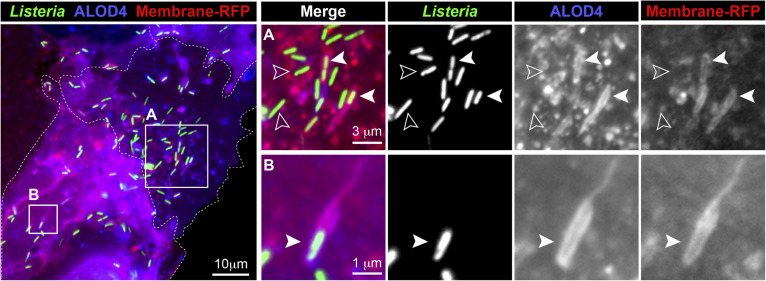
Listeria monocytogenes causes listeriosis, a foodborne disease with high mortality rates in vulnerable populations. This Gram-positive intracellular pathogen disseminates through mammalian tissues via actin-based motility and subsequent formation of host plasma membrane (PM) protrusions to cross cellular junctions. Recently, we found that 25-hydroxycholesterol (25HC) potently suppressed L. monocytogenes infection by internalizing a specific pool of cholesterol, termed “accessible cholesterol,” from the PM, preventing induction of membrane protrusions and cell-to-cell spread (1). Accessible cholesterol is a mobile fraction of PM cholesterol that has been previously shown to regulate cholesterol homeostasis and Hedgehog signaling (2–4). The development of ALOD4, a toxin-based biosensor of accessible cholesterol, has allowed for visualization of this signaling lipid on the surface of cultured cells (2).
This widefield microscopy image shows binding of ALOD4 (blue) to Caco-2 intestinal epithelial cells (dashed lines outline contact borders) that were preinfected with L. monocytogenes expressing GFP (green). Bacteria that formed membrane protrusions were identified by their encapsulation by the PM marker Membrane-RFP (red) (1). Image analysis revealed high concentrations of ALOD4 decorating the Membrane-RFP positive protrusions encapsulating L. monocytogenes that are poised to cross over from one cell to another (Boxes A and B, closed arrows). In contrast, cytosolic bacteria did not colocalize with either ALOD4 or Membrane-RFP (Box A, open arrows). When combined with a previous result showing that accessible cholesterol is enriched in microvilli of CHO cells (3), this image suggests that accessible cholesterol may be important for generating highly curved membrane structures on the PM.
EQUIPMENT: Observer Z1 fluorescent microscope (Zeiss)
METHODS: Caco-2 cells stably expressing the PM marker Membrane-RFP were seeded at a density of 90,000 cells onto 25 mm glass coverslips in 6-well plates (1). After 3 days, cells were infected with L. monocytogenes-GFP (multiplicity of infection = 0.1) for 60 min. The cells were washed with PBS, and fresh lipoprotein-rich media (10% FBS) supplemented with 50 μg/ml gentamicin was added to kill extracellular L. monocytogenes. After 5 h, cells were washed twice with PBS and incubated with a 3 μM solution of ALOD4-647 (ALOD4 labeled at its lone cysteine with Alexa Fluor 647) for 20 min at 37°C. Samples were then fixed in 3.7% formaldehyde, washed with PBS, and mounted onto slides with Prolong Gold. Images were acquired and analyzed with Zen 2 Pro software. A merged image with all three fluorescent channels is shown (left panel). Magnified views of specific regions (Boxes A and B), with all channels (merge) and individual channels are shown (right panels).
REFERENCES
- 1.Abrams M. E., Johnson K. A. Perelman S. S. Zhang L. S. Endapally S. Mar K. B. Thompson B. M. McDonald J. G. Schoggins J. W. Radhakrishnan A. Alto N. M.. 2020. Oxysterols provide innate immunity to bacterial infection by mobilizing cell surface accessible cholesterol. Nat. Microbiol. 5: 929–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Infante R. E., and Radhakrishnan A.. 2017. Continuous transport of a small fraction of plasma membrane cholesterol to endoplasmic reticulum regulates total cellular cholesterol. eLife. 6: e25466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.He C., Hu X. Weston T. A. Jung R. S. Sandhu J. Huang S. Heizer P. Kim J. Ellison R. Xu J. et al. 2018. Macrophages release plasma membrane-derived particles rich in accessible cholesterol. Proc. Natl. Acad. Sci. USA. 115: E8499–E8508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kinnebrew M., Iverson E. J. Patel B. B. Pusapati G. V. Kong J. H. Johnson K. A. Luchetti G. Eckert K. M. McDonald J. G. Covey D. F. et al. 2019. Cholesterol accessibility at the ciliary membrane controls hedgehog signaling. eLife. 8: e50051. [DOI] [PMC free article] [PubMed] [Google Scholar]