Observational evidence suggests that mask wearing mitigates SARS-CoV-2 transmission. It is uncertain if this observed association arises through protection of uninfected wearers (protective effect), via reduced transmission from infected mask wearers (source control), or both. This randomized controlled trial investigates whether recommending surgical mask use when outside the home reduces wearers' risk for SARS-CoV-2 infection in a setting where masks were uncommon and not among recommended public health measures.
Visual Abstract. Effectiveness of Mask Recommendation for Preventing SARS-CoV-2 Infection.
Observational evidence suggests that mask wearing mitigates SARS-CoV-2 transmission. It is uncertain if this observed association arises through protection of uninfected wearers (protective effect), via reduced transmission from infected mask wearers (source control), or both. This randomized controlled trial investigates whether recommending surgical mask use when outside the home reduces wearers' risk for SARS-CoV-2 infection in a setting where masks were uncommon and not among recommended public health measures.
Abstract
Background:
Observational evidence suggests that mask wearing mitigates transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). It is uncertain if this observed association arises through protection of uninfected wearers (protective effect), via reduced transmission from infected mask wearers (source control), or both.
Objective:
To assess whether recommending surgical mask use outside the home reduces wearers' risk for SARS-CoV-2 infection in a setting where masks were uncommon and not among recommended public health measures.
Design:
Randomized controlled trial (DANMASK-19 [Danish Study to Assess Face Masks for the Protection Against COVID-19 Infection]). (ClinicalTrials.gov: NCT04337541)
Setting:
Denmark, April and May 2020.
Participants:
Adults spending more than 3 hours per day outside the home without occupational mask use.
Intervention:
Encouragement to follow social distancing measures for coronavirus disease 2019, plus either no mask recommendation or a recommendation to wear a mask when outside the home among other persons together with a supply of 50 surgical masks and instructions for proper use.
Measurements:
The primary outcome was SARS-CoV-2 infection in the mask wearer at 1 month by antibody testing, polymerase chain reaction (PCR), or hospital diagnosis. The secondary outcome was PCR positivity for other respiratory viruses.
Results:
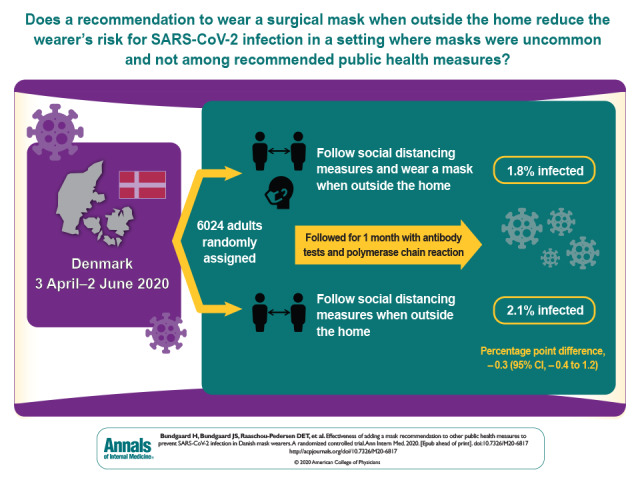
A total of 3030 participants were randomly assigned to the recommendation to wear masks, and 2994 were assigned to control; 4862 completed the study. Infection with SARS-CoV-2 occurred in 42 participants recommended masks (1.8%) and 53 control participants (2.1%). The between-group difference was −0.3 percentage point (95% CI, −1.2 to 0.4 percentage point; P = 0.38) (odds ratio, 0.82 [CI, 0.54 to 1.23]; P = 0.33). Multiple imputation accounting for loss to follow-up yielded similar results. Although the difference observed was not statistically significant, the 95% CIs are compatible with a 46% reduction to a 23% increase in infection.
Limitation:
Inconclusive results, missing data, variable adherence, patient-reported findings on home tests, no blinding, and no assessment of whether masks could decrease disease transmission from mask wearers to others.
Conclusion:
The recommendation to wear surgical masks to supplement other public health measures did not reduce the SARS-CoV-2 infection rate among wearers by more than 50% in a community with modest infection rates, some degree of social distancing, and uncommon general mask use. The data were compatible with lesser degrees of self-protection.
Primary Funding Source:
The Salling Foundations.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the cause of coronavirus disease 2019 (COVID-19), has infected more than 54 million persons (1, 2). Measures to impede transmission in health care and community settings are essential (3). The virus is transmitted person-to-person, primarily through the mouth, nose, or eyes via respiratory droplets, aerosols, or fomites (4, 5). It can survive on surfaces for up to 72 hours (6), and touching a contaminated surface followed by face touching is another possible route of transmission (7). Face masks are a plausible means to reduce transmission of respiratory viruses by minimizing the risk that respiratory droplets will reach wearers' nasal or oral mucosa. Face masks are also hypothesized to reduce face touching (8, 9), but frequent face and mask touching has been reported among health care personnel (10). Observational evidence supports the efficacy of face masks in health care settings (11, 12) and as source control in patients infected with SARS-CoV-2 or other coronaviruses (13).
An increasing number of localities recommend masks in community settings on the basis of this observational evidence, but recommendations vary and controversy exists (14). The World Health Organization (WHO) and the U.S. Centers for Disease Control and Prevention (15) strongly recommend that persons with symptoms or known infection wear masks to prevent transmission of SARS-CoV-2 to others (source control) (16). However, WHO acknowledges that we lack evidence that wearing a mask protects healthy persons from SARS-CoV-2 (prevention) (17). A systematic review of observational studies reported that mask use reduced risk for SARS, Middle East respiratory syndrome, and COVID-19 by 66% overall, 70% in health care workers, and 44% in the community (12). However, surgical and cloth masks were grouped in preventive studies, and none of the 3 included non–health care studies related directly to COVID-19. Another systematic review (18) and American College of Physicians recommendations (19) concluded that evidence on mask effectiveness for respiratory infection prevention is stronger in health care than community settings.
Observational evidence suggests that mask wearing mitigates SARS-CoV-2 transmission, but whether this observed association arises because masks protect uninfected wearers (protective effect) or because transmission is reduced from infected mask wearers (source control) is uncertain. Here, we report a randomized controlled trial (20) that assessed whether a recommendation to wear a surgical mask when outside the home among others reduced wearers' risk for SARS-CoV-2 infection in a setting where public health measures were in effect but community mask wearing was uncommon and not recommended.
Methods
Trial Design and Oversight
DANMASK-19 (Danish Study to Assess Face Masks for the Protection Against COVID-19 Infection) was an investigator-initiated, nationwide, unblinded, randomized controlled trial (ClinicalTrials.gov: NCT04337541). The trial protocol was registered with the Danish Data Protection Agency (P-2020-311) (Part 10 of the Supplement) and published (21). The researchers presented the protocol to the independent regional scientific ethics committee of the Capital Region of Denmark, which did not require ethics approval (H-20023709) in accordance with Danish legislation (Parts 11 and 12 of the Supplement). The trial was done in accordance with the principles of the Declaration of Helsinki.
Participants and Study Period
During the study period (3 April to 2 June 2020), Danish authorities did not recommend use of masks in the community and mask use was uncommon (<5%) outside hospitals (22). Recommended public health measures included quarantining persons with SARS-CoV-2 infection, social distancing (including in shops and public transportation, which remained open), limiting the number of persons seen, frequent hand hygiene and cleaning, and limiting visitors to hospitals and nursing homes (23, 24). Cafés and restaurants were closed during the study until 18 May 2020.
Eligible persons were community-dwelling adults aged 18 years or older without current or prior symptoms or diagnosis of COVID-19 who reported being outside the home among others for at least 3 hours per day and who did not wear masks during their daily work. Recruitment involved media advertisements and contacting private companies and public organizations. Interested citizens had internet access to detailed study information and to research staff for questions (Part 3 of the Supplement). At baseline, participants completed a demographic survey and provided consent for researchers to access their national registry data (Parts 4 and 5 of the Supplement). Recruitment occurred from 3 through 24 April 2020. Half of participants were randomly assigned to a group on 12 April and half on 24 April.
Intervention
Participants were enrolled and data registered using Research Electronic Data Capture (REDCap) software (25). Eligible participants were randomly assigned 1:1 to the mask or control group using a computer algorithm and were stratified by the 5 regions of Denmark (Supplement Table 1). Participants were notified of allocation by e-mail, and study packages were sent by courier (Part 7 of the Supplement). Participants in the mask group were instructed to wear a mask when outside the home during the next month. They received 50 three-layer, disposable, surgical face masks with ear loops (TYPE II EN 14683 [Abena]; filtration rate, 98%; made in China). Participants in both groups received materials and instructions for antibody testing on receipt and at 1 month. They also received materials and instructions for collecting an oropharyngeal/nasal swab sample for polymerase chain reaction (PCR) testing at 1 month and whenever symptoms compatible with COVID-19 occurred during follow-up. If symptomatic, participants were strongly encouraged to seek medical care. They registered symptoms and results of the antibody test in the online REDCap system. Participants returned the test material by prepaid express courier.
Written instructions and instructional videos guided antibody testing, oropharyngeal/nasal swabbing, and proper use of masks (Part 8 of the Supplement), and a help line was available to participants. In accordance with WHO recommendations for health care settings at that time, participants were instructed to change the mask if outside the home for more than 8 hours. At baseline and in weekly follow-up e-mails, participants in both groups were encouraged to follow current COVID-19 recommendations from the Danish authorities.
Antibody and Viral PCR Testing
Participants tested for SARS-CoV-2 IgM and IgG antibodies in whole blood using a point-of-care test (Lateral Flow test [Zhuhai Livzon Diagnostics]) according to the manufacturer's recommendations and as previously described (26). After puncturing a fingertip with a lancet, they withdrew blood into a capillary tube and placed 1 drop of blood followed by 2 drops of saline in the test chamber in each of the 2 test plates (IgM and IgG). Participants reported IgM and IgG results separately as “1 line present” (negative), “2 lines present” (positive), or “I am not sure, or I could not perform the test” (treated as a negative result). Participants were categorized as seropositive if they had developed IgM, IgG, or both. The manufacturer reported that sensitivity was 90.2% and specificity 99.2%. A previously reported internal validation using 651 samples from blood donors before November 2019 and 155 patients with PCR-confirmed SARS-CoV-2 infection estimated a sensitivity of 82.5% (95% CI, 75.3% to 88.4%) and specificity of 99.5% (CI, 98.7% to 99.9%) (26). We (27) and others (28) have reported that oropharyngeal/nasal swab sampling for SARS-CoV-2 by participants, as opposed to health care workers, is clinically useful. Descriptions of RNA extraction, primer and probe used, reverse transcription, preamplification, and microfluidic quantitative PCR are detailed in Part 6 of the Supplement.
Data Collection
Participants received 4 follow-up surveys (Parts 4 and 5 of the Supplement) by e-mail to collect information on antibody test results, adherence to recommendations on time spent outside the home among others, development of symptoms, COVID-19 diagnosis based on PCR testing done in public hospitals, and known COVID-19 exposures.
Outcomes
The primary outcome was SARS-CoV-2 infection, defined as a positive result on an oropharyngeal/nasal swab test for SARS-CoV-2, development of a positive SARS-CoV-2 antibody test result (IgM or IgG) during the study period, or a hospital-based diagnosis of SARS-CoV-2 infection or COVID-19. Secondary end points included PCR evidence of infection with other respiratory viruses (Supplement Table 2).
Sample Size Calculations
The sample size was determined to provide adequate power for assessment of the combined composite primary outcome in the intention-to-treat analysis. Authorities estimated an incidence of SARS-CoV-2 infection of at least 2% during the study period. Assuming that wearing a face mask halves risk for infection, we estimated that a sample of 4636 participants would provide the trial with 80% power at a significance level of 5% (2-sided α level). Anticipating 20% loss to follow-up in this community-based study, we aimed to assign at least 6000 participants.
Statistical Analysis
Participants with a positive result on an antibody test at baseline were excluded from the analyses. We calculated CIs of proportions assuming binomial distribution (Clopper–Pearson).
The primary composite outcome (intention-to-treat) was compared between groups using the χ2 test. Odds ratios and confidence limits were calculated using logistic regression. We did a per protocol analysis that included only participants reporting complete or predominant use of face masks as instructed. A conservative sensitivity analysis assumed that participants with a positive result on an antibody test at the end of the study who had not provided antibody test results at study entrance had had a positive result at entrance. To further examine the uncertainty of loss to follow-up, we did (post hoc) 200 imputations using the R package smcfcs, version 1.4.1 (29), to impute missing values of outcome. We included sex, age, type of work, time out of home, and outcome in this calculation.
Prespecified subgroups were compared by logistic regression analysis. In a post hoc analysis, we explored whether there was a subgroup defined by a constellation of participant characteristics for which a recommendation to wear masks seemed to be effective. We included sex, age, type of work, time out of home, and outcome in this calculation.
Two-sided P values less than 0.05 were considered statistically significant. Analyses were done using R, version 3.6.1 (R Foundation).
Role of the Funding Source
An unrestricted grant from the Salling Foundations supported the study, and the BESTSELLER Foundation donated the Livzon tests. The funders did not influence study design, conduct, or reporting.
Results
Participants
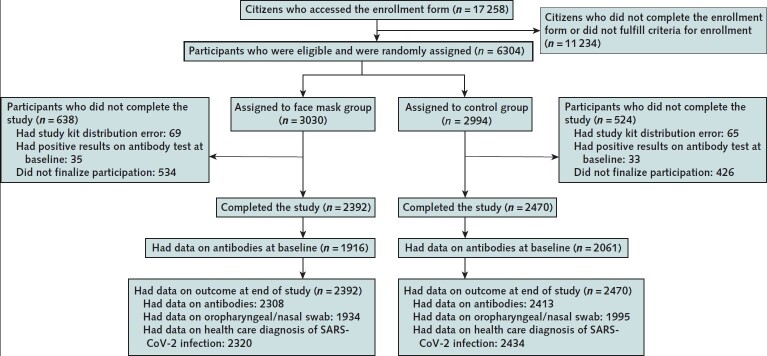
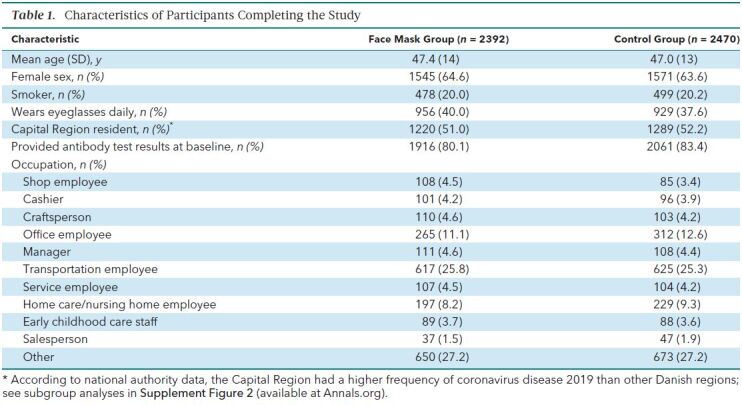
A total of 17 258 Danish citizens responded to recruitment, and 6024 completed the baseline survey and fulfilled eligibility criteria. The first participants (group 1; n = 2995) were randomly assigned on 12 April 2020 and were followed from 14 to 16 April through 15 May 2020. Remaining participants (group 2; n = 3029) were randomly assigned on 24 April 2020 and were followed from 2 to 4 May through 2 June 2020. A total of 3030 participants were randomly assigned to the recommendation to wear face masks, and 2994 were assigned not to wear face masks (Figure); 4862 participants (80.7%) completed the study. Table 1 shows baseline characteristics, which were well balanced between groups. Participants reported having spent a median of 4.5 hours per day outside the home.
Figure. Study flow diagram.
Inclusion and exclusion criteria are described in the Methods section, and criteria for completion of the study are given in the Supplement. SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
Table 1. Characteristics of Participants Completing the Study.
Adherence
Based on the lowest adherence reported in the mask group during follow-up, 46% of participants wore the mask as recommended, 47% predominantly as recommended, and 7% not as recommended.
Primary Outcome
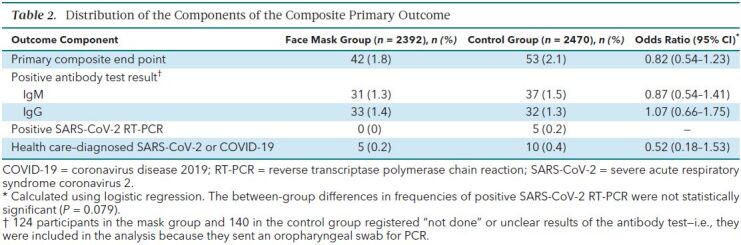
The primary outcome occurred in 42 participants (1.8%) in the mask group and 53 (2.1%) in the control group. In an intention-to-treat analysis, the between-group difference was −0.3 percentage point (CI, −1.2 to 0.4 percentage point; P = 0.38) (odds ratio [OR], 0.82 [CI, 0.54 to 1.23]; P = 0.33) in favor of the mask group (Supplement Figure 1). When this analysis was repeated with multiple imputation for missing data due to loss to follow-up, it yielded similar results (OR, 0.81 [CI, 0.53 to 1.23]; P = 0.32). Table 2 provides data on the components of the primary end point, which were similar between groups.
Table 2. Distribution of the Components of the Composite Primary Outcome.
In a per protocol analysis that excluded participants in the mask group who reported nonadherence (7%), SARS-CoV-2 infection occurred in 40 participants (1.8%) in the mask group and 53 (2.1%) in the control group (between-group difference, −0.4 percentage point [CI, −1.2 to 0.5 percentage point]; P = 0.40) (OR, 0.84 [CI, 0.55 to 1.26]; P = 0.40). Supplement Figure 2 provides results of the prespecified subgroup analyses of the primary composite end point. No statistically significant interactions were identified.
In the preplanned sensitivity analysis, those who had a positive result on an antibody test at 1 month but had not provided antibody results at baseline were considered to have had positive results at baseline (n = 18)—that is, they were excluded from the analysis. In this analysis, the primary outcome occurred in 33 participants (1.4%) in the face mask group and 44 (1.8%) in the control group (between-group difference, −0.4 percentage point [CI, −1.1 to 0.4 percentage point]; P = 0.22) (OR, 0.77 [CI, 0.49 to 1.22]; P = 0.26).
Three post hoc (not preplanned) analyses were done. In the first, which included only participants reporting wearing face masks “exactly as instructed,” infection (the primary outcome) occurred in 22 participants (2.0%) in the face mask group and 53 (2.1%) in the control group (between-group difference, −0.2 percentage point [CI, −1.3 to 0.9 percentage point]; P = 0.82) (OR, 0.93 [CI, 0.56 to 1.54]; P = 0.78). The second post hoc analysis excluded participants who did not provide antibody test results at baseline; infection occurred in 33 participants (1.7%) in the face mask group and 44 (2.1%) in the control group (between-group difference, −0.4 percentage point [CI, −1.4 to 0.4 percentage point]; P = 0.33) (OR, 0.80 [CI, 0.51 to 1.27]; P = 0.35). In the third post hoc analysis, which investigated constellations of patient characteristics, we did not find a subgroup where face masks were effective at conventional levels of statistical significance (data not shown).
A total of 52 participants in the mask group and 39 control participants reported COVID-19 in their household. Of these, 2 participants in the face mask group and 1 in the control group developed SARS-CoV-2 infection, suggesting that the source of most observed infections was outside the home. Reported symptoms did not differ between groups during the study period (Supplement Table 3).
Secondary Outcomes
In the mask group, 9 participants (0.5%) were positive for 1 or more of the 11 respiratory viruses other than SARS-CoV-2, compared with 11 participants (0.6%) in the control group (between-group difference, −0.1 percentage point [CI, −0.6 to 0.4 percentage point]; P = 0.87) (OR, 0.84 [CI, 0.35 to 2.04]; P = 0.71). Positivity for any virus, including SARS-CoV-2, occurred in 9 mask participants (0.5%) versus 16 control participants (0.8%) (between-group difference, −0.3 percentage point [CI, −0.9 to 0.2 percentage point]; P = 0.26) (OR, 0.58 [CI, 0.25 to 1.31]; P = 0.19).
Discussion
In this community-based, randomized controlled trial conducted in a setting where mask wearing was uncommon and was not among other recommended public health measures related to COVID-19, a recommendation to wear a surgical mask when outside the home among others did not reduce, at conventional levels of statistical significance, incident SARS-CoV-2 infection compared with no mask recommendation. We designed the study to detect a reduction in infection rate from 2% to 1%. Although no statistically significant difference in SARS-CoV-2 incidence was observed, the 95% CIs are compatible with a possible 46% reduction to 23% increase in infection among mask wearers. These findings do offer evidence about the degree of protection mask wearers can anticipate in a setting where others are not wearing masks and where other public health measures, including social distancing, are in effect. The findings, however, should not be used to conclude that a recommendation for everyone to wear masks in the community would not be effective in reducing SARS-CoV-2 infections, because the trial did not test the role of masks in source control of SARS-CoV-2 infection. During the study period, authorities did not recommend face mask use outside hospital settings and mask use was rare in community settings (22). This means that study participants' exposure was overwhelmingly to persons not wearing masks.
The observed infection rate was similar to that reported in other large Danish studies during the study period (26, 30). Of note, the observed incidence of SARS-CoV-2 infection was higher than we had estimated when planning a sample size that would ensure more than 80% power to detect a 50% decrease in infection. The intervention lasted only 1 month and was carried out during a period when Danish authorities recommended quarantine of diagnosed patients, physical distancing, and hand hygiene as general protective means against SARS-CoV-2 transmission (23). Cafés and restaurants were closed through 18 May, but follow-up of the second randomized group continued through 2 June.
The first randomized group was followed while the Danish society was under lockdown. Reopening occurred (18 May 2020) during follow-up of the second group of participants, but it was not reflected in the outcome because infection rates were similar between groups (Supplement Figure 2). The relative infection rate between mask wearers and those not wearing masks would most likely be affected by changes in applied protective means or in the virulence of SARS-CoV-2, whereas the rate difference between the 2 groups would probably not be affected solely by a higher—or lower—number of infected citizens.
Although we saw no statistically significant difference in presence of other respiratory viruses, the study was not sufficiently powered to draw definite conclusions about the protective effect of masks for other viral infections. Likewise, the study had limited power for any of the subgroup analyses.
The primary outcome was mainly defined by antibodies against SARS-CoV-2. This definition was chosen because the viral load of infected patients may be only transiently detectable (31, 32) and because approximately half of persons infected with SARS-CoV-2 are asymptomatic (33, 26). Masks have been hypothesized to reduce inoculum size (34) and could increase the likelihood that infected mask users are asymptomatic, but this hypothesis has been challenged (35). For these reasons, we did not rely solely on identification of SARS-CoV-2 in oropharyngeal/nasal swab samples. As mentioned in the Methods section, an internal validation study estimated that the point-of-care test has 82.5% sensitivity and 99.5% specificity (26).
The observed rate of incident SARS-CoV-2 infection was similar to what was estimated during trial design. These rates were based on thorough screening of all participants using antibody measurements combined with PCR, whereas the observed official infection rates relied solely on PCR test–based estimates during the period. In addition, authorities tested only a small subset of primarily symptomatic citizens of the entire population, yielding low incidence rates. On this basis, the infection rates we report here are not comparable with the official SARS-CoV-2 infection rates in the Danish population. The eligibility requirement of at least 3 hours of exposure to other persons outside the home would add to this difference. Between 6 April and 9 May 2020, we found a similar seroprevalence of SARS-CoV-2 of 1.9% (CI, 0.8% to 2.3%) in Danish blood donors using the Livzon point-of-care test and assessed by laboratory technicians (36). Testing at the end of follow-up, however, may not have captured any infections contracted during the last part of the study period, but this would have been true in both the mask and control groups and was not expected to influence the overall findings.
The face masks provided to participants were high-quality surgical masks with a filtration rate of 98% (37). A published meta-analysis found no statistically significant difference in preventing influenza in health care workers between respirators (N95 [American standard] or FFP2 [European standard]) and surgical face masks (38). Adherence to mask use may be higher than observed in this study in settings where mask use is common. Some mask group participants (14%) reported adverse reactions from other citizens (Supplement Table 4). Although adherence may influence the protective effect of masks, sensitivity analyses had similar results across reported adherence.
How SARS-CoV-2 is transmitted—via respiratory droplets, aerosols, or (to a lesser extent) fomites—is not firmly established. Droplets are larger and rapidly fall to the ground, whereas aerosols are smaller (≤5 μm) and may evaporate and remain in the air for hours (39). Transmission of SARS-CoV-2 may take place through multiple routes. It has been argued that for the primary route of SARS-CoV-2 spread—that is, via droplets—face masks would be considered effective, whereas masks would not be effective against spread via aerosols, which might penetrate or circumnavigate a face mask (37, 39). Thus, spread of SARS-CoV-2 via aerosols would at least partially explain the present findings. Lack of eye protection may also have been of importance, and use of face shields also covering the eyes (rather than face masks only) has been advocated to halt the conjunctival route of transmission (40, 41). We observed no statistically significant interaction between wearers and nonwearers of eyeglasses (Supplement Figure 2). Recent reports indicate that transmission of SARS-CoV-2 via fomites is unusual (42), but masks may alter behavior and potentially affect fomite transmission.
The present findings are compatible with the findings of a review of randomized controlled trials of the efficacy of face masks for prevention (as personal protective equipment) against influenza virus (18). A recent meta-analysis that suggested a protective effect of face masks in the non–health care setting was based on 3 observational studies that included a total of 725 participants and focused on transmission of SARS-CoV-1 rather than SARS-CoV-2 (12). Of 725 participants, 138 (19%) were infected, so the transmission rate seems to be higher than for SARS-CoV-2. Further, these studies focused on prevention of infection in healthy mask wearers from patients with a known, diagnosed infection rather than prevention of transmission from persons in their surroundings in general. In addition, identified comparators (control participants) not wearing masks may also have missed other protective means. Recent observational studies that indicate a protective association between mandated mask use in the community and SARS-CoV-2 transmission are limited by study design and simultaneous introduction of other public health interventions (14, 43).
Several challenges regarding wearing disposable face masks in the community exist. These include practical aspects, such as potential incorrect wearing, reduced adherence, reduced durability of the mask depending on type of mask and occupation, and weather. Such circumstances may necessitate the use of multiple face masks during the day. In our study, participants used a mean of 1.7 masks per weekday and 1.3 per weekend day (Supplement Table 4). Wearing a face mask may be physically unpleasant, and psychological barriers and other side effects have been described (44). “Face mask policing” between citizens might reinforce use of masks but may be challenging. In addition, the wearer of a face mask may change to a less cautious behavior because of a false sense of security, as pointed out by WHO (17); accordingly, our face mask group seemed less worried (Supplement Table 4), which may explain their increased willingness to wear face masks in the future (Supplement Table 5). These challenges, including costs and availability, may reduce the efficacy of face masks to prevent SARS-CoV-2 infection.
The potential benefits of a community-wide recommendation to wear masks include combined prevention and source control for symptomatic and asymptomatic persons, improved attention, and reduced potential stigmatization of persons wearing masks to prevent infection of others (17). Although masks may also have served as source control in SARS-CoV-2–infected participants, the study was not designed to determine the effectiveness of source control.
The most important limitation is that the findings are inconclusive, with CIs compatible with a 46% decrease to a 23% increase in infection. Other limitations include the following. Participants may have been more cautious and focused on hygiene than the general population; however, the observed infection rate was similar to findings of other studies in Denmark (26, 30). Loss to follow-up was 19%, but results of multiple imputation accounting for missing data were similar to the main results. In addition, we relied on patient-reported findings on home antibody tests, and blinding to the intervention was not possible. Finally, a randomized controlled trial provides high-level evidence for treatment effects but can be prone to reduced external validity.
Our results suggest that the recommendation to wear a surgical mask when outside the home among others did not reduce, at conventional levels of statistical significance, the incidence of SARS-CoV-2 infection in mask wearers in a setting where social distancing and other public health measures were in effect, mask recommendations were not among those measures, and community use of masks was uncommon. Yet, the findings were inconclusive and cannot definitively exclude a 46% reduction to a 23% increase in infection of mask wearers in such a setting. It is important to emphasize that this trial did not address the effects of masks as source control or as protection in settings where social distancing and other public health measures are not in effect.
Reduction in release of virus from infected persons into the environment may be the mechanism for mitigation of transmission in communities where mask use is common or mandated, as noted in observational studies. Thus, these findings do not provide data on the effectiveness of widespread mask wearing in the community in reducing SARS-CoV-2 infections. They do, however, offer evidence about the degree of protection mask wearers can anticipate in a setting where others are not wearing masks and where other public health measures, including social distancing, are in effect. The findings also suggest that persons should not abandon other COVID-19 safety measures regardless of the use of masks. While we await additional data to inform mask recommendations, communities must balance the seriousness of COVID-19, uncertainty about the degree of source control and protective effect, and the absence of data suggesting serious adverse effects of masks (45).
Supplementary Material
Footnotes
This article was published at Annals.org on 18 November 2020
References
- 1. Helmy YA , Fawzy M , Elaswad A , et al. The COVID-19 pandemic: a comprehensive review of taxonomy, genetics, epidemiology, diagnosis, treatment, and control. J Clin Med. 2020;9. [PMID: ] doi: 10.3390/jcm9041225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Worldometer. Pandemic, COVID-19 coronavirus. 2020. Accessed at www.worldometers.info/coronavirus on 29 October 2020.
- 3. Qualls N , Levitt A , Kanade N , et al. CDC Community Mitigation Guidelines Work Group. Community mitigation guidelines to prevent pandemic influenza — United States, 2017. MMWR Recomm Rep. 2017;66:1-34. [PMID: ] doi: 10.15585/mmwr.rr6601a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Richard M , Kok A , de Meulder D , et al. SARS-CoV-2 is transmitted via contact and via the air between ferrets. Nat Commun. 2020;11:3496. [PMID: ] doi: 10.1038/s41467-020-17367-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liu Y , Ning Z , Chen Y , et al. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. 2020;582:557-560. [PMID: ] doi: 10.1038/s41586-020-2271-3 [DOI] [PubMed] [Google Scholar]
- 6. van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1 [Letter]. N Engl J Med. 2020;382:1564-1567. [PMID: ] doi: 10.1056/NEJMc2004973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kwok YL , Gralton J , McLaws ML . Face touching: a frequent habit that has implications for hand hygiene. Am J Infect Control. 2015;43:112-4. [PMID: ] doi: 10.1016/j.ajic.2014.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lucas TL , Mustain R , Goldsby RE . Frequency of face touching with and without a mask in pediatric hematology/oncology health care professionals. Pediatr Blood Cancer. 2020:e28593. [PMID: ] doi: 10.1002/pbc.28593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen YJ , Qin G , Chen J , et al. Comparison of face-touching behaviors before and during the coronavirus disease 2019 pandemic. JAMA Netw Open. 2020;3:e2016924. [PMID: ] doi: 10.1001/jamanetworkopen.2020.16924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rebmann T , Carrico R , Wang J . Physiologic and other effects and compliance with long-term respirator use among medical intensive care unit nurses. Am J Infect Control. 2013;41:1218-23. [PMID: ] doi: 10.1016/j.ajic.2013.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang X , Ferro EG , Zhou G , et al. Association between universal masking in a health care system and SARS-CoV-2 positivity among health care workers. JAMA. 2020. [PMID: ] doi: 10.1001/jama.2020.12897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chu DK , Akl EA , Duda S , et al. COVID-19 Systematic Urgent Review Group Effort (SURGE) study authors. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet. 2020;395:1973-1987. [PMID: ] doi: 10.1016/S0140-6736(20)31142-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Leung NHL , Chu DKW , Shiu EYC , et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat Med. 2020;26:676-680. [PMID: ] doi: 10.1038/s41591-020-0843-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lyu W , Wehby GL . Community use of face masks and COVID-19: evidence from a natural experiment of state mandates in the US. Health Aff (Millwood). 2020;39:1419-1425. [PMID: ] doi: 10.1377/hlthaff.2020.00818 [DOI] [PubMed] [Google Scholar]
- 15. Centers for Disease Control and Prevention. CDC calls on Americans to wear masks to prevent COVID-19 spread. 14 July 2020. Accessed at www.cdc.gov/media/releases/2020/p0714-americans-to-wear-masks.html on 29 October 2020.
- 16. Brooks JT , Butler JC , Redfield RR . Universal masking to prevent SARS-CoV-2 transmission—the time is now. JAMA. 2020. [PMID: ] doi: 10.1001/jama.2020.13107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. World Health Organization. Advice on the use of masks in the context of COVID-19: interim guidance. 5 June 2020.
- 18. Xiao J , Shiu EYC , Gao H , et al. Nonpharmaceutical measures for pandemic influenza in nonhealthcare settings—personal protective and environmental measures. Emerg Infect Dis. 2020;26:967-975. [PMID: ] doi: 10.3201/eid2605.190994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Qaseem A , Etxeandia-Ikobaltzeta I , Yost J , et al. Use of N95, surgical, and cloth masks to prevent COVID-19 in health care and community settings: living practice points from the American College of Physicians (version 1). Ann Intern Med. 2020;173:642-649. doi: 10.7326/M20-3234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ford I , Norrie J . Pragmatic trials. N Engl J Med. 2016;375:454-63. [PMID: ] doi: 10.1056/NEJMra1510059 [DOI] [PubMed] [Google Scholar]
- 21. Bundgaard H , Bundgaard JS , Raaschou-Pedersen DET , et al. Face masks for the prevention of COVID-19 - rationale and design of the randomised controlled trial DANMASK-19. Dan Med J. 2020;67. [PMID: ] [PubMed] [Google Scholar]
- 22. YouGov. Personal measures taken to avoid COVID-19. 2020. Accessed at https://yougov.co.uk/topics/international/articles-reports/2020/03/17/personal-measures-taken-avoid-covid-19 on 27 October 2020.
- 23. Danish Health Authority. COVID-19: prevention of the spread of infection. 2 October 2020. Accessed at www.sst.dk/da/Udgivelser/2020/COVID-19-Forebyggelse-af-smittespredning on 26 October 2020.
- 24. Danish Health Authority. General guidance. 22 October 2020. Accessed at www.sst.dk/en/English/Corona-eng/Prevent-infection/General-guidance on 27 October 2020.
- 25. Harris PA , Taylor R , Thielke R , et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-81. [PMID: ] doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Iversen K , Bundgaard H , Hasselbalch RB , et al. Risk of COVID-19 in health-care workers in Denmark: an observational cohort study. Lancet Infect Dis. 2020. [PMID: ] doi: 10.1016/S1473-3099(20)30589-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Therchilsen JH, von Buchwald C, Koch A, et al. Self-collected versus healthcare worker-collected swabs in the diagnosis of severe acute respiratory syndrome coronavirus 2. Diagnostics. 2020;10:678. doi:10.3390/diagnostics10090678 [DOI] [PMC free article] [PubMed]
- 28. Tu YP , Jennings R , Hart B , et al. Swabs collected by patients or health care workers for SARS-CoV-2 testing [Letter]. N Engl J Med. 2020;383:494-496. [PMID: ] doi: 10.1056/NEJMc2016321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bartlett J, Keogh R, Ekstrøm CT, et al. Multiple imputation of covariates by substantive model compatible fully conditional specification. Version 1.4.1. CRAN; 2020.
- 30. Pedersen OB , Nissen J , Dinh KM , et al. SARS-CoV-2 infection fatality rate among elderly retired Danish blood donors - a cross-sectional study. Clin Infect Dis. 2020. [PMID: ] doi: 10.1093/cid/ciaa1627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pan Y , Zhang D , Yang P , et al. Viral load of SARS-CoV-2 in clinical samples [Letter]. Lancet Infect Dis. 2020;20:411-412. [PMID: ] doi: 10.1016/S1473-3099(20)30113-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wölfel R , Corman VM , Guggemos W , et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465-469. [PMID: ] doi: 10.1038/s41586-020-2196-x [DOI] [PubMed] [Google Scholar]
- 33. Oran DP , Topol EJ . Prevalence of asymptomatic SARS-CoV-2 infection. A narrative review. Ann Intern Med. 2020;173:362-367. doi: 10.7326/M20-3012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gandhi M , Rutherford GW . Facial masking for covid-19. Reply [Letter]. N Engl J Med. 2020;383. [PMID: ] doi: 10.1056/NEJMc2030886 [DOI] [PubMed] [Google Scholar]
- 35. Rasmussen AL , Escandón K , Popescu SV . Facial masking for covid-19 [Letter]. N Engl J Med. 2020;383. [PMID: ] doi: 10.1056/NEJMc2030886 [DOI] [PubMed] [Google Scholar]
- 36. Erikstrup C , Hother CE , Pedersen OBV , et al. Estimation of SARS-CoV-2 infection fatality rate by real-time antibody screening of blood donors. Clin Infect Dis. 2020. [PMID: ] doi: 10.1093/cid/ciaa849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rengasamy S , Miller A , Eimer BC , et al. Filtration performance of FDA-cleared surgical masks. J Int Soc Respir Prot. 2009;26:54-70. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
- 38. Long Y , Hu T , Liu L , et al. Effectiveness of N95 respirators versus surgical masks against influenza: a systematic review and meta-analysis. J Evid Based Med. 2020;13:93-101. [PMID: ] doi: 10.1111/jebm.12381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Klompas M , Baker MA , Rhee C . Airborne transmission of SARS-CoV-2: theoretical considerations and available evidence. JAMA. 2020;324:441-442. [PMID: ] doi: 10.1001/jama.2020.12458 [DOI] [PubMed] [Google Scholar]
- 40. Perencevich EN , Diekema DJ , Edmond MB . Moving personal protective equipment into the community: face shields and containment of COVID-19. JAMA. 2020;323:2252-2253. [PMID: ] doi: 10.1001/jama.2020.7477 [DOI] [PubMed] [Google Scholar]
- 41. Marra AR , Edmond MB , Popescu SV , et al. Examining the need for eye protection for coronavirus disease 2019 (COVID-19) prevention in the community. Infect Control Hosp Epidemiol. 2020:1-2. [PMID: ] doi: 10.1017/ice.2020.314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Meyerowitz EA , Richterman A , Gandhi RT , et al. Transmission of SARS-CoV-2. A review of viral, host, and environmental factors. Ann Intern Med. 2020. doi: 10.7326/M20-5008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mitze T, Kosfeld R, Rode J, et al. Face masks considerably reduce COVID-19 cases in Germany: a synthetic control method approach. IZA Discussion Papers. June 2020. [DOI] [PMC free article] [PubMed]
- 44. Lazzarino AI , Steptoe A , Hamer M , et al. Covid-19: important potential side effects of wearing face masks that we should bear in mind [Letter]. BMJ. 2020;369:m2003. [PMID: ] doi: 10.1136/bmj.m2003 [DOI] [PubMed] [Google Scholar]
- 45. Javid B , Weekes MP , Matheson NJ . Covid-19: should the public wear face masks? [Editorial]. BMJ. 2020;369:m1442. [PMID: ] doi: 10.1136/bmj.m1442 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.