Abstract
We investigated humoral immune response to influenza A(H1N1)pdm infection and found 32 (22%) of the infected individuals identified by PCR failed to produce a ≥4-fold hemagglutinin inhibition assay (HAI) response; a subset of 18 (56%) produced an alternate antibody response (against full-length HA, HA stalk, or neuraminidase). These individuals had lower pre-existing HAI antibody titers and showed a pattern of milder illness. An additional subset of 14 (44%) did not produce an alternate antibody response, had higher pre-existing antibody titers against full-length & stalk HA, and were less sick. These findings demonstrate that some individuals mount an alternate antibody response to influenza infection. In order to design more broadly protective influenza vaccines it may be useful to target these alternate sites. These findings support that there are influenza cases currently being missed by solely implementing HAI assays, resulting in an underestimation of the global burden of influenza infection.
Keywords: influenza, antibodies, neuraminidase, hemagglutination inhibition
As part of an ongoing effort to improve influenza vaccines and develop our understanding of the dynamics of the immune response to infection, there is a great deal of interest in investigating alternate correlates of protection against influenza [1, 2].
The influenza virus has two surface glycoproteins; hemagglutinin (HA) and neuraminidase (NA) [3]. Most individuals experience a strong hemagglutination-inhibition (HAI) response to infection with influenza virus, which is currently the only generally accepted correlate of protection for influenza [3–5]. There is variation in response levels, however, and some individuals do not produce a strong HAI antibody response to infection [6].
Importantly, HAI only measures a subset of antibodies that target the HA head. Additional antibody responses can be captured by using enzyme-linked immunosorbent assays (ELISA) against HA stalk region, full-length HA protein, and NA [2,3,7]. These regions are all potential universal influenza vaccine targets, due to their conserved nature and impact on virus fitness and spread [2, 4]. Here we assess whether individuals with a limited HAI response after natural influenza infection produce alternate immune responses to the HA stalk, full-length HA, or NA, and examine how these atypical responders differ from those presenting a typical HAI response to infection.
Materials and Methods
Study Design
To investigate the immune response patterns to HAI and potential alternate correlates of protection, a case-ascertainment study of naturally occurring influenza virus transmission was performed in households in Managua, Nicaragua. Study design has been previously described [7, 8]. Subjects provided daily symptom assessment, and respiratory swabs (nasal and oropharyngeal) were taken every 2–3 days over a 10–14 day period. Blood samples were collected at enrollment and 3–5 weeks later. Households eligible for inclusion in the study were those with ≥2 individuals and an index case that experienced acute respiratory infection (ARI) symptom onset within 48 hours and tested positive for influenza. For this analysis, 66 RT-PCR confirmed influenza A(H1N1)pdm index cases from the 2013 and 2015 influenza seasons and their 423 household contacts were considered. 123 participants were excluded due to absence of paired blood samples for testing, resulting in a final analysis group of 366 individuals. This study received ethical approval from the institutional review boards at the Ministry of Health of Nicaragua and the University of Michigan. Informed consent was collected for all participants and verbal assent obtained from children ≥6 years.
Laboratory Methods
Respiratory samples were tested at the Nicaraguan National Virology Laboratory via real-time RT-PCR following U.S. Centers for Disease Controls and Prevention protocols. Samples were tested for influenza A virus; positive samples were then subtyped as H1N1 or H3N2, with RT-PCR for both universal A and subtype repeated for initially unsubtypable samples to reduce probability of false positive. Hemagglutinin inhibition assays were conducted to measure HAI titers; ELISAs were performed to measure anti-HA stalk, full-length HA, and NA antibodies as previously described [7]. Full-length recombinant HA constructs corresponded to vaccine strains from the respective seasons (2013: H1 A/California/4/09, 2015: H1 A/Michigan/45/15) were used. To measure HA stalk antibodies, a recombinant chimeric HA with the head domain from an H6 HA (A/mallard/Sweden/81/02) and a stalk domain from A/California/4/09 was used (cH6/1); to measure NA antibodies, a recombinant NA of A/California/4/09 was used [7].
Statistical Analysis
The main outcomes of this study were PCR-confirmed influenza virus infection, seroconversion by HAI (defined as a ≥4-fold rise in antibody titer), and the ratio of antibody response comparing the post- and pre-infection measurements for HA stalk, full-length HA, and NA antibodies. “HAI responders” were defined as individuals with PCR-confirmed influenza virus infection and a ≥4-fold rise in HAI titer. “HAI non-responders” were defined as individuals with PCR-confirmed influenza infection who did not exhibit a ≥4-fold rise in HAI titer. “Alternate responders” were defined as “HAI non-responders” who had a ≥4-fold response to full-length HA, HA stalk and/or NA. All definitions were established prior to the statistical analysis of the data. While there is some debate in the literature as to whether a 2-fold response to HAI can truly be considered non-response, we elected to define our seroconversions by the currently accepted standard of a fold rise [9]. Chi-squared analyses, t-tests, and ANOVA modeling were used to compare groups. Statistical analyses were conducted using SAS. Graphics were constructed in R using the packages ggplot, plotly, and reshape2.
Results
Study Population
Among the total study population (n=366), 149 (41%) individuals experienced PCR-confirmed influenza virus infection and 147 (40%) individuals had serological evidence of infection using traditional criteria of a ≥4-fold rise in HAI titer (Table S1). Antibody levels (both pre- and post-exposure) were more strongly correlated between HAI, full-length HA, HA stalk, and NA among children than among adults (Fig. S1). HAI, full-length HA, and HA stalk antibodies were most strongly correlated irrespective of age category or exposure timing. Among both children and adults, NA titer correlation to HAI and full-length HA increased post-exposure; among children the correlation between NA titer and HA stalk also increased while among adults correlation slightly decrease. Children displayed a higher proportion of HAI responders as well as increased correlation of HA stalk and NA compared to adults.
Characteristics of HAI responders
A total of 117 (79%) PCR-positive participants experienced a ≥4-fold HAI antibody titer rise. Among these “HAI responders”, 52 (44%) were male, 79 (68%) were children (defined as ≤14 years of age), and 95 (81%) exhibited a symptom profile for influenza-like illness (ILI) (Table 1). 39% exhibited ≥4-fold rise in anti-HA stalk antibodies, 51% exhibited ≥4-fold rise in anti-full-length HA antibodies, and 32% exhibited ≥4-fold rise in anti-NA antibodies (Table S2).
Table 1.
HAI Responder versus Non-Responder Characteristics
| PCR-positive HAI respondera (n = 117) | PCR-positive HAI non-responderb (n=32) | P-value | |
|---|---|---|---|
| Male | 52 (44%) | 14 (44%) | 0.94 |
| Age | 0.39 | ||
| 0–14 years | 79 (68%) | 19 (59%) | |
| 15+ years | 38 (32%) | 13 (41%) | |
| Symptoms | |||
| Feverc | 99 (85%) | 22 (69%) | <0.05 |
| ILId | 95 (81%) | 20 (65%) | <0.01 |
| ARIe | 108 (92%) | 23 (72%) | <0.05 |
| Index case | 55 (44%) | 10 (31%) | 0.11 |
| Symptom duration (mean days, SD) | |||
| Cough | 7.39 (3.54) | 5.83 (4.17) | <0.05 |
| Shedding | 4.23 (3.07) | 3.28 (2.50) | 0.11 |
Data are no. individuals witd characteristic/no. of individuals.
Abbreviations: ILI, influenza-like illness; ARI, acute respiratory infection; SD, standard deviation.
HAI responder is an individual who generated a 4-fold or greater HAI response.
HAI non-responder is an individual who failed to generate a 4-fold or greater HAI response.
Fever is reported fever or measured temperature ≥ 37.5 °C.
ILI is fever as described above plus eitder cough or sore tdroat.
ARI is defined as any of tde following symptoms: fever, cough, sore tdroat, runny nose.
Characteristics of HAI non-responders
The “HAI non-responders” group consisted of 18 (56%) individuals who had a ≥4-fold response against HA stalk, full-length HA, and/or NA, hereafter termed “alternate responder”, and 14 (44%) individuals who had ≤4 fold response, hereafter termed “no response”. Among the “alternate responder” group, 6 (33%) were male, 12 (67%) were children, and 13 (72%) exhibited a symptom profile for ILI (Table 2). Among this “alternate responder” population, 11 (61%) exhibited ≥4-fold antibody response to HA stalk, 14 (78%) exhibited ≥4-fold antibody response to full-length HA, and 4 (22%) exhibited ≥4-fold response to NA (Table S2). Among the “no response” group, 8 (57%) were male, 7 (50%) were children, and 7 (50%) exhibited a symptom profile for ILI (Table 2).There was no evidence of differences by age (p=0.44), sex (p=0.96), or symptoms (p=0.14) between those who developed an alternate antibody response and those who did not, although these comparisons are limited in power by the sample size of n=32 (Table S3).
Table 2.
HAI Responders versus Alternate responders versus No response
| PCR-positive HAI respondersa (n=117) | PCR-positive HAI non-respondersa with alternate responseb (n=18) | PCR-positive No response (n=14) | P-value | |
|---|---|---|---|---|
| Male | 52 (44%) | 6 (33%) | 8 (57%) | 0.4037 |
| Age | 0.4248 | |||
| 0–14 years | 79 (68%) | 12 (67%) | 7 (50%) | |
| 15+ years | 38 (32%) | 6 (33%) | 7 (50%) | |
| Symptoms | ||||
| Feverc | 99 (85%) | 13 (72%) | 9 (64%) | 0.1070 |
| ILId | 95 (81%) | 13 (72%) | 7 (50%) | 0.0274 |
| ARIe | 108 (92%) | 15 (83%) | 8 (57%) | 0.0006 |
| Illness duration (mean days, SD) | ||||
| Cough | 7.39 (3.54) | 6.14 (4.00) | 5.41 (4.49) | 0.0933 |
| Shedding | 4.23 (3.07) | 3.72 (2.87) | 2.71 (1.90) | 0.1732 |
| Pre-exposure antibody levels (mean level, SD) | ||||
| HAI | 11.41 (16.28) | 5.27 (1.18) | 19.23 (28.64) | 0.0757 |
| HA full | 41.59 (71.69) | 38.45 (101.14) | 104.78 (99.81) | 0.0173 |
| HA stalk | 36.57 (54.96) | 27.21 (47.57) | 116.75 (138.87) | 0.0001 |
| NA | 13.11 (19.44) | 9.90 (8.34) | 23.93 (31.88) | 0.1131 |
Data are no. individuals with characteristic/no. of individuals.
Abbreviations: ILI, influenza-like illness; ARI, acute respiratory infection; SD, standard deviation; HAI, hemagglutinin inhibition assay; HA, hemagglutinin; NA, neuraminidase.
HAI non-responder is an individual who failed to generate a 4-fold or greater HAI response; HAI responder is an individual who generated a 4-fold or greater HAI response.
Alternate response is a 4-fold or greater response to HA full, HA stalk, or NA.
Fever is reported fever or measured temperature ≥ 37.5 °C.
ILI is fever as described above plus either cough or sore throat.
ARI is defined as any of the following symptoms: fever, cough, sore throat, runny nose.
HAI non-responders compared to HAI responders
Among PCR-positive individuals, 32 (22%) did not exhibit a ≥4-fold HAI antibody response. “HAI non-responders” did not differ significantly from “HAI responders” by age (p=0.3894), sex (p=0.94), or index patient status (p=0.11) (Table 1). “HAI non-responders” were significantly less sick then “HAI responders”, as they were less likely to report fever (p<0.05), be classified as an ILI (p<0.01) or ARI episode (p<0.05; Table 1). There was a significant difference in coughing duration (Table 1). “HAI non-responders” also had lower fold-change antibody titers to full-length HA and NA than “HAI responders” across all age groups (Fig. S2a, S2c). The HA stalk response was similar for “HAI responders” and “HAI non-responders” under 40 years of age (Fig. S2b).
No response individuals versus HAI and alternate responders
When this analysis was further subset into three groups (“HAI responders”, “alternate responders”, “no response”), significant patterns emerged. There was a difference in both symptoms and pre-exposure antibody levels between the “no response” population and the HAI and alternate responders. “No response” individuals were less ill than HAI responders and alternate responders, with only 64% exhibiting fever (p-value = 0.1070), 50% exhibiting ILI (p-value 0.0274), and 57% exhibiting ARI (p-value = 0.0006). “No response” individuals also had significantly higher levels of pre-existing full-length HA (mean titer 104.78, p-value 0.0173) and stalk antibodies (mean titer 116.75, p-value 0.0001) than HAI responders or alternate responders. There was no significant difference in age or sex amongst the three groups.
Alternate responders compared to HAI responders
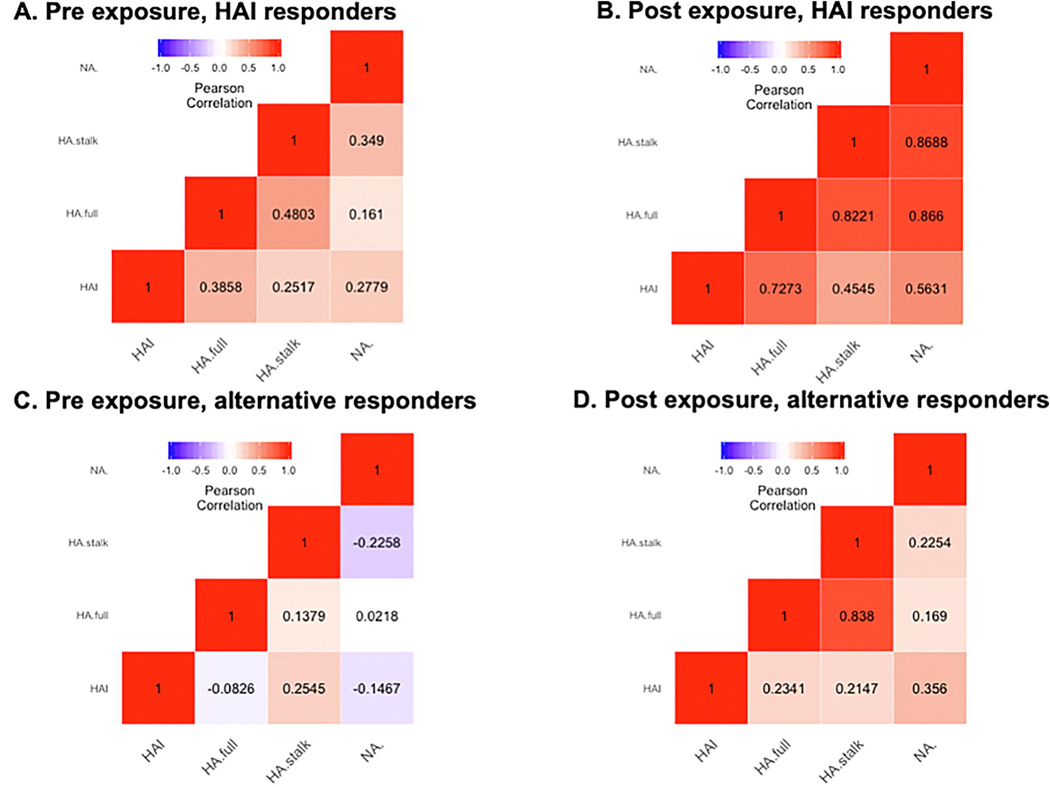
The “alternate responders” were less symptomatic and had shorter duration of symptoms than “HAI responders”, but otherwise did not differ (Table S4). There was no significant difference in pre-exposure antibody levels between the “alternate responders” and the “HAI responders” except for the HAI pre-titer (Table S4). Among the “alternate responders”, only 1 (6%) had a detectable HAI pre-titer; among the “HAI responders”, 30 (26%) had a detectable HAI pre-titer. Antibody titers (both pre- and post-exposure) had lower correlation on average among “alternate responders” than among “HAI responders” (Fig. 1). In general, these “alternate responders” had a pattern of increased correlation in their post-exposure titers, similar to the magnitude of change in correlation pre- and post-exposure among the “HAI responders”. To visually examine potential patterns in antibody responses, individuals were mapped onto a 3D plot reflecting their relative responses to each of the antibodies (Fig. S3). “Alternate responders” varied in their antibody response and did not exhibit a consistent pattern outside of their low HAI response compared to “HAI responders”. To display the distribution of how “alternate responders” overlap each other, a pie chart was created (Fig. S4); the greatest percentage responded to both HA stalk and full HA (34%), followed by those who responded only to full HA (19%) or only NA (19%). We assessed whether alternate response patterns among the “HAI responders” made any additional benefits (Table S5) and found that HAI responders who also produced an “alternate response” experienced more severe symptoms (p-value 0.0193) and were more likely to be under 15 years of age (p-value 0.0035) and male (p-value 0.0272). A tree diagram depicting each subpopulation analysis presented across tables in the study has been included in the Supplemental Materials (Fig. S5).
Figure 1. Pee-exposure and post-exposure correlation plots among HAI responders & alternative responders.
Correlation of antibody levels as measured by Pearson correlation coefficient, comparing pre (A,C) and post (B,D) exposure levels for both HAI responders and non-responders, Brighter colors indicate greater correlation, Red colors indicate positive correlation, purple colors indicate negative correlation, HA = hemagglutinin, NA = neuraminidase, HAI = hemagglutination inhibition assay.
Discussion
Here we found that within the overall population of influenza A(H1N1)pdm PCR-positive individuals, there are those who produce a ≥4-fold HAI response (“HAI responders”) but also a sub-population that fails to produce the ≥4-fold rise typically associated with infection (“HAI non-responders”). These “HAI non-responders” were less sick and had shorter symptom duration than the “HAI responders”. Within this “HAI non-responder” population, there were individuals who produced a ≥4-fold response to one of the alternate antibody targets (HA, HA stalk, NA), who we designated as “alternate responders”. These “alternate responders” did not differ significantly from the “HAI responders” by any individual variables but did display an overall pattern of less severe symptoms. These findings broadly provide evidence that some individuals with limited HAI response after influenza virus infection produce alternate immune responses.
We also identified individuals who were confirmed positive for influenza by PCR but exhibited no serologic response to infection. Notably, this population had higher pre-existing HAI titers and higher pre-existing full-length and stalk HA antibody titers. The fact that these antibody markers have been associated with protection [6,7] might explain why this population tends to exhibit less symptoms that the “HAI responders” or the “alternate responders”. Alternatively, the level of influenza exposure among those individuals may have been too weak to induce any immune response. Prior exposure to influenza could have an impact on whether individuals exhibit an alternate response to subsequent infection as it has been well-established in the literature that memory B cells produced in response to prior infection have an advantage generating antibodies over naive B cells [10].
These findings are consistent with prior research which identified alternative responses to influenza infection [6, 11]. Additionally, the level of HAI non-response in this study is similar to other studies [5, 12–13]. While there have been studies identifying non-responders to HAI [6], and studies highlighting alternate correlates of protection that may be used in assessing immune response to influenza [7, 14–15], there has not been an extensive examination of the overlap between these two areas. What these findings demonstrate is that among the previously identified population of those with limited response to HAI, there is a sub-population responding meaningfully to one of the alternate markers of infection previously identified in other studies. With regards to the differential results between adults and children, while it is known that HA stalk and NA antibody levels increase with age, children exhibiting higher correlation in this analysis doesn’t indicate that their actual antibody levels are higher, so this finding does not contradict the established literature [12,13]. Strengths of this study include the intensive nature of follow-up. Limitations include the fairly small number of non-responders identified, decreasing the statistical power of the study; we also did not assess any potential contribution of cellular immune responses.
Prior studies have identified HA stalk and NA as alternative and independent correlates of protection for influenza and suggested that inclusion of these correlates could result in development of a more robustly protective influenza vaccine [14–18]. In particular, Huang et al. found in a cohort study in New Zealand that among those infected with influenza, nearly a third only seroconverted in response to NA, not hemagglutinin [18]. Their work highlights the importance of considering other antigen responses in determining the overall burden of influenza, as they noted distinct patterns of NA-only response based on age and virus type; within their study, children under 5 and those infected with influenza B were significantly more likely to experience only NAI seroconversion. Our work expands on these findings by including assays for HA stalk and full HA (in addition to HAI and NA), and examining these associations in response to influenza A(H1N1)pdm, which the Huang study was unable to do as they were limited to a single influenza season with low H1N1 circulation. We find that in addition to individuals responding to NA, there are also influenza-infected individuals responding to the HA stalk or other regions of the HA not detected by traditional HAI.
Our findings support the conclusions of prior studies in this area and suggest that including these alternative correlates as serological markers of infection would allow us to capture influenza cases currently being missed by the serology standard of HAI, which results in both an underestimation of influenza infections and a bias in cases identified. Additionally, these findings indicate that designing influenza vaccines to elicit immune responses such as those that occur in the “alternate responders”, may result in broader protection for the general population and that HAI antibodies are not required to clear influenza infection in all individuals.
Supplementary Material
Highlights.
Not all influenza-infected people generate hemagglutination inhibition antibodies
A subset of individuals respond exclusively to alternate viral targets of infection
Including neuraminidase and hemagglutinin stalk could improve the influenza vaccine
Acknowledgments
Financial Support
This work was supported by the National Institute for Allergy and Infectious Diseases (award no. R01 AI120997 to A.G. and contract nos. HHSN272201400008C to F.K. and HHSN272201400006C to A.G.)
We thank the many dedicated study personnel in Nicaragua at the Centro Nacional de Diagnóstico y Referencia and the Sócrates Flores Vivas Health Center.
Footnotes
Conflicts of interest
All authors report no conflicts of interest.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lee VJ, Ho ZJ, Goh EH, Campbell H, Cohen C, Cozza V, et al. Advances in measuring influenza burden of disease. Influenza Other Respi Viruses 2018; 12:3–9. doi: 10.1111/irv.12533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Erbelding EJ, Post DJ, Stemmy EJ, Roberts PC, Augustine AD, Ferguson S, et al. A universal influenza vaccine: the strategic plan for the National Institute of Allergy and Infectious Diseases. The Journal of Infectious Diseases 2018; 218(3), 347–54. doi: 10.1093/infdis/jiy103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reber A, Katz J. Immunological assessment of influenza vaccines and immune correlates of protection. Expert Review of Vaccines 2013; 12(5), 519–36, doi: 10.1586/erv.13.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jacobsen H, Rajendran M, Choi A, Sjursen H, Brokstad KA, Cox RJ, et al. Influenza virus hemagglutinin stalk-specific antibodies in human serum are a surrogate marker for in vivo protection in a serum transfer mouse challenge model. MBio 2017; 8. doi: 10.1128/mBio.01463-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katz JM, Hancock K, Xu X. Serologic assays for influenza surveillance, diagnosis and vaccine evaluation. Expert Review of Anti-infective Therapy 2011; 9(6), 669–83. doi: 10.1586/eri.11.51 [DOI] [PubMed] [Google Scholar]
- 6.Hermans D, Webby RJ, Wong S. Atypical antibody responses to influenza. Journal of Thoracic Disease 2018; 10(S9). doi: 10.21037/jtd.2017.12.122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ng S, Nachbagauer R, Balmaseda A, Stadlbauer D, Ojeda S, Patel M, et al. Novel correlates of protection against pandemic H1N1 influenza A virus infection. Nature Medicine 2019; 25, 962–7. doi: 10.1038/s41591-019-0463-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gordon A, Tsang TK, Cowling BJ, Kuan G, Ojeda S, Sanchez N, et al. Influenza transmission dynamics in urban households, Managua, Nicaragua, 2012–2014. Emerging Infectious Diseases 2018; 24(10), 1882–88. doi: 10.3201/eid2410.161258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cauchemez S, Horby P, Fox A, Mai LQ, Thanh LT, Thai PQ, et al. Influenza infection rates, measurement errors and the interpretation of paired serology. PLoS Pathog 2012; 8:e1003061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Waffarn EE, & Baumgarth N Protective B cell responses to flu--no fluke!. Journal of immunology 2011; 186(7), 3823–3829. doi: 10.4049/jimmunol.1002090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tete SM, Krammer F, Lartey S, Bredholt G, Wood J, Skrede S, Cox RJ. Dissecting the hemagglutinin head and stalk-specific IgG antibody response in healthcare workers following pandemic H1N1 vaccination. Npj Vaccines 2016; 1(1). doi: 10.1038/npjvaccines.2016.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mcelhaney JE, Ewen C, Zhou Kane KP, Xie D, Hager WD, et al. Granzyme B: Correlates with protection and enhanced CTL response to influenza vaccination in older adults. Vaccine 2009; 27(18), 2418–25. doi: 10.1016/j.vaccine.2009.01.136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forrest BD, Pride MW, Dunning AJ, Capeding MR, Chotpitayasunondh T, Tam JS, et al. Correlation of cellular immune responses with protection against culture-confirmed influenza virus in young children. Clinical and Vaccine Immunology 2008; 15(7), 1042–53. doi: 10.1128/cvi.00397-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christensen SR, Toulmin SA, Griesman T, Lamerato LE, Petrie JG, Martin ET, et al. Assessing the protective potential of H1N1 influenza virus hemagglutinin head and stalk antibodies in humans. Journal of Virology 2019; 93(8). doi: 10.1128/jvi.02134-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Couch RB, Atmar RL, Franco LM, Quarles JM, Wells J, Arden N, et al. Antibody correlates and predictors of immunity to naturally occurring influenza in humans and the importance of antibody to the neuraminidase. The Journal of Infectious Diseases 2013; 207(6), 974–981. doi: 10.1093/infdis/jis935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krammer F, Fouchier R, Eichelberger M, Webby RJ, Shaw-Saliba K, Wan H, et al. NAction! how can neuraminidase-based immunity contribute to better influenza virus vaccines? mBio 2018; 9 (2) e02332–17. doi: 10.1128/mBio.02332-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zarnitsyna VI, Lavine J, Ellebedy A, Ahmed R, Antia R. Multi-epitope models explain how pre-existing antibodies affect the generation of broadly protective responses to Influenza. PLoS pathogens 2016; 12(6), e1005692. doi: 10.1371/journal.ppat.1005692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang QS, Bandaranayake D, Wood T, Newbern EC, Seeds R, Ralston J, et al. Southern Hemisphere Influenza and Vaccine Effectiveness Research and Surveillance (SHIVERS) Investigation Team, Risk Factors and Attack Rates of Seasonal Influenza Infection: Results of the SouthernHemisphere Influenza and Vaccine Effectiveness Research and Surveillance (SHIVERS) Seroepidemiologic Cohort Study. The Journal of Infectious Diseases 2019; 219(3), 347–57. doi: 10.1093/infdis/jiy443 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.