. Consistent with a shift from fatty acid synthesis to lipolysis, a fat body-expressed lipase (CG7367) was upregulated in females. In males, the fat-body-expressed lipase 4 was downregulated, but lipase 4 is also strongly expressed in the digestive system, suggesting that overall downregulation of this lipase primarily reflects the reduced requirement for digestion of nutritional fats. Conspicuously, many other genes that showed a reduced response to starvation in LipinΔNLS are predominantly or exclusively expressed in the digestive system, especially the midgut (Table 2, supplemental Table S2). This suggests that Lipin regulates genes in the gut that are involved in the uptake and processing of nutrients. Among these genes is p38c, which encodes a MAPK functioning in intestinal lipid homeostasis. Flies deficient in p38c accumulate neutral lipids in the gut, suggesting that the kinase controls lipid processing by the tissue (44). Another example is Nplp2, a gene that is important for dietary lipid extraction and effective lipid storage in the fat body (45).
Interestingly, our data show that the upregulation under starvation conditions of lipase 3 (Lip3), another lipase that is expressed in the digestive system, is considerably inhibited in LipinΔNLS flies (Table 2). In the fed state, Lip3 is negatively regulated by Lipin (Table 1). Lip3 is regulated by the fatty acid-activated nuclear receptor HNF4, which upregulates Lip3 under starvation conditions (46, 47). Thus, the reduced upregulation of Lip3 in LipinΔNLS flies suggests that HNF4 and Lipin cooperate in Lip3 activation. In mammals, lipin 1 directly interacts with HNF4α to regulate lipid metabolic genes (15). The Drosophila HNF4 orthologue has an important role during starvation when it activates genes that act in fatty acid β-oxidation and lipolysis, such as Lip3 (47). We therefore asked whether the two proteins share additional potential target genes. HNF4 expression is not changed in the LipinΔNLS mutant either under fed or starved conditions. Thus, potential responses of HNF4 target genes to Lipin should be independent of HNF4. Genes regulated by HNF4 in Drosophila have been identified by microarray studies using third-instar larvae (47). When we compare these data with our RNA-seq data, we find that, in the fed state, HNF4 and Lipin share no target genes in females and only four genes in males. However, in the starved state, this number increases for males and females together to 22 genes, corresponding to 7% of the genes that respond to HNF4 under starvation conditions (supplemental Table S12). Among the shared targets under both fed and fasting conditions is the trimethyllysine dioxygenase encoded by CG4335 and, under fed conditions, the γ-butyrobetaine dioxygenase encoded by CG5321 and a predicted lipase (CG6295). The transmembrane receptor gene mthl8, which is upregulated in fed LipinΔNLS flies, is upregulated in both LipinΔNLS and HNF4 mutant flies under starvation conditions as well (supplemental Table S12).
In summary, our data reveal complex changes in the starvation response of genes involved in lipid metabolism that depend on Lipin. Overall, these changes seem to promote lipolysis while lowering fatty acid synthesis, as one would expect based on the energy demands of starved flies.
In addition to genes involved in lipid metabolism, a similar number of genes involved in carbohydrate and mitochondrial energy metabolism showed a reduced response to starvation in the LipinΔNLS mutant. Among these are genes promoting glycogen breakdown (Gbs-76, CG9485) and sugar transport (CG9657, CG17930) that are downregulated during starvation in males. CG9485, which encodes an orthologue of the glycogen debranching enzyme AGL, is also downregulated when insulin signaling is reduced in the larval fat body of Drosophila (48). Another gene associated with glycogen storage, Acer, is reduced in starved females. Acer mutants of Drosophila have reduced glycogen stores. It is therefore somewhat surprising that these mutants exhibit slightly increased starvation resistance (49). Thus, reduced downregulation of Acer in the LipinΔNLS mutant may contribute to the starvation sensitivity of the mutant. The upregulation of several genes by starvation that promote energy production by glycolysis and oxidative phosphorylation, including 6-phosphofructo-2-kinase, phosphoglucose isomerase, and klumpfuss (50), was impaired in LipinΔNLS (Table 2). Thus, combined, the data obtained for fed and fasting conditions suggest that it is the basic function of nuclear Lipin to stimulate catabolic processes and energy production.
Consistent with the observed effects of Lipin on genes controlling feeding behavior in the fed state, our data suggest that Lipin also contributes to changes in feeding behavior induced by starvation (Table 2). FIT, a satiety hormone produced by the fat body that is essential for feeding control in females and strongly downregulated during starvation (51), shows reduced downregulation in the LipinΔNLS mutant. Likewise, reduced expression of Root, a gene that is required for the normal function of sugar-sensing neurons, is blunted in the mutant (52). Thus, our data support a role of Lipin in controlling feeding behavior in both fed and starved flies.
Interestingly, for some genes, the starvation response was enhanced in the LipinΔNLS mutant, indicating that Lipin normally limits the starvation response of these genes. Enriched among these genes in males were, again, genes involved in lipid metabolism (Table 2, supplemental Table S3). The lipase Brummer and two other lipases were more strongly induced during starvation, suggesting that accelerated depletion of fat reserves may contribute to the reduced starvation resistance of LipinΔNLS males. Interestingly, a target of the nutrient-responsive TORC1 pathway, the translational repressor 4E-BP1 (Thor), was more strongly activated in the LipinΔNLS mutant. Equally noteworthy is the enhanced downregulation of Gpdh1, which encodes cytosolic glycerol-3-phosphate dehydrogenase, a key enzyme linking carbohydrate and lipid metabolism. Finally, genes encoding antimicrobial peptidoglycan-recognition proteins showed an enhanced response in the mutant, adding to the suite of immune response genes whose starvation response depends on Lipin (supplemental Tables S2, S3).
Metabolic rate is reduced in LipinΔNLS flies
The RNA-seq analyses indicated that interference with Lipin’s ability to translocate into the cell nucleus has broad effects on the expression of genes involved in energy homeostasis. In particular, they suggested a shift to reduced β-oxidation in fed LipinΔNLS flies that predicts that energy production by oxidative phosphorylation is reduced in these flies. To test this hypothesis, we examined whether LipinΔNLS and control flies exhibited differences in their metabolic rates. The metabolic rate in male and female flies was measured as CO2 production by open-flow respirometry. We found that the metabolic rates were indeed significantly reduced by 8% in male and by 9% in female flies (Fig. 5). These results confirm our prediction that energy production is throttled in LipinΔNLS flies and are consistent with our observation that LipinΔNLS flies lived longer and thus seemed healthier than control flies.
Fig. 5.
Metabolic rate is decreased in LipinΔNLS flies. The metabolic rate of adult ad libitum fed flies of the indicated genotypes was measured as CO2 production. Differences between mutant flies and control flies were statistically significant (P = 0.021; two-way ANOVA).
DISCUSSION
We created three different Lipin mutants, LipinKO, LipinD812E, and LipinΔNLS, to distinguish between requirements for different activities of the Lipin protein during development and different metabolic states. We had previously shown that animals homozygous for a hypomorphic allele of Lipin exhibit delayed development and late-larval and pupal lethality (17). Our data with the newly generated LipinKO and LipinD812E mutants now show that zygotically expressed Lipin and its enzymatic activity are absolutely required for larval development beyond the L1 stage. This result is consistent with our previous observation that the expression of LipinD812E from a transgene does not rescue Lipin mutant phenotypes (12). Rescue experiments show that ubiquitous, but not fat-body-restricted expression, of wild-type Lipin can fully rescue the Lipin null mutant (except for fertility), indicating that Lipin has essential functions outside of the fat body. Flies rescued by ubiquitous expression appeared healthy and had a life expectancy that was comparable to that of wild-type flies, whereas most of the flies rescued by fat-body expression died shortly after eclosion. Interestingly, however, all rescued flies remained sterile, indicating a requirement of Lipin for reproduction that is sensitive to the timing and/or amount of Lipin expression in one or more tissues. In contrast to Lipin’s PAP activity, interference with the ability of the protein to translocate into the cell nucleus does not impair survival under normal feeding conditions. Animals that rely entirely on Lipin without an NLS are not only viable but also appear healthier and live longer than wild-type flies. This finding is consistent with our previous observation that LipinΔNLS expressed from a transgene rescues lethality and larval fat-body phenotypes of Lipin mutants to a similar extent as wild-type Lipin (12). The improved life expectancy of LipinΔNLS flies correlates with changes in the expression levels of metabolic genes. However, in stark contrast to the improved survival under fed conditions, the lack of the NLS proved to be detrimental under starvation conditions. The requirement of nuclear translocation to resist starvation is consistent with earlier data showing that Lipin translocates into the cell nucleus when nutrients are scarce and activity of the TORC1 pathway is low (12).
Our RNA-seq analysis provides a first insight into the genomic response to a loss of Lipin function in Drosophila. Previous studies in yeast, mice, and C. elegans that identified candidate genes that respond to the loss of lipin (14) were based on overexpression of the protein (15) or addressed lipin-dependent changes in response to a sugar-rich diet (16). Overexpression of lipin 1 in mouse liver led to the changed expression of almost 4,000 genes (15). Among the upregulated genes were the genes encoding nuclear receptors PPARα and HNF4α, which were also shown to physically interact with lipin 1 (15). Thus, at least some of the response to lipin 1 is likely mediated by these two transcription factors and lipin 1 in a cooperative fashion. PPARs, which are known as key regulators of lipid and energy metabolism in mammals, have not been identified in Drosophila, whereas an HNF4 orthologue has. Drosophila HNF4 acts predominantly during starvation, activating genes involved in lipolysis and fatty acid β-oxidation. Because target genes of Drosophila HNF4 are similar to target genes of PPARα, it has been proposed that HNF4 may, at least in part, functionally substitute for PPARα in Drosophila (47). A comparison of our RNA-seq data with microarray data obtained for an HNF4 null mutant did not indicate a substantial overlap between potential target genes of Lipin and HNF4. However, it must be emphasized that the data were obtained with different source materials, whole third-instar larvae in the case of HNF4 and adult flies in the case of Lipin. Therefore, they do not allow firm conclusions with respect to the extent of cooperation between HNF4 and Lipin in Drosophila, although negative results of genetic interaction studies do not support such a cooperation either (Q. Chen, unpublished observations). Still, the potential relationship between the two proteins warrants further investigation. Our data indicate that functions of Lipin and HNF4 may converge at some points, especially the carnitine synthesis pathway and the regulation of Lip3. Whereas both Lipin and HNF4 stimulate Lip3 expression during starvation, only Lipin has a negative effect on Lip3 in the fed state, leading to strong upregulation of Lip3 in LipinΔNLS flies (Table 1). Because high levels of Lip3 expression cause lipotoxicity (53), the effect of Lipin on Lip3 may help protect cells from the detrimental effects of an overload of free fatty acids in the fed state.
Our RNA-seq data provide a first glimpse into the organism-wide genomic response to Lipin under feeding and fasting conditions. A large number of genes were differentially expressed in the fed state in both males and females between wild-type and LipinΔNLS flies (n = 413). This does not come as a surprise because Lipin shows partial nuclear localization in the fed state in some tissues, such as the gut and the brain, and preferential nuclear localization in at least one tissue, the Malpighian tubules (17). Strikingly, male and female flies showed a vastly different response to wild-type and mutant Lipin with an overlap of only about 20% of the genes that responded at least 1.5-fold in both sexes. This result is in accordance with an increasing number of studies that find substantial differences in metabolic gene expression between the sexes (54–56). Especially in insects, with their yolk-rich eggs and extensive resource allocation for reproduction, sex-specific differences in metabolic gene expression do not come as a surprise. Despite these differences, the biological processes affected (Fig. 6) are similar in both males and females. The majority of the affected genes have functions in energy metabolism, in particular lipid and carbohydrate metabolism.
Fig. 6.
Biological processes that respond to interference with Lipin’s ability to migrate into the cell nucleus.
A prediction resulting from the RNA-seq data was that energy production is reduced in fed LipinΔNLS flies. We confirmed this by showing that the flies indeed exhibit a lowered metabolic rate. Reductions in reactive oxygen species associated with low metabolic rates are being discussed as determining factors of aging and longevity (57, 58). Thus, the reduced metabolic rate may contribute to the observed increase in life expectancy of LipinΔNLS flies. However, although it does not refute this possibility, it should be noted that Drosophila strains selected for longevity exhibit normal metabolic rates (59, 60).
In contrast to the fed state, the ability of Lipin to translocate into the cell nucleus clearly plays an essential role under conditions of nutrient deprivation. Both male and female LipinΔNLS flies die significantly earlier under starvation conditions than control flies. The genomic response to starvation is substantially altered in LipinΔNLS flies. The enhanced up- or reduced downregulation of several lipases, including Brummer in starved LipinΔNLS males, suggests that these flies break down fat stores more rapidly than control flies (Table 2). At the same time, the data suggest that they also exhaust glycogen faster. Downregulation of a gene encoding a glycogen debranching enzyme is attenuated in LipinΔNLS males, as is downregulation of Gbs-76A, which encodes a putative protein phosphatase 1 (PP-1) inhibitory subunit. PP-1 plays a critical role in glycogenolysis by inhibiting glycogen phosphorylase (61). Interestingly, the conserved NLIP domain of Lipin contains an HVRF motif that constitutes a binding site for the catalytic subunit of PP-1 (62). This raises the possibility that Gbs-76A contributes to the regulation of Lipin phosphorylation. Female LipinΔNLS flies less efficiently downregulate Acer, a gene that is also involved in glycogen metabolism (49), and they less efficiently upregulate glycolytic enzymes. This suggests that, similar to males, females cannot make efficient use of energy reserves, although lipolytic enzymes are not as much affected in females as they are in males. Together with the presence of an additional energy source provided by degenerating oocytes, the latter may explain why starvation resistance is not as severely affected in females as it is in males (Fig. 3). The reduced activity predicted by the data of several acyl-CoA synthetases in starved LipinΔNLS males and females and of cytosolic glycerol-3-phosphate dehydrogenase in males is consistent with the interpretation that LipinΔNLS flies deplete energy reserves more quickly than control flies.
The transcriptional upregulation of ACC during starvation, which is dampened in LipinΔNLS females, was somewhat unexpected. ACC produces malonyl-CoA from acetyl-CoA for the de novo synthesis of fatty acids. Malonyl-CoA also has the property of blocking the carnitine shuttle that transports fatty acids into mitochondria for β-oxidation. Thus, upregulation of ACC has the potential of counteracting the shift from lipogenesis to β-oxidation that occurs during starvation. However, it is important to note that the activity of ACC is tightly regulated at the posttranslational level, in particular by AMPK (63). Moreover, malonyl-CoA produced by ACC is not only used for fatty acid synthesis but also for protein malonylation (64). Interestingly, activity of the nutrient-sensitive TOR kinase can be reduced by malonylation, which may contribute to the inhibition of TORC1 during starvation (65). The possibility of increased inhibition of the carnitine shuttle after 24 h of starvation, which must be considered as long-term starvation for flies, is not only suggested by upregulation of ACC but also by decreased expression of two enzymes acting in the carnitine synthesis pathway, trimethyllysine dioxygenase and γ-butyrobetaine dioxygenase. It is possible that after this extended period of starvation flies start to conserve energy stores for long-term survival.
An interesting and novel observation was that Lipin is involved in the control of immune response genes. Starvation-induced changes in the expression of a number of immune response genes depend on Lipin (supplemental Tables S2, S3). Recent evidence suggests that interactions between Lipin and the immune response are dynamic. Lipin transcript levels decrease by 50% when an immune response is stimulated by the expression of a constitutively active Toll receptor in the fat body. This decrease is associated with a reduction in TAG storage (66). Together, these observations further strengthen previously observed links between lipid metabolism and the immune response in Drosophila (67).
The improved life expectancy of normally fed flies that results from interference with nuclear translocation of Lipin is of particular interest in light of the recent finding that high levels of glucose induce nuclear translocation of C. elegans Lipin 1 and that decreased levels of Lipin 1 increase glucose toxicity (16). Misregulation in LipinΔNLS flies of the α-glucosidase Tobi as well as Root and the Mthl8 receptor, which is regulated by the sugar-sensitive Cabut transcription factor, suggest a similar role of Drosophila Lipin in the metabolic response to glucose. These observations support the idea that nuclear translocation of lipins is sensitive to specific nutrients and that lipins mediate or block the genomic effects of these nutrients. It will be interesting to further investigate how the nuclear translocation of Drosophila Lipin is fine-tuned in this manner and how this translates into healthy or unhealthy metabolic outcomes.
Data availability
Data have been deposited with the Gene Expression Omnibus under accession number GSE158189. All other data are contained and described herein and in the supplemental material.
Supplementary Material
Acknowledgments
We thank Steven J. Beaupre for providing access to the Sable system for metabolic-rate measurements and Jae Park and the Bloomington Drosophila Stock Center for providing fly stocks.
Footnotes
Author contributions—M.L. study design and concept; S.E.H., Q.C., J.O., and M.L. experimental design; S.E.H., X.V.K., Q.C., J.S., J.O., and M.L. experiments; J.O. and M.L. data and statistical analysis; S.E.H., J.O., and M.L. visualization; M.L. writing-original draft; S.E.H., X.V.K., J.S., and J.O. writing-review and editing.
This article contains supplemental data.
Funding and additional information—This work was supported by National Institutes of Health Grant 1R15DK114748-01 and the Arkansas Biosciences Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest—The authors declare that they have no conflicts of interest with the contents of this article.
Author ORCIDs—Michael Lehmann https://orcid.org/0000-0002-8736-6990
Abbreviations—
- ACC
- acetyl-CoA carboxylase
- GO
- gene ontology
- Lip3
- lipase 3
- mth8
- methuselah-like 8
- NLS
- nuclear localization signal
- PAP
- phosphatidic acid phosphatase
- PP-1
- protein phosphatase 1
- TAG
- triacylglycerol
- Tobi
- target of brain insulin
- TORC1
- target of rapamycin complex 1
Manuscript received July 30, 2020, and in revised form September 21, 2020. Published, JLR Papers in Press, September 28, 2020, DOI 10.1194/jlr.RA120001051.
REFERENCES
- 1.Owusu-Ansah E., and Perrimon N.. 2014. Modeling metabolic homeostasis and nutrient sensing in Drosophila: implications for aging and metabolic diseases. Dis. Model. Mech. 7: 343–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heier C., and Kühnlein R. P.. 2018. Triacylglycerol metabolism in Drosophila melanogaster. Genetics. 210: 1163–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han G-S., Wu W-I., and Carman G. M.. 2006. The Saccharomyces cerevisiae Lipin homolog is a Mg2+-dependent phosphatidate phosphatase enzyme. J. Biol. Chem. 281: 9210–9218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Péterfy M., Phan J., Xu P., and Reue K.. 2001. Lipodystrophy in the fld mouse results from mutation of a new gene encoding a nuclear protein, lipin. Nat. Genet. 27: 121–124. [DOI] [PubMed] [Google Scholar]
- 5.Pillai A. N., Shukla S., and Rahaman A.. 2017. An evolutionarily conserved phosphatidate phosphatase maintains lipid droplet number and endoplasmic reticulum morphology but not nuclear morphology. Biol. Open. 6: 1629–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lehmann M. 2018. Endocrine and physiological regulation of neutral fat storage in Drosophila. Mol. Cell. Endocrinol. 461: 165–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siniossoglou S. 2013. Phospholipid metabolism and nuclear function: roles of the lipin family of phosphatidic acid phosphatases. Biochim. Biophys. Acta. 1831: 575–581. [DOI] [PubMed] [Google Scholar]
- 8.Harris T. E., and Finck B. N.. 2011. Dual function lipin proteins and glycerolipid metabolism. Trends Endocrinol. Metab. 22: 226–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Csaki L. S., and Reue K.. 2010. Lipins: multifunctional lipid metabolism proteins. Annu. Rev. Nutr. 30: 257–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reue K., and Dwyer J. R.. 2009. Lipin proteins and metabolic homeostasis. J. Lipid Res. 50 (Suppl): S109–S114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reue K., and Brindley D. N.. 2008. Thematic Review Series: Glycerolipids. Multiple roles for lipins/phosphatidate phosphatase enzymes in lipid metabolism. J. Lipid Res. 49: 2493–2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmitt S., Ugrankar R., Greene S. E., Prajapati M., and Lehmann M.. 2015. Drosophila Lipin interacts with insulin and TOR signaling pathways in the control of growth and lipid metabolism. J. Cell Sci. 128: 4395–4406. [DOI] [PubMed] [Google Scholar]
- 13.Peterson T. R., Sengupta S. S., Harris T. E., Carmack A. E., Kang S. A., Balderas E., Guertin D. A., Madden K. L., Carpenter A. E., Finck B. N., et al. 2011. mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell. 146: 408–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Santos-Rosa H., Leung J., Grimsey N., Peak-Chew S., and Siniossoglou S.. 2005. The yeast lipin Smp2 couples phospholipid biosynthesis to nuclear membrane growth. EMBO J. 24: 1931–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finck B. N., Gropler M. C., Chen Z., Leone T. C., Croce M. A., Harris T. E., Lawrence J. C., and Kelly D. P.. 2006. Lipin 1 is an inducible amplifier of the hepatic PGC-1alpha/PPARalpha regulatory pathway. Cell Metab. 4: 199–210. [DOI] [PubMed] [Google Scholar]
- 16.Jung Y., Kwon S., Ham S., Lee D., Park H-E. H., Yamaoka Y., Jeong D-E., Artan M., Altintas O., Park S., et al. 2020. Caenorhabditis elegans Lipin 1 moderates the lifespan-shortening effects of dietary glucose by maintaining ω-6 polyunsaturated fatty acids. Aging Cell. 19: e13150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ugrankar R., Liu Y., Provaznik J., Schmitt S., and Lehmann M.. 2011. Lipin is a central regulator of adipose tissue development and function in Drosophila melanogaster. Mol. Cell. Biol. 31: 1646–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee G., and Park J. H.. 2004. Hemolymph sugar homeostasis and starvation-induced hyperactivity affected by genetic manipulations of the adipokinetic hormone-encoding gene in Drosophila melanogaster. Genetics. 167: 311–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maggert K. A., Gong W. J., and Golic K. G.. 2008. Methods for homologous recombination in Drosophila. Methods Mol. Biol. 420: 155–174. [DOI] [PubMed] [Google Scholar]
- 20.Tennessen J. M., Barry W. E., Cox J., and Thummel C. S.. 2014. Methods for studying metabolism in Drosophila. Methods. 68: 105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ritchie M. E., Phipson B., Wu D., Hu Y., Law C. W., Shi W., and Smyth G. K.. 2015. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43: e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu G., Wang L-G., Han Y., and He Q-Y.. 2012. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 16: 284–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chintapalli V. R., Wang J., and Dow J. A. T.. 2007. Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat. Genet. 39: 715–720. [DOI] [PubMed] [Google Scholar]
- 24.Leader D. P., Krause S. A., Pandit A., Davies S. A., and Dow J. A. T.. 2018. FlyAtlas 2: a new version of the Drosophila melanogaster expression atlas with RNA-Seq, miRNA-Seq and sex-specific data. Nucleic Acids Res. 46: D809–D815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colinet H., and Renault D.. 2012. Metabolic effects of CO(2) anaesthesia in Drosophila melanogaster. Biol. Lett. 8: 1050–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lighton J. R. B. 2008. Oxford University Press, Oxford, UK. [Google Scholar]
- 27.Garrido D., Rubin T., Poidevin M., Maroni B., Le Rouzic A., Parvy J-P., and Montagne J.. 2015. Fatty acid synthase cooperates with glyoxalase 1 to protect against sugar toxicity. PLoS Genet. 11: e1004995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chung H., Loehlin D. W., Dufour H. D., Vaccarro K., Millar J. G., and Carroll S. B.. 2014. A single gene affects both ecological divergence and mate choice in Drosophila. Science. 343: 1148–1151. [DOI] [PubMed] [Google Scholar]
- 29.Lin W-S., Yeh S-R., Fan S-Z., Chen L-Y., Yen J-H., Fu T-F., Wu M-S., and Wang P-Y.. 2018. Insulin signaling in female Drosophila links diet and sexual attractiveness. FASEB J. 32: 3870–3877. [DOI] [PubMed] [Google Scholar]
- 30.Gutierrez E., Wiggins D., Fielding B., and Gould A. P.. 2007. Specialized hepatocyte-like cells regulate Drosophila lipid metabolism. Nature. 445: 275–280. [DOI] [PubMed] [Google Scholar]
- 31.Chung H., and Carroll S. B.. 2015. Wax, sex and the origin of species: dual roles of insect cuticular hydrocarbons in adaptation and mating. BioEssays. 37: 822–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chertemps T., Duportets L., Labeur C., Ueda R., Takahashi K., Saigo K., and Wicker-Thomas C.. 2007. A female-biased expressed elongase involved in long-chain hydrocarbon biosynthesis and courtship behavior in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA. 104: 4273–4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buch S., Melcher C., Bauer M., Katzenberger J., and Pankratz M. J.. 2008. Opposing effects of dietary protein and sugar regulate a transcriptional target of Drosophila insulin-like peptide signaling. Cell Metab. 7: 321–332. [DOI] [PubMed] [Google Scholar]
- 34.Bartok O., Teesalu M., Ashwall-Fluss R., Pandey V., Hanan M., Rovenko B. M., Poukkula M., Havula E., Moussaieff A., Vodala S., et al. 2015. The transcription factor Cabut coordinates energy metabolism and the circadian clock in response to sugar sensing. EMBO J. 34: 1538–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiao Y., Moon S. J., Wang X., Ren Q., and Montell C.. 2008. Gr64f is required in combination with other gustatory receptors for sugar detection in Drosophila. Curr. Biol. 18: 1797–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Masek P., and Keene A. C.. 2013. Drosophila fatty acid taste signals through the PLC pathway in sugar-sensing neurons. PLoS Genet. 9: e1003710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williams M. J., Goergen P., Rajendran J., Zheleznyakova G., Hägglund M. G., Perland E., Bagchi S., Kalogeropoulou A., Khan Z., Fredriksson R., et al. 2014. Obesity-linked homologues TfAP-2 and Twz establish meal frequency in Drosophila melanogaster. PLoS Genet. 10: e1004499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meunier N., Belgacem Y. H., and Martin J-R.. 2007. Regulation of feeding behaviour and locomotor activity by takeout in Drosophila. J. Exp. Biol. 210: 1424–1434. [DOI] [PubMed] [Google Scholar]
- 39.Sarov-Blat L., So W. V., Liu L., and Rosbash M.. 2000. The Drosophila takeout gene is a novel molecular link between circadian rhythms and feeding behavior. Cell. 101: 647–656. [DOI] [PubMed] [Google Scholar]
- 40.Redhai S., Pilgrim C., Gaspar P., Giesen L. V., Lopes T., Riabinina O., Grenier T., Milona A., Chanana B., Swadling J. B., et al. 2020. An intestinal zinc sensor regulates food intake and developmental growth. Nature. 580: 263–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu X., Gopalacharyulu P., Seppänen-Laakso T., Ruskeepää A-L., Aye C. C., Carson B. P., Mora S., Orešič M., and Teleman A. A.. 2012. Insulin signaling regulates fatty acid catabolism at the level of CoA activation. PLoS Genet. 8: e1002478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thimgan M. S., Kress N., Lisse J., Fiebelman C., and Hilderbrand T.. 2018. The acyl-CoA Synthetase, pudgy, Promotes Sleep and Is Required for the Homeostatic Response to Sleep Deprivation. Front. Endocrinol. (Lausanne). 9: 464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parvy J-P., Napal L., Rubin T., Poidevin M., Perrin L., Wicker-Thomas C., and Montagne J.. 2012. Drosophila melanogaster Acetyl-CoA-carboxylase sustains a fatty acid-dependent remote signal to waterproof the respiratory system. PLoS Genet. 8: e1002925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chakrabarti S., Poidevin M., and Lemaitre B.. 2014. The Drosophila MAPK p38c regulates oxidative stress and lipid homeostasis in the intestine. PLoS Genet. 10: e1004659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rommelaere S., Boquete J-P., Piton J., Kondo S., and Lemaitre B.. 2019. The exchangeable apolipoprotein Nplp2 sustains lipid flow and heat acclimation in Drosophila. Cell Rep. 27: 886–899.e6. [DOI] [PubMed] [Google Scholar]
- 46.Becker T., Loch G., Beyer M., Zinke I., Aschenbrenner A. C., Carrera P., Inhester T., Schultze J. L., and Hoch M.. 2010. FOXO-dependent regulation of innate immune homeostasis. Nature. 463: 369–373. [DOI] [PubMed] [Google Scholar]
- 47.Palanker L., Tennessen J. M., Lam G., and Thummel C. S.. 2009. Drosophila HNF4 regulates lipid mobilization and beta-oxidation. Cell Metab. 9: 228–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamada T., Habara O., Kubo H., and Nishimura T.. 2018. Fat body glycogen serves as a metabolic safeguard for the maintenance of sugar levels in Drosophila. Development. 145: dev158865. [DOI] [PubMed] [Google Scholar]
- 49.Glover Z., Hodges M. D., Dravecz N., Cameron J., Askwith H., Shirras A., and Broughton S. J.. 2019. Loss of angiotensin-converting enzyme-related (ACER) peptidase disrupts behavioural and metabolic responses to diet in Drosophila melanogaster. J. Exp. Biol. 222: jeb194332. [DOI] [PubMed] [Google Scholar]
- 50.Chen J., Shi X., Padmanabhan R., Wang Q., Wu Z., Stevenson S. C., Hild M., Garza D., and Li H.. 2008. Identification of novel modulators of mitochondrial function by a genome-wide RNAi screen in Drosophila melanogaster. Genome Res. 18: 123–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun J., Liu C., Bai X., Li X., Li J., Zhang Z., Zhang Y., Guo J., and Li Y.. 2017. Drosophila FIT is a protein-specific satiety hormone essential for feeding control. Nat. Commun. 8: 14161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen J. V., Kao L-R., Jana S. C., Sivan-Loukianova E., Mendonça S., Cabrera O. A., Singh P., Cabernard C., Eberl D. F., Bettencourt-Dias M., et al. 2015. Rootletin organizes the ciliary rootlet to achieve neuron sensory function in Drosophila. J. Cell Biol. 211: 435–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bülow M. H., Wingen C., Senyilmaz D., Gosejacob D., Sociale M., Bauer R., Schulze H., Sandhoff K., Teleman A. A., Hoch M., et al. 2018. Unbalanced lipolysis results in lipotoxicity and mitochondrial damage in peroxisome-deficient Pex19 mutants. Mol. Biol. Cell. 29: 396–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chella Krishnan K., Mehrabian M., and Lusis A. J.. 2018. Sex differences in metabolism and cardiometabolic disorders. Curr. Opin. Lipidol. 29: 404–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lopes-Ramos C. M., Chen C-Y., Kuijjer M. L., Paulson J. N., Sonawane A. R., Fagny M., Platig J., Glass K., Quackenbush J., and DeMeo D. L.. 2020. Sex differences in gene expression and regulatory networks across 29 human tissues. Cell Rep. 31: 107795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Norheim F., Hasin-Brumshtein Y., Vergnes L., Chella Krishnan K., Pan C., Seldin M. M., Hui S. T., Mehrabian M., Zhou Z., Gupta S., et al. 2019. Gene-by-sex interactions in mitochondrial functions and cardio-metabolic traits. Cell Metab. 29: 932–949.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lesnefsky E. J., and Hoppel C. L.. 2006. Oxidative phosphorylation and aging. Ageing Res. Rev. 5: 402–433. [DOI] [PubMed] [Google Scholar]
- 58.Riera C. E., Merkwirth C., De Magalhaes Filho C. D., and Dillin A.. 2016. Signaling networks determining life span. Annu. Rev. Biochem. 85: 35–64. [DOI] [PubMed] [Google Scholar]
- 59.van Voorhies W. A., Khazaeli A. A., and Curtsinger J. W.. 2003. Selected contribution: long-lived Drosophila melanogaster lines exhibit normal metabolic rates. J. Appl. Physiol. 95: 2605–2613. [DOI] [PubMed] [Google Scholar]
- 60.Khazaeli A. A., Van Voorhies W., and Curtsinger J. W.. 2005. Longevity and metabolism in Drosophila melanogaster: genetic correlations between life span and age-specific metabolic rate in populations artificially selected for long life. Genetics. 169: 231–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Petersen M. C., Vatner D. F., and Shulman G. I.. 2017. Regulation of hepatic glucose metabolism in health and disease. Nat. Rev. Endocrinol. 13: 572–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kok B. P. C., Skene-Arnold T. D., Ling J., Benesch M. G. K., Dewald J., Harris T. E., Holmes C. F. B., and Brindley D. N.. 2014. Conserved residues in the N terminus of lipin-1 are required for binding to protein phosphatase-1c, nuclear translocation, and phosphatidate phosphatase activity. J. Biol. Chem. 289: 10876–10886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hardie D. G., and Pan D. A.. 2002. Regulation of fatty acid synthesis and oxidation by the AMP-activated protein kinase. Biochem. Soc. Trans. 30: 1064–1070. [DOI] [PubMed] [Google Scholar]
- 64.Hirschey M. D., and Zhao Y.. 2015. Metabolic regulation by lysine malonylation, succinylation, and glutarylation. Mol. Cell. Proteomics. 14: 2308–2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bruning U., Morales-Rodriguez F., Kalucka J., Goveia J., Taverna F., Queiroz K. C. S., Dubois C., Cantelmo A. R., Chen R., Loroch S., et al. 2018. Impairment of angiogenesis by fatty acid synthase inhibition involves mTOR malonylation. Cell Metab. 28: 866–880.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Martínez B. A., Yeudall S., Hoyle R. G., Castle J. D., Leitinger N., and Bland M. L.. 2020. Innate immune signaling in Drosophila shifts anabolic lipid metabolism from triglyceride storage to phospholipid synthesis in an ER stress-dependent manner. bioRxiv. 10.1101/2020.03.01.972208. [DOI] [PMC free article] [PubMed]
- 67.DiAngelo J. R., Bland M. L., Bambina S., Cherry S., and Birnbaum M. J.. 2009. The immune response attenuates growth and nutrient storage in Drosophila by reducing insulin signaling. Proc. Natl. Acad. Sci. USA. 106: 20853–20858. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data have been deposited with the Gene Expression Omnibus under accession number GSE158189. All other data are contained and described herein and in the supplemental material.