Abstract
Familial LCAT deficiency (FLD) is a rare genetic disorder of HDL metabolism, caused by loss-of-function mutations in the LCAT gene and characterized by a variety of symptoms including corneal opacities and kidney failure. Renal disease represents the leading cause of morbidity and mortality in FLD cases. However, the prognosis is not known and the rate of deterioration of kidney function is variable and unpredictable from patient to patient. In this article, we present data from a follow-up of the large Italian cohort of FLD patients, who have been followed for an average of 12 years. We show that renal failure occurs at the median age of 46 years, with a median time to a second recurrence of 10 years. Additionally, we identify high plasma unesterified cholesterol level as a predicting factor for rapid deterioration of kidney function. In conclusion, this study highlights the severe consequences of FLD, underlines the need of correct early diagnosis and referral of patients to specialized centers, and highlights the urgency for effective treatments to prevent or slow renal disease in patients with LCAT deficiency.
Keywords: lecithin:cholesterol acyltransferase, renal disease, lipoproteins, high density lipoprotein, cholesterol/metabolism, familial lecithin:cholesterol acyltransferase deficiency, kidney transplantation
Familial LCAT deficiency (FLD) is a very rare autosomal recessive disorder of lipid metabolism caused by loss-of-function mutations in the LCAT gene (1). FLD patients have severe hypoalphalipoproteinemia and impairment of cholesterol esterification in plasma (2). Besides lipid abnormalities, homozygous and compound heterozygous FLD patients present corneal opacification, hemolytic anemia, and renal disease, which represents the primary cause of morbidity and mortality (3). Proteinuria can develop as early as in the second decade of life (4), and it unpredictably progresses to renal insufficiency and eventually to kidney failure (5, 6). FLD patients are candidates for renal transplantation, but the disease can rapidly reoccur in the transplanted kidney within only a few years, as shown in a single reported anecdotal case (7). Although chronic kidney disease (CKD) etiopathogenesis is not completely understood, lipoprotein X (LpX), an abnormal lipoprotein enriched in unesterified cholesterol and relatively poor in other lipids and apolipoproteins, is involved in causing renal damage (8). Currently, no effective treatment is available for FLD. Intervention is limited to the correction of lipid profile, through lipid-lowering drugs, and to the prevention and delay of renal failure, through renoprotective agents (9). However, there are no data about the real efficacy of these interventions. Hence, the prognosis of renal disease is not known and the rate of deterioration of kidney function is variable and unpredictable. Here, we report the follow-up of the Italian cohort of FLD patients (1, 2, 10), one of the largest cohorts described, followed for up to 24 years.
MATERIALS AND METHODS
Subjects
We collected clinical data from 18 Caucasian subjects (12 males and 6 females) with molecular confirmation of FLD, all belonging to the Italian cohort (1, 2, 10). The group was comprised of 13 homozygous and 5 compound heterozygous for mutations in the LCAT gene. All procedures were followed in accordance with the ethical standards of the local institutional committees on human experimentation and according to tenets of the Helsinki Declaration of 1964, as revised in 2013. The study was approved by the internal ethics committee (approval number 446-092014). All patients provided written informed consent. In the case of deceased patients, consent was waived according to the Italian Data Protection Authority (Deliberation Number 85 1 March 2012).
Data collection
Demographic characteristics and medical history (with specific reference to renal disease), both at referral (baseline) and during follow-up, and information on new renal events were collected. CKD was classified according to the KDIGO guidelines (11). Estimated glomerular filtration rate (eGFR) was calculated using the 2009 CKD-EPI creatinine equation (12).
The follow-up period was defined as the time between the first and the last available contact. The mean follow-up was 12 ± 8.5 years (range: 1–24 years). Event of interest was defined as any of the following: 1) dialysis, 2) kidney transplantation, or 3) death for renal complications.
Biochemical analyses
Blood samples were collected after an overnight fast and plasma separated by low-speed centrifugation at 4°C. Plasma total cholesterol, HDL-cholesterol, triglyceride, and apolipoprotein levels were determined with certified methods using a Roche Integra c311 autoanalyzer (Roche Diagnostics). LDL-cholesterol was calculated using the Friedewald’s formula. When triglycerides were >400 mg/dl (two cases), LDL-cholesterol was assessed using direct measurement. LDL-cholesterol levels were virtually identical when calculated by the Martin-Hopkins equation compared with the Friedewald’s formula (91.8 ± 61.0 mg/dl and 91.7 ± 61.0 mg/dl, respectively). Unesterified cholesterol was determined by using a previously described enzymatic technique (13). Plasma LCAT concentration was measured by a specific competitive enzyme-linked immunoassay (14). Plasma cholesterol esterification rate and LCAT activity, which reflect the ability of endogenous LCAT to esterify cholesterol within endogenous lipoproteins and exogenous HDL, respectively, were assessed by using previously described methods (2).
The presence of proteinuria was assessed by protein reagent strip and then confirmed by spot test and/or 24 h urine collection.
Statistical analysis
Descriptive statistics such as mean ± SD were estimated for all variables. Comparisons between groups were performed by independent t-test or Chi-square as appropriate. Nonnormally distributed variables were log-transformed before proceeding to the analysis. We estimated the time to first renal event by using Kaplan-Meier survival curves. Survival curves stratified by sex and median unesterified cholesterol were compared by using the log-rank test. Due to the limited sample size, no adjusted analyses were carried out (15).
All statistical analyses were performed using SPSS software version 26.0 (SPSS Inc., Chicago, IL). Tests were two-sided and P values <0.05 were considered as statistically significant.
RESULTS
Baseline characteristics of Italian FLD cohort
Eighteen Caucasian subjects (6 females and 12 males) with molecular confirmation of FLD were included in the analysis. Included subjects were either homozygous or compound heterozygous carriers of mutations in the LCAT gene. FLD cohort characteristics at diagnosis are summarized in Table 1. Median age at diagnosis was 32.5 years (range: 20–70 years); twelve were male. FLD patients showed the typical lipid profile, characterized by very low HDL-cholesterol, ApoA-I, and ApoA-II levels, and high triglycerides. As expected in the total absence of LCAT activity, assayed on both exogenous and endogenous lipoproteins (LCAT activity and cholesterol esterification rate, respectively), plasma cholesterol was mainly unesterified, although with a certain variability. Included cases carried 15 different mutations in the LCAT gene and belonged to 14 families. No major differences were observed between females and males, except for a significantly higher prevalence of hypertension in males.
TABLE 1.
Demographic and lipid/lipoprotein characteristics of FLD patients at diagnosis
| All (n = 18) | Females (n = 6) | Males (n = 12) | Reference Values | |
| Age at diagnosis (years) | 37.5 ± 14.8 | 38.2 ± 15.1 | 37.2 ± 15.3 | — |
| Body mass index (kg/m2) | 23.8 ± 3.0 | 21.9 ± 2.7 | 24.2 ± 3.1 | 18–25 |
| Total cholesterol (mg/dl) | 156.1 ± 81.4 | 134.3 ± 61.1 | 168 ± 91.0 | <200 |
| Unesterified cholesterol (mg/dl) | 140.1 ± 73.2 | 113.7 ± 45.8 | 154.5 ± 82.9 | <60 |
| Unesterified/total cholesterol | 0.91 ± 0.11 | 0.86 ± 0.10 | 0.94 ± 0.11 | <0.30 |
| LDL-cholesterol (mg/dl) | 91.7 ± 61.0 | 68.8 ± 23.8 | 104.2 ± 72.0 | <130 |
| HDL-cholesterol (mg/dl) | 7.2 ± 3.4 | 7.5 ± 3.3 | 7.1 ± 3.5 | >40 |
| Triglycerides (mg/dl) | 266.9 ± 179.1 | 282.2 ± 209 | 258.5 ± 170.8 | <150 |
| Phospholipids (mg/dl) | 292.6 ± 118.0 | 261.2 ± 79.2 | 306.9 ± 133.0 | <200 |
| ApoA-I (mg/dl) | 36.4 ± 8.8 | 31.7 ± 6.1 | 38.9 ± 133.0 | 115–180 |
| ApoA-II (mg/dl) | 6.9 ± 4.0 | 8.1 ± 6.1 | 6.6 ± 3.6 | 26–51 |
| ApoB (mg/dl) | 50.7 ± 26.9 | 60 ± 23.8 | 44.6 ± 28.3 | 70–150 |
| LCAT mass (µg/ml) | 1.55 ± 1.38 | 2.35 ± 1.76 | 1.06 ± 0.86 | 3.1–6.7 |
| Cholesterol esterification rate (nmol/ml/h) | 0 ± 0 | 0 ± 0 | 0 ± 0 | 30–60 |
| LCAT activity (nmol/ml/h) | 0 ± 0 | 0 ± 0 | 0 ± 0 | 25–55 |
| Hemoglobin (g/dl) | 10.2 ± 2.1 | 10.7 ± 1.5 | 9.9 ± 2.6 | >12 |
| Presence of proteinuria [yes, n (%)] | 7 (38) | 2 (33) | 5 (42) | Absent |
| Hypertension [yes, n (%)] | 12 (67) | 1 (17) | 11 (92)a | No |
Data are reported as mean ± SD or number (%).
P < 0.05 versus females.
Twelve FLD patients (three females and nine males, mean age 41.7 ± 15.0 years) already had CKD at diagnosis; seven had proteinuria (moderately to severely increased), four were on hemodialysis, and one already had kidney transplantation. Mean eGFR in these patients was 36.2 ± 36.4 ml/min/1.73 m2. The remaining six patients (three females and three males) had normal renal function at diagnosis (eGFR 117.0 ± 16.8 ml/min/1.73 m2 and absence of proteinuria) and were indeed younger (mean age 29.2 ± 11.3 years). Change of eGFR over time for one representative patient with normal renal function and one representative patient with proteinuria was reported in supplemental Fig. S1. Interestingly, unesterified cholesterol was higher in FLD patients with CKD at diagnosis (156.9 ± 78.9 mg/dl vs. 99.7 ± 37.6 mg/dl). Use of renoprotective agents (ACE inhibitors) at diagnosis was recorded in seven patients.
Evolution of renal disease in Italian FLD patients
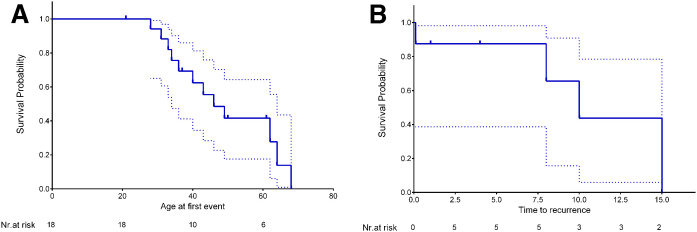
Mean follow-up was 12 ± 8.5 years (range: 1–24 years). During follow-up, 16 renal events (dialysis, kidney transplant, or death for renal complications) were recorded, giving an overall incidence of cumulative events of 88.8% (7.8% per year). Ten new events were observed in FLD patients with normal renal function at diagnosis. Survival analysis was performed to assess the median age of occurrence of any renal outcome (i.e., dialysis, kidney transplantation, or death for renal complications). Kaplan-Meier survival analyses showed that the overall median event-free survival in the FLD cohort was 46 years (Fig. 1A) [95% CI 35.1–56.9]. Five patients had a second kidney failure caused by the disease (as confirmed by renal biopsy) that required dialysis, and three underwent a second kidney transplant. Kaplan-Meier analysis for event recurrence showed that median time to a second event (dialysis, kidney transplant, or death for renal complications) was 10 years [95% CI 5.0–14.1] (Fig. 1B). By the sixth decade of age, more than two-thirds of FLD patients had established CKD or were dead.
Fig. 1.
Kaplan-Meier survival curves for first (A) and recurrent (B) renal event (dialysis, kidney transplantation, or death for renal complications) in FLD patients. Dashed lines indicate the 95% CIs.
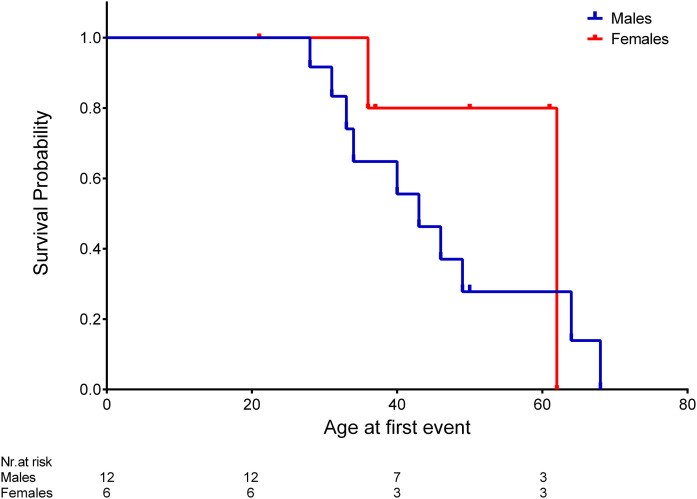
Survival curves categorized for sex showed that event-free survival time was extended in females compared with males (median time 62 vs. 43 years in females and males, respectively), although it did not reach statistical significance (log-rank test: P = 0.308) (Fig. 2) and a confounding effect of hypertension could not be ruled out. However, Kaplan-Meier survival analyses showed that, in this cohort, hypertension was not associated with renal events (supplemental Fig. S2).
Fig. 2.
Kaplan-Meier survival curves for renal event (dialysis, kidney transplantation, or death for renal complications) according to sex. Log-rank test P = 0.308 according to male (blue line) or female (red line) sex.
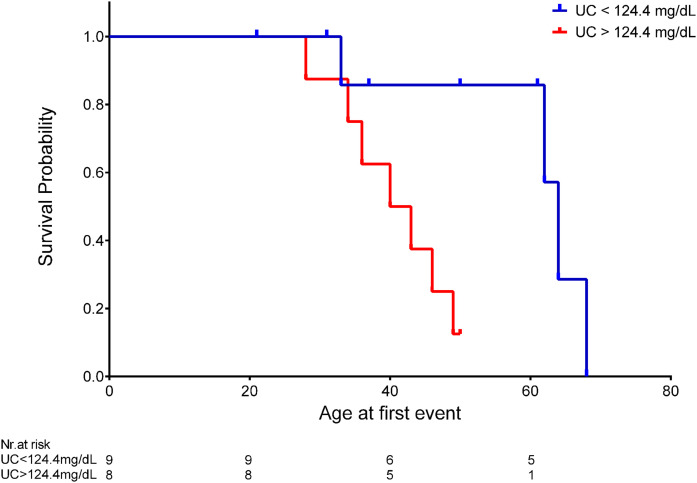
Interestingly, when subjects were categorized according to unesterified cholesterol over or under median plasma levels at diagnosis (124.4 mg/dl), event-free survival was significantly lower in patients with unesterified cholesterol values above the median (median time 40 vs. 64 years for unesterified cholesterol over and under the median, respectively; log-rank test: P = 0.016) (Fig. 3). A sensitivity analysis was performed by computing Kaplan-Meyer curves stratified for unesterified cholesterol level after including only hypertensive subjects (the majority of the sample). The resulting log-rank test was still significant, thus indicating that a confounding effect by hypertension is rather unlikely (supplemental Fig. S3).
Fig. 3.
Kaplan-Meier survival curves for renal event (dialysis, kidney transplantation, or death for renal complications) according to plasma unesterified cholesterol (UC) levels at diagnosis. Log-rank test P = 0.016 according to UC level under (blue line) or above (red line) the median.
DISCUSSION
Clinical manifestations of FLD include corneal opacification, which represents a hallmark of the disease but is rarely associated with visual impairment, hemolytic anemia, and renal insufficiency. Renal disease represents the major cause of morbidity and mortality in FLD cases; proteinuria can develop early in life and can unpredictably progress to renal insufficiency and eventually to end-stage renal disease. Due to the rarity of the disease, the natural history of FLD is still largely unknown. In the past 20 years, we have collected a large cohort of carriers of LCAT mutations, which comprises 33 unrelated families. Thanks to the availability of a large number of FLD carriers, followed in some cases for more than two decades, we showed that half of the FLD patients had the first renal event, i.e., kidney failure or kidney transplantation or death for renal complications, by the age of 46 years. Moreover, we showed that median time to a second kidney failure, kidney transplant, or death for renal complications was 10 years, and by the sixth decade of age more than two-thirds of FLD patients had established CKD or were dead.
Appearance and progression of renal damage in FLD patients is highly variable, even within the same family (2), suggesting that several genetic and environmental factors can impact on kidney deterioration. The biochemical phenotype, which is quite heterogenous in FLD patients, also depending on modifier genes such as APOE (16), is likely a major determinant of kidney failure (17). The role of circulating lipids in renal damage is suggested by the accumulation of oxidized phospholipids in the glomeruli (18) and by the entrapment in capillary loops of abnormal lipoprotein particles, namely LpX, made of phospholipids and unesterified cholesterol (3). Interestingly, in our cohort, unesterified cholesterol level at diagnosis was the only plasma lipid parameter able to identify patients with rapid deterioration of kidney function. Increased unesterified cholesterol plasma level is the leading feature of LCAT deficiency; the excess unesterified cholesterol cannot accommodate in circulating lipoproteins and it thus accumulates in the abnormal LpX, a vesicle or a multilamellar vesicle comprised of phospholipid/cholesterol bilayers surrounding an aqueous core. LpX has been shown to be cytotoxic and pro-inflammatory in cell culture studies (19), it accumulates in the kidney and thus could account for the lipid deposition in mesangial cells, one of the main pathologic findings in the kidneys of FLD patients (3).
Renal transplantation represents an option in severe FLD cases with kidney failure; however, because transplantation does not correct the underlying enzymatic defect, disease can rapidly reoccur. As for other systemic diseases characterized by renal involvement, e.g., amyloid light-chain amyloidosis, organ transplant improves life expectancy, but graft survival is limited (20). Indeed, in our cohort of 18 FLD patients, 5 had a second renal failure that required dialysis, and 3 underwent a second kidney transplant within 15 years.
The cure of FLD should thus focus on correcting the systemic disorder, and specifically on reducing the circulating amount of the toxic unesterified cholesterol. Enzyme replacement therapy with recombinant LCAT, able to restore LCAT activity and eventually reduce circulating unesterified cholesterol, is certainly an option, and it is currently under clinical development (21, 22). A second option is represented by gene therapy (23), which is at present very far from being tested in humans. Finally, small molecule activators of LCAT, eventually orally active, have been tested in vitro and proved to be able to activate not only wild-type but also some mutant LCAT (24).
In conclusion, the present study highlights the severe consequences of FLD, which leads to kidney failure before the fifth decade of age, and points-out the need of early diagnosis and referral to specialized centers, and the urgency for effective treatments to prevent or slow the renal disease in FLD patients.
Data availability
The datasets generated and/or analyzed during the current study are available from Laura Calabresi (Università degli Studi di Milano, laura.calabresi@unimi.it) upon reasonable request.
Supplementary Material
Footnotes
This article contains supplemental data.
Author contributions—L.C. study concept; C.P., A.O., M.A., L.D., L.G.,T.L., T.S., and G.B. data acquisition; C.P. and L.C. writing-original draft; F.V. and C.P. statistical analysis; C.P., M.A., L.D., T.L., T.S., L.G., G.B., A.O. and L.C. writing-review and editing. All authors approved the final version.
Author ORCIDs—Chiara Pavanello https://orcid.org/0000-0001-5892-9857
Funding and additional information—This work was supported, in part, by funding from Telethon-Italy (GG14125 to L.C.) and Fondazione Cariplo (2011-0628 to L.C.).
Conflict of interest—Laura Calabresi has received research grants from MedImmune, Daiichi Sankyio, and Cerenis Therapeutics.
Abbreviations—
- CKD
- chronic kidney disease
- eGFR
- estimated glomerular filtration rate
- FLD
- familial LCAT deficiency
- LpX
- lipoprotein X
Manuscript received June 19, 2020, and in revised form September 25, 2020. Published, JLR Papers in Press, September 30, 2020, DOI 10.1194/jlr.P120000976.
REFERENCES
- 1.Calabresi L., Simonelli S., Gomaraschi M., and Franceschini G.. 2012. Genetic lecithin:cholesterol acyltransferase deficiency and cardiovascular disease. Atherosclerosis. 222: 299–306. [DOI] [PubMed] [Google Scholar]
- 2.Calabresi L., Pisciotta L., Costantin A., Frigerio I., Eberini I., Alessandrini P., Arca M., Bon G. B., Boscutti G., Busnach G., et al. . 2005. The molecular basis of lecithin:cholesterol acyltransferase deficiency syndromes: a comprehensive study of molecular and biochemical findings in 13 unrelated Italian families. Arterioscler. Thromb. Vasc. Biol. 25: 1972–1978. [DOI] [PubMed] [Google Scholar]
- 3.Santamarina-Fojo S., Hoeg J. M., Assmann G., and Brewer H. B. J.. 2001. Lecithin cholesterol acyltransferase deficiency and fish eye disease. In The Metabolic and Molecular Bases of Inherited Diseases. C. R. Scriver, A. L. Beaudet, W. S. Sly, et al., editors. McGraw-Hill, New York. 2817–2833. [Google Scholar]
- 4.Holleboom A. G., Kuivenhoven J. A., van Olden C. C., Peter J., Schimmel A. W., Levels J. H., Valentijn R. M., Vos P., Defesche J. C., Kastelein J. J., et al. . 2011. Proteinuria in early childhood due to familial LCAT deficiency caused by loss of a disulfide bond in lecithin:cholesterol acyl transferase. Atherosclerosis. 216: 161–165. [DOI] [PubMed] [Google Scholar]
- 5.Sessa A., Battini G., Meroni M., Daidone G., Carnera I., Brambilla P. L., Viganò G., Giordano F., Pallotti F., Torri Tarelli L., et al. . 2001. Hypocomplementemic type II membranoproliferative glomerulonephritis in a male patient with familial lecithin-cholesterol acyltransferase deficiency due to two different allelic mutations. Nephron. 88: 268–272. [DOI] [PubMed] [Google Scholar]
- 6.Imbasciati E., Paties C., Scarpioni L., and Mihatsch M. J.. 1986. Renal lesions in familial lecithin-cholesterol acyltransferase deficiency. Ultrastructural heterogeneity of glomerular changes. Am. J. Nephrol. 6: 66–70. [DOI] [PubMed] [Google Scholar]
- 7.Strøm E. H., Sund S., Reier-Nilsen M., Dorje C., and Leren T. P.. 2011. Lecithin:cholesterol acyltransferase (LCAT) deficiency: renal lesions with early graft recurrence. Ultrastruct. Pathol. 35: 139–145. [DOI] [PubMed] [Google Scholar]
- 8.Ossoli A., Neufeld E. B., Thacker S. G., Vaisman B., Pryor M., Freeman L. A., Brantner C. A., Baranova I., Francone N. O., Demosky S. J. Jr., et al. . 2016. Lipoprotein X causes renal disease in LCAT deficiency. PLoS One. 11: e0150083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aranda P., Valdivielso P., Pisciotta L., Garcia I., Garcã A-Arias C., Bertolini S., Martã N-Reyes G., Lez-Santos G., and Calandra S.. 2008. Therapeutic management of a new case of LCAT deficiency with a multifactorial long-term approach based on high doses of angiotensin II receptor blockers (ARBs). Clin. Nephrol. 69: 213–218. [DOI] [PubMed] [Google Scholar]
- 10.Bigazzi F., Dal Pino B., Pavanello C., Sbrana F., Aquaro G. D., Napoli V., Palmieri C., Barison A., Calabresi L., and Sampietro T.. Familial LCAT deficiency and cardiovascular disease: the game is not over. A case of dramatic multivessel atherosclerosis. Minerva Med. Epub ahead of print. May 29, 2020; doi:10.23736/S0026-4806.20.06633-1. . [DOI] [PubMed] [Google Scholar]
- 11.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. 2013. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. Suppl. 3: 1–150. [Google Scholar]
- 12.Levey A. S., Stevens L. A., Schmid C. H., Zhang Y. L., Castro A. F. III, Feldman H. I., Kusek J. W., Eggers P., Van Lente F., Greene T., et al. ; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). 2009. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 150: 604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomaraschi M., Ossoli A., Castelnuovo S., Simonelli S., Pavanello C., Balzarotti G., Arca M., Di Costanzo A., Sampietro T., Vaudo G., et al. . 2017. Depletion in LpA-I:A-II particles enhances HDL-mediated endothelial protection in familial LCAT deficiency. J. Lipid Res. 58: 994–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murakami T., Michelagnoli S., Longhi R., Gianfranceschi G., Pazzucconi F., Calabresi L., Sirtori C. R., and Franceschini G.. 1995. Triglycerides are major determinants of cholesterol esterification/transfer and HDL remodeling in human plasma. Arterioscler. Thromb. Vasc. Biol. 15: 1819–1828. [DOI] [PubMed] [Google Scholar]
- 15.D’Erasmo L., Minicocci I., Nicolucci A., Pintus P., Roeters Van Lennep J. E., Masana L., Mata P., Sánchez-Hernández R. M., Prieto-Matos P., Real J. T., et al. . 2018. Autosomal recessive hypercholesterolemia: long-term cardiovascular outcomes. J. Am. Coll. Cardiol. 71: 279–288. [DOI] [PubMed] [Google Scholar]
- 16.Baass A., Wassef H., Tremblay M., Bernier L., Dufour R., and Davignon J.. 2009. Characterization of a new LCAT mutation causing familial LCAT deficiency (FLD) and the role of APOE as a modifier gene of the FLD phenotype. Atherosclerosis. 207: 452–457. [DOI] [PubMed] [Google Scholar]
- 17.Lamiquiz-Moneo I., Civeira F., Gómez-Coronado D., Blanco-Vaca F., Villafuerte-Ledesma H. M., Gil M., Amigó N., Mateo-Gallego R., and Cenarro A.. 2019. Lipid profile rather than the LCAT mutation explains renal disease in familial LCAT deficiency. J. Clin. Med. 8: 1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jimi S., Uesugi N., Saku K., Itabe H., Zhang B., Arakawa K., and Takebayashi S.. 1999. Possible induction of renal dysfunction in patients with lecithin:cholesterol acyltransferase deficiency by oxidized phosphatidylcholine in glomeruli. Arterioscler. Thromb. Vasc. Biol. 19: 794–801. [DOI] [PubMed] [Google Scholar]
- 19.Lynn E. G., Choy P. C., and Magil A. O. K.. 1997. Uptake and metabolism of lipoprotein-X in mesangial cells. Mol. Cell. Biochem. 175: 187–194. [DOI] [PubMed] [Google Scholar]
- 20.Angel-Korman A., Stern L., Sarosiek S., Sloan J. M., Doros G., Sanchorawala V., and Havasi A.. 2019. Long-term outcome of kidney transplantation in AL amyloidosis. Kidney Int. 95: 405–411. [DOI] [PubMed] [Google Scholar]
- 21.Shamburek R. D., Bakker-Arkema R., Auerbach B. J., Krause B. R., Homan R., Amar M. J., Freeman L. A., and Remaley A. T.. 2016. Familial lecithin:cholesterol acyltransferase deficiency: First-in-human treatment with enzyme replacement. J. Clin. Lipidol. 10: 356–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shamburek R. D., Bakker-Arkema R., Shamburek A. M., Freeman L. A., Amar M. J., Auerbach B., Krause B. R., Homan R., Adelman S. J., Collins H. L., et al. . 2016. Safety and tolerability of ACP-501, a recombinant human lecithin:cholesterol acyltransferase, in a phase 1 single-dose escalation study. Circ. Res. 118: 73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amar M. J., Shamburek R. D., Vaisman B., Knapper C. L., Foger B., Hoyt R. F. Jr., Santamarina-Fojo S., Brewer H. B. Jr., and Remaley A. T.. 2009. Adenoviral expression of human lecithin-cholesterol acyltransferase in nonhuman primates leads to an antiatherogenic lipoprotein phenotype by increasing high-density lipoprotein and lowering low-density lipoprotein. Metabolism. 58: 568–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freeman L. A., Demosky S. J. Jr., Konaklieva M., Kuskovsky R., Aponte A., Ossoli A. F., Gordon S. M., Koby R. F., Manthei K. A., Shen M., et al. . 2017. Lecithin:cholesterol acyltransferase activation by sulfhydryl-reactive small molecules: role of cysteine-31. J. Pharmacol. Exp. Ther. 362: 306–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from Laura Calabresi (Università degli Studi di Milano, laura.calabresi@unimi.it) upon reasonable request.